Abstract
When scanning faces, individuals with autism spectrum disorder (ASD) have shown reduced visual attention (e.g., less time on eyes) and atypical autonomic responses (e.g., heightened arousal). To understand how these differences might explain sub-clinical variability in social functioning, 9-month-olds, with or without a family history of ASD, viewed emotionally-expressive faces, and gaze and pupil diameter (a measure of autonomic activation) were recorded using eye-tracking. Infants at high-risk for ASD with no subsequent clinical diagnosis (HRA-) and low-risk controls (LRC) showed similar face scanning and attention to eyes and mouth. Attention was overall greater to eyes than mouth, but this varied as a function of the emotion presented. HRA- showed significantly larger pupil size than LRC. Correlations between scanning at 9 months, pupil size at 9 months, and 18-month social-communicative behavior, revealed positive associations between pupil size and attention to both face and eyes at 9 months in LRC, and a negative association between 9-month pupil size and 18-month social-communicative behavior in HRA-. The present findings point to heightened autonomic arousal in HRA-. Further, with greater arousal relating to worse social-communicative functioning at 18 months, this work points to a mechanism by which unaffected siblings might develop atypical social behavior.
Keywords: high-risk infants, emotion processing, eye-tracking, autism spectrum disorders, pupillometry
The ability to recognize and respond appropriately to facial expressions of emotion is a hallmark of face processing and social competence, and a growing body of work has focused on characterizing the behavioral and neural correlates of emotion processing (e.g., Batty & Taylor, 2003; Morris et al., 1998; Nelson & de Haan, 1996; Peltola, Leppanen, Maki, & Hietanen, 2009; Pourtois et al., 2004). This research has spanned infancy through adulthood (for reviews, see Leppanen & Nelson, 2009, 2012, and Righi & Nelson, 2013), and has recently expanded to include atypically-developing populations, such as children with autism spectrum disorder (for a review, see Nuske et al., 2013).
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by social and emotional impairments in conjunction with communicative difficulties and restricted interests and patterns of behavior. Individuals with ASD show atypicalities in the behavioral processing of emotionally-expressive faces, including impaired emotion recognition (e.g., Ashwin et al., 2006; Baron-Cohen et al., 1997; Celani et al., 1999). Eye-tracking evidence indicates that they spend less time looking at core features (e.g., eyes, mouth) when scanning emotional faces (e.g., Dalton et al., 2005; de Wit et al., 2008; Pelphrey et al., 2002; for a review, see Guillon et al., 2014), and this has been proposed as a mechanism by which individuals with ASD might develop difficulty interpreting social and emotional information (Pelphrey et al., 2002).
Recent research has further posited that atypical regulation of the autonomic nervous system (ANS) in children with ASD could contribute to difficulties with social processing. The sympathetic and parasympathetic branches of the ANS work together to promote appropriate physiological responses. The polyvagal theory, put forth by Porges (e.g., 2007), discusses how the balance in these two systems contributes to social engagement. For example, increased levels of parasympathetic control can modulate sympathetic arousal, resulting in a calm physiological state that allows for improved social responses. Studies have found atypicalities in individuals with ASD which reflect an imbalance in sympathetic and parasympathetic influences on the ANS, including larger tonic pupil size and lower levels of salivary alpha-amylase, an enzyme reflecting one of the key components of pupillary responses, the norepinephrine system (Anderson & Colombo, 2009; Anderson et al., 2013). Abnormalities have also been found in ASD for cardiac parasympathetic activity, including increased heart rate and reduced respiratory sinus arrhythmia (RSA; e.g., Bal et al., 2010; Ming et al., 2005; Van Hecke et al., 2009). Bal, Van Hecke, and others discuss the important relations between high RSA and improved control of the sympathetic influences to the ANS, and in line with the polyvagal theory, children with ASD who have higher RSA showed better social skills (Van Hecke et al., 2009) and faster emotion recognition (Bal et al., 2010; see Quintana et al., 2012, for a parallel finding in healthy adults).
Work has also looked at atypicalities in the sympathetic responses of individuals with ASD, with several studies suggesting heightened physiological states, or hyperarousal, in ASD (e.g., Dalton et al., 2005; Joseph et al., 2008; Kushki et al., 2013; Kushki et al., 2014). Joseph et al. (2008) measured skin conductance responses (SCRs) in older children with ASD as they viewed faces and found that the ASD group showed greater SCRs in response to faces and a negative correlation between arousal and face recognition, whereby larger SCRs related to worse task performance. Relatedly, Kaartinen et al. (2012) found that increased SCRs in response to faces with direct gaze in children with ASD were associated with greater impairments in social skills. The amygdala, a neuroanatomical structure known to play a crucial role in emotional reactivity, has also been examined for relations between arousal and social motivation and behavior. Dalton et al. (2005) studied brain responses of older children with ASD during an fMRI task of face processing and found larger responses in the amygdala in ASD as compared to controls. Kleinhans et al. (2010) used fMRI in adults with ASD and found increased amygdala activity in response to emotional faces was related to increased social anxiety. These studies suggest heightened sympathetic arousal in individuals with ASD, as well as links between increased physiological states and social abilities in ASD. Some studies have also found profiles of hypoarousal, or decreased physiological responses, in ASD (e.g, Hirstein et al., 2001; Schoen et al., 2008), and continued work is needed to clarify what causal roles hyperarousal and hypoarousal might have on social impairments in individuals with ASD (see Keehn et al., 2013 and Senju & Johnson, 2009 for further discussion).
With behavioral and physiological evidence pointing to atypicalities in face processing in children and adults with ASD, questions remain about when these patterns emerge and whether these early traits might be present even in those individuals without a diagnosis but with familial vulnerability to ASD. Atypicalities in social and emotional processing have been identified in first-degree relatives of individuals with ASD (e.g., Adolphs, et al., 2008; Baron-Cohen & Hammer, 1997; Dalton et al., 2007; Dawson et al., 2005; Dorris et al., 2004; Wallace et al., 2010). Dorris et al. (2004) found that unaffected siblings of individuals with ASD showed impairments in using information conveyed in the eyes to identify emotional expressions as compared to control participants. Dawson et al. (2005) found that parents of children with ASD showed atypicalities in electrophysiological responses to faces that were similar to those found in children and adults with ASD. Work by Dalton et al. (2007) found that unaffected siblings of individuals with ASD showed significantly less time fixating on eyes as compared to controls, and showed significantly decreased activation in the right fusiform gyrus than controls as measured using fMRI, a region that is highly involved in face processing in typically-developing adults (e.g., Kanwisher, et al., 1997; Tong et al. 2000).
To understand the development of impairments in emotional processing in ASD and those with increased familial risk of ASD, work has begun to focus on infant siblings of children with ASD, who have as high as a 1 in 5 chance of developing the disorder (e.g., Elsabbagh & Johnson, 2010; Ozonoff et al., 2011), as compared to 1 in 68 in the general population (Autism and Developmental Disabilities Monitoring Network, 2014). Longitudinal studies with this at-risk group offer a unique opportunity to ask whether and when early differences in development are evident in high-risk infants, and much of the research has explored whether some patterns are predictive of an ASD outcome (e.g., Zwaigenbaum et al., 2005; for reviews, see Jones et al., 2014, and Tager-Flusberg, 2010). On the other hand, two large-scale recent studies have redirected focus onto children who do not develop ASD (Messinger et al., 2013; Ozonoff et al., 2014), instead exploring how other patterns of early development might predict the range of possible outcomes, spanning typical development, developmental delays, and the broader autism phenotype (e.g., Baron-Cohen et al., 2001; Bolton et al., 1994).
A growing number of studies have examined visual attention to faces in at-risk infants during the first year of life, and the majority of results reveal similarities in visual scanning and attention to core features of the face in high- and low-risk infants (e.g., Dundas et al., 2012; Key & Stone, 2012; Merin, et al., 2007; Young et al., 2009), with significant group differences not emerging before 12 months (Jones & Klin, 2013). Only one study thus far has examined autonomic responses in high-risk infants to look for differences in physiological reactivity. Nyström and colleagues (Nyström et al., 2015) studied the pupillary light reflex (PLR) in 10-month-old infants at high risk for ASD. The PLR is an index of the integrity of the cholinergic system (for discussion, see Fotiou et al., 2009) that has shown abnormalities in children with ASD (Fan et al., 2009). Nyström et al. (2015) found that, while there were no pupil size differences between high-risk and low-risk infants during a baseline period, the high-risk group showed heightened sensitivity in the PLR, marked by stronger and faster responses.
Among studies with high-risk infants, several have longitudinally examined how early social attention might relate to later ASD outcome and development more generally. Young et al. (2009) examined face scanning at 6 months and ASD symptoms at 18 and 24 months and found no relations, though this study found that greater attention to the mouth at 6 months predicted higher levels of expressive language at 24 months (see also Elsabbagh et al., 2014 for a related finding). Work by de Klerk et al. (2014) also found relations between early eye-tracking measures and later social skills, with high-risk infants showing a significant negative association between looking to faces at 7 months in a face pop-out task and face recognition at 3 years of age. This finding might suggest that difficulties modulating attention and/or arousal in high-risk infants during the first year of life can lead to difficulties in social abilities in later development, but how this differs based on later outcome is not yet known, as all high-risk infants, regardless of outcome status, were included in this correlational analysis.
The present study aimed to extend previous work linking early development with non-ASD outcome by examining visual scanning and autonomic responses to socially-relevant stimuli in infants at risk for ASD during the first year of life. At 9 months, infant siblings of children with ASD were presented with static images of emotionally-salient faces, and gaze and pupil size were measured. Additionally, these infants were followed longitudinally and social-communicative behavior was measured at 18 months. The present study only included infants without a subsequent clinical diagnosis, allowing this work to focus on predicting variability in early social development that is not accounted for by a diagnosis of ASD or other non-spectrum disorder, with the goal of better understanding the range of outcomes observed in high-risk infants.
Two major questions were explored in the present study. The first question asked whether there are group differences between high- and low-risk infants in visual attention and autonomic responses while viewing emotional faces. Based on past work with high-risk infants (e.g., Key & Stone, 2012), few differences in visual scanning were expected between groups; however, predictions were less clear for autonomic activity, as measured by pupil size, since little work with high-risk infants has examined these physiological responses (though work by Nyström et al., 2015 studying reflexive pupil responses suggests group differences could be found). An exploratory question was also posed regarding visual attention and pupil responses in each group of infants, specifically, how might these measures be correlated. The second major aim of the present study was to ask whether visual attention and pupil responses at 9 months predict social-communicative development at 18 months. Work with both high- and low-risk infants has found relations between early attention to faces and later social and language development (e.g., de Klerk et al., 2014; Wagner, Luyster, Yim, Tager-Flusberg, & Nelson, 2013; Young et al., 2009), so associations were predicted for both groups. Finally, while concurrent associations have been found between physiological responses and social ability in older individuals with ASD, this has not been examined longitudinally in high-risk infants, so there were no strong predictions for this analysis.
Method
Participants
The final sample consisted of 49 9-month-old infants: 20 low-risk controls (LRC) with a typically-developing older sibling and no family history of ASD (mean age = 280 days, SD = 9. days; 13 male) and 29 infants at high risk for ASD (HRA) by virtue of having an older sibling diagnosed with ASD (mean age = 282 days, SD = 10 days; 17 male). Family history of ASD was queried during a pre-enrollment phone screen. Diagnosis of ASD in the HRA proband (and confirmation that the LRC proband did not have ASD) was corroborated via parent report using an age-appropriate screener prior to enrollment: for probands over 4 years old, the Social Communication Questionnaire was used (SCQ; Rutter et al., 2003); for probands under 4 years old, the Pervasive Developmental Disorders Screening Test-II was used (PDDST-II; Siegel, 2004).
After this initial screening, participants were enrolled in a longitudinal infant sibling project and asked to participate regularly until 36 months in various tasks, with data collected through parent-report, behavioral, eye-tracking, electrophysiological, genetic, and fNIRS measures, some of which have already been discussed in other work (e.g., Keehn et al., 2013; Luyster et al., 2014; Nelson et al., 2015; Talbott et al., 2015). To be considered in the present sample, infants needed to participate at 9 months, be followed longitudinally, and have received an Autism Diagnostic Observation Schedule (Lord et al., 1999) assessment from a research-reliable experimenter at 24 or 36 months. Thirty-seven infants were excluded for a) not having a lab visit at 24 or 36 months (14 LRC, 15 HRA), or b) administration issues with standardized assessments (4 LRC; 4 HRA). Of the remaining infants, 17 were excluded for meeting the following two conditions: 1) met classification on the ADOS on their most recent visit at 24 or 36 months, and 2) received a clinical judgment of ASD or non-spectrum disorder (i.e., anxiety or developmental delays) by a staff clinician based on all available information (2 LRC, 15 HRA). With confirmation that all HRA infants did not have ASD or another disorder at their most recent lab visit at 24 or 36 months, this group is referred to as HRA- to indicate HRA infants who did not receive a clinical diagnosis. Table 1 illustrates scores on standardized assessments for the included sample of LRC and HRA-.
Table 1.
Sample means (standard deviations) on standardized tests for LRC and HRA-
| LRC | HRA- | p Value | |
|---|---|---|---|
| CSBS-DP (18 months) | n = 17 | n = 26 | |
| Social Percentile | 73.47 (19.78) | 48.92 (29.08) | .002 |
| Range | 25–99 | 2–99 | |
| Total Percentile | 72.59 (22.49) | 44.73 (30.87) | .001 |
| Range | 18–94 | 3–98 | |
| MSEL (18 months) | n = 20 | n = 28 | |
| ELC | 107.60 (14.62) | 102.14 (14.07) | .19 |
| Range | 78–133 | 72–125 | |
| ADOS Module 1 | n = 5 | n = 10 | |
| Social Total | 1.20 (1.30) | 1.50 (.71) | .57 |
| Range | 0–2 | 0–2 | |
| Communication Total | 1.40 (1.67) | 2.20 (1.48) | .36 |
| Range | 0–4 | 0–5 | |
| Soc+Comm Total | 2.60 (2.70) | 3.70 (1.83) | .36 |
| Range | 0–6 | 2–7 | |
| ADOS Module 2 | n = 15 | n = 19 | |
| Social Total | 1.07 (1.10) | 1.89 (1.85) | .14 |
| Range | 0–6 | 0–8 | |
| Communication Total | 1.13 (1.13) | 2.21 (2.44) | .12 |
| Range | 0–4 | 0–9 | |
| Soc+Comm Total | 2.20 (1.74) | 4.11 (3.90) | .089 |
| Range | 0–6 | 0–17 | |
Note: LRC: Low-risk controls; HRA-: High-risk autism with no clinical diagnosis; CSBS-DP: Communication and Symbolic Behavior Scales - Developmental Profile; MSEL: Mullen Scales of Early Learning; ELC: Early Learning Composite; ADOS: Autism Diagnostic Observation Schedule; ADOS data are from the most recent ADOS administration (either 24 or 36 months). Final sample included n = 20 for LRC and n = 29 for HRA-; CSBS-DP at 18mo missing for three LRC and three HRA-; MSEL at 18mo missing for one HRA- (see Method for more detail).
Twelve additional infants were excluded (7 LRC, 5 HRA-) because they accumulated insufficient eye-tracking data at their 9-month visit (less than 10% of the presentation time for one or more of the three emotion faces), due to movement outside of the appropriate spatial window for successful eye-tracking acquisition (left/right and forward/backward) and/or looks away from the screen. The use of a generous looking time criteria (minimum of 10% of time to each emotion) was intended to maximize the number of infants included and to account for the fact that this was the second eye-tracking task of the 9-month visit and included only static images with no attention-getters in between images. Included infants attended to the faces, on average, 40% of the time they were presented on the screen (i.e., mean time on faces was 7.9s out of 20s, SD = 3.2s). Project approval was obtained from the Institutional Review Boards of Boston Children’s Hospital and Boston University and informed consent was obtained from the parents of each infant participant.
Stimuli
Based on the procedure used by Peltola and colleagues (Peltola, Leppanen, Vogel-Farley, Hietanen, & Nelson, 2009), four female faces comprised the final stimulus set. Each infant saw color images of one of the four female faces expressing three different emotions: fearful (open mouth), happy (open mouth), and neutral (closed mouth; see Figure 1). The four female faces were taken from the NimStim library (Tottenham et al., 2009).
Figure 1.
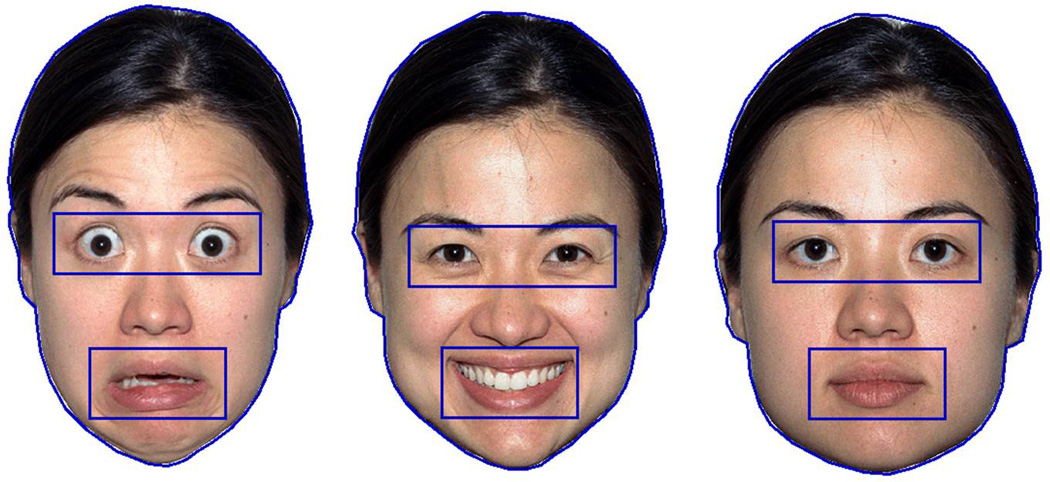
Examples of the fearful, happy, and neutral facial expressions used from the NimStim set with the face, eyes, and mouth AOIs drawn.
Apparatus
Infants were seated on a caregiver’s lap and presented with face images on a 17” TFT Tobii T60 monitor using Clearview software (Tobii Technology AB; www.tobii.com). The Tobii monitor recorded gaze and pupil diameter of both eyes at 60Hz based on the reflection of near-infrared light from the cornea and pupil. The monitor specifications include accuracy of 0.5 degrees of the visual angle, and a tolerance of head movements within the range of 44 × 22 × 30cm.
Procedure
As part of a battery of eye-tracking, electrophysiological, and behavioral measures at the 9-month visit, infants were brought into a dimly lit testing room and seated on their caregiver’s lap. Ambient lighting conditions were identical for all participants: the overhead light was off and a small desk lamp was illuminated behind a black curtain that hung behind the eye-tracking monitor. Infants were seated with their eyes positioned approximately 60 cm from the Tobii T60 monitor. Before the session began, a calibration procedure was used to confirm that the infant and monitor positions allowed for satisfactory gaze tracking. During calibration, a blue and white checkered sphere appeared in the four corners of the monitor and the center of the screen. Following the 5-location calibration procedure, the Clearview program reported whether the eye-tracker successfully tracked eye gaze in these five locations. If successful, the testing session began. If unsuccessful, infant position and monitor position were adjusted and calibration was repeated until successful calibration was achieved.
During testing, infants were presented with two blocks of three 10-second trials containing a female face expressing fearful, happy, and neutral expressions on the center of the screen. The order of the three expressions was randomized across each of the two blocks, and infants were randomly assigned to see one of four female faces across all six trials (2 fearful, 2 happy, and 2 neutral trials). A blank screen preceded each face that was presented, and the interstimulus-interval varied depending on infant attention. The experimenter triggered stimulus presentation when the infant’s gaze was fixating on the blank screen in between trials. The faces measured 6.5” in height and 4” at their widest point. With infants positioned at 60 cm from the screen, this resulted in faces subtending a visual angle of roughly 9.7 degrees.
Social Communication Measure at 18 Months
When infants in the present sample were 18 months, parents were asked to complete the Communication and Symbolic Behavior Scales Developmental Profile (CSBS-DP, Wetherby et al., 2002). The CSBS-DP is a norm-referenced measure used to capture the early communicative competence of young children, including 45 questions covering seven domains of social communication and symbolic development: emotion and eye gaze, communication, gestures, sounds, words, understanding, and object use. Scoring yields three composite scores: Social (comprised of the Emotion and Eye Gaze, Communication, and Gestures clusters), Speech (comprised of the Sounds and Words clusters) and Symbolic (comprised of the Understanding and Object Use clusters). An overall Total score, which captures performance across the three composites, is also obtained. Each raw score is assigned a standard score and percentile rank according to previously established norms (Wetherby et al., 2002).
Cognitive Assessment at 18 Months
The Mullen Scales of Early Learning (MSEL; Mullen, 1995) was administered by an experimenter during the lab visit at 18 months for LRC and HRA-. The MSEL evaluates cognitive functioning for children from birth to 68 months of age. Standardized domain scores (T-scores: M = 50, SD = 10) are calculated for five subtests (gross motor, fine motor, visual reception, receptive language, and expressive language), and these domain scores (excluding the gross motor subtest) are used to generate one overall composite score, termed the Early Learning Composite (ELC; M = 100, SD = 15).
Data Processing and Analyses
Infant eye-tracking at 9 months
Gaze and pupil data were collected throughout the testing session. Before these data were exported from the Clearview software, areas of interest (AOIs) were drawn to allow for subsequent analysis of duration of gaze and pupil diameter in conjunction with specific regions of the image. For each of the three emotional expressions, AOIs were drawn for the overall face, the eyes, and the mouth (see Figure 1). The size of the eyes AOI and the mouth AOI were kept constant across the three emotional expressions: the eyes AOI measured .94” tall × 2.98” wide, corresponding to a visual angle of 7.23 degrees with the infant seated 60 cm from the monitor; the mouth AOI measured 1” tall × 2” wide, corresponding to a visual angle of 4.85 degrees.
Eye-tracking data exported for each subject resulted in a series of text files containing information about the × and y coordinates and pupil diameter of the right and left eyes throughout the test session, with samples identified by the Clearview software when gaze fell within the pre-defined AOIs. Export settings in Clearview included a fixation radius of 10 pixels and a minimum fixation duration of 15 ms, effectively allowing identification of all samples of gaze data that fell in an AOI, since the sampling rate of 60Hz resulted in a sample every 16.7 ms. The present study focused on total samples in a given AOI rather than requiring minimum fixation lengths in order to give infant participants the best chance to contribute meaningful data without being penalized for potential sample loss by the eye-tracking system (for examples of developmental eye-tracking work looking at attention to AOIs independent of fixations, see Chawarska & Shic, 2009; de Klerk et al., 2014; Norwood et al., 2014).
A custom-made Python script (Python Programming Language; www.python.org) was used to extract duration of gaze and pupil diameter for each AOI for the three emotional faces. Duration of gaze was calculated for each trial as the total number of samples spent in each AOI. Duration of gaze was then combined for same-emotion trials to calculate total duration of gaze for each emotion for the overall face, eyes, and mouth, and from these values, proportion of time spent on the eyes and the mouth was calculated as a function of total time on the face.
Pupil diameter was calculated for each trial as the average pupil diameter while gaze fell within one of the three AOIs. Analysis of pupil responses focused on the face AOI, as this allowed for inclusion of all infants (i.e., if an infant spent no time on the mouth or eyes AOI for a given emotion, no pupil size could be calculated for that AOI for the infant and they would be excluded from an analysis of pupil size compared across the eye and mouth regions). A weighted average was used to combine pupil data across each child’s same-emotion trials. For example, if total gaze on the first trial fearful face was 2000 ms and pupil diameter was 3.5 mm, and if gaze to the second trial fearful face was 4000 ms and pupil diameter was 3.8 mm, the pupil diameter for the fearful face AOI was calculated as (2000/(2000+4000))*3.5+(4000/(4000+2000))*3.8.
CSBS-DP at 18 months
The present analyses examined social and communicative development using the CSBS-DP percentile ranks for the Social composite score and the Total score. CSBS-DP scores were unavailable for a subset of children due to failure of parents to return the completed questionnaire (3 LRC, 3 HRA-).
MSEL at 18 months
The present analyses examined cognitive ability with the MSEL Early Learning Composite (ELC) score. MSEL ELC scores were unavailable for one HRA- child at 18 months due to administration issues.
Results
Eye-tracking (9 Months)
Eye-tracking results focused on three sets of analyses using group (LRC, HRA-) as a between-subjects variable and emotional expression (fearful, happy, neutral) as a within-subjects variable: 1) duration of time on the face AOI, 2) proportion of time on the eyes AOI and mouth AOI (calculated out of total time on the face AOI), and 3) pupil diameter in response to the face AOI. These three analyses addressed the first major aim of the present work, exploring group differences in visual attention and autonomic activity in response to emotional faces. A preliminary regression analysis examined total time on the face AOIs as a predictor of average pupil diameter to the faces and found no significant influence (β = .162, p = .27). See Table 2 for raw gaze and pupil data, standardized gaze data, and skew and kurtosis for all eye-tracking variables for LRC and HRA- for each emotion.
Table 2.
Mean (SD), skewness, kurtosis for gaze and pupil data in LRC and HRA- for each emotion
| LRC | HRA- | |||||
|---|---|---|---|---|---|---|
| Fearful | Happy | Neutral | Fearful | Happy | Neutral | |
| Pupil to Face | ||||||
| Raw size in mm | 3.08 (.39) | 3.10 (.35) | 3.09 (.36) | 3.41 (.53) | 3.43 (.58) | 3.32 (.59) |
| Skew; Kurtosis | .30; −1.04 | .58; −.85 | .37; −.75 | .012; −.74 | .16; −.83 | .23; −.70 |
| Time on Face | ||||||
| Raw time in ms | 8382 (3426) | 8261 (4108) | 8497 (3728) | 7518 (4114) | 7716 (4222) | 7669 (3813) |
| Skew; Kurtosis | .43; −.17 | .87; −.17 | .08; −.48 | .75; −.02 | .83; −.26 | .84; .12 |
| Standardized % | .42 (.17) | .41 (.21) | .42 (.19) | .38 (.21) | .39 (.21) | .38 (.19) |
| Skew; Kurtosis | .43; −.17 | .87; −.17 | .08; −.48 | .75; −.02 | .83; −.26 | .84; .12 |
| Time on Eyes | ||||||
| Raw time in ms | 4685 (3541) | 3933 (7341) | 4185 (3866) | 4548 (3887) | 3497 (3621) | 4016 (3021) |
| Skew; Kurtosis | .90; .53 | 1.38; 2.99 | 1.30; .51 | 1.65; 2.54 | 2.11; 4.29 | .89; .25 |
| Standardized % | .52 (.30) | .44 (.28) | .47 (.29) | .56 (.26) | .44 (.28) | .49 (.26) |
| Skew; Kurtosis | .08; −1.15 | .27; −.42 | .28; −1.34 | −.38; −. 66 | .33; −1.22 | −.08; −.37 |
| Time on Mouth | ||||||
| Raw time in ms | 640 (962) | 1005 (1224) | 716 (1519) | 598 (934) | 1305 (2121) | 777 (1360) |
| Skew; Kurtosis | 1.57; 1.12 | 1.25; .37 | 2.70; 7.00 | 2.21; 4.43 | 2.88; 9.21 | 2.40; 5.39 |
| Standardized % | .10 (.15) | .14 (.17) | .09 (.17) | .08 (.09) | .15 (.18) | .08 (.13) |
| Skew; Kurtosis | 1.59; 1.63 | 1.47; 1.97 | 2.39; 5.31 | 1.10; −.11 | 1.28; 1.01 | 2.68; 8.99 |
Note: LRC: Low-risk controls; HRA-: High-risk autism with no clinical diagnosis; For Time on Face, Standardized % is time on face divided by total time each face was presented (2 × 10 sec = 20 sec per emotion); For Time on Eyes and Time on Mouth, Standardized % is time on eyes/mouth divided by total time on face.
Duration of time on face
A 3 (Emotion: fearful, happy, neutral) × 2 (Group: LRC, HRA-) repeated-measures ANOVA examining time on the face AOI resulted in no effect of emotion (F(2,94) = .037, p = .96), no effect of group (F(1,47) = .57, p = .45), and no interaction between emotion and group (F(2,94) = .06, p = .94). The two groups showed similar time spent looking to the faces (LRC: M = 8380ms, SD = 3047; HRA-: M = 7635ms, SD = 3615).
Proportion of time on eyes and mouth
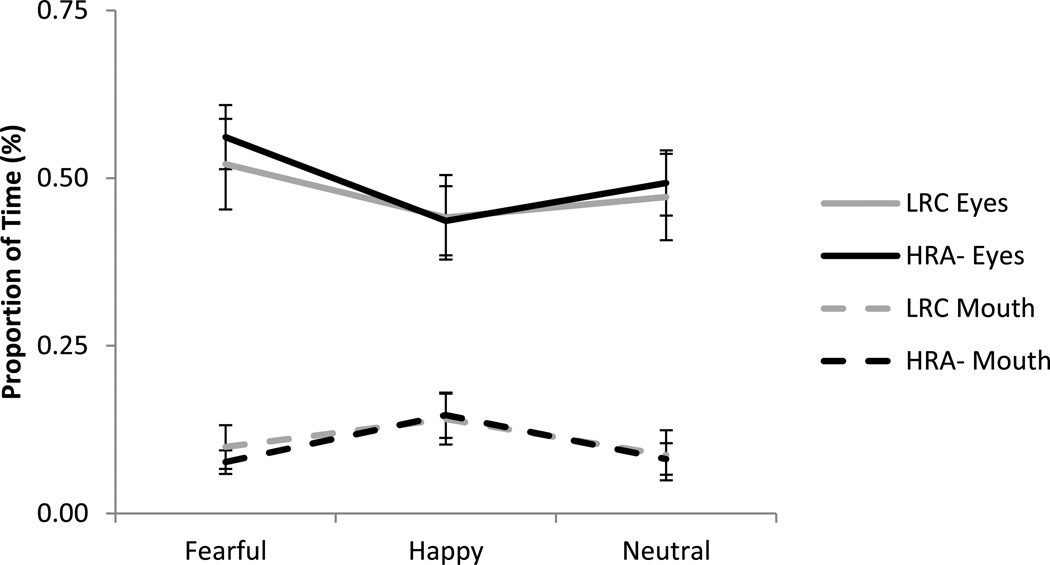
Proportion of time on eyes and mouth (out of total time spent looking at the face) was examined using a 3 (Emotion: fearful, happy, neutral) × 2 (Region: eyes, mouth) × 2 (Group: LRC, HRA-) repeated-measures ANOVA, with emotion and region as the within-subjects factors and group as the between-subjects factor. A main effect of region was found, F(1,47) = 60.77, p < .001, ηp2 = .56, with a significantly greater proportion of time spent on the eyes (M = .49, SD = .25) than the mouth (M = .10, SD = .13). Additionally, a significant interaction between emotion and region was found, F(2,94) = 8.48, p < .001, ηp2 = .15, This interaction showed that while there was consistently greater attention to eyes than mouth overall (see Figure 2), for proportion of time spent on the eye region, infants showed significantly greater attention to eyes for fearful faces (M = .54, SD = .27) as compared to happy faces (M = .44, SD = .28; t(48) = 3.94, p < .001, d = .37) and marginally more attention to eyes for fearful faces as compared to neutral (M = .48, SD = .27; t(48) = 1.95, p = .057, d = .22). In contrast, for the mouth region, infants showed a significantly greater proportion of time for happy faces (M = .14, SD = .17) as compared to neutral faces (M = .08, SD = .14; t(48) = 3.09, p = .003, d = .39) and fearful faces (M = .09, SD = .12; t(48) = 3.57, p = .001, d =.34; see Figure 2). There were no other significant main effects (emotion: F(2,94) = 2.48, p = .09; group: F(1,47) = .03, p = .86) and no other significant interactions (emotion × group: F(2,94) = .05, p = .95; region × group: F(1,47) = .07, p = .79; emotion × region × group: F(2,94) = .45, p = .64)
Figure 2.
Proportion of time looking to the eyes and mouth for HRA- and LRC. There were no group differences or interactions with group. Overall, infants spent a significantly greater proportion of time scanning the eyes than the mouth (p < .001), and this varied as a function of emotional expression (p < .001). For the eye region, infants showed greater attention for fearful faces than happy faces. For the mouth region, infants showed greater attention for happy faces than both neutral and fearful faces. Error bars represent standard error.
Pupil response to face
A 3 (Emotion: fearful, happy, neutral) × 2 (Group: LRC, HRA-) repeated-measures ANOVA examined pupil size while viewing the face AOI. Results revealed a main effect of group, F(1,47)= 5.69, p = .021, ηp2 = .11, with HRA- showing larger pupil size (M = 3.43, SD = .56) as compared to LRC (M = 3.09, SD = .36). There was no main effect of emotion (F(2,94) = .47, p = .63) or interaction between emotion and group (F(2,94) = .074, p = .93).
Relations between face scanning and pupil size
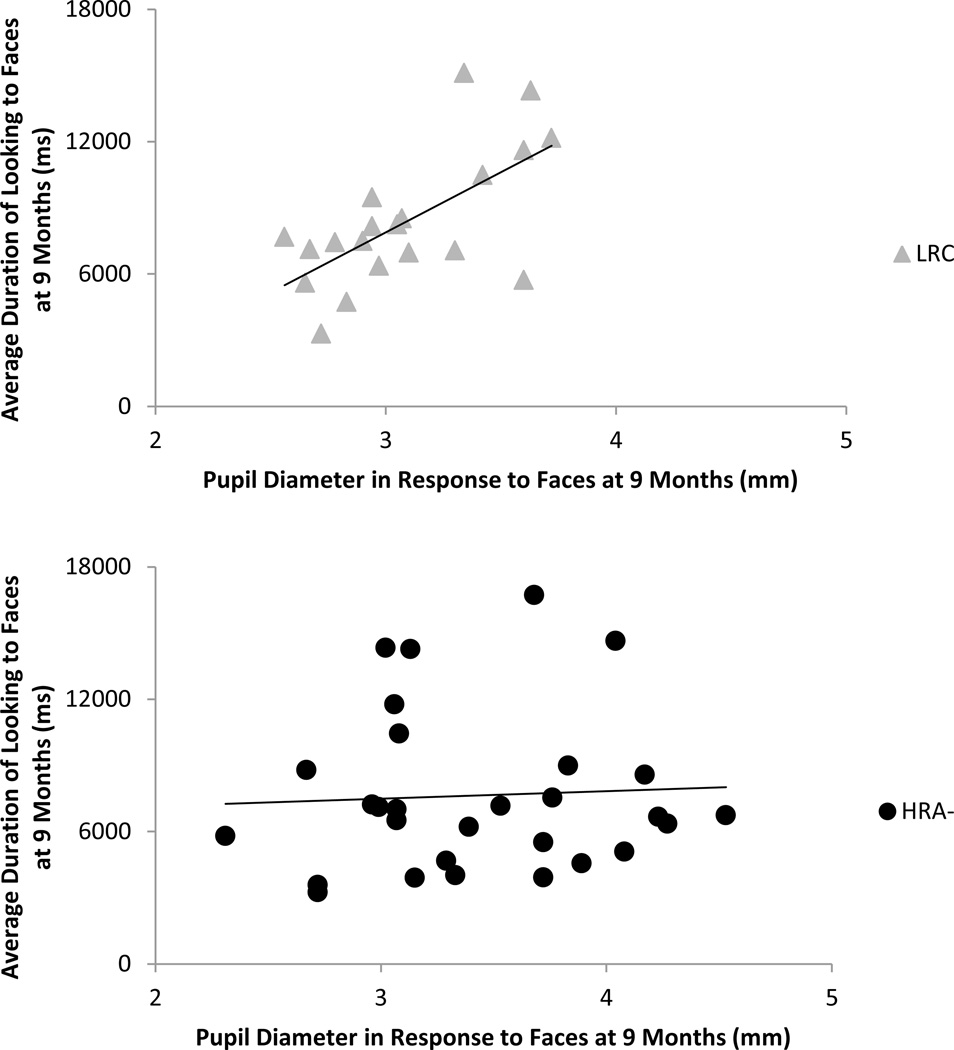
A set of correlations examined the relations at 9 months between visual attention and pupil size for LRC and HRA- separately. The face scanning measures included 1) average time on faces, 2) average proportion of time on eyes, and 3) average proportion of time on mouth. The pupil measure was average pupil size to faces. For LRC, significant positive associations were found between average pupil size and attention to faces (r(18) = .65, p = .002; see Figure 3) and proportion of time on eyes (r(18) = .45, p = .045). In HRA-, no significant associations were found.
Figure 3.
Correlation between average duration of time spent on the face and average pupil size in response to emotional faces at 9 months for LRC and HRA-. LRC showed a significant positive association, r(18)= .65, p = .002, with larger pupil diameter associated with increased attention to the face. HRA- showed no significant relation (p = .79).
Associations Between Eye-tracking (9 Months) and CSBS-DP (18 Months)
To address the second major aim of the present study, which asked whether early eye-tracking measures are associated with later social-communicative development, a series of correlations were performed to examine the relations between gaze and pupil responses to faces at 9 months and social-communicative behavior as assessed through the CSBS-DP at 18 months. The face scanning and pupil measures were identical to those included in the prior correlations. The CSBS-DP measures included percentile rank for 1) the social composite score and 2) the total score. Partial correlations, controlling for IQ using the MSEL ELC score at 18 months, were run between eye-tracking measures and CSBS-DP measures separately for each group. Additionally, regression models were run separately for each group with all four eye-tracking variables and the MSEL ELC as predictors of CSBS-DP scores.
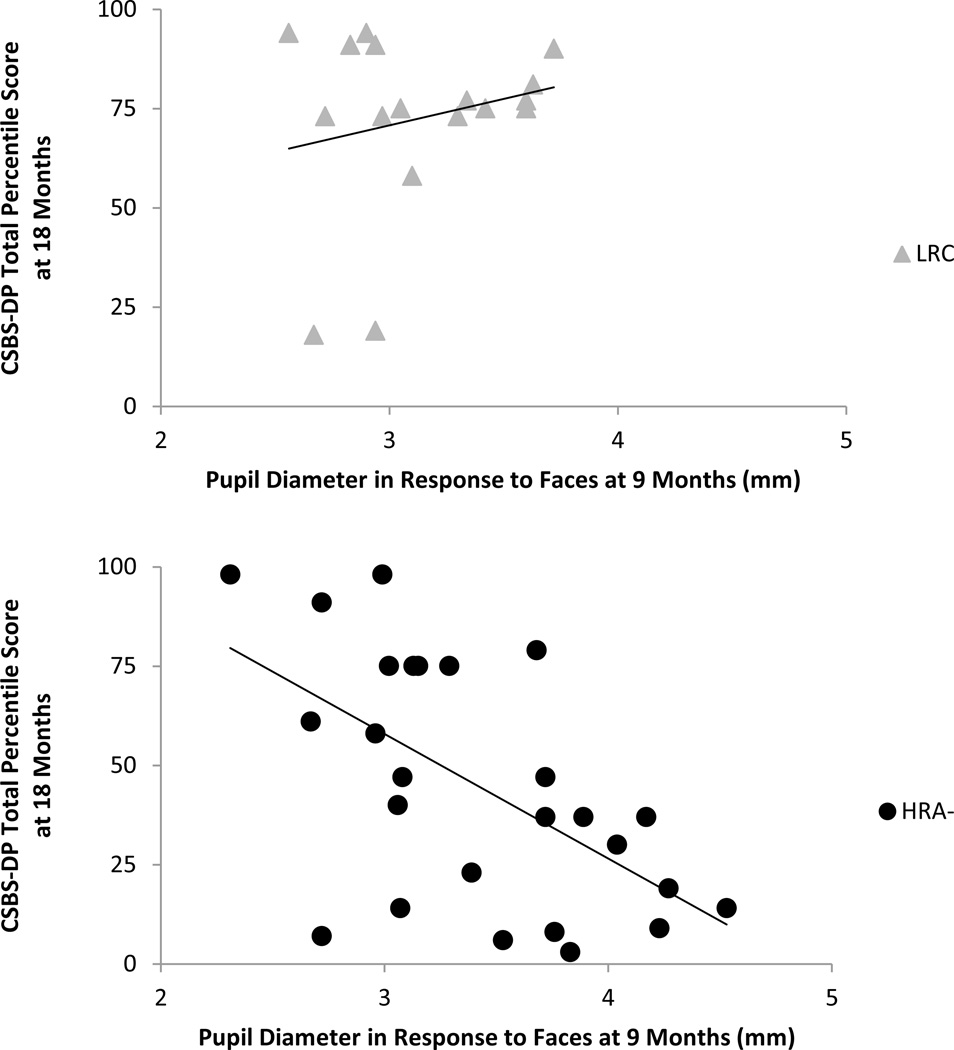
For the correlational analyses, in LRC, a significant positive relation was found between proportion of time spent on the eyes at 9 months and CSBS-DP social scores at 18 months, r(14) = .51, p = .044.. Analyses with HRA- infants showed a significant negative correlation between pupil size in response to faces at 9 months and CSBS-DP total scores at 18 months, r(22) = −.47, p = .021 (see Figure 4). No other associations were significant. Table 3 presents all correlations found for LRC and HRA-.
Figure 4.
Correlation between average pupil size in response to emotional faces at 9 months and CSBS-DP total percentile score at 18 months for LRC and HRA- after partialing out MSEL ELC at 18 months. HRA- showed a significant negative association, r(22) = −.47, p = .021, with larger pupil diameter at 9 months associated with worse social-communicative outcomes at 18 months. LRC showed no significant relation (p = .95).
Table 3.
Correlations between visual scanning at 9 months, pupil responses at 9 months, and CSBS scores at 18 months for LRC and HRA-
| CSBS at 18 months |
|||
|---|---|---|---|
| Pupil Response to Face at 9 months | CSBS Social Percentile | CSBS Total Percentile | |
| LRC | |||
| Time on Face | .65 (.002)** | . 23 (.40) | −. 03 (.92) |
| Prop. on Eyes | .45 (.045)* | .51 (.044)* | .12 (.66) |
| Prop. on Mouth | −. 36 (.12) | −.14 (.61) | −.44 (.092)^ |
| Pupil to Face | . 23 (.39) | .02 (.95) | |
| HRA- | |||
| Time on Face | .05 (.79) | . 10 (.64) | .13 (.56) |
| Prop. on Eyes | −.02 (.92) | −.03 (.89) | .24 (.26) |
| Prop. on Mouth | .17 (.37) | .06 (.79) | −.13 (.55) |
| Pupil to Face | −.18 (.40) | −.47 (.021)** | |
Note: LRC: Low-risk controls; HRA-: High-risk autism with no clinical diagnosis; CSBS: Communication and Symbolic Behavior Scales. All eye-tracking variables averaged across the three emotional faces. Mullen Scales of Early Learning Early Learning Score (MSEL ELC) partialed out of all CSBS correlations. Correlations (r) shown with p-value in parentheses.
p<0.1
p<0.05
p<0.025
Regression analyses were used to predict CSBS-DP scores from the eye-tracking variables of interest. For both LRC and HRA-, MSEL ELC was a significant positive predictor of CSBS-DP total scores (LRC: β = .62, p = .01; HRA-: β = .50, p = .008) and was therefore included in the model as a control variable. In HRA-, pupil response was a significant negative predictor of CSBS-DP total scores after controlling for the other eye-tracking variables and the MSEL ELC (β = −.37, p = .042). No other predictors were significant for CSBS-DP total scores for either group. For CSBS-DP social scores, regression analyses showed MSEL ELC as a marginal positive predictor for HRA- (β = .43, p = .053). In LRC, proportion of time on eyes was a marginal positive predictor of CSBS-DP social scores (β = .64, p = .09). No other predictors were significant for CSBS-DP social scores for either group (ps > .34).
To further explore the significant relation between pupil size at 9 months and CSBS-DP total score at 18 months in HRA- but not LRC, a univariate ANOVA was conducted. CSBS-DP total score was the dependent variable, and group and pupil size, as well as the interaction between these two variables, were tested as potential explanatory variables. This model found a significant interaction between group and average pupil response, F(1,39)= 5.64, p = .023, ηp2 = .13, confirming that the relation between pupil responses at 9 months and CSBS-DP total scores at 18 months differs for LRC and HRA-. For the remaining explanatory factors, group was a marginal predictor of CSBS-DP total scores (p = .053; see Table 1 for overall comparisons between LRC and HRA- on the CSBS-DP), but pupil size on its own was not a significant predictor (p = .35).
Discussion
In the present study we focused on two groups of infants who were followed longitudinally and received no clinical judgment of ASD or non-spectrum disorder at their most recent visit at 24 or 36 months: 1) infants with an older sibling with ASD (HRA-) and 2) infants with a typically-developing older sibling (LRC). By studying infants with and without familial risk for ASD who did not receive a subsequent clinical outcome, our findings focus on variability that is subclinical in nature and could therefore reflect an endophenotype in the HRA- infants. Scanning and pupil diameter in response to emotional faces was examined at 9 months and social-communicative behavior was examined at 18 months, and the following findings were revealed: 1) HRA- and LRC infants showed no differences in scanning patterns to emotionally-salient faces; 2) 9-month-old infants in both groups showed greater attention to eyes than mouths, and within each region, attention differed by emotion; 3) HRA- showed larger pupil size than LRC in response to emotional faces, 4) LRC infants at 9 months showed positive associations between pupil size and attention to face and eyes, and 5) associations between early eye-tracking measures and later social-communicative behavior revealed that HRA- infants showed a negative association between 9-month pupil size in response to faces and 18-month social-communicative outcome after controlling for IQ.
Studies with unaffected siblings in adolescence have shown atypicalities in behavioral and neural attention to faces akin to individuals with ASD (e.g., Dalton et al., 2007; Spencer et al., 2011); however the present study shows that HRA- and LRC infants show no overall differences in visual attention to faces, including attention to the eyes and mouth. This finding is in line with prior work with 6- to 11-month HRA infants showing similar attention to eyes, mouth, and faces overall as compared to LRC during viewing of static faces (Dundas et al., 2012; Key & Stone, 2012) and face-to-face interactions (Merin et al., 2007). Based on recent work by Jones and Klin (2013), it might be hypothesized that changes in attention over time would be a stronger measure for distinguishing groups based on a single visual attention measure, and future research should examine emergent differences in scanning patterns over the first year of life.
Consistent with past work, the present study found that when infants viewed static images of faces, they spent more time scanning the eyes than the mouth (e.g., Haith et al., 1977; Maurer & Salapatek, 1976; Peltola, Leppanen, Vogel-Farley, Hietanen, & Nelson, 2009; Wagner, Luyster, Yim, Tager-Flusberg, & Nelson, 2013). Further, it was found that attention to the eyes and mouth was modulated by the emotional expression being viewed. For looking to eyes, infants showed greatest attention to fearful faces; however, for the mouth, they showed greatest attention for happy faces. This work contributes to reports in infants, preschoolers, and adults that point to contrasting attention for positive versus negative emotional expressions (Hunnius et al., 2011; de Wit et al., 2008). For example, work by de Wit et al. (2008) found that typically-developing preschoolers, as well as those with ASD, spend more time on the eyes for negative emotions as compared with positive ones, theorizing that these differential scanning patterns could reflect attention to the areas conveying information most relevant for a particular emotional expression (i.e., the mouth for a happy expression; see also Dimberg & Petterson, 2000).
While several researchers have found atypicalities in autonomic responses in children and adolescents with ASD (e.g., Anderson & Colombo, 2009; Anderson et al., 2013; Bal et al., 2010; Hirstein et al., 2001; Joseph et al., 2008; Nuske, Vivanti, & Dissanayake, 2014; Nuske, Vivanti, Hudry, & Dissanayake, 2014; Van Hecke et al., 2009), only Nyström et al. (2015) has looked at autonomic activity in infants at high risk for developing ASD. Their study found heightened sensitivity in the PLR in a group of 10-month-old HRA infants as compared to LRC. The present study also found differences in pupil responses, with 9-month-old HRA- showing significantly larger pupil diameter when viewing emotionally-salient faces as compared to LRC. Non-reflexive pupillary responses have been discussed in terms of both the sympathetic and parasympathetic nervous system, with increased pupil diameter found in relation to 1) emotionally-arousing stimuli (e.g., Bradley et al., 2008; Siegle et al., 2003), processes mediated by the sympathetic nervous system, and 2) increased cognitive load or attention (e.g., Porter et al., 2007; Siegle et al., 2004), processes mediated by the parasympathetic nervous system. Work by Bradley et al. (2008) presented neurotypical adults with emotional faces while monitoring pupil diameter, heart rate, and skin conductance. By measuring pupil responses alongside two other measures of autonomic activity, Bradley et al. (2008) confirmed that pupil diameter in response to emotionally-salient faces is moderated by the sympathetic nervous system. Based on this past work, our finding suggests that larger pupil size in HRA- infants is related to heightened reactivity/arousal in sympathetic responses; however, future developmental studies will need to look at additional measures of autonomic functioning in order to identify the sympathetic and/or parasympathetic influences on emotional face processing in infants and young children.
In addition to increased pupil size in response to emotional faces in HRA- at 9 months as a group, a series of correlational tests revealed that HRA- infants with larger pupil size at 9 months showed lower social-communicative functioning at 18 months, pointing to a mechanism by which over-arousal in response to emotionally-salient stimuli might lead to cascading difficulties in social development for this high risk group. No previous work has examined predictive relations between autonomic arousal and later social abilities in unaffected siblings of children with ASD, however several studies have found concurrent relations between these measures in older children and adolescents with ASD, and together, this work suggests that successful modulation of sympathetic responses relates to better social functioning (e.g., Bal et al., 2010; Joseph et al., 2008; Van Hecke et al., 2009). Studies of the structure and function of the amygdala have also found associations between activation of the amygdala to faces and visual social attention (Dalton et al., 2005) as well as between the size of the amygdala and ASD symptomology (Schumann et al., 2009). Overall, this body of work with individuals with ASD raises questions of whether the regulation of these neural and physiological responses might not only have an impact on emotional reactivity during social situations, but whether individual differences in these responses might be associated with variability in social-communicative functioning (Van Hecke et al., 2009).
One additional notable finding from the present study was a positive association between pupil size and attention to face and eyes in 9-month-old LRC. While HRA- and LRC infants did not differ in overall attention to faces, individual differences among LRC showed that larger pupil size throughout the viewing of emotional faces was associated with greater time spent on the face and greater attention to the eyes. This finding suggests that LRC infants who have higher states of physiological arousal are more actively seeking out social information on the screen. Interestingly, in preschoolers with ASD, Nuske and colleagues (Nuske, Vivanti, & Dissanayake, 2014) found similar concurrent associations between amplitude of pupil responses and visual attention to face and eyes when viewing a familiar face expressing fear, while research on adolescents with ASD by Wagner et al. (Wagner, Hirsch, Vogel-Farley, Redcay, & Nelson, 2013) found a positive association between pupil size while viewing emotionally-salient faces and looking to the mouth region, a potential sign of aversion to eye gaze. These findings and the findings of others (e.g., Joseph et al., 2008) suggest that while heightened arousal might be adaptive in motivating social attention in typically-developing populations, hyperarousal in individuals with ASD, as well as those who share genetic risk, might lead to a decrease in successful social attention and functioning.
One important limitation of the present work is that there was no consistent baseline period administered during the eye-tracking session and therefore very little pre-task pupil data was available. Future work should include a fixed baseline period, allowing for more robust measures of baseline autonomic activation that could then be used to calculate a pupil difference score between baseline and task. Relatedly, with a difference in luminance levels between the inter-stimulus interval (i.e., the white screen) and the face stimuli on the white background, as well as differences in luminance levels between the testing room and the screen with the face stimuli, the present pupil findings might have been influenced by PLRs, as pupil size was calculated by averaging across all pupil data for looking in the face AOI for a given 10-second trial. Unlike studies with adults where participants can be instructed to remain still and maintain gaze on the screen for the duration of a trial, infant attention is less consistent (i.e., as reported above, infants spent, on average, 40% of their time attending to the faces). Without video recordings of infant behavior, to accompany eye-tracking data in the present study, there was no systematic way to determine if lost data samples within a given trial were due to an infant looking off the screen vs. an infant moving out of view of the eye-tracker while still looking at the face, among other possibilities. These lost samples make it difficult to identify when PLRs might be occurring, both those related to stimulus onset and those related to infant shifts of gaze off screen and back to the stimuli. With the findings of Nyström et al. (2015) showing stronger PLRs in HRA, it is important for future infant eye-tracking work to utilize advanced analysis methods alongside video recordings to separately examine the contributions of reflexive pupil responses and the contributions of content-driven pupil responses.
An additional limitation was that the present study did not quantitatively verify the calibration accuracy of the eye-tracker for each participant, and future studies should consider this important step to reach higher precision and robustness within the eye-tracking data. Finally, for the present analyses, it was deemed too conservative to correct the correlations for multiple comparisons based on the sample size, so follow-up work with larger samples will be needed to extend the correlational results.
The current work offers an important contribution to our understanding of how autonomic responses relate to the range of outcomes observed in unaffected family members of children with ASD (Bolton et al., 1994). This includes both heightened states of physiological arousal and predictive relations between this autonomic arousal in infancy and social-communicative difficulties nine months later. This predictive relation provides an important starting point for understanding mechanisms by which some unaffected siblings show more subclinical characteristics of ASD than others. Future work should continue to delineate the role of early autonomic responsivity in predicting later social difficulties as well as more ASD-specific characteristics.
Acknowledgments
This research was made possible, in part, by grants from Autism Speaks, the Simons Foundation, and the NIDCD (R21 DC 08637 & R01 DC 10290 to CAN and HTF), as well as the NIMH-funded Stuart T. Hauser Research Training Program in Biological and Social Psychiatry (to JBW).
References
- Adolphs R, Spezio ML, Parlier M, Piven J. Distinct face-processing strategies in parents of autistic children. Current Biology. 2008;18:1090–1093. doi: 10.1016/j.cub.2008.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CJ, Colombo J. Larger tonic pupil size in young children with autism spectrum disorder. Developmental Psychobiology. 2009;51:207–211. doi: 10.1002/dev.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CJ, Colombo J, Unruh KE. Pupil and salivary indicators of autonomic dysfunction in autism spectrum disorder. Developmental Psychobiology. 2013;55:465–482. doi: 10.1002/dev.21051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwin C, Chapman E, Colle L, Baron-Cohen S. Impaired recognition of negative basic emotions in autism: A test of the amygdala theory. Social Neuroscience. 2006;1:349–363. doi: 10.1080/17470910601040772. [DOI] [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network. Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR Surveillance Summary. 2014;63:1–21. [PubMed] [Google Scholar]
- Bal E, Harden E, Lamb D, Vaughan Van Hecke A, Denver JW, Porges SW. Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders. 2010;40:358–370. doi: 10.1007/s10803-009-0884-3. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Jolliffe T. Is there a “language of the eyes”? Evidence from normal adults, and adults with autism or Asperger Syndrome. Visual Cognition. 1997;4:311–331. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The Autism-spectrum Quotient (AQ): Evidence from Asperger syndrome/high functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Hammer J. Parents of children with Asperger syndrome: What is the cognitive phenotype? Journal of Cognitive Neuroscience. 1997;9:548–554. doi: 10.1162/jocn.1997.9.4.548. [DOI] [PubMed] [Google Scholar]
- Batty M, Taylor MJ. Early processing of the six basic facial emotional expressions. Cognitive Brain Research. 2003;17:613–620. doi: 10.1016/s0926-6410(03)00174-5. [DOI] [PubMed] [Google Scholar]
- Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, Bailey A, Rutter M. A case-control family history study of autism. Journal of Child Psychology & Psychiatry. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celani G, Battacchi MW, Arcidiacono L. The understanding of emotional meaning of facial expressions in people with autism. Journal of Autism and Developmental Disorders. 1999;29:57–66. doi: 10.1023/a:1025970600181. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Shic F. Looking but not seeing: Atypical visual scanning and recognition of faces in 2 and 4-year-old children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;39:1663–1672. doi: 10.1007/s10803-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biological Psychiatry. 2007;61:512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gemsbacher MA, Goldsmith HH, Davidson RJ. Gaze-fixation and the neural circuity of face processing in autism. Nature Reviews Neuroscience. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb S, Wijsman E, Schellenberg G, Estes A, Munson J, Faja S. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: Implications for a model of abnormal development of social brain circuitry in autism. Development and Psychopathology. 2005;17:679–697. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- de Klerk CCJM, Gliga T, Charman T, Johnson MH The BASIS. Face engagement during infancy predicts later face recognition ability in younger siblings of children with autism. Developmental Science. 2014;17:596–611. doi: 10.1111/desc.12141. [DOI] [PubMed] [Google Scholar]
- de Wit TC, Falck-Ytter T, von Hofsten C. Young children with autism spectrum disorder look differently at positive versus negative emotional faces. Research in Autism Spectrum Disorders. 2008;2:651–659. [Google Scholar]
- Dimberg U, Petterson M. Facial reactions to happy and angry facial expressions: Evidence for right hemisphere dominance. Psychophysiology. 2000;37:693–696. [PubMed] [Google Scholar]
- Dundas E, Gastgeb H, Strauss MS. Left visual field biases when infants process faces: A comparison of infants at high- and low-risk for autism spectrum disorder. Journal of Autism and Developmental Disorders. 2012;42:2659–2668. doi: 10.1007/s10803-012-1523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris L, Espie CAE, Knott F, Salt J. Mindreading difficulties in the siblings of people with Asperger’s syndrome: Evidence for a genetic influence in the abnormal development of a specific cognitive domain. Journal of Child Psychology and Psychiatry. 2004;45:412–418. doi: 10.1111/j.1469-7610.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Johnson MH. Getting answers from babies about autism. Trends in Cognitive Science. 2010;14:81–87. doi: 10.1016/j.tics.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Bedford R, Senju A, Charman T, Pickles A, Johnson MH BASIS Team. What you see is what you get: Contextual modulation of face scanning in typical and atypical development. Social Cognitive and Affective Neuroscience. 2014;9:538–543. doi: 10.1093/scan/nst012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Miles JH, Takahashi N, Yao G. Abnormal transient pupillary light reflex in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:1499–1508. doi: 10.1007/s10803-009-0767-7. [DOI] [PubMed] [Google Scholar]
- Fotiou DF, Stergiou V, Tsiptsios D, Lithari C, Nakou M, Karlovasitou A. Cholinergice deficiency in Alzheimer’s and Parkinson’s disease: Evaluation with pupillometry. International Journal of Psychophysiology. 2009;73:143–149. doi: 10.1016/j.ijpsycho.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Guillon Q, Hadjikhani N, Baduel S, Roge B. Visual social attention in autism spectrum disorders: Insights from eye tracking studies. Neuroscience and Biobehavioral Reviews. 2014;42:279–297. doi: 10.1016/j.neubiorev.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Haith MM, Bergman T, Moore MJ. Eye contact and face scanning in early infancy. Science. 1977;198:853–855. doi: 10.1126/science.918670. [DOI] [PubMed] [Google Scholar]
- Hirstein W, Iverson P, Ramachandran VS. Autonomic responses of autistic children to people and objects. Proceedings of the Royal Society of London B. 2001;268:1883–1888. doi: 10.1098/rspb.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnius S, de Wit TCJ, Vrins S, von Hofsten C. Facing threat: Infants’ and adults’ visual scanning of faces with neutral, happy, sad, angry, and fearful emotional expressions. Cognition and Emotion. 2011;25:193–205. doi: 10.1080/15298861003771189. [DOI] [PubMed] [Google Scholar]
- Jones EJH, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience and Biobehavioral Reviews. 2014;39:1–33. doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Klin A. Attention to eyes in present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013;504:427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R, Ehrman K, McNally R, Keehn B. Affective response to eye contact and face recognition ability in children with ASD. Journal of the International Neuropsychological Society. 2008;14:947–955. doi: 10.1017/S1355617708081344. [DOI] [PubMed] [Google Scholar]
- Kaartinen M, Puura K, Makela T, Rannisto M, Lemponen R, Helminen M, Hietanene JK. Autonomic arousal to direct gaze correlates with social impairments among children with ASD. Journal of Autism and Developmental Disorders. 2012;42:1917–1927. doi: 10.1007/s10803-011-1435-2. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Muller R, Townsend J. Atypical attentional networks and the emergence of autism. Neuroscience and Biobehavioral Reviews. 2013;37:164–183. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Wagner JB, Tager-Flusberg H, Nelson CA. Functional connectivity in the first year of life in infants at-risk for autism: A preliminary near infrared spectroscopy study. Frontiers in Human Neuroscience. 2013;7:444. doi: 10.3389/fnhum.2013.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key APF, Stone W. Processing of novel and familiar faces in infants at average and high risk for autism. Developmental Cognitive Neuroscience. 2012;2:244–255. doi: 10.1016/j.dcn.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Weaver K, Johnson LC, Greenson J, Dawson G, Aylward E. Association between amygdala response to emotional faces and social anxiety in autism spectrum disorders. Neuropsychologia. 2010;48:3665–3670. doi: 10.1016/j.neuropsychologia.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushki A, Drumm E, Mobarak MP, Tanel N, Dupuis A, Chau T, Anagnostou E. Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS ONE. 2013;8:e59730. doi: 10.1371/journal.pone.0059730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushki A, Brian J, Dupuis A, Anagnostou E. Functional autonomic nervous system profile in children with autism spectrum disorder. Molecular Autism. 2014;5:39. doi: 10.1186/2040-2392-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen JM, Nelson CA. Tuning the developing brain to social signals of emotions. Nature Reviews Neuroscience. 2009;10:37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen JM, Nelson CA. Early development of fear processing. Current Directions in Psychological Science. 2012;21:200–204. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule-WPS (ADOS-WPS) Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Luyster R, Powell C, Tager-Flusberg H, Nelson CA. Neural measures of social attention across the first years of life: Characterizing typical development and markers of autism risk. Developmental Cognitive Neuroscience. 2014;8:131–143. doi: 10.1016/j.dcn.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Salapatek P. Developmental changes in the scanning of faces by young infants. Child Development. 1976;47:523–527. [PubMed] [Google Scholar]
- Merin N, Young G, Ozonoff S, Rogers S. Visual fixation patterns during reciprocal social interaction distinguish a subgroup of 6-month-old infants at-risk for autism from comparison infants. Journal of Autism and Developmental Disorders. 2007;37:108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, Sigman M. Beyond autism: A Baby Siblings Research Consortium study of high-risk children at three years of age. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:300–308. doi: 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Julu POO, Brimacombe M, Connor S, Daniels ML. Reduced cardiac parasympathetic activity in children with autism. Brain and Development. 2005;27:509–516. doi: 10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen scales of early learning. Circle Pines, MN: American Guidance Service, Inc; 1995. [Google Scholar]
- Nelson CA, de Haan M. Neural correlates of infants’ visual responsiveness to facial expressions of emotion. Developmental Psychobiology. 1996;29:577–595. doi: 10.1002/(SICI)1098-2302(199611)29:7<577::AID-DEV3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Varcin K, Coman N, DeVivo I, Tager-Flusberg H. Shortened telomeres in families with a propensity to autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2015;54:588–594. doi: 10.1016/j.jaac.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Norwood A, Wagner JB, Motley C, Hirsch SB, Vogel-Farley VK, Nelson CA. Behavioral and electrophysiological indices of memory in typically-developing and hypoxic-ischemic injured infants. Infancy. 2014;19:28–52. [Google Scholar]
- Nuske HJ, Vivanti G, Dissanayake C. Are emotion impairments unique to, universal, or specific in autism spectrum disorder? A comprehensive review. Cognition & Emotion. 2013;27:1042–1061. doi: 10.1080/02699931.2012.762900. [DOI] [PubMed] [Google Scholar]
- Nuske HJ, Vivanti G, Dissanayake C. Reactivity to fearful expressions of familiar and unfamiliar people in children with autism: An eye-tracking pupillometry study. Journal of Neurodevelopmental Disorders. 2014;6:1–14. doi: 10.1186/1866-1955-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuske HJ, Vivanti G, Hudry K, Dissanayake C. Pupillometry reveals reduced unconscious emotional reactivity in autism. Biological Psychology. 2014;101:24–35. doi: 10.1016/j.biopsycho.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Nyström P, Gredebäck G, Bölte S, Falck-Ytter T EASE team. Hypersensitive pupillary light reflex in infants at risk for autism. Molecular Autism. 2015;6:10. doi: 10.1186/s13229-015-0011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Stone WL. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 2011;128:488–495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hutman T, Johnson S, Iosif A. The broader autism phenotype in infancy: When does it emerge? Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:398–407. doi: 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. Journal of Autism and Developmental Disorders. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Peltola MJ, Leppanen JM, Maki S, Hietanen JK. Emergence of enhanced attention to fearful faces between 5 and 7 months of age. Social Cognitive and Affective Neuroscience. 2009;4:134–142. doi: 10.1093/scan/nsn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola MJ, Leppanen JM, Vogel-Farley VK, Hietanen JK, Nelson CA. Fearful faces but not fearful eyes alone delay attention disengagement in 7-month-old infants. Emotion. 2009;9:560–565. doi: 10.1037/a0015806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter G, Troscianki T, Gilchrist ID. Effort during visual search and counting: Insights from pupillometry. Quarterly Journal of Experimental Psychology. 2007;60:211–229. doi: 10.1080/17470210600673818. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vulleumier P. Eletrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex. 2004;14:619–633. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ, Outhred T, Hickie IB, Kemp AH. Heart rate variability is associated with emotion recognition: Direct evidence for a relationship between the autonomic nervous system and social cognition. International Journal of Psychophysiology. 2012;86:168–172. doi: 10.1016/j.ijpsycho.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Righi G, Nelson CA. The neural architecture and developmental course of face processing. In: Rakic P, Rubenstein J, editors. Comprehensive developmental neuroscience. San Diego, CA: Elsevier Press; 2013. pp. 331–349. [Google Scholar]
- Rutter M, Bailey A, Lord C. Social communication questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Schoen SA, Miller LJ, Brett-Green B, Hepburn SL. Psychophysiology of children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2008;2:417–429. [Google Scholar]
- Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biological Psychiatry. 2009;66:942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Johnson MH. Atypical eye contact in autism: Models, mechanisms, and development. Neuroscience and Biobehavioral Reviews. 2009;33:1204–1214. doi: 10.1016/j.neubiorev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Siegel B. Pervasive developmental disorders screening test -II. San Antonio, TX: PsychCorp; 2004. [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil diameter in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003;27:365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME. Pupillary assessment and computational modeling of the Stroop task in depression. International Journal of Psychophysiology. 2004;52:63–76. doi: 10.1016/j.ijpsycho.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Holt RJ, Chura LR, Suckling J, Calder AJ, Bullmore ET, Baron-Cohen S. A novel functional brain imaging endophenotype of autism: The neural response to facial expression of emotion. Translational Psychiatry. 2011;1:e19. doi: 10.1038/tp.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H. The origins of social impairments in autism spectrum disorder: Studies of infants at risk. Neural Networks. 2010;23:1072–1076. doi: 10.1016/j.neunet.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott MR, Nelson CA, Tager-Flusberg H. Maternal gesture use and language development in infant siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2015;45:4–14. doi: 10.1007/s10803-013-1820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong F, Nakayama K, Moscovitch M, Weinrib O, Kanwisher N. Response properties of the human fusiform face area. Cognitive Neuropsychology. 2000;17:257–279. doi: 10.1080/026432900380607. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey B, Nelson CA. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn Van Hecke A, Lebow J, Bal E, Lamb D, Harden E, Kramer A, Denver J, Bazhenova O, Porges SW. Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Development. 2009;80:1118–1133. doi: 10.1111/j.1467-8624.2009.01320.x. [DOI] [PubMed] [Google Scholar]
- Wagner JB, Luyster RJ, Yim JY, Tager-Flusberg H, Nelson CA. The role of early visual attention in social development. International Journal of Behavioral Development. 2013;37:118–124. doi: 10.1177/0165025412468064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JB, Hirsch SB, Vogel-Farley V, Redcay E, Nelson CA. Eye-tracking, autonomic, and electrophysiological correlates of emotional face processing in adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2013;43:188–199. doi: 10.1007/s10803-012-1565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace S, Sebastian C, Pellicano E, Parr J, Bailey A. Face processing abilities in relatives of individuals with ASD. Autism Research. 2010;3:345–349. doi: 10.1002/aur.161. [DOI] [PubMed] [Google Scholar]
- Wetherby AM, Allen L, Cleary J, Kublin K, Goldstein H. Validity and reliability of the communication and symbolic behavior scales developmental profile with very young children. Journal of Speech and Hearing Research. 2002;45:1202–1218. doi: 10.1044/1092-4388(2002/097). [DOI] [PubMed] [Google Scholar]
- Young G, Merin N, Rogers S, Ozonoff S. Gaze behavior and affect at 6 months: Predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;12:798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]