Abstract
Translation regulation largely occurs during initiation, which features ribosome assembly onto mRNAs and selection of the translation start site. Short, upstream ORFs (uORFs) located in the 5′-leader of the mRNA can be selected for translation. Multiple transcripts associated with stress amelioration are preferentially translated through uORF-mediated mechanisms during activation of the integrated stress response (ISR) in which phosphorylation of the α subunit of eIF2 results in a coincident global reduction in translation initiation. This review presents key features of uORFs that serve to optimize translational control that is essential for regulation of cell fate in response to environmental stresses.
Keywords: eukaryotic translation initiation, stress response, translation, translation control, translation regulation
Introduction
Multiple genome-wide analyses, including those utilizing ribosome and polysome profiling and mass spectrometry approaches, have provided evidence demonstrating that translation is a major regulator of gene expression (1–5). In addition to protein coding sequences (CDSs),2 another class of ORFs suggested to be translated at high frequency consists of short, upstream ORFs (uORFs) that are located within the 5′-leader of mRNAs (3–6). Over 40% of mammalian mRNAs contain uORFs, illustrating that uORFs are prevalent genome-wide and can serve as major regulators of translation (5, 7, 8). Approximation of uORF prevalence has relied upon the use of an AUG to denote the uORF start codon; however, recent ribosome profiling studies indicate that non-canonical initiation codons (e.g. CUG, UUG, and GUG) can also serve as competent sites of translation initiation (3, 4, 6). These findings suggest that the magnitude of uORF prevalence and the contribution of uORF translation in the regulation of gene expression have likely been underestimated.
Typically, uORFs are considered to be inhibitors of downstream translation initiation at CDSs. The inhibitory effect of uORFs is attributed to the fact that in eukaryotes the 43S preinitiation complex binds to the 5′-cap structure of the mRNA, then scans processively 5′ to 3′ and initiates translation at the first encountered initiation codon that is in an optimal context (9). The 43S preinitiation complex is composed of multiple factors including eIF3, eIF1, eIF1A, the eIF2·GTP·Met-tRNAiMet ternary complex, and the small 40S ribosomal subunit (10). Disassociation of the eIF2 ternary complex and other critical initiation factors during translation of constitutively repressing uORFs is suggested to be the cause of the low levels of subsequent translation reinitiation at downstream coding sequences.
Although uORFs can serve as repressors of CDS translation in a constitutive manner, there are also examples of uORFs that serve as dampeners in a controlled fashion or even promote translation initiation at the CDS in response to environmental stresses (4, 5, 11). Based on these studies, uORFs can have the following core properties that are critical for translational control (Fig. 1). 1) They enhance reinitiation after uORF translation, allowing for degrees of translation initiation at the downstream CDS. For example, translation of short uORFs can allow for retention of critical initiation factors, such as eIF3, which facilitate efficient translation reinitiation (12, 13). 2) They demonstrate direct ribosome elongation stalling during translation of the uORF and, as a consequence, thwart translation at the downstream CDS. Pauses in elongation can be reliant upon RNA secondary structure, codon usage bias, or polypeptide sequences encoded in the uORF, as well as interaction of trans-acting factors (12, 14–17). 3) They promote ribosome dissociation from the mRNA and therefore diminish subsequent CDS translation. Ribosome dissociation from the mRNA post-uORF translation can also be regulated by nucleotide sequences or the polypeptide encoded in the uORF (18–23). 4) They position uORFs out-of-frame with CDSs, resulting in ribosome termination downstream of CDS start codons. Translating ribosomes are suggested to only “back up” in a 3′ to 5′ fashion for a small number of nucleotides, resulting in low translation of the CDS (24–26). 5) They allow for scanning ribosomes to bypass the uORF either in a largely constitutive fashion or upon induction of physiological signals. Ribosome bypass is thought to occur at least in part due to the nucleotide sequences flanking the initiation codon (9, 18, 27, 28). These uORF properties, which can be integrated individually or in combination within mRNAs, help determine the specific mechanism of translational control of a given gene (Fig. 1). This review highlights the mechanisms by which uORFs can modulate translation at CDSs, and the processes by which uORFs with these diverse properties can be integrated individually or in combination into mRNAs to facilitate differential regulation of translation.
FIGURE 1.
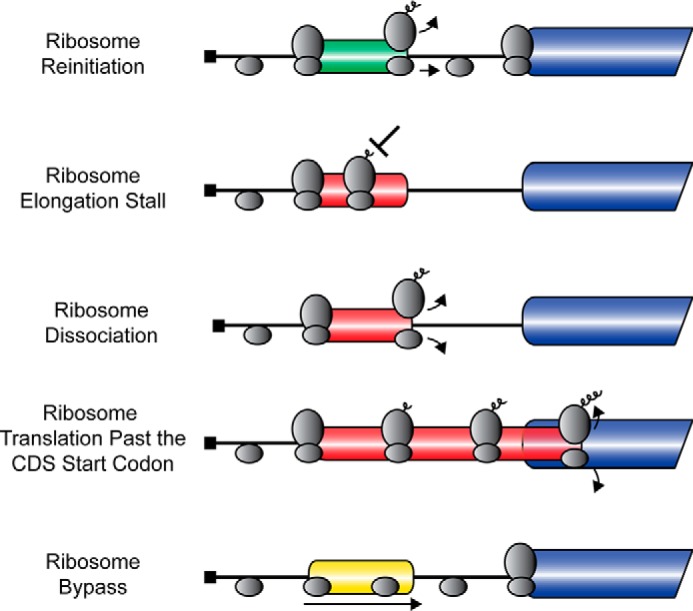
uORFs regulate downstream CDS translation. uORFs can have multiple core properties, including promoting ribosome reinitiation after uORF translation, ribosome elongation stalling while translating the uORF, ribosome dissociation from the mRNA, ribosome translation of uORFs past the CDS start codon, or ribosome bypass of the uORF. CDSs are indicated by the blue bar; positive-acting uORFs are indicated by a green bar; negative-acting uORFs are indicated by a red bar; and uORFs that have no effect on downstream translation are indicated by a yellow bar. Scanning and elongating ribosomes are illustrated by the gray ovals.
Translation Regulation during the Integrated Stress Response
Phosphorylation of eIF2 on its α subunit at serine 51 (eIF2α-P) inhibits the activity of the guanine nucleotide exchange factor eIF2B that results in a decrease in the exchange of GDP for GTP, thus lowering formation of the 43S preinitiation complex that triggers a global reduction in translation initiation (Fig. 2A) (29–31). Because eIF2α-P can direct translational control in response to a range of different environmental stresses, this pathway is often referred to as the integrated stress response (ISR) (32). Lowered protein synthesis would allow cells to conserve nutrients and energy and facilitate reprogramming of gene expression to alleviate stress damage. To aid in reprogramming of gene expression during eIF2α-P, a subset of mRNAs is preferentially translated via uORF-mediated mechanisms. uORFs are similarly distributed among mRNAs that are repressed, resistant, or preferentially translated during cellular stress and eIF2α-P, emphasizing that it is the specific properties of the uORFs that are critical for their regulatory capabilities in translation (Fig. 2A) (5). Furthermore, the proper mixing and matching of these uORF features are critical for uORF-mediated translational control mechanisms that appropriately regulate gene expression.
FIGURE 2.
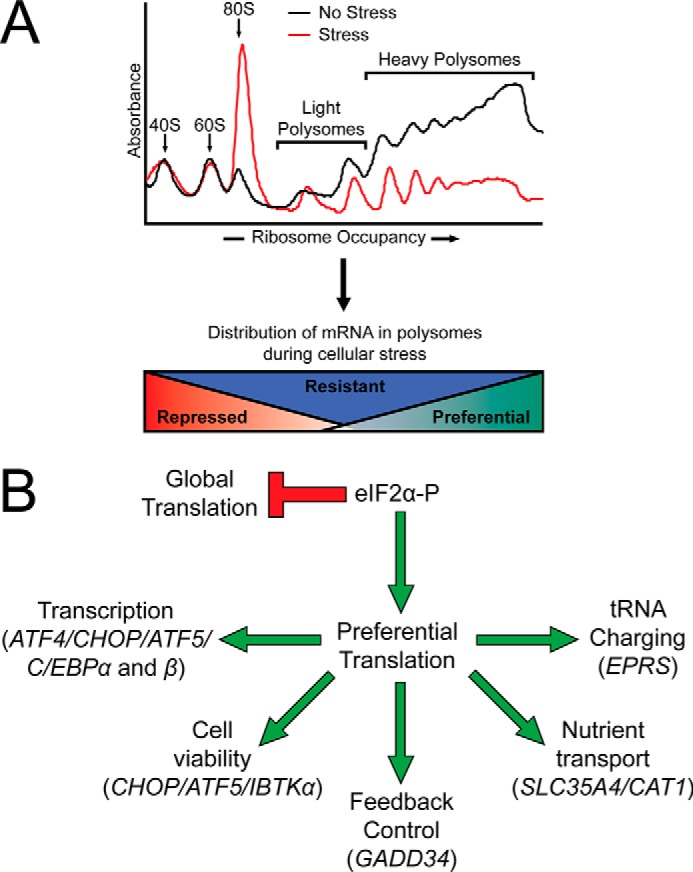
The integrated stress response features a global reduction in translation initiation concomitant with the preferential translation of stress remediation transcripts. A, depiction of polysome profiles as measured by sucrose gradient analyses of lysates prepared from mouse embryonic fibroblast cells that were left untreated (black line) or subjected to the ER stress inducer thapsigargin (red line). Basal polysome profiles feature distinctive peaks for the 40S and 60S ribosomal subunits and the 80S monosome, with large peaks observed for heavy polysomes, indicative of high levels of global translation. Polysome profiles from cells subjected to ER stress feature decreased heavy polysomes and an elevated 80S monosome peak that is indicative of inhibition of global translation initiation during eIF2α-P. Those mRNAs that are preferentially translated during cellular stress are largely associated with heavy polysomes, whereas those mRNAs that are repressed during cellular stress are largely associated with 80S monosomes and light polysomes. The mRNAs that are translated constitutively are associated with polysomes independent of stress. B, depiction of the preferentially translated mRNAs and their function in stress remediation. Multiple preferentially translated mRNAs encode transcription factors that promote stress alleviation (ATF4, C/EBPα, and C/EBPβ). If the cellular stress is too great to overcome, a subset of transcription factors promotes a pro-apoptotic signaling cascade (CHOP and ATF5). Feedback dephosphorylation occurs through the activity of the preferentially translated GADD34. Priming of the cell for resumption of global translation occurs through the activity of the preferentially translated nutrient transporters SLC35A4 and CAT1, as well as the glutamyl-prolyl tRNA synthetase EPRS. Cell fate regulator IBTKα is also preferentially translated through an uORF-mediated mechanism.
Encoded CDS products of mRNAs that are preferentially translated through uORF-mediated mechanisms play diverse roles in remediation of cellular stress (Fig. 2B). Included among the ISR preferentially translated gene transcripts are ATF4 (CREB2), CHOP (DDIT3/GADD153), ATF5, and C/EBPα and C/EBPβ that each encode basic leucine zipper transcription factors that modify gene expression programs to address cellular stress (24, 27, 33–37). GADD34 (PPP1R15A) combines with the catalytic subunit of protein phosphatase 1 (PP1c) to regulate dephosphorylation of eIF2α-P and restore protein synthesis (Fig. 2B) (18, 38–41). Nutrient transporters SLC35A4 and CAT1, as well as the bifunctional glutamyl-prolyl tRNA synthetase EPRS, serve to increase available nutrients and prime the cell for resumption of protein synthesis once cellular stress is remediated (Fig. 2B) (11, 26). Finally, the cell fate regulator IBTKα was shown to be subject to preferential translation through a mechanism involving uORFs (Fig. 2B) (5).
Ribosome Reinitiation in the ISR
The capacity for the ribosome to reinitiate translation downstream has been attributed largely to the ability of the scanning ribosome to retain or reacquire critical initiation factors following uORF translation (42). Extended distance between the uORF stop codon and CDS initiation codon allows more time for the scanning 40S to reacquire a new eIF2·GTP·Met-tRNAiMetcomplex, a significant feature of the delayed translation reinitiation model that was originally identified in the yeast Saccharomyces cerevisiae for the transcriptional activator GCN4 (19, 21, 43) and subsequently suggested for the related mammalian ATF4 (24, 33, 34).
Delayed Translation Reinitiation
Both yeast GCN4 and mammalian ATF4 encode transcription factors that increase expression of genes involved in nutrient import, metabolism, and alleviation of oxidative stress (32, 33, 44–48). The 5′-leader of ATF4 contains two uORFs: the 5′-proximal uORF1 that is three codons in length and a 59-codon-long uORF2 that overlaps out-of-frame with the ATF4 coding region (Fig. 3A) (24, 34). The GCN4 5′-leader contains four uORFs, each encoding polypeptides two to three residues in length (19, 21). In the delayed translation reinitiation model, the 5′-proximal uORF1 in the ATF4 and GCN4 5′-leaders acts as a positive element that promotes downstream translation reinitiation (19, 20, 24). During nonstressed conditions, the 40S ribosome resumes scanning after translation of uORF1 and reacquires a new eIF2·GTP·Met-tRNAiMet complex in sufficient time to reinitiate translation at the next uORF initiation codon. In the case of ATF4, translation initiation at the overlapping out-of-frame uORF2 results in translation termination and ribosome dissociation 3′ of the ATF4 CDS, thereby reducing synthesis of ATF4 protein (Fig. 3A) (24). Translation of the downstream GCN4 uORFs 3 or 4 also thwarts expression of the GCN4 coding region during nonstressed conditions (see Fig. 4C) (21, 49). The inhibitory property of uORF4 relies upon a 10-nucleotide sequence 3′ of the uORF4 stop codon that is suggested to interact with the 40S ribosomal subunit to promote ribosome dissociation (49).
FIGURE 3.
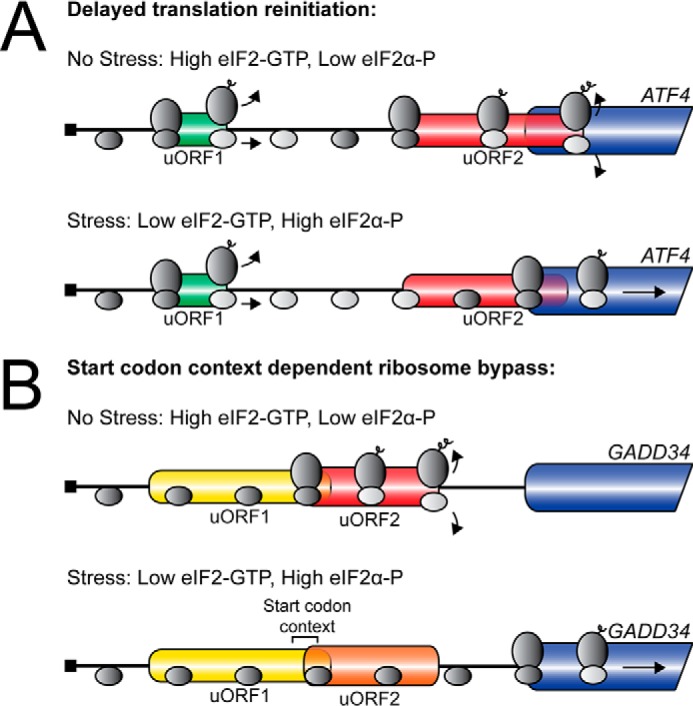
uORFs regulate mRNA translation through diverse mechanisms. A, depiction of the ATF4 mechanism of preferential translation. During nonstressed conditions, there are low levels of eIF2α-P and high levels of eIF2-GTP. Ribosomes scanning the ATF4 mRNA initiate at the 5′-proximal uORF1, and following termination, quickly reacquire a new eIF2 ternary complex. Competent 40S scanning ribosomes (dark gray oval) then reinitiate translation at uORF2, which overlaps out-of-frame with the ATF4 CDS. Translation of uORF2 results in ribosome termination and dissociation 3′ of the ATF4 initiation codon, resulting in low ATF4 expression. During cellular stress, elevated eIF2α-P results in low levels of eIF2-GTP. Ribosomes scanning the ATF4 mRNA initiate at uORF1 and post-uORF translation resume scanning. Due to the low levels of eIF2 ternary complex, the 40S ribosome (light gray oval) scans pass the initiation codon of the inhibitory uORF2 before reacquiring a new ternary complex (dark gray oval). Delayed acquisition of the eIF2 ternary complex results in translation initiation at the ATF4 CDS and an increase in ATF4 expression during cellular stress. B, depiction of the GADD34 mechanism of preferential translation. During nonstressed conditions, scanning ribosomes bypass the GADD34 uORF1 due to its poor start codon context and initiate translation at uORF2. Translation of a Pro-Pro-Gly peptide sequence juxtaposed to the uORF2 stop codon results in an inefficient ribosome termination event that increases ribosome release from the mRNA and causes low levels of basal GADD34 expression. During cellular stress, elevated eIF2α-P results in a ribosomal bypass of uORF1 due to its poor start codon context and uORF2 due to its moderate Kozak consensus sequence. Bypass of the inhibitory uORF2 by a portion of scanning ribosomes results in increased translation initiation at the GADD34 CDS and an increase in GADD34 expression.
FIGURE 4.
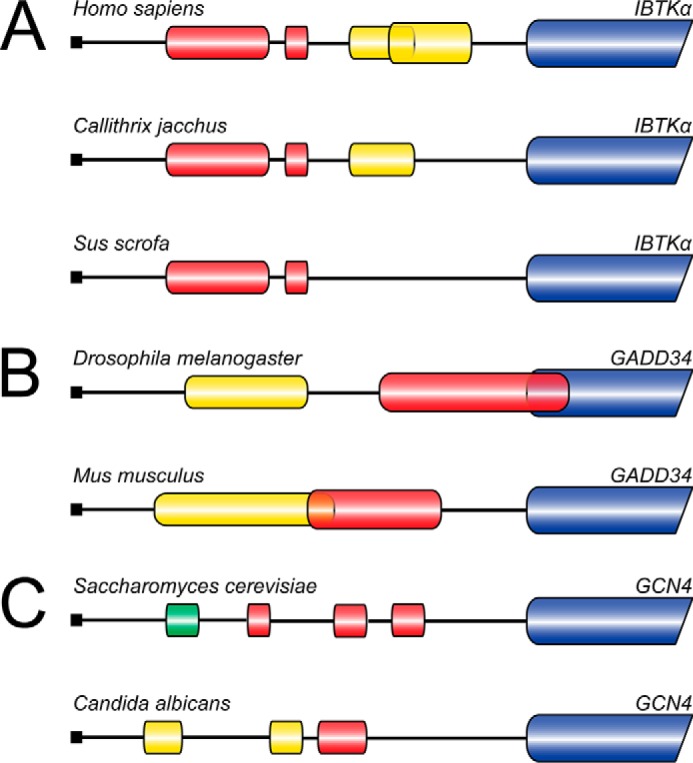
uORF mechanisms of translation control are evolutionarily conserved. A, illustration of the IBTKα 5′-leader in multiple species including: H. sapiens, Callithrix jacchus, and Sus scrofa. Translation of IBTKα mRNA is regulated by a bypass mechanism. The inhibitory uORFs 1 and 2 (red bars) repress IBTKα CDS translation during nonstressed conditions. The inhibitory effects of uORF1 and 2 are overcome during eIF2α-P, facilitating the preferential translation of IBTKα (blue bar). uORF 3 and 4 (yellow bars) are considered to be dispensable for IBTKα translation control and are not conserved between species. B, depiction of the 5′-leaders for D. melanogaster and M. musculus GADD34 mRNAs. The 5′-leader of GADD34 mRNA in both species contains a dispensable uORF1 (yellow bar) that is largely bypassed independent of cellular stress. uORF2 (red bar) in both mRNAs is translated during basal conditions and is inhibitory to downstream GADD34 CDS translation. uORF2 in D. melanogaster overlaps out-of-frame with the GADD34 CDS (blue bar) and promotes ribosome dissociation from the mRNA 3′ of the initiation codon of the GADD34 CDS. The M. musculus uORF contains an inhibitory Pro-Pro-Gly sequence juxtaposed to the uORF2 termination codon that promotes inefficient termination that increases ribosome dissociation from the mRNA. During cellular stress, the inhibitory uORF2 in either D. melanogaster or M. musculus is bypassed, resulting in increased translation initiation at the GADD34 CDS and increased GADD34 protein synthesis. Bypass of M. musculus uORF2 relies upon its moderate start codon context, whereas bypass of D. melanogaster uORF2 may rely upon additional factors. C, illustration of the 5′-leader of GCN4 in fungal species S. cerevisiae and C. albicans. Translation control of S. cerevisiae GCN4 relies on a delayed translation reinitiation model in which translation of the positive acting uORF1 (green bar) promotes translation reinitiation at downstream uORFs. Translation of the following uORFs 2, 3, and 4 (red bars) in the S. cerevisiae GCN4 5′-leader are inhibitory to downstream translation by promoting ribosome dissociation from the mRNA in nonstressed conditions. During cellular stress, low levels of eIF2 ternary complex levels allow the scanning 40S ribosome to scan through the inhibitory uORFs in GCN4 post-uORF1 translation, resulting in translation initiation at the GCN4 CDS (blue bar). C. albicans translation control relies on a bypass mechanism in which only uORF3 (red bar) is required for regulation of GCN4 expression. In nonstressed conditions, translation of uORF3 precludes the ribosome from initiating translation at the C. albicans GCN4 CDS, presumably through ribosome dissociation from the mRNA. During cellular stress, eIF2α-P promotes bypass of the inhibitory uORF3, thereby facilitating an increase in translation of the GCN4 coding region (blue bar).
During cellular stress, eIF2α-P results in lowered levels of eIF2·GTP required for delivery of Met-tRNAiMet for reinitiating ribosomes. As a consequence, after translation of uORF1, the scanning 40S ribosomal subunit takes a longer amount of time to reacquire a new eIF2 ternary complex that is required for recognition of the next translation initiation codon in the 5′-leaders of the ATF4 and GCN4 mRNAs. The delay in the acquisition of eIF2 ternary complex allows the 40S ribosomal subunit to scan through the inhibitory uORFs in the two mRNAs and instead promote translation initiation at the ATF4 or GCN4 CDS, promoting production of ATF4 and GCN4 proteins that serve to transcriptionally enhance genes important for remediation of the stress damage (Fig. 3A). Lack of appropriate GCN4 and ATF4 expression renders cells susceptible to nutrient deficiencies and oxidative damage (32, 46, 50).
Translation Reinitiation and Differential CDS Translation
Levels of eIF2α-P and the ensuing reduction of eIF2·GTP levels also play a role in start codon selection that regulates the abundance of protein isoforms encoded in the same mRNA. For example, C/EBPβ mRNA has four AUG initiation codons that encode three different protein isoforms and one uORF (37). The protein isoforms encoded in C/EBPβ are transcription factors that regulate adipogenesis, immunity, and functions of bone and liver tissues (51, 52). All three protein isoforms contain the basic leucine zipper domain, but the two larger isoforms, liver-enriched activating proteins (LAP* and LAP), also contain an N-terminal domain that activates transcription of target genes, which is missing in the smaller isoform, liver-enriched inhibitory protein (LIP) (37, 53). As a consequence, the LAP* and LAP isoforms can dimerize and serve as activators of transcription, whereas production of LIP serves as a transcriptional repressor (52).
The largest protein isoform, LAP*, is translated beginning at the most 5′-proximal AUG (37). The next AUG that follows the LAP* initiation site encodes an out-of-frame uORF. Importantly, the uORF termination codon is located 5′ of the initiation sites for both LAP and LIP and plays a central role in the regulation of translational expression of the LAP and LIP isoforms (37). After translation of the uORF, a number of 40S ribosomes are suggested to scan past the initiation codon of the LAP isoform before reacquiring a new eIF2·GTP·Met-tRNAiMetcomplex and begin translation at the initiation codon of LIP. LIP expression is also elevated after UV irradiation, suggesting that decreased eIF2 ternary complex levels during cellular stress exacerbate this phenomenon (54). The related gene C/EBPα and its encoded protein isoforms are also translationally regulated by a 5′-proximal uORF (28, 37, 55).
Ribosome Bypass in the ISR
Selection of the translation start site relies largely on the nucleotide context surrounding the start codon. The optimal sequence, termed the Kozak consensus sequence, is GCC(A/G)CCAUGG of which the most important residues are the purines in the −3 and +4 positions, and the initiation codon is underlined (9, 56). These two residues have been shown to interact with the eIF2α subunit and the 18S rRNA contained within the small ribosomal subunit and are proposed to promote recognition of the initiation codon (57). Additionally, poor uORF start codon context has been associated with those mRNAs that are preferentially translated, whereas mRNAs that are repressed during eIF2α-P typically contain an uORF in a strong Kozak consensus sequence (5). These findings suggest that start codon context plays a significant role in uORF-mediated translation regulation.
Ribosome Bypass Is Dependent on Start Codon Context
An important example in the ISR of ribosome bypass of an uORF is that of GADD34 (18, 38). GADD34 functions in feedback control of the ISR (39–41). The GADD34 mRNA contains two uORFs (Fig. 3B). The uORF1 is considered to be a constitutive, modest dampener of downstream GADD34 expression (18, 38). uORF1 is in a poor Kozak consensus sequence and is largely bypassed both basally and during stress conditions. Translation of uORF2, however, results in a significant decrease in translation initiation at the CDS, indicating that uORF2 is largely inhibitory to downstream translation (Fig. 3B) (18, 38). The inhibitory nature of GADD34 uORF2 relies in part upon a Pro-Pro-Gly sequence juxtaposed to the stop codon (18). Translation of the Pro-Pro-Gly sequence in uORF2 is suggested to result in an inefficient termination that increases ribosome release from the mRNA, promoting low levels of GADD34 expression during basal conditions (Fig. 3B). This was an interesting finding, because polyproline and Pro-Pro-Gly sequences have been shown to require the activity of an additional factor, eIF5A, for efficient translation (58).
Preferential translation of GADD34 in response to eIF2α-P and cellular stress occurs by scanning ribosome bypass of uORF2 by a process involving, at least in part, the poor start codon context of this inhibitory uORF (Fig. 3B) (18). Appropriate inhibition of GADD34 translation by uORF2 and its regulated bypass is critical for the magnitude and duration of translational control during the progression of a stress response. For example, deletion of the inhibitory GADD34 uORF2 results in an inappropriate increase in GADD34 expression even in the absence of stress that serves to sharply diminish levels of eIF2α-P even upon induction of the ISR (18). As a consequence, there are continued high levels of protein synthesis and increased cell sensitivity to cellular stress.
Ribosomal bypass of an uORF is also central for preferential translation of CHOP in the ISR (27, 35). Prolonged expression of CHOP during times of chronic stress and continued eIF2α-P can induce apoptosis (59–62). Analogous to GADD34, bypass of the inhibitory CHOP uORF during eIF2α-P is also suggested to rely at least in part upon poor start codon context of the uORF (14, 27). The inhibitory nature of the CHOP uORF during basal conditions is centered upon an elongation stall at an encoded polypeptide segment (14). Emphasizing the importance of the uORF for appropriate CHOP expression in the ISR, mutations rendering the uORF nonfunctional lead to overexpression of CHOP and its downstream pro-apoptotic target genes, enhancing cell death during stress (14).
We do not yet understand the mechanisms by which selection of translation start sites can be affected by eIF2α-P. It was suggested that eIF2α-P disrupts the stability of the interaction between the scanning ribosomal subunit and the mRNA at the −3 position of the start codon (63). Another possibility is that eIF2α-P may modify the nature of the interaction between eIF2 and the Met-tRNAiMet that regulates start site selection (63). Alternatively, the effects of eIF2α-P on start codon selection may be indirect. For example, eIF2α-P may alter the expression and stoichiometry of other initiation factors, such as eIF1, which play a role in translation start site selection (64). The recently characterized small molecule ISRIB (integrated stress response inhibitor), which renders eIF2B largely insensitive to eIF2α-P (65–67), may provide an important tool for discerning genome-wide changes in initiation codon selection afforded by eIF2α-P.
Ribosome Bypass and Non-canonical Start Codons
Recent ribosome profiling studies have illustrated that previously uncharacterized uORFs with non-canonical initiation codons are more commonly translated than previously thought (3, 4, 6). This observation has been followed by the characterization of multiple mechanisms involving bypass of uORFs due to their non-canonical initiation codons that regulate gene-specific translation during cellular stress conditions (4, 26, 68).
One such example is the translation mechanism for glutamyl-prolyl tRNA synthetase, EPRS. Although EPRS expression is required for normal protein homeostasis, preferential translation of EPRS is suggested to increase the appropriately charged tRNA pool and prime the cell for resumption of protein synthesis once the cellular stress is remediated (26, 69). Of the five non-canonical initiation codons in the 5′-leader of EPRS only the 5′-proximal UUG and the subsequent CUG are considered to be the main regulators of EPRS preferential translation (26). The uORF containing the CUG initiation codon overlaps out-of-frame with the EPRS CDS and was shown to be inhibitory to EPRS expression, presumably by ribosome dissociation from the mRNA past the initiation codon for the CDS (26). The uORF encoded by the UUG, on the other hand, terminates 51 nucleotides 5′ of the CDS start codon, and was suggested to allow for a portion of the translating ribosomes to reinitiate at the downstream EPRS CDS (26). Importantly, both inhibitory uORFs are bypassed due to their non-canonical initiation codons to a moderate extent during basal conditions and experience increased bypass efficiency during eIF2α-P and stress (26).
Another gene recently identified as containing a functional uORF with a non-canonical initiation codon is that of GADD45G, which regulates cell growth and apoptosis (4). The GADD45G mRNA contains an overlapping out-of-frame uORF with a CUG initiation codon. The uORF serves as a barrier to downstream translation during nonstressed conditions and is bypassed due to its noncanonical initiation codon during eIF2α-P (4). Also recently described is the model of translation control for BiP (GRP78/HSPA5) that participates in protein folding in the ER (68). The 5′-leader of the BiP mRNA features two uORFs that are both encoded by non-canonical initiation codons, as well as possible internal ribosome entry sequences (IRES), which may function in conjunction with uORFs to facilitate BiP translation during cellular stress (68, 70–72).
Other Complexities of Gene Expression Regulated by uORFs
The observation that the 5′-leader of BiP mRNA contains both uORFs and an IRES that coordinate expression of BiP emphasizes that other mRNA structural features also contribute to translation regulation involving uORFs. The mRNA encoding the cationic amino acid transporter CAT1 contains both an uORF and an IRES that are required for induced expression of the CAT1 CDS during cellular stress (73, 74). The 5′-leader of CAT1 forms a stable secondary structure that prevents translation during nonstressed conditions. However, translation of the uORF during eIF2α-P is suggested to unfold the RNA structure, yielding an IRES that serves to increase translation of the CAT1 CDS (75).
The mRNA encoding the HER2 proto-oncogene contains an uORF that represses translation initiation at the primary HER2 initiation codon due to insufficient time for the scanning 40S ribosomal subunit to reacquire a new eIF2 ternary complex (76, 77). Instead translation reinitiation occurs at alternative downstream initiation codons that lead to the synthesis of N-terminally truncated HER2 protein isoforms (77). However, full-length HER2 expression is enhanced in in both breast and ovarian cancers (76). This regulatory scheme is suggested to occur via interactions between RNA secondary structures in the 3′-UTR of the HER2 mRNA and the terminating ribosome at the uORF stop codon. This interaction is thought to retain the terminating ribosome and associated initiation factors on the HER2 mRNA until a new eIF2 ternary complex is acquired, thereby facilitating translation at the most immediate 5′-proximal AUG that leads to full-length HER2 protein synthesis (76).
The three major regulators of mRNA abundance, transcription, mRNA processing, and mRNA degradation, also play major roles in uORF-mediated regulation of ISR-induced preferential translation (54, 78–80). For example, ATF4 expression is potently induced during endoplasmic reticulum stress, but there are only low levels of ATF4 expression during UV irradiation (54, 81). Both conditions induce robust eIF2α-P, and preferential translation of ATF4 mRNA occurs with either stress (81). However, increased LIP expression and binding to the ATF4 promoter during UV irradiation repress ATF4 transcription (54). Lowered levels of ATF4 mRNA available for translation during UV stress thus result in negligible ATF4 protein and transcriptional activity. These findings illustrate that ATF4 mRNAs with identical 5′-leaders and uORF configurations have sharply different induction capabilities in response to different stress conditions despite having comparable levels of eIF2α-P.
Expression of the ISR transcriptional regulator ATF5 features two different mRNA isoforms (79). The more abundant transcript, ATF5α, contains two uORFs that serve to promote preferential translation by delayed translation reinitiation (36, 80). ATF5β encodes the same ATF5 CDS, but contains an alternative 5′-leader that does not contain any conserved uORFs and is not translationally regulated in a stress-dependent manner (80). Thus, expression of ATF5 can also be modulated through the differential production of mRNA isoforms (79). Recent genome-wide evidence has suggested that regulation of translation through mRNA splicing also plays a significant role in the presence of different 5′-leaders in mRNAs that can affect translational expression (78).
uORF translation can also result in activation of the mRNA decay pathways, thus adding another layer to the mechanisms in which uORFs can negatively regulate downstream translation (82). For example, CHOP was identified as a target of the nonsense-mediated mRNA decay pathway that recognizes the presence of a premature termination codon (83). Depletion of the nonsense-mediated mRNA decay machinery from cells results in the stabilization of CHOP mRNA levels (84). CHOP mRNA half-life was also increased more than 2-fold in cells in which the two initiating codons of the CHOP uORF were mutated (14). Combined, these studies suggest that the presence of an uORF can also serve to repress expression of the CDS through mechanisms involving mRNA decay.
Evolutionary Conservation of uORF-mediated Translation Mechanisms
Many uORF-mediated translation control schemes that rely on eIF2α-P are conserved in eukaryotes. For example, the uORF-mediated translational control mechanism for IBTKα is suggested to be largely conserved among mammals (Fig. 4A) (5). Interestingly, the Homo sapiens IBTKα mRNA has four uORFs, but only the two key uORFs that confer IBTKα translational control are consistently conserved among mammals (Fig. 4A) (5). This finding indicates that those uORFs that are retained throughout species likely maintain functional significance. This idea is emphasized in genome-wide analyses of uORF conservation in which the presence of an uORF and the regulatory nature of uORF(s) is suggested to be conserved throughout species (6, 7, 85–87).
An example in which the regulatory function of an uORF is retained, but the underlying mechanisms vary among species is the inhibitory uORF located in the 5′-leader of GADD34 mRNA (18, 38, 88). The 5′-leaders of the Drosophila melanogaster and Mus musculus GADD34 transcripts each contain two uORFs, with the first uORF considered to be largely dispensable for GADD34 translation control (Fig. 4B) (18, 38, 88). uORF2 in D. melanogaster overlaps out-of-frame with the GADD34 CDS and is considered to be inhibitory by promoting uORF translation termination 3′ of the start codon for the GADD34 CDS (88). By comparison, M. musculus uORF2, which terminates 23 nucleotides upstream of the GADD34 CDS, prohibits reinitiating downstream due to the inefficient translation termination (Fig. 4B) (18). Both inhibitory uORFs have been suggested to require ribosome bypass during cellular stress for preferential translation of GADD34 (18, 88).
Assessment of the GCN4 mRNAs in 12 fungal species revealed that uORFs range from three to six in number and are not positionally conserved (25, 89). Furthermore, the mechanism of GCN4 translation control for Candida albicans is reliant upon bypass of a single inhibitory uORF whereas GCN4 in S. cerevisiae relies on a mechanism involving delayed translation reinitiation that features multiple uORFs (Fig. 4C) (19, 25). These findings suggest that even among gene orthologs in different species, different uORF configurations and mechanisms can be implemented to achieve preferential translation in response to eIF2α-P.
These examples of the evolutionary conservation of uORF-mediated translation control emphasize that there are multiple features of uORFs that can be combined in specific ways to generate uORFs of similar functions in regulation of translation. Furthermore, the proper composition and position of uORFs and their features are critical for uORF-mediated translation control mechanisms that direct regulated gene expression for optimal adaptation to environmental stress.
A future challenge is the application of key tenets from uORF-mediated mechanisms described herein to genome-wide assessments of translation. The ubiquitous placement of uORFs throughout the genome indicates that the specific properties of uORFs themselves determine whether uORFs regulate gene expression in a positive or negative fashion. How can the specific features of uORFs be used to predict patterns of translation control? Furthermore, although uORFs regulate mRNA translation in a cis manner, can some uORF peptides function in trans to facilitate cellular homeostasis? Finally, base modifications in RNA, such as N6-methyladenosine, can occur differentially in the 5′-leaders of mRNAs in response to cellular stress (90, 91). Additionally, N6-methyladenosine can promote translation of select mRNAs by binding to eIF3 and recruiting ribosomes independent of the 5′-cap. Can RNA base modifications in specific mRNAs alter ribosome scanning and uORF utilization affecting translational control in the ISR?
This work was supported by National Institutes of Health Grant GM049164 (to R. C. W.) and the Ralph W. and Grace M. Showalter Research Trust Fund. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- CDS
- coding sequence
- uORF
- upstream open reading frame
- ISR
- integrated stress response
- LAP
- liver-enriched activating protein
- LIP
- liver-enriched inhibitory protein
- IRES
- internal ribosome entry sequence
- ER
- endoplasmic reticulum.
References
- 1. Schwanhäusser B., Gossen M., Dittmar G., and Selbach M. (2009) Global analysis of cellular protein translation by pulsed SILAC. Proteomics 9, 205–209 [DOI] [PubMed] [Google Scholar]
- 2. Aviner R., Geiger T., and Elroy-Stein O. (2013) Novel proteomic approach (PUNCH-P) reveals cell cycle-specific fluctuations in mRNA translation. Genes Dev. 27, 1834–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ingolia N. T., Ghaemmaghami S., Newman J. R., and Weissman J. S. (2009) Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324, 218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gao X., Wan J., Liu B., Ma M., Shen B., and Qian S. B. (2015) Quantitative profiling of initiating ribosomes in vivo. Nat. Methods 12, 147–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baird T. D., Palam L. R., Fusakio M. E., Willy J. A., Davis C. M., McClintick J. N., Anthony T. G., and Wek R. C. (2014) Selective mRNA translation during eIF2 phosphorylation induces expression of IBTKα. Mol. Biol. Cell 25, 1686–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fritsch C., Herrmann A., Nothnagel M., Szafranski K., Huse K., Schumann F., Schreiber S., Platzer M., Krawczak M., Hampe J., and Brosch M. (2012) Genome-wide search for novel human uORFs and N-terminal protein extensions using ribosomal footprinting. Genome Res. 22, 2208–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iacono M., Mignone F., and Pesole G. (2005) uAUG and uORFs in human and rodent 5′untranslated mRNAs. Gene 349, 97–105 [DOI] [PubMed] [Google Scholar]
- 8. Calvo S. E., Pagliarini D. J., and Mootha V. K. (2009) Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc. Natl. Acad. Sci. U.S.A. 106, 7507–7512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kozak M. (1989) The scanning model for translation: an update. J. Cell Biol. 108, 229–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jackson R. J., Hellen C. U., and Pestova T. V. (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andreev D. E., O'Connor P. B., Fahey C., Kenny E. M., Terenin I. M., Dmitriev S. E., Cormican P., Morris D. W., Shatsky I. N., and Baranov P. V. (2015) Translation of 5′ leaders is pervasive in genes resistant to eIF2 repression. Elife 4, e03971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kozak M. (2001) Constraints on reinitiation of translation in mammals. Nucleic Acids Res. 29, 5226–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szamecz B., Rutkai E., Cuchalová L., Munzarová V., Herrmannová A., Nielsen K. H., Burela L., Hinnebusch A. G., and Valásek L. (2008) eIF3a cooperates with sequences 5′ of uORF1 to promote resumption of scanning by post-termination ribosomes for reinitiation on GCN4 mRNA. Genes Dev. 22, 2414–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Young S. K., Palam L. R., Wu C., Sachs M. S., and Wek R. C. (2016) Ribosome elongation stall directs gene-specific translation in the integrated stress response. J. Biol. Chem. 291, 6546–6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Law G. L., Raney A., Heusner C., and Morris D. R. (2001) Polyamine regulation of ribosome pausing at the upstream open reading frame of S-adenosylmethionine decarboxylase. J. Biol. Chem. 276, 38036–38043 [DOI] [PubMed] [Google Scholar]
- 16. Col B., Oltean S., and Banerjee R. (2007) Translational regulation of human methionine synthase by upstream open reading frames. Biochim. Biophys. Acta 1769, 532–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei J., Wu C., and Sachs M. S. (2012) The arginine attenuator peptide interferes with the ribosome peptidyl transferase center. Mol. Cell. Biol. 32, 2396–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Young S. K., Willy J. A., Wu C., Sachs M. S., and Wek R. C. (2015) Ribosome reinitiation directs gene-specific translation and regulates the integrated stress response. J. Biol. Chem. 290, 28257–28271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hinnebusch A. G. (2005) Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 59, 407–450 [DOI] [PubMed] [Google Scholar]
- 20. Grant C. M., and Hinnebusch A. G. (1994) Effect of sequence context at stop codons on efficiency of reinitiation in GCN4 translational control. Mol. Cell. Biol. 14, 606–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abastado J. P., Miller P. F., Jackson B. M., and Hinnebusch A. G. (1991) Suppression of ribosomal reinitiation at upstream open reading frames in amino acid-starved cells forms the basis of GCN4 translational control. Mol. Cell. Biol. 11, 486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pöyry T. A., Kaminski A., Connell E. J., Fraser C. S., and Jackson R. J. (2007) The mechanism of an exceptional case of reinitiation after translation of a long ORF reveals why such events do not generally occur in mammalian mRNA translation. Genes Dev. 21, 3149–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zinoviev A., Hellen C. U., and Pestova T. V. (2015) Multiple mechanisms of reinitiation on bicistronic calicivirus mRNAs. Mol. Cell 57, 1059–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vattem K. M., and Wek R. C. (2004) Reinitiation involving upstream open reading frames regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 101, 11269–11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sundaram A., and Grant C. M. (2014) A single inhibitory upstream open reading frame (uORF) is sufficient to regulate Candida albicans GCN4 translation in response to amino acid starvation conditions. RNA 20, 559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Young S. K., Baird T. D., and Wek R. C. (2016) Translation regulation of the glutamyl-prolyl-tRNA synthetase gene EPRS through bypass of upstream open reading frames with noncanonical initiation codons. J. Biol. Chem. 291, 10824–10835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palam L. R., Baird T. D., and Wek R. C. (2011) Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J. Biol. Chem. 286, 10939–10949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calkhoven C. F., Bouwman P. R., Snippe L., and Ab G. (1994) Translation start site multiplicity of the CCAAT/enhancer binding protein α mRNA is dictated by a small 5′ open reading frame. Nucleic Acids Res. 22, 5540–5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rowlands A. G., Panniers R., and Henshaw E. C. (1988) The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation factor 2. J. Biol. Chem. 263, 5526–5533 [PubMed] [Google Scholar]
- 30. Jennings M. D., and Pavitt G. D. (2014) A new function and complexity for protein translation initiation factor eIF2B. Cell Cycle 13, 2660–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jennings M. D., Zhou Y., Mohammad-Qureshi S. S., Bennett D., and Pavitt G. D. (2013) eIF2B promotes eIF5 dissociation from eIF2·GDP to facilitate guanine nucleotide exchange for translation initiation. Genes Dev. 27, 2696–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harding H. P., Zhang Y., Zeng H., Novoa I., Lu P. D., Calfon M., Sadri N., Yun C., Popko B., Paules R., Stojdl D. F., Bell J. C., Hettmann T., Leiden J. M., and Ron D. (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 [DOI] [PubMed] [Google Scholar]
- 33. Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., and Ron D. (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 [DOI] [PubMed] [Google Scholar]
- 34. Lu P. D., Harding H. P., and Ron D. (2004) Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167, 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jousse C., Bruhat A., Carraro V., Urano F., Ferrara M., Ron D., and Fafournoux P. (2001) Inhibition of CHOP translation by a peptide encoded by an open reading frame localized in the chop 5′UTR. Nucleic Acids Res. 29, 4341–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou D., Palam L. R., Jiang L., Narasimhan J., Staschke K. A., and Wek R. C. (2008) Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J. Biol. Chem. 283, 7064–7073 [DOI] [PubMed] [Google Scholar]
- 37. Calkhoven C. F., Müller C., and Leutz A. (2000) Translational control of C/EBPα and C/EBPβ isoform expression. Genes Dev. 14, 1920–1932 [PMC free article] [PubMed] [Google Scholar]
- 38. Lee Y. Y., Cevallos R. C., and Jan E. (2009) An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2α phosphorylation. J. Biol. Chem. 284, 6661–6673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harding H. P., Zhang Y., Scheuner D., Chen J. J., Kaufman R. J., and Ron D. (2009) Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 α (eIF2α) dephosphorylation in mammalian development. Proc. Natl. Acad. Sci. U.S.A. 106, 1832–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Novoa I., Zeng H., Harding H. P., and Ron D. (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153, 1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Connor J. H., Weiser D. C., Li S., Hallenbeck J. M., and Shenolikar S. (2001) Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol. Cell. Biol. 21, 6841–6850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kozak M. (1987) Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol. Cell. Biol. 7, 3438–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mueller P. P., and Hinnebusch A. G. (1986) Multiple upstream AUG codons mediate translational control of GCN4. Cell 45, 201–207 [DOI] [PubMed] [Google Scholar]
- 44. Jiang H. Y., Wek S. A., McGrath B. C., Lu D., Hai T., Harding H. P., Wang X., Ron D., Cavener D. R., and Wek R. C. (2004) Activating transcription factor 3 (ATF3) is integral to the eIF2 kinase stress response. Mol. Cell. Biol. 24, 1365–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mascarenhas C., Edwards-Ingram L. C., Zeef L., Shenton D., Ashe M. P., and Grant C. M. (2008) Gcn4 is required for the response to peroxide stress in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 19, 2995–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Natarajan K., Meyer M. R., Jackson B. M., Slade D., Roberts C., Hinnebusch A. G., and Marton M. J. (2001) Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol. Cell. Biol. 21, 4347–4368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hinnebusch A. G., and Natarajan K. (2002) Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryot. Cell 1, 22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Staschke K. A., Dey S., Zaborske J. M., Palam L. R., McClintick J. N., Pan T., Edenberg H. J., and Wek R. C. (2010) Integration of general amino acid control and target of rapamycin (TOR) regulatory pathways in nitrogen assimilation in yeast. J. Biol. Chem. 285, 16893–16911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller P. F., and Hinnebusch A. G. (1989) Sequences that surround the stop codons of upstream open reading frames in GCN4 mRNA determine their distinct functions in translational control. Genes Dev. 3, 1217–1225 [DOI] [PubMed] [Google Scholar]
- 50. Fusakio M. E., Willy J. A., Wang Y., Mirek E. T., Al Baghdadi R. J., Adams C. M., Anthony T. G., and Wek R. C. (2016) Transcription factor ATF4 directs basal and stress-induced gene expression in the unfolded protein response and cholesterol metabolism in the liver. Mol. Biol. Cell 27, 1536–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tsukada J., Yoshida Y., Kominato Y., and Auron P. E. (2011) The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine 54, 6–19 [DOI] [PubMed] [Google Scholar]
- 52. Nerlov C. (2007) The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 17, 318–324 [DOI] [PubMed] [Google Scholar]
- 53. Wethmar K., Bégay V., Smink J. J., Zaragoza K., Wiesenthal V., Dörken B., Calkhoven C. F., and Leutz A. (2010) C/EBPβΔuORF mice: a genetic model for uORF-mediated translational control in mammals. Genes Dev. 24, 15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dey S., Savant S., Teske B. F., Hatzoglou M., Calkhoven C. F., and Wek R. C. (2012) Transcriptional repression of ATF4 gene by CCAAT/enhancer-binding protein β (C/EBPβ) differentially regulates integrated stress response. J. Biol. Chem. 287, 21936–21949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang C., Chen X., Wang Y., Gong J., and Hu G. (2007) C/EBPαp30 plays transcriptional regulatory roles distinct from C/EBPαp42. Cell Res. 17, 374–383 [DOI] [PubMed] [Google Scholar]
- 56. Kozak M. (1986) Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 44, 283–292 [DOI] [PubMed] [Google Scholar]
- 57. Pisarev A. V., Kolupaeva V. G., Pisareva V. P., Merrick W. C., Hellen C. U., and Pestova T. V. (2006) Specific functional interactions of nucleotides at key −3 and +4 positions flanking the initiation codon with components of the mammalian 48S translation initiation complex. Genes Dev. 20, 624–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gutierrez E., Shin B. S., Woolstenhulme C. J., Kim J. R., Saini P., Buskirk A. R., and Dever T. E. (2013) eIF5A promotes translation of polyproline motifs. Mol. Cell 51, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zinszner H., Kuroda M., Wang X., Batchvarova N., Lightfoot R. T., Remotti H., Stevens J. L., and Ron D. (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rutkowski D. T., Arnold S. M., Miller C. N., Wu J., Li J., Gunnison K. M., Mori K., Sadighi Akha A. A., Raden D., and Kaufman R. J. (2006) Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 4, e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marciniak S. J., Yun C. Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H. P., and Ron D. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Teske B. F., Fusakio M. E., Zhou D., Shan J., McClintick J. N., Kilberg M. S., and Wek R. C. (2013) CHOP induces activating transcription factor 5 (ATF5) to trigger apoptosis in response to perturbations in protein homeostasis. Mol. Biol. Cell 24, 2477–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hussain T., Llácer J. L., Fernández I. S., Munoz A., Martin-Marcos P., Savva C. G., Lorsch J. R., Hinnebusch A. G., and Ramakrishnan V. (2014) Structural changes enable start codon recognition by the eukaryotic translation initiation complex. Cell 159, 597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pestova T. V., and Kolupaeva V. G. (2002) The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev. 16, 2906–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sidrauski C., Acosta-Alvear D., Khoutorsky A., Vedantham P., Hearn B. R., Li H., Gamache K., Gallagher C. M., Ang K. K., Wilson C., Okreglak V., Ashkenazi A., Hann B., Nader K., Arkin M. R., et al. (2013) Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife 2, e00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sidrauski C., McGeachy A. M., Ingolia N. T., and Walter P. (2015) The small molecule ISRIB reverses the effects of eIF2α phosphorylation on translation and stress granule assembly. Elife 4, 10.7554/eLife.05033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sekine Y., Zyryanova A., Crespillo-Casado A., Fischer P. M., Harding H. P., and Ron D. (2015) Mutations in a translation initiation factor identify the target of a memory-enhancing compound. Science 348, 1027–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Starck S. R., Tsai J. C., Chen K., Shodiya M., Wang L., Yahiro K., Martins-Green M., Shastri N., and Walter P. (2016) Translation from the 5′ untranslated region shapes the integrated stress response. Science 351, aad3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ibba M., and Soll D. (2000) Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 69, 617–650 [DOI] [PubMed] [Google Scholar]
- 70. Cho S., Park S. M., Kim T. D., Kim J. H., Kim K. T., and Jang S. K. (2007) BiP internal ribosomal entry site activity is controlled by heat-induced interaction of NSAP1. Mol. Cell. Biol. 27, 368–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Johannes G., and Sarnow P. (1998) Cap-independent polysomal association of natural mRNAs encoding c-myc, PiP, and eIF-4G conferred by internal ribosome entry sites. RNA 4, 1500–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jackson R. J. (2013) The current status of vertebrate cellular mRNA IRESs. Cold Spring Harb. Perspect. Biol. 5, a011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fernandez J., Yaman I., Huang C., Liu H., Lopez A. B., Komar A. A., Caprara M. G., Merrick W. C., Snider M. D., Kaufman R. J., Lamers W. H., and Hatzoglou M. (2005) Ribosome stalling regulates IRES-mediated translation in eukaryotes, a parallel to prokaryotic attenuation. Mol. Cell 17, 405–416 [DOI] [PubMed] [Google Scholar]
- 74. Fernandez J., Yaman I., Sarnow P., Snider M. D., and Hatzoglou M. (2002) Regulation of internal ribosomal entry site-mediated translation by phosphorylation of the translation initiation factor eIF2α. J. Biol. Chem. 277, 19198–19205 [DOI] [PubMed] [Google Scholar]
- 75. Yaman I., Fernandez J., Liu H., Caprara M., Komar A. A., Koromilas A. E., Zhou L., Snider M. D., Scheuner D., Kaufman R. J., and Hatzoglou M. (2003) The zipper model of translational control: a small upstream ORF is the switch that controls structural remodeling of an mRNA leader. Cell 113, 519–531 [DOI] [PubMed] [Google Scholar]
- 76. Mehta A., Trotta C. R., and Peltz S. W. (2006) Derepression of the Her-2 uORF is mediated by a novel post-transcriptional control mechanism in cancer cells. Genes Dev. 20, 939–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Spevak C. C., Park E. H., Geballe A. P., Pelletier J., and Sachs M. S. (2006) her-2 upstream open reading frame effects on the use of downstream initiation codons. Biochem. Biophys. Res. Commun. 350, 834–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Floor S. N., and Doudna J. A. (2016) Tunable protein synthesis by transcript isoforms in human cells. Elife 5, e10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hansen M. B., Mitchelmore C., Kjaerulff K. M., Rasmussen T. E., Pedersen K. M., and Jensen N. A. (2002) Mouse ATF5: molecular cloning of two novel mRNAs, genomic organization, and odorant sensory neuron localization. Genomics 80, 344–350 [DOI] [PubMed] [Google Scholar]
- 80. Watatani Y., Ichikawa K., Nakanishi N., Fujimoto M., Takeda H., Kimura N., Hirose H., Takahashi S., and Takahashi Y. (2008) Stress-induced translation of ATF5 mRNA is regulated by the 5′-untranslated region. J. Biol. Chem. 283, 2543–2553 [DOI] [PubMed] [Google Scholar]
- 81. Dey S., Baird T. D., Zhou D., Palam L. R., Spandau D. F., and Wek R. C. (2010) Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J. Biol. Chem. 285, 33165–33174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gardner B. M., Pincus D., Gotthardt K., Gallagher C. M., and Walter P. (2013) Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 5, a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mendell J. T., Sharifi N. A., Meyers J. L., Martinez-Murillo F., and Dietz H. C. (2004) Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat. Genet. 36, 1073–1078 [DOI] [PubMed] [Google Scholar]
- 84. Gardner L. B. (2008) Hypoxic inhibition of nonsense-mediated RNA decay regulates gene expression and the integrated stress response. Mol. Cell. Biol. 28, 3729–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Neafsey D. E., and Galagan J. E. (2007) Dual modes of natural selection on upstream open reading frames. Mol. Biol. Evol. 24, 1744–1751 [DOI] [PubMed] [Google Scholar]
- 86. Bazzini A. A., Johnstone T. G., Christiano R., Mackowiak S. D., Obermayer B., Fleming E. S., Vejnar C. E., Lee M. T., Rajewsky N., Walther T. C., and Giraldez A. J. (2014) Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. EMBO J. 33, 981–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Johnstone T. G., Bazzini A. A., and Giraldez A. J. (2016) Upstream ORFs are prevalent translational repressors in vertebrates. EMBO J. 35, 706–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Malzer E., Szajewska-Skuta M., Dalton L. E., Thomas S. E., Hu N., Skaer H., Lomas D. A., Crowther D. C., and Marciniak S. J. (2013) Coordinate regulation of eIF2α phosphorylation by PPP1R15 and GCN2 is required during Drosophila development. J. Cell Sci. 126, 1406–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cvijović M., Dalevi D., Bilsland E., Kemp G. J., and Sunnerhagen P. (2007) Identification of putative regulatory upstream ORFs in the yeast genome using heuristics and evolutionary conservation. BMC Bioinformatics 8, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Meyer K. D., Patil D. P., Zhou J., Zinoviev A., Skabkin M. A., Elemento O., Pestova T. V., Qian S. B., and Jaffrey S. R. (2015) 5′ UTR m6A promotes cap-independent translation. Cell 163, 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhou J., Wan J., Gao X., Zhang X., Jaffrey S. R., and Qian S. B. (2015) Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594 [DOI] [PMC free article] [PubMed] [Google Scholar]


