Abstract
Tumor necrosis factor (TNF)-like cytokine 1A (TL1A) is expressed on antigen presenting cells and provides co-stimulatory signals to activated lymphocytes that bear its functional receptor, death receptor 3 (DR3). TL1A/DR3 signaling is involved in the pathogenesis of human and experimental inflammatory bowel disease (IBD). In the present study, we investigated the role of this cytokine/receptor pair in acute intestinal injury/repair pathways. We demonstrate that intact DR3 signaling protected mice from acute DSS colitis, as DR3−/− mice showed more severe mucosal inflammation and increased mortality. DR3−/− mice were compromised in their ability to maintain adequate numbers of CD4+CD25+Foxp3+ T regulatory cells (Tregs) in response to acute mucosal damage. This defect in immune regulation led to a non-specific upregulation of effector pro-inflammatory pathways, which was most prominent for the Th17 immunophenotype. TL1A−/− mice were similarly more susceptible to DSS colitis, although without mortality and with delayed kinetics compared to DR3−/− mice, and also displayed significantly reduced numbers of Tregs. Infection of DR3−/− mice with Salmonella typhimurium was associated with defective microbial clearance and elevated bacterial load. Taken together, our findings indicate a novel protective role for the TL1A/DR3 axis in the regulation of mucosal homeostasis during acute intestinal injury/repair, which contrasts with its known pathogenic function during chronic intestinal inflammation.
Introduction
Recent evidence supports a pivotal role for innate immunity in the fine-tuning of intestinal mucosal responses towards both homeostasis or inflammation (1). Interestingly, it has been shown that “innate” cytokines may have opposite roles depending on the specific setting, conferring protection during gut homeostasis and contributing to tissue injury under inflammatory conditions (2). This may be of particular pathogenic and therapeutic importance for inflammatory bowel disease (IBD), inasmuch as there appears to be a distinction between early triggering events, which represent a failure of repair mechanisms after acute mucosal injury, and chronic perpetual inflammation, which signifies a dysregulation between pro-inflammatory and regulatory adaptive immune responses (3). The latter are also influenced by cellular and soluble mediators of innate immunity that provide the necessary polarizing signals for the development of adaptive responses, which then become dominant.
The shaping of effective adaptive immunity following antigen presentation critically depends on the provision of co-stimulatory signals to T-lymphocytes (4). During this process, interactions between proteins of the TNF and TNF receptor superfamilies (TNFSF and TNFRSF, respectively) play decisive roles. TNFSF proteins are expressed on antigen-presenting cells and bind to their cognate receptors on CD4+ and CD8+ lymphocytes; these include OX40 ligand (TNFSF4) and OX40 (TNFRSF4), CD70 (TNFSF7) and CD27 (TNFRSF7), 4-1BBL (TNFSF9) and 4-1BB (TNFRSF9), and TNF-like ligand 1A (TL1A or TNFSF15) and death receptor 3 (DR3 or TNFRSF25) (5). Expression of these molecules is highly upregulated during inflammatory conditions. Several recent publications have revealed the critical role of TNFSF/TNFRSF signaling in the generation and maintenance of chronic mucosal inflammation, which characterizes the two main forms of IBD, ulcerative colitis and Crohn’s disease.
DR3 is the TNFRSF member that shares the highest homology to the prototype molecule, TNFR (6, 7). It is expressed on activated lymphocytes, and its only known ligand, TL1A, has a variety of cellular sources, including dendritic cells, macrophages, plasma cells, lymphocytes and endothelial cells (8–11). Binding of TL1A to DR3 generates co-stimulatory signals for activated T cells, leading to proliferation and cytokine secretion (9). Although TL1A was originally described as a Th1-specific factor, it is now established that TL1A/DR3 signaling leads to a generalized, non-specific stimulation of all major T-cell effector pathways, i.e. Th1, Th2 and Th17 (12–14). Moreover, recent reports have suggested that DR3 may also contribute to the functional expansion of T regulatory cells (Tregs) (15). TL1A and DR3 have been implicated in the pathogenesis of inflammation in experimental models of diverse conditions, including rheumatoid arthritis (16), asthma (17) and IBD (18). Regarding the latter, elevated expression of TL1A and DR3 has been found in several murine models of colitis and ileitis, wherein administration of neutralizing antibodies against TL1A ameliorated disease severity (19). Furthermore, transgenic expression of TL1A in mice has been shown to lead to development of chronic intestinal inflammation (20–22). In humans, elevated expression of local and systemic TL1A has been reported in IBD patients (23, 24), and genetic polymorphisms of TNFSF15, the gene encoding the TL1A protein, modify the risk for developing IBD in both Caucasian and Asian populations (25, 26).
Although the significance of the TL1A/DR3 system for T cell-mediated chronic inflammation is clearly well supported, the role of the TL1A/DR3 axis in preserving gut homeostasis following acute injury has not been explored. To address this question, in the present study we assessed the severity of dextran sodium sulfate (DSS)-induced acute colitis in both DR3- and TL1A-deficient mice. We discovered that DSS-induced colitis is more severe in either DR3−/− or TL1A−/− mice compared to their wild-type (WT) controls, indicating a protective role for this cytokine system during acute intestinal injury/repair. We further demonstrate that the increased severity of DSS colitis in DR3−/− mice may be mediated through defective expansion of the Treg cellular pool and a concomitant aggravation of pro-inflammatory effector T cell responses, mainly of the Th1 and Th17 type. Finally, we show that TL1A or DR3 deficiency leads to differential kinetics of DSS colitis, raising the possibility for the existence of additional ligands and/or receptors for these proteins.
MATERIALS AND METHODS
Reagents, proteins, and antibodies
Anti-CD3e (2C11) and anti-CD28 (37.51) antibodies were purchased from BD Biosciences (San Diego, CA). Anti-CD4 (RM4–5), anti-CD25 (PC61.5) and anti-Foxp3 (FJK-16s) antibodies contained in a Mouse Regulatory T-cell Staining Kit were purchased from eBiosciences (San Diego, CA). Recombinant mouse TL1A was purchased from R&D Systems, Inc. (Minneapolis, MN). DSS was purchased from TdB Consultancy AB (Uppsala, Sweden). Cell culture medium and reagents were all purchased from Invitrogen (Grand Island, NY).
Mice
DR3−/− mice were kindly provided by Dr. Eddie C.Y. Wang, Cardiff University, Wales, UK. TL1A−/− mice were kindly provided by Dr. Linda Burkly from Biogen Idec Inc. (Cambridge, MA). Mice were genotyped by PCR-based assays of genomic tail DNA. Severe combined immunodeficiency (SCID) (C.B17) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were maintained under specific pathogen-free conditions in the Animal Resource Center at Case Western Reserve University. All experimental procedures were approved by the Institutional Animal Care and Use Committee and Animal Resource Center at Case Western Reserve University.
Induction of DSS-colitis
To induce colitis, sterilized 3% (w/v) DSS was added to the drinking water of mice for 7 days. Adult wild-type DR3−/− and TL1A−/− mice were allowed ad libitum access to water. Mice returned to normal drinking water after 7 days of DSS administration. Daily monitoring was performed for body weight, fecal bleeding and the presence of loose stools. Mice were sacrificed on days 0, 7 and 21 for experiments. In order to control for cage-to-cage variability that could be attributable to gut microbiota changes, fresh fecal samples from the experimental cages were mixed and equally distributed back to the original cages, 7 days before the induction of DSS colitis.
Induction of colitis by DSS administration in SCID mice and T-cell transfer
SCID mice were injected i.p. with either PBS (vehicle), 3x106 CD4+ cells, 3x106 CD4+CD25− cells, or both 3x106 CD4+CD25− cells and 0.5x106 CD4+CD25+ cells combined. The respective populations were sorted by FACS (≥90–95% purity). Immediately after transfer, SCID mice were administered DSS in their drinking water for 6 days and then sacrificed for experiments (see experimental design schematic in Figure 4A).
Histology
Mouse colons were removed and placed in Bouin’s fixative solution (Fisher Scientific, Pittsburgh, PA), embedded in paraffin and sectioned. Sections of 3µm were stained with hematoxylin and eosin (H&E). Histological evaluation of inflammation in the colon was performed using a semi-quantitative scoring system. In brief, scores were given for % ulceration (0–3), % re-epithelialization (0–4), active inflammation (0–3), chronic inflammation (0–3) and transmural inflammation (0–3). The total inflammatory score was calculated by adding the individual scores. Histological scoring was performed by a pathologist who was blinded to the experimental design.
Isolation and culture of lamina propria mononuclear cell (LPMC)
Colons were removed from experimental mice, cleaned in ice-cold PBS and cut into ~0.5 cm pieces. To remove epithelial cells and intraepithelial lymphocytes, pieces were placed in 25 ml HBSS (without Ca++ and Mg++) supplemented with 5 mM EDTA and 1 mM DTT and shaken 2x30 min at 250 rpm at 37°C. The remaining tissues were finely chopped, placed in 30 ml of RPMI with 10% FBS containing 0.8 µg/ml of dispase and 0.1 µg/ml of collagenase D (Roche, Indianapolis, IN), and digested for 1 h at 37°C. Cells were collected by centrifugation at 1300 rpm for 5 min at room temperature. Cell pellets were re-suspended in 2 ml of 20% Percoll per tube then overlaid on 40% Percoll. Samples were centrifuged at 2500 rpm at room temperature for 20 min without brake. LPMCs located in the interface between the 40% and 70% Percoll were collected, washed two times, and re-suspended in complete medium. LPMC cells (2x 106 ) were placed in 24 well plates which were pre-coated with 0.5µg/ml anti-CD 3e (Cat# 553058 BD biosciences ) in 1x PBS overnight at 4 ° C. The LPMC cells were cultured in 1ml RPMI medium supplement with 10% FBS, 1x glutamine, 2x pen-strap. 0.1 mM β-mercaptoethanol, anti-CD28 (5 µg/ml) (Cat# 55329 BD biosciences), TGF-β1 (1ng/ml) (R&D system), IL-6 (20 ng/ml) (R&D system) and IL-2 (20 U/ml) (eBioscience Cat# 14–8021) for 72 h. The medium were changed once on day 3. The supernatants were used for measuring cytokines secretion. For measurement of IL-17A secreting cells on day 3, LPMC cells were stimulated with PMA (50ng/ml) (sigma), Ionomycin (1 µg/ml) (sigma) and 1 x GolgiStop (Cat# 554724 BD Biosciences) 37 °C for 4 h.
Isolation and culture of CD4+ T cells
CD4+ cells from mesenteric lymph nodes (MLNs) were isolated by magnetic sorting, using a CD4+ T cell isolation kit from Miltenyi Biotec Inc. (Auburn, CA). Cell culture was performed as previously reported (8). Briefly, purified CD4+ cells were cultured in 96-well round-bottom plates (2x105 cells/well) with plate-bound anti-CD3e (0.5 µg/ml) and soluble anti-CD28 (1 µg/ml) in RPMI 1640 medium with 10% FBS, 2 mM L-glutamine, 10 mM β-mercaptoethanol, and 1% penicillin/streptomycin. Supernatants were collected after incubation for 72 h at 37°C and 5% CO2, and stored at −80°C until further use.
Cytokine measurements
Cytokine production was measured by the Th1/Th2/Th17 cytokine BD Cytometric Bead Array (BD Biosciences, San Diego, CA) or the Q-Plex Array Mouse cytokine screen IR Quansys 16-Plex kit (Quansys Biosciences, Logan, UT). IL-2, IL-10, TGFβ, and IL-17A were detected by ELISA (eBioscience) according to the manufacturer’s instructions at day 7 after colitis induction.
Quantitative real-time RT-PCR
Total RNA was isolated from homogenized colonic tissues by use of the RNeasy Mini kit (Qiagen, Valencia, CA). cDNA was generated by maize mosaic virus random hexamers (1 µg of total RNA in a final reaction volume of 20 µl) (Invitrogen, Grand Island, NY). Amplification and quantification of the target genes was done by real-time RT-PCR using either SYBR Green or TaqMan methodology. The following oligonucleotides were used: β-actin: F5’-CAGGGTGTGATGGTGGGAATG-3’, R5’-GTAGAAGGTGTGGTGCCAGATC-3’; IL-6: F5’-GAGGATACCACTCCCAACAGACC-3’, R5’-AAGTGCATCATCGTTGTTCATACA-3’; TGF-β: F5’-TGACGTCACTGGAGTTGTACGG-3’, R5’-GGTTCATGTCATGGATGGTGC-3’. TaqMan primers and probes for Csf3, E-selectin, and Gapdh (reference gene) were purchased from Applied Biosystems (Grand Island, NY). All reactions were carried out in duplicate in an ABI Step-One Plus system. Relative expression of each target gene was calculated by the ΔΔct method.
Flow cytometry
To identify CD4+CD25+Foxp3+ Tregs, freshly isolated MLN cells were stained with a mouse regulatory T cell staining kit (eBioscience), according to the manufacturer’s protocol. Briefly, isolated MLN lymphocytes were sequentially incubated with affinity purified anti-mouse CD16/32 (to block FcγR), FITC anti-mouse CD4, and APC anti-mouse CD25, each for 30 min at 4°C in the dark. After washing, cells were incubated in 1x fixation/permeabilization buffer for 30 min at 4°C in the dark. Cells were then washed with permeabilization buffer and stained with PE anti-mouse Foxp3 for 30 min at 4°C in the dark (FJK-16s). FACS data were acquired and analyzed on an LSRII (BD Biosciences). To identify IL-17A secreting cells LPMCs were collected and stained for IL-17A–PE (eBioscience) for FACS analysis.
Salmonella infection model
The Barthel’s streptomycin method was followed (27). All mice were 6-wk-old WT DR3+/+ or DR3−/− female mice. Mice were gavaged with 1.0x108 CFU of Salmonella enterica serovar Typhimurium in 75 µl of PBS for 48 h, and then with 20 mg/100 µl PBS with streptomycin for 24 h. Animals were sacrificed by CO2 asphyxiation, and intestinal tissues, MLNs, and colonic fecal pellets were collected for analysis.
Statistical Analysis
All data were analyzed using the Student’s t-test (two-tailed). Differences with a P value ≤ 0.05 were considered statistically significant. Two-way ANOVA and Log-rank (Mantel-Cox) tests were also used as indicated in the results section.
Results
DR3−/− mice demonstrate increased susceptibility to acute DSS-colitis
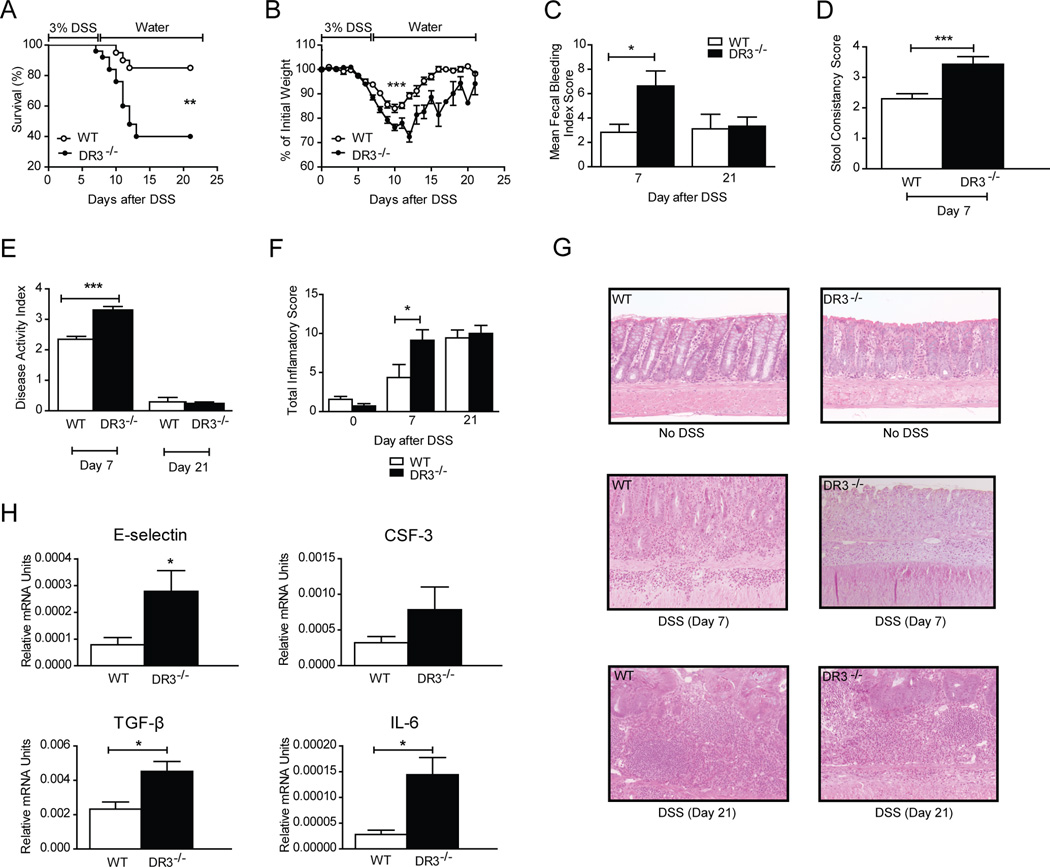
Administration of DSS in drinking water for 7 days leads to acute colitis in mice, followed by spontaneous restitution; hence, this model offers the opportunity to study immunological mechanisms that follow acute injury to the colonic mucosa, as well as repair mechanisms leading to tissue healing (28). To investigate the role of DR3 during this process, we induced acute DSS colitis in DR3−/− mice and determined its clinical and pathological characteristics in comparison to that induced in WT DR3+/+ littermates. Our findings clearly demonstrated that DSS colitis was more severe in DR3−/− mice, as indicated by both higher mortality and significant increases in markers of colitis severity (Fig. 1). In particular, 85% (17/20) of WT mice survived acute colitis, which was significantly higher than the 40% survival rate (10/25) in DR3−/− mice (Fig. 1A). Furthermore, DR3−/− mice lost significantly more body weight as compared to controls (Fig. 1B), and had higher scores for rectal bleeding (Fig. 1C) and loose stools (Fig. 1D). Maximum differences were seen in the acute injury phase (i.e., by day 7; P < 0.05 for all parameters). By day 21, no significant differences were observed between the two groups. These findings may be attributable to the higher mortality rate observed in DR3−/− mice and reflect the fact that mice with the most severe colitis likely succumbed to death during the acute phase and were not included in the assessment at the later time point. Nevertheless, DR3−/− mice that survived demonstrated more severe injury and slower recovery, as indicated by the consistently lower weight of DR3−/− mice at time points after day 7 (Fig. 1B). The DSS colitis disease activity index (DAI) was significantly increased in DR3−/− mice at day 7 compared to WT littermates (3.3 ± 0.1 vs. 2.3 ± 0.1, P<0.0001) (Fig. 1E). We also compared the histological indices for colitis severity between the two groups of mice. As depicted in Fig. 1F, DR3−/− mice had significantly higher colonic total inflammatory scores than WT DR3+/+ littermates at day 7. Similar to body weight and bleeding scores, there was no difference in histological scores by day 21. Representative histology is shown in Figure 1G, demonstrating more severe lesions in DR3−/− mice. In fact, DR3−/− mice at day 7 show almost complete loss of the epithelium, with increased infiltration of inflammatory cells in the lamina propria (left middle panel); by comparison WT DR3+/+ mice at day 7 still maintain cryptal architecture with slightly decreased inflammatory cells in laminar propria (right middle panel). DR3−/− and WT mice at day 21 show comparable severity of colitis (left and right lower panels).
FIGURE 1. Increased severity of acute DSS colitis in DR3−/− mice.
DSS (3% w/v) was added to the drinking water of DR3−/− (n=25) and WT DR3+/+ (n=20) mice for 7 days. A, Increased mortality, and B, increased loss of body weight in DR3−/− compared to WT littermates. C-F, Significant differences between DR3−/− and WT mice were observed by day 7 post-DSS administration, but not day 21, in mean fecal bleeding index scores (C), stool consistency scores (D), disease activity index scores (E), and total inflammatory score (F), calculated by adding the values for individual semi-quantitative histological scoring indices for % ulceration, % re-epithelialization, and active, chronic, and transmural inflammation. G, Representative H&E-stained sections depict the histologic characteristics of colonic tissues from DR3−/− and WT mice, with and without induction of DSS-colitis. DR3−/− mice at day 7 show almost complete loss of the epithelium, with increased infiltration of inflammatory cells in the lamina propria (left middle panel); by comparison WT DR3+/+ mice at day 7 still maintain cryptal architecture with slightly decreased inflammatory cells in laminar propria (right middle panel). DR3−/− and WT mice at day 21 show comparable severity of colitis (left and right lower panels).
Original magnification of photomicrographs, 20x. H, Total RNA was extracted from colonic specimens of DSS-treated DR3−/− and WT DR3+/+ mice. Mucosal mRNA expression of the indicated factors was quantified by real-time PCR. The relative expression of each target gene was normalized to the relative expression of GAPDH in the same sample. All data presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Traditionally, DSS colitis has been considered to be mediated by innate immunity inflammatory pathways. Intestinal inflammation in this model develops in response to chemical-injury-mediated loss of epithelial barrier integrity and the subsequent influx of bacterial-derived factors within the colonic lamina propria. Therefore, we compared WT and DR3−/− mice with DSS colitis for colonic mRNA expression of several inflammatory mediators with an established role in mucosal inflammation, primarily focusing on components of innate immunity. Our comparative analysis showed that mRNA expression of adhesion molecule E-selectin and growth factor CSF, as well as of cytokines TGFβ and IL-6 were significantly upregulated in DR3−/− mice with DSS colitis in comparison to WT controls (Fig. 1H). Nevertheless, expression of other pivotal innate immunity factors, such as IL-1 and TNF did not differ between the two groups (data not shown).
These data indicate that DR3 plays a protective role during the early stage of acute DSS colitis. The most important impact of DR3 deficiency appears to be extensive acute injury from which mice are unable to recover, leading to higher mortality compared with WT DR3+/+, mice.
DR3 deficiency is associated with impaired expansion of regulatory T cells in acute DSS colitis
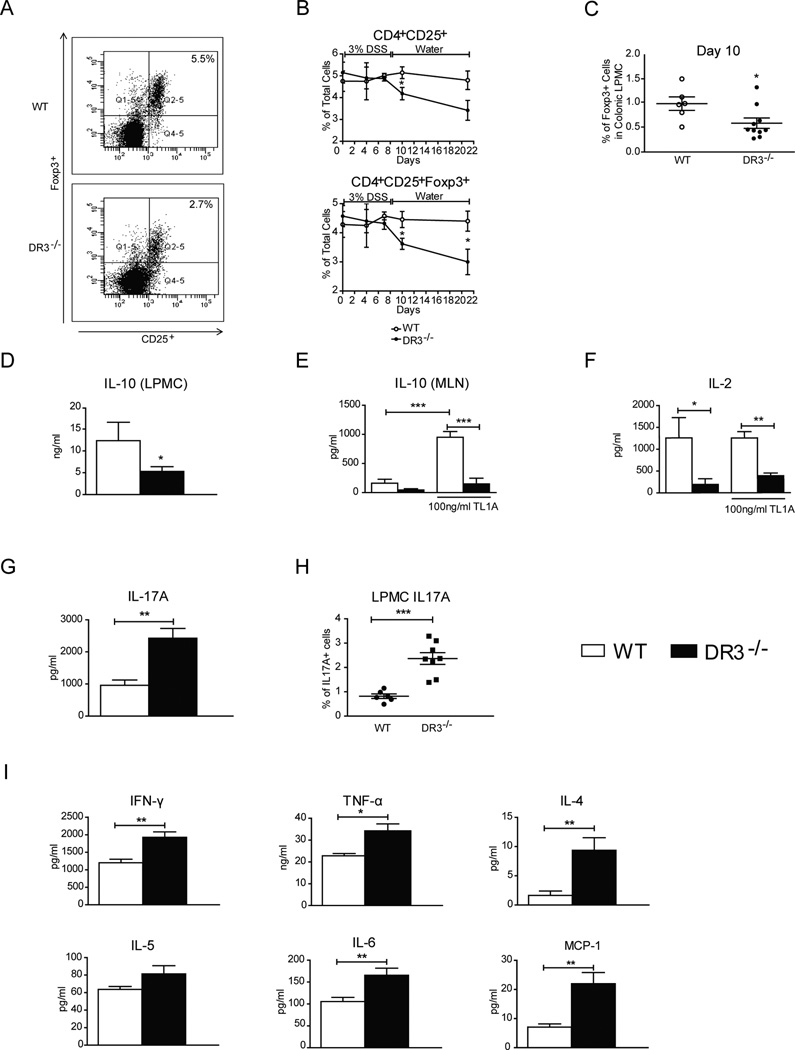
Our comparative analysis of mRNA expression at the intestinal mucosa of WT and DR3−/− mice (Figure 1H) could not by itself explain the differential effect of DSS administration between the two strains. Therefore, we sought to determine alternative immunological mechanism(s) that may underlie the increased severity of DSS colitis in DR3−/− mice. As such, although innate immunity is critical for the pathogenesis of DSS colitis, adaptive immune responses also develop in the course of this disease (29). As DR3 is primarily expressed on lymphocytes, we hypothesized that following DSS administration, DR3−/− mice may demonstrate dysregulation of secondary adaptive immune responses. Recent evidence supports a pivotal role of regulatory T-cells (FoxP3+) in the maintenance of mucosal homeostasis after DSS-inflicted acute injury to the intestinal mucosa (30). As TL1A/DR3 function was recently shown to positively affect the proliferation of Tregs, we tested whether DR3−/− mice fail to develop adequate regulatory immune responses following the induction of acute mucosal injury. We used flow cytometry to quantify the frequency of Tregs within the MLN and lamina propria of mice with DSS-induced colitis. Tregs were defined as cells bearing a CD4+CD25+Foxp3+ phenotype (Fig. 2A). First, we analyzed MLN cells obtained at days 0, 4, 7, 10 and 21 after DSS administration. As shown in Figure 2A and B, we observed a significant decrease in the proportion of CD4+CD25+Foxp3+ Tregs in DR3−/− mice as compared to WT DR3+/+ littermates. Our time course study showed that a notable difference in Treg numbers was evident by day 7. The difference became statistically significant by day 10 and remained so through day 21. We also looked at isolated LPMCs from mice with DSS colitis and found a similar significant decrease in Foxp3+ Tregs by day 10 (Fig. 2C). As IL-10 is a pivotal mediator of the regulatory function of CD4+CD25+Foxp3+ Tregs, we measured the mucosal expression of IL-10 in DR3−/− mice with DSS colitis. In line with the changes in Treg number, CD4+-enriched LPMCs obtained from DR3−/− mice exhibited suppressed secretion of IL-10 in response to TCR-mediated stimulation (Fig. 2D). By comparison, cells from both DR3−/− and DR3+/+ mice produced low levels of IL-10, and the DR3−/−-derived cells were unresponsive with regards to IL-10 production upon stimulation with recombinant TL1A (Fig. 2E). In contrast, when TL1A was added to cultures of WT cells, there was a significant elevation of IL-10 secretion. Therefore, the secretion of IL-10 from CD4+ cells is both TL1A- and DR3-dependent. One possible explanation for the reduced expansion of Tregs in DR3−/− mice may be defective in mucosal production of IL-2, as it is known that the latter acts as a growth factor for Tregs and induces their proliferation. To test this possibility, we compared IL-2 secretion by stimulated CD4+ cells from MLNs. We found significantly reduced production of IL-2 by cells from DR3−/− as compared to WT DR3+/+ mice (Fig. 2F). The addition of TL1A to the culture media did not affect IL-2 secretion in WT or DR3−/− mice, indicating that this is a TL1A-independent, but DR3-dependent, phenomenon.
FIGURE 2. DR3−/− mice with acute DSS-colitis have reduced frequency of mucosal Tregs and augmented pro-inflammatory responses.
MLN cells and LPMCs were purified from DR3−/− and WT mice before and at sequential time points after the administration of DSS. Flow cytometry was used to determine the percentages of CD4+ cells that express the Treg markers, CD25 and Foxp3. A, Representative FACS histogram. Lymphocytes were gated for CD4+ cells and expression of CD25 and Foxp3 were analyzed. B, Time course study of the percentages of CD4+CD25+ and CD4+CD25+Foxp3+ Tregs in MLN cells isolated from DR3−/− and WT DR3+/+ mice treated with DSS-colitis (n = 4–9 mice per time point). C, Percentages of CD4+CD25+Foxp3+ Tregs in freshly isolated LPMCs from DR3−/− and WT DR3+/+ mice treated with DSS-colitis at day 10 (n = 5–10 mice per group). D, Freshly isolated LPMCs were stimulated with anti-CD3 and anti-CD28, and IL-10 protein concentration was measured in culture supernatants. E, F, CD4+ cells were purified from MLNs of DR3−/− and WT DR3+/+ mice with acute DSS-colitis and stimulated via TCR with and without the addition of TL1A to the culture media. The concentration of IL-10 (E) and IL-2 (F) proteins in the culture supernatants was measured by ELISA. G, H, Secretion of IL-17 by TCR-stimulated LPMCs (G) and number of IL-17A expressing LPMCs (H) in DR3−/− and WT DR3+/+ mice with acute DSS-colitis. I, Protein concentrations for the indicated cytokines were measured in supernatants of cultures of TCR-stimulated LPMCs. All data expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
In addition to Tregs, TL1A/DR3 interactions are also involved in Th17 pathways (31). Tregs and Th17 cells are intertwined by their mutual dependence upon the presence of TGF-β1. Thus, we asked whether the suppression of Tregs in DR3−/− mice was coupled with an opposite effect on the expression of IL-17. Indeed, we observed that TCR-stimulated LPMC secretion of IL-17A, the prototypic Th17 cytokine, was significantly higher in DR3−/− mice than in their WT DR3+/+ littermates (DR3+/+: 959.4 ± 165.9 pg/ml vs. DR3−/− : 2426 ± 304.1.36 pg/ml, P ≤ 0.01) (Fig. 2G). In addition, we measured the percentage of IL-17-producing cells in freshly isolated LPMCs by flow-cytometry. Our comparative study showed that a higher percentage of IL-17+ cells were detected in LPMC preparations from DR3−/− versus WT DR3+/+ mice during acute DSS-induced colitis (Fig. 2H).
Our previous results imply that, besides Th17, the other arms of effector immunity may also expand in DR3−/− mice in the absence of the inhibitory control of Tregs. To test this hypothesis, we studied pro-inflammatory cytokine secretion by TCR-stimulated LPMCs, which were isolated from mice with acute colitis (i.e., 7 days post-DSS). As shown in Fig. 2I, several cytokines of all major arms of adaptive T-cell effector responses were significantly elevated in DR3−/− mice as compared to their WT littermates. Elevations included IFNγ (DR3+/+: 1,198 ± 103.3 pg/ml vs. DR3−/−: 1,926 ± 154 pg/ml, P < 0.01), TNF (DR3+/+: 22.77 ng/ml ± 1.07 ng/ml vs. DR3−/−: 33.66 ± 3.74 pg/ml, P < 0.05), IL-6 (DR3+/+: 105.5 ± 9.39 pg/ml; DR3−/−: 165.5 ± 16.25 pg/ml, P < 0.01), IL-4 (DR3+/+: 1.65 ± 0.75 pg/ml; DR3−/−: 7.81 ± 2.35 pg/ml, P ≤ 0.01), and MCP-1 (DR3+/+: 7.08 pg/ml ± 1.11 pg/ml vs. DR3−/−: 21.99 ± 3.81 pg/ml, P < 0.05) (Fig. 2A). Taken together, results from this part of our study support the hypothesis that a major effect of DR3 deficiency during DSS colitis is defective expansion of the Treg pool. In turn, this may lead to inadequate control of all effector responses (Th1, Th2, and Th17).
DR3 deficiency is not associated with functional impairment of Tregs
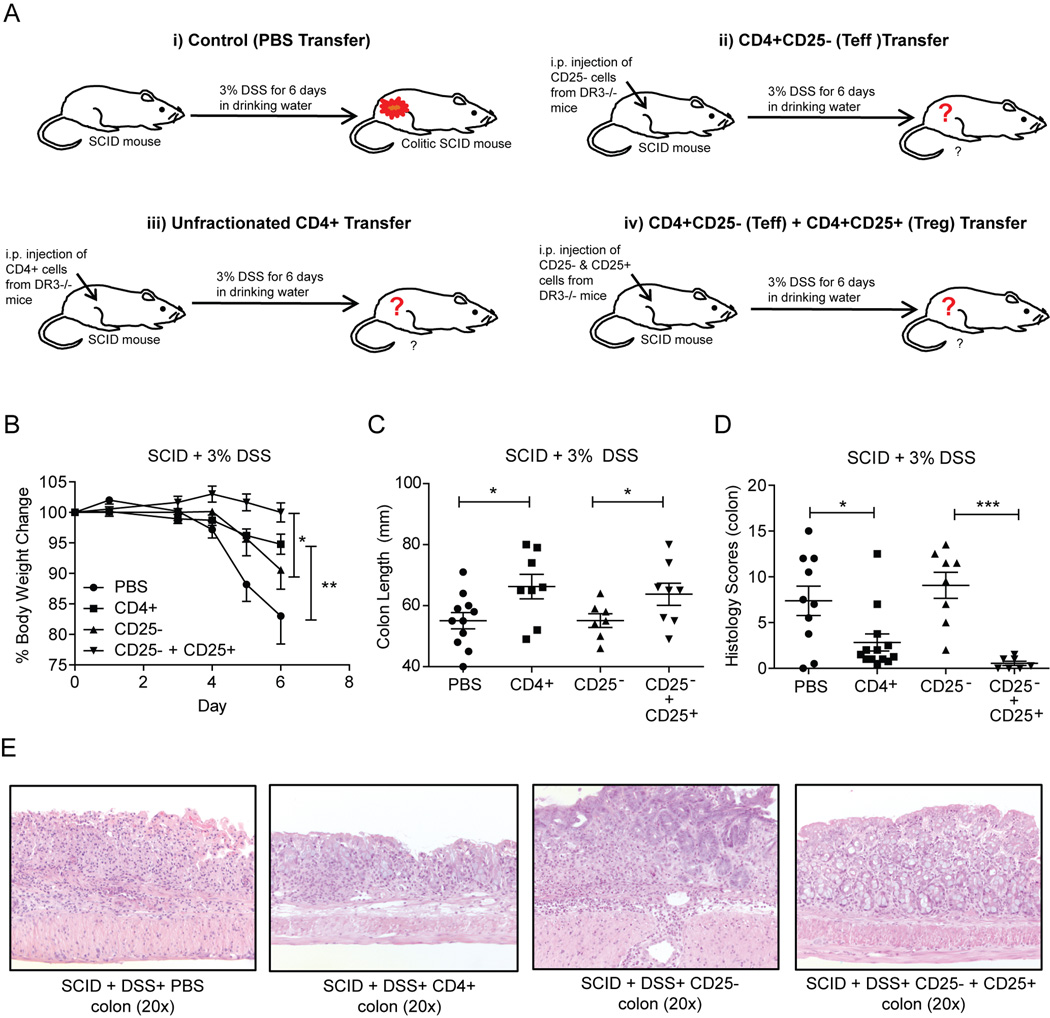
Our previous results indicate that DR3 deficient mice fail to generate an adequate number of Tregs in response to mucosal injury. Nonetheless, the functional capacity of these cells was not addressed. To investigate whether Tregs from DR3−/− mice retained their immunosuppressive properties, we used a novel approach that combined the adoptive transfer of various lymphocytic populations into SCID mice, with the subsequent induction of DSS colitis (Fig. 3A). DSS administration to control SCID mice led to severe colitis, as indicated by disease activity indices for weight loss (Fig. 3B), colon length (Fig. 3C), and histological severity (Fig. 3D). Transfer of effector CD4+CD25− cells from DR3−/− mice did not alter the severity of DSS colitis. In contrast, adoptive transfer of unfractionated CD4+ cells resulted in a significant reduction in DSS colitis severity. This result showed that unfractionated CD4+ cells contain a population of Tregs that prevent the development of DSS colitis. We further validated this observation by showing that DSS colitis was almost completely prevented when regulatory CD4+CD25+ cells were co-transferred with the CD4+CD25− population before the administration of DSS (Fig. 3B–D).
FIGURE 3. Tregs from DR3−/− mice retain their ability to suppress effector responses.
A, Experimental design of the adoptive transfer experiments used in our study. DR3−/− and WT DR3+/+ littermates were used as donors, and SCID mice as recipients. Transferred populations included unfractionated CD4+ cells, CD4+CD25- (Teff) cells, and the combination of CD4+CD25- Teff and CD4+CD25+ Treg cells. After transfer of the respective populations, SCID mice were administered DSS in the drinking water for 6 days (PBS, n = 5; CD4+, n = 14; CD4+CD25-, n = 7; CD4+25-CD25+, n = 8). B, Time course of body weight loss following adoptive transfer of T-cell populations into SCID recipient mice and administration of DSS. C, Colon length of recipient SCID mice after adoptive transfer of T-cell populations and treatment with DSS. D, Semi-quantitative histological measurement of total inflammation (inflammatory score) calculated by adding the values for individual indices for % ulceration, % re-epithelialization, and active, chronic, and transmural inflammation. E, Representative photomicrographs of H&E stained colon sections (20x) from the different experimental groups. Data expressed as mean ± SEM. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
TL1A−/− mice develop severe acute DSS-colitis with delayed kinetics
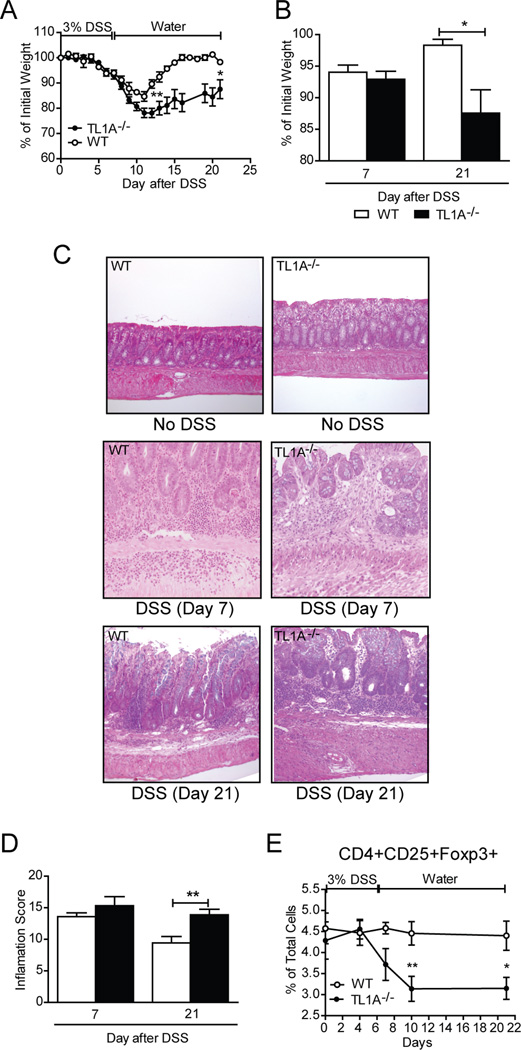
Presently, TL1A is the only known ligand for DR3. In mice, TL1A has only been shown to bind to DR3 as there is no murine analogue for the human decoy receptor 3 (DcR3), which competes with DR3 for TL1A binding in humans (9). Therefore, we examined the characteristics of DSS colitis in TL1A−/− mice in order to determine whether a similar phenotype occurs as in DR3−/− mice. Our experiments revealed both similarities and differences. Similar to DR3−/− mice, TL1A−/− mice also developed more severe colitis compared to WT TL1A+/+ controls. However, TL1A−/− mice showed no mortality after DSS treatment. The kinetics of disease also differed between the two strains, as the major differences in inflammatory markers were seen at later time points in colitic TL1A−/− mice versus DR3−/− mice (Fig. 4). In particular, increased weight loss in TL1A−/− mice compared to their WT littermates was observed by day 12 and persisted through day 21 (Fig. 4A, B). This stands in contrast to DR3 deficiency, which showed the highest effect at day 7. Moreover, unlike mice with DR3 deficiency, there was no difference in colonic inflammatory scores between TL1A−/− and TL1A+/+ mice at day 7 and the greatest differences were observed at day 21 (Fig. 4C, D). Although DR3−/− mice did not show a similarly persistent increase in colitis severity through day 21 (according to body weight or inflammatory score), the results for DR3 deficiency may be confounded by the fact that the most severely diseased DR3−/− mice had died by these later time points. Finally, we wanted to test whether the decrease in the number of Tregs seen in DR3−/− mice was mediated through TL1A/DR3 signaling. As shown in Fig. 4E, similar to DR3−/− mice, the proportion of CD4+CD25+Foxp3+ cells in the MLNs was also decreased in TL1A−/− mice with acute DSS colitis.
FIGURE 4. TL1A−/− mice develop acute DSS-colitis of increased severity and delayed kinetics.
DSS (3% w/v) was added in the drinking water of TL1A−/− (n=9) and WT TL1A+/+ (n=8) mice for 7 days. A, Time course of body weight loss in TL1A−/− mice and TL1A+/+ littermates after induction of DSS-colitis. B, Significant body weight loss was observed between TL1A−/− and WT mice at late (day 21) but not early (day 7) time points after DSS administration. C, H&E-stained sections depict the histological characteristics of colon tissues from TL1A−/− and WT TL1A+/+ mice, with and without induction of DSS colitis on days 7 and 21. Original magnification of photomicrographs, 20x. D, Semi-quantitative histological measurement of total inflammation (inflammatory score) calculated by adding the values for individual indices for % ulceration, % re-epithelialization, and active, chronic, and transmural inflammation. E, Time course study of the percentages of CD4+CD25+Foxp3+ Tregs in MLN cells isolated from TL1A−/− mice and WT TL1A+/+ littermates treated with DSS-colitis. All data presented as mean ± SEM; *P<0.05, **P <0.01.
Altogether, these data are consistent with protective TL1A/DR3 signaling in acute mucosal injury, which may be related to the maintenance of adequate numbers of functional Tregs in the colonic mucosa. Nevertheless, they also point to involvement of other pathways, as the effects of TL1A and DR3 deficiency in DSS colitis were similar, but not identical.
DR3−/− mice demonstrate compromised Th1 responses and defective bacterial clearance in experimental Salmonella infection
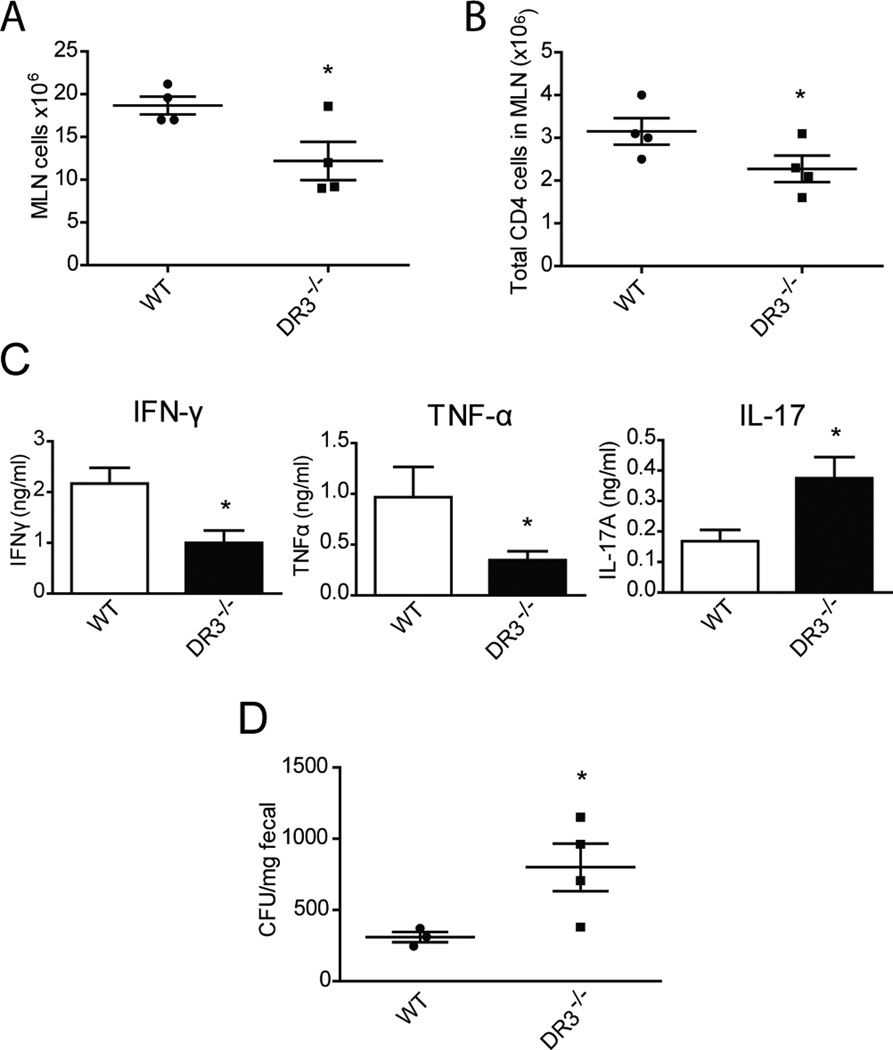
Our previous results were compatible with a generalized dysregulation of mucosal immune responses in DR3−/− mice in the presence of a mucosal insult, (i.e. DSS-induced injury). Along this same line of thought, we tested whether such dysregulation would affect mucosal protection against invasion with a single pathogenic microorganism. To accomplish our aim, we infected WT and DR3−/− mice with Salmonella enterica serovar Typhimurium and compared the microbiological and immunological responses between the two strains. Our results showed that DR3−/− mice demonstrated reduced expansion of MLN cells in response to Salmonella infection, which was accompanied by reduced numbers of CD4+ lymphocytes (Fig. 5A, B). As Salmonella infection leads to accumulation of effector lymphocytes in the mucosa, we also measured TCR-stimulated secretion of cytokines by MLN cells (Fig. 5C). Unlike in DSS colitis, MLN cells from DR3−/− mice infected with Salmonella produced significantly lower amounts of IFNγ compared to WT controls (DR3+/+: 2.17 ± 0.31 ng/ml vs. DR3−/−: 1.00 ± 0.24 ng/ml, P ≤ 0.05). Secretion of TNF was also lower (DR3+/+: 1.09 ± 0.23 ng/ml vs. DR3−/−: 0.29 ± 0.08 ng/ml, P ≤ 0.05). These results indicate a compromise in the generation of Th1 responses in DR3−/− mice infected with Salmonella. In contrast, similar to the DSS colitis model, Th17 responses were significantly enhanced in the absence of DR3, as indicated by elevated secretion of IL-17 by lymphocytes isolated from DR3−/− mice (DR3+/+: 0.17 ± 0.04 ng/ml vs. DR3−/−: 0.38 ± 0.07 ng/ml, P ≤ 0.05). The secretion of IL-10 or IL-13, as well as the percentage of Foxp3+ cells, in MLN cells did not differ between DR3−/− and WT mice (Supplemental Figure 1). To see if these altered mucosal responses had an effect on clearance of the infectious agent, we measured the concentration of Salmonella microorganisms in the feces. As demonstrated in Figure 5D, DR3−/− mice had a 2.5-fold higher CFU/mg fecal load in the colon as compared to WT controls (DR3+/+: 265.0 ± 51.1 CFU/mg vs. DR3−/− 798.8 ± 167.1 CFU/mg, P = 0.022).
FIGURE 5. Compromised Th1 responses and defective bacterial clearance in DR3−/− mice after Salmonella infection.
DR3−/− and WT DR3+/+ mice were infected with Salmonella enterica serovar Typhimurium as described in Methods. A, The total number of cells in MLNs was significantly reduced in DR3−/− mice after Salmonella infection. B, The number of CD4+ lymphocytes in MLNs was significantly lower in DR3−/− mice after Salmonella infection as compared to WT DR3+/+ mice. C, Freshly isolated cells from the MLNs of DR3−/− (n=4) and WT (n=4) mice were cultured under stimulation with anti-CD3 and anti-CD28, and the concentrations of IFN-γ, TNF-α, and IL-17 protein were measured in the supernatants. D, Concentration of Salmonella in the feces of DR3−/− and WT mice expressed in CFU/mg of feces. All data are expressed as mean ± SEM; *P<0.05.
These results indicate that DR3−/− mice have an impaired ability to mount an effective Th1 response after infection with Salmonella, which leads to defective clearance of the microorganism.
DISCUSSION
In the present study, we report a novel protective role of the TL1A/DR3 cytokine system in acute DSS colitis in mice. Such an effect stands in contrast to the pro-inflammatory role that has been attributed to TL1A/DR3 in chronic clinical and experimental inflammation (12). Regarding their role in IBD, these proteins have been associated with intestinal inflammation in several murine models, such as the SAMP1/YitFc and TNFΔARE models of Crohn’s-like ileitis (8), as well as in the Gαi2−/− T-cell transfer model of colitis (19). This functional “paradox” is most likely explained by the different mechanisms of intestinal inflammation in each case. Acute DSS administration induces a transient disturbance of the epithelial barrier, followed by spontaneous mucosal repair (28). Consequently, the natural history of DSS colitis depends on the integrity of mucosal homeostatic control. With that perspective, our study clearly shows that intact TL1A/DR3 signaling is required for preservation of gut homeostasis, as TL1A- and DR3-deficient mice both develop exaggerated colitis compared to their wild-type counterparts. Our results are in line with several recent reports that implicate “classical” pro-inflammatory cytokines of the innate immune system in mucosal protection against acute injury. Such dichotomous effects have been reported for members of the IL-1 family (32), the “master” pro-inflammatory transcription factor NF-κB (33), as well as TNF (34, 35). TNF-deficient mice suffer more severe acute DSS colitis, whereas the integrity of TNFR1-signaling in colonic myeloid lineage cells contributes to suppression of acute, damage-associated mortality. We have shown that preventive treatment of SAMP1/YitFc mice with probiotics protects these ileitis-prone mice from inflammation by stimulating intestinal epithelial TNF production (36). Hence, it may be of pathogenic importance that TL1A was recently reported to act upstream of TNF and induce its expression (37). It should also be noted that, in addition to intestinal disease, DR3 may exert both pathogenic and protective roles in renal inflammation (38). Our results are different from those reported by Meylan et al., which reported no difference in body weight loss in DR3−/− mice following DSS administration (20). This discrepancy may be attributed to differences in DSS administration protocols or gut microbiota composition in different animal facilities.
Contrary to acute inflammation, chronic intestinal inflammation is dominated by adaptive immune responses with heavy infiltration of the lamina propria by activated T-lymphocytes that express surface DR3 abundantly. Signaling through DR3 results in T-cell proliferation and secretion of effector cytokines (39, 40). It is therefore conceivable that abrogation of TL1A/DR3 function would ameliorate chronic intestinal inflammation. Interestingly, a recent study reported that when DSS colitis becomes chronic after repeated cycles of administration, blockade of TL1A leads to disease improvement (19). This study, combined with our present findings, strongly supports functional diversity for the TL1A/DR3 system. On the one hand, TL1A/DR3 signaling is necessary for maintaining gut homeostasis, whereas on the other, it amplifies established chronic pro-inflammatory mucosal responses.
The mechanisms underlying such functional diversity are not clear. Nevertheless, several explanations are possible. First, ligand availability may be a critical factor. TL1A is not constitutively expressed under normal conditions (9). However, during chronic inflammation it becomes highly and sustainably upregulated after stimulation via the Fc receptor or TLRs (12, 18). Accordingly, mice with sustained, transgenic expression of TL1A develop intestinal inflammation (20–22). Second, during chronic inflammation, there is mucosal accumulation of memory lymphocytes with alternatively spliced DR3 mRNA, leading to selective expression of the full-length, transmembrane DR3 protein and augmented pro-inflammatory signaling (8, 41). Third, it was recently shown that intestinal lymphoid cells and innate-like T cells abundantly express DR3 and respond to TL1A (42, 43). As these cells are critical for mucosal homeostasis, DR3 deficiency may be associated with their functional impairment and inability to handle an acute injury. Finally, the type of antigenic stimuli may be of importance, as immune function of DR3 may be different in response to signals from microbiota than from pathogens. Our results provide support for this hypothesis. In DSS colitis, disruption of the epithelial barrier results in invasion of the lamina propria by commensal bacteria. In the absence of DR3, exaggerated immune responses were observed, indicating that DR3 signaling restrains mucosal over-reactivity to microbiota and controls acute inflammation. Due to the highly variable antigenic composition of the commensal flora, a generalized overexpression of effector immunity was observed, which included elements of Th1, Th2, and Th17 responses. On the other hand, in acute Salmonella infection, intact DR3 function was necessary for mounting an effective mucosal response against this single pathogenic microorganism. In this situation, although DR3 deficiency led to over-reactivity of Th17 responses similar to that seen in DSS colitis, it also resulted in selective suppression of IFNγ and TNF (both Th1 responses), a finding that is compatible with a limited repertoire of antigenic stimuli derived from a single pathogen. Our results are consistent with those reported by Buchan et al. demonstrating deficient bacterial clearance and fewer Th1 cells in DR3 deficient mice challenged by i.p. infection with Salmonella (44). However, to our knowledge, our study is the first to demonstrate a role for DR3 in protection against oral Salmonella infection, which is the physiological route of infection for this agent.
Innate immunity components are not only responsible for acute injury and repair, but also dictate the generation of adaptive immune responses. Our present findings indicate that DR3 signaling may affect the balance between regulatory and effector adaptive immunity, as shown by the development of a pro-IL-17 and anti-Treg mucosal environment in DR3−/− mice. Th17 and Treg responses may be interconnected as they both require TGFβ1 to develop (45). When TGFβ1 acts alone, it induces the generation of Tregs, but in the presence of innate pro-inflammatory factors, in particular IL-6, immune responses are directed towards a Th17 phenotype. Further functional diversity is promoted by IL-2, which stimulates the proliferation of T cells with regulatory properties, while simultaneously suppressing the expansion of Th17 lymphocytes (46). This may be of relevance during acute DSS colitis as we showed that, in the absence of DR3, a mucosal environment that was rich in IL-6 and TGFβ1, and poor in IL-2, was generated. This scenario favored the expansion of Il-17 producing cells and enhanced secretion of IL-17 by LPMCs. Although the specific cellular source of IL-17A was not determined in our study, we speculate that the secretion of IL-17A in LPMC can occur by both T cells and non-T cells, possibly innate lymphoid cells. At the same time, we noted reduced numbers of mucosal CD4+CD25+Foxp3+ Tregs and decreased IL-10 secretion. Interestingly, IL-17 enrichment was noted in both DSS- and Salmonella infection-induced acute injury, indicating a stable immunophenotype for DR3−/− mice under these conditions. This is supportive of the recently reported inhibition of IL-17 secretion via TL1A/DR3 (31). However, another report by Pappu et al. demonstrated that IL-17 is negatively regulated by TL1A/DR3 in a chronic model of EAE (47). These opposite results may be explained by the dichotomous role of TL1A /DR3 in acute versus chronic inflammatory conditions. Nevertheless, an imbalance between Tregs and Th17 cells has been reported in both IBD and experimental colitis (48, 49).
Our results suggest that contraction of the Treg pool is a major effect of deficient TL1A/DR3 signaling. This leads to primary loss of counter-regulatory control of effector responses, which is supported by the generalized, nonspecific up-regulation of all major pro-inflammatory pathways in DR3−/− mice. The number of CD4+CD25+Foxp3+ cells was decreased in both TL1A−/− and DR3−/− mice; thus, development of this Treg population is dependent upon ligand/receptor association. Supporting our findings, converging recent evidence supports an important role of TL1A/DR3 signaling in the control of Tregs. Stimulation of lymphocytes with either an agonistic anti-DR3 antibody, a fusion TL1A-Ig protein, or the natural ligand, TL1A, results in significant expansion and activation of Tregs (15, 22, 50). Interestingly, this enhancement in proliferation appears to be coupled with an attenuation of the suppressive potential of Tregs upon DR3 crosslinking. Our results confirm such proliferation/suppression diversity. DR3 deficiency resulted in reduced numbers CD4+CD25+Foxp3+ cells. Nevertheless, these DR3−/− Tregs retained the ability to prevent the development of colitis upon adoptive transfer of Teff to SCID recipients. In our study we did not test the suppressive activities of WT Tregs since DR3−/− Tregs display almost complete suppression of colitis in the adoptive transfer experiment. It is possible that ligand availability may be of pivotal importance under such conditions. In a previous study, TL1A-Ig augmented TCR-mediated proliferation of Foxp3+ Tregs, while at the same time, inhibited their suppressive capacity (48). Nevertheless, suppressive function was fully established in ex vivo Tregs upon removal of TL1A from the culture conditions. Taken together, our present findings, as well as previous studies, clearly indicate that the TL1A/DR3 pathway is involved in both effector and regulatory T-cell development and function. This is a common complexity for co-stimulatory pathways and should be taken into consideration before attempting to therapeutically modify their actions for clinical purposes, as clearly demonstrated by the deterioration of Crohn’s disease after blocking co-stimulatory CTL4A-B7-mediated interactions (51). The possibility of adverse effects should also be taken into account as, during an intestinal infection, a temporary halt of Treg function may be necessary to allow clearance of the offensive agent by effector cells.
At present, TL1A is the only known ligand to DR3 in both humans and mice. Therefore, it was anticipated that TL1A−/− mice would have similar immune response to acute DSS colitis as DR3−/− mice. Our studies revealed that the two models were indeed similar, and had more severe inflammation than their respective WT littermates. Nevertheless, their responses were not identical, as TL1A−/− mice showed no lethality and delayed kinetics in the development of colitis compared to DR3−/− mice. This diversity raises the possibility that additional ligands for DR3 may exist, which may partially compensate for TL1A deficiency. We are actively seeking these alternatives in our laboratory.
In conclusion, we demonstrate a novel protective role for TL1A/DR3 in acute intestinal inflammation following chemical injury or infection. Our findings point to a central role of DR3 signaling in the shaping of secondary adaptive immune responses within the intestinal mucosa, the primary abnormality being a contraction of the Treg pool in DR3−/− mice, which leads to augmented effector responses. The efficacy of pharmaceutical manipulation of the TL1A/DR3 pathway will critically depend upon the ability to restrict effector responses, while concomitantly preserving the anti-inflammatory function of Tregs.
Supplementary Material
Acknowledgments
The authors would like to thank Mitchell Guanzon and Wei Xin for their technical support and expertise in histological assessment of intestinal inflammation. A special thanks for the support received from the Administrative, Mouse Models and Histology/Imaging Cores of the Cleveland Digestive Diseases Research Core Center.
Footnotes
Grant Support: This work was supported by the National Institutes of Health: DK042191 (FC and TTP), DK055812 (FC), and DK097948 (FC).
Abbreviations used in this paper: IBD, inflammatory bowel disease; LPMC, lamina propria mononuclear cell; MLN, mesenteric lymph node; SCID, severe combined immunodeficiency, Teff, effector T-cell; Treg, regulatory T-cell; TNFSF, tumor necrosis factor superfamily; TNFRSF, tumor necrosis factor receptor superfamily; WT, wild-type
The authors have no conflicting financial interests.
REFERENCES
- 1.Harrison OJ, Maloy KJ. Innate immune activation in intestinal homeostasis. J Innate Immun. 2011;3:585–593. doi: 10.1159/000330913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamias G, Corridoni D, Pizarro TT, Cominelli F. New insights into the dichotomous role of innate cytokines in gut homeostasis and inflammation. Cytokine. 2012;59:451–459. doi: 10.1016/j.cyto.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamias G, Nyce MR, De La Rue SA, Cominelli F. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143:895–904. doi: 10.7326/0003-4819-143-12-200512200-00007. [DOI] [PubMed] [Google Scholar]
- 4.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 6.Chinnaiyan AM, O’Rourke K, Yu GL, Lyons RH, Garg M, Duan DR, Xing L, Gentz R, Ni J, Dixit VM. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274:990–992. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 7.Kitson J, Raven T, Jiang YP, Goeddel DV, Giles KM, Pun KT, Grinham CJ, Brown R, Farrow SN. A death-domain-containing receptor that mediates apoptosis. Nature. 1996;384:372–375. doi: 10.1038/384372a0. [DOI] [PubMed] [Google Scholar]
- 8.Bamias G, Mishina M, Nyce M, Ross WG, Kollias G, Rivera-Nieves J, Pizarro TT, Cominelli F. Role of TL1A and its receptor DR3 in two models of chronic murine ileitis. Proc Natl Acad Sci U S A. 2006;103:8441–8446. doi: 10.1073/pnas.0510903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, Cho YH, Ullrich S, Kanakaraj P, Carrell J, Boyd E, Olsen HS, Hu G, Pukac L, Liu D, Ni J, Kim S, Gentz R, Feng P, Moore PA, Ruben SM, Wei P. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 10.Prehn JL, Mehdizadeh S, Landers CJ, Luo X, Cha SC, Wei P, Targan SR. Potential role for TL1A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin Immunol. 2004;112:66–77. doi: 10.1016/j.clim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Prehn JL, Thomas LS, Landers CJ, Yu QT, Michelsen KS, Targan SR. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J Immunol. 2007;178:4033–4038. doi: 10.4049/jimmunol.178.7.4033. [DOI] [PubMed] [Google Scholar]
- 12.Meylan F, Richard AC, Siegel RM. TL1A and DR3, a TNF family ligand-receptor pair that promotes lymphocyte costimulation, mucosal hyperplasia, and autoimmune inflammation. Immunol Rev. 2011;244:188–196. doi: 10.1111/j.1600-065X.2011.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bamias G, Jia LG, Cominelli F. The tumor necrosis factor-like cytokine 1A/death receptor 3 cytokine system in intestinal inflammation. Curr Opin Gastroenterol. 2013;29:597–602. doi: 10.1097/MOG.0b013e328365d3a2. [DOI] [PubMed] [Google Scholar]
- 14.Schreiber TH, Podack ER. Immunobiology of TNFSF15 and TNFRSF25. Immunol Res. 2013;57:3–11. doi: 10.1007/s12026-013-8465-0. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber TH, Wolf D, Tsai MS, Chirinos J, Deyev VV, Gonzalez L, Malek TR, Levy RB, Podack ER. Therapeutic Treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. 2010;120:3629–3640. doi: 10.1172/JCI42933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Wang X, Fahmi H, Wojcik S, Fikes J, Yu Y, Wu J, Luo H. Role of TL1A in the pathogenesis of rheumatoid arthritis. J Immunol. 2009;183:5350–5357. doi: 10.4049/jimmunol.0802645. [DOI] [PubMed] [Google Scholar]
- 17.Fang L, Adkins B, Deyev V, Podack ER. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008;205:1037–1048. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shih DQ, Michelsen KS, Barrett RJ, Biener-Ramanujan E, Gonsky R, Zhang X, Targan SR. Insights into TL1A and IBD pathogenesis. Adv Exp Med Biol. 2011;691:279–288. doi: 10.1007/978-1-4419-6612-4_29. [DOI] [PubMed] [Google Scholar]
- 19.Takedatsu H, Michelsen KS, Wei B, Landers CJ, Thomas LS, Dhall D, Braun J, Targan SR. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135:552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meylan F, Song YJ, Fuss I, Villarreal S, Kahle E, Malm IJ, Acharya K, Ramos HL, Lo L, Mentink-Kane MM, Wynn TA, Migone TS, Strober W, Siegel RM. The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal Immunol. 2011;4:172–185. doi: 10.1038/mi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih DQ, Barrett R, Zhang X, Yeager N, Koon HW, Phaosawasdi P, Song Y, Ko B, Wong MH, Michelsen KS, Martins G, Pothoulakis C, Targan SR. Constitutive TL1A (TNFSF15) expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. PLoS One. 2011;6:e16090. doi: 10.1371/journal.pone.0016090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taraban VY, Slebioda TJ, Willoughby JE, Buchan SL, James S, Sheth B, Smyth NR, Thomas GJ, Wang EC, Al-Shamkhani A. Sustained TL1A expression modulates effector and regulatory T-cell responses and drives intestinal goblet cell hyperplasia. Mucosal Immunol. 2011;4:186–196. doi: 10.1038/mi.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bamias G, Kaltsa G, Siakavellas SI, Gizis M, Margantinis G, Zampeli E, Vafiadis-Zoumboulis I, Michopoulos S, Daikos GL, Ladas SD. Differential expression of the TL1A/DcR3 system of TNF/TNFR-like proteins in large vs. small intestinal Crohn’s disease. Dig Liver Dis. 2012;44:30–36. doi: 10.1016/j.dld.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Bamias G, Kaltsa G, Siakavellas SI, Papaxoinis K, Zampeli E, Michopoulos S, Zouboulis-Vafiadis I, Ladas SD. High intestinal and systemic levels of decoy receptor 3 (DcR3) and its ligand TL1A in active ulcerative colitis. Clin Immunol. 2010;137:242–249. doi: 10.1016/j.clim.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Thiebaut R, Kotti S, Jung C, Merlin F, Colombel JF, Lemann M, Almer S, Tysk C, O’Morain M, Gassull M, Binder V, Finkel Y, Pascoe L, Hugot JP. TNFSF15 polymorphisms are associated with susceptibility to inflammatory bowel disease in a new European cohort. Am J Gastroenterol. 2009;104:384–391. doi: 10.1038/ajg.2008.36. [DOI] [PubMed] [Google Scholar]
- 26.Yang SK, Lim J, Chang HS, Lee I, Li Y, Liu J, Song K. Association of TNFSF15 with Crohn’s disease in Koreans. Am J Gastroenterol. 2008;103:1437–1442. doi: 10.1111/j.1572-0241.2007.01752.x. [DOI] [PubMed] [Google Scholar]
- 27.Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boehm F, Martin M, Kesselring R, Schiechl G, Geissler EK, Schlitt HJ, Fichtner-Feigl S. Deletion of Foxp3+ regulatory T cells in genetically targeted mice supports development of intestinal inflammation. BMC Gastroenterol. 2012;12:97. doi: 10.1186/1471-230X-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones GW, Stumhofer JS, Foster T, Twohig JP, Hertzog P, Topley N, Williams AS, Hunter CA, Jenkins BJ, Wang EC, Jones SA. Naive and activated T cells display differential responsiveness to TL1A that affects Th17 generation, maintenance, and proliferation. FASEB J. 2011;25:409–419. doi: 10.1096/fj.10-166843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopetuso LR, Chowdhry S, Pizarro TT. Opposing Functions of Classic and Novel IL-1 Family Members in Gut Health and Disease. Front Immunol. 2013;4:181. doi: 10.3389/fimmu.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 34.Mizoguchi E, Hachiya Y, Kawada M, Nagatani K, Ogawa A, Sugimoto K, Mizoguchi A, Podolsky DK. TNF receptor type I-dependent activation of innate responses to reduce intestinal damage-associated mortality. Gastroenterology. 2008;134:470–480. doi: 10.1053/j.gastro.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 35.Naito Y, Takagi T, Handa O, Ishikawa T, Nakagawa S, Yamaguchi T, Yoshida N, Minami M, Kita M, Imanishi J, Yoshikawa T. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J Gastroenterol Hepatol. 2003;18:560–569. doi: 10.1046/j.1440-1746.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 36.Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci U S A. 2010;107:454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin S, Chin J, Seeber S, Niewoehner J, Weiser B, Beaucamp N, Woods J, Murphy C, Fanning A, Shanahan F, Nally K, Kajekar R, Salas A, Planell N, Lozano J, Panes J, Parmar H, DeMartino J, Narula S, Thomas-Karyat DA. TL1A/TNFSF15 directly induces proinflammatory cytokines, including TNFalpha, from CD3+CD161+ T cells to exacerbate gut inflammation. Mucosal Immunol. 2013;6:886–899. doi: 10.1038/mi.2012.124. [DOI] [PubMed] [Google Scholar]
- 38.Al-Lamki RS, Wang J, Tolkovsky AM, Bradley JA, Griffin JL, Thiru S, Wang EC, Bolton E, Min W, Moore P, Pober JS, Bradley JR. TL1A both promotes and protects from renal inflammation and injury. J Am Soc Nephrol. 2008;19:953–960. doi: 10.1681/ASN.2007060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bamias G, Martin C, 3rd, Marini M, Hoang S, Mishina M, Ross WG, Sachedina MA, Friel CM, Mize J, Bickston SJ, Pizarro TT, Wei P, Cominelli F. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171:4868–4874. doi: 10.4049/jimmunol.171.9.4868. [DOI] [PubMed] [Google Scholar]
- 40.Papadakis KA, Prehn JL, Landers C, Han Q, Luo X, Cha SC, Wei P, Targan SR. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol. 2004;172:7002–7007. doi: 10.4049/jimmunol.172.11.7002. [DOI] [PubMed] [Google Scholar]
- 41.Twohig JP, Marsden M, Cuff SM, Ferdinand JR, Gallimore AM, Perks WV, Al-Shamkhani A, Humphreys IR, Wang EC. The death receptor 3/TL1A pathway is essential for efficient development of antiviral CD4(+) and CD8(+) T-cell immunity. FASEB J. 2012;26:3575–3586. doi: 10.1096/fj.11-200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahn YO, Weeres MA, Neulen ML, Choi J, Kang SH, Heo DS, Bergerson R, Blazar BR, Miller JS, Verneris MR. Human group3 innate lymphoid cells express DR3 and respond to TL1A with enhanced IL-22 production and IL-2-dependent proliferation. Eur J Immunol. 2015;45:2335–2342. doi: 10.1002/eji.201445213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmkvist P, Roepstorff K, Uronen-Hansson H, Sanden C, Gudjonsson S, Patschan O, Grip O, Marsal J, Schmidtchen A, Hornum L, Erjefalt JS, Hakansson K, Agace WW. A major population of mucosal memory CD4+ T cells, coexpressing IL-18Ralpha and DR3, display innate lymphocyte functionality. Mucosal Immunol. 2015;8:545–558. doi: 10.1038/mi.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchan SL, Taraban VY, Sleboda TJ, James S, Cunnigham AF, Al-Shamkhani A. Death receptor 3 is essential for generating optimal protective CD4+ T-cell immunity agaunst Salmonella. Eur J Immunol. 2012;42:580–588. doi: 10.1002/eji.201041950. [DOI] [PubMed] [Google Scholar]
- 45.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 46.Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol. 2011;12:551–559. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pappu BP, Borodovosky A, Zheng TS, Yang X, Wu P, Dong X, Weng S, Browing B, Scott ML, Ma L, Su L, Tian Q, Schneider P, Flavell RA, Dong C, Burkly LC. TL1A-Dr3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008;205:1049–1062. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishimaru N, Yamada A, Kohashi M, Arakaki R, Takahashi T, Izumi K, Hayashi Y. Development of inflammatory bowel disease in Long-Evans Cinnamon rats based on CD4+CD25+Foxp3+ regulatory T cell dysfunction. J Immunol. 2008;180:6997–7008. doi: 10.4049/jimmunol.180.10.6997. [DOI] [PubMed] [Google Scholar]
- 49.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. Journal Clinical Immunol. 2010;30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 50.Khan SQ, Tsai MS, Schreiber TH, Wolf D, Deyev VV, Podack ER. Cloning, expression, and functional characterization of TL1A-Ig. J Immunol. 2013;190:1540–1550. doi: 10.4049/jimmunol.1201908. [DOI] [PubMed] [Google Scholar]
- 51.Sandborn WJ, Colombel JF, Sands BE, Rutgeerts P, Targan SR, Panaccione R, Bressler B, Geboes K, Schreiber S, Aranda R, Gujrathi S, Luo A, Peng Y, Salter-Cid L, Hanauer SB. Abatacept for Crohn’s disease and ulcerative colitis. Gastroenterology. 2012;143:62–69. doi: 10.1053/j.gastro.2012.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.