Abstract
Rationale
Human genome wide association studies (GWAS) have revealed novel genetic loci that are associated with coronary heart disease (CHD). One such locus resides in LNK/SH2B3 which in mice is expressed in hematopoietic cells and suppresses thrombopoietin (TPO) signaling via its receptor MPL. However, the mechanisms underlying the association of LNK snps with CHD are poorly understood.
Objective
To understand the functional effects of LNK snps and explore the mechanisms whereby LNK loss of function impacts atherosclerosis and thrombosis.
Methods and Results
Using human cord blood, we show that the common TT risk genotype (R262W) of LNK is associated with expansion of hematopoietic stem cells and enhanced megakaryopoiesis, demonstrating reduced LNK function and increased MPL signaling. In mice hematopoietic Lnk deficiency leads to accelerated arterial thrombosis and atherosclerosis, but only in the setting of hypercholesterolemia. Hypercholesterolemia acts synergistically with LNK deficiency to increase IL-3/GM-CSF receptor signaling in bone marrow myeloid progenitors, while in platelets cholesterol loading combines with Lnk deficiency to increase activation. Platelet LNK deficiency increases MPL signaling and AKT activation, while cholesterol loading decreases SHIP-1 phosphorylation, acting convergently to increase AKT and platelet activation. Together with increased myelopoiesis, platelet activation promotes pro-thrombotic and pro-atherogenic platelet/leukocyte aggregate formation.
Conclusions
LNK (R262W) is a loss of function variant that promotes TPO/MPL signaling, platelet and leukocyte production. In mice, LNK deficiency is associated with both increased platelet production and activation. Hypercholesterolemia acts in platelets and hematopoietic progenitors to exacerbate thrombosis and atherosclerosis associated with LNK deficiency.
Keywords: LNK/SH2B3, Hypercholesterolemia, atherosclerosis, thrombosis, platelet, cholesterol
Subject Terms: Lipids and Cholesterol, Stem Cells, Platelets, Atherosclerosis, Thrombosis
INTRODUCTION
In human epidemiological studies, increased monocyte, neutrophil and platelet counts predict risk of myocardial infarction and thrombotic stroke.1–3 Augmented production of platelets and myeloid cells, formation of platelet-leukocyte aggregates and increased generation of platelet and leukocyte inflammatory mediators4 are potential underlying mechanisms that contribute to accelerated athero-thrombotic disease. Platelet-leukocyte interactions are thought to trigger a series of events that contribute to the inflammatory reaction of the vessel wall and promotion of atherogenesis and thrombosis.4
LNK (also called SH2B3) is a member of the SH2B family of adaptor proteins primarily expressed in hematopoietic and endothelial cells5. In hematopoietic cells, LNK functions as a negative regulator of cytokine signaling and cell proliferation5, 6. Rare loss of function mutations in LNK give rise to myeloproliferative neoplasms characterized by platelet and leukocyte overproduction7–9. Targeted deletion of LNK in mice causes expansion of hematopoietic stem cells (HSC), increased myelopoiesis, megakaryopoiesis, thrombocytosis and leukocytosis5, 10, 11, suggesting lack of negative feedback regulation of TPO and MPL signaling5,6. In human GWAS, the LNK SNP, rs3184504 causing a missense mutation at position 262 (p.R262W, c.784T>C), is associated with an increased risk of CHD12; the same SNP is also associated with increased platelet counts and leukocytosis13, which are risk factors for CHD11, 28, 29, suggesting that the risk allele of LNK might worsen CHD via its effects on hematopoiesis. However, rs3184504 is associated with pleiotropic effects including autoimmunity, Type 1 diabetes and hypertension14–17. Moreover, it is not known if this SNP directly affects LNK expression or hematopoietic functions, and the relationship of LNK germline genomic variation to atherosclerosis and thrombosis has not been directly investigated. In this study, we aim to assess the impact of rs3184504 on hematopoiesis and use Lnk−/− mice as a model to assess the effects of LNK loss of function on atherothrombosis.
METHODS
Full Methods are provided in the Online Data Supplement.
RESULTS
The TT risk allele of LNK increases human HSC and megakaryopoiesis
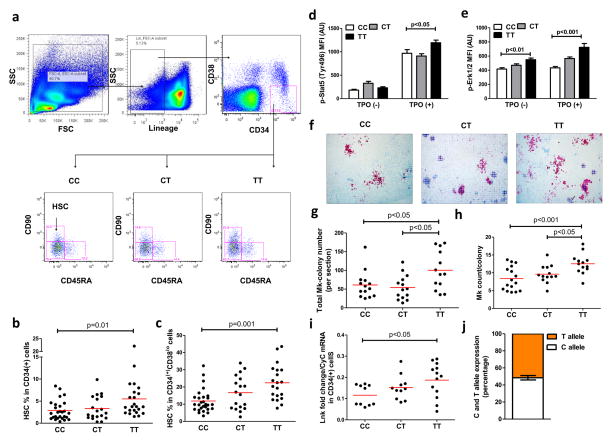
In order to assess the potential impact of rs3184504 on hematopoietic functions, we characterized the HSC compartment in human cord blood samples based on LNK genotype. This revealed a significant increase in the HSC-containing fraction (CD90+CD45RA−)18 in subjects carrying the TT risk genotype (Fig. 1a–c). Using phospho-flow cytometry, we showed increased phosphorylation of STAT5 and ERK1/2, but not AKT, in response to TPO in TT HSCs (Fig. 1d,e, Online Fig. I), suggesting that HSCs were increased as a result of increased TPO/MPL signaling. Moreover, the number of megakaryocyte colonies derived from the cord blood CD34+ cells and the overall megakaryocyte counts per colony were increased in association with the TT genotype (Fig. 1f–h), indicating increased megakaryopoiesis. Since LNK functions as a negative regulator of cytokine signaling in hematopoietic cells, including TPO/MPL signaling, these findings suggest that the T allele is associated with reduced LNK function which could result from altered protein structure/activity or decreased expression of LNK in HSPCs and megakaryocyte progenitors (MkPs). R262 is not conserved in mice, and overexpression studies with LNK R262W in cell lines have not been informative7. Thus, an expression quantitative trait loci (eQTL) at rs3184504 or another SNP in linkage disequilibrium with rs3184504 could be responsible for reduced LNK function. We assessed LNK mRNA levels in cord blood CD34+ cells. Paradoxically, LNK mRNA levels were increased in subjects with the TT genotype (Fig. 1i), which is inconsistent with an eQTL causing reduced LNK activity via a reduction in LNK mRNA. ATXN2, PTPN11 and TRAFD1 are neighboring genes of LNK/SH2B3 on chromosome 12 and there is evidence of linkage disequilibrium (LD) of rs3184504 with SNPs in these genes19. Thus, we measured ATXN2, PTPN11 and TRAFD1 mRNA levels in a new set of cord blood CD34+ cells but found no significant difference between TT vs CC individuals, while LNK/SH2B3 mRNA levels were reproducibly higher in association with TT(Online Fig. II). TPO/MPL signaling increases LNK transcription in human hematopoietic cells20. Since we detected increased TPO/MPL signaling in hematopoietic cells of the TT genotype (Fig. 1d, e), the increase in LNK mRNA may be explained by LNK loss of function and disruption of a LNK/MPL negative feedback loop by the LNK R262W variant.
Figure 1. Association of the LNK TT risk SNP with HSC expansion and increased megakaryopoiesis.
Flow cytometry overview of HSCs (Lin−CD34+CD38loCD90+CD45RA−) (a) and HSC percentage in CD34+ (b) or Lin−CD34+CD38lo (c) cord blood cell fractions. p-STAT5 (d) and p-ERK1/2 (e) levels in CD34+ cells. Megakaryocyte (Mk) colony number (f,g) and Mk counts per colony (h). Normalized Lnk mRNA levels in CD34+ cells (i). j) Allele-specific LNK expression of human cord blood CD34+ cells.
We also carried out studies to assess alternative possibilities that LNK mRNA levels were increased due to distinct regulation of the T allele specific expression by cis-regulatory or post-transcriptional regulatory effects. This was based on a novel approach described in a recent study21 which quantified the allele-specific (in our case, T or C specific) expression of LNK. Since we hypothesized that the increased LNK mRNA levels associated with T allele is due to increased transcription as a result of feedback regulation to compensate for the functional deficiency of LNK, we expect this regulatory impact to be equal for both T- and C-specific LNK alleles in heterozygous samples. Thus, a 1:1 allele-specific expression ratio is expected from these samples despite an increase in total LNK mRNA level. A ratio different from 1:1 would suggest allele-specific cis-regulatory effects or allele-specific post-transcriptional regulation and refute our hypothesis. We first showed, using samples with fixed ratios of TT or CC cDNAs, that the measured allele-specific expression of the two alleles closely correlated with the expected ratio (Online Fig. IIIa), validating this assay. Then, we assessed the relative T or C allele specific expression of the heterozygous samples and the ratio of the allele-specific expression was determined. The average of this ratio was indeed 1:1 (Online Fig. IIIb), consistent with our hypothesis. Together these findings show for the first time that LNK acts as a brake on TPO/MPL signaling in humans, and suggest that R262W causes a functional defect of LNK that leads to increased TPO/MPL signaling.
LNK loss of function interacts with hypercholesterolemia to promote myelopoiesis
While R262 is not conserved in mice, Lnk−/− mice show increased hematopoietic stem cells and megakaryocytes in bone marrow (BM) and increases in blood platelets and leukocytes5, 10, 11, similar to the effects of the T allele in humans. Thus, Lnk−/− mice appear to be a suitable model to assess the effects of reduced LNK function on cardiovascular disease. Humans have much higher levels of LDL cholesterol than mice and some studies have suggested that increased LDL cholesterol increases platelet reactivity22. We therefore assessed the impact of LNK deficiency on hematopoietic functions, thrombosis and atherosclerosis in both normo- and hyper-cholesterolemic backgrounds. We transplanted BM from WT or Lnk−/− mice into irradiated WT or Ldlr−/− mice, followed by feeding either a chow diet (normocholesterolemic WT recipients) or WTD (hypercholesterolemic Ldlr−/− recipients) for 12 weeks.
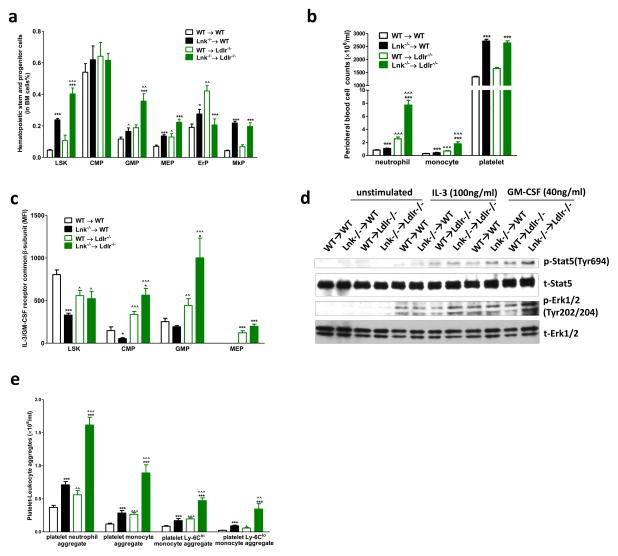
Similar to chow-fed Lnk−/− mice5, 10, 11, Lnk−/− BM recipients displayed expansion of hematopoietic stem and progenitor cells (HSPCs, defined as Lin−, Sca-1+, c-Kit+ LSK cells), myeloid and megakaryocyte progenitors in BM (Fig. 2a) and monocytosis and neutrophilia on both diets (Fig.2b, Online Fig.IV). Interestingly, hypercholesterolemic Lnk−/− BM recipients also showed markedly more pronounced neutrophilia and monocytosis compared to WT or chow-fed Lnk−/− recipients (Fig. 2b), reflecting an increase in the BM LSK and GMP populations (Fig. 2a).
Figure 2. Hypercholesterolemia markedly increases myelopoiesis in Lnk−/− BM recipient.
Hematopoietic stem and progenitor cells in BM (a), peripheral neutrophil, monocyte and platelet counts (b), cell surface CBS levels (c) and platelet neutrophil/monocyte aggregates (e). n=14–15. (d) p-Erk and p-Stat levels in BM cells with and without stimulation of IL-3 (100ng/ml) and GM-SCF (40ng/ml).
Black bars represent chow feeding and green bars represent WTD feeding for 12 weeks. *, **, *** denote p<0.05, <0.01 and <0.001 for WTD-fed Lnk −/− vs WT or chow-fed Lnk −/− vs WT. ^, ^^, ^^^ denote p<0.05, <0.01 and <0.001 for chow-fed Lnk −/− vs WTD-fed Lnk −/− or chow-fed WT vs WTD-fed WT.
This suggested an interaction of LNK deficiency with hypercholesterolemia to expand BM myeloid progenitors. There is evidence that LNK negatively regulates IL-3 signaling 10. Moreover, cholesterol accumulation in HSPCs has been associated with expansion of HSPCs and increased signaling via the IL-3/GM-CSF receptor due to increased levels of the common-beta subunit (CBS) of this receptor on the cell surface23, 24. CBS deletion reversed HSPC expansion and the associated leukocytosis25. Cell surface CBS levels were decreased in HSPCs in chow-fed Lnk−/− BM recipients (Fig. 2c), consistent with increased IL-3/GM-CSF signaling and feedback down-regulation of the CBS26 as a result of disrupted negative feedback regulation by LNK. This decrease in CBS was reversed in HSPCs and the CBS levels were further increased in Lnk−/− GMPs in the setting of hypercholesterolemia (Fig. 2c). Consistent with increased CBS levels, hematopoietic cell signaling in response to IL-3 or GM-CSF treatment, using phosphorylated STAT5 or ErK1/2 as the readout, was increased by the combination of LNK deficiency and hypercholesterolemia (Fig. 2d). These findings explain at least in part the marked increase in neutrophilia and monocytosis in hypercholesterolemic mice with hematopoietic LNK deficiency.
LNK loss of function interacts with hypercholesterolemia to increase platelet activation
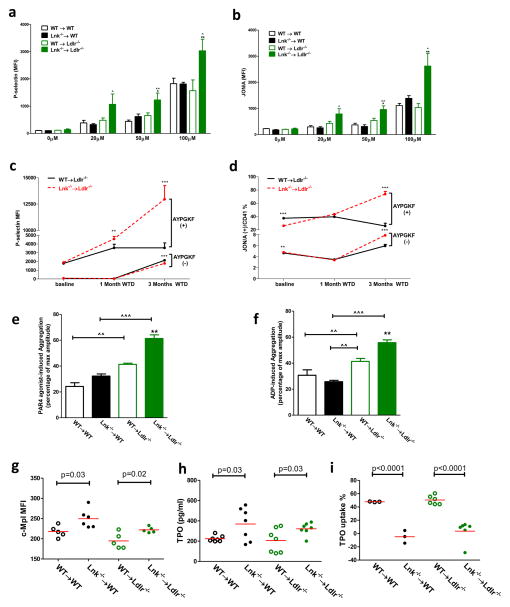
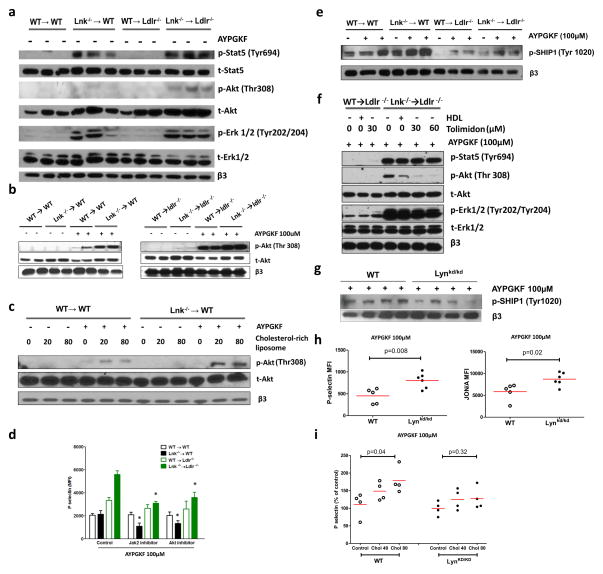
Platelet/leukocyte aggregates are thought to have an important role in promoting both atherosclerosis and thrombosis in states of leukocyte and platelet overproduction as exemplified in myeloproliferative neoplasms27. Platelet/leukocyte aggregates were markedly increased by the combination of hypercholesterolemia and hematopoietic LNK deficiency, involving both increased platelet/neutrophil and increased platelet/monocyte aggregates (Fig. 2e). In contrast to leukocytosis, thrombocytosis in Lnk−/− BM recipients was similar in both normo- and hypercholesterolemic backgrounds (Fig. 2b), paralleling similar levels of the BM MkP population (Fig. 2a). Formation of platelet/leukocyte aggregates is promoted by platelet activation28. Thus, we further assessed parameters of platelet function. In the resting state, Lnk−/− platelets from either normo- or hypercholesterolemic mice did not show significant alteration of surface presentation of P-selectin or active integrin αIIbβ3(JON/A), markers of platelet activation, relative to the WT BM recipients (Fig. 3a, b). However, Lnk−/− platelets from hypercholesterolemic mice showed markedly increased surface exposure of these molecules when activated by PAR4 agonist AYPGKF, while Lnk−/− platelets from normocholesterolemic mice showed similar activation (Fig. 3a, b), indicating an interaction of LNK deficiency with hypercholesterolemia to promote platelet activation. To further assess the impact of hypercholesterolemia on WT or Lnk−/− platelets, we performed a time course study. As the mice on the WTD developed progressive hypercholesterolemia (Online Fig. V), there was a marked increase in cell surface P-selectin and active integrin αIIbβ3 in response to PAR4 activation in Lnk−/− platelets but not in WT platelets (Fig. 3c, d). Similarly, aggregation of Lnk−/− platelets in response to PAR4 agonist or ADP was markedly increased only when mice became hypercholesterolemic (Fig. 3e, f, Online Fig. VI). Together, these findings indicate a profound interaction of the Lnk−/− genotype with hypercholesterolemia to increase platelet activation.
Figure 3. Hypercholesterolemia markedly increases platelet reactivity in Lnk−/− BM recipient.
Surface P-selectin (a) and active integrin αIIbβ3 (JON/A) (b) levels on washed platelets with or without PAR4 agonist (AYPGKF, 100 μM) stimulation (n=5). Surface P-selectin (c) and active integrin αIIbβ3 (JON/A) (d) levels on platelets in whole blood with or without AYPGKF (100 μM) stimulation (n=5). Washed platelet aggregation upon AYPGKF (100μM) (e) or ADP (20 μM) (f) stimulation (n=4–5). TPO receptor (Mpl) levels on platelet surface (g), platelet TPO internalization (h) and plasma TPO levels (i) in WT or Lnk−/− BM recipient mice. (n=3–7)
For 3a–3f, black bars represent chow feeding and green bars represent WTD feeding for 12 weeks. *, **, *** denote p<0.05, <0.01 and <0.001 for WTD-fed Lnk −/− vs WT or chow-fed Lnk −/− vs WT. ^, ^^, ^^^ denote p<0.05, <0.01 and <0.001 for chow-fed Lnk −/− vs WTD-fed Lnk −/− or chow-fed WT vs WTD-fed WT.
We next considered that there might be a cell intrinsic effect of cholesterol enrichment of LNK deficient platelets. We found increased cell surface MPL levels in Lnk−/− platelets (Fig. 3g), reflecting a defect in internalization of MPL (Fig. 3i), suggesting that LNK negatively regulates MPL signaling in part by promoting MPL internalization. Plasma TPO levels were increased in mice with hematopoietic LNK deficiency (Fig. 3h), likely reflecting the decreased internalization and turnover of MPL and TPO in platelets. Hepatic, splenic or bone marrow TPO mRNA levels showed no difference between wild type and Lnk−/− mice, with or without hypercholesterolemia (Online Fig. VII). This novel observation suggests that in addition to the known cell intrinsic effects of LNK deficiency in HSCs5, increased plasma TPO levels may contribute to HSC expansion and megakaryopoiesis in hematopoietic LNK deficiency. However, the increase in plasma TPO levels was similar in normocholesterolemic and hypercholesterolemic mice, consistent with similar numbers of MkPs and platelets in these mice. Thus, while an increase in plasma TPO levels likely promoted platelet production, and an increase in platelet MPL might lead to increased MPL signaling and priming of platelets, this did not explain the effects of hypercholesterolemia on platelet activation.
Cholesterol modulates Lnk−/− platelet activation
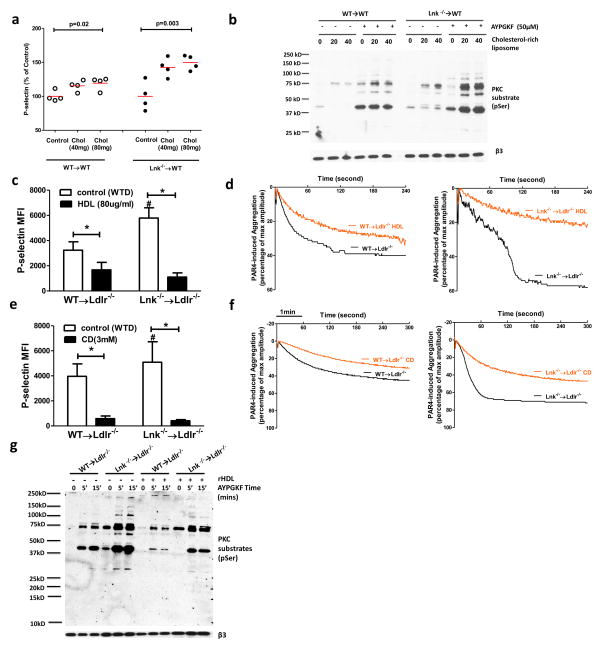
We next undertook experiments to determine if cholesterol enrichment or depletion acted directly in platelets to alter their activation. While the WTD increased total cholesterol mass in platelets from both WT and Lnk−/− mice, there was no difference in total and free cholesterol content or in plasma membrane free cholesterol content as assessed by filipin staining and flow cytometry between platelets of the two genotypes (Online Fig. VIIIa–c). To model the effect of hypercholesterolemia-induced cholesterol accumulation in platelets of either genotype,, we cholesterol-loaded platelets from chow-fed WT mice that had been transplanted with WT or Lnk−/− BM, using cholesterol-rich liposomes29. Cholesterol loading increased both WT and Lnk−/− platelet P-selectin exposure after PAR4 activation but the increase was more pronounced and occurred at a lower threshold in Lnk−/− platelets (Fig. 4a). Increased cell surface levels of P-selectin in Lnk−/− platelets indicated increased platelet degranulation. Protein kinase C (PKC)-mediated signaling is a major pathway downstream of PAR4 activation, regulating platelet degranulation30. An increase in PKC activation after PAR4 activation was observed in Lnk−/− platelets only after cholesterol loading, providing an explanation for the synergistic effect of platelet LNK deficiency and cholesterol loading on P-selectin exposure (Fig 4b). Consistently, PKC activation in response to PAR4 agonist AYPGKF was much more pronounced in Lnk−/− platelets relative to WT platelets from WTD-fed Ldlr−/− recipients but the difference was less in platelets from chow-fed WT recipients (Online Fig. IX). To assess the effects of cholesterol removal, we treated platelets with cholesterol-poor reconstituted HDL (rHDL), which has been shown to promote cholesterol efflux and decrease platelet activation in diabetic humans31. HDL treatment decreased P-selectin exposure in response to PAR4 activation in both Lnk−/− and WT platelets and abolished their differential response (Fig. 4c). The heightened Lnk−/− platelet aggregation induced by PAR4 activation was reversed by HDL treatment (Fig 4d). We also employed cyclodextrin, which nonspecifically promotes cellular cholesterol efflux32. Cyclodextrin, like HDL, reversed elevation of P-selectin exposure and platelet aggregation in LNK deficiency (Fig 4e,f). Consistent with the reversal of Lnk−/− platelet hyperreactivity by HDL, HDL also significantly reduced serine phosphorylation of PKC protein substrates in response to PAR4 activation in WT and Lnk−/− platelets (Fig 4g). Together, these findings indicate that, in hypercholesterolemic mice with LNK deficiency, increased platelet cholesterol content heightens the response to PAR4 activator, leading to increased PKC signaling, P-selectin exposure and integrin αIIbβ3 activation.
Figure 4. Cholesterol loading increases while cholesterol unloading decreases platelet and PKC activity.
(a) Platelets from the chow-fed recipients were loaded with or without cholesterol and then stimulated with AYPGKF (100μM). Surface P-selectin levels were shown (n=4). (b) Platelets from the chow-fed recipients were loaded with or without cholesterol and then stimulated with or without AYPGKF (50μM). PKC activity was estimated (n=3). AYPGKF-induced surface P-selectin exposure (c) or aggregation (d) of platelets from the WTD-fed recipients, with or without rHDL (50 μg/ml) treatment. (e, f) similar as in (c) or (d) except that cyclodextrin (CD) (3mM) replaced HDL for the treatment (n=4). (g) PKC activity in platelets from WTD-fed recipients was estimated, with and without AYPGKF (100μM) and HDL (50μg/ml) treatment (n=3).
For 3c and 3e, * p<0.05 between indicated groups; # p<0.05 between control (WTD) and CD or HDL treatment groups.
LNK loss of function and cholesterol enrichment increase platelet AKT activation
STATs, ERK1/2 and AKT are the major downstream signaling pathways in TPO/MPL signaling33. Consistent with increased TPO/MPL signaling, basal p-STAT5, p-ERK1/2 and p-AKT levels were increased in Lnk−/− platelets from the chow- or WTD-fed mice (Fig. 5a). Interestingly, hypercholesterolemia further increased p-AKT but not p-STAT5 or p-ERK1/2 levels (Fig. 5a, Online Fig. Xa), suggesting convergent effects of LNK deficiency and hypercholesterolemia on AKT activation. AKT has a critical role regulating platelet degranulation and aggregation34. PAR4 activation increases AKT phosphorylation and activation and AKT also is an important node in the TPO/MPL signaling pathway33, 35. Thus, we next focused on assessing the role of AKT in the heightened activation of Lnk−/− platelets from hyperlipidemic mice.
Figure 5. LNK loss of function and cholesterol enrichment increase platelet AKT activation. (a).
TPO/Mpl signaling in resting platelets of WT and Lnk −/− BM recipient mice without TPO treatment. (b) AKT activity in platelets from WT and Lnk −/− BM recipient mice with and without platelet agonist AYPGKF stimulation. (c) effect of ex ex vivo cholesterol loading on AKT activity from platelets of chow fed mice. (d) effect of Jak2 and AKT inhibitors on platelets of WT and Lnk −/− BM recipient mice (n=4). (e) p-SHIP1 level in platelets of WT and Lnk −/− BM recipient mice with and without AYPGKF stimulation. (f) change of AKT activity after HDL and Lyn tyrosine kinase activator Tolimidone treatment in Lnk−/− platelets from WTD fed mice. (g) p-SHIP1 levels in WT and Lynkd/kd platelets upon AYPGKF stimulation. (h) platelet activity of Lynkd/kd mice upon AYPGKF stimulation. (i) cholesterol loading effect on platelet activity of Lynkd/kd mice.
AKT phosphorylation induced by PAR4 activation was increased in both WT and Lnk−/− platelets from hypercholesterolemic mice compared to normocholestrolemic mice, with a more pronounced effect in the Lnk−/− platelets (Fig. 5b and Online Fig. Xa). Cholesterol-loading increased AKT phosphorylation in response to PAR4 activation in both WT and Lnk−/− platelets (Fig. 5c), with a more pronounced effect in Lnk−/− platelets (Fig. 5c). Conversely, removal of cholesterol from platelets by HDL reversed the increased AKT phosphorylation associated with hypercholesterolemia (Fig. 5f). To assess the functional impact of increased AKT phosphorylation and activation on platelets, WT or Lnk−/− platelets from the chow-fed WT or WTD-fed Ldlr−/− recipient were pretreated with ruxolitinib, a JAK2 inhibitor36, or an AKT inhibitor MK2206 followed by PAR4 activation. JAK2 acts upstream of AKT in TPO/MPL signaling33. The increased exposure of surface P-selectin in Lnk−/− platelets from the hypercholesterolemic recipient was reversed by inhibition of JAK2 or AKT (Fig. 5d), indicating a critical role of AKT in the heightened platelet activation.
PI3K mediates AKT activation by generating PI(3,4,5)P3, while SHIP-1 reduces AKT activation by converting PI(3,4,5)P3 to PI(3,4)P237. SHIP-1 phosphorylation was increased in Lnk−/− versus wild type platelets from normocholesterolemic mice (Fig. 5e and Online Fig. Xb), consistent with increased MPL signaling and activation of a negative feedback effects on AKT phosphorylation38; however SHIP-1 phosphorylation was markedly decreased in both WT and Lnk−/− platelets from hypercholesterolemic mice, relative to the normocholesterolemic mice. Ex vivo cholesterol loading of platelets from normocholesterolemic WT or Lnk−/− BM recipient mice also reduced SHIP-1 phosphorylation (Online Fig. XI). Together, these results suggest that cholesterol enrichment increases AKT phosphorylation and activation by decreasing SHIP-1 phosphatase activity.
Src family kinases including LNY Kinase mediate SHIP-1 tyrosine phosphorylation and activation39. We showed previously that cellular cholesterol accumulation in MkPs inhibited LYN kinase activation and increased AKT phosphorylation and activation in response to TPO40. Thus, we assessed the potential role of LYN Kinase in SHIP-1 and AKT signaling in platelets. Tolimidone, a LYN kinase activator41, markedly reduced AKT phosphorylation in response to PAR4 activation in Lnk−/− platelets from the hypercholesterolemic mice (Fig. 5f and Online Fig. XII). This idea was further assessed in genetic models expressing a kinase dead mutant of LYN (Lynkd/kd)42. SHIP-1 phosphorylation was increased in response to PAR4 activation in platelets (not shown) but the increase was markedly reduced in Lynkd/kd platelets (Fig. 5g and Online Fig. XIII).Together, these results indicate a rate limiting role of LYN kinase in mediating SHIP-1 phosphorylation in platelets. The functional impact on platelet activation was also assessed: Lynkd/kd platelets showed increased P-selectin exposure and integrin αIIββ3 activation in response to PAR4 activation (Fig. 5h). Importantly, the priming effect of cholesterol enrichment was abolished in Lynkd/kd platelets (Fig.5i), suggesting that cholesterol enrichment acts to reduce LYN Kinase activity, which decreases SHIP-1 phosphorylation and increases AKT activation in response to PAR4 agonists.
Together, these studies suggest that LNK deficiency increases AKT activation by enhancing TPO/MPL signaling, while cholesterol enrichment increases AKT activation by reducing LYN kinase and SHIP-1 activation. Thus, LNK deficiency and cholesterol enrichment act independently but converge on AKT to promote platelet activation.
LNK loss of function promotes thrombosis and atherosclerosis
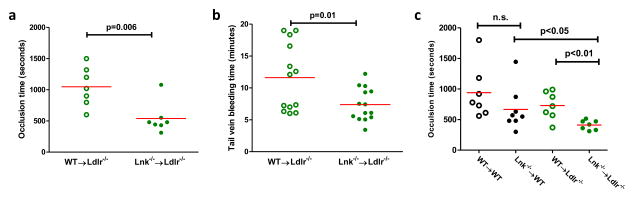
Increased platelet reactivity in association with thrombocytosis in the hypercholesterolemic Lnk−/− BM recipients would be expected to accelerate thrombosis. A previous study suggested that LNK functions to stabilize thrombus formation and LNK deficiency retards arterial thrombosis; notably, however, this study was conducted in normocholesterolemic mice43. Mice do not develop spontaneously ruptured atherosclerotic plaques giving rise to athero-thrombosis. Thus, to evaluate the in vivo impact of LNK deficiency on arterial thrombosis in our hypercholesterolemic model, we used FeCl3-induced carotid artery thrombosis40. Carotid artery occlusion was markedly accelerated in the WTD-fed Lnk−/− BM recipients relative to controls (Fig. 6a). Consistently, tail vein bleeding time was significantly shortened compared to controls (Fig. 6b). The accelerated thrombosis in WTD-fed Lnk−/− BM recipients could be the consequence of thrombocytosis or platelet hyperreactivity or both. Next, we assessed carotid artery thrombosis in chow or WTD-fed WT or Lnk−/− BM recipients. On the chow diet, LNK deficiency did not significantly alter thrombosis although the chow-fed Lnk−/− BM recipients showed marked thrombocytosis (Fig. 6c). In contrast, the induced thrombosis was significantly accelerated in the WTD-fed Lnk−/− recipients (Fig. 6c). These results suggest that the hyperreactivity of cholesterol-loaded Lnk platelets likely has a major role in the accelerated arterial thrombosis in vivo.
Figure 6. LNK deficiency accelerates thrombosis.
FeCl3-induced carotid artery thrombotic occlusion (a) or tail vein bleeding (b) in WT→Ldlr−/− vs. Lnk −/−→ Ldlr−/− recipients fed WTD for 10 weeks. (c) FeCl3-induced carotid artery thrombotic occlusion in chow-fed (black symbols) or WTD-fed (green symbols) recipient mice (n=7–8).
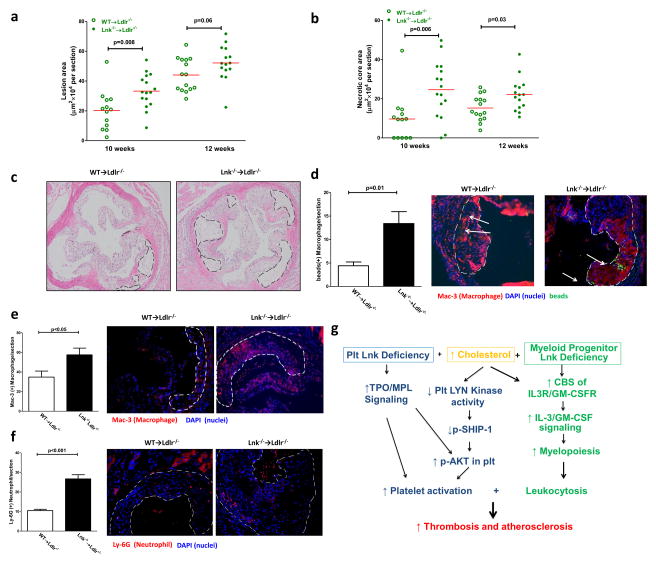
Activated platelets, monocytosis, neutrophilia, and platelet-leukocyte aggregates all promote atherogenesis44–47. To assess atherogenesis, a similar BM transplantation protocol to that described above was employed, however, normocholesterolemic controls were not included as they do not develop atherosclerosis. Plasma non-HDL and HDL lipoprotein cholesterol levels were similar in the WTD-fed Ldlr−/− mice receiving WT or Lnk−/− BM (not shown). Hematopoietic LNK deficiency in combination with angiotensin II injection increases blood pressure48. However, systolic and diastolic blood pressure in WTD-fed Lnk−/− and WT BM recipients showed no difference (Online Fig. XIV), likely because angiotensin II injection was required to show increased blood pressure in LNK deficiency48. We also measured blood insulin and glucose levels from the fasting mice which showed no difference between chow- or WT-fed WT and Lnk−/− mice (Online Fig. XV). Atherosclerotic lesion size was significantly increased in aortic roots of the Ldlr−/− mice receiving Lnk−/− BM (Fig. 7a), after feeding the WTD for 10 weeks. A separate study showed larger lesions and a trend to larger size in the Lnk−/− BM recipient after feeding WTD for 12 weeks (p=.06). (Fig. 7a). The latter was confirmed as aortic arch oil-red O stained en face lesion area was significantly increased in Ldlr−/− mice receiving Lnk−/− BM relative to WT BM after feeding WTD for 16 weeks (p<0.01). We also assessed necrotic core area, which is thought to be an index of plaque stability. Necrotic core area was markedly increased both in the 10 and 12 week samples (Fig. 7b,c). These results indicate that hematopoietic LNK deficiency accelerates atherogenesis.
Figure 7.
(a) Atherosclerotic lesion area and (b) necrotic core area at aortic roots in Ldlr−/− recipients fed with WTD for 10 and 12 weeks respectively (n=14–16). (c) Representative images of H&E staining of aortic root atherosclerosis lesions of WT and Lnk −/− BM recipient mice. (d) Accumulation of fluorescence bead labeled monocytes in atherosclerotic lesions. Atherosclerotic lesional macrophage (e) and neutrophil (f) staining from mice on WTD for 10 weeks. N=14–16. (g) Schematic summary. Hypercholesterolemia enriches platelet membrane, including lipid rafts, with cholesterol and inhibits LYN Kinase activity. Together with increased TPO/MPL signaling due to LNK deficiency, hypercholesterolemia and LNK deficiency act convergently to activate AKT and platelets in a 2 hit model.
The presence of neutrophilia, monocytosis and increased platelet-monocyte or platelet-neutrophil aggregates suggested increased neutrophil and monocyte recruitment into the lesion. Moreover, plasma levels of MCP-1, a potent chemokine for monocytes49, were increased in WTD-fed Lnk−/− relative to WT BM recipients (Online Fig. XVI). We assessed Ly6Chi monocyte recruitment into atherosclerotic lesions in Lnk−/− vs WT BM recipients50. Ly6Chi monocyte recruitment was significantly increased in hypercholesterolemic Lnk−/− BM recipients compared to controls (Fig. 7d). Consistently, lesional macrophages were markedly increased in Lnk−/− BM recipients (Fig. 7e). Lesional neutrophils were also increased in Lnk−/− BM recipients (Fig. 7f). While circulating T cell counts were markedly increased in Lnk−/− BM recipients (Online Fig. IV), the number of lesional T cells was not altered (Online Fig. XVII).
DISCUSSION
Human GWAS have revealed many genetic loci associated with CHD. While a number of these loci act by increasing LDL cholesterol or triglyceride level, the majority act through unknown mechanisms12, 51. By analyzing human cord blood samples, we found that a common genetic variant in LNK that has been linked to CHD in human GWAS is associated with HSC expansion, increased TPO/MPL signaling and increased megakaryopoiesis, suggesting that the T allele is a loss of function variant that promotes platelet production and myelopoiesis. This likely explains the association of LNK with leukocyte and platelet counts13, 19. Our studies in mice provide the first direct evidence that Lnk loss of function promotes atherogenesis and arterial thrombosis and thus suggest that LNK acts at least in part through a similar mechanism in humans. Accelerated atherosclerosis, thrombosis and hematopoietic abnormalities associated with Lnk deficiency were markedly exacerbated by hyperlipidemia, reflecting increased activation of Lnk−/− platelets by cholesterol loading as well as enhanced myelopoiesis. Increased MPL signaling in LNK deficient platelets and reduced SHIP-1 activity due to hypercholesterolemia combined to increase AKT activation and enhance the response of platelets to activation signals.
While rs3184504 is associated with an increased risk of CAD, whether R262W LNK is a causal variant has been contentious. This snp appears to have arisen 1000 to 1500 years ago in European populations as part of a large linkage disequilibrium block that may have endowed an enhanced immune response and protection from infections such as the plague at the expense of increased susceptibility to autoimmune and cardiovascular diseases19. Snps in multiple genes within this LD block are associated with CHD19. This leaves open the question of whether rs3184504 is in LD with other snp(s) which alter expression of several genes in the region. A recent study suggested that phenotypic associations of rs3184504 could be mediated through altered expression of the neighboring gene ATXN252. However, in another study53, the authors analyzed 1000 Genomes CEU and ENCODE databases and showed that no other SNPs in LD with rs3184504 [T] would cause nonsynonymous amino acid changes or were associated with an eQTL. Moreover, we showed that the other genes in this region that are expressed in hematopoietic tissues i.e. ATXN2, PTPN11, TRAFD119 did not show altered expression in cord blood CD34+ cells, whereas LNK mRNA was increased. Moreover, since LNK acts to inhibit hematopoietic functions6, including in the cord blood samples we analyzed, the directionality of change was opposite to that expected for an eQTL effect. This was explained by our functional studies which showed increased TPO/MPL signaling and megakaryopoiesis in association with rs3184504 [T], indicating reduced LNK inhibition of hematopoiesis. Since TPO/MPL signaling increases LNK gene expression20, our findings suggest that the increase in LNK mRNA is secondary to reduced LNK function due to an amino acid change. While our study shows several similarities between LNK(R262W) in humans and LNK deficiency in mice, including increased TPO signaling, HSC expansion, increased myelopoiesis, megakaryopoiesis and accelerated atherosclerosis and arterial thrombosis, the effects on hematopoiesis appear to be more pronounced in Lnk−/− mice, suggesting that human LNK(R262W) may represent a partial loss of function.
The increase in atherosclerosis and arterial thrombosis in hypercholesterolemic mice with hematopoietic LNK deficiency is likely explained in part by the increase in leukocytes, enhanced platelet activation and increased platelet/ leukocyte aggregates. While our study does not differentiate amongst these different factors, there is considerable evidence to implicate each of these mechanisms in accelerated atherosclerosis and thrombosis46, 50, 54, 55. Infusion of activated platelets into Apoe−/− mice resulted in increased atherosclerosis, reflecting formation of platelet/leukocyte aggregates which bind to arterial endothelium over atherosclerotic plaques where they release inflammatory chemokines and cytokines, and thereby promote the entry of monocytes and neutrophils into the plaque.46, 56 The formation of platelet/leukocyte aggregates is mediated by the interaction of platelet P-selectin with P-selectin ligand on leukocytes57. Thus the marked increase in platelet P-selectin exposure (Fig. 3a) as well as the increase in circulating leukocytes (Fig. 2b) may explain the increase in platelet/leukocyte aggregate formation and the increased entry of monocytes and neutrophils into plaques (Figs 2e, 7d, 7f). Even though increased activation of platelets ex vivo required exposure to agonists, platelet-leukocyte aggregates were significantly increased in the basal state, likely contributing to accelerated atherogenesis. The marked increase in induced arterial thrombosis likely reflects the enhanced activation of platelets via thrombin generation secondary to tissue factor exposure in the injured vessel.58–60 While hypertension, erythrocytosis and hyperglycemia were not present in our hypercholesterolemic Lnk−/− mice, rs3184504 is associated with these risk factors in humans, which likely also contribute to atherothrombosis.
Hyperlipidemia as exemplified by familial hypercholesterolemia is associated with increased platelet activation and an underlying pro-coagulant state61, 62, likely reflecting multiple underlying mechanisms62–64. Earlier studies show that in vitro cholesterol-loading increases human platelet activation29 while HDL infusion reduces platelet activation in diabetic subjects, likely by promoting cholesterol efflux from platelets31. There is also markedly increased platelet activation and thrombosis in Scarb1−/− mice64, 65. Scarb1−/− mice have an unusually high plasma unesterified-to-total cholesterol ratio, reflecting impaired delivery of cholesterol to the liver66. Platelet cholesterol overload but not intrinsic SR-BI deficiency in platelets is responsible for the heightened platelet activation in Scarb1−/− mice64. The heightened Lnk−/− platelet activation due to HC is distinct from the Scarb1−/− model in that LNK deficiency does not affect platelet cholesterol content and moreover the heightened activation requires intrinsic hematopoietic Lnk deficiency.
Our studies suggest that increased platelet MPL levels and signaling due to LNK deficiency lead to increased AKT activation which when combined with effects of platelet cholesterol loading to reduce SHIP-1 activation further increases AKT activation and platelet priming (Fig 7g), which is well known to enhance the response to agonists34. As a result there is increased PKC activation in response to agonists such as thrombin, leading to increased platelet degranulation and P-selectin exposure. Moreover, as suggested in an earlier study40, LYN Kinase may act as a membrane cholesterol sensor in platelets, and our studies suggest that inhibition of LYN Kinase in cholesterol loaded platelets may contribute to reduced SHIP-1 phosphorylation and increased AKT activation (Fig 5f–i).
On a therapeutic level our study suggests that individuals with common genetic variants such as the LNK TT genotype that lead to overproduction of platelets and leukocytes could benefit from early identification and aggressive treatment of LDL cholesterol levels. A recent study showed that individuals with a high genetic risk score for CAD (which included rs3184504 in LNK) were threefold more likely to benefit from statin therapy compared to those with a low genetic risk score67. Novel strategies to decrease leukocyte overproduction1 and platelet activation68 such as JAK/STAT or AKT inhibitors69, or LYN Kinase activators, could also reduce the risk of athero-thrombotic disease in individuals with the LNK TT variant.
Supplementary Material
Novelty and Significance.
What Is Known?
Human GWAS have shown that a common SNP in LNK/SH2B3 (R262W) is associated with leukocytosis, thrombocytosis and an increased risk of coronary heart disease and stroke.
LNK inhibits signaling via various cytokine receptors especially in hematopoietic cells suppressing cell proliferation.
In mice hematopoietic Lnk deficiency leads to increased production of leukocytes and platelets, reflecting increased signaling of thrombopoietin via its receptor (MPL) in hematopoietic stem cells (HSCs)
What New Information Does This Article Contribute?
Studies using human cord blood HSCs show that the risk SNP reduces LNK function, leading to increased signaling of thrombopoietin via MPL and enhanced megakaryopoiesis.
Mice with hematopoietic LNK deficiency on an Ldlr−/− background display HSC and myeloid progenitor expansion, increased production of leukocytes, platelets and platelet-leukocyte aggregates, and accelerated arterial thrombosis and atherosclerosis.
In the setting of hypercholesterolemia, Lnk−/− platelets display increased MPL signaling, which is amplified by the effects of cholesterol loading and reduced SHIP-1 activation, leading to increased AKT and platelet priming
LNK is a scaffolding protein that reduces signaling via various cytokine receptors in hematopoietic cells, limiting proliferative responses. Our studies in human cord blood stem cells show that a common SNP in LNK/SH2B3 results in reduced LNK function and thus increased signaling of thrompopoietin via its receptor (MPL) and enhanced production of leukocytes and platelets. These functions could explain the association of the risk SNP with leukocytosis, thrombocytosis and atherothrombosis. In mice with hematopoietic LNK deficiency in the background of LDL receptor deficiency, hypercholesterolemia combines with LNK deficiency to augment HSC, myeloid and megakaryocyte progenitor expansion, leading to thrombocytosis, leukocytosis, platelet-leukocyte aggregates, accelerated atherosclerosis and thrombosis. LNK deficient platelets show enhanced activation in response to agonists, reflecting convergent activating effects of increased MPL signaling and membrane cholesterol loading on AKT.
Acknowledgments
The authors wish to thank Dr. Margaret L. Hibbs for kindly providing Lynkd/kd mice for this study.
SOURCES OF FUNDING
This study was supported the National Institutes of Health Grant RO1 HL107653 (to A. R. Tall) and RO1 HL118567 (to N. Wang). The Columbia University CCTI Flow Cytometry Core, supported in part by the Office of the Director, NIH, under awards S10RR027050 and S10OD020056, was used for this study.
Nonstandard Abbreviations and Acronyms
- LNK
lymphocyte specific adapter protein
- CHD
coronary heart disease
- GWAS
genome wide association study
- MPL
myeloproliferative leukemia virus oncogene
- TPO
thrombopoietin
- SHIP-1
SH2 domain-containing inositol phosphatase 1
- HSC
hematopoietic stem cell
- HSPC
hematopoietic stem and progenitor cell
- MkP
megakaryocyte progenitor
- BM
bone marrow
- WTD
Western type diet
Footnotes
DISCLOSURES
Dr. Tall is a consultant for Amgen and CSL Behring.
References
- 1.Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler Thromb Vasc Biol. 2005;25:658–70. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- 2.Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. American journal of epidemiology. 2001;154:758–64. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Wu H, Mueller C, Gibson CM, Murphy S, Shi Y, Xu G, Yang J. Baseline platelet count and clinical outcome in acute coronary syndrome. Circ J. 2012;76:704–11. doi: 10.1253/circj.cj-11-0707. [DOI] [PubMed] [Google Scholar]
- 4.Totani L, Evangelista V. Platelet-leukocyte interactions in cardiovascular disease and beyond. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2357–61. doi: 10.1161/ATVBAHA.110.207480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bersenev A, Wu C, Balcerek J, Tong W. Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J Clin Invest. 2008;118:2832–44. doi: 10.1172/JCI35808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong W, Lodish HF. Lnk inhibits Tpo-mpl signaling and Tpo-mediated megakaryocytopoiesis. J Exp Med. 2004;200:569–80. doi: 10.1084/jem.20040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMullin MF, Wu C, Percy MJ, Tong W. A nonsynonymous LNK polymorphism associated with idiopathic erythrocytosis. Am J Hematol. 2011;86:962–4. doi: 10.1002/ajh.22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viny AD, Levine RL. Genetics of myeloproliferative neoplasms. Cancer J. 2014;20:61–5. doi: 10.1097/PPO.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bersenev A, Wu C, Balcerek J, Jing J, Kundu M, Blobel GA, Chikwava KR, Tong W. Lnk constrains myeloproliferative diseases in mice. J Clin Invest. 2010;120:2058–69. doi: 10.1172/JCI42032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velazquez L, Cheng AM, Fleming HE, Furlonger C, Vesely S, Bernstein A, Paige CJ, Pawson T. Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med. 2002;195:1599–611. doi: 10.1084/jem.20011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takaki S, Morita H, Tezuka Y, Takatsu K. Enhanced hematopoiesis by hematopoietic progenitor cells lacking intracellular adaptor protein, Lnk. J Exp Med. 2002;195:151–60. doi: 10.1084/jem.20011170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Consortium CAD; Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, Konig IR, Cazier JB, Johansson A, Hall AS, Lee JY, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikainen LP, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL, Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi-Boehm S, Cox D, Dimitriou M, Do R, Doney AS, El Mokhtari N, Eriksson P, Fischer K, Fontanillas P, Franco-Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han BG, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C, Langford C, Leander K, Lokki ML, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Muller-Nurasyid M, Mu TC, Nikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S, Rubin D, Rumpf MP, Schafer A, Sivananthan M, Song C, Stewart AF, Tan ST, Thorgeirsson G, van der Schoot CE, Wagner PJ, Wells GA, Wild PS, Yang TP, Amouyel P, Arveiler D, Basart H, Boehnke M, Boerwinkle E, Brambilla P, Cambien F, Cupples AL, de Faire U, Dehghan A, Diemert P, Epstein SE, Evans A, Ferrario MM, Ferrieres J, Gauguier D, Go AS, Goodall AH, Gudnason V, Hazen SL, Holm H, Iribarren C, Jang Y, Kahonen M, Kee F, Kim HS, Klopp N, Koenig W, Kratzer W, Kuulasmaa K, Laakso M, Laaksonen R, Lee JY, Lind L, Ouwehand WH, Parish S, Park JE, Pedersen NL, Peters A, Quertermous T, Rader DJ, Salomaa V, Schadt E, Shah SH, Sinisalo J, Stark K, Stefansson K, Tregouet DA, Virtamo J, Wallentin L, Wareham N, Zimmermann ME, Nieminen MS, Hengstenberg C, Sandhu MS, Pastinen T, Syvanen AC, Hovingh GK, Dedoussis G, Franks PW, Lehtimaki T, Metspalu A, Zalloua PA, Siegbahn A, Schreiber S, Ripatti S, Blankenberg SS, Perola M, Clarke R, Boehm BO, O’Donnell C, Reilly MP, Marz W, Collins R, Kathiresan S, Hamsten A, Kooner JS, Thorsteinsdottir U, Danesh J, Palmer CN, Roberts R, Watkins H, Schunkert H, Samani NJ Consortium D, Consortium C, Wellcome Trust Case Control C. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, Thorleifsson G, Helgadottir H, Steinthorsdottir V, Stefansson H, Williams C, Hui J, Beilby J, Warrington NM, James A, Palmer LJ, Koppelman GH, Heinzmann A, Krueger M, Boezen HM, Wheatley A, Altmuller J, Shin HD, Uh ST, Cheong HS, Jonsdottir B, Gislason D, Park CS, Rasmussen LM, Porsbjerg C, Hansen JW, Backer V, Werge T, Janson C, Jonsson UB, Ng MC, Chan J, So WY, Ma R, Shah SH, Granger CB, Quyyumi AA, Levey AI, Vaccarino V, Reilly MP, Rader DJ, Williams MJ, van Rij AM, Jones GT, Trabetti E, Malerba G, Pignatti PF, Boner A, Pescollderungg L, Girelli D, Olivieri O, Martinelli N, Ludviksson BR, Ludviksdottir D, Eyjolfsson GI, Arnar D, Thorgeirsson G, Deichmann K, Thompson PJ, Wjst M, Hall IP, Postma DS, Gislason T, Gulcher J, Kong A, Jonsdottir I, Thorsteinsdottir U, Stefansson K. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–7. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 14.Hunt KA, Zhernakova A, Turner G, Heap GA, Franke L, Bruinenberg M, Romanos J, Dinesen LC, Ryan AW, Panesar D, Gwilliam R, Takeuchi F, McLaren WM, Holmes GK, Howdle PD, Walters JR, Sanders DS, Playford RJ, Trynka G, Mulder CJ, Mearin ML, Verbeek WH, Trimble V, Stevens FM, O’Morain C, Kennedy NP, Kelleher D, Pennington DJ, Strachan DP, McArdle WL, Mein CA, Wapenaar MC, Deloukas P, McGinnis R, McManus R, Wijmenga C, van Heel DA. Newly identified genetic risk variants for celiac disease related to the immune response. Nat Genet. 2008;40:395–402. doi: 10.1038/ng.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Kottgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O’Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–87. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Doring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O’Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O’Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvanen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dorr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Volker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Volzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB Wellcome Trust Case Control C. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–76. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smyth DJ, Plagnol V, Walker NM, Cooper JD, Downes K, Yang JH, Howson JM, Stevens H, McManus R, Wijmenga C, Heap GA, Dubois PC, Clayton DG, Hunt KA, van Heel DA, Todd JA. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008;359:2767–77. doi: 10.1056/NEJMoa0807917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635–45. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soranzo N, Spector TD, Mangino M, Kuhnel B, Rendon A, Teumer A, Willenborg C, Wright B, Chen L, Li M, Salo P, Voight BF, Burns P, Laskowski RA, Xue Y, Menzel S, Altshuler D, Bradley JR, Bumpstead S, Burnett MS, Devaney J, Doring A, Elosua R, Epstein SE, Erber W, Falchi M, Garner SF, Ghori MJ, Goodall AH, Gwilliam R, Hakonarson HH, Hall AS, Hammond N, Hengstenberg C, Illig T, Konig IR, Knouff CW, McPherson R, Melander O, Mooser V, Nauck M, Nieminen MS, O’Donnell CJ, Peltonen L, Potter SC, Prokisch H, Rader DJ, Rice CM, Roberts R, Salomaa V, Sambrook J, Schreiber S, Schunkert H, Schwartz SM, Serbanovic-Canic J, Sinisalo J, Siscovick DS, Stark K, Surakka I, Stephens J, Thompson JR, Volker U, Volzke H, Watkins NA, Wells GA, Wichmann HE, Van Heel DA, Tyler-Smith C, Thein SL, Kathiresan S, Perola M, Reilly MP, Stewart AF, Erdmann J, Samani NJ, Meisinger C, Greinacher A, Deloukas P, Ouwehand WH, Gieger C. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat Genet. 2009;41:1182–90. doi: 10.1038/ng.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baran-Marszak F, Magdoud H, Desterke C, Alvarado A, Roger C, Harel S, Mazoyer E, Cassinat B, Chevret S, Tonetti C, Giraudier S, Fenaux P, Cymbalista F, Varin-Blank N, Le Bousse-Kerdiles MC, Kiladjian JJ, Velazquez L. Expression level and differential JAK2-V617F-binding of the adaptor protein Lnk regulates JAK2-mediated signals in myeloproliferative neoplasms. Blood. 2010;116:5961–71. doi: 10.1182/blood-2009-12-256768. [DOI] [PubMed] [Google Scholar]
- 21.Soldner F, Stelzer Y, Shivalila CS, Abraham BJ, Latourelle JC, Barrasa MI, Goldmann J, Myers RH, Young RA, Jaenisch R. Parkinson-associated risk variant in distal enhancer of alpha-synuclein modulates target gene expression. Nature. 2016;533:95–9. doi: 10.1038/nature17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harmon JT, Tandon NN, Hoeg JM, Jamieson GA. Thrombin binding and response in platelets from patients with dyslipoproteinemias: increased stimulus-response coupling in type II hyperlipoproteinemia. Blood. 1986;68:498–505. [PubMed] [Google Scholar]
- 23.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–93. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–49. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Subramanian M, Abramowicz S, Murphy AJ, Gonen A, Witztum J, Welch C, Tabas I, Westerterp M, Tall AR. Interleukin-3/granulocyte macrophage colony-stimulating factor receptor promotes stem cell expansion, monocytosis, and atheroma macrophage burden in mice with hematopoietic ApoE deficiency. Arterioscler Thromb Vasc Biol. 2014;34:976–84. doi: 10.1161/ATVBAHA.113.303097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Moczygemba M, Huston DP. Proteasomal regulation of betac signaling reveals a novel mechanism for cytokine receptor heterotypic desensitization. J Clin Invest. 2001;108:1797–806. doi: 10.1172/JCI13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tefferi A, Pardanani A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol. 2015;1:97–105. doi: 10.1001/jamaoncol.2015.89. [DOI] [PubMed] [Google Scholar]
- 28.Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, Nacher M, Pitaval C, Radovanovic I, Fukui Y, McEver RP, Filippi MD, Lizasoain I, Ruiz-Cabello J, Zarbock A, Moro MA, Hidalgo A. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–8. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shattil SJ, Anaya-Galindo R, Bennett J, Colman RW, Cooper RA. Platelet hypersensitivity induced by cholesterol incorporation. J Clin Invest. 1975;55:636–43. doi: 10.1172/JCI107971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerrard JM, Beattie LL, Park J, Israels SJ, McNicol A, Lint D, Cragoe EJ., Jr A role for protein kinase C in the membrane fusion necessary for platelet granule secretion. Blood. 1989;74:2405–13. [PubMed] [Google Scholar]
- 31.Calkin AC, Drew BG, Ono A, Duffy SJ, Gordon MV, Schoenwaelder SM, Sviridov D, Cooper ME, Kingwell BA, Jackson SP. Reconstituted high-density lipoprotein attenuates platelet function in individuals with type 2 diabetes mellitus by promoting cholesterol efflux. Circulation. 2009;120:2095–104. doi: 10.1161/CIRCULATIONAHA.109.870709. [DOI] [PubMed] [Google Scholar]
- 32.Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270:17250–6. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 33.Kaushansky K. Thrombopoiesis. Semin Hematol. 2015;52:4–11. doi: 10.1053/j.seminhematol.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Woulfe DS. Akt signaling in platelets and thrombosis. Expert Rev Hematol. 2010;3:81–91. doi: 10.1586/ehm.09.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S, Jin J, Kunapuli SP. Relative contribution of G-protein-coupled pathways to protease-activated receptor-mediated Akt phosphorylation in platelets. Blood. 2006;107:947–54. doi: 10.1182/blood-2005-07-3040. [DOI] [PubMed] [Google Scholar]
- 36.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, Vaddi K, Levy R, Tefferi A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–27. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gratacap MP, Severin S, Chicanne G, Plantavid M, Payrastre B. Different roles of SHIP1 according to the cell context: the example of blood platelets. Adv Enzyme Regul. 2008;48:240–52. doi: 10.1016/j.advenzreg.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Kaushansky K. Molecular mechanisms of thrombopoietin signaling. J Thromb Haemost. 2009;7(Suppl 1):235–8. doi: 10.1111/j.1538-7836.2009.03419.x. [DOI] [PubMed] [Google Scholar]
- 39.Phee H, Jacob A, Coggeshall KM. Enzymatic activity of the Src homology 2 domain-containing inositol phosphatase is regulated by a plasma membrane location. J Biol Chem. 2000;275:19090–7. doi: 10.1074/jbc.M001093200. [DOI] [PubMed] [Google Scholar]
- 40.Murphy AJ, Bijl N, Yvan-Charvet L, Welch CB, Bhagwat N, Reheman A, Wang Y, Shaw JA, Levine RL, Ni H, Tall AR, Wang N. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nature medicine. 2013;19:586–94. doi: 10.1038/nm.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ochman AR, Lipinski CA, Handler JA, Reaume AG, Saporito MS. The Lyn kinase activator MLR-1023 is a novel insulin receptor potentiator that elicits a rapid-onset and durable improvement in glucose homeostasis in animal models of type 2 diabetes. J Pharmacol Exp Ther. 2012;342:23–32. doi: 10.1124/jpet.112.192187. [DOI] [PubMed] [Google Scholar]
- 42.Verhagen AM, Wallace ME, Goradia A, Jones SA, Croom HA, Metcalf D, Collinge JE, Maxwell MJ, Hibbs ML, Alexander WS, Hilton DJ, Kile BT, Starr R. A kinase-dead allele of Lyn attenuates autoimmune disease normally associated with Lyn deficiency. J Immunol. 2009;182:2020–9. doi: 10.4049/jimmunol.0803127. [DOI] [PubMed] [Google Scholar]
- 43.Takizawa H, Nishimura S, Takayama N, Oda A, Nishikii H, Morita Y, Kakinuma S, Yamazaki S, Okamura S, Tamura N, Goto S, Sawaguchi A, Manabe I, Takatsu K, Nakauchi H, Takaki S, Eto K. Lnk regulates integrin alphaIIbbeta3 outside-in signaling in mouse platelets, leading to stabilization of thrombus development in vivo. J Clin Invest. 2010;120:179–90. doi: 10.1172/JCI39503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–16. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nahrendorf M, Swirski FK. Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res. 2015;116:884–94. doi: 10.1161/CIRCRESAHA.116.303550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–7. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 47.Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, Bergmeier W, Richter T, Lorenz M, Konrad I, Nieswandt B, Gawaz M. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–96. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest. 2015;125:1189–202. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zernecke A, Weber C. Chemokines in atherosclerosis: proceedings resumed. Arterioscler Thromb Vasc Biol. 2014;34:742–50. doi: 10.1161/ATVBAHA.113.301655. [DOI] [PubMed] [Google Scholar]
- 50.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–94. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Consortium CAD. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–30. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braenne I, Civelek M, Vilne B, Di Narzo A, Johnson AD, Zhao Y, Reiz B, Codoni V, Webb TR, Foroughi Asl H, Hamby SE, Zeng L, Tregouet DA, Hao K, Topol EJ, Schadt EE, Yang X, Samani NJ, Bjorkegren JL, Erdmann J, Schunkert H, Lusis AJ Leducq Consortium CADGd. Prediction of Causal Candidate Genes in Coronary Artery Disease Loci. Arterioscler Thromb Vasc Biol. 2015;35:2207–17. doi: 10.1161/ATVBAHA.115.306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flister MJ, Hoffman MJ, Lemke A, Prisco SZ, Rudemiller N, O’Meara CC, Tsaih SW, Moreno C, Geurts AM, Lazar J, Adhikari N, Hall JL, Jacob HJ. SH2B3 Is a Genetic Determinant of Cardiac Inflammation and Fibrosis. Circ Cardiovasc Genet. 2015;8:294–304. doi: 10.1161/CIRCGENETICS.114.000527. [DOI] [PubMed] [Google Scholar]
- 54.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–6. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 56.von Hundelshausen P, Koenen RR, Sack M, Mause SF, Adriaens W, Proudfoot AE, Hackeng TM, Weber C. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood. 2005;105:924–30. doi: 10.1182/blood-2004-06-2475. [DOI] [PubMed] [Google Scholar]
- 57.Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2005;25:1321–4. doi: 10.1161/01.ATV.0000166521.90532.44. [DOI] [PubMed] [Google Scholar]
- 58.Day SM, Reeve JL, Myers DD, Fay WP. Murine thrombosis models. Thromb Haemost. 2004;92:486–94. [PubMed] [Google Scholar]
- 59.Day SM, Reeve JL, Pedersen B, Farris DM, Myers DD, Im M, Wakefield TW, Mackman N, Fay WP. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–8. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 60.Denis CV, Wagner DD. Platelet adhesion receptors and their ligands in mouse models of thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:728–39. doi: 10.1161/01.ATV.0000259359.52265.62. [DOI] [PubMed] [Google Scholar]
- 61.Carvalho AC, Colman RW, Lees RS. Platelet function in hyperlipoproteinemia. N Engl J Med. 1974;290:434–8. doi: 10.1056/NEJM197402212900805. [DOI] [PubMed] [Google Scholar]
- 62.Owens AP, 3rd, Passam FH, Antoniak S, Marshall SM, McDaniel AL, Rudel L, Williams JC, Hubbard BK, Dutton JA, Wang J, Tobias PS, Curtiss LK, Daugherty A, Kirchhofer D, Luyendyk JP, Moriarty PM, Nagarajan S, Furie BC, Furie B, Johns DG, Temel RE, Mackman N. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012;122:558–68. doi: 10.1172/JCI58969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086–95. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korporaal SJ, Meurs I, Hauer AD, Hildebrand RB, Hoekstra M, Cate HT, Pratico D, Akkerman JW, Van Berkel TJ, Kuiper J, Van Eck M. Deletion of the high-density lipoprotein receptor scavenger receptor BI in mice modulates thrombosis susceptibility and indirectly affects platelet function by elevation of plasma free cholesterol. Arterioscler Thromb Vasc Biol. 2011;31:34–42. doi: 10.1161/ATVBAHA.110.210252. [DOI] [PubMed] [Google Scholar]
- 65.Ma Y, Ashraf MZ, Podrez EA. Scavenger receptor BI modulates platelet reactivity and thrombosis in dyslipidemia. Blood. 2010;116:1932–41. doi: 10.1182/blood-2010-02-268508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trigatti BL, Krieger M, Rigotti A. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1732–8. doi: 10.1161/01.ATV.0000091363.28501.84. [DOI] [PubMed] [Google Scholar]
- 67.Mega JL, Stitziel NO, Smith JG, Chasman DI, Caulfield MJ, Devlin JJ, Nordio F, Hyde CL, Cannon CP, Sacks FM, Poulter NR, Sever PS, Ridker PM, Braunwald E, Melander O, Kathiresan S, Sabatine MS. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264–71. doi: 10.1016/S0140-6736(14)61730-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.FitzGerald GA. Translational therapeutics at the platelet vascular interface. Summary. Arterioscler Thromb Vasc Biol. 2008;28:s51–2. doi: 10.1161/ATVBAHA.108.162206. [DOI] [PubMed] [Google Scholar]
- 69.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D’Andrea A, Frohling S, Dohner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ, Gilliland DG. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.