Abstract
Morquio A syndrome features systemic skeletal dysplasia. To date, there has been no curative therapy for this skeletal dysplasia. No systemic report on a long-term effect of hematopoietic stem cell transplantation (HSCT) for Morquio A has been described.
We conducted HSCT for 4 cases with Morquio A (age at HSCT: 4–15 years, mean 10.5 years) and followed them at least 10 years (range 11–28 years; mean 19 years). Current age ranged between 25 and 36 years of age (mean 29.5 years). All cases had a successful full engraftment of allogeneic bone marrow transplantation without serious GVHD. Transplanted bone marrow derived from HLA-identical siblings (three cases) or HLA-identical unrelated donor. The levels of the enzyme activity in the recipient’s lymphocytes reached the levels of donors’ enzyme activities within two years after HSCT.
For the successive over 10 years post-BMT, GALNS activity in lymphocytes was maintained at the same level as the donors. Except one case who had osteotomy in both legs one year later post BMT, other three cases had no orthopedic surgical intervention. All cases remained ambulatory, and three of them could walk over 400 m. Activity of daily living (ADL) in patients with HSCT was better than untreated patients. The patient who underwent HSCT at four years of age showed the best ADL score.
In conclusion, the long-term study of HSCT has demonstrated therapeutic effect in amelioration of progression of the disease in respiratory function, ADL, and biochemical findings, suggesting that HSCT is a therapeutic option for patients with Morquio A.
Keywords: Morquio A syndrome, hematopoietic stem cell transplantation, respiratory function, GVHD, ADL
1. Introduction
Mucopolysaccharidosis IVA (MPS IVA; Morquio A syndrome) (OMIM #253000) is a lysosomal storage disorder with an autosomal recessive trait. Morquio A syndrome is caused by defective N-acetylgalactosamine-6-sulfate sulfatase (GALNS). GALNS deficiency causes accumulation of keratan sulfate (KS) and chondroitin-6-sulfate (C6S) in lysosomes and excessive excretion of these substrates in blood and urine [1].
Patients with Morquio A have unique skeletal manifestations [1–7]. Patients with Morquio A appear healthy at birth although some patients may have lumbar gibbous and another minor bone deformity. Major signs and symptoms in most patients are usually seen before one year of age, including kyphosis, protrusion of the chest, and prominent forehead [8]. Walking alone often delays. Patients with Morquio A are clinically diagnosed during the second year of life for unique skeletal features including knock-knee, growth retardation, cervical instability, laxity of joints (hand, fingers, cervical spine, hip), and abnormal gait with a tendency to fall in addition to kyphosis, protrusion of the chest, prominent forehead. These clinical features with preservation of intelligence distinguish Morquio A from other types of MPS.
The most life-threatening feature in patients with Morquio A at an early stage is that odontoid hypoplasia with ligamentous laxity and extradural GAGs deposition can lead to atlantoaxial subluxation and/or cervical stenosis with or without cord compression, cervical myelopathy or even death [2–7]. Later in life above a teenage a primary issue is related to pulmonary compromise. Most patients have difficulty with anesthesia due to a narrow obstructive airway and a small, restrictive lung, and sleep apnea increases with progress. The difficulty with both upper and lower airways escalates as the disease advances and greatly enhances the risk of anesthesia and sedation [9]. Mean lifespan in patients with a severe form of Morquio A is the 3rd decade of life if untreated [2–7, 10].
In general multiple surgical procedures including cervical decompression/fusion, lower cervical to thoracic fusion, osteotomy of legs, and knee and hip reconstruction are required through the life of patients with Morquio A. Without surgical interventions, cervical myelopathy and successive paralysis of extremities as well as inability of walking and successive wheelchair bound life often occur in the second decade.
Therapies for Morquio A have been developed for the last two decades experimentally and clinically. These include enzyme replacement therapy (ERT), gene therapy, and hematopoietic stem cell transplantation (HSCT) [11–14]. ERT is approved for use in patients with Morquio A in several countries. Patients with Morquio A treated with ERT showed clinical improvement in 6 min walk test and improved quality of life. However, there are several limitations with current ERT: i) limited effect on skeletal symptoms [15, 16], ii) rapid clearance from the circulation, and iii) high cost compared with therapeutic efficacy [11].
HSCT has been used to treat patients with poor-risk hematological malignancies and other diseases of the hematopoietic system including severe aplastic anemia and fatal congenital immunodeficiencies. Since the first bone marrow transplantation (BMT) was reported in a patient suffering from a severe form of mucopolysaccharidosis I (Hurler syndrome) [17], approximately 1,000 patients with MPS I, II, VI, and VII have received BMT. Remarkable clinical improvement and/or maintenance in visceral organs and central nervous system has been reported until now especially if HSCT for patients with MPS I is conducted at an early stage [18–20]. Therefore, it is widely accepted as a standard therapy for Hurler syndrome. It is also proved that HSCT for patients with MPS II provides a better outcome in ADL than conventional ERT [21–23]. Bone marrow (BM) including hematopoietic stem cells can produce a large amount of leukocytes and macrophages that contain the enzyme deficient in patients with MPS. After achieving engraftment, peripheral leukocytes and macrophages (or microglia) begin to produce the deficient enzyme responsible for the disorder. The enzyme is secreted into circulation and transported into damaged organs. Or the transplanted cells themselves can migrate into a variety of tissues including CNS and secrete the enzyme on the site. However, despite the improvement of biochemical variables obtained by newly produced enzyme after HSCT, the clinical benefits are various by clinical phenotype of the patient, type of MPS, and stage (or age) of disease at the time of BMT. Especially improvement of skeletal dysplasia remains a challenge.
To date, there has been only one case report of the systemic clinical consequence on HSCT for MPS IVA [4, 5, 29, 30].
This report summarizes therapeutic effects and limitations in four HSCT-treated cases with Morquio A, who have been followed up at least over 10 years.
2. Subjects and Methods
Subjects
All four patients were diagnosed by the enzyme assay of GALNS at Department of Pediatrics, Gifu University. Genotyping was also performed at Gifu University by PCR amplification of all 14 exons and exon/intron boundaries, followed by direct sequencing [24, 25]. We conducted HSCT for four patients with Morquio A (age at HSCT: 4–15 years, mean 10.5 years) and followed them at least 10 years (range 11–28 years; mean 19 years). Current age ranged between 25 and 36 years of age (mean 29.5 years). We defined the clinical phenotype by height according to the growth chart of Morquio A [3, 6]. Among four HSCT-treated patients, three patients (2 males and 1 female) had a severe phenotype (their height < 75th percentile on the growth chart of Morquio A patients), and one male patient had an attenuated phenotype (their height > 75th percentile on the growth chart of Morquio A patients) (Table 1).
Table 1.
Summary of patients with Morquio A
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Gender | F | M | M | M |
| Present age | 25 years | 36 years | 31 years | 26 years |
| Diagnosis | 1.5 years | 4 years | 12 years | 14 years |
| Age at transplantation | 4 years | 8 years | 15 years | 15 years |
| Follow up duration | 21 years | 28 years | 16 years | 11 years |
| Regimen of BMT | busulfan (20 mg/kg ), cyclophosphamide (200 mg/kg), anti T- lymphocytic globulin (10 mg/kg/day) | cyclophosphamide (200 mg/kg), thoracoabdominal irradiation 8 Gy | Detailed data not avialble (Performed at Nippon Medical School) | busulfan (4 mg/kg), cyclophosphamide (50 mg/kg), anti T-lymphocytic globulin (15 mg/kg/day) |
| Genotype | p.M318R/undefined | Double deletion/p.Q148X | ins ex1/undefined | Undefined |
| Donor | HLA-identical sibling | HLA-identical unrelated donor | HLA-identical heterozygous sibling | HLA-identical heterozygous sibling |
| GVHD | grade I | grade I | None | None |
| Engraftment | Full | Full | Full | Full |
| Enzyme activity | Normal | Normal | carrier level | carrier level |
| Walking | walk independently | walk independently | walk independently | walk independently |
| Height | 113 cm at 20 years | 115 cm at 24 years | 137 cm at 18 years | 103 cm at 25 years |
| ADL score (full score 60: higher is better) | 59 | 33 | 55 | 34 to 46* |
| Other MPS Symptoms (full score 60: higher is better) | 50 | 42.5 | 51 | 44 to 56* |
| Orthopedic Surgical Intervention post BMT | ND | ND | ND | Osteotomies of both femurs at 16 years |
ND: not done
pre-BMT to post-BMT
Questionnaires for Activity of Daily Living (ADL) and MPS symptoms
The questionnaires for ADL and MPS symptoms were described previously for patients with Hunter syndrome [21]. Briefly, the questionnaire (12 items) consisted of three domains (“Movement,” “Movement with Cognition,” and “Cognition”); each with four subcategories scored from 0 – 5, 0 being the inability to perform task without maximum assistance, and 5 being the ability to perform a task without any assistance. “Movement” comprised basic motor skills needed for normal daily function with the subcategories: 1) walking, 2) movement on stairs, 3) grasping/finger movement, and 4) endurance in a 6-minute walk (6 MWT). “Movement with Cognition,” daily activities that required some level of cognitive awareness, comprised 1) toileting, 2) changing clothes, 3) bathing, and 4) eating. For “Cognition,” subcategories included 1) understanding of everyday conversation, 2) conversation and speaking with others, 3) social participation and 4) problem solving. The maximum possible score that could be obtained was 20 per domain with a total of 60 points.
In another questionnaire, twelve symptoms specific to MPS were assessed including: work/study, behavioral problems, sleep, pain, joint flexion, respiratory status, infection, vision, hearing, skin, hair, and appetite. These were all scored on 0 – 5 scales and total score were 60.
Questionnaires were sent to families, completed directly by the patient and/or the patient’s parent/guardian, and then returned to the study group. Three of 4 patients here and 16 untreated patients over 20 years of age answered the questionnaires. Sixteen untreated patients ranged between 20 and 43 years of age (mean; 32.7 ± 7.4 years). The scores from healthy controls (0.33 – 50 years) were described previously [21].
3. Clinical course in patients with Morquio A
Case 1
Clinical history
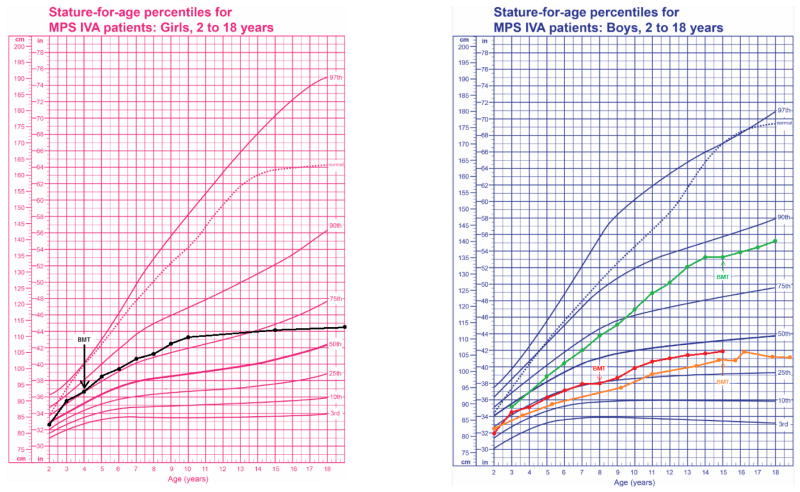
A female case with a severe phenotype is 25 years of age (Table 1). The patient was diagnosed at the age of 1-year-old biochemically. Her genotype was p.M318R/undefined. She received allogeneic BMT from human leukocyte antigen (HLA)-identical sibling at the age of 4 years. She was preconditioned by 20 mg/kg of busulfan (Bu), 200 mg/kg of cyclophosphamide (CY) and 10 mg/kg of anti T-lymphocytic globulin (ATG), and was administered cyclosporin A (CsA) for the prophylaxis of graft-versus-host disease (GVHD). Hematopoietic recovery was prompt, and DNA polymorphism confirmed engraftment. Acute GVHD grade I developed, easily treated with standard dose of prednisolone. GALNS activity in her peripheral leucocytes reached normal range. Her final height was 113 cm with 26 kg body weight at 20 years of age. Her cross-sectional growth chart shows that her growth in height continues post-BMT (Fig. 1). The mild hearing loss has been noticed, but the conversation is possible without tubing.
Figure 1. Growth charts for patients with Morquio A.
The baseline of growth charts in untreated patients with Morquio A was revised from Montano et al. (2008) and Tomatsu et al. (2012). The average of the final height in patients with Japanese Morquio A is 90 cm (female) and 95 cm (male).
Her ADL score is 59 out of 60, deemed as equivalent to normal controls (the healthy control group above 20 years of age: 60, n = 10) and far better than untreated patients over 20 years (mean 38.5 ± 11.8, n = 16) (Fig. 1, Video 1). Her movement and movement accompanied by cognitive performance were superior to those in untreated patients. Cognitive performance was similar between HSCT-treated and untreated groups. After 21 years follow-up post-BMT, she can walk independently more than 1,000 meters easily and can climb and down stairs. She can also do a squat and perform a regular range of motion exercises smoothly, in which the age-matched patients with a severe form of Morquio A impairs such movements (Video 1).
She still has several Morquio-related symptoms such as short stature, mild arthralgia, laxity of joint with deformity of bone including knock-knee, mild shortness of breath after walking, and mild hearing loss. The patient has no abnormal findings in visceral organs and heart function except mild restrictive lung function. Her current MPS symptom score was 51.5 out of 60 (the healthy control group: 56, n = 10) while untreated patients over 20 years (n = 16) had 41.3 ± 8.1. She has never experienced any surgical intervention in her life and is working as a technical dentist. Her spirometry at 15 years of age showed a reduced FVC (73.9%) in the absence of reduction of a predicted FEV1 (101.3%), suggesting a mild restrictive lung problem but no obstructive lung.
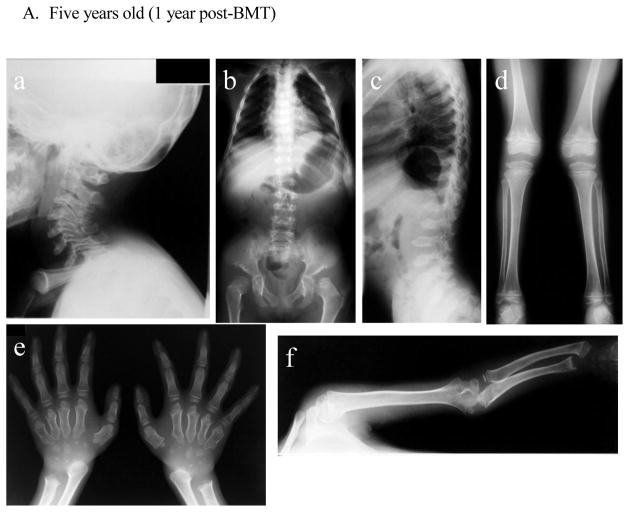
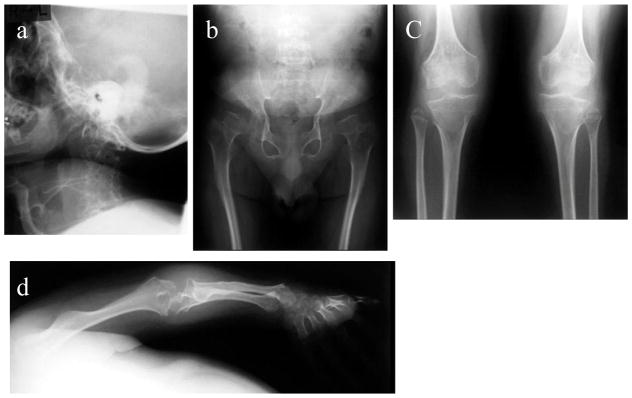
Radiographs (Fig. 3)
Figure 3. X-ray and MRI photographs after BMT at five years, 16 years, 25 years (Case 1: 1 year, 12 years, and 21 years post-BMT).
- Cervical spine: Dysmorphic changes (platyspondyly and oval shape with anterior beaking) of the cervical vertebra with widening of disc spaces are observed. The thickness of the cervical spine itself is reduced in order from the second cervical vertebra, and intervertebral distance is narrowed. No evidence of cervical spine instability is seen.
- Chest and hip: The upper thorax is narrow. The thorax is also squeezed to the left diaphragm elevated. Formation of the acetabular bottom is wrong. The femoral head is recessed in the femoral neck that has been reduced itself. Both femoral heads with dysmorphic changes are in a position of subluxation.
- Spine: Thoracolumbar body is thin and flat with marked osteodysplasia. Tongue-like anterior beaking of the lumbar vertebrae appears. The vertebrae spaces are wide.
- Lower extremities: Genu valgum is observed. Expansion of both distal end of the femur and the proximal end of the tibia are seen.
- Hands: Hand phalanges, metacarpals, and carpal have marked dysplasia. Bilateral hand radiographs show the cone-shaped tapering of the distal portion of metacarpals 2 through 5, expansion of the radius distal end, and shortening of the ulna distal end.
- Upper extremity: Both proximal and distal ends of the humerus and the forearm bones are swollen, and the forearm bones are curved. The hands take on a characteristic with tilting of the radial epiphysis towards the ulna, resulting from a combination of metaphyseal deformities, hypoplasia of the bones, and degradation of connective tissues.
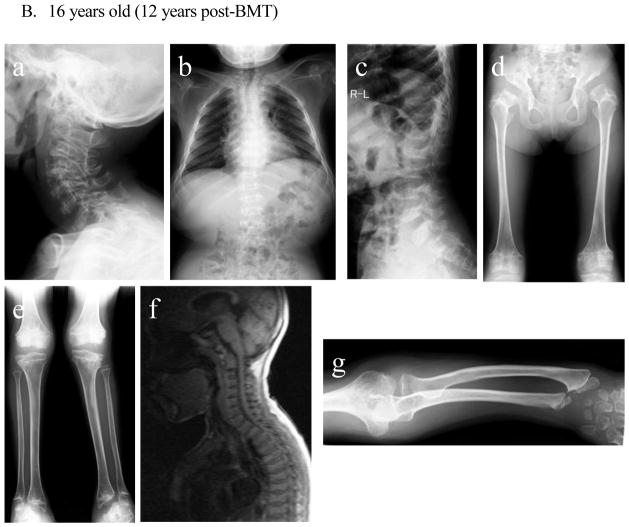
- Cervical spine: Compared to that at 5 years old, ossification failure of the entire cervical spine has become more sophisticated. Dysmorphic changes (platyspondyly and oval shape with anterior beaking) of the cervical vertebra with widening of disc spaces are not changed.
- Chest: Oar shaped ribs remain wide and are over constricted in their paravertebral portions.
- Spine: Bony changes of the lumbar spine with anterior inferior beaking and platyspondyly of the vertebrae remain evident.
- Hip and Femur: Acetabular space is more expanded in a position of subluxation, and the femoral cap is not observed. There are an oblique acetabular roof with coxa valgus deformity and flared iliac wings. Coxa valga appears, and genu valgum is improving.
- Knee and tibia: Persistent genu valgum is seen (left leg is more severe than right leg). The medial tilt of the ankle mortise is noted.
- There is no evidence of cervical myelopathy due to compression of cervical spinal cord in the first and second cervical vertebrae. Platyspondyly of cervical vertebrae is observed.
- The tapered irregular distal radius and ulna are observed with tilting of the radial epiphysis towards the ulna. Remarkable swelling of the humeral and radial distal end is prominent. Ossification failure of carpal is notable.
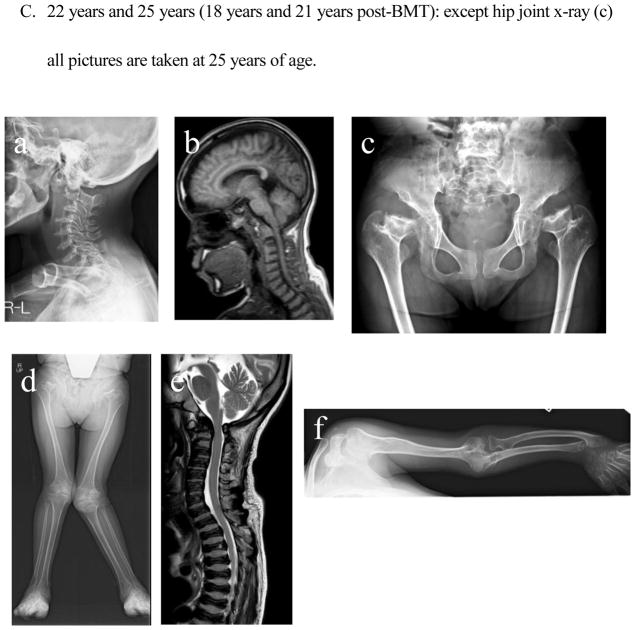
- Cervical spine: Dysmorphic changes of the cervical vertebra with widening of disc spaces remain present. Cervical vertebrae become thinner, but the extent of ossification is kept. No evidence of cervical spine instability is seen.
- Cervical spinal cord: Mild compression of the spinal cord is seen, but cervical myelopathy is not noticed.
- The femoral neck has been damaged and shortened. Ossification of the entire pelvis is well maintained. Oblique acetabular roof with coxa valgus deformity and flared iliac wings are observed. Subluxation of the hip joints is seen.
- Bilateral genu valgus can be seen, and intense deformation and swelling of the knee joint are observed. The mild curvature of the femur can be seen.
- MRI of spine shows slight compression of the cervical spinal cord. Universal platyspondyly with anterior beaking is observed in cervical and thoracic vertebrae.
- An elbow joint is swollen, and its destruction is in marked. The curvature of the ulna and radius is seen with tilting of the radial epiphysis towards the ulna in the distal end. Cortical thinning and mild widening of the diaphysis of the humerus are visible.
There was no radiograph available prior to BMT. At five years of age (1 year post-BMT), dysmorphic changes (platyspondyly and oval shape with anterior beaking) of the cervical vertebra with widening of disc spaces are seen without evidence of cervical spine instability. MRI pictures of the cervical spine at 16 years of age showed anterior subluxation of the atlas secondary to odontoid hypoplasia and mild compression of the spinal cord without any sign and symptom. Bony changes of the thoracic and lumbar spine with anterior beaking and platyspondyly of the vertebrae are seen. Small carpal bones and tilting of the radial epiphysis towards the ulna led to hypermobile joints in hands. Oar shaped ribs remained wide anteriorly and laterally and over constricted in their paravertebral portions. Both femoral heads with some dysmorphic changes are in a position of subluxation. In the pelvis, an oblique acetabular roof with coxa valgus deformity, flared iliac wings, and widely opened joint spaces were found. With age, an erect composite view of the hips, pelvis, and lower extremities demonstrates genu valgum with open knee joint spaces. Most bony deformities remain still unchanged with age, compared with the findings at five years of age. Genu valgum is slowly progress with irregular and flattened femoral heads.
Overall, her health condition has been kept well with the normal ADL over 20 years post-BMT. Compared with age-matched untreated patients with Morquio A, functional ADL remains much better (Fig. 1, Video 1).
Case 2
Clinical history
A 36-year-old male case with a severe phenotype is 115 cm tall (Table 1). The patient was diagnosed as Morquio A at four years of age with a typical skeletal feature; marked short stature (92 cm), prominent chest, abnormal walking, and kyphoscoliosis. His genotype was double gene deletion/p.Q148X. The patient received BMT from HLA-identical unrelated donor when he was eight years old at a progressive stage. The preconditioning regimen consisted of thoracoabdominal irradiation (TAI) 8 Gy and 200 mg/kg of CY, and GVHD prophylaxis comprised short-term methotrexate (MTX), CsA, and 2 mg/kg/day of prednisolone. HLA typing confirmed full engraftment. Acute GVHD grade I developed, resolved by additional immunosuppression. The activity of GALNS in peripheral blood was elevated to normal range after BMT. He never underwent any surgical procedure post HSCT. His height had stopped when he was seven years old; however, he re-grew after BMT until he was 15 years old (Fig. 4). His final height is 115 cm, and body weight is 32 kg. Both corneal clouding and kyphosis laxity of joints (knock knee, floppy hands) remains present. He can walk independently at home and walk further several hundred meters with a walking frame. His hearing is kept normal with bilateral tubing, and cardiac function is also kept normal. He was graduated from college at 22 years of age; however, he could not obtain the job and stayed at home, leading to low ADL score, 33. The patient has the ability of walking, climbing stairs, toileting, changing clothes, bathing, and eating and has no respiratory issue until now.
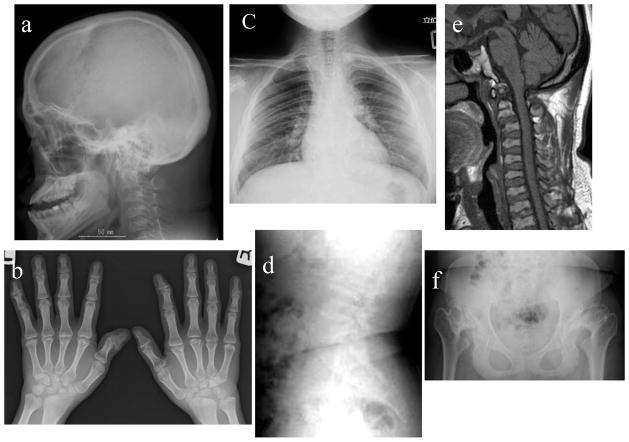
Figure 4. X-ray and MRI photographs after BMT at 17 years of age (Case 2: 9 years post-BMT).
- Cervical spine: The picture depicts anterior subluxation of the atlas secondary to odontoid hypoplasia.
- Hip: the femoral head is not seen as collapse, the acetabular cover is destroyed, and hypoplasia of the lower iliac with flared iliac wings is marked. Coxa valga is maintained.
- Knee: Knee joint is swollen (distal femoral end and tibial proximal end). Synovial membrane surface is irregular, and cartilage is destroyed.
- Humerus and forearm bones are shortened. Swelling of the elbow is marked, and destruction of the elbow joint causes a strong contracture, leading to the extension limit. The irregular distal radius and ulna and tilting of the radial epiphysis towards the ulna are observed.
Radiographs (Fig. 4)
There was no X-ray picture available prior to BMT. X-ray pictures (9 years post-BMT at 17 years) demonstrated anterior subluxation of the atlas secondary to odontoid hypoplasia with platyspondyly of cervical vertebrae. Multiple abnormalities were present in the pelvis, including spondylosis-epiphyseal dysplastic femoral heads and moderate oblique acetabular roof with mild coxa valgus deformity and flared iliac wings. Both femoral heads were subluxated although covered by their respective acetabula. Mild dysplasia of epiphysis in bilateral femur and tibia remained in place. Bilateral genu valgus was observed. The irregular distal radius and ulna and tilting of the radial epiphysis towards the ulna were also seen. Overall, his health condition has been well kept post-BMT until 36 years of age without surgical intervention and tracheal obstruction and can live independently.
Case 3
Clinical history
A 31-year-old male case is defined as an attenuated phenotype with the final height of 137 cm (Fig. 1, Table 1). Knock-knee was initially noticed at four years of age. He underwent tonsillectomy and adenoidectomy at five years of age. The patient was diagnosed as Morquio A at 12 years. His genotype was ins ex1/undefined.
He underwent BMT at 15 years of age with the height of 130 cm when he stopped growing. The donor was a HLA-matched carrier, sibling. He received a full engraftment without GVHD. The enzyme activity of GALNS reached the donor’s level.
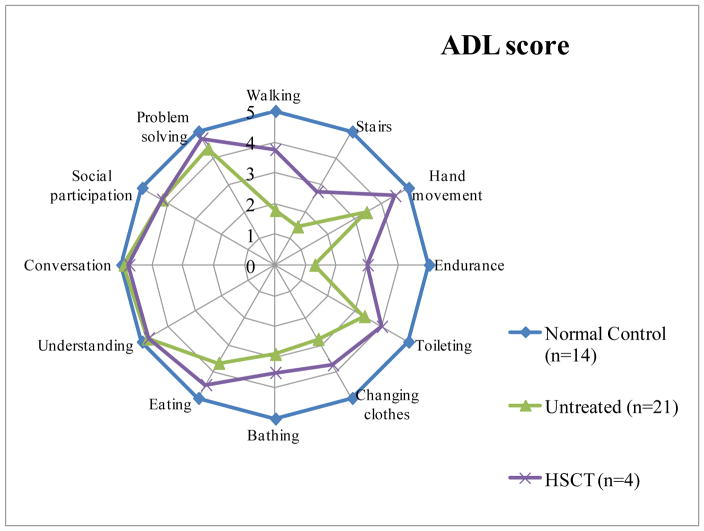
His cross-sectional growth chart showed that his growth in height continues post-HSCT (Fig. 1). At present, he can walk independently more than 800 meters although there is some limitation of endurance. He can climb and down stairs. His ADL score is 55 out of 60, as nearly equivalent to normal healthy controls (Fig. 2).
Figure 2. ADL score in patients with Morquio A with or without HSCT. The graph shows the mean of the score for each item (HSCT, n = 4; untreated, n = 21; normal controls, n = 14).
All subjects are over 20 years of age.
Current MPS symptom score was 51 out of 60. After 16 years follow-up post-BMT, he has several Morquio-related symptoms such as moderate short stature and short neck, moderate arthralgia, restriction of range of motion, and mild corneal clouding. The patient has no abnormal finding in visceral organs and heart function. His respiratory condition remains stable without any problem. He has no significant surgical intervention in the bone until now.
Radiographs (Fig. 5)
Figure 5. X-ray and MRI photographs after BMT at 24 years of age (Case 3: 9 years post-BMT).
- Cervical spine: Skull is thickened, and Sella Tunica is not enlarged. The picture shows no anterior subluxation of the atlas with ossification of the odontoid process. Mild anterior central beaking of the vertebra is observed with a round shape. The cervical spine is thin, and intervertebral space is expanding. There is neither compression of the cervical spinal cord, nor cervical myelopathy.
- Chest: Development of the rib cage is almost normal, and interval of ribs is also close to normal. Deformation of the ribs itself is not seen almost.
- Spinal Cord: MRI of cervical spine shows no subluxation of the neck vertebrae and compression of the spinal cord. Vertebral bodies are mildly affected.
- Hands: Bilateral hand radiographs in a patient aged six years. Tapering of the proximal portion of metacarpals is not seen with ossified carpal bones. The distal portion of the radius tilted toward the ulna is observed mildly. Metacarpal bones of the second to fifth fingers are slightly shortened, and cone-shaped variation of the proximal end is mild.
- Spine: X-ray of the thoracolumbar transition shows that thickness of vertebral bodies and intervertebral space are kept normal, corresponding to an intermediate type of MPS IVA.
- Pelvis: Mild spondylosis-epiphyseal dysplastic femoral heads and oblique acetabular roof are observed. Both femoral heads are well positioned in hip joints.
There was no X-ray picture available prior to BMT. X-ray picture of the cervical spine (9 years post-BMT at 24 years) showed no anterior subluxation of the atlas with partial ossification of the odontoid process. Mild anterior central beaking of the vertebra is observed with an oval shape. Mild abnormal and small ribcage is found with slight oar-shaped ribs. MRI of cervical spine shows no slippage (subluxation) of the neck vertebrae and compression of the spinal cord. Tapering of the proximal portion of metacarpals is not seen with ossified carpal bones. A mild distal portion of the radius tilted toward the ulna is observed. The irregularity and anterior beaking of vertebral bodies are observed mildly. Mild spondylo-epiphyseal dysplastic femoral heads and oblique acetabular roof are seen. Both femoral heads are well positioned in hip joints.
Overall, his health condition has been kept well with nearly normal ADL, and he can live independently.
Case 4
Clinical history
This male case was described previously [13]. The patient is now 26 years of age with the final height of 103 cm and defined as severe phenotype (Table 1). By 1.5 years of age, the patient had Morquio-like clinical features including the short neck, prominent chest, short trunk, genu valgum, kyphosis, and hypermobile joints of fingers and wrist. At eight years of age, the patient underwent cervical decompression/fusion surgery, because of spinal cord compression. He could not assume a half-sitting posture, squeeze a towel, or sit without support. He had orthopnea all night, loud snoring, postural dyspnea, occasional shortness of breath, and required a wheelchair. At 14 years of age, the patient was diagnosed as Morquio A biochemically. No mutation was found in all the 14 exons and each exon-intron boundary region in the GALNS gene.
At 15 years and 8 months, BMT with marrow from an HLA-identical elder sister was conducted. The patient’s height and weight were height 103 cm and 40.7 kg, respectively. Conditioning consisted of Bu (4 mg/kg/day × 4 days), CY (50 mg/kg/day × 4 days), and ATG (15 mg/kg/day × 4 days). After BMT, CsA (1.5 mg/kg × 2 div/day × 30 days) and MTX (7~10 mg/day × 3/one week) were prescribed. He received a full engraftment without GVHD.
Two years after BMT, the enzyme activity of GALNS in white blood cells reached half of normal level as observed in his carrier donor sister. The level of uronic acid decreased around 35% from pre-BMT (15 years seven months) to post-BMT (17 years 9 months) and was maintained at the same level during the following period. At 25 years of age, the latest level of uronic acid was reduced to 21% of a pre-BMT level (7.8 mg/dl cre). Three years post-BMT, vital capacity (VC) increased from 1.08 to 1.31 (L), %VC from 43 to 48.2 (%), peak expiratory flow (PEF) from 2.03 to 2.36 (L/sec), and one second forced expiratory volume (FEV1.0) increased from 1.08 to 1.12 (L). In the following 11 years post-BMT, the level of the pulmonary function was maintained, showing that %VC was 48.6 and FEV1.0% was 87.8 with the pattern of the restrictive lung but no obstructive airway. Bone mineral density (BMD) of the lumbar spine by dual-energy X-ray absorptiometry (DXA) showed that BMD at L2–4 increased from 0.372 to 0.548 (g/cm2) and was maintained at the level of 0.48 ± 0.054 until now. Annual echocardiography showed no valvular involvement.
Thirteen months later post-BMT, the patient underwent osteotomies of both femurs without any complication of surgical and anesthesia procedures. After correction osteotomies for knock-knee, the patient could walk for 100 meters by ankle-foot orthoses and for 400 meters by hip-knee-ankle-foot orthoses. Orthopnea, loud snoring, and postural dyspnea have 1disappeared and no difficult airway is observed..
At 26 years of age, he is working as a designer of computer graphics. His height and weight were 103 cm and 34 kg, respectively and his BMI was improved to 30.2 (obese; > 30) compared with pre-BMT status (103 cm, 41 kg, BMI; 38.6). His grip strength is still weak with 3.5 kilograms by the right hand and 3.2 kilograms by the left hand (47.5 kg on the average of the age-matched Japanese male controls). ADL score was a 34 at pre-BMT while he scored a 46 at 11 years later post-BMT (Fig. 2). The patient scored a 40 in MPS symptom at pre-BMT, while the score of MPS symptom improved to 56 at post-BMT. He can walk over 400 m independently (Video 2).
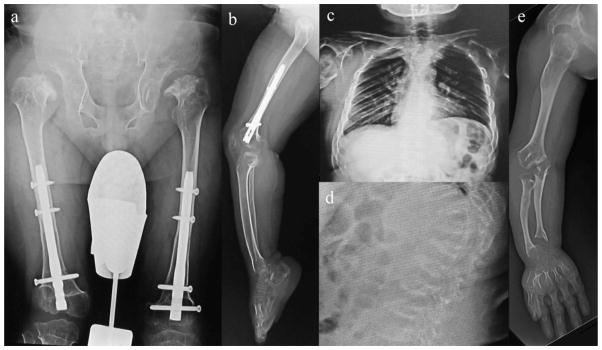
Radiographs (Fig. 6)
Figure 6. X-ray photographs at 26 years of age (Case 4: 11 years post-BMT).
- Hip: Femoral head is fitted into the femoral neck, and femoral neck is rudimentary. The acetabular roof is destroyed, and the iliac has been scraped off the top with flared iliac wings. Coxa valga is seen. Both femoral heads are well positioned.
- Knee and lower extremity: Knee joint is hypoplasia of the bone, defined as severe. Femur and the entire lower leg have ossification failure.
- Chest: Thorax diaphragm is lifted, and chest cavity is compressed. The space between the ribs is also narrow, and the deformation of the ribs is seen, leading to a reduction in air content of the thoracic cavity inferred.
- Thoracolumbar spine: Thoracolumbar vertebral bodies are flattening, and anteriorly beaking like tongue-shaped deformation. The intervertebral body space is enlarged. Platyspondyly and anterior beaking of thoracolumbar vertebra were almost unchanged 11 years later post-BMT.
- Upper extremity: Due to a high degree of dwarfism, humerus and forearm bone are shortened, and swelling of the elbow is marked. Destruction of elbow joint leads to a strong contracture and the extension limit. Shortening of the metacarpal bone and carpal ossification of failure are marked.
Radiographs showed that platyspondyly and anterior beaking of thoracolumbar vertebra increased slightly in size, while the margin of vertebra became clear. Ulnar and radial deviation is observed. Eleven years later post-BMT at 26 years of age, these figures including the upper limbs remained steady. Both femoral heads are well positioned. Overall, his health condition is well maintained post-BMT and can live and walk independently.
Discussion
We have demonstrated that 1) all 4 patients (3 severe and one attenuated) had a full engraftment after HSCT without a serious adverse effect and obtained the donor’s level of the enzyme activity, 2) HSCT provides substantial improvement and maintenance of ADL and the highest score of ADL equivalent to normal controls is provided with the youngest patient, Case 1, at HSCT procedure, 3) no orthopedic surgical intervention except the bilateral osteotomy in Case 4 (1 year post-BMT) has been performed in any patient over 11 years after HSCT, 4) dysmorphic changes of the bones are ameliorated or slowed, and 4) less impact is provided on growth and laxity of joints in severe phenotypic cases. These findings indicate that HSCT is effective for Morquio A, keeping their ADL better, compared with untreated patients, and that early introduction of HSCT provides a more benefit.
Cases 1 and 2 reached normal enzyme activity while cases 3 and 4 obtained a carrier’s level of the enzyme activity. There was no proof that a carrier enzyme activity impacts late outcomes clinically although more HSCT cases are required to conclude the effect of the enzyme activity by the donor.
The procedure of HSCT consisted of preconditioning that enables engraftment of infused graft, hematopoietic stem cell infusion, prophylaxis of GVHD, and supportive therapy including prevention of infection, both red blood cell, and platelet transfusions.
Preconditioning is the most important process for the success of HSCT in patients with MPS. At the current standard protocol, preconditioning for MPS consists predominantly of CY and Bu. Total body irradiation (TBI) or total lymph node irradiation (TLI) may not be recommended because of adverse effect on the growth and endocrine system. We reported the engraftment rate in 35 stem cell-graft recipients with congenital metabolic diseases who received different conditioning regimens [26]. We concluded that the conditioning, which consists of 560 to 600 mg/m2 of Bu + 200 mg/kg of CY + ATG, is enough to obtain durable engraftment.
HSCT for MPS patients has been conducted before ERT has started; however, initial attempts of HSCT were controversial because of a high mortality rate. HSCT has a risk for the development of mortality by GVHD, infections, and additional complications. One of the reasons why the mortality rate of HSCT was high during initial attempts in 1980–1990 is based on the fact that patients, who underwent HSCT, were already at an advanced or even a terminal stage of disease progression. It is not optimal for patients with advanced-stage disease to execute the rigorous regimen of HSCT. Regimens for HSCT have been markedly improved in each medical facility, and well-trained staffs contribute to the least mortality of HSCT. As part of conditioning, Busulfan is used as a standard drug prior to HSCT, leading to substantial reduction of the mortality rate of HSCT.
In Japan, 107 patients with MPS from 1986 to 2012 have been treated by allogeneic BMT and registered to The Japan Society for Hematopoietic Cell Transplantation [27, unreported]. Although the detail concerning the efficacy is not analyzed until now, the result of the survival is promising; 5-year survival rate after HSCT in MPS were 87%. Since 1999, at Tokai University treated 21 MPS patients by HSCT, and except one case with MPS II there was no fatality due to complications of HSCT. This case failed in 1st HSCT in a local hospital, but achieved durable engraftment after 2nd HSCT performed in our hospital. He died of EBV-related lymphoproliferative disorder seven months after 2nd HSCT at local hospital because of profound immunosuppressive condition due to repeated transplantation.
In Europe, since 2005 new international HSCT guidelines for MPS disorders were proposed [28]. The survival and graft outcomes of MPS patients receiving HCT according to these guidelines in two European centers of expertise were evaluated based on conditioning regimens including busulfan. A non-carrier matched sibling donor, matched unrelated cord blood, or matched unrelated donor were considered to be preferred donors. Study of 62 MPS patients receiving HSCT at median age 13.5 months (range, 3 to 44 months) showed high overall survival (95.2%) and event-free survival (90.3%) with only low toxicity similar to our data.
Thus, HSCT is a therapeutic option for selected cases of MPS with careful pre-transplantation counseling and clinical evaluation at the well-trained facility.
It is known that busulfan may still induce a severe adverse effect such as toxicity in lung and liver and can not be used for the infants less than six months of age. Treosulfan, an alkylating agent, is often applied as a chemotherapy drug with less toxicity in the treatment of ovarian cancer and is increasingly used for HSCT, preferably in non-malignant diseases.
In Europe, treosulfan is used effectively and safely in pediatric patients with HSCT [329–32]. Pediatric patients with MPS I, who received a conditioning regimen consisting of treosulfan, achieved stable hematopoietic engraftment and stable donor chimerism without GVHD. The regimen with treosulfan could be an additional option when unrelated donor HSCT is considered for a patient with MPS [33]. A pilot study of treosulfan in our facility showed the better outcomes for pediatric patients with metabolic disorders treated by HSCT. Thus, HSCT for neonatal or infant less than six months of age could be conducted safely as a main therapy for patients with MPS if treosulfan regimen is established in each type of MPS.
With the advanced technology and awareness of the disease, newborn screening for MPS is also being conducted or under development. Such newborn screening programs for patients with MPS in combination with a new regimen may offer an opportunity to consider HSCT even in the first months of life before the appearance of signs and symptoms [12, 34].
The clinical outcomes of HSCT in patients with MPS have varied considerably. Factors that affect the outcome of HSCT consist of the type of the MPS disorder, the genotype and HLA typing of the donor, the degree of clinical involvement, preparative regimen and complications by HSCT, and the age at the time of transplantation [35]. Treatments of HSCT are effective, as for MPS I [36, 37] and MPS II [23, 38], to prevent deterioration of CNS if HSCT is conducted at an early stage. Cases with MPS VI [39] and MPS VII [40] have been also reported with improvements of visceral organs. These types of MPS are thought to be clinically impacted by HSCT. Meanwhile, there has been only one case with MPS IVA that describes the detailed clinical consequence until now [4, 5, 13, 41, 42].
Improvement of pre-existing skeletal phenotype by HSCT in the present and reported cases with MPS remains partial and unmet challenges because bone abnormalities are irreversible at the time of the transplant. During the perinatal life, patients with MPS appear nearly normal at the skeletal development [5, 11, 12, 21, 34, 43]. Therefore, one can speculate that the first months of life is the best window of opportunity for preventing bone deformities in patients with MPS. According to this perspective, a long-term clinical outcome of patients with MPS depends upon whether HSCT prevents the progression of the disorder and provides maximal benefit when performed early in life [5, 11, 12, 21, 34, 43].
For the past years, bone marrow has been used as a primary donor source for HSCT, but the availability of donors has been limited. To date, cord blood is recognized as another useful source of HSCT for MPS. Full-donor chimerism and normal enzyme levels are frequent during the follow-up period [37, 44]. HSCT with cord blood has several advantages such as 1) easy procurement, 2) no risk to donors, 3) low risk of transmitting infections, 4) immediate availability, and 5) immune tolerance allowing successful transplantation despite HLA disparity [45]. Cord blood transplantation also improves neurocognitive development in children with Hurler syndrome [37].
Patients with MPS IVA often require surgical interventions such as cervical fusion, decompression of spinal cord, osteotomy, and hip replacement through their lifetime. In general more than four times surgical interventions are required for most Morquio A patients until mid-twenties [2, unpublished data]. Therefore, a substantial reduction in the number of surgical interventions in treated patients here indicates a marked therapeutic effect of HSCT.
Achievement of walking was improved or stabilized in three cases without any surgical intervention and spinal cord compression which requires surgical intervention has not been recognized in any case. One case required the surgical intervention of osteotomy one year later and was maintained in a stable condition. All four cases can live and walk independently while 70% of patients with MPS IVA become wheelchair-bound and crawling in the teenage years, as did Case 4 at pre-BMT [2]. Clinical course post HSCT improved over pre-HSCT status in the disappearance of orthopnea and loud snoring as well as ADL.
Overall, the progression of skeletal dysplasia is slowed and prevented.
The pattern of growth in patients with MPS IVA is characterized by impaired growth velocity by the first year of age that later progresses throughout life. Growth stops in patients with MPS IVA up to 3–8 years of age, as observed in the patient with a severe phenotype here [2–6]. Therefore, this accounts for why growth impact is not enough evident for the present cases even if we consider the mean final height of Japanese patients with MPS IVA (male Morquio; 107.5 ± 21cm, n = 12, female Morquio; 96.5 cm ± 11 cm, n = 10). Thus, in spite of improvement or maintenance of ADL and MPS symptoms, impact on the growth remains limited since HSCT was conducted at the age of 4–15 years.
Symptoms and signs in patients with Morquio A would worsen year by year with progressive motor and respiratory dysfunctions. Respiratory failure with tracheal obstruction and restrictive lung is one of the most important causes of morbidity and mortality in patients with MPS IVA [10, 46]. None of patient treated with HSCT in this study showed any significant tracheal obstruction or respiratory problem until now. Thus, our results indicated that respiratory function has been improved without any evidence of tracheal obstruction and that no immediate life-threatening issue is observed.
There are several limitations to evaluate 4 cases: 1) still hard to assess therapeutic efficacy precisely because of variability of the clinical severity, the disease stage, and age at HSCT in each case, 2) small number of 4 cases as described here may not be applied to other patients, and 3) direct comparison between treated and untreated clinical status in the same patients is impossible. Therefore, it is required to accumulate more data of the HSCT-treated patients retrospectively as well as prospectively.
When compared with ERT, the advantages of HSCT are 1) that it is a one-time permanent treatment if fully engrafted, 2) that the enzyme can be supplied and circulated at the same level as the donor’s level for the lifetime continuously, 3) that the enzyme expressed in bone marrow will access the targeting site, bone, and cartilage readily, and 4) that cost is effective.
It has been thought that the HSCT procedure provides a high mortality rate in patients with MPS. There has been no mortality in patients with MPS IVA treated by HSCT in our study with minimum adverse effect. With the improved protocol, and with consideration of the selection and condition of the patient and the type of donor, the risk of HSCT could be minimized.
In conclusion, results of successful HSCT in four patients with Morquio A indicate that HSCT should be a therapeutic option for patients with Morquio A. We cannot conclude whether HSCT under the current regime is effective in entire patients with Morquio A, but long-term evaluation of patients as described here and accumulation of data from additional patients receiving HSCT should clarify this issue and define indication of HSCT.
Supplementary Material
Activity and range of motion in Case 1 (25 years old)
Walking, sitting, and range of motion are described. Compared with Case 4, range of motion is wide, and walking and sitting are performed better and faster.
Activity and range of motion in Case 4 (26 years old)
Walking, sitting, and range of motion are described.
Highlights.
Successful four cases of HSCT are presented.
Effect and limitation of HSCT for MPS IVA are described.
Early HSCT provides a better therapeutic effect.
Established bone lesion remains an unmet challenge.
HSCT should start at a very early stage prior to the irreversible bone lesion.
Acknowledgments
This work was supported by grants from Austrian MPS Society and International Morquio Organization (Carol Ann Foundation). S.T. was supported by National Institutes of Health grant 1R01HD065767-01 and 5 P20 RR020173-07. The sponsors have not influenced the content of the article. We thank the Morquio A families for kindly providing the consent for the clinical pictures. Michelle Stofa provided editorial assistance to the manuscript at Nemours/Alfred I. duPont Hospital for Children.
Footnotes
Conflict of Interest:
All the authors contributed to this article and had no conflict of interest with any other party.
Hiromasa Yabe, Akemi Tanaka, Yasutsugu Chinen, Shunichi Kato, Kazuki Sawamoto, Eriko Yasuda, Haruo Shintaku, Yasuyuki Suzuki, Tadao Orii, Shunji Tomatsu declare that they have no conflict of interests.
Contributions to the project:
Hiromasa Yabe is a Principal Investigator for this article and has contributed to performance of HSCT, follow up with the patient, the planning of the article, collection of data on HSCT, and reporting of the work described.
Akemi Tanaka is a Co-Principal Investigator for this article and has contributed to performance of HSCT, follow up with the patient, the planning of the article, collection of data on HSCT, and reporting of the work described.
Yasutsugu Chinen is a Co-Principal Investigator for this article and has contributed to performance of HSCT, follow up with the patient, the planning of the article, collection of data on HSCT, and reporting of the work described.
Shunichi Kato contributed has contributed to performance of HSCT, follow up with the patient, the planning of the article, collection of data on HSCT, and reporting of the work described.
Kazuki Sawamoto contributed to the planning of the article, a collection of data on HSCT, and reporting of the work described.
Eriko Yasuda contributed to the planning of the article, a collection of data on HSCT, and reporting of the work described.
Haruo Shintaku contributed to the planning of the article, a collection of data on HSCT, and reporting of the work described.
Yasuyuki Suzuki contributed to the planning of the article, a collection of data on HSCT, and reporting of the work described.
Tadao Orii has contributed to the diagnosis of the patients, the concept of the manuscript, planning of the article, a collection of data, and reporting of the work described.
Shunji Tomatsu is a Co-Principal Investigator for this article and has contributed to the concept and planning of the article, a collection of data, and reporting of the work described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dorfman A, Arbogast B, Matalon R. The enzymic defects in Morquio and Maroteaux-Lamy syndrome. Adv Exp Med Biol. 1976;68:261–276. doi: 10.1007/978-1-4684-7735-1_18. [DOI] [PubMed] [Google Scholar]
- 2.Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 3.Montaño AM, Tomatsu S, Brusius A, Smith M, Orii T. Growth charts for patients affected with Morquio A disease. Am J Med Genet A. 2008;146:1286–1295. doi: 10.1002/ajmg.a.32281. [DOI] [PubMed] [Google Scholar]
- 4.Tomatsu S, Montaño AM, Oikawa H, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Cur Pharm Biotech. 2011;12:931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- 5.Tomatsu S, Mackenzie WG, Theroux MC, et al. Current and emerging treatments and surgical interventions for Morquio A Syndrome: a review. Res Rep Endocr Disord. 2012a;2:65–77. doi: 10.2147/RRED.S37278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomatsu S, Montaño AM, Oikawa H, et al. Impairment of body growth in mucopolysaccharidoses. 2091–2116. In: Preedy VR, editor. Handbook of Growth and Growth Monitoring in health and disease. © Springer science+Business media, LLC 2012b. [DOI] [Google Scholar]
- 7.Hendriksz CJ, Harmatz P, Beck M, et al. Review of clinical presentation and diagnosis of mucopolysaccharidosis. IVA Mol Genet Metab. 2013;110:54–64. doi: 10.1016/j.ymgme.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohashi A, Montaño AM, Colón JE, Oguma T, Luisiri A, Tomatsu S. Sacral dimple: incidental findings from newborn evaluation. Mucopolysaccharidosis IVA disease. Acta Paediatr. 2009;98:768–769. 910–912. doi: 10.1111/j.1651-2227.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 9.Theroux MC, Nerker T, Ditro C, Mackenzie WG. Anesthetic care and perioperative complications of children with Morquio syndrome. Paediatr Anaesth. 2012;22:901–907. doi: 10.1111/j.1460-9592.2012.03904.x. [DOI] [PubMed] [Google Scholar]
- 10.Lavery C, Hendriksz C. Mortality in patients with morquio syndrome a. JIMD Rep. 2015;15:59–66. doi: 10.1007/8904_2014_298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomatsu S, Sawamoto K, Alméciga-Díaz CJ, et al. Impact of enzyme replacement therapy and hematopoietic stem cell transplantation in patients with Morquio A syndrome. Drug Des Devel Ther. 2015 Apr 1;9:1937–53. doi: 10.2147/DDDT.S68562. eCollection 2015. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomatsu S, Alméciga-Díaz CJ, Montaño AM, et al. Therapies for the bone in mucopolysaccharidoses. Mol Genet Metab. 2015;114:94–109. doi: 10.1016/j.ymgme.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinen Y, Higa T, Tomatsu S, Suzuki Y, Orii T, Hyakuna N. Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA. Mol Genet Metab Rep. 2014;1:31–41. doi: 10.1016/j.ymgmr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomatsu S, Yasuda E, Patel P, et al. Morquio A syndrome: diagnosis and current and future therapies. Pediatr Endocrinol Rev. 2014;12(suppl 1):141–151. [PMC free article] [PubMed] [Google Scholar]
- 15.Connock M, Juarez-Garcia A, Frew E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type I. Health Technol Assess. 2006;10:iii–iv. ix-113. doi: 10.3310/hta10200. [DOI] [PubMed] [Google Scholar]
- 16.Rohrbach M, Clarke JT. Treatment of lysosomal storage disorders : progress with enzyme replacement therapy. Drugs. 2007;67:2697–2716. doi: 10.2165/00003495-200767180-00005. [DOI] [PubMed] [Google Scholar]
- 17.Hobbs JR, Hugh-Jones K, Byrom N, et al. Reversal of clinical features of Hurler’s disease and biochemical improvement after treatment by bone marrow transplantation. Lancet. 1981;2:709–712. doi: 10.1016/s0140-6736(81)91046-1. [DOI] [PubMed] [Google Scholar]
- 18.Hobbs JR. Correction of 34 genetic diseases by displacement bone marrow transplantation. Plasma Ther Transfus Technol. 1985;6:221–246. [Google Scholar]
- 19.Hoogerbrugge PM, Brouwer OF, Bordigoni P, et al. Allogeneic bone marrow transplantation for lysosomal storage diseases. Lancet. 1995;345:1398–1402. doi: 10.1016/s0140-6736(95)92597-x. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda E, Mackenzie WG, Ruhnke K, et al. Long-term follow-up of post haematopoietic stem cell transplantation for the patient with Hurler syndrome: correlation between pathological phenotype, biomarker and clinical improvement. Mol Genet Metab Rep. 2015;2:65–76. doi: 10.1016/j.ymgmr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanjuakio J, Suzuki Y, Patel P, et al. Activities of Daily Living in patients with Hunter syndrome: impact of enzyme replacement therapy and hematopoietic stem cell transplantation. Mol Genet Metab. 2015;114:161–169. doi: 10.1016/j.ymgme.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel P, Suzuki Y, Tanaka A, et al. Impact of enzyme replacement therapy and hematopoietic stem cell therapy on growth in patients with Hunter syndrome. Mol Genet Metab Rep. 2014;1:184–196. doi: 10.1016/j.ymgmr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka A, Okuyama T, Suzuki Y, et al. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol Genet Metab. 2012;107:513–520. doi: 10.1016/j.ymgme.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Tomatsu S, Montaño AM, Nishioka T, et al. Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A) Hum Mutat. 2005;26:500–512. doi: 10.1002/humu.20257. [DOI] [PubMed] [Google Scholar]
- 25.Tomatsu S, Montaño AM, Lopez P, et al. Determinant factors of spectrum of missense variants in mucopolysaccharidosis IVA gene. Mol Genet Metab. 2006;89:139–149. doi: 10.1016/j.ymgme.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Yabe H, Yabe M, Hattori K, et al. Preconditioning regimens for bone marrow transplantation in patients with congenital metabolic diseases. Exp Hematol. 2001;29(suppl):94. [Google Scholar]
- 27.Japan Society for Hematopoietic Cell Transplantation Monograph. 2002;7:39. [Google Scholar]
- 28.Aldenhoven M, Jones SA, Bonney D, Borrill RE, Coussons M, Mercer J, Bierings MB, Versluys B, van Hasselt PM, Wijburg FA, van der Ploeg AT, Wynn RF, Boelens JJ. Hematopoietic cell transplantation for mucopolysaccharidosis patients is safe and effective: results after implementation of international guidelines. Biol Blood Marrow Transplant. 2015;21:1106–1109. doi: 10.1016/j.bbmt.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Bernardo ME, Piras E, Vacca A, et al. Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood. 2012;120:473–476. doi: 10.1182/blood-2012-04-423822. [DOI] [PubMed] [Google Scholar]
- 30.Slatter MA, Rao K, Amrolia P, et al. Treosulfan-based conditioning regimens for hematopoietic stem cell transplantation in children with primary immunodeficiency: United Kingdom experience. Blood. 2011;117:4367–4375. doi: 10.1182/blood-2010-10-312082. [DOI] [PubMed] [Google Scholar]
- 31.Strocchio L, Zecca M, Comoli P, et al. Treosulfan-based conditioning regimen for allogeneic haematopoietic stem cell transplantation in children with sickle cell disease. Br J Haematol. 2015;169:726–736. doi: 10.1111/bjh.13352. [DOI] [PubMed] [Google Scholar]
- 32.Wachowiak J, Sykora KW, Cornish J, et al. Treosulfan-based preparative regimens for allo-HSCT in childhood hematological malignancies: a retrospective study on behalf of the EBMT pediatric diseases working party. Bone Marrow Transplant. 2011;46:1510–1518. doi: 10.1038/bmt.2010.343. [DOI] [PubMed] [Google Scholar]
- 33.Schwinger W, Sovinz P, Benesch M, et al. Unrelated CD3/CD19-depleted peripheral stem cell transplantation for Hurler syndrome. Pediatr Hematol Oncol. 2014;31:723–730. doi: 10.3109/08880018.2014.939794. [DOI] [PubMed] [Google Scholar]
- 34.Tomatsu S, Fujii T, Fukushi M, et al. Newborn screening and diagnosis of mucopolysaccharidoses. Mol Genet Metab. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Diseases. McGraw-Hill; New York: 2001. pp. 3421–3452. [Google Scholar]
- 36.Peters C, Shapiro EG, Anderson J, et al. Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. The Storage Disease Collaborative Study Group. Blood. 1998;91:2601–2608. [PubMed] [Google Scholar]
- 37.Staba SL, Escolar ML, Poe M, et al. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 38.Guffon N, Bertrand Y, Forest I, Fouilhoux A, Froissart R. Bone marrow transplantation in children with Hunter syndrome: outcome after 7 to 17 years. J Pediatr. 2009;154:733–737. doi: 10.1016/j.jpeds.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 39.Krivit W. Allogeneic stem cell transplantation for the treatment of lysosomal and peroxisomal metabolic diseases. Springer Semin Immunopathol. 2004;26:119–132. doi: 10.1007/s00281-004-0166-2. [DOI] [PubMed] [Google Scholar]
- 40.Yamada Y, Kato K, Sukegawa K, et al. Treatment of MPS VII (Sly disease) by allogeneic BMT in a female with homozygous A619V mutation. Bone Marrow Transplant. 1988;21:629–634. doi: 10.1038/sj.bmt.1701141. [DOI] [PubMed] [Google Scholar]
- 41.Orchard PJ, Blazar BR, Wagner J, Charnas L, Krivit W, Tolar J. Hematopoietic cell therapy for metabolic disease. J Pediatr. 2007;151:340–346. doi: 10.1016/j.jpeds.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 42.Belinson S, Mauger Rothenberg B, Chopra R, Aronson N. Future research needs for hematopoietic stem-cell transplantation in the pediatric population: identification of future research needs from comparative effectiveness review No. 48 [Internet] Rockville (MD): Agency for Healthcare Research and Quality (US); 2012. [PubMed] [Google Scholar]
- 43.Tomatsu S, Alméciga-Díaz CJ, Barbosa Hector, Montaño AM, et al. Therapies of Mucopolysaccharidosis IVA (Morquio A Syndrome) Expert Opin Orphan Drugs. 2013;1:805–818. doi: 10.1517/21678707.2013.846853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boelens JJ, Aldenhoven M, Purtill D, et al. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121:3981–3987. doi: 10.1182/blood-2012-09-455238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tse W, Laughlin MJ. Umbilical cord blood transplantation: a new alternative option. Hematology Am Soc Hematol Educ Program. 2005:377–383. doi: 10.1182/asheducation-2005.1.377. [DOI] [PubMed] [Google Scholar]
- 46.Tomatsu S, Averill LW, Sawamoto K, Mackenzie WG, Bober MB, Pizarro C, Goff CJ, Xie L, Orii T, Theroux M. Obstructive airway in Morquio A syndrome, the past, the present and the future. Mol Genet Metab. doi: 10.1016/j.ymgme.2015.09.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Activity and range of motion in Case 1 (25 years old)
Walking, sitting, and range of motion are described. Compared with Case 4, range of motion is wide, and walking and sitting are performed better and faster.
Activity and range of motion in Case 4 (26 years old)
Walking, sitting, and range of motion are described.