SUMMARY
iNKT cells are innate T lymphocytes recognizing endogenous and foreign lipid antigens presented in the MHC-like molecule CD1d. The semi-invariant iNKT cell TCR can detect certain bacterial and parasitic lipids, and drive iNKT cell responses. How iNKT cells respond to fungi, however, is unknown. We found that CD1d-deficient mice, which lack iNKT cells, poorly control infection with the fungal pathogen Aspergillus fumigatus. Furthermore, A. fumigatus rapidly activates iNKT cells in vivo and in vitro in the presence of APCs. Surprisingly, despite a requirement for CD1d recognition, the anti-fungal iNKT cell response does not require fungal lipids. Instead, Dectin-1 and MyD88-mediated responses to β-1,3 glucans, major fungal cell-wall polysaccharides, trigger IL-12 production by APCs that drives self-reactive iNKT cells to secrete IFN-γ. Innate recognition of β-1,3 glucans also drives iNKT cell responses against Candida, Histoplasma and Alternaria, suggesting that this mechanism may broadly define the basis for anti-fungal iNKT cell responses.
INTRODUCTION
Invariant Natural Killer T (iNKT) cells are a subset of innate-like T lymphocytes recognizing lipid antigens presented in the non-polymorphic MHC-like molecule CD1d (Cohen et al., 2009). Like other innate lymphocytes, iNKT cells are self-reactive and express a semi-clonal T cell receptor (TCR) with a canonical TCRα chain paired with a limited set of TCRVβ chains. Following development, iNKT cells emerge from the thymus and circulate in a state of partial activation that phenotypically resembles that of effector/memory T cells (Godfrey et al., 2010). Thus poised for rapid response, iNKT cells release large quantities of cytokines such as IFN-γ, IL-2, TNF-α, IL-4, IL-13, IL-17 and GM-CSF within minutes to hours after primary TCR-mediated stimulation. As a result, iNKT cells act as powerful modulators of many biological processes including inflammation, autoimmunity, tumor rejection and anti-microbial immunity (Bendelac et al., 2007). During infection, rapid iNKT cell activation can contribute to shaping both ongoing innate responses and developing adaptive ones, with important consequences for microbial clearance. This is evidenced by the increased susceptibility of iNKT cell-deficient mice to a range of bacterial, viral and parasitic infections (Cohen et al., 2009; Tupin et al., 2007).
It remains unclear how iNKT cells can be activated by a broad range of microbes given the essentially clonal nature of their TCR repertoire and the lack of polymorphism of CD1d (Brigl and Brenner, 2010; Kronenberg and Kinjo, 2009). A number of bacteria and parasites have been found to synthesize glycolipids that can be presented in CD1d and trigger anti-microbial iNKT cell responses. Organisms in which iNKT cell antigens have been defined include Sphingomonas α-proteobacteria, the pathogenic bacteria Borrelia burgdoferi and Streptococcus pneumoniae, as well as the parasite Leishmania donovani (Amprey et al., 2004; Burrows et al., 2009; Fischer et al., 2004; Kinjo et al., 2006; Kinjo, 2005; Mattner et al., 2005; Sriram et al., 2005). Thus, it is now clear that CD1d can function as a microbial antigen-presenting molecule, and that iNKT cells are able to detect a number of microbial lipids. However, the range of microbes able to synthesize lipids recognized by iNKT cells remains uncertain. Furthermore, it is apparent that in the context of viral infection iNKT cell activation must occur in the absence of foreign lipid recognition. Indeed, in some cases, inflammatory cytokines such as type I IFN, IL-12 and IL-18 elicited by Toll-like receptor (TLR) mediated innate responses, usually in combination with weak self-reactive TCR-signals, are sufficient to drive iNKT cell responses in the absence of foreign lipids (Brigl et al., 2003; Mattner et al., 2005; Nagarajan and Kronenberg, 2007; Paget et al., 2009; Paget et al., 2007; Salio et al., 2007).
While these findings have illustrated how iNKT cell responses may be mounted against bacteria, parasites and viruses, the iNKT cell response to fungi, a group of pathogens with growing clinical importance, has remained entirely uncharacterized. Fungi synthesize a variety of lipids that they use in cellular and pathogenic processes including membrane biogenesis, energy storage, inter- and intra-cellular signaling and virulence (Kaneko et al., 1976; Singh and Del Poeta, 2011). While some fungal lipids are found in mammalian cells as well, fungal biosynthesis pathways also give rise to many lipid species not found in mammals and that could potentially act as microbial-specific iNKT cell antigens (Itoh and Kaneko, 1975; Shea et al., 2006). Furthermore, evidence suggests that iNKT cells may help orchestrate successful anti-fungal responses. Specifically, studies have found that clearance of the opportunistic fungal pathogen Cryptococcus neoformans (C.n.) from the lungs is delayed in iNKT cell-deficient mice, and that the DTH response to the fungus is obliterated (Kawakami et al., 2001a). In addition, treatment of systemically infected mice with the lipid α-galactosylceramide (α-GalCer), a potent artificial iNKT cell antigen, diminishes the fungal burden in the lungs and spleen of infected mice, and promotes protective Th1 responses (Kawakami et al., 2001b; Kawakami et al., 2001c). These data indicate that iNKT cells may occupy an important position in the mammalian anti-fungal arsenal, and underscore the value of defining the mechanisms leading to their deployment.
In this study, we sought to characterize the iNKT cell response to the fungus Aspergillus fumigatus (A.f.), a ubiquitous mold responsible for a spectrum of human illnesses including fatal invasive disease in immune-compromised hosts. We found that CD1d−/− mice are impaired in their ability to rapidly control A.f. infection. Furthermore, iNKT cells become activated by A.f. both in vivo following infection, and in vitro in the presence of antigen-presenting cells (APC). Unexpectedly, while CD1d recognition was required for driving iNKT cell anti-fungal responses, fungal lipid antigens were not. Instead, we define a broadly applicable and distinct mechanism that links innate recognition of fungal β-glucans, structural polysaccharides shared by all fungi, with iNKT cell activation, bypassing the need for cognate TCR-mediated recognition of fungal lipid antigens.
RESULTS
Mice deficient in CD1d-restricted T cells are impaired in early fungal clearance
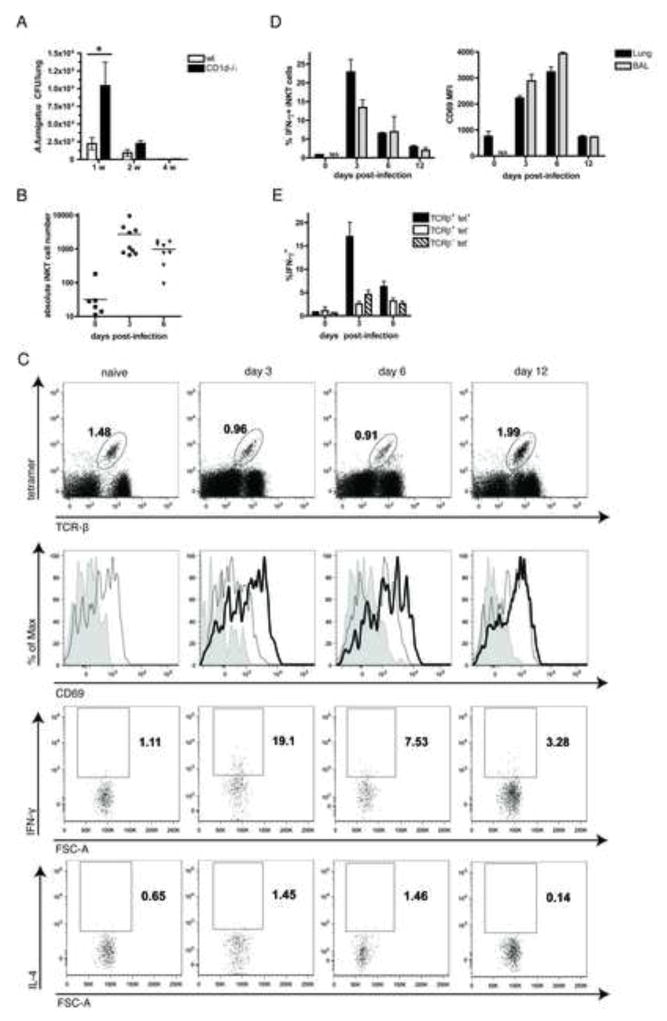
In order to determine if CD1d-restricted T cells promote anti-fungal immune responses, we infected wild type (WT) and CD1d−/− B6 mice with live A.f. conidia intra-tracheally (i.t.) and monitored clearance of the fungi from the lungs. While both WT and CD1d−/− mice eventually eliminated the infection and neither group developed invasive disease characterized by the formation of fungal hyphae, CD1d−/− animals exhibited significantly higher CFUs one week post-infection (p=0.0286), indicating that early clearance of fungal spores was impaired (Fig. 1a and data not shown). In addition, observation of stained lung sections and flow cytometric analysis of infected lungs revealed that CD1d−/− mice developed exacerbated pulmonary inflammation characterized by higher numbers of inflammatory monocytes, DCs and neutrophils compared to WT mice (Sup. Fig. 1 ac). These data suggest that mice lacking CD1d-restricted T cells develop less effective early anti-fungal responses and are compromised in their ability to rapidly clear A.f. from their lungs.
Fig. 1. CD1d-restricted T cells participate in the immune response against A.f.
WT or CD1d−/− B6 mice were infected i.t. with 1×107 (a) or 4×107 (b–e) live A.f. conidia and euthanized at the indicated timepoints. (a) Fungal CFU in lung homogenates. Bars, mean ± SEM; n=4–5 mice/group, data representative of 3 independent experiments. (b) Absolute number of iNKT cells (CD45+, CD19-, TCR-β+, α-GalCer-loaded CD1d tetramer+) in the BAL. Data pooled from 3 independent experiments. (c) Lung iNKT cell percentage (top row), CD69 MFI (second row) and cytokine secretion (bottom rows) following infection. Shaded histogram, isotype control; grey line, naïve mouse; black line, infected mouse. (d) Average %IFN-γ+ iNKT and CD69 MFI following infection. N/A, not applicable (to few cells). Bars, mean ± SEM, n=3 mice. Data representative of ≥ 4 independent experiments. (e) Average %IFN-γ+ iNKT cells, tet− T cells, and NK cells, (TCR-β−tet−) following infection. Data pooled from 4 independent experiments, Bars, mean ± SEM, n=9–12 mice.
A.f. rapidly activates iNKT cells in vivo during pulmonary infection
In order to determine whether iNKT cells specifically respond to A.f. infection, we assessed the kinetics and activation of iNKT cells in the airways of A.f.-infected mice by flow cytometry. Large numbers of iNKT cells appeared in the bronchio-alveolar lavage fluid (BAL) of infected mice as early as 3 days post-infection, and the cells persisted at least until day 6 (Fig. 1b). Furthermore, iNKT cells in both the lungs and the BAL rapidly became activated in response to the fungus. Levels of the activation marker CD69 expressed on iNKT cells were increased three days following infection, and reached their peak by day 6. Similarly, iNKT cells in the airways rapidly secreted IFN-γ (Fig. 1c, d). IFN-γ secretion occurred as early as 24 hours post infection (data not shown), but the percentage of IFN-γ+ iNKT cells peaked at day 3, reaching a mean of 22.9±3.3% in the lung and a mean of 13.4±1.9% in the BAL. While iNKT cells were not found to secrete significant levels of IL-4 or up-regulate CD25 in the context of A.f. infection, in some experiments, a small number of iNKT cells did produce IL-17, but the fraction of IL-17+ iNKT cells was typically under 5% (Fig. 1c, Sup. Fig. 1d and data not shown). Lymphocytes other than iNKT cells also secreted IFN-γ at early timepoints following infection. Although CD19− TCR- β+ tet− cells (mostly MHC-restricted T cells) and CD19−TCR-β−tet− cells (mostly NK cells) and represent a larger fraction of total lymphocytes, only 2.5±0.6% and 4.5±0.9% of these populations, respectively, were IFN-γ+ at day 3 post-infection (Fig. 1e, Sup. Table 1). Thus, following A.f. infection, a large proportion of iNKT cells become rapidly activated, are largely Th1-polarized and accumulate in airways of infected mice.
A.f. activates iNKT cells in vitro in the presence of CD1d-expressing DCs
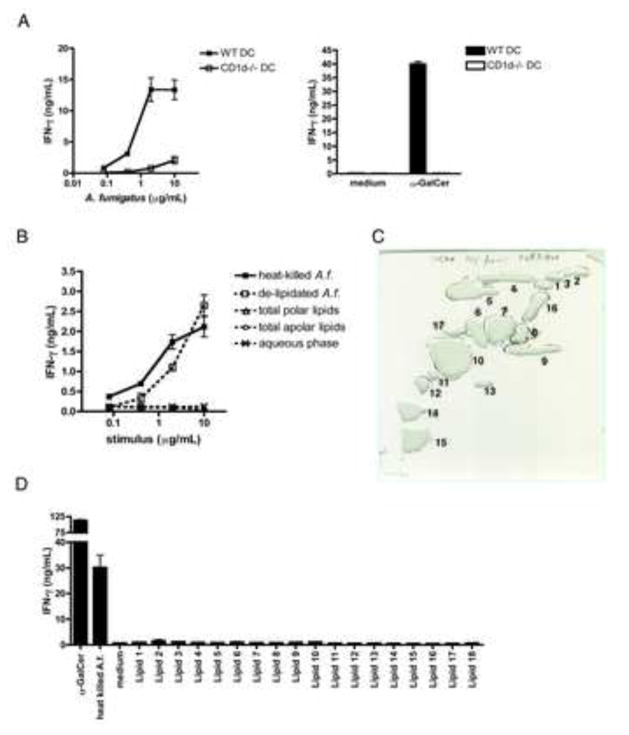
In order to dissect the mechanisms leading to iNKT cell activation in response to fungal infection, we established an in vitro co-culture system using iNKT cell lines and primary bone marrow-derived DCs (DC) as APCs (Chiba et al., 2009), and tested the ability of A.f. to activate iNKT cells in vitro in this system. iNKT cells were co-cultured overnight with DCs in the presence of increasing concentrations of heat-killed A.f. hyphae. Co-culture supernatants were then analyzed by ELISA for the presence of secreted cytokines. Consistent with our in vivo observations, we found that iNKT cells were able to secrete abundant quantities of IFN-γ in response to A.f. in the presence of APCs (Fig. 2a). The ability to react to A.f. was not restricted to a single iNKT cell line as several independently derived lines demonstrated comparable responses to fungal stimulation in the presence of WT DCs (Sup. Fig. 2). While iNKT cells were not found to secrete detectable amounts of IL-17, significant levels of IL-4 and IL-13 were detected in the supernatants of some assays in vitro, but secretion of these cytokines was more variable compared to IFN-γ, which was consistently robust (data not shown). Importantly, iNKT cells did not secrete cytokines in response to A.f. in the absence of DCs (data not shown). In addition, INF-γ secretion by iNKT cell in this system was strongly dependent on CD1d expression by APCs. In co-cultures containing CD1d−/− DCs, the mean IFN-γ detected following exposure to 2μg/mL of fungus was decreased on average by 94.8 ±2.2% compared to WT DC co-cultures (Fig. 2a). Thus, A.f. potently activates iNKT cells in vitro in a manner dependent on the presence of CD1d-expressing APCs.
Fig. 2. Non-lipidic components of the A.f. cell wall drive iNKT cell activation in vitro.
105 iNKT cells and 104 CD11c+ DCs were co-cultured for 16–20hrs with the indicated stimuli. IFN-γ in the supernatants was measured by ELISA. (a) IFN-γ secreted in response to heat-killed A.f. hyphae, α-GalCer (100ng/mL) or medium in co-cultures of iNKT cells and WT or CD1d−/− DCs. (b) IFN-γ secreted in response to lipidic and non-lipidic fungal fractions extracted from hyphae in co-cultures of iNKT cells and WT DCs. (c) Preparative 2D-TLC purification of A.f. polar lipids. (d) IFN-γ secreted in response to α-GalCer (10ng/mL), A.f. (20μg/mL) or 2D TLC-purified polar lipid species (20μg/mL) in co-cultures containing iNKT cells and WT DCs. (a–d) Error bars, ± SEM, data representative of ≥ 3 independent experiments.
A.f. retains its stimulatory activity for iNKT cells following fungal lipid extraction
In order to determine whether fungal lipids might act as CD1d-presented antigens and thus account for iNKT cell activation in this system, we extracted polar and apolar lipids from live fungal hyphae using chloroform, methanol and petroleum ether based solvent systems (Dobson et al., 1985). Lipid extracts, the aqueous phase and delipidated hyphae were then tested for their ability to stimulate murine iNKT cells in vitro in the presence of APCs. Lipid extracts did not trigger detectable iNKT cell activation (Fig. 2b). We further separated the polar lipid extract into individual lipid species by preparative 2D thin layer chromatography (TLC), but none of the purified lipids stimulated significant production of IFN-γ or IL-13 by iNKT cells in our co-culture system (Fig. 2c, d and data not shown). In contrast, the delipidated cell fraction retained a similar ability as the unextracted fungus to stimulate cytokine secretion by iNKT cells in vitro (Fig. 2b). These data suggest that fungal lipids are likely not primarily responsible for fungal-driven iNKT cell activation in this model.
IL-12p70 is required for A. fumigatus-driven iNKT cell activation
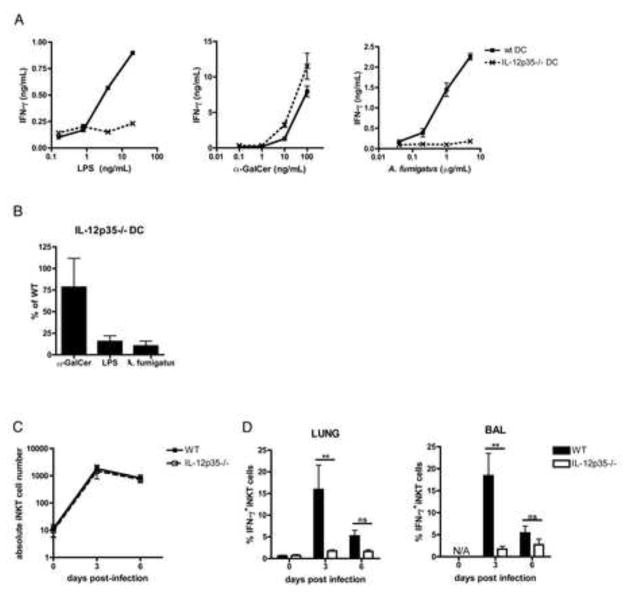
Cytokines such as IL-12, which is secreted by activated APCs, can play a key role in triggering iNKT cell responses against bacterial or viral TLR agonists (Brigl et al., 2003; Brigl et al., 2011; Paget et al., 2009). To determine whether IL-12 is required for fungal-driven iNKT cell activation in vitro, we tested the ability of IL-12p35−/− DCs to stimulate iNKT cells in the presence of fungus. Strikingly, IL-12p35−/− DCs were unable to support fungal-driven IFN-γ secretion by iNKT cells, which was impaired by more 89.8±5.6% on average in co-cultures containing IL-12p35−/− compared with WT DCs (Fig. 3a). As expected, LPS-driven iNKT cell activation was similarly impaired when IL-12p35−/− DCs were used. In contrast, antigen-driven iNKT cell activation was not affected by APC deficiency in IL-12p35, since α-GalCer was able to elicit potent cytokine secretion regardless whether or not the DCs used could produce IL-12 (Fig. 3a, b). Like IL-12p35−/− DCs, we found that DCs deficient in the p40 subunit of IL-12 were also unable to stimulate IFN-γ secretion by iNKT cells in the presence of either LPS or A.f., while their ability to support lipid antigen-driven iNKT cell responses was unaffected (data not shown). In order to determine if the anti-fungal iNKT cell response in vivo also required IL-12, we assessed iNKT cell accumulation and activation in the airways of WT or IL-12p35−/− mice following fungal infection. While accumulation of iNKT cells in the BAL was not affected by IL-12p35-deficiency (Fig. 3c), consistent with our in vitro observations, iNKT cells in the airways of IL-12p35−/− animals failed to secrete IFN-γ. 3 days post-infection, the %IFN-γ+ iNKT cells in the lungs and BAL was on average reduced by 84.1±5.9% (p=0.0022) and 87.2±4.9% (p=0.0043), respectively. In addition, up-regulation of CD69 was blunted in both lung and BAL iNKT cells (Fig. 3d and data not shown). Thus, the anti-fungal iNKT cell response is profoundly impaired in the absence of IL-12, both in vitro and in vivo.
Fig. 3. A.f.-driven iNKT cell activation is IL-12p70-dependent in vitro and in vivo.
(a) IFN-γ in the supernatant of co-cultures containing iNKT cells and WT or IL-12p35−/− DCs in the presence of the indicated stimuli, error bars, ± SEM. (b) Average IFN-γ in supernatants of co-cultures containing α-GalCer (100ng/mL), LPS (20ng/mL) or A.f. (5μg/mL), iNKT cells and IL-12p35−/− DCs as a percentage of the IFN-γ in co-cultures containing WT DCs, data pooled from 3 independent experiments, error bars, ± SEM. (c) Absolute number and (d) average %IFN-γ-secreting iNKT cells in the airways of WT or IL-12p35−/− mice post-infection. Bars, mean ± SEM, n=5–6 mice, data pooled from 2 independent experiments.
Fungal β1,3-glucans drive iNKT cell activation
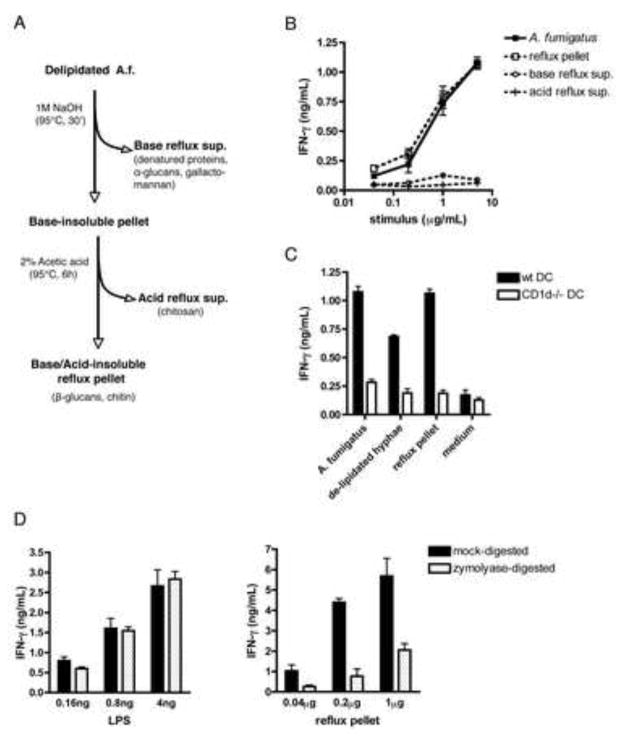
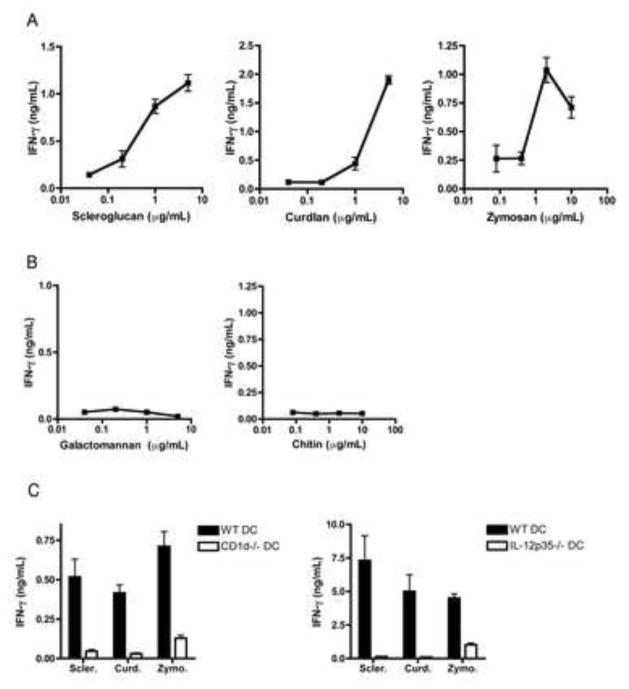
The ability of delipidated fungus to activate iNKT cells together with the requirement for IL-12 suggests that innate, APC-mediated recognition of non-lipidic fungal components drives iNKT cell activation in response to A.f. The fungal cell wall is comprised of a complex network of polysaccharides, a number of which can be recognized by mammalian pattern recognition receptors (PRR) (Bernard and Latge, 2001; Latge, 2010). Many of these compounds can be distinguished on the basis of their solubility in, or sensitivity to, heated base or acid treatment. To determine the identity of the fungal polysaccharides responsible for iNKT cell activation, we subjected the delipidated A.f. to sequential base and acid treatments and tested the activity of the acid or base-soluble and insoluble fractions (Fig. 4a). The active compound in the A.f. cell wall was resistant to boiling in 1:40 (w:v) 1M NaOH at 95°C for 30 minutes followed by refluxing in 1:100 (w:v) 2% acetic acid at 95°C for 6 hours, as the CD1d-dependent activity of the original A.f. hyphal preparation was entirely retained in the acid/base treated insoluble pellet, while the reflux supernatants were not active (Fig. 4b, c). These data rule out acid-soluble fungal components such as chitosan, base-soluble elements like α-glucans and galactomannans, as well as proteins and nucleic acids (which should be destroyed by the procedure) as iNKT cell stimulatory compounds in our system (Hu et al., 1999; Sugawara et al., 2004; Wu et al., 2004). However, β1,3-glucans, glucose polymers integral to the cell wall of most fungi and particularly abundant in A.f. hyphae, are insoluble in 1M NaOH and 2% acetic acid (Bernard and Latge, 2001). We thus reasoned that β1,3-glucans should comprise an important part of the active pellet obtained following acid/base refluxing of delipidated A.f. hyphae. Consistent with this prediction, combined gas chromatography and mass spectrometry analysis of sugar composition revealed that 49.7% of the sugars detected in the iNKT cell stimulating refluxed material were glucose residues, and that most of these residues were in a 1,3-linked configuration. The remaining material was mostly made up of N-acetyl-glucosamine residues, the building blocks of chitin, with trace amounts of galactose and mannose (Sup. Table 2 and data not shown). Furthermore, we found that scleroglucan, curdlan and zymosan, which are β-glucan-enriched preparations from Sclerotium rolfsii, Agrobacteria biobar and Saccharomyces cervisiae, respectively, were all able to drive IFN-γ secretion by iNKT cells in vitro in a dose-dependent manner in the presence of WT but not CD1d−/− or IL-12p35−/− APCs (Fig. 5a, c) while other fungal polysaccharides such as galactomannan and chitin were inactive (Fig. 5b). Thus, β-glucans can drive iNKT cell activation. To further confirm that β1,3-glucans were responsible for driving the iNKT cell response to A.f., we digested the A.f. reflux pellet with zymolyase, an enzyme specific for β1,3-glucans, and tested the activity of the digests in our co-culture assay. While control zymolyase digestion of LPS did not affect its ability to activate iNKT cells in the presence of DCs, the activity of the zymolyase-digested A.f. reflux pellet was on average reduced by 83.1±7.6% compared to the mock digested material (Fig. 4d). Together, these data indicate that β1,3-glucans in the cell wall of A.f. are necessary for eliciting iNKT cell responses.
Fig. 4. Cell wall β1,3-glucans are responsible for A.f.-driven iNKT cell activation in vitro.
(a) Fungal polysaccharide fractionation protocol. (b) Activity of reflux supernatants and pellet in co-culture assay containing iNKT cells and WT DCs. (c) IFN-γ secretion in co-cultures containing iNKT cells, either wild type or CD1d−/− DC and 5μg/mL of the indicated fractions. (d) IFN-γ secretion in co-cultures containing LPS or the A.f. reflux pellet digested overnight with zymolyase. (a–d) Error bars, ± SEM, data representative of 2–4 independent experiments.
Fig. 5. Microbial β-glucans activate iNKT cells in the presence of CD1d- and IL-12-sufficient APCs.
(a–c) IFN-γ secretion in iNKT cells co-cultures containing the indicated polysaccharides in increasing concentrations (a, b) or at 2–5μg/mL (c), error bars, ± SEM, data representative of 2–4 independent experiments.
Dectin-1 and TLR-mediated recognition of A.f. by APCs stimulates iNKT cells
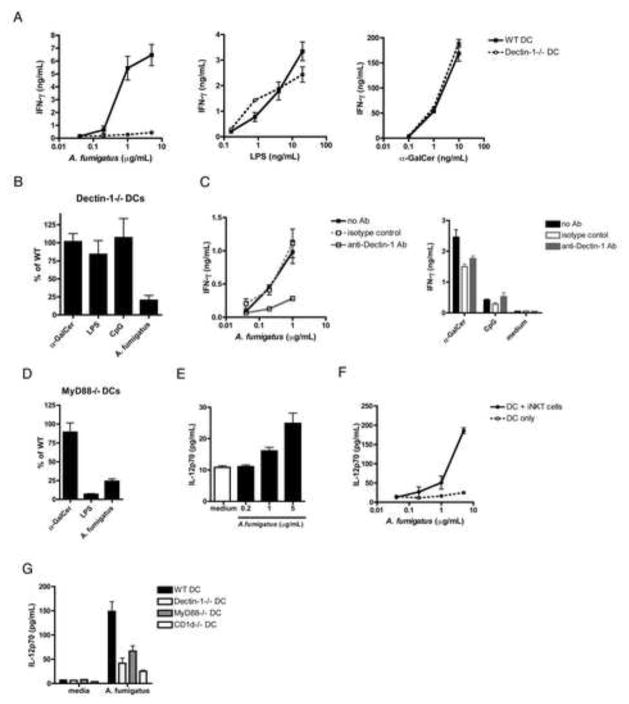
The C-type lectin Dectin-1 is a PRR known to specifically detect fungal β1,3-glucans (Goodridge et al., 2009), and is critical for survival of A.f. infection both in mice and immunocompromised humans (Cunha et al., 2010; Saijo et al., 2007; Taylor et al., 2007; Werner et al., 2009). To determine if Dectin-1-mediated innate recognition of A.f. by DCs promotes iNKT cell activation, we assessed the ability of Dectin-1−/− DCs or DCs treated with Dectin-1-blocking mAb to drive iNKT cell cytokine secretion in the presence of A.f. Strikingly, in the absence of Dectin-1 expression by APCs, the iNKT cell response to A.f. was reduced by 79.7±6.6% on average. In contrast, LPS, CpG and antigen-driven stimulation were unaffected (Fig. 6a, b). Similar results were obtained by incubating DCs with Dectin-1 blocking mAb prior to co-culturing with iNKT cells (Fig. 6c). Dectin-1 mediated recognition of fungal β-glucan often requires collaborative TLR signaling (Dennehy et al., 2008; Luther et al., 2007; Reid et al., 2009). Indeed, we found that iNKT cell responses were also dependent on MyD88, as IFN-γ secretion in response to A.f. was inhibited by 75.8±6.0% in co-cultures containing MyD88−/− DCs (Fig. 6d). Thus, A.f.-driven activation of iNKT cells requires β-glucan recognition by APCs via Dectin-1 combined with MyD88 co-signaling likely mediated by TLRs.
Fig. 6. A.f.-driven iNKT cell activation depends on APC-mediated fungal recognition via the C-type lectin Dectin-1 and MyD88 signaling.
(a) IFN-γ detected in the supernatants of co-cultures containing iNKT cells, WT or Dectin-1−/− DCs and the indicated stimuli. (b) Average IFN-γ in iNKT cell co-cultures containing α-GalCer (10ng/mL), CpG (1μg/mL), LPS (4ng/mL) or A.f. (5μg/mL) and Dectin-1−/− DCs or (d) MyD88−/− DCs as a percentage of the IFN-γ measured in co-cultures containing WT DCs, data pooled from 3 independent experiments. (c) Co-culture performed in the presence of 2.5μg/mL of anti-Dectin-1 blocking mAb, an isotype control, or no antibody, and heat-killed A.f. hyphae (left), α-GalCer (10ng/mL), CpG (1μg/mL) or medium (right). Data representative of 2 independent experiments. (e) IL12p70 detected in the supernatants of WT DCs co-cultured with the indicated amounts of heat-killed A.f. in the absence or (f) presence of iNKT cells. (g) IL12p70 detected in the supernatants of co-cultures containing DCs, iNKT cells and either medium or A.f. (5μg/mL). Data representative of 2–3 independent experiments. (a–g) Error bars, ± SEM.
iNKT cells amplify IL-12p70 secretion by APCs activated by A.f. via PRRs
Our data highlight a critical role for IL-12 in fungal-driven iNKT cell activation. However, since several reports suggest that Dectin-1 is not a potent inducer of IL-12, even in the context of TLR co-signaling, we sought to clarify the mechanism underlying IL-12 production by DCs in response to A.f. (Dennehy et al., 2009; Huang et al., 2009). We first incubated DCs with A.f. and measured IL-12 secretion in the supernatant by ELISA. We found that A.f.-stimulated DCs secrete low levels of IL-12p70 in a dose dependent manner (Fig. 6e). Strikingly, the amount of IL-12p70 secreted by DCs in the presence of A.f. was dramatically increased when iNKT cells were added to the co-culture. 5μg/mL of A.f. stimulated an average of 24.9±3.3pg/mL by DCs alone, versus 185.9±7.7pg/mL in the presence of iNKT cells, suggesting that iNKT cells promote IL-12p70 secretion by activated APCs (Fig. 6f). Furthermore, the enhancement of IL-12 secretion was blunted if Dectin-1−/−, MyD88−/− or CD1d−/− DCs were used instead of WT DCs (Fig. 6g), suggesting that iNKT cell-mediated amplification of IL-12 secretion requires DCs to be stimulated via PRRs, and is also dependent on CD1d-mediated DC-iNKT cell interactions. Together, these data suggest that PRR-mediated recognition of A.f. potentiates APC secretion of IL-12, which is then amplified following CD1d-dependent interactions with iNKT cells.
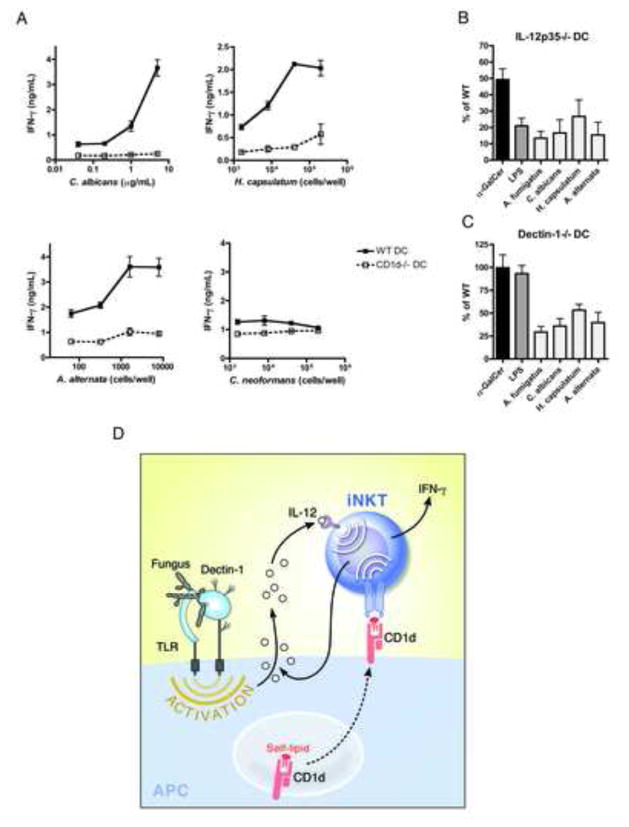
Candida albicans, Histoplasma capsulatum and Alternaria alternata activate iNKT cells following innate Dectin-1-mediated recognition and IL-12 secretion by activated CD1d-expressing APCs
Because β-glucans are integral to the cell wall of most fungi, we next sought out to determine whether IL-12 secretion resulting from innate recognition of fungal β-glucans from pathogens other than A.f. also triggered iNKT cell activation. While C.n., a fungal pathogen whose glucan-containing cell wall is shielded by a thick glucuronoxylomannan capsule, failed to activate iNKT cells in vitro, we found that like A.f., the pathogenic yeast C. albicans (C.a.), the dimorphic fungus H. capsulatum (H.c.), and the opportunistic mold Alternaria alternata (A.a.) all elicited dose-dependent IFN-γ secretion by iNKT cells in the presence of CD1d-expressing but not CD1d-deficient APCs. Furthermore, the iNKT cell response to C.a., H.c. and A.a. was reduced by 83.4±8%, 73.3±10% and 84.6±7.8% when IL-12p35−/− DCs were used and by 64.0±8%, 46.7±6.5% and 60.3±11% when WT DCs were substituted for Dectin-1−/− cells, respectively (Fig. 7b, c). Thus, innate Dectin-1-mediated recognition of fungal β-glucans and subsequent secretion of IL-12p70 drives iNKT cell responses against several fungal pathogens and likely represents a broadly applicable pathway for driving iNKT cell responses against fungi (Fig. 7d).
Fig. 7. Dectin-1-mediated recognition of pathogenic fungi and IL-12 secretion by activated APCs drives iNKT cell activation.
(a) IFN-γ detected in the supernatants of co-cultures containing iNKT cells, WT or CD1d−/− DCs and heat-killed fungi. (b) Average IFN-γ in iNKT cell co-cultures containing α-GalCer (1ng/mL), LPS (20ng/mL), A.f. (5μg/mL), C.a. (5μg/mL), H.c. (8–40×103 cells/well), A.a. (1.6×103 cells/well) and IL-12p35−/− or (c) Dectin-1−/− DCs as a percentage of the IFN-γ in co-cultures containing WT DCs, data pooled from 3–4 independent experiments. (a–c) Error bars, ± SEM. (d) Model mechanism for fungal-driven iNKT cell activation.
DISCUSSION
While a number of innate and adaptive processes at the basis of anti-microbial immunity have been delineated, the mechanism of action of innate-like lymphocytes such as γδT cell, Mucosal-associated invariant T cells and iNKT cells, all evolutionarily conserved lineages with broad anti-microbial functions, is poorly understood. One of the most puzzling features these cells share is the highly limited diversity of their TCR repertoires. How do semi-clonal lymphocyte populations detect vastly diverse microorganisms?
While iNKT cells have long been known to recognize various mammalian self-lipids, whether they could also detect microbial lipids was unknown until fairly recently, when a small number of bacteria and parasites were found to synthesize lipids that could be presented by CD1d and elicit iNKT cell responses (Kinjo et al., 2006; Kinjo, 2005; Mattner et al., 2005; Sriram et al., 2005). This suggested that at least some microbes possess antigens for iNKT cells. In addition, because several of these microbial lipids shared an unusual α-anomeric glycan linkage with α-GalCer, it has been postulated that α-linked glycolipids may represent a microbial motif recognized specifically by the iNKT cell TCR (Tupin et al., 2007). However, many microbes may not produce such antigens, and to date they have only been detected in three bacterial species. The possibility that even microbes lacking α-linked glycolipids may stimulate iNKT cell responses is underscored by the examples of viruses and purified or synthetic TLR agonists that potently activate iNKT cells in the absence of exogenous microbial lipids (Brigl and Brenner, 2010; Salio et al., 2007).
Here, we sought to characterize the response of iNKT cells to fungi, an important group of pathogens whose detection by iNKT cells has never been described. While there is evidence that iNKT cells may play an important role in anti-fungal immunity (Kawakami et al., 2001a; Kawakami et al., 2001b; Kawakami et al., 2001c), whether or not fungi produce lipid antigens capable of driving iNKT cell responses is unknown. We found that murine iNKT cells mount a potent Th1-polarized response against pulmonary infection with the filamentous fungus A.f. In addition, fungal-driven iNKT cell responses in vitro require recognition of CD1d on APCs, suggesting that TCR-mediated signals are needed. Surprisingly, fractionation experiments indicated that fungal cell wall carbohydrates, not lipids, however, were responsible for driving anti-fungal iNKT cell responses. While suggestive, initial lipid extractions could not entirely rule out the possibility that fungal lipid antigens were driving iNKT cell responses, as some compounds may have resisted chemical extraction. Despite this possibility, several additional lines of evidence support our hypothesis. First, we found that the inflammatory cytokine IL-12 is absolutely required for iNKT cell cytokine secretion in response to A.f., both in vitro and in vivo. Thus, even if fungal lipids are presented to iNKT cells, alone, they are not sufficient to drive iNKT cell activation. Second, we found that the active compound in the A.f. cell wall was not only resistant to organic solvent extraction, but also to boiling in NaOH and acetic acid, treatments likely to destroy any remaining lipid. In fact, the insoluble fungal material remaining after acid/base refluxing is comprised of highly resistant polysaccharides comprising the structural scaffold of fungal cell walls: β1,4-N-acetylglucosamine (chitin) and β1,3-glucans (Latge, 2007; Latge et al., 2005). Large chitin polymers are thought to be immunologically inert, and while smaller chitin fragments derived from crab shells have been found to stimulate the immune system (Da Silva et al., 2008), recent studies suggest that fungal chitin purified from C.a. does not elicit cytokine responses from mammalian cells, and can even block innate recognition of more stimulatory fungal components (Chai et al., 2011; Mora-Montes et al., 2011). In contrast, fungal β1,3-glucans are well known as potent immune-stimulants. β1,3-glucans are recognized by several mammalian PRRs, and drive leukocytes to exert various antimicrobial functions (Goodridge et al., 2009). Consistent with these observations, we found that enzymatic digestion of β1,3-glucans in the delipidated, acid/base refluxed A.f. material strongly impaired iNKT cell activation. Furthermore, like A.f. cell walls, enriched β1,3-glucans from other microbial sources also potently activated iNKT cells in the presence of CD1d-expressing APCs, but chitin or galactomannan did not. Finally, we found that expression of Dectin-1, a known β1,3-glucan receptor, was required for A.f. driven iNKT cell activation. While we can not fully rule out that A.f. chitin is not also playing a role in driving the iNKT cell response, together, our data strongly suggest that β1,3-glucans, not lipids, are the major fungal component responsible for activating iNKT cells in response to A. fumigatus.
Thus, we define a widely applicable model for fungal-driven iNKT cell activation whereby innate recognition of fungal β1,3-glucans potentiates IL-12 production by APCs, which, in concert with weak TCR signals provided by self-lipid:CD1d complexes, drives iNKT cell activation (Fig 7d). Our data indicate that both Dectin-1 and MyD88-mediated signals contribute to fungal recognition. This finding is consistent with several reports suggesting that detection of fungal β1,3-glucans by Dectin-1 requires collaborative TLR-signaling (Balloy and Chignard, 2009; Dennehy et al., 2008; Luther et al., 2007; Reid et al., 2009). Our data further suggest that activated iNKT cells enhance IL-12 secretion by APCs, initiating a positive feedback loop (Kitamura et al., 1999; Vincent et al., 2002). Most importantly, we show that like A.f., the yeast C.a., the dimorphic fungal pathogen H.c. and the opportunistic filamentous fungus A.a., all activate iNKT cells in a manner dependent on innate fungal β1,3-glucan recognition and the resulting IL-12. While C.n. did not activate iNKT cells in vitro, likely due to the capsule shielding of its cell wall glucans, it is likely that innate responses absent from our in vitro system (eg. complement activation) drive in vivo IL-12 responses resulting in similarly innate-driven iNKT cell responses (Kozel, 1993) (Decken et al., 1998) (Kawakami et al., 2001a). Thus, the model suggested here may represent a general mechanism for activation of iNKT cell responses against diverse fungi.
Our findings extend reports indicating that TLR-stimulated APCs can drive iNKT cell responses in the absence of foreign lipid antigens (Brigl and Brenner, 2010). In the context of A.f. responses, we find that Dectin-1 and MyD88 collaboratively drive cellular responses to fungal β-glucans. Both TLRs and Dectin-1 are known to play an important role in early innate responses to A.f. spores in the lung (Bretz et al., 2008; Werner et al., 2009) and may play redundant role in activating airway iNKT cell responses, as we have found that the absence of Dectin-1 alone does not significantly impair iNKT cell cytokine secretion following infection (data not shown). Nevertheless, our model of innate-driven activation is strongly supported by the heavy dependence of iNKT cell activation on IL-12 in vitro and in vivo. In addition, TCR-mediated CD1d-recognition was required despite the absence of exogenous lipids, suggesting self-lipids may be recognized. Whether anti-fungal inflammatory responses alter the nature or antigenic potential of self-lipids available for iNKT cell recognition, as has been documented in other contexts (Darmoise et al., 2010; Paget et al., 2007; Salio et al., 2007), remains to be determined.
Finally, our data suggest that CD1d-restricted T cells are important for early fungal elimination. These observations are in line with a large body of literature implicating CD1d-restricted T cells as powerful modulators of pulmonary immune responses (De Santo et al., 2008; Ho et al., 2008; Johnson et al., 2002; Joyee et al., 2008; Kinjo et al., 2002; Nieuwenhuis, 2002; Sada-Ovalle et al., 2010). Activated iNKT cells can enhance innate airway responses and promote recruitment and activation of neutrophils, as well as phagocytosis and killing of intracellular microbes by alveolar macrophages (Kim et al., 2008; Nieuwenhuis, 2002; Sada-Ovalle et al., 2008), processes essential for fungal clearance (Brakhage et al., 2010; Schaffner et al., 1982). Our data indicate that recruitment of inflammatory infiltrates is enhanced in mice lacking CD1d. Thus, while mobilization of innate effector cells is not impaired, the efficacy of early responses may be diminished in CD1d−/− mice, resulting in a higher fungal load and the enhanced inflammatory response observed.
In conclusion, our findings indicate that CD1d-restricted T cells are important for anti-fungal immunity and that the potent iNKT cell response mounted against A.f. does not require recognition of microbial lipid antigens. Instead, anti-fungal iNKT cell cytokine secretion is strongly dependent on IL-12 derived from innate recognition of fungal β1,3-glucans. Importantly, we show that this pathway could represent a general mechanism for driving anti-fungal iNKT cell responses.
EXPERIMENTAL PROCEDURES
Mice
Wild type C57BL/6 (B6) and IL-12p35−/− mice were obtained from Jackson laboratories. CD1d−/− and Dectin-1−/− mice were kindly provided by Mark Exley (Exley et al., 2003) and Yoichiro Iwakura (Saijo et al., 2007), respectively. Mice were maintained under SPF conditions in the DFCI animal facility. This study was approved by the DFCI’s office for the protection of research subjects.
Fungus preparation
A.f. conidia were grown on SDA at 37°C for 7 days, then dislodged, re-suspended in 0.025%Tween/PBS, filtered twice through 70μm strainers and counted. SD broth was inoculated with 107 conidia/mL and incubated on a shaker at 37°C overnight to generate hyphae, which were collected by filtration and washed in sterile LPS-free PBS. For in vitro assays, hyphae were autoclaved at 121°C for 15 minutes then homogenized by sonication in media. Hyphae did not contain detectable endotoxin levels, as assessed by limulus assay. See supplemental material for A.a., C.a., C.n. and H.c. preparation.
Intra-tracheal infection, histology, fungal burden assessment and flow cytometry
8–12 week old mice anaesthetized using isofluorane were injected i.t. with 1–4×107 conidia in 50μl of 0.025%Tween/PBS. BAL was collected from euthanized mice in 5%FBS/PBS. For histology, lungs were perfused with 4% PFA, fixed at 25°C, embedded in paraffin, sectioned, and stained using H&E. Morphometric analysis was conducted using ImageScope (Aperio). For fungal burden assessment and flow cytometry, lungs were perfused with PBS, minced, and digested in 3mg/mL Type IV collagenase and 10μg/mL DNAse I in 5%FBS/PBS for 1hr at 37°C. Digests were filtered through 70μm strainers and washed. Diluted lung digests were plated on SDA plates and incubated at 37°C until fungal CFU could be counted. For flow cytometry, cells were stained with antibodies following an FcR blocking step. Cytokine secretion assays (Miltenyi) were performed according to the manufacturer’s instructions. Data were collected on an LSRII cytometer and analyzed using FlowJo Software (Treestar).
Chemical extractions, 2DTLC and enzymatic digestions
To extract polar and apolar lipids, lyophilized hyphae were treated with methanolic saline and petroleum ether. The upper phase (apolar lipids) was removed and the remaining biomass and lower phase were treated with chloroform, methanol and saline, then biphased with a chloroform and saline solution to yield polar lipid extract and an aqueous phase (Dobson et al., 1985). For in vitro testing, dry lipidic and non-lipidic fractions were resuspended in culture medium by sonication. Polar lipids were separated by 2D-TLC on plastic-backed plates of silica gel 60 F254 using CHCl3, CH3OH, H2O (60 30:6, v/v/v) in the first direction and CHCl3/CH3COOH/CH3OH/H2O (40: 25:3:6, v/v/v/v) in the second direction (Tatituri et al., 2007). TLC plates were sprayed with 0.01% 1,6-diphenyl-1,3,5-hexatriene dissolved in petroleum ether/acetone (9:1, v/v), and lipids were identified under UV light. Lipid species were scraped off the plates and extracted from the silica gel using CHCl3/CH3OH (2:1, v/v). For carbohydrate fractionation, delipidated hyphae were refluxed in 1:40 (w/v) 1M NaOH at 95°C for 30 minutes. The alkali soluble material in the supernatant was collected by centrifugation. The alkali-insoluble material was refluxed in 1:100 (w/v) 2% acetic acid at 95°C for 6 hours and centrifuged to separate insoluble pellet and the acid-soluble supernatant. Enzymatic digestions were performed by incubating substrates (1mg/mL) with zymolyase (100μg/mL) in LPS-free PBS at overnight at 37°C. Enzymes were then inactivated by incubation at 65°C for 30 minutes.
In vitro co-culture assays
For co-culture experiments, 105 iNKT cells were cultured overnight with 104 CD11c+ DCs and stimuli in round-bottom 96-well plates at 37°C. Cytokines in the supernatants were quantified by ELISA. See supplemental material for the derivation of DCs and iNKT cells.
Statistical analysis
Statistical analyses were performed using a two-sided Wilcoxon Rank Sums Test (SAS); *, p<0.05; **, p<0.01.
Supplementary Material
HIGHLIGHTS.
CD 1d-restricted T cells participate in immunity against A. fumigatus
Anti-fungal iNKT cell responses do not require fungal lipid recognition
Innate recognition of β-1,3 glucans by APCs drives anti-fungal iNKT cell activation
Fungal-driven iNKT cell activation requires CD1d and IL-12
Acknowledgments
We thank the NIH for providing CD1d tetramers; G. Ostroff for help with polysaccharide purification and analysis and for providing reagents; G. Deepe and C. Steele for H.c. samples, H. Kita for A.a isolates, D. Perlin, Y. Zhao, A. Tuli, M.W. Painter, H. Kim, D. Moody, S. Turley, N. Letvin, J. Cohen and E. Pamer for their critical input or technical help. This work was supported by the NIH (grant AI063428-06A1, M.B. Brenner; K08AI077795, M. Brigl), the Department of Energy-funded (DE-FG09-93ER-20097) Center for Plant and Microbial Complex Carbohydrates and the Sandler Foundation (grant 06-0058, M. B. Brenner).
Footnotes
The authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amprey JL, Im JS, Turco SJ, Murray HW, Illarionov PA, Besra GS, Porcelli SA, Spath GF. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med. 2004;200:895–904. doi: 10.1084/jem.20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balloy V, Chignard M. The innate immune response to Aspergillus fumigatus. Microbes Infect. 2009;11:919–927. doi: 10.1016/j.micinf.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Bernard M, Latge JP. Aspergillus fumigatus cell wall: composition and biosynthesis. Med Mycol. 2001;39(Suppl 1):9–17. [PubMed] [Google Scholar]
- Brakhage AA, Bruns S, Thywissen A, Zipfel PF, Behnsen J. Interaction of phagocytes with filamentous fungi. Curr Opin Microbiol. 2010;13:409–415. doi: 10.1016/j.mib.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Bretz C, Gersuk G, Knoblaugh S, Chaudhary N, Randolph-Habecker J, Hackman RC, Staab J, Marr KA. MyD88 signaling contributes to early pulmonary responses to Aspergillus fumigatus. Infection and immunity. 2008;76:952–958. doi: 10.1128/IAI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigl M, Brenner MB. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin Immunol. 2010;22:79–86. doi: 10.1016/j.smim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011 doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows PD, Kronenberg M, Taniguchi M. NKT cells turn ten. Nat Immunol. 2009;10:669–671. doi: 10.1038/ni0709-669. [DOI] [PubMed] [Google Scholar]
- Chai LY, Vonk AG, Kullberg BJ, Verweij PE, Verschueren I, van der Meer JW, Joosten LA, Latge JP, Netea MG. Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes Infect. 2011;13:151–159. doi: 10.1016/j.micinf.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Chiba A, Cohen N, Brigl M, Brennan PJ, Besra GS, Brenner MB. Rapid and reliable generation of invariant natural killer T-cell lines in vitro. Immunology. 2009;128:324–333. doi: 10.1111/j.1365-2567.2009.03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NR, Garg S, Brenner MB. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, D’Angelo C, Pierini A, Pitzurra L, Falzetti F, et al. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood. 2010;116:5394–5402. doi: 10.1182/blood-2010-04-279307. [DOI] [PubMed] [Google Scholar]
- Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008;181:4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmoise A, Teneberg S, Bouzonville L, Brady RO, Beck M, Kaufmann SH, Winau F. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 2010;33:216–228. doi: 10.1016/j.immuni.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately MK, Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infection and immunity. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennehy KM, Willment JA, Williams DL, Brown GD. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur J Immunol. 2009;39:1379–1386. doi: 10.1002/eji.200838543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson G, Minnikin DE, Minnikin SM, Parlett M, Goodfellow M, Ridell M, Magnusson M. Systematic analysis of complex mycobacterial lipids. London: Academic Press, Inc; 1985. [Google Scholar]
- Exley MA, Bigley NJ, Cheng O, Shaulov A, Tahir SM, Carter QL, Garcia J, Wang C, Patten K, Stills HF, et al. Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology. 2003;110:519–526. doi: 10.1111/j.1365-2567.2003.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, Hurwitz R, Kursar M, Bonneville M, Kaufmann SH, et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci U S A. 2004;101:10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LP, Denney L, Luhn K, Teoh D, Clelland C, McMichael AJ. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur J Immunol. 2008;38:1913–1922. doi: 10.1002/eji.200738017. [DOI] [PubMed] [Google Scholar]
- Hu KJ, Yeung KW, Ho KP, Hu JL. Rapid extraction of high-quality chitosan from mycelia of Absidia glauca. J Food Biochem. 1999;23:187–196. [Google Scholar]
- Huang H, Ostroff GR, Lee CK, Wang JP, Specht CA, Levitz SM. Distinct patterns of dendritic cell cytokine release stimulated by fungal beta-glucans and toll-like receptor agonists. Infect Immun. 2009;77:1774–1781. doi: 10.1128/IAI.00086-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Kaneko H. The distribution of pyrophosphatidic acid in nature. J Biochem. 1975;78:817–820. doi: 10.1093/oxfordjournals.jbchem.a130971. [DOI] [PubMed] [Google Scholar]
- Johnson TR, Hong S, Van Kaer L, Koezuka Y, Graham BS. NK T cells contribute to expansion of CD8(+) T cells and amplification of antiviral immune responses to respiratory syncytial virus. J Virol. 2002;76:4294–4303. doi: 10.1128/JVI.76.9.4294-4303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyee AG, Qiu H, Fan Y, Wang S, Yang X. Natural Killer T Cells are Critical for Dendritic Cells to Induce Immunity in Chlamydial Pneumonia. Am J Respir Crit Care Med. 2008 doi: 10.1164/rccm.200804-517OC. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Hosohara M, Tanaka M, Itoh T. Lipid composition of 30 species of yeast. Lipids. 1976;11:837–844. doi: 10.1007/BF02532989. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Kinjo Y, Uezu K, Yara S, Miyagi K, Koguchi Y, Nakayama T, Taniguchi M, Saito A. Monocyte chemoattractant protein-1-dependent increase of V alpha 14 NKT cells in lungs and their roles in Th1 response and host defense in cryptococcal infection. J Immunol. 2001a;167:6525–6532. doi: 10.4049/jimmunol.167.11.6525. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Kinjo Y, Yara S, Koguchi Y, Uezu K, Nakayama T, Taniguchi M, Saito A. Activation of Valpha14(+) natural killer T cells by alpha-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect Immun. 2001b;69:213–220. doi: 10.1128/IAI.69.1.213-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Kinjo Y, Yara S, Uezu K, Koguchi Y, Tohyama M, Azuma M, Takeda K, Akira S, Saito A. Enhanced gamma interferon production through activation of Valpha14(+) natural killer T cells by alpha-galactosylceramide in interleukin-18-deficient mice with systemic cryptococcosis. Infect Immun. 2001c;69:6643–6650. doi: 10.1128/IAI.69.11.6643-6650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, Benoit LA, Byers DE, Alevy Y, Tucker J, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjo Y, Kawakami K, Yamamoto N, Miyagi K, Kinjo T, Uezu K, Nakasone C, Nakayama T, Taniguchi M, Saito A. The role for Va14+ NKT cells in the early host defense against pulmonary infection with Streptococcus pneumoniae. Paper presented at: 2nd NK T cell & CD1 workshop; Woods Hole, MA, USA. 2002. [Google Scholar]
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Wu D, Kim G, Xing GW, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434 doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR. Activation of the complement system by the capsule of Cryptococcus neoformans. Curr Top Med Mycol. 1993;5:1–26. [PubMed] [Google Scholar]
- Kronenberg M, Kinjo Y. Innate-like recognition of microbes by invariant natural killer T cells. Curr Opin Immunol. 2009;21:391–396. doi: 10.1016/j.coi.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latge JP. The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol. 2007;66:279–290. doi: 10.1111/j.1365-2958.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- Latge JP. Tasting the fungal cell wall. Cellular microbiology. 2010;12:863–872. doi: 10.1111/j.1462-5822.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- Latge JP, Mouyna I, Tekaia F, Beauvais A, Debeaupuis JP, Nierman W. Specific molecular features in the organization and biosynthesis of the cell wall of Aspergillus fumigatus. Med Mycol. 2005;43(Suppl 1):S15–22. doi: 10.1080/13693780400029155. [DOI] [PubMed] [Google Scholar]
- Luther K, Torosantucci A, Brakhage AA, Heesemann J, Ebel F. Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the dectin-1 beta-glucan receptor and Toll-like receptor 2. Cellular microbiology. 2007;9:368–381. doi: 10.1111/j.1462-5822.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- Mora-Montes HM, Netea MG, Ferwerda G, Lenardon MD, Brown GD, Mistry AR, Kullberg BJ, O’Callaghan CA, Sheth CC, Odds FC, et al. Recognition and Blocking of Innate Immunity Cells by Candida albicans. Chitin Infect Immun. 2011 doi: 10.1128/IAI.01282-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis EFea. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from the lung. Nat Med. 2002;8:588–593. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- Paget C, Bialecki E, Fontaine J, Vendeville C, Mallevaey T, Faveeuw C, Trottein F. Role of invariant NK T lymphocytes in immune responses to CpG oligodeoxynucleotides. J Immunol. 2009;182:1846–1853. doi: 10.4049/jimmunol.0802492. [DOI] [PubMed] [Google Scholar]
- Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Reid DM, Gow NA, Brown GD. Pattern recognition: recent insights from Dectin-1. Curr Opin Immunol. 2009;21:30–37. doi: 10.1016/j.coi.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS pathogens. 2008;4:e1000239. doi: 10.1371/journal.ppat.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada-Ovalle I, Skold M, Tian T, Besra GS, Behar SM. Alpha-galactosylceramide as a therapeutic agent for pulmonary Mycobacterium tuberculosis infection. Am J Respir Crit Care Med. 2010;182:841–847. doi: 10.1164/rccm.200912-1921OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, Besra GS, Platt FM, Cerundolo V. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci U S A. 2007;104:20490–20495. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982;69:617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea JM, Henry JL, Del Poeta M. Lipid metabolism in Cryptococcus neoformans. FEMS Yeast Res. 2006;6:469–479. doi: 10.1111/j.1567-1364.2006.00080.x. [DOI] [PubMed] [Google Scholar]
- Singh A, Del Poeta M. Lipid signalling in pathogenic fungi. Cellular microbiology. 2011;13:177–185. doi: 10.1111/j.1462-5822.2010.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Takahashi S, Osumi M, Ohno N. Refinement of the structures of cell-wall glucans of Schizosaccharomyces pombe by chemical modification and NMR spectroscopy. Carbohydr Res. 2004;339:2255–2265. doi: 10.1016/j.carres.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Tatituri RV, Illarionov PA, Dover LG, Nigou J, Gilleron M, Hitchen P, Krumbach K, Morris HR, Spencer N, Dell A, et al. Inactivation of Corynebacterium glutamicum NCgl0452 and the role of MgtA in the biosynthesis of a novel mannosylated glycolipid involved in lipomannan biosynthesis. J Biol Chem. 2007;282:4561–4572. doi: 10.1074/jbc.M608695200. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, Brenner MB. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3:1163–1168. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Zivanovic S, Draughon FA, Sams CE. Chitin and chitosan--value-added products from mushroom waste. J Agric Food Chem. 2004;52:7905–7910. doi: 10.1021/jf0492565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.