Recent advances in targeted therapies have bypassed triple-negative breast cancer because of its tremendous heterogeneity and the lack of defined molecular targets. This article reviews molecular characterization, current treatment paradigms, and the emerging role of newer agents in triple-negative breast cancer.
Keywords: Cancer biomarkers, Cancer chemotherapy agents, Cancer genetics, Triple-negative breast neoplasms
Abstract
Triple-negative breast cancer (TNBC) accounts for 15% of all breast cancers and is associated with poor long-term outcomes compared with other breast cancer subtypes. Because of the lack of approved targeted therapy, at present chemotherapy remains the mainstay of treatment for early and advanced disease. TNBC is enriched for germline BRCA mutation, providing a foundation for the use of this as a biomarker to identify patients suitable for treatment with DNA-damaging agents. Inherited and acquired defects in homologous recombination DNA repair, a phenotype termed "BRCAness," may be present in a large proportion of TNBC cases, making it an attractive selection and response biomarker for DNA-damaging therapy. Triple-negative breast cancer is a diverse entity for which additional subclassifications are needed. Increasing understanding of biologic heterogeneity of TNBC has provided insight into identifying potentially effective systemic therapies, including cytotoxic and targeted agents. Numerous experimental approaches are under way, and several encouraging drug classes, such as immune checkpoint inhibitors, poly(ADP-ribose) polymerase inhibitors, platinum agents, phosphatidylinositol-3-kinase pathway inhibitors, and androgen receptor inhibitors, are being investigated in TNBC. Molecular biomarker-based patient selection in early-phase trials has the potential to accelerate development of effective therapies for this aggressive breast cancer subtype. TNBC is a complex disease, and it is likely that several different targeted approaches will be needed to make meaningful strides in improving the outcomes.
Implications for Practice:
Triple-negative breast cancer (TNBC) is an aggressive subtype that is associated with poor outcomes. This article reviews clinical features and discusses the molecular diversity of this unique subtype. Current treatment paradigms, the role of germline testing, and platinum agents in TNBC are reviewed. Results and observations from pertinent clinical trials with potential implications for patient management are summarized. This article also discusses the clinical development and ongoing clinical trials of novel promising therapeutic agents in TNBC.
Abstract
摘要
三阴性乳腺癌 (TNBC) 占所有乳腺癌的15%, 其长期转归比其他乳腺癌亚型都要差。由于目前尚无靶向疗法获批, 目前化疗仍然是早期和晚期TNBC的主要治疗手段。TNBC中生殖系BRCA突变发生率很高, 这是将BRCA突变作为鉴别适合DNA损伤剂治疗的患者的生物标记物的理论基础。DNA同源重组修复的遗传性和获得性缺陷 (即BRCAness表型) 可能存在于很大比例的TNBC病例中, 因此将其作为DNA损伤治疗的选择和应答生物标记物颇具吸引力。三阴性乳腺癌是一种多样性疾病, 有必要进一步细分亚组。对TNBC生物学异质性的不断了解已经使得我们对找到潜在的有效系统治疗 (包括细胞毒性药物和靶向制剂) 有了深入的认识。大量的实验研究正在开展之中, 对包括免疫检查点抑制剂、多 (ADP-核糖) 聚合酶抑制剂、铂类制剂、磷脂酰肌醇-3-激酶通路抑制剂以及雄激素受体抑制剂在内的众多令人振奋的药物种类在TNBC中的应用进行研究。在早期阶段的临床试验中, 基于分子生物标记物对患者进行选择, 有可能加速针对这种侵袭性乳腺癌亚型的有效疗法的研发。TNBC是一种复杂的疾病, 很可能需要对多个不同的靶向途径加以干预方能在改善转归方面取得重大进展。The Oncologist 2016;21:1059–1062
对临床实践的提示: 三阴性乳腺癌 (TNBC) 是一种转归不佳的侵袭性乳腺癌亚型。本文回顾了这一独特亚型的临床特征, 并就其分子多样性进行了讨论。本文还回顾了TNBC目前的治疗方案、生殖系检测的作用以及铂类制剂的应用。此外, 本文对可能对患者管理具有意义的相关临床试验的结果和观察数据进行了总结, 还对在 TNBC 中颇具前景的新药的临床研发和正在开展的临床试验进行了讨论。
Introduction
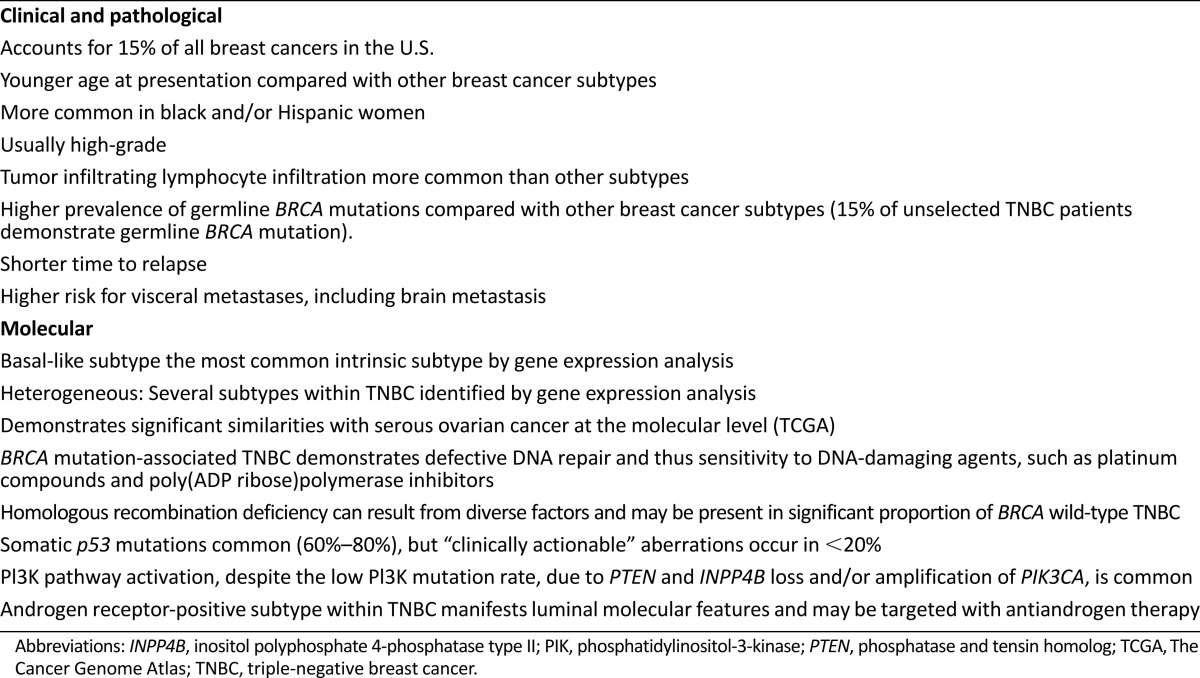
Triple-negative breast cancer (TNBC), which is defined by the lack of expression of estrogen receptor (ER) and progesterone receptor (PgR) and absence of ERBB2 (HER2) overexpression and/or gene amplification, accounts for 15% of all breast cancers in the U.S. [1–4]. TNBC is the most fatal subtype of breast cancer and is associated with poor long-term outcomes compared with other breast cancer subtypes [5–7]. TNBC demonstrates some unique clinical and molecular characteristics, which are summarized in Table 1. Compared with other breast cancer subtypes, TNBC usually demonstrates high pathologic grade, more frequently affects younger women, is more prevalent in black women, and shows a higher prevalence of germline BRCA mutation [8–12]. During the past two decades, institution and/or enhancement of targeted therapies has improved the outcomes of HER2-amplified and hormone-positive breast cancers. However, these recent advances in targeted therapies have bypassed triple-negative breast cancer because of its tremendous heterogeneity and the lack of defined molecular targets. This article reviews molecular characterization, current treatment paradigms, and the emerging role of newer agents in TNBC.
Table 1.
Clinical and molecular characteristics of triple-negative breast cancer
Molecular Characterization of TNBC
In recent years, significant progress has been made in unraveling the biological diversity of TNBC and linking gene expression patterns to distinct molecular subtypes with potential therapeutic associations [13–16].
Molecular Subtypes
The seminal work by Perou et al. categorized breast cancer by gene expression profiling into four intrinsic subtypes [13]. The basal-like subtype comprises a group of tumors characterized by the absence or low levels of expression of estrogen receptors, very low prevalence of HER2 overexpression, and expression of genes usually found in the basal or myoepithelial cells of the human breast [13]. Although most TNBCs fall into the basal-like intrinsic subtype on the PAM50 intrinsic subtyping assay, the overlap between immunohistochemically defined TNBC and basal-like molecular subtype is not complete. Various studies demonstrate that 70%–80% of TNBCs are basal-like on molecular profiling and 20%–30% of non-triple-negative breast cancers are basal-like on molecular profiling [15, 17, 18]. Thus, caution should be used when using the term "basal-like" to refer to TNBCs at large. Further refinement of the original Perou-Sorlie gene expression profiling has identified a claudin-low subset within the basal-like subtype. Claudin-low tumors are characterized by the absence of luminal differentiation markers, enrichment for epithelial-mesenchymal-transition markers, immune response genes, low proliferation, cancer stem cell-like features, and poor prognosis. However, the therapeutic implications of the claudin-low subset are not yet clear [19].
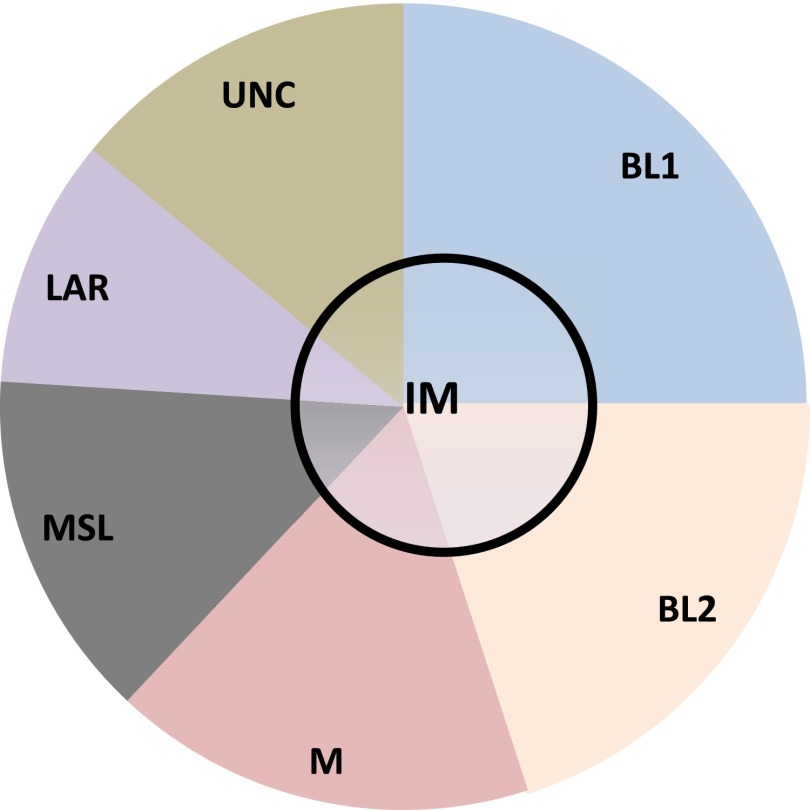
Triple-negative breast cancer is a diverse entity for which additional subclassifications may be needed, and grouping TNBC into basal and nonbasal subtypes may be oversimplifying the molecular heterogeneity of this disease. Using gene expression from publicly available data sets, Lehmann et al. classified TNBC into seven molecular subtypes: basal-like 1, basal-like 2, mesenchymal (M), mesenchymal stem cell-like (MSL), immunomodulatory (IM), luminal androgen receptor (AR)-like (LAR), and unclassified [14]. On the basis of identification of a cell line corresponding to each subtype, they also demonstrated that these subtypes may be responsive to different targeted therapies (Fig. 1). The methods of molecular classification used by Lehmann et al. have recently been simplified to an RNA-seq platform to better fit individual clinical samples (TNBCtype; InsightGenetics, Nashville, TN, http://www.insightgenetics.com) [20]. There is a modest degree of overlap between the subtypes identified by these different gene expression investigations. The MSL and M subtypes closely correspond to the previously described “claudin-low” subtype, the LAR subtype may fit more closely with the “luminal” intrinsic type, and the IM subtype may in fact reflect the tumor microenvironment rather than the tumor itself [15, 18].
Figure 1.
Proposed molecular subtypes of TNBC.
Abbreviations: BL1, basal-like 1; BL2, basal-like 2; IM, immunomodulatory (is likely distributed within all TNBC subtypes); LAR, luminal androgen receptor/luminal-like; M, mesenchymal; MSL, mesenchymal-stem cell-like; TNBC, triple-negative breast cancer; UNC, unknown classification.
It is speculated that heterogeneity of both the tumor and the microenvironment contributes to the transcriptome diversity noted in TNBC. Furthermore, some of this diversity could also stem from the discrete global methylation patterns in TNBC. For example, recent methylome sequencing of The Cancer Genome Atlas (TCGA) samples has identified three prognostically distinct methylation clusters in TNBC [21]. Despite variation in the number/types of subclasses identified by different transcriptome analysis, one common theme has emerged—there are biologically distinct subsets within TNBC. These subclasses respond differently to standard chemotherapy and will likely also display differential responses to novel targeted agents.
Targetable Alterations Are Not Common
Development and refinement of next-generation sequencing have improved our understanding of the prevalence of somatic mutations in various cancers. Mutation or loss of TP53 occurs at a high frequency in TNBC. In TGCA, 68% of primary TNBC tumors were found to have TP53 mutation, with an additional 3% demonstrating homozygous deletion of the gene [22]. These findings were confirmed by Shah et al., who reported that on exome sequencing of 102 primary TNBCs, TP53 mutation was the most frequent clonal event (53.8%), followed by PIK3CA mutations (10.7%) [16]. To date, there are no available effective agents to target TP53 mutations, although efforts to develop such agents are ongoing [23]. Absence of high-frequency, targetable oncogenic drivers in TNBC has hindered the development of successful therapeutic strategies [16, 24]. The frequency and coexistence of various genomic alterations in TNBC also evolve under the pressure of systemic chemotherapy. For example, profiling of residual TNBC tumor tissue after neoadjuvant chemotherapy revealed higher frequency of several potentially targetable alterations compared with basal-like primary breast cancers in TCGA. These included alteration in the phosphatase and tensin homolog (PTEN)/phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) pathway (noted in 40% of samples); amplifications of JAK2; and CDK6, CCND1, CCND2, and CCND3 amplification. Therefore, there are promising opportunities for studying targeted therapy in appropriately selected patients with residual disease after neoadjuvant chemotherapy. Several ongoing phase I/II studies are investigating phosphatidylinositol-3-kinase (PI3K) inhibitors in advanced TNBC, and early-phase studies are also assessing Janus kinase 2 and cyclin-dependent kinase inhibitors in hormone-negative breast cancer.
Molecular Similarities Between Basal-Like Breast Cancers and Serous Ovarian Cancer
Interestingly, TCGA analysis noted striking contrast between the basal-like breast cancers and luminal/human epidermal growth receptor 2 (HER2) breast cancer subtypes. However, comparison of basal-like breast cancers with high-grade serous ovarian cancers demonstrated prominent molecular similarities (BRCA1 inactivation, RB1 loss, high expression of AKT1, high frequency of TP53 mutation, and MYC amplification) [22]. This is an important observation and suggests that common therapeutic strategies should be explored for serous ovarian cancer and TNBC (e.g., platinum agents, poly[ADP-ribose] polymerase [PARP] inhibitors [PARPi]).
Diagnosis and Clinical Behavior
Diagnosis of TNBC requires ER, PgR, and HER2 status testing. Testing and the cutoffs for ER, PR, and HER2 status were developed to determine the likelihood of response to endocrine and HER2-directed therapy, respectively, and not to specifically identify the “triple-negative” phenotype. Thus, during the past decade ER, PR, and HER2 cutoffs used to describe TNBC have varied. Most contemporary studies are now using the current American Society of Clinical Oncology–College of American Pathologists guidelines for determining ER/PgR and HER2 negativity (ER and PgR nuclear staining of less than 1% by immunohistochemistry [IHC] and HER2 IHC staining of 0 to 1+ or fluorescent in situ hybridization <2.0 if IHC 2+ or IHC not performed [2, 3].
TNBC is associated with not only higher but also an earlier risk for relapse. Hazard rates for distant recurrence are highest for TNBC in the first 2 years after diagnosis, and relapses after 5 years are uncommon [6, 25]. Compared with hormone-positive breast cancer, TNBC is characterized by a higher proportion of visceral relapse and short survival after development of metastatic disease [7, 26]. Median survival of patients with metastatic TNBC is only 12–18 months, compared with 5 years among patients with metastatic HER2-positive breast cancer, highlighting the pressing need for identification of more effective systemic therapies for this subgroup [27].
TNBC and Germline BRCA Mutation
Compared with other subtypes of breast cancers, women with TNBC have a higher prevalence of germline BRCA mutations [11, 12, 24, 28]. Various studies have demonstrated that 15%–20% of women with TNBC carry germline BRCA1/2 mutations. Most genetic testing guidelines include TNBC subtype as an independent criterion for hereditary breast and/or ovarian cancer syndrome (HBOC) counseling and testing recommendation. The National Comprehensive Cancer Network guidelines recommend genetic risk assessment of all TNBC patients and HBOC testing for all TNBC patients aged ≤60 years regardless of family history (http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf). Despite these recommendations, financial constraints, insurance coverage, and access to genetic counseling/testing continue to be important challenges for optimal use of HBOC testing in the clinical setting [11].
Current State of Management of Early-Stage Disease
Systemic Chemotherapy
Because of the lack of molecular targets, chemotherapy is the only available systemic treatment for TNBC, and therefore adjuvant chemotherapy is recommended for TNBC patients with stage I (tumor size >0.5 cm)–III disease [4, 29–31]. Currently, the chemotherapy recommendations for early-stage TNBC are not unique to this subtype but are identical to the recommendations for other breast cancer subtypes. Most guidelines recommend anthracycline-taxane-based chemotherapy for stage I–III TNBC.
Despite receiving standard anthracycline-taxane-based chemotherapy, a substantial proportion (30%–40%) of patients with early-stage TNBC develop metastatic disease and die of the cancer [32–34]. Even with overall poor outcomes, it is evident that a subset of TNBC patients respond well to standard-of-care chemotherapy combinations and that patients who achieve pathological complete response (pCR) after neoadjuvant chemotherapy have excellent long-term survival. However, despite achieving higher rates of pCR with conventional chemotherapy, TNBC phenotype is associated with higher relapse rates than hormone receptor-positive and HER2-positive breast cancers, a phenomenon known as the triple-negative paradox [5–7, 35]. This paradox is primarily driven by very high relapse rates in the subgroup of TNBC patients with residual disease after neoadjuvant chemotherapy. Therefore, there is a need to develop predictive markers to identify TNBC patients who are likely to have excellent outcomes with standard chemotherapy so that research efforts can be focused on patients who are most likely to recur after standard neoadjuvant therapy.
Even with overall poor outcomes, it is evident that a subset of TNBC patients respond well to standard-of-care chemotherapy combinations and that patients who achieve pathological complete response after neoadjuvant chemotherapy have excellent long-term survival.
Local Therapy: Surgery and Radiation
Several large retrospective analyses from individual clinical trials have demonstrated that tumor infiltrating lymphocytes (TILs) are prognostic in early-stage TNBC [36–39]. Presence and increasing percentage of TILs are associated with better response to anthracycline-based neoadjuvant chemotherapy and improved long-term survival in TNBC patients treated with adjuvant anthracycline chemotherapy. However, it is not yet clear whether the prognostic effect of TILs reflects underlying favorable tumor biology or whether TILs can in fact predict improved response to certain chemotherapy drugs. TILs are not yet part of routine clinical pathology reports, although efforts to standardize pathological evaluation and reporting of TILs are ongoing [40]. Prospectively integrating TILs into neoadjuvant trials for TNBC may help to better stratify patients and identify good-prognosis subgroups that may not need therapy intensification. Future research efforts will also assess the clinical utility of TILs in the setting of immune checkpoint inhibitors (programmed death 1 [PD-1] and programmed death ligand 1 [PD-L1] blockade). In the past decade, several retrospective transcriptional gene expression profiling investigations have sought to identify multigene signatures that can predict response to chemotherapy with anthracycline or taxane or both in TNBC [41–43]. One such signature is being prospectively evaluated in the setting of a neoadjuvant study (https://clinicaltrials.gov/ct2/show/NCT02276443). Currently, we do not have any clinically available biomarkers to identify TNBC patients who are likely to have excellent response and outcome with standard anthracycline/taxane neoadjuvant therapy, although efforts to identify such markers are aggressively being pursued.
Local Therapy: Surgery and Radiation
The principles for local therapy (surgery and radiation) for breast cancer are applied in a similar fashion for all breast cancer subtypes, and there are no TNBC-specific recommendations for local management. During the past decade, mastectomy rates in women with breast cancer have been rising in the U.S., and this trend has been noted with TNBC as well. Some of the recent studies suggest that more than 50% of women with operable TNBC are choosing to undergo mastectomy [44, 45]. High prevalence of germline mutations, family history of breast cancer, and availability of acceptable reconstruction options are all factors that likely contribute to the high mastectomy rates, especially in younger women with TNBC.
Role of Novel Chemotherapy and Biologics in Early-Stage Disease
Attempts to improve upon the fourth-generation adjuvant anthracycline/taxane chemotherapy regimens in TNBC have thus far been unsuccessful. Studies have demonstrated that addition of a fourth chemotherapy drug (gemcitabine) to an anthracycline/cyclophosphamide/taxane backbone or substitution of paclitaxel for novel chemotherapy (ixabepilone) does not improve outcomes in early-stage TNBC [46–48]. Angiogenesis is considered to be an important target for cancer therapy. Of agents in this class, bevacizumab has been the most widely studied in breast cancer.
In 2008, the U.S. Food and Drug Administration (FDA) approved bevacizumab (Avastin, a vascular endothelial growth factor inhibitor; Genentech, South San Francisco, CA, https://www.gene.com) in combination with paclitaxel as a first-line treatment for metastatic HER2-negative breast cancer based on the progression-free survival improvement noted with addition of bevacizumab to weekly paclitaxel in the Eastern Cooperative Oncology Group 2100 trial [49]. However, subsequent trials assessing addition of bevacizumab to first-line chemotherapy (AVADO and RIBBON-1 trials) failed to show a significant benefit in overall survival despite small improvements in progression-free survival [50, 51]. A meta-analysis of phase III trials with bevacizumab as first-line treatment for metastatic breast cancer demonstrated improved progression-free survival; however, no significant improvement in overall survival was observed, and addition of bevacizumab was associated with a significant increase in grade 3–4 toxicities [52]. On the basis of these data, the FDA revoked the metastatic breast cancer approval of bevacizumab in 2011. Subgroup analysis of some trials that added bevacizumab or sorafenib (an oral multikinase inhibitor, with antiproliferative and antiangiogenic activity) to chemotherapy showed a hint of greater benefit in TNBC patients [53, 54]. Unfortunately, randomized studies of adjuvant bevacizumab have failed to demonstrate improvement in overall survival in patients with TNBC [55, 56]. It is possible that a subgroup of TNBC may benefit from antiangiogenesis therapy, but lack of markers to predict benefit from such an approach and modest toxicity associated with antiangiogenesis agents have limited further development of this class of agents for TNBC.
Role of Platinum Agents
Sporadic and germline cases of BRCA mutation-associated TNBC share several pathological and molecular similarities [32, 57, 58]. The phenotypic and molecular similarities between BRCA1 mutation-associated and sporadic TNBC have led many to surmise that a significant proportion of BRCA wild-type TNBCs may involve BRCA1 pathway dysfunction through alternative mechanisms. Thus, BRCA1-directed therapeutic approaches (such as platinum agents and PARPi are being explored for a general population of patients with TNBC. Platinum agents are not new to the treatment of breast cancer. In the 1980s, cisplatin was evaluated in advanced breast cancer in two phase II studies and demonstrated significant single-agent frontline activity, with response rates in the range of 50%–54% [59, 60]. Because of its toxicity, cisplatin was subsequently abandoned and replaced by other active agents with more favorable toxicity profiles (taxanes, fluoropyridines). However, more recently there has been renewed interest in exploring platinum agents in TNBC and BRCA mutation-associated breast cancers.
Repair of platinum-induced interstrand crosslinks invokes BRCA1-mediated homologous recombination (HR), and there is abundant clinical and in vitro evidence that BRCA1-deficient cells are hypersensitive to platinum agents [61–63]. Observational, small neoadjuvant, and metastatic studies have demonstrated that BRCA mutation-associated breast cancers are sensitive to platinum agents [61, 63–67]. In a phase II study, single-agent cisplatin yielded an impressive 80% response rate in BRCA1 mutation-associated metastatic breast cancer [63]. A recent randomized phase III trial demonstrated that in unselected metastatic TNBC, carboplatin and docetaxel were equal in efficacy as first-line treatment [65]. However, in BRCA mutation-associated TNBC, carboplatin yielded a superior response rate and progression-free survival compared with docetaxel.
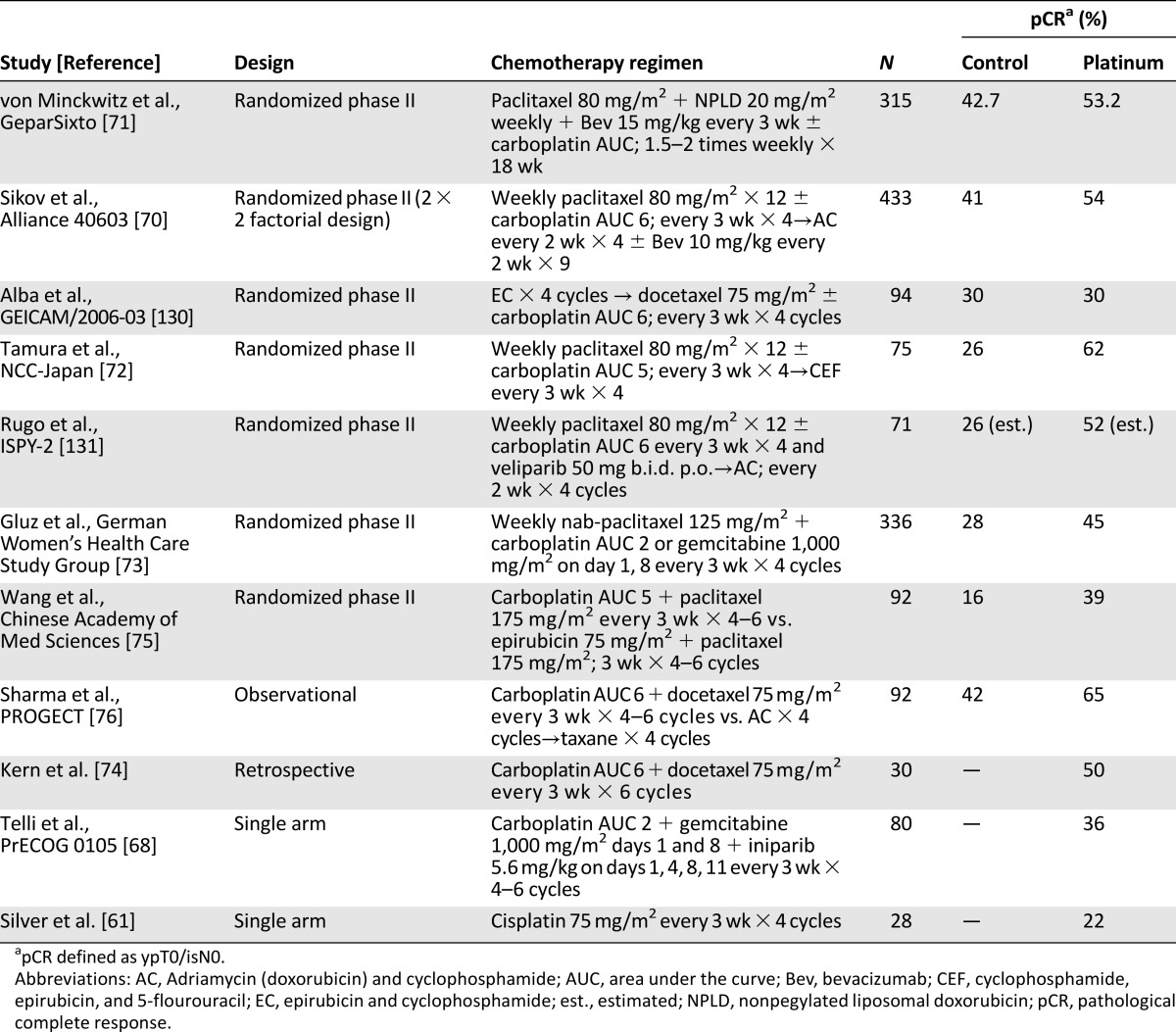
Growing evidence suggests that platinum compounds may be active in a significantly larger number of TNBC patients beyond germline BRCA mutation carriers [68, 69]. Recent studies have focused on the role of platinum agents when used as a component of neoadjuvant therapy (Table 2). Three randomized studies have demonstrated that the addition of neoadjuvant carboplatin to anthracycline/taxane-based chemotherapy improves pCR in patients with stage I–III TNBC (pCR improved from 41% to 54% with addition of carboplatin) [70–72]. Other investigators studying anthracycline-free platinum regimens have reported encouraging pCR rates ranging from 36% to 65% [68, 73–76].
Table 2.
Neoadjuvant clinical trials with platinum agents in triple-negative breast cancer
The improvement in pCR attained with the addition of carboplatin to anthracycline/taxane chemotherapy comes at the cost of increase in toxicity. In both Cancer and Leukemia Group B (CALGB) 40603 and GeparSixto, dose reductions or omissions were needed in 40%–50% of patients. Furthermore, the long-term outcomes from the addition of platinum in the neoadjuvant setting are not yet clear. Event-free survival (EFS) and overall survival (OS) data from CALGB 40603 and GeparSixto clinical trials were recently presented [77, 78]. In GeparSixto, 3-year EFS improved 44% with the addition of concurrent carboplatin to an anthracycline + taxane + bevacizumab chemotherapy backbone. On the other hand, in CALGB 40603 the addition of sequential carboplatin did improve pCR rate, but 3-year EFS or OS did not significantly improve. In both trials, the positive effect of pCR on long-term outcomes (EFS and OS) was confirmed and the hazard ratios for 3-year EFS favored carboplatin. However, neither of these two trials was powered sufficiently for EFS and OS endpoints and thus cannot be considered definitive studies to answer the question of clinical utility of platinum agents for early-stage TNBC.
We need adequately powered studies to determine the long-term benefits of platinum agents in early-stage TNBC. The optimal dose, sequence, and chemotherapy backbone for efficacious incorporation of platinum into treatment of early-stage TNBC are also not yet known. Several ongoing randomized phase III trials are evaluating various schedules and combinations of platinum in early-stage TNBC. NRG-BR003 (NCT02488967), Chinese TPPC (NCT02455141), and the Korean PEARLY (NCT02441933) studies are all evaluating efficacy of sequential adjuvant platinum (platinum vs. placebo) when added to Adriamycin (doxorubicin) and cyclophosphamide (AC)/epirubicin and cyclophosphamide followed by taxane chemotherapy backbone. Eastern Cooperative Oncology Group–American College of Radiology Imaging Network 1131 (NCT02445391) will study adjuvant platinum in TNBC patients who have basal-like residual disease after neoadjuvant anthracycline/taxane chemotherapy. Neoadjuvant Brightness (NCT02032277) is assessing addition of carboplatin or carboplatin + PARPi (veliparib) to AC, followed by paclitaxel in TNBC patients stratified by germline BRCA status. GeparOcto (NCT02125344) will evaluate addition of weekly carboplatin, bevacizumab, or both to neoadjuvant anthracycline/taxane backbone. Another phase II neoadjuvant study is comparing AC followed by paclitaxel plus carboplatin to anthracycline-free docetaxel plus carboplatin regimen (NCT02413320).
While we await the completion and outcomes from these randomized studies, oncologists are still faced with decisions about the utility of platinum agents for TNBC in day-to-day practice. The ideal approach for patients and physicians is to seek participation in one of the many ongoing platinum trials. If a suitable trial is not available, the decision for incorporation of platinum into neoadjuvant treatment of a patient with TNBC should be individualized. Although long-term outcome data are not clear, the individual patient benefit from attainment of pCR may still justify use of neoadjuvant platinum in select patients. Most important, given the molecular heterogeneity of TNBC, it is very likely that platinum agents will benefit only a subgroup of patients with TNBC. Ongoing and future translational studies (described in the following section) are focusing on identifying TNBC patients most likely to benefit from platinum therapy.
Homologous Recombination Defects and DNA-Damaging Therapy
HR is a DNA repair mechanism responsible for repair of double-strand DNA breaks. BRCA1/2 and other Fanconi anemia pathway genes (RAD51D, NBN, ATM) are key components of the HR-mediated DNA repair. Germline BRCA1/2 mutations are the prototype molecular alterations that confer homologous recombination deficiency and sensitivity to DNA damaging therapy.
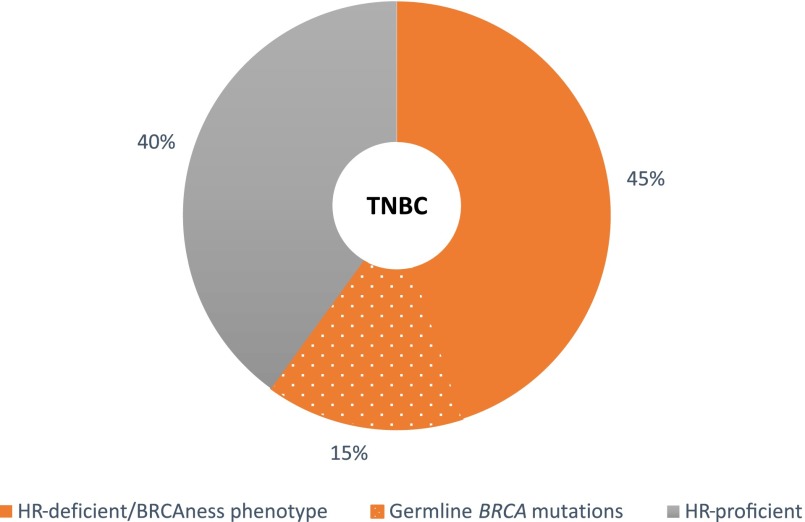
Inherited and acquired defects in homologous recombination, a phenotype called, as mentioned earlier, "BRCAness," may lead to therapeutic exploitation in breast cancer. To this end, development and clinical evaluation of platforms to identify markers of BRCAness have been a subject of intense investigation, especially in TNBC, a subtype thought to be enriched for BRCAness [58, 79–83]. Approximately 10%–20% of TNBCs harbor detectable germline BRCA1/2 mutations [12, 22, 24, 61, 84]. However, DNA repair may be altered through other mechanisms, such as somatic or germline mutation in other genes, DNA methylation, or attenuated mRNA expression. It is estimated that if these factors beyond germline BRCA mutations are comprehensively evaluated, 50%–60% of TNBC will demonstrate HR deficiency or BRCAness, making it an attractive selection and response biomarker for DNA-damaging therapy, such as platinum compounds and PARPi (Fig. 2) [58, 68, 80, 81, 85]. It is speculated that DNA-damaging therapy may be most active in tumors with germline BRCA mutations and in BRCA wild-type tumors that harbor the BRCAness phenotype.
Figure 2.
HR deficiency in triple-negative breast cancer.
Abbreviations: HR, homologous recombination; TNBC, triple-negative breast cancer.
Germline BRCA mutation status is beginning to emerge as an important predictive marker of response to platinum agents in TNBC. The randomized TNT study demonstrated that in the metastatic setting, patients with germline BRCA1 or BRCA2 mutation experienced significantly greater response and progression-free survival with carboplatin compared with docetaxel [65]. A smaller nonrandomized study also demonstrated that rate of response to platinum (first-/second-line treatment) in metastatic TNBC was significantly higher in germline BRCA1/2 carriers than in noncarriers [69]. In the GeparSixto neoadjuvant study, TNBC patients with germline BRCA1/2 or Rad 50/51c (another gene involved in DNA repair) mutations had higher overall pCR rates and larger increments in the pCR rate with the addition of carboplatin [86]. The significance of germline BRCA mutation status to predict selective response to platinum agents is being prospectively evaluated in an ongoing randomized neoadjuvant trial (INFORM) that is comparing four cycles of cisplatin with four cycles of doxorubicin/cyclophosphamide in patients with germline BRCA mutations (NCT01670500).
Interestingly, in the GeparSixto study, a strongly positive family history of breast and/or ovarian cancer, even in the absence of an identifiable mutation, was also associated with a higher incremental increase in pCR rate with the addition of carboplatin.
Interestingly, in the GeparSixto study, a strongly positive family history of breast and/or ovarian cancer, even in the absence of an identifiable mutation, was also associated with a higher incremental increase in pCR rate with the addition of carboplatin. This latter group accounted for 30% of patients enrolled in the trial. This observation supports the notion that other multigenic alterations (beyond germline BRCA mutations) affecting HR-DNA repair pathway are present in a substantial proportion of TNBC patients.
Currently, a standard platform for detecting HR deficiency or BRCAness beyond germline BRCA mutations has not reached routine clinical application. However, several promising assays are emerging and have been retrospectively evaluated. The homologous recombination deficiency (HRD) assay developed by Myriad Genetics Inc. (Salt Lake City, UT, https://www.myriad.com) evaluates tumor genome loss of heterozygosity, telomeric allelic imbalance, and large-scale state transitions, which are all indirect measures of tumor genomic instability. High HRD scores are highly correlated with defects in BRCA1/2 and are associated with sensitivity to neoadjuvant platinum-based chemotherapy in TNBC [68, 83]. An array comparative genomic hybridization (aCGH) signature resembling BRCA1- and BRCA2-mutant breast cancers has also been reported to predict response to high-dose platinum therapy in a retrospective study [87, 88]. In addition to genomic instability, tumors with BRCAness may also exhibit characteristic gene expression patterns. A 44-gene DNA-damage response deficiency signature, which was developed in cohorts enriched for germline BRCA1/2 and Fanconi anemia mutations, predicted favorable response to chemotherapy with 5-flourouracil, epirubicin, and cyclophosphamide in patients with triple-negative breast cancer [81]. All of the assays described here are compatible with formalin-fixed, paraffin-embedded tissues, making them suitable for evaluation in prospective studies. An ongoing neoadjuvant trial (TBCRC 030, NCT01982448) is randomly assigning patients to cisplatin or weekly paclitaxel to assess the ability of the HRD assay to predict pathological complete response with platinum versus taxane in TNBC patients without a BRCA mutation. Another ongoing study is using the aCGH BRCA-like assay to determine whether neoadjuvant intensified alkylating chemotherapy improves the response rates in tumors with HRD (NCT01057069). An upcoming randomized phase II trial (S1416) will use multiple BRCAness markers to predict benefit from addition of PARPi to platinum chemotherapy in metastatic TNBC.
Using functional measures of HR pathway deficiency, rather than relying on documented changes in specific genes, should identify more patients who might benefit from DNA-damaging therapies. If appropriately validated, HRD assays could have a tremendous effect on treatment of TNBC by identifying patients most likely to benefit from DNA-damaging agents, such as platinum salts and/or PARPi.
Identification and Development of Novel Targeted Agents: Promise on the Horizon
Immune Checkpoint Inhibitors
As our understanding of the relationship between breast cancer biology and immunity is expanding, it is allowing for new advances in immunotherapy for breast cancer patients. Cancers use multiple mechanisms to evade the immune response. PD-1 is an antigen expressed on activated T cells, pro-B cells, natural killer cells, dendritic cells, and monocytes. PD-1 and its ligands, PD-L1 and PD-L2, play a major role in maintenance of T-cell tolerance [89, 90]. PD-1 and PD-L1 are aberrantly expressed in basal-like breast cancer [91, 92]. Their expression parallels that of the TILs, suggesting negative feedback activation as part of the immune reaction. Preclinical data support the concept that blockade of immune checkpoints may be an effective treatment strategy for TNBC. Supporting these preclinical findings, there is now emerging evidence of the clinical efficacy of agents targeting PD-1/PD-L1 in TNBC.
Pembrolizumab (MK-3475), a monoclonal antibody specific for PD-1, was evaluated in a phase I study of 32 TNBC patients. In this heavily pretreated population, pembrolizumab led to an overall response rate of 18.5%, including one complete response and four partial responses. Another phase I clinical trial evaluated atezolizumab (MPDL3280A), an anti-PD-L1 monoclonal antibody, in nine metastatic TNBC patients and demonstrated a similar overall response rate of 33%, including one complete response and two partial responses [93, 94]. On the basis of these encouraging phase I data, both of these antibodies are now being evaluated in larger studies. A randomized phase III trial will assess nab-paclitaxel with or without atezolizumab (MPDL3280A) in patients with previously untreated metastatic TNBC (IMpassion130, NCT02425891). A phase III study will evaluate the addition of neoadjuvant atezolizumab (MPDL3280A) to carboplatin and nab-paclitaxel in patients with locally advanced TNBC (NCT02620280). An upcoming randomized phase III trial (Southwestern Oncology Group [SWOG] 1418) will assess efficacy of adjuvant pembrolizumab compared with placebo in TNBC patients who have residual disease after neoadjuvant chemotherapy. Another phase II study is assessing pembrolizumab plus doxorubicin in patients with metastatic TNBC (NCT02648477). Nivolumab (another PD-1 antibody) is being studied in combination with various chemotherapy drugs and radiation in advanced TNBC in the TONIC trial (NCT02499367). Studies with these promising immune checkpoint inhibitors are still at their beginning in TNBC, with many interesting clinical trials ongoing. Results of these ongoing trials will direct the future application of immune therapy in TNBC.
PARPi
PARP enzymes recognize DNA damage and facilitate DNA repair to maintain genomic stability. Preclinical studies demonstrate that PARP inhibition in the presence of BRCA deficiency leads to synthetic lethality. PARPi have shown preclinical and clinical activity in targeting tumors with pre-existing DNA repair defects, in particular BRCA1- and BRCA2-deficient advanced breast and ovarian tumors [95–102]. The FDA has recently approved monotherapy with olaparib, a PARPi, as a first-in-class drug to treat germline BRCA mutation-associated advanced refractory ovarian cancers (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427554.htm). Several ongoing studies are assessing the activity of PARPi alone or in combination with chemotherapy for germline BRCA-associated metastatic and early-stage breast cancers. A randomized phase III (Olympia AD NCT02032823) study is evaluating adjuvant olaparib in patients with germline BRCA-associated TNBC or high-risk hormone-positive breast cancer. An ongoing phase II/III trial is comparing carboplatin and paclitaxel with or without ABT-888 (veliparib) in patients with BRCA mutation-associated advanced breast cancer (NCT01506609). Two phase III randomized trials (OlympiaAD and EMBRACA) and are comparing chemotherapy of physician’s choice with the single-agent PARPi olaparib and talazoparib (BMN-673), respectively, in BRCA mutation-associated advanced breast cancer (NCT01945775, NCT02000622).
Because a substantial proportion of TNBCs are thought to harbor DNA repair defects, it might be possible to extend the observation of PARPi sensitivity of germline BRCA-associated tumors to BRCA wild-type TNBCs that harbor a BRCAness phenotype. Accordingly, PARPi are being explored in the general population of patients with TNBC. As monotherapy, PARPi have demonstrated limited activity in breast cancer not associated with BRCA mutation [101, 103]. The efficacy of PARPi in BRCA wild-type TNBC is likely to be observed only in tumors with a BRCAness phenotype and not in all BRCA wild-type TNBC. Furthermore, BRCA wild-type TNBC with a BRCAness phenotype may harbor only partial homologous recombination defects, and PARPi monotherapy may lead to “synthetic sickness” rather than synthetic lethality, necessitating the presence of robust DNA-damaging chemotherapy with PARP inhibition to achieve cell death. Thus, several studies are also looking at combination of PARPi and platinum-based DNA-damaging chemotherapy in TNBC. The Brightness study (NCT02032277) will assess the activity of veliparib in combination with carboplatin in neoadjuvant setting in both BRCA-associated and wild-type TNBC. SWOG 1416 will use a combination of PARPi and cisplatin to test for PARPi activity in both BRCA-associated and BRCAness phenotype metastatic TNBC.
PI3K/AKT/mTOR Pathway Inhibitors
Inhibition of PI3K and the downstream components AKT and mTOR are recognized as promising targets for treatment of breast cancer. Activating mutations in PIK3CA are noted in 9% of primary basal-like breast cancers [22]. However, inferred PI3K pathway activation, through loss of PTEN and inositol polyphosphate 4-phosphatase type II (INPP4B), frequently occur in basal-like breast cancers [22, 104–106].
This high frequency (approximately 50%) of PI3K pathway alteration in TNBC makes this pathway a promising target for therapeutics, and inhibitors of PI3K, AKT, and/or mTOR are in clinical development. Two randomized phase II studies are evaluating AKT inhibitors (AZD5363, GDC-0068) in combination with paclitaxel as front-line treatment for metastatic triple-negative breast cancer (PAKT/NCT02423603, NCT02162719). Another randomized phase II study will assess the efficacy of preoperative GDC-0068 in combination with paclitaxel in stage I–III TNBC (NCT02301988).
A phase II, single-arm study of BYL719 (a selective PI3K α inhibitor) monotherapy in the second-line setting for advanced metastatic breast cancer (NCT02506556) is under way. Another ongoing phase I/II study is evaluating combination of BYL719 with nab-paclitaxel in HER2-negative metastatic breast cancer (NCT02379247).
PI3K blockade promotes HR deficiency by downregulating BRCA1/2 and thus sensitizing BRCA-proficient tumors to PARP inhibition [107, 108]. To capitalize on these findings, a phase I study of the pan-PI3K inhibitor BKM120 in combination with the PARPi olaparib in patients with metastatic TNBC is ongoing (NCT01623349).
Heat Shock Protein 90 and Histone Deacetylase Inhibitors
Histone deacetylase (HDAC) modulates the transcription rate and the protein levels of several components of the DNA-damage response cascade [109–114]. Heat-shock protein 90 (HSP90) chaperones “client” proteins into their native conformations, regulating multiple aspects of protein function. Multiple components of the HR and nonhomologous end joining DNA repair machinery (e.g., CHK1, BRCA1, BRCA2, RAD51, FANCA) are clients of HSP90 [115–117]. HDAC inhibitors induce hyperacetylation of HSP90 and dissociate client proteins, such as BRCA1, from the chaperone. In vitro studies have also demonstrated that HDAC inhibitor vorinostat and HSP90 inhibitor AUY922 were ranked near the top for inducing the HRD-like gene expression profiles in TNBC cell lines [118]. Thus, treatment with HDAC inhibitors can increase the therapeutic efficacy of DNA-damaging agents, such as platinum compounds in TNBC. Indeed, in vitro studies show that cotreatment with a pan-HDAC inhibitor and cisplatin synergistically induced apoptosis of both BRCA1-mutant and BRCA1-proficient cell lines and HDAC inhibitor treatment induces synergistic lethality with PARPi and cisplatin in triple-negative breast cancer cell lines [119–121].
Clinical studies of HSP90 and HDAC inhibitors are in early stages right now. An ongoing phase I study is assessing safety and dosing of an HSP90 inhibitor (AT13387) and paclitaxel combination in advanced TNBC (NCT02474173). An upcoming preoperative trial is studying combination of ganetespib (HSP90 inhibitor) with paclitaxel (NCT02637375). On the basis of preclinical synergy of HSP90 and PARPi, an upcoming phase I study will assess combination of PARPi (BMN 673) and HSP90 inhibitor (AT13387) in advanced solid tumors, including TNBC (NCT 02627430). A phase II trial investigating combination treatment of entinostat (HDAC inhibitor) and the DNA methyltransferase inhibitor azacitidine in patients with chemotherapy-resistant advanced TNBC is also under way (NCT01349959). An ongoing phase I/II study is evaluating combination of cisplatin with romidepsin (class I HDAC inhibitor) in metastatic TNCB or BRCA mutation-associated HER2-negative metastatic breast cancer (NCT02393794).
Androgen-Targeted Therapy
On immunohistochemistry, approximately 10%–15% of TNBCs express AR [122–124]. On gene expression analysis, 12% of TNBCs (LAR or highly enriched for AR and classified as the LAR subtype) [14]. AR IHC is often used as a surrogate for the LAR subtype. Compared with other TNBC subtypes, the AR+ subtype appears to be relatively chemoresistant but displays better long-term prognosis. Primary tumor AR analysis has demonstrated that AR+ TNBC patients exhibit lower pCR rates with anthracycline/taxane chemotherapy, however, and have better disease-free survival and overall survival compared with AR− TNBC patients [125]. Similarly, a retrospective neoadjuvant analysis also demonstrated that the LAR molecular subtype is associated with lower pCR rates compared with other TNBC subtypes [126]. Preclinical data demonstrates that pharmacological inhibition of AR by bicalutamide greatly decreased cell viability and tumor growth [14, 16]. The TBCRC001 phase II proof-of-concept trial of bicalutamide (oral AR inhibitor) in 26 patients with metastatic AR+ (IHC ≥ 10%) TNBC demonstrated a clinical benefit rate of 19% at 24 weeks [127]. A recent study reported encouraging activity of a next-generation, novel androgen-targeted drug, enzalutamide, in AR+ TNBC. This trial enrolled 118 women with AR+ TNBC. More than 50% of the patients received enzalutamide as a first- or second-line therapy for their metastatic disease. Of the 75 patients who could be evaluated for response, the response rate and clinical benefit rate were 8% and 35%, respectively [128]. In addition to AR IHC, this trial also reported on the positive association of molecular assays (PREDICT AR) for identification of TNBC patients most likely to benefit from this approach [129]. Together, these emerging data provide a strong rationale for prospectively identifying AR+ TNBC patients and aligning these patients to clinical trials of androgen-targeted therapies.
In addition to AR dependency, LAR TNBC cell lines commonly harbor activating mutation in the kinase domain of PIK3CA and display sensitivity to PIK3CA inhibitors [14]. Thus, the antiandrogen and PI3K inhibitor combination is also being explored in a phase I/II study of taselisib (GDC-0032) and enzalutamide in patients with AR+ (≥10%) metastatic TNBC (NCT02457910)
Other Agents
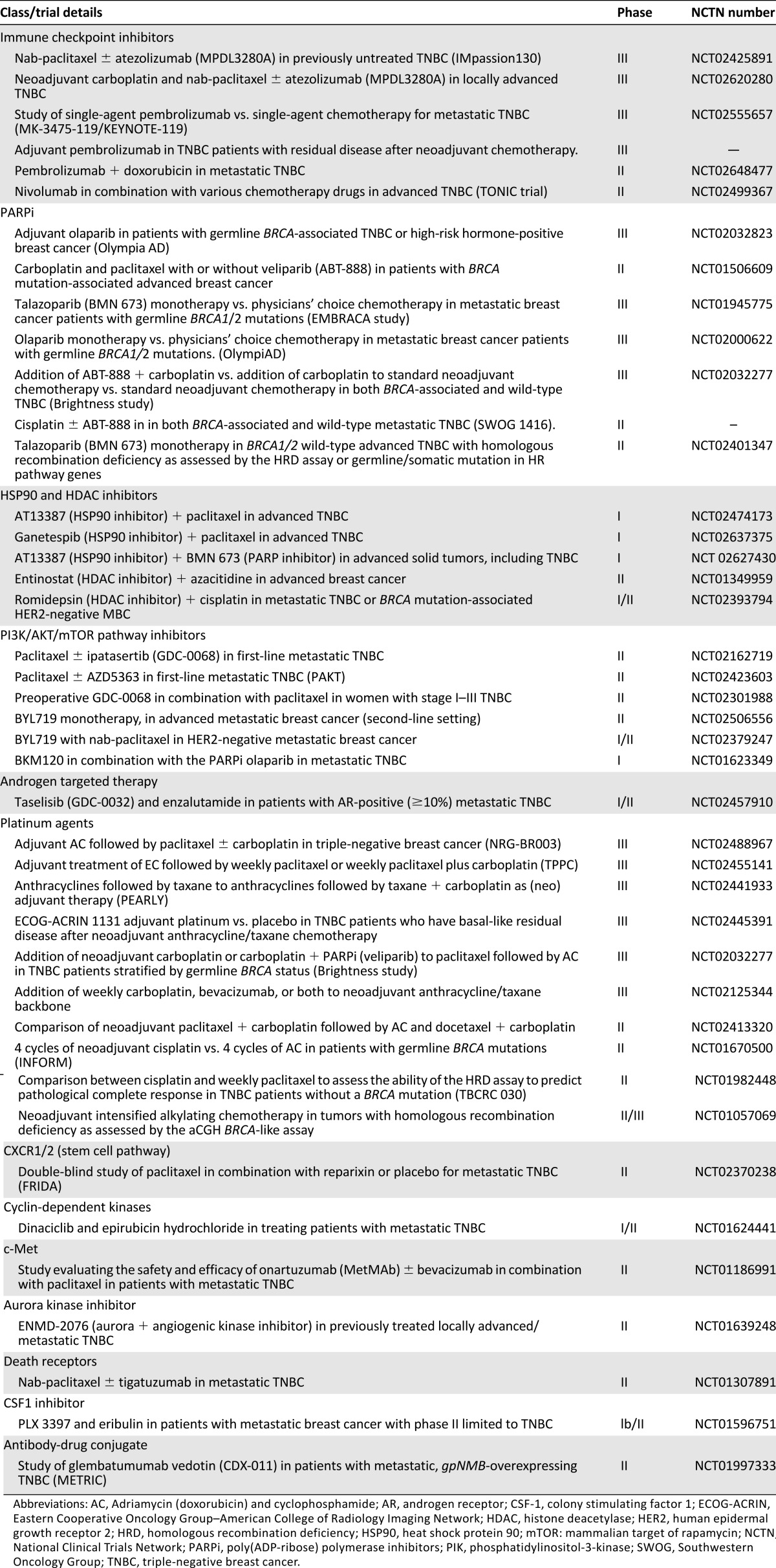
In addition to the agents described here, other targeted agents are also under development in TNBC (Table 3). Reparixin (inhibitor of interleukin-8 activation of CXCR1/CXCR2 chemokine receptors) is being tested in combination with paclitaxel in a randomized phase II study. Antibody drug conjugate CDX-011 (glembatumumab vedotin) is being compared with capecitabine in a randomized phase II study in patients with glycoprotein NMB-overexpressing, metastatic TNBC (the METRIC study). Several other agents are also being investigated in early-phase studies (Table 3).
Table 3.
Selected active clinical trials of novel agents in treatment of triple negative breast cancer.
Conclusion
TNBC is a small but heterogeneous subtype of breast cancer. Because of the lack of approved targeted therapy, chemotherapy remains the mainstay of treatment for early and advanced disease. Modern technology platforms have contributed immensely to our current understanding of the molecular diversity of this subtype. These molecular advances have enabled us to start ascertaining promising therapeutic targets in TNBC. Numerous experimental approaches are under way, and several encouraging drug classes, such as immune checkpoint inhibitors, PARPi, platinum agents, and PI3K inhibitors, are being investigated in clinical trials. Current research efforts are focused on optimizing the traditional drugs by applying them to patients and tumors that will benefit the most, and by studying newer drugs in biologically selected patient subgroups. New-generation TNBC trials are beginning to embed the concept of heterogeneity, and investigations in smaller molecularly identified subgroups of TNBC are emerging. TNBC is a complex disease, and it is likely that several different targeted approaches will be needed to make meaningful strides in improving the outcomes.
Disclosures
Priyanka Sharma: Novartis, Celgene (RF).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 2.Hammond ME, Hayes DF, Wolff AC, et al. American Society Of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff AC, Hammond MEH, Hicks DG, et al. American Society of Clinical Oncology. College of American Pathologists Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 4.Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 6.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 7.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 8.Trivers KF, Lund MJ, Porter PL, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009;20:1071–1082. doi: 10.1007/s10552-009-9331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livasy CA, Karaca G, Nanda R, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Modern Pathol . 2006;19:264–271. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Klemp JR, Kimler BF, et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: Implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treat. 2014;145:707–714. doi: 10.1007/s10549-014-2980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Angulo AM, Timms KM, Liu S, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17:1082–1089. doi: 10.1158/1078-0432.CCR-10-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5:5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah SP, Roth A, Goya R, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastien RR, Rodríguez-Lescure Á, Ebbert MT, et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genomics. 2012;5:44. doi: 10.1186/1755-8794-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prat A, Adamo B, Cheang MC, et al. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. The Oncologist. 2013;18:123–133. doi: 10.1634/theoncologist.2012-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ring BZ, Hout DR, Morris SW, et al. Generation of an algorithm based on minimal gene sets to clinically subtype triple negative breast cancer patients. BMC Cancer. 2016;16:143. doi: 10.1186/s12885-016-2198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stirzaker C, Zotenko E, Song JZ, et al. Methylome sequencing in triple-negative breast cancer reveals distinct methylation clusters with prognostic value. Nat Commun. 2015;6:5899. doi: 10.1038/ncomms6899. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leijen S, Schellens JH, Shapiro G, et al. A phase I pharmacological and pharmacodynamic study of MK-1775, a Wee1 tyrosine kinase inhibitor, in monotherapy and combination with gemcitabine, cisplatin, or carboplatin in patients with advanced solid tumors. J Clin Oncol. 2010 doi: 10.1200/JCO.2016.67.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartman AR, Kaldate RR, Sailer LM, et al. Prevalence of BRCA mutations in an unselected population of triple-negative breast cancer. Cancer. 2012;118:2787–2795. doi: 10.1002/cncr.26576. [DOI] [PubMed] [Google Scholar]
- 25.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 26.Dent R, Hanna WM, Trudeau M, et al. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 27.Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couch FJ, Hart SN, Sharma P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;33:304–311. doi: 10.1200/JCO.2014.57.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senkus E, Kyriakides S, Penault-Llorca F, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi7–23. doi: 10.1093/annonc/mdt284. [DOI] [PubMed] [Google Scholar]
- 30.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies-improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): breast cancer. 2014. Available at http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. [DOI] [PubMed]
- 32.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 33.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 34.Tan DS, Marchió C, Jones RL, et al. Triple negative breast cancer: Molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Res Treat. 2008;111:27–44. doi: 10.1007/s10549-007-9756-8. [DOI] [PubMed] [Google Scholar]
- 35.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 36.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHer trial. Ann Oncol 2014;25:1544–1550. [DOI] [PubMed]
- 38.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieci MV, Criscitiello C, Goubar A et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: A retrospective multicenter study. Ann Oncol 2015;26:1518. [DOI] [PMC free article] [PubMed]
- Salgado R, Denkert C, Demaria S et al. The evaluation of tumor-infiltrating lymphocytes (tils) in breast cancer: Recommendations by an international TILS working group 2014. Ann Oncol 2015;26:259–271. [DOI] [PMC free article] [PubMed]
- 41.Hatzis C, Pusztai L, Valero V, et al. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA. 2011;305:1873–1881. doi: 10.1001/jama.2011.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayers M, Symmans WF, Stec J, et al. Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer. J Clin Oncol. 2004;22:2284–2293. doi: 10.1200/JCO.2004.05.166. [DOI] [PubMed] [Google Scholar]
- 43.Gianni L, Zambetti M, Clark K, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005;23:7265–7277. doi: 10.1200/JCO.2005.02.0818. [DOI] [PubMed] [Google Scholar]
- 44.Connor CS, Kimler BF, Mammen JM, et al. Impact of neoadjuvant chemotherapy on axillary nodal involvement in patients with clinically node negative triple negative breast cancer. J Surg Oncol. 2015;111:198–202. doi: 10.1002/jso.23790. [DOI] [PubMed] [Google Scholar]
- 45.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: Surgical results from CALGB 40603 (Alliance) Ann Surg. 2015;262:434–439; discussion 438–439. doi: 10.1097/SLA.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swain SM, Tang G, Geyer CE, Jr, et al. Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: The NSABP B-38 trial. J Clin Oncol. 2013;31:3197–3204. doi: 10.1200/JCO.2012.48.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yardley DAB, Bosserman LD, Keaton MR, et al. TITAN: Phase III study of doxorubicin/cyclophosphamide (AC) followed by ixabepilone (Ixa) or paclitaxel (Pac) in early-stage, triple-negative breast cancer (TNBC) J Clin Oncol. 2015:33:1000a. [Google Scholar]
- 48.Wolfgang J, Schneeweiss A, Haeberle A, et al. Adjuvant gemcitabine for high-risk breast cancer (BC) patients: Final survival results of the randomized phase III SUCCESS-A study. J Clin Oncol. 2014:32:1010a. [Google Scholar]
- 49.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 50.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 51.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- Rossari JR, Metzger-Filho O, Paesmans M, et al. Bevacizumab and breast cancer: A meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J Oncol 2012;2012:417673. [DOI] [PMC free article] [PubMed]
- 53.Brufsky A, Valero V, Tiangco B, et al. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: Subgroup analysis of the RIBBON-2 trial. Breast Cancer Res Treat. 2012;133:1067–1075. doi: 10.1007/s10549-012-2008-6. [DOI] [PubMed] [Google Scholar]
- 54.Baselga J, Segalla JG, Roché H, et al. Sorafenib in combination with capecitabine: An oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol. 2012;30:1484–1491. doi: 10.1200/JCO.2011.36.7771. [DOI] [PubMed] [Google Scholar]
- 55.Miller K, O'Neill AM, Dang CT, et al. Bevacizumab (Bv) in the adjuvant treatment of HERr2-negative breast cancer: Final results from Eastern Cooperative Oncology Group E5103. J Clin Oncol. 2014:32:500a. [Google Scholar]
- 56.Cameron D, Brown J, Dent R, et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): Primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14:933–942. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 57.Prat A, Cruz C, Hoadley KA, et al. Molecular features of the basal-like breast cancer subtype based on BRCA1 mutation status. Breast Cancer Res Treat. 2014;147:185–191. doi: 10.1007/s10549-014-3056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lips EH, Mulder L, Oonk A, et al. Triple-negative breast cancer: BRCAness and concordance of clinical features with BRCA1-mutation carriers. Br J Cancer. 2013;108:2172–2177. doi: 10.1038/bjc.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kolarić K, Roth A, Jelicic I, et al. Phase II clinical trial of cis dichlorodiammine platinum (Cis DDP) in metastatic brain tumors. J Cancer Res Clin Oncol. 1982;104:287–293. doi: 10.1007/BF00406247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sledge GW, Jr, Loehrer PJ, Sr, Roth BJ, et al. Cisplatin as first-line therapy for metastatic breast cancer. J Clin Oncol. 1988;6:1811–1814. doi: 10.1200/JCO.1988.6.12.1811. [DOI] [PubMed] [Google Scholar]
- 61.Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santarosa M, Del Col L, Tonin E, et al. Premature senescence is a major response to DNA cross-linking agents in BRCA1-defective cells: Implication for tailored treatments of BRCA1 mutation carriers. Mol Cancer Ther. 2009;8:844–854. doi: 10.1158/1535-7163.MCT-08-0951. [DOI] [PubMed] [Google Scholar]
- 63.Byrski T, Dent R, Blecharz P, et al. Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res. 2012;14:R110. doi: 10.1186/bcr3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hurley J, Reis IM, Rodgers SE, et al. The use of neoadjuvant platinum-based chemotherapy in locally advanced breast cancer that is triple negative: Retrospective analysis of 144 patients. Breast Cancer Res Treat. 2013;138:783–794. doi: 10.1007/s10549-013-2497-y. [DOI] [PubMed] [Google Scholar]
- Tutt A, Ellis P, Kilburn L et al. The TNT trial: A randomized phase III trial of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer (CRUK/07/012). J Cancer Res 2015;75:S3-01.
- 66.von Minckwitz G, Blohmer JU, Costa SD, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31:3623–3630. doi: 10.1200/JCO.2012.45.0940. [DOI] [PubMed] [Google Scholar]
- 67.Sharma PS, Stecklein S, Kimler BF et al. BRCA1 insufficiency is predictive of superior survival in patients with triple negative breast cancer treated with platinum based chemotherapy. Cancer Res. 2012;72:PD09–02. [Google Scholar]
- 68.Telli ML, Jensen KC, Vinayak S, et al. Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor-based measure of genomic instability: Precog 0105. J Clin Oncol. 2015;33:1895–1901. doi: 10.1200/JCO.2014.57.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Isakoff SJ, Mayer EL, He L, et al. Tbcrc009: A multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33:1902–1909. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 72.Tamura K, Hashimoto J, Tsuda H, et al. Randomized phase II study of weekly paclitaxel with and without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIia HER2-negative breast cancer. J Clin Oncol. 2014:32:1017a. [Google Scholar]
- Gluz O, Nitz U, Liedtke C et al. Comparison of 12 weeks neoadjuvant nab-paclitaxel combined with carboplatinum vs. gemcitabine in triple-negative breast cancer: WSG-ADAPT TN randomized phase II trial. Cancer Res 2016;15:S6-07.
- 74.Kern P, Kalisch A, Kolberg HC, et al. Neoadjuvant, anthracycline-free chemotherapy with carboplatin and docetaxel in triple-negative, early-stage breast cancer: A multicentric analysis of feasibility and rates of pathologic complete response. Chemotherapy. 2013;59:387–394. doi: 10.1159/000362756. [DOI] [PubMed] [Google Scholar]
- 75.Wang J, Xu B, Li Q, et al. Differential response of neoadjuvant chemotherapy with taxane-carboplatin versus taxane-epirubicin in patients with locally advanced triple-negative breast cancer. J Clin Oncol. 2014:32:1105a. [Google Scholar]
- 76.Sharma P, Stecklein SR, Kimler BF, et al. Efficacy of neoadjuvant carboplatin/docetaxel chemotherapy in sporadic and BRCA-associated triple-negative breast cancer (TNBC) J Clin Oncol. 2014:32:1022a. [Google Scholar]
- Sikov WM, Berry DA, Perou CM et al. Event-free and overall survival following neoadjuvant weekly paclitaxel and dose-dense AC +/− carboplatin and/or bevacizumab in triple-negative breast cancer: Outcomes from CALGB 40603 (Alliance). Cancer Res 2016;76:S2-05
- Von Minckwitz G, Loibl S, Schneeweiss A et al. Early survival analysis of the randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). Presented at the 2015 San Antonio Breast Cancer Symposium; December 8–12, 2015; San Antonio, TX. Abstract S2–04.
- 79.Stecklein SR, Sharma P. Tumor homologous recombination deficiency assays: Another step closer to clinical application? Breast Cancer Res. 2014;16:409. doi: 10.1186/s13058-014-0409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharma P, Stecklein SR, Kimler BF, et al. The prognostic value of BRCA1 promoter methylation in early stage triple negative breast cancer. J Cancer Ther Res. 2014;3:1–11. doi: 10.7243/2049-7962-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mulligan JM, Hill LA, Deharo S, et al. Identification and validation of an anthracycline/cyclophosphamide-based chemotherapy response assay in breast cancer. J Natl Cancer Inst. 2014;106:djt335. doi: 10.1093/jnci/djt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watkins J, Weekes D, Shah V, et al. Genomic complexity profiling reveals that hormad1 overexpression contributes to homologous recombination deficiency in triple-negative breast cancers. Cancer Discov. 2015;5:488–505. doi: 10.1158/2159-8290.CD-14-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms KM, Abkevich V, Neff C et al. Association between BRCA1/2 status and DNA-based assays for homologous recombination deficiency in breast cancer. Cancer Res 2013;suppl 73:P6-05-10.
- 84.Sharma P, Khan QJ, Kimler BF, et al. Results of a phase II study of neoadjuvant platinum/taxane based chemotherapy and erlotinib for triple negative breast cancer. Cancer Res. 2010;70:P1–11-07. [Google Scholar]
- 85.Abkevich V, Timms KM, Hennessy BT, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107:1776–1782. doi: 10.1038/bjc.2012.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Von Minckwitz GH, Hahnen E, Fasching PA, et al. Pathological complete response (pCR) rates after carboplatin-containing neoadjuvant chemotherapy in patients with germline BRCA (gBRCA) mutation and triple-negative breast cancer (TNBC): Results from GeparSixto. J Clin Oncol. 2014:32:1005a. [Google Scholar]
- 87.Vollebergh MA, Lips EH, Nederlof PM, et al. Genomic patterns resembling BRCA1- and BRCA2-mutated breast cancers predict benefit of intensified carboplatin-based chemotherapy. Breast Cancer Res. 2014;16:R47. doi: 10.1186/bcr3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips EH, Mulder L, Hannemann J et al. Indicators of homologous recombination deficiency in breast cancer and association with response to neoadjuvant chemotherapy. Ann Oncol 2011;22:870–876. [DOI] [PubMed]
- 89.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One. 2014;9:e88557. doi: 10.1371/journal.pone.0088557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda R, Chow LQ, Dees EC et al. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. Cancer Res 2015;75:S1-09.
- Emens LA, Braiteh FS, Cassier P et al. Inhibition of PD-l1 by MPDL3280A leads to clinical activity in patients with metastatic triple- negative breast cancer. Cancer Res 2015;75:PD1-6.
- 95.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 96.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 97.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 98.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 99.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 100.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sandhu SK, Schelman WR, Wilding G, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- Lea M. Bmn673 is a PARP inhibitor in clinical development for the treatment of breast cancer patients with deleterious germline brca1 and 2 mutations. Paper presented at: San Antonio Breast Cancer Symposium; December 10–14, 2013; San Antonio, TX. [Google Scholar]
- 103.Pahuja SB, Beumer JH, Appleman LJ, et al. Outcome of BRCA 1/2-mutated (BRCA+) and triple-negative, BRCA wild type (BRCA-wt) breast cancer patients in a phase i study of single-agent veliparib (V) J Clin Oncol. 2014:32:135a. [Google Scholar]
- 104.Gewinner C, Wang ZC, Richardson A, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marty B, Maire V, Gravier E, et al. Frequent PTEN genomic alterations and activated phosphatidylinositol 3-kinase pathway in basal-like breast cancer cells. Breast Cancer Res. 2008;10:R101. doi: 10.1186/bcr2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fedele CG, Ooms LM, Ho M, et al. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc Natl Acad Sci USA. 2010;107:22231–22236. doi: 10.1073/pnas.1015245107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ibrahim YH, García-García C, Serra V, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2:1036–1047. doi: 10.1158/2159-8290.CD-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Juvekar A, Burga LN, Hu H, et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012;2:1048–1063. doi: 10.1158/2159-8290.CD-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 110.Adimoolam S, Sirisawad M, Chen J, et al. HDAC inhibitor PCI-24781 decreases RAD51 expression and inhibits homologous recombination. Proc Natl Acad Sci USA. 2007;104:19482–19487. doi: 10.1073/pnas.0707828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botrugno OA, Robert T, Vanoli F et al. Molecular pathways: Old drugs define new pathways: Non-histone acetylation at the crossroads of the DNA damage response and autophagy. Clin Cancer Res 2012;18:2436–2442. [DOI] [PubMed]
- 112.Brazelle W, Kreahling JM, Gemmer J, et al. Histone deacetylase inhibitors downregulate checkpoint kinase 1 expression to induce cell death in non-small cell lung cancer cells. PLoS One. 2010;5:e14335. doi: 10.1371/journal.pone.0014335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi A, Kurland JF, Nishikawa T et al. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res 2005;11:4912–4922. [DOI] [PubMed]
- 114.Zhang J, Powell SN. The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol Cancer Res. 2005;3:531–539. doi: 10.1158/1541-7786.MCR-05-0192. [DOI] [PubMed] [Google Scholar]
- 115.Stecklein SR, Kumaraswamy E, Behbod F, et al. BRCA1 and HSP90 cooperate in homologous and non-homologous DNA double-strand-break repair and G2/M checkpoint activation. Proc Natl Acad Sci USA. 2012;109:13650–13655. doi: 10.1073/pnas.1203326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;410:439–453. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- 117.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 118.Peng G, Chun-Jen Lin C, Mo W, et al. Genome-wide transcriptome profiling of homologous recombination DNA repair. Nat Commun. 2014;5:3361. doi: 10.1038/ncomms4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bhalla KN, Rao R, Das Gupta S, et al. Treatment with histone deacetylase inhibitors creates ‘BRCAness’ and sensitizes human triple negative breast cancer cells to PARP inhibitors and cisplatin. Cancer Res. 2012;72(suppl 24):S3–S7. [Google Scholar]
- 120.Weberpals JI, O’Brien AM, Niknejad N, et al. The effect of the histone deacetylase inhibitor M344 on BRCA1 expression in breast and ovarian cancer cells. Cancer Cell Int. 2011;11:29. doi: 10.1186/1475-2867-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ha K, Fiskus W, Choi DS, et al. Histone deacetylase inhibitor treatment induces ‘BRCAness’ and synergistic lethality with PARP inhibitor and cisplatin against human triple negative breast cancer cells. Oncotarget. 2014;5:5637–5650. doi: 10.18632/oncotarget.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gucalp A, Traina TA. Triple-negative breast cancer: role of the androgen receptor. Cancer J. 2010;16:62–65. doi: 10.1097/PPO.0b013e3181ce4ae1. [DOI] [PubMed] [Google Scholar]
- 123.Farmer P, Bonnefoi H, Becette V, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 124.Doane AS, Danso M, Lal P, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25:3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
- 125.Loibl S, Müller BM, von Minckwitz G, et al. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;130:477–487. doi: 10.1007/s10549-011-1715-8. [DOI] [PubMed] [Google Scholar]
- Masuda H, Baggerly KA, Wang Y et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res 2013;19:5533–5540. [DOI] [PMC free article] [PubMed]
- Gucalp A, Tolaney S, Isakoff SJ et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res 2013;19:5505–5512. [DOI] [PMC free article] [PubMed]
- 128.Traina TA, Miller K, Yardley DA, et al. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC) J Clin Oncol. 2015:33:1003a. [Google Scholar]
- 129.Parker JS, Peterson AC, Tudor IC, Hoffman J, Uppal H, et al. A novel biomarker to predict sensitivity to enzalutamide (ENZA) in TNBC. J Clin Oncol. 2015:33:1083a. [Google Scholar]
- Alba E, Calvo L, Albanell J et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: Results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol 2012;23:3069–3074. [DOI] [PubMed]
- Rugo HS, Olopade O, DeMichele A et al. Veliparib/carboplatin plus standard neoadjuvant therapy for high-risk breast cancer: First efficacy results from the i-spy2 trial. Cancer Res 2013;73:S5-02