Abstract
α-Synuclein accumulation and mitochondrial dysfunction have both been strongly implicated in the pathogenesis of Parkinson’s disease (PD), and the two appear to be related. Mitochondrial dysfunction leads to accumulation and oligomerization of α-synuclein, and increased levels of α-synuclein cause mitochondrial impairment, but the basis for this bidirectional interaction remains obscure. We now report that certain post-translationally modified species of α-synuclein bind with high-affinity to the TOM20 presequence receptor of the mitochondrial protein import machinery, prevent its interaction with its co-receptor, TOM22, and impair mitochondrial protein import. As a consequence, there is deficient mitochondrial respiration, enhanced ROS production and loss of mitochondrial membrane potential. Examination of postmortem PD tissue reveals an aberrant α-synuclein:TOM20 interaction in nigrostriatal neurons that is associated with loss of imported mitochondrial protein, thereby confirming this pathogenic process in the human disease. Modest knockdown of endogenous α-synuclein was sufficient to maintain mitochondrial protein import in an in vivo model of PD; furthermore, in in vitro systems, overexpression of TOM20 or a mitochondrial targeting signal peptide had beneficial effects and preserved protein import. This study defines a new pathogenic mechanism in PD, identifies toxic species of wildtype α-synuclein, and reveals new therapeutic strategies for neuroprotection.
Parkinson’s disease (PD) is a common neurodegenerative disorder that results in motor impairment, cognitive and psychiatric symptoms and autonomic dysfunction (1). Both genetic and environmental factors have been implicated in PD pathogenesis, and it appears that mitochondrial defects and accumulation of the synaptic protein, α-synuclein, are common to most forms of the disease (2). Moreover, there is evidence of a bidirectional interaction between mitochondrial dysfunction and α-synuclein accretion and aggregation. Inhibition of mitochondrial complex I leads to accumulation and oligomerization of α-synuclein (3–5), and increased levels of α-synuclein cause mitochondrial impairment and production of reactive oxygen species (ROS) (6). The nature of the interaction between α-synuclein and mitochondria remains obscure, as does the basis for the vulnerability of dopamine neurons of the nigrostriatal tract. Furthermore, it is unclear whether unmodified monomeric α-synuclein is responsible for these effects or whether post-translational modifications which have been implicated in pathogenesis, such as oligomerization, dopamine modification, phosphorylation or nitration are important.
Although mitochondria contain their own genome, it encodes only 13 proteins (7). Since mitochondria may contain up to 1500 distinct proteins (8), they must import roughly 99% of these. The mitochondrial protein import and sorting machinery is complex and highly regulated (9, 10), and its components and mechanisms vary by the compartment to which a protein is to be sorted. The best characterized system is one by which nuclear-encoded, matrix-targeted proteins that contain a mitochondrial targeting signal (MTS; presequence) are recognized by the translocase of the outer membrane (TOM) receptors and translocated through the outer membrane to the translocase of the inner membrane (TIM) and into the matrix, where the MTS is cleaved to yield the mature protein (10). In this system, import of a presequence-containing pre-protein starts with recognition of the MTS via interaction of its hydrophobic face with the TOM20 receptor (10). Subsequently, the hydrophilic side of the presequence is recognized by TOM22. Given the normally close proximity of TOM20 and TOM22, it is also possible that both receptors recognize the MTS simultaneously. However, recent cryo-EM studies suggest that, in addition to the central TOM complex core, there are ‘peripheral’ TOM20 components, which are in a dynamic equilibrium with the preassembled TOM complex (11).
Although monomeric α-synuclein is an intrinsically disordered protein in solution, in association with anionic lipids in membranes it forms an amphipathic helix (12) similar to known MTS motifs. In this context, we hypothesized that, under some conditions, α-synuclein might interact with TOM20 and interfere with import of mitochondrially-targeted proteins. As such, this may represent a new pathogenic mechanism and a potential target for therapeutic intervention in PD.
Results
α-Synuclein-TOM20 interaction
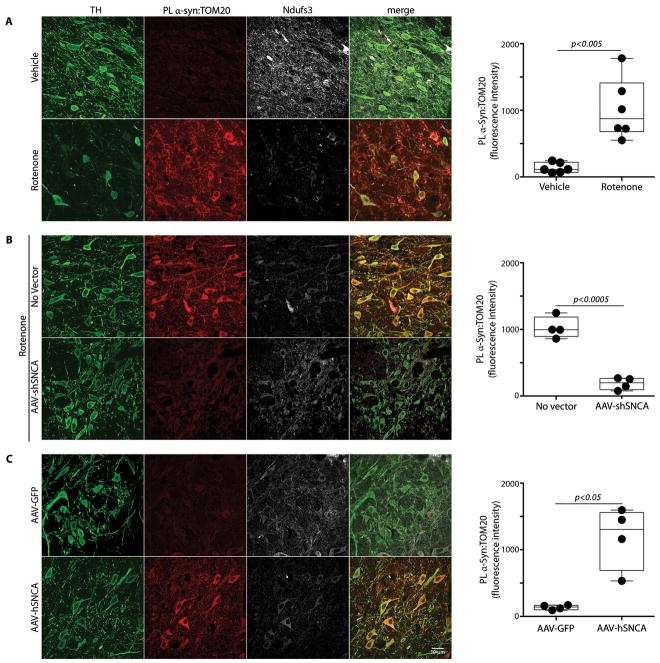
Systemic defects in mitochondrial complex I have been described repeatedly in PD, and systemic inhibition of complex I with rotenone in rodents reproduces many features of PD, importantly including Lewy pathology and accumulation and oligomerization of α-synuclein in the substantia nigra (4, 5, 13). We have also found that in vivo rotenone treatment increases S129-phosphorylation of α-synuclein in nigrostriatal neurons (213% of control, p<0.0001, 2-tailed unpaired t-test; Figure S1). Moreover, occupational exposure to rotenone is a risk factor for developing the disease (14). Therefore, we used the rotenone model to assess potential interactions between α-synuclein and mitochondrial protein import machinery. Using proximity ligation (PL) assays (15), we demonstrated a marked increase in the interaction between α-synuclein and TOM20 in nigrostriatal dopamine neurons of rats treated with rotenone relative to rats treated with vehicle (Figure 1A; N=6/group; p<0.001, 2-tailed unpaired t-test, or p<0.005 with Welch’s correction for unequal variances). There was no such interaction between α-synuclein and TOM22 or TOM40, or a component of the translocase of the inner membrane, TIM23 (Figure S2). As an initial assessment of whether this apparent interaction between α-synuclein and TOM20 results in loss of mitochondrially targeted protein, we examined levels of the nuclear-encoded, mitochondrially-targeted subunit of complex I, Ndufs3, and found a decrease in this imported protein after rotenone treatment. Further, the remaining Ndufs3 immunoreactivity was more diffuse than punctate, as verified by a drop in the TOM20:Ndufs3 Pearson index from 0.77 ± 0.02 in control animals to 0.23 ± 0.02 in rotenone-treated rats (P<0.0001, 2-tailed unpaired t-test; Figure S3).
Figure 1.
Ex vivo proximity ligation between α-synuclein and TOM20 is associated with decreased mitochondrial import of the complex I subunit, Ndufs3, in nigrostriatal neurons in vivo in the rotenone and α-synuclein overexpression models of PD. (A) In a vehicle-treated rat (top row), there is little α-synuclein:TOM20 PL signal and there is intense punctate staining of Ndufs3 in mitochondria of nigrostriatal neurons. In contrast, in a rotenone-treated rat (bottom row), there is a strong α-synuclein:TOM20 PL signal, which is associated with loss of mitochondrial Ndufs3 staining. In the box plot, mean nigrostriatal cellular PL fluorescence values for individual animals (vehicle- or rotenone-treated) are indicated by black circles. In each animal, PL signal was measured in 35–50 nigrostriatal neurons per hemisphere. Statistical testing by 2-tailed unpaired t-test with Welch’s correction. (B) In a rat that received a unilateral injection of AAV-shSNCA, the rotenone-induced α-synuclein:TOM20 PL signal was largely prevented, and mitochondrial Ndufs3 staining was preserved. In the box plot, mean nigrostriatal cellular PL fluorescence values for each hemisphere (control or AAV-shSNCA-injected) of individual animals are indicated by black circles. For each animal, PL signal was measured in 50–70 nigrostriatal neurons per hemisphere. Statistical testing by 2-tailed paired t-test with Welch’s correction. (C) In a rat that received unilateral injection of an α-synuclein overexpression vector (AAV-hSNCA), the α-synuclein-injected hemisphere shows a strong α-synuclein:TOM20 PL signal with an associated loss of Ndufs3 staining. In the box plot, mean nigrostriatal cellular PL fluorescence values for each hemisphere (control AAV-GFP or AAV-SNCA-injected) of individual animals are indicated by black circles. For each animal, PL signal was measured in 50–70 nigrostriatal neurons per hemisphere. Statistical testing by 2-tailed unpaired t-test with Welch’s correction. TH, tyrosine hydroxylase. Scale bar = 30 μm.
α-Synuclein levels in nigrostriatal dopamine neurons increase with normal aging, in Parkinson’s disease, and in rats treated with rotenone (4, 16). Because the amount of oligomer formation and other post-translational modifications are dependent on the concentration of α-synuclein, we examined the in vivo effects of reducing α-synuclein levels on its interaction with TOM20 in rotenone-treated rats. Three weeks prior to rotenone treatment, rats received a unilateral injection into substantia nigra of AAV2 virus containing an shRNA specifically targeting rat α-synuclein (17). Quantitative confocal immunofluorescence showed a 30–40% knockdown of endogenous α-synuclein protein in transduced dopamine neurons 3 weeks after injection (17). Using tissue from this previous study, we found that after rotenone treatment, in the untransduced hemisphere, there was a strong PL signal between α-synuclein and TOM20, which was almost completely prevented by the modest reduction in α-synuclein levels in the AAV2-injected hemisphere (Figure 1B; N=4; p<0.0001, 2-tailed paired t-test, or p<0.0005 with Welch’s correction). Consistent with the decreased interaction of α-synuclein with TOM20, knockdown was associated with a preservation of punctate (mitochondrial) Ndufs3 staining, suggesting normal protein import. Importantly, knockdown of endogenous α-synuclein provided behavioral protection and preservation of nigrostriatal neurons, terminals and dendrites (17), possibly indicating the importance of reduced mitochondrial protein import.
To validate our finding of an in vivo interaction between α-synuclein and TOM20, we used another model of PD that does not rely on a neurotoxin, namely, AAV2 viral-mediated overexpression of human α-synuclein in nigrostriatal neurons. Quantitative immunofluorescence indicated a 3-fold overexpression of α-synuclein in the transduced nigral neurons (P<0.01) along with a marked increase in S129-phosphorylation (394% of control, p<0.005, paired t-test; Figure S1). In this model, which induces α-synuclein oligomerization (18) and causes delayed and progressive neurodegeneration (19), we also found a strong PL signal between α-synuclein and TOM20 with a parallel loss of mitochondrial Ndufs3 in dopamine neurons in the vector-injected hemisphere, but not in the control hemisphere (Figure 1C; N=4; p<0.005, 2-tailed paired t-test, or p<0.05 with Welch’s correction).
Relevance to the human disease
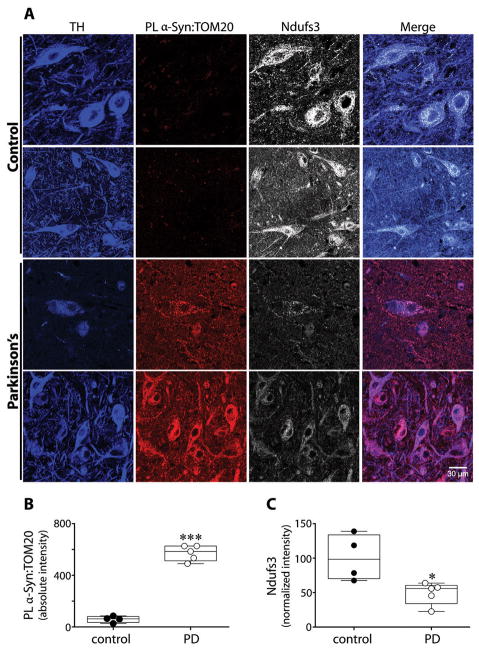
Our results from rotenone-treated and hSNCA-overexpressing rats demonstrated an interaction between α-synuclein and TOM20, which is associated with reduced import and a decrease in mitochondrially localized Ndufs3. To determine whether this process is relevant to idiopathic human PD, we performed PL assays (α-synuclein:TOM20) and immunocytochemistry for the Ndufs3 subunit in blinded postmortem substantia nigra sections from individuals with PD (N=5) and from controls (N=4). Compared to controls, nigrostriatal dopamine neurons from all PD cases had a strong PL signal (p<0.0001), indicating an interaction between α-synuclein and TOM20 (Figure 2). Further, relative to the controls, there was a prominent loss of mitochondrial Ndufs3 staining (p<0.02, or p<0.05 with Welch’s correction) – and the staining that remained tended to be diffuse rather than punctate. This strongly suggests that α-synuclein-induced impairment of mitochondrial protein import occurs in the human disease.
Figure 2.
Evidence of impaired mitochondrial protein import in human dopaminergic substantia nigra neurons in Parkinson’s disease. (A) In TH+ dopamine neurons from PD cases, there was an intense α-synuclein:TOM20 PL signal and a marked loss of Ndufs3 immunoreactivity. In PD cases, remaining Ndufs3 staining was rather diffuse instead of punctate. (B) Quantification of the α-synuclein:TOM20 PL signal in control vs. PD dopamine neurons. (C) Quantification of Ndufs3 immunoreactivity in control vs. PD dopamine neurons. The Ndufs3 signal was normalized to the TH signal, which tends to minimize the apparent differences. ***p<0.0001; *p <0.05; 2-tailed unpaired t-test with Welch’s correction for unequal variances.
Effects of α-synuclein on import
To explore in more detail the functional significance of the α-synuclein-TOM20 interaction, we turned to cellular and in vitro assays of mitochondrial protein import in the presence or absence of various forms of monomeric, oligomeric or otherwise post-translationally-modified recombinant α-synuclein. For this purpose, we used (1) confocal imaging of dopaminergic SH-SY5Y cells expressing mitochondrially-targeted GFP (MTS-GFP), (2) direct assays of mitochondrial protein import in isolated mitochondria, and (3) confocal measurements of mitochondrial localization of endogenous, presequence-containing, nuclear encoded, imported proteins. α-Synuclein binds to lipid membranes and can easily cross the plasma membrane, a property we took advantage of in in vitro studies (20). Control experiments using fluorescently labeled α-synuclein confirmed that each species of α-synuclein used in subsequent experiments entered cells to an equivalent extent (Figure S4). Furthermore, when cells were treated with exogenous α-synuclein species (200 nM monomer equivalent), there was no detectable change in the total cellular content of α-synuclein, consistent with data suggesting that endogenous intraneuronal concentrations are in the range of 2–5 μM (21), or even higher (22).
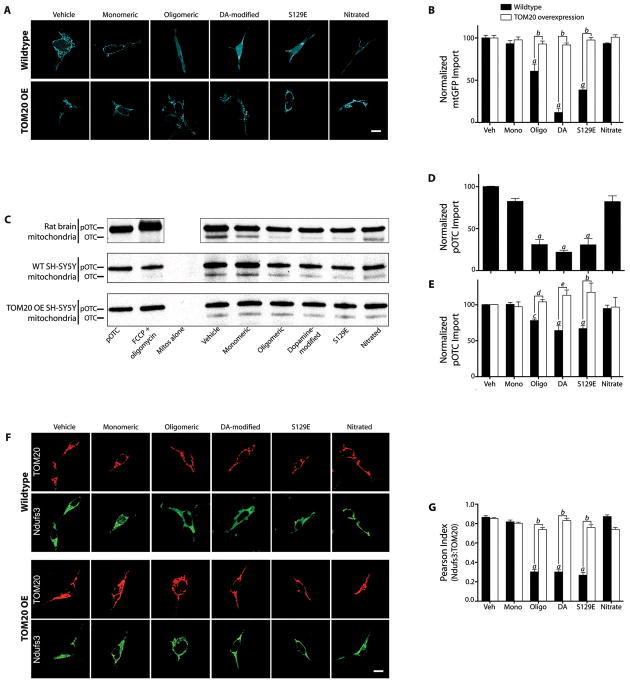
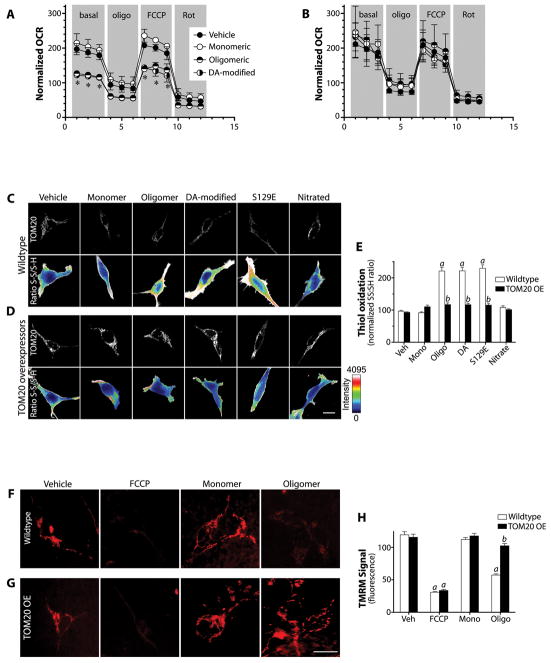
Treatment of SH-SY5Y cells with 200 nM monomeric α-synuclein for 24 or 48h had no effect on import (mitochondrially localized GFP), but the same amount of α-synuclein, in the form of small oligomers, potently inhibited import by about 50% (p<0.0001; 2-way ANOVA; N=3; Figures 3A & B). Dopamine stably modifies α-synuclein, and although the molecular nature of this modification is controversial, this form of α-synuclein has been implicated in pathogenesis (23, 24). When applied to SH-SY5Y cells, dopamine-modified α-synuclein also potently inhibited import (p<0.0001). Similarly, S129E α-synuclein, a phosphomimetic mutant, strongly inhibited MTS-GFP import (p<0.0001). In contrast, unlike the other post-translational modifications, nitrated α-synuclein behaved like the monomeric, unmodified protein and did not inhibit import. Additionally, thioflavin-T-positive fibrils of α-synuclein had no effect on import (Figure S5A–D).
Figure 3.
Post-translationally-modified α-synuclein binds to TOM20 and inhibits mitochondrial protein import. (A) mtGFP import in intact wildtype and TOM20 overexpressing (OE) SH-SY5Y cells exposed to various forms of α-synuclein. In wildtype cells treated with oligomeric, dopamine-modified or S129E α-synuclein, note the diffuse pattern of staining compared to vehicle. In TOM20 overexpressing cells, mtGFP maintained its mitochondrial localization despite α-synuclein treatment. (B) Quantification of mtGFP import in wildtype and TOM20 OE cells. For each condition in each experiment, mtGFP localization was determined in 5–10 ROIs in zoomed confocal images from 5–10 cells, and 3 or 4 independent experiments were performed. (C) Autoradiographs of in vitro import of pre-OTC into mitochondria isolated from rat brain (top), wildtype (WT) SH-SY5Y cells (middle), and TOM20 OE SH-SY5Y cells (bottom) after exposure to various forms of α-synuclein (30 min @ 4°C). Upper band represents 35S-labeled pre-OTC and lower band represents imported, cleaved (mature) OTC. (D) Quantification of OTC import into brain mitochondria. Results were normalized to the vehicle-treated control and FCCP + oligomycin was used to collapse membrane potential and define zero import. N=3 independent experiments. (E) Quantification of OTC import into mitochondria from wildtype and TOM20 overexpressing SH-SY5Y cells. N=3–4 independent experiments. (F) Immunolocalization of TOM20 and Ndufs3 in wildtype and TOM20 overexpressing HEK293 cells exposed to various forms of α-synuclein. In wildtype cells exposed to oligomeric, dopamine-modified or S129E α-synuclein, Ndufs3 localization is diffuse rather than mitochondrial (i.e., Ndufs3 redistributed outside of mitochondria as defined by TOM20). This effect is prevented in TOM20 overexpressing cells. (G) Correlation (Pearson Index) of the localizations of TOM20 and Ndufs3 in wildtype vs. TOM20 overexpressing cells. TOM20 overexpression rescues the normal localization of Ndufs3. For each experimental condition, at least 100 cells were analyzed in each of 3 or 4 independent experiments.
Statistical analyses were by by 1- or 2-way ANOVA followed by pairwise testing and correction for multiple comparisons. a – p<0.0001 vs. monomer; b – p<0.0001 vs. wildtype cells; c – p<0.005 vs. monomer; d – p<0.05 vs. wildtype cells; e – p<0.002 vs. wildtype cells. Scale bars = 5 μm.
Given the critical role of TOM20 in the import of mitochondrially targeted proteins containing an N-terminal targeting signal, we examined whether overexpression of TOM20 might ameliorate the deleterious effects of α-synuclein on protein import. In cells transiently or stably overexpressing TOM20 (~4-fold over endogenous), the inhibitory effects of oligomeric, dopamine-modified and S129E α-synuclein on import of MTS-GFP were completely prevented (p<0.0001; Figure 3A & B). Overexpression of another component of the TOM complex, TOM5, did not preserve import (Figure S6C).
Although α-synuclein inhibited mitochondrial protein import in cells, this effect might be indirect. Therefore, we used isolated rat brain mitochondria to examine direct effects of various forms of α-synuclein on import. After a 30 minute pre-incubation of 200 nM α-synuclein with mitochondria at 4°C, we found that oligomeric, dopamine-modified, and S129E α-synuclein each inhibited import of radiolabeled pre-ornithine transcarbamylase (pre-OTC) by more than 50% (1-way ANOVA, p<0.0001; N=3–4), but monomeric and nitrated α-synuclein did not (Figures 3C & D). Fibrillar α-synuclein was without effect (Figure S5). A time course experiment revealed that the reduction in import was not simply a slowing of import rate but instead reflected a true decrease in import capacity (Fig S7). α-Synuclein had identical effects in mitochondria isolated from human dopaminergic SH-SY5Y cells (Figure 3C & E). To rule out a nonspecific effect of α-synuclein on mitochondrial membrane potential (ΔΨm), and hence, on import, we monitored ΔΨm with TMRM under the same conditions, and found that it was unchanged for the duration of the experiment (Figure S8). Thus, it is clear that the effect of α-synuclein on mitochondrial import is direct, and not due to nonspecific disruption of ΔΨm. Further, unlike in control mitochondria, protein import in mitochondria isolated from TOM20 overexpressing SH-SY5Y cells was not inhibited in vitro by post-translationally-modified α-synuclein (Figure 3C & E).
To examine the effects of α-synuclein on import of an endogenous, nuclear-encoded mitochondrial protein, we treated HEK293 cells with various forms of α-synuclein for 24 h and then used confocal microscopy to localize the complex I subunit, Ndufs3. Treatment with oligomeric, dopamine-modified and S129E α-synuclein decreased the import of the Ndufs3 subunit. As a result, the mitochondrial localization (Pearson index with TOM20) of Ndufs3 decreased from about 0.9 to 0.3 (p<0.0001, 2-way ANOVA with Sidak correction for multiple comparisons; N=4 experiments), with an obvious increase in the diffuse cytosolic localization of this mitochondrial protein (Figure 3F & G). The impaired import of endogenous Ndufs3, with redistribution to the cytosol, was prevented by overexpression of TOM20 (p<0.0001; Figure 3F & G).
To determine if the import of other presequence-containing proteins was also disrupted by α-synuclein, we assessed the distributions of endogenous SDHA (a subunit of complex II), COX4 (a subunit of complex IV), and mtHSP70 (a matrix chaperone). As with Ndufs3, there was a cytosolic redistribution of each of these endogenous proteins after treatment of cells with oligomeric, dopamine-modified and S129E α-synuclein, but not after monomeric or nitrated species (Figure S9). Additionally, we monitored the expression and distribution of 2 proteins encoded by the mitochondrial genome (which do not require import). ND1 (a subunit of complex I) and mtCO1 (a subunit of complex IV) were unaffected by α-synuclein during the time course of this experiment.
Binding of α-synuclein to TOM20
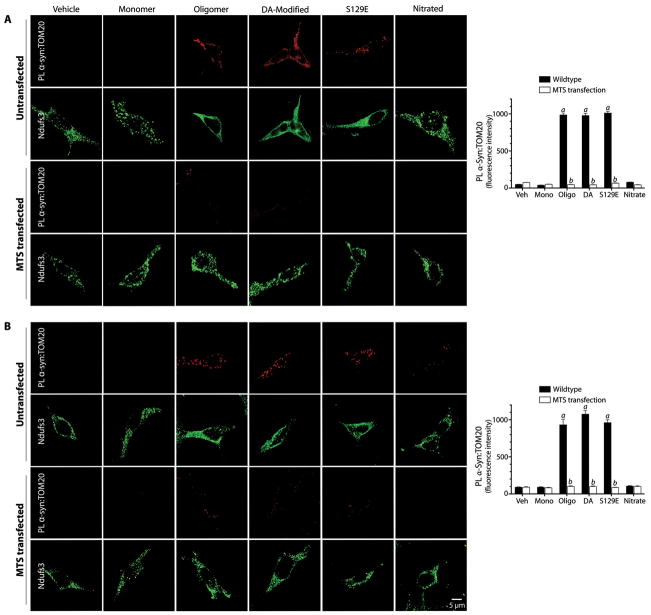
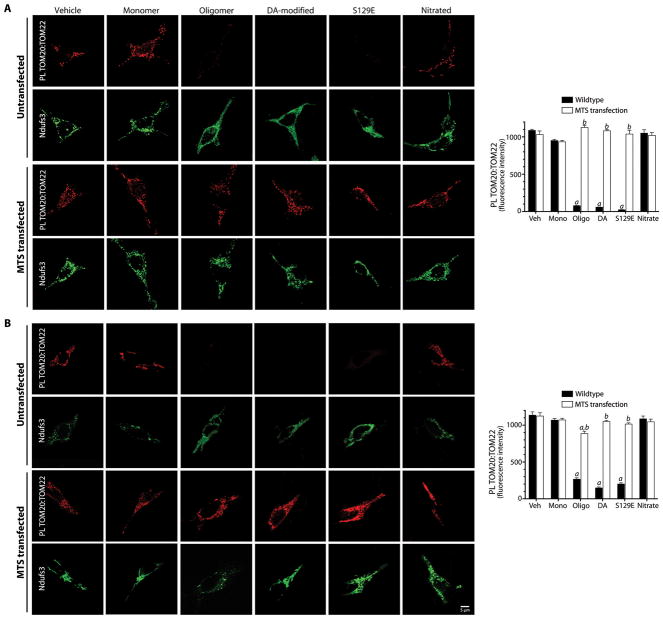
In order to determine whether the inhibitory effects of post-translationally modified forms of α-synuclein on mitochondrial protein import correlate with their interactions with TOM20, we performed proximity ligation assays in cells exposed to various forms of α-synuclein. There was strong PL signal between TOM20 and oligomeric, dopamine-modified and S129E α-synuclein, but not monomeric or nitrated α-synuclein (ANOVA, p<0.0001; N=3; Figure 4A). Control experiments showed that the α-synuclein:TOM20 PL signal localized to mitochondria (Figure S10). Fibrillar α-synuclein did not produce a PL signal with TOM20 (Figure S5E). Expression of a ‘naked’ MTS peptide (COX8 presequence) prevented proximity ligation between post-translationally modified α-synuclein and TOM20, suggesting that α-synuclein binds to a site on TOM20 that overlaps the MTS receptor site (Figures 4A). Moreover, the MTS was able to reverse the α-synuclein:TOM20 PL interaction when transfection occurred 24h after α-synuclein treatment (Figure 4B).
Figure 4.
Proximity ligation of post-translationally modified α-synuclein and TOM20 and Ndufs3 localization in HEK293 cells. (A) In untransfected cells, there is proximity ligation between TOM20 and oligomeric, dopamine-modified and S129E α-synuclein, but not monomeric or nitrated species. This is associated with a cytosolic redistribution of Ndufs3. Fibrillar α-synuclein did not interact with TOM20 (figure S5). When cells were transfected with an MTS expression vector prior to treatment with α-synuclein, the TOM20:α-synuclein interaction was blocked, indicating that the α-synuclein binding site overlaps the MTS binding site on TOM20. MTS transfection also preserved the punctate (mitochondrial) distribution of Ndufs3. (B) When cells were transfected with the MTS expression vector 24h after α-synuclein treatment, the TOM20:α-synuclein interaction was reversed. Bar graphs show quantification of the α-synuclein:TOM20 PL signal in mock transfected (black bars) and MTS-overexpressing cells. At least 100 cells were analyzed for each condition in every independent experiment (N=3). a, p<0.0001 vs vehicle; b, p<0.0001 vs mock transfected; 2-way ANOVA. Scale bar = 5 μm.
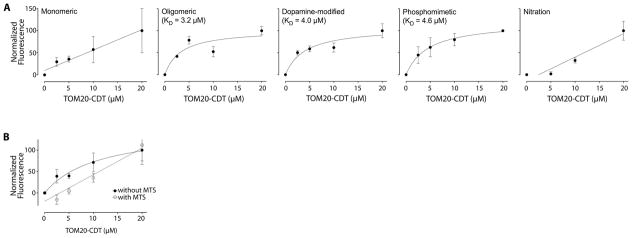
To confirm that the α-synuclein:TOM20 PL signals represent true protein-protein interactions, we measured binding between recombinantly purified α-synuclein and TOM20 (C-terminal cytosolic fragment (25)) using fluorescence spectroscopy and a ‘pseudo-wildtype’ tryptophan mutant of α-synuclein to impart fluorescence (26). We found saturable, specific binding of oligomeric, dopamine-modified and S129E α-synuclein, with KDs of approximately 5 μM (Figure 5A). As binding of monomeric and nitrated α-synuclein was not saturable, we conclude there was not a specific interaction in this assay, which is consistent with our PL and import results. Inclusion of an MTS peptide (COX8 presequence) at a concentration of 250 μM (~10 x KD of a native MTS (25)) in this in vitro assay abolished specific, saturable binding of α-synuclein to TOM20 and thereby confirmed that the MTS and α-synuclein bind to the same site on TOM20 (Figure 5B).
Figure 5.
Binding curves of TOM20 to various forms of α-synuclein. (A) There was saturable binding of oligomeric, dopamine-modified and S129E α-synuclein, but not the monomeric or nitrated species. N = 3. (B) The COX8 MTS peptide inhibits binding of oligomeric α-synuclein to TOM20. When binding is performed in the presence of excess MTS (250 μM), specific binding is markedly reduced or abolished; nonlinear curve fitting yielded an affinity of >7 × 1014 μM when the MTS was present. Similar results were obtained with dopamine-modified and S129E α-synuclein. The overall effect of the MTS was significant (p<0.02) by 2-way ANOVA. N=3.
Disruption of TOM20-TOM22 interactions
As described above, it is believed that the presequence of mitochondrially-targeted proteins is first recognized by TOM20 in a hydrophobic interaction and subsequently, by TOM22 in a hydrophilic interaction before the pre-protein is imported (27). The existence of ‘peripheral’ TOM20 receptors at some distance from the core TOM complex (11) raises the possibility that TOM20-bound pre-proteins may be trafficked from the periphery to TOM22 in the core translocase complex. In unchallenged cells in culture, we found a strong proximity ligation signal between TOM20 and TOM22, indicating that at least a portion of these 2 proteins is normally in close approximation (Figure 6A). After treatment with forms of α-synuclein that bind TOM20 (and thereby produce a TOM20:α-synuclein PL signal), the TOM20:TOM22 PL signal was lost (ANOVA, p<0.0001; N=3). This result suggests that binding of α-synuclein to TOM20 prevents its association with TOM22 and this, together with specific blockade of presequence binding sites on TOM20 by α-synuclein likely accounts for loss of import activity. The reasons for loss of association of TOM20 and TOM22 are unclear, but steric interference with the PL assay is unlikely since control experiments show antibody binding to TOM20 and TOM22 is not occluded by α-synuclein binding. It is possible that the normal mobility of TOM20 in the outer membrane might be impeded by the affinity of α-synuclein for membrane lipids (28). In cells overexpressing a ‘naked’ MTS, the TOM20:TOM22 PL signal was maintained even in the presence of oligomeric, dopamine-modified and S129E α-synuclein (Figure 6A). Moreover, expression of the MTS 24 h after treatment with oligomeric α-synuclein was able to restore the TOM20:TOM22 PL signal (Figure 6B), further indicating that the effects of α-synuclein on protein import machinery are reversible.
Figure 6.
α-Synuclein interaction with TOM20 prevents the normal interaction between TOM20 and TOM22 in HEK293 cells. (A) Under basal conditions (vehicle), proximity ligation detects an interaction between TOM20 and TOM22. This is blocked by oligomeric, dopamine-modified and S129E α-synuclein, but not monomeric or nitrated species. Loss of the TOM20:TOM22 PL signal is associated with relocalization of Ndufs3 to the cytosol. In cells overexpressing a ‘naked’ MTS (COX8 presequence), the TOM20:TOM22 PL signal is maintained even after treatment with oligomeric, dopamine-modified and S129E α-synuclein. (B) When cells were treated with α-synuclein and then transfected with the MTS 24 h later, the TOM20:TOM22 PL signal could be restored. Graphs show quantification of TOM20:TOM22 signal in mock transfected (black bars) and MTS-overexpressing cells (white bars). a, p<0.0001 vs vehicle; b, p<0.0001 vs mock transfected; 2-way ANOVA. At least 100 cells were analyzed per condition in each independent experiment. N=3. Scale bar = 5 μm.
Downstream consequences of import impairment
Certain complex I subunits, such as Ndufs3, can normally turn over completely in less than 24h (29), and reduced levels of Ndufs3 may lead to metabolic deficits and excessive production of ROS (30). To determine the functional consequence of α-synuclein-induced impairment of mitochondrial protein import, we measured mitochondrial respiration. Results indicated that monomeric α-synuclein had no effect on respiration, but oligomeric and dopamine-modified species depressed both basal and FCCP-stimulated respiration by 30 – 40% (p<0.01; 1-way ANOVA; N=3; Figure 7A). These deleterious effects were prevented by overexpression of TOM20 (Figure 7B) or a ‘naked’ MTS (Figure S11E).
Figure 7.
Downstream effects of α-synuclein on mitochondria. (A) In wildtype SH-SY5Y cells, a 24 h exposure to oligomeric or dopamine-modified α-synuclein reduced basal and FCCP-stimulated mitochondrial respiration. Monomeric α-synuclein was without effect. oligo – oligomycin, FCCP - carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone, Rot – rotenone. *p<0.01 vs vehicle; 1-way ANOVA; N=3. (B) In SH-SY5Y cells overexpressing TOM20, the deleterious effects of oligomeric and dopamine-modified α-synuclein were prevented. N=3. (C) In wildtype HEK293cells, a 24 h exposure to oligomeric, dopamine-modified or S129E α-synuclein induced oxidation of protein thiols; exposure to monomeric or nitrated α-synuclein did not. (D, E) In HEK293 cells overexpressing TOM20, α-synuclein did not induce oxidative stress. At least 100 cells were quantified per condition in each experiment. a, p<0.001 vs vehicle; b, p<0.001 vs mock transfected cells; 2-way ANOVA; N=3. (F) TMRM fluorescence in SH-SY5Y cells (as an index of mitochondrial membrane potential) is reduced by oligomeric but not monomeric α-synuclein. (G, H) In SH-SY5Y cells overexpressing TOM20, oligomeric α-synuclein does not significantly impact mitochondrial membrane potential. 30–50 cells were analyzed for each treatment in each of 3 independent experiments. a – p<0.001 vs. vehicle; b – p< 0.001 vs. wildtype cells; 2-way ANOVA. Scale bars = 10 μm.
We next examined protein thiol oxidation (oxidative damage) after exposure to various forms of α-synuclein. Treatment with oligomeric, dopamine-modified and S129E α-synuclein induced protein thiol oxidation (p<0.001; 2-way ANOVA; N=3), but monomeric and nitrated α-synuclein did not (Figure 7C,E). α-Synuclein-induced oxidative damage was prevented by overexpression of TOM20 (Figure 7D,E) or a ‘naked’ MTS (Figure S11A,B). Similarly, after 24h exposure to oligomeric α-synuclein, there was a significant drop in ΔΨm (p<0.001; 2-way ANOVA; N=4) that was not seen with monomeric α-synuclein (Figure 7F,H), and which was prevented by overexpression of TOM20 (Figure 7F–H) or a ‘naked’ MTS (Figure S11C,D).
It is notable that in cells over-expressing TOM20, its distribution was mitochondrial, not ectopic, and mitochondrial morphology was unchanged. Moreover, even though such cells were protected against α-synuclein-induced import defects and resultant respiratory defects, oxidative stress and depolarization, the mitochondrial α-synuclein:TOM20 PL signal remained intact. The mechanism by which overexpression of TOM20 is protective is not entirely clear, but it appears to enhance the efficiency of import by increasing the initial rate of mitochondrial protein import. Additionally, the protective effect is saturable, since higher concentrations of toxic α-synuclein species (1.7 or 3.4 μM vs. 200 nM) can overcome the beneficial effects of enhanced TOM20 expression (Figure S12).
Toxic species of α-synuclein
Numerous lines of evidence presented here suggest that oligomeric, dopamine-modified and S129E α-synuclein impair protein import, while monomeric, nitrated and fibrillar forms do not. To look for possible structural explanations for these differences, we used circular dichroism spectroscopy to examine the α-synuclein species used in biological experiments (Figure S13A & B). However, this did not reveal clear differences between the toxic and non-toxic species; all were predominantly in a random coil conformation with a similar small component of alpha-helix conformation. As expected, only the fibril preparation had a significant amount of beta-sheet structure. In contrast, there were some apparent differences when the species were separated by SDS-PAGE (Figure S13C). As intended, the nontoxic monomer preparation was predominantly monomer, with a small component of dimer. The nitrated species was composed mostly of monomer and dimer and high molecular weight material, with little trimer or tetramer. On the other hand, each of the toxic species – oligomeric, dopamine-modified and S129E α-synuclein – had relatively large amounts of trimer and tetramer, about 25–35% of the total (p<0.05–0.001 vs monomer or nitrated species; ANOVA; Figure S13D). Under conditions of our preparation, the fibril sample was a continuous ‘smear’ and individual bands could not be resolved. From these experiments, we tentatively conclude that a trimeric and/or tetrameric structure may be important for mitochondrial toxicity of α-synuclein. It is also important to note that each species of α-synuclein was used in our experiments at a concentration of 200 nM (monomer equivalent), so the actual concentration of oligomer was substantially lower.
Selective vulnerability of dopamine neurons?
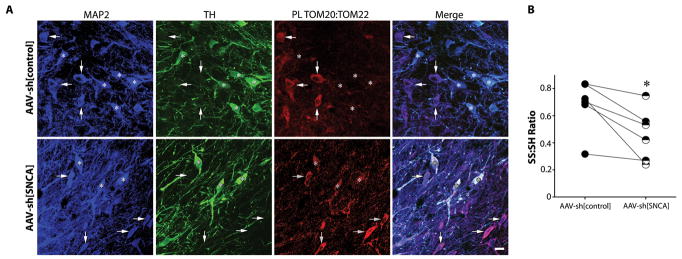
As noted, in cultured cells, there is a strong PL signal between TOM20:TOM22, which can be disrupted by post-translationally modified-α-synuclein. Similar TOM20:TOM22 interactions are seen in neurons in rat brain sections. However, there is a conspicuous absence of the TOM20:TOM22 PL signal in nigrostriatal dopamine neurons in control animals. In the midbrain, these neurons have by far the highest levels of SNCA mRNA (17), raising the possibility that endogenous α-synuclein (perhaps combined with dopamine-modification) may be sufficient to impair import in these neurons at baseline. Consistent with this hypothesis, knockdown of endogenous α-synuclein in control rats ‘restored’ a TOM20:TOM22 PL signal in nigrostriatal neurons relative to the untransduced hemisphere (Figure 8A). Further, we have reported previously that, under basal conditions, nigrostriatal neurons exist in a more oxidized state than cortical or other dopaminergic neurons (31) – and results in figure 7 suggest that α-synuclein may contribute to this process. Here, in a separate cohort of animals, we found that AAV-sh[SNCA]-mediated knockdown of endogenous α-synuclein decreased basal levels of protein thiol oxidation in nigrostriatal neurons relative to the contralateral hemisphere, which received AAV-sh[control] (p<0.05; Figure 8B). Thus, neuron-specific differences in endogenous α-synuclein levels (or post-translational state) may confer differences in mitochondrial protein import efficiency and possibly, selective vulnerability.
Figure 8.
The normal TOM20:TOM22 PL signal seen in most neurons is absent in nigrostriatal dopamine neurons in vivo, but is restored by knockdown of endogenous α-synuclein. (A) In the untreated hemisphere (top row), MAP2+/TH- non-dopaminergic neurons (arrows) show a strong TOM20:TOM22 PL signal, which is absent in TH+ dopaminergic cells (asterisks). In the hemisphere that received AAV2-shSNCA (bottom row), there was emergence of a strong TOM20:TOM22 PL signal in the TH+ dopaminergic neurons. Scale bar = 30 μm. (B) Consistent with in vitro data (figure 5C–E), α-synuclein knockdown was associated with decreased levels of protein thiol oxidation. Filled circles, -S-S-/-SH ratio of nigral neurons in the control hemisphere; half-filled circles, -S-S-/-SH ratio of nigral neurons in the SNCA knockdown hemisphere; lines connect the means from each hemisphere in each animal. *p<0.05, Wilcoxon matched-pairs signed rank test. Scale bar = 30 μm.
Discussion
Our results tie together two central pathogenic mechanisms in PD: α-synuclein accumulation and mitochondrial impairment. We have found that certain species of α-synuclein bind specifically to TOM20, prevent its interaction with the co-receptor, TOM22, and inhibit mitochondrial protein import. This leads to impairment of mitochondrial function, with reduced respiration and excessive ROS production. Our data suggest this process is operative in human PD and may contribute to pathogenesis. These findings help to explain the deleterious effects of α-synuclein on mitochondria, and they suggest new therapeutic strategies.
There have been no previous direct studies of the mitochondrial protein import system in PD, or models thereof. There is a single report that α-synuclein is a substrate for the import machinery and is imported to the matrix, where it binds to and inhibits complex I (32). To the best of our knowledge, this has not been replicated, and our results suggest there is no specific interaction between monomeric α-synuclein and the TOM machinery. Another report suggests that TOM40 levels are reduced when α-synuclein is overexpressed, but import was not measured, and no mechanism was elucidated (33). Finally, it has been reported that efficient import prevents PINK1 from accumulating on the mitochondrial surface, where it can recruit parkin and promote autophagic mitochondrial clearance (34). We hypothesize that by inhibiting protein import, α-synuclein may also alter PINK1-parkin signaling, and this is under investigation.
In the current study, we used complementary techniques (proximity ligation, multiple assays of mitochondrial protein import and fluorescence spectroscopy) to provide convergent evidence that certain post-translationally modified forms of α-synuclein (soluble oligomers, dopamine-modification, and S129E) bind to TOM20 and impair mitochondrial import of endogenous or exogenous presequence-containing nuclear encoded proteins. Our binding assays indicate that these species of α-synuclein bind to TOM20 with affinities of about 5 μM. By contrast, the affinity of a normal TOM20 substrate, the aldehyde dehydrogenase presequence, is lower – about 20 μM (25). Interestingly, the intraneuronal concentration of α-synuclein has been estimated to be 2 – 5 μM (21) and, in our experiments, α-synuclein species were applied to cells or isolated mitochondria at a concentration equivalent to 200 nM monomeric protein. Control experiments confirmed that all forms of α-synuclein used here entered cells to an equivalent extent and did not alter total α-synuclein levels in treated cells. Thus, we conclude that local conditions that predispose to relatively minor oligomer formation, dopamine-modification or S129 phosphorylation (or some combination thereof) are likely to have pronounced effects on protein import.
While binding assays provided strong evidence that α-synuclein interacts specifically with TOM20, the fact that overexpression of a ‘naked’ MTS (COX8 presequence) blocked the α-synuclein:TOM20 PL signal indicates that the α-synuclein binding site overlaps the MTS recognition site on TOM20. Direct inhibition of α-synuclein binding to TOM20 by the MTS peptide confirms this conclusion. Thus, one mechanism by which α-synuclein inhibits protein import appears to involve direct competition for TOM20 presequence receptor sites. Moreover, when the MTS was overexpressed after α-synuclein was already bound to TOM20, it was able to normalize the α-synuclein:TOM20 PL signal, indicating that the interaction of α-synuclein with TOM20 is reversible.
The presequence of mitochondrially-targeted proteins must be recognized by both TOM20 and TOM22 (either sequentially or simultaneously) before translocation through the TOM40 pore. α-Synuclein binds to TOM20, but we found no evidence for binding to TOM22. Cryo-electron microscopy studies suggest that, in addition to the central TOM complex core, there are ‘peripheral’ TOM20 components, which are in a dynamic equilibrium with the preassembled TOM complex (11). Whether these peripheral TOM20 receptors bind the presequences of pre-proteins and traffic them to the central TOM complex (containing TOM22) for import is unclear. Under basal conditions, our PL studies indicate that at least some portion of TOM20 interacts with TOM22, but this interaction is prevented by toxic species of α-synuclein. Interference with the normal TOM20:TOM22 interaction may represent a second mechanism by which α-synuclein impairs import.
The mechanism by which α-synuclein prevents the TOM20:TOM22 interaction is unclear. However, if ‘peripheral’ TOM20 components must normally traffic to the central TOM complex, it is possible that the affinity of TOM20-bound α-synuclein for lipid membranes may impede lateral movement of TOM20 in the outer mitochondrial membrane and reduce formation of a functional import complex. We found that overexpression of a ‘naked’ MTS in the setting of exogenous α-synuclein, or knockdown of endogenous α-synuclein in nigrostriatal neurons in vivo, was able to restore this normal TOM20:TOM22 interaction.
Overall, these studies provide compelling evidence for a specific interaction between certain species of wildtype α-synuclein and the TOM20 presequence receptor. Further, we have delineated two plausible and related mechanisms by which this interaction impairs mitochondrial protein import.
The relative loss of mitochondrial protein import caused by α-synuclein has several deleterious downstream effects. Basal and FCCP-induced mitochondrial respiration are reduced, and protein thiol oxidation is increased. Additionally, because of impaired electron transport complex activity, mitochondrial membrane potential declines. The fact that each of these effects can be prevented by overexpression of TOM20 (or an MTS peptide) confirms that they result from defective import. Together, these findings indicate that α-synuclein-induced impairment of mitochondrial protein import has the potential to produce senescent, inefficient mitochondria that produce less energy and more ROS.
Our studies have used a wide variety of assays, including proximity ligation, direct import assays, fluorescence spectroscopic binding assays and respiratory measurements, and these have provided consistent results about which species of α-synuclein are toxic to mitochondria. Monomeric, wildtype α-synuclein appears to have no effect, but oligomeric, dopamine-modified and S129E phosphomimetic species potently impair import function. While the phosphomimetic mutant behaved like oligomeric and dopamine-modified species, it is important to recognize that the S129E mutation does not always behave identically to bona fide phosphorylated α-synuclein (35). Interestingly, nitrated α-synuclein and amyloid fibrils of α-synuclein were essentially inert in our assays. Our limited structural studies suggest a trimeric or tetrameric conformation may be important for toxicity. If so, we would anticipate that these conformations are distinct from the endogenous tetramers posited by Selkoe and colleagues (36). Although the current study focused on post-translationally modified wildtype α-synuclein in the context of idiopathic (sporadic) PD, it will be of interest to examine in future studies the impact of mutant α-synuclein, and post-translational modifications thereof, on mitochondrial protein import.
To determine the relevance of our findings to the human disease, we examined postmortem brain specimens from controls and individuals who died with PD. Just as in rats treated with rotenone or injected with an AAV[SNCA] overexpression vector, we found that nigrostriatal dopamine neurons from PD cases showed a marked increase in the α-synuclein:Tom20 PL signal compared to controls. Similarly, this α-synuclein:Tom20 PL signal was associated with a relative loss and cytosolic redistribution of the endogenous, imported complex I subunit, Ndufs3. It is noteworthy that another recent study reported a large loss of a presequence-containing imported protein (Ndufb8) relative to a mitochondrially-encoded protein (COX1) in nigral neurons in PD (37). This discovery of an apparent protein import defect in human PD adds to the list of findings that have been predicted by the rotenone rat model of PD (13, 38, 39).
The results of this study may also shed light on the basis for the selective vulnerability of nigrostriatal neurons to degeneration in PD. At baseline, these cells express more SNCA mRNA than surrounding midbrain neurons and they exist in a higher state of oxidation (17, 31). We found that these neurons do not show the normal TOM20:TOM22 interaction seen in other neurons (and presumably needed for efficient protein import); however, when endogenous α-synuclein was knocked down in vivo, the TOM20:TOM22 PL signal emerged. Further, the knockdown of endogenous α-synuclein decreased the oxidation state of these neurons. Thus, under basal conditions, it appears that endogenous α-synuclein levels may be sufficient to impact protein import function – and this situation is likely exacerbated in aging as α-synuclein accumulates in these cells (16). Moreover, elevated α-synuclein levels increase redistribution of dopamine from vesicles to the cytosol (40) where it can modify α-synuclein – in turn, dopamine-modified α-synuclein is particularly potent at inhibiting mitochondrial protein import.
While the results of our studies are unanticipated and provide new insights into pathogenesis, there are limitations and unknowns, as well. For example, thus far we have not identified the structural characteristics that define the toxic species of α-synuclein, although a trimeric or tetrameric structure may be important. We also found that nitrated and fibrillar species of α-synuclein were essentially inert in our system; however, this does not exclude the possibility that they might exert toxicity by other mechanisms. Moreover, our study focused on wildtype α-synuclein, so we do not yet know how pathogenic α-synuclein mutations impact mitochondrial protein import. Finally, although we showed that mitochondrial protein import impairment caused cellular toxicity in the form of reduced respiration, oxidative damage and mitochondrial depolarization, the relative extent to which this specific mechanism contributes to the overall neurotoxicity of α-synuclein remains to be determined.
Despite these caveats, the results presented here have several important therapeutic implications. First, together with the recent report by Zharikov et al (17), they suggest that even modest reduction of endogenous α-synuclein levels has beneficial effects on mitochondrial function and nigral redox state – and may be neuroprotective in PD. Given that multiple post-translational modifications render α-synuclein toxic to mitochondria, it appears that strategies aimed at a general reduction of α-synuclein, rather than targeting specific modifications or aggregation states, may be most efficacious.
Additionally, we found that overexpression of TOM20 prevented α-synuclein-induced impairment of mitochondrial protein import, as well as its downstream consequences, such as respiratory defects, ROS production and loss of ΔΨm. The mechanism by which a moderate (2–3-fold) increase in TOM20 protein levels is protective is not yet clear, but preliminary studies indicate that TOM20-overexpressing mitochondria have a faster initial rate of protein import. This result is consistent with previous work showing that TOM20 overexpression can increase import (41). Additionally, however, we found that mitochondria isolated from TOM20 overexpressing cells and assayed in vitro were resistant to the inhibitory effects of the otherwise toxic species of α-synuclein. In this context, it will be of interest to determine whether overexpression of TOM20 in vivo is protective in models of PD.
Finally, contrary to our expectations, overexpression of a ‘naked’ MTS had beneficial effects. We had anticipated that expression of the MTS would be similar to α-synuclein, competing for TOM20 presequence binding sites and inhibiting import. However, while the MTS blocked the α-synuclein:TOM20 PL signal as expected, it also preserved the mitochondrial import of the endogenous Ndufs3 subunit. In addition, MTS overexpression was able to prevent or even reverse the loss of the TOM20:TOM22 interaction induced by α-synuclein. As a consequence, overexpression of the ‘naked’ MTS prevented downstream toxic effects of α-synuclein, such as reduced respiration, increased ROS production and mitochondrial depolarization. On this basis, examination of an MTS peptide as a neuroprotective strategy is warranted.
In conclusion, we have defined a mechanism by which α-synuclein impairs a critical mitochondrial function, protein import, and have shown that this likely occurs in human PD. Our findings lead logically to new therapeutic strategies, which are currently being tested.
Materials and Methods
α-Synuclein expression and purification
Human α-synuclein cDNA (WT or S129E) was transfected into BL21-DE3 E. coli and α-synuclein was purified as described by Volles and Lansbury (42).
α-synuclein modification
Oligomers: monomeric α-synuclein was diluted to a concentration of 5mg/ml and shaken at ~300–500 RPM at 37°C for 3 days. Dopamine-modified: as described by Martinez-Vicente (23). Nitration: monomeric α-synuclein was diluted to a concentration of 5mg/ml and 50μL of 1% tetranitromethane was added per 500μL of α-synuclein solution. The solution was vortexed twice for 10 min. S-129 Phosphomimetic: S129E mutant SNCA cDNA was transfected into E. coli as described above. All samples (monomers, oligomers, dopamine-modified, nitrated, S129E) received the following treatment after modification: dialysis in the dark in 4L of PBS with gentle stirring overnight (10 kDa MW cutoff). Following dialysis, all samples were spun at 14,000g for 5 min to pellet any fibrils that formed. The supernatant contained soluble oligomers and monomers as confirmed by SDS-PAGE, and the pellet contained fibrils. Samples were prepared fresh weekly.
Proximity Ligation Assay
PLA was performed in 4% PFA-fixed tissue or cells. Samples were incubated with specific primary antibodies to the proteins to be detected. Secondary antibodies conjugated with oligonucleotides were added to the reaction and incubated. Ligation solution, consisting of two oligonucleotides and ligase, was added. In this assay, the oligonucleotides hybridize to the two PLA probes and join to a closed loop if they are in close proximity. Amplification solution, consisting of nucleotides and fluorescently labeled oligonucleotides, was added together with polymerase. The oligonucleotide arm of one of the PLA probes acts as a primer for “rolling-circle amplification” using the ligated circle as a template, and this generates a concatemeric product. Fluorescently labeled oligonucleotides hybridize to the RCA product. The PL signal was visible as a distinct fluorescent spot and was analyzed by confocal microscopy (Duolink; Sigma Aldrich). Control experiments included routine immunofluorescence staining of the proteins of interest under identical experimental conditions.
Fluorescence measurements
Quantitative fluorescence measurements were made with an Olympus upright 3-laser scanning confocal microscope, taking care to ensure that images contained no saturated pixels. For quantitative comparisons, all imaging parameters (e.g., laser power, exposure, pinhole) were held constant across specimens.
Statistical analyses
Each result presented here was derived from 3–6 independent experiments. For simple comparisons of 2 experimental conditions, 2-tailed, unpaired t-tests were used. Where variances were not equal, Welch’s correction was used. When virus was injected into 1 hemisphere of the brain and the other hemisphere was used as a control, 2-tailed paired t-tests or Wilcoxon matched-pair signed rank tests were employed. For comparisons of multiple experimental conditions, 1-way or 2-way ANOVA was used, and if significant overall, post hoc corrections (Bonferroni or Sidak) for multiple pairwise comparisons were made. P-values less than 0.05 were considered significant. All bar graphs show mean ± SEM.
Other methods are described in Supplemental Materials and Methods.
Supplementary Material
Text. Additional Materials and Methods.
Fig. S1. Levels of α-synuclein and S129-phospho- α-synuclein in vivo.
Fig. S2. Positive and negative PL interactions with α-synuclein in substantia nigra pars compacta.
Fig. S3. Rotenone induces a loss of mitochondrial localization of the nuclear-encoded, imported protein, Ndufs3.
Fig. S4. All species of α-synuclein used in this study enter cells to an equivalent extent, and when added at 200 nM, they do not change intracellular concentrations of α-synuclein or its localization.
Fig. S5. Fibrillar α-synuclein does not affect mitochondrial protein import.
Fig. S6. Overexpression of TOM20 and TOM5.
Fig. S7. Time course of isolated brain mitochondrial protein import in the absence or presence of monomeric and oligomeric α-synuclein.
Fig. S8. Lack of mitochondrial depolarization by α-synuclein during import assays.
Fig. S9. Effects of α-synuclein on import and localization of other mitochondrial proteins.
Fig. S10. The α-synuclein:TOM20 PL signal colocalizes with mitochondria.
Fig. S11. Downstream effects of α-synuclein on mitochondria are blocked by MTS overexpression.
Fig. S12. The protective effects of TOM20 overexpression on mitochondrial protein import can be overcome by increased concentrations of α-synuclein.
Fig. S13. Structural analysis of the α-synuclein species used in this study.
Acknowledgments
We thank Dr. Hiroko Yano for the pGEM-3Zf(+)-pOTC plasmid.
Funding: This work was supported by research grants from the DSF Charitable Foundation, the Ri.MED Foundation, the Consolidated Anti-Aging Foundation, the National Institutes of Health (NS095387, NS059806, ES022644, ES020718, ES020327, NS065789, AG026389, P50AG005133), the United States Department of Veterans’ Affairs (1I01BX000548), the Blechman Foundation, the American Parkinson Disease Association and the Department of Biotechnology, Government of India.
Footnotes
Author contributions: RDM and PJB designed, performed and analyzed most experiments and edited the manuscript; EKH, CB, AZ, AB, XH and JM performed and analyzed experiments; CTC supervised human neuropathological studies; EAB and TGH designed and analyzed experiments and edited the manuscript; JTG supervised the project, designed and analyzed the experiments and wrote the paper.
Competing interests: The authors declare that they have no competing financial interests.
References and notes
- 1.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015 Apr 17; doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 2.Zaltieri M, et al. Mitochondrial Dysfunction and alpha-Synuclein Synaptic Pathology in Parkinson’s Disease: Who’s on First? Parkinsons Dis. 2015;2015:108029. doi: 10.1155/2015/108029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betarbet R, et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000 Dec;3:1301. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 4.Betarbet R, et al. Intersecting pathways to neurodegeneration in Parkinson’s disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006 May;22:404. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Cannon JR, et al. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol Dis. 2009 May;34:279. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu LJ, et al. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol. 2000 Aug;157:401. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schon EA, DiMauro S, Hirano M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nat Rev Genet. 2012 Dec;13:878. doi: 10.1038/nrg3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elstner M, et al. MitoP2: an integrative tool for the analysis of the mitochondrial proteome. Molecular biotechnology. 2008 Nov;40:306. doi: 10.1007/s12033-008-9100-5. [DOI] [PubMed] [Google Scholar]
- 9.Koehler CM. Protein translocation pathways of the mitochondrion. FEBS Lett. 2000 Jun 30;476:27. doi: 10.1016/s0014-5793(00)01664-1. [DOI] [PubMed] [Google Scholar]
- 10.Schulz C, Schendzielorz A, Rehling P. Unlocking the presequence import pathway. Trends in cell biology. 2015 May;25:265. doi: 10.1016/j.tcb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Model K, Meisinger C, Kuhlbrandt W. Cryo-electron microscopy structure of a yeast mitochondrial preprotein translocase. J Mol Biol. 2008 Nov 28;383:1049. doi: 10.1016/j.jmb.2008.07.087. [DOI] [PubMed] [Google Scholar]
- 12.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998 Apr 17;273:9443. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 13.Greenamyre JT, Cannon JR, Drolet R, Mastroberardino PG. Lessons from the rotenone model of Parkinson’s disease. Trends Pharmacol Sci. 2010 Jan 21;31:141. doi: 10.1016/j.tips.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanner CM, et al. Rotenone, Paraquat and Parkinson’s Disease. Environ Health Perspect. 2011 doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koos B, et al. Analysis of protein interactions in situ by proximity ligation assays. Curr Top Microbiol Immunol. 2014;377:111. doi: 10.1007/82_2013_334. [DOI] [PubMed] [Google Scholar]
- 16.Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol Dis. 2007 Jan;25:134. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Zharikov A, et al. shRNA targeting α-synuclein prevents neurodegeneration in a Parkinson’s disease model. J Clin Invest. 2015;125 doi: 10.1172/JCI64502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimant H, et al. Direct detection of alpha synuclein oligomers in vivo. Acta neuropathologica communications. 2013;1:6. doi: 10.1186/2051-5960-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decressac M, Mattsson B, Lundblad M, Weikop P, Bjorklund A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiol Dis. 2012 Mar;45:939. doi: 10.1016/j.nbd.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Ahn KJ, Paik SR, Chung KC, Kim J. Amino acid sequence motifs and mechanistic features of the membrane translocation of alpha-synuclein. J Neurochem. 2006 Apr;97:265. doi: 10.1111/j.1471-4159.2006.03731.x. [DOI] [PubMed] [Google Scholar]
- 21.Westphal CH, Chandra SS. Monomeric synucleins generate membrane curvature. J Biol Chem. 2013 Jan 18;288:1829. doi: 10.1074/jbc.M112.418871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theillet FX, et al. Structural disorder of monomeric alpha-synuclein persists in mammalian cells. Nature. 2016 Jan 25; doi: 10.1038/nature16531. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Vicente M, et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008 Feb;118:777. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001 Nov 9;294:1346. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 25.Abe Y, et al. Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell. 2000 Mar 3;100:551. doi: 10.1016/s0092-8674(00)80691-1. [DOI] [PubMed] [Google Scholar]
- 26.Lee JC, Langen R, Hummel PA, Gray HB, Winkler JR. Alpha-synuclein structures from fluorescence energy-transfer kinetics: implications for the role of the protein in Parkinson’s disease. Proc Natl Acad Sci U S A. 2004 Nov 23;101:16466. doi: 10.1073/pnas.0407307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz C, Schendzielorz A, Rehling P. Unlocking the presequence import pathway. Trends in cell biology. 2014 Dec 23; doi: 10.1016/j.tcb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Ramakrishnan M, Jensen PH, Marsh D. Association of alpha-synuclein and mutants with lipid membranes: spin-label ESR and polarized IR. Biochemistry. 2006 Mar 14;45:3386. doi: 10.1021/bi052344d. [DOI] [PubMed] [Google Scholar]
- 29.Dieteren CE, et al. Subunit-specific incorporation efficiency and kinetics in mitochondrial complex I homeostasis. J Biol Chem. 2012 Dec 7;287:41851. doi: 10.1074/jbc.M112.391151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suhane S, Kanzaki H, Arumugaswami V, Murali R, Ramanujan VK. Mitochondrial NDUFS3 regulates the ROS-mediated onset of metabolic switch in transformed cells. Biology open. 2013 Mar 15;2:295. doi: 10.1242/bio.20133244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horowitz MP, et al. Single-Cell Redox Imaging Demonstrates a Distinctive Response of Dopaminergic Neurons to Oxidative Insults. Antioxid Redox Signal. 2011 Jun 6; doi: 10.1089/ars.2010.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008 Apr 4;283:9089. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bender A, et al. TOM40 mediates mitochondrial dysfunction induced by alpha-synuclein accumulation in Parkinson’s disease. PLoS One. 2013;8:e62277. doi: 10.1371/journal.pone.0062277. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Bertolin G, et al. The TOMM machinery is a molecular switch in PINK1 and PARK2/PARKIN-dependent mitochondrial clearance. Autophagy. 2013 Nov 1;9:1801. doi: 10.4161/auto.25884. [DOI] [PubMed] [Google Scholar]
- 35.Paleologou KE, et al. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem. 2008 Jun 13;283:16895. doi: 10.1074/jbc.M800747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dettmer U, et al. Parkinson-causing alpha-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun. 2015;6:7314. doi: 10.1038/ncomms8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grunewald A, et al. Quantitative quadruple-label immunofluorescence of mitochondrial and cytoplasmic proteins in single neurons from human midbrain tissue. J Neurosci Methods. 2014 Jul 30;232:143. doi: 10.1016/j.jneumeth.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mastroberardino PG, et al. A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson’s disease. Neurobiol Dis. 2009 Jun;34:417. doi: 10.1016/j.nbd.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanders LH, et al. Mitochondrial DNA damage: molecular marker of vulnerable nigral neurons in Parkinson’s disease. Neurobiology of disease. 2014 Oct;70:214. doi: 10.1016/j.nbd.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosharov EV, et al. Alpha-synuclein overexpression increases cytosolic catecholamine concentration. J Neurosci. 2006 Sep 6;26:9304. doi: 10.1523/JNEUROSCI.0519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yano M, et al. Visualization of mitochondrial protein import in cultured mammalian cells with green fluorescent protein and effects of overexpression of the human import receptor Tom20. J Biol Chem. 1997 Mar 28;272:8459. doi: 10.1074/jbc.272.13.8459. [DOI] [PubMed] [Google Scholar]
- 42.Volles MJ, Lansbury PT., Jr Relationships between the sequence of alpha-synuclein and its membrane affinity, fibrillization propensity, and yeast toxicity. J Mol Biol. 2007 Mar 9;366:1510. doi: 10.1016/j.jmb.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schleiff E, Shore GC, Goping IS. Interactions of the human mitochondrial protein import receptor, hTom20, with precursor proteins in vitro reveal pleiotropic specificities and different receptor domain requirements. J Biol Chem. 1997 Jul 11;272:17784. doi: 10.1074/jbc.272.28.17784. [DOI] [PubMed] [Google Scholar]
- 44.Chu CT, et al. Ubiquitin immunochemistry as a diagnostic aid for community pathologists evaluating patients who have dementia. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2000 Apr;13:420. doi: 10.1038/modpathol.3880072. [DOI] [PubMed] [Google Scholar]
- 45.Alafuzoff I, et al. Staging/typing of Lewy body related alpha-synuclein pathology: a study of the BrainNet Europe Consortium. Acta Neuropathol. 2009 Jun;117:635. doi: 10.1007/s00401-009-0523-2. [DOI] [PubMed] [Google Scholar]
- 46.Di Maio R, Mastroberardino PG, Hu X, Montero LM, Greenamyre JT. Thiol oxidation and altered NR2B/NMDA receptor functions in in vitro and in vivo pilocarpine models: implications for epileptogenesis. Neurobiol Dis. 2013 Jan;49:87. doi: 10.1016/j.nbd.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Horowitz MP, et al. Single-cell redox imaging demonstrates a distinctive response of dopaminergic neurons to oxidative insults. Antioxid Redox Signal. 2011 Aug 15;15:855. doi: 10.1089/ars.2010.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text. Additional Materials and Methods.
Fig. S1. Levels of α-synuclein and S129-phospho- α-synuclein in vivo.
Fig. S2. Positive and negative PL interactions with α-synuclein in substantia nigra pars compacta.
Fig. S3. Rotenone induces a loss of mitochondrial localization of the nuclear-encoded, imported protein, Ndufs3.
Fig. S4. All species of α-synuclein used in this study enter cells to an equivalent extent, and when added at 200 nM, they do not change intracellular concentrations of α-synuclein or its localization.
Fig. S5. Fibrillar α-synuclein does not affect mitochondrial protein import.
Fig. S6. Overexpression of TOM20 and TOM5.
Fig. S7. Time course of isolated brain mitochondrial protein import in the absence or presence of monomeric and oligomeric α-synuclein.
Fig. S8. Lack of mitochondrial depolarization by α-synuclein during import assays.
Fig. S9. Effects of α-synuclein on import and localization of other mitochondrial proteins.
Fig. S10. The α-synuclein:TOM20 PL signal colocalizes with mitochondria.
Fig. S11. Downstream effects of α-synuclein on mitochondria are blocked by MTS overexpression.
Fig. S12. The protective effects of TOM20 overexpression on mitochondrial protein import can be overcome by increased concentrations of α-synuclein.
Fig. S13. Structural analysis of the α-synuclein species used in this study.