Abstract
Streptococcus suis serotype 2 (S. suis 2)-induced sepsis and meningitis are often accompanied by bacteremia. The evasion of polymorphonuclear leukocyte-mediated phagocytic clearance is central to the establishment of bacteremia caused by S. suis 2 and is facilitated by the ability of factor H (FH)-binding protein (Fhb) to bind FH on the bacterial surface, thereby impeding alternative pathway complement activation and phagocytic clearance. Here, C3b/C3d was found to bind to Fhb, along with FH, forming a large immune complex. The formation of this immune complex was mediated by domain II of Fhb via electrostatic and hydrophobic interactions, which, to our knowledge, is a new type of interaction. Interestingly, Fhb was found to be associated with the cell envelope and also present in the culture supernatant, where secreted Fhb inhibited complement activation via interactions with domain II, thereby enhancing antiphagocytic clearance by polymorphonuclear leukocytes. Thus, Fhb is a multifunctional bacterial protein, which binds host complement component C3 as well as FH and interferes with innate immune recognition in a secret protein manner. S. suis 2 therefore appears to have developed a new strategy to combat host innate immunity and enhance survival in host blood.
Keywords: complement, host defense, infection, secretion, Streptococcus, Streptococcus suis, Fhb, Factor H;complement 3, immune evasion, streptococcus, streptococcus suis
Introduction
Streptococcus suis serotype 2 (S. suis 2)3 infection is one of the major causes of septicemia and meningitis in humans and pigs (1, 2). Severe sepsis can lead to streptococcal toxic shock syndrome, which has a high incidence of morbidity and mortality, despite antibiotic therapy (3). The septicemia and meningitis caused by S. suis 2 are often accompanied by bacteremia (4–6). To cause bacteremia, bacterial pathogens must survive in human blood and disseminate while evading polymorphonuclear leukocyte (PMN)-mediated phagocytosis. However, little is known of how this is achieved by S. suis 2.
To survive in human blood, bacteria have to evade host defense mechanisms, including complement- and PMN-mediated phagocytosis. The alternative pathway of complement is important in fast innate recognition, opsonization, and elimination of pathogens. Activation and amplification of the alternative pathway is dependent on spontaneous C3b, which deposits on bacterial surfaces and augments phagocytosis (7). To avoid complement attack of self-structures, the alternative pathway has to be kept under strict regulation by factor H (FH) to avoid excess complement activation (8, 9). A variety of pathogenic microbes use different mechanisms to avoid complement attack. For example, increasing numbers of microbes have been shown to recruit host FH to their surface to inhibit complement activation and protect themselves against complement attack (10). The FH-binding proteins (Fhbs) in Gram-positive bacteria include PspC of Streptococcus pneumonia (11), M-protein and Scl of Streptococcus pyogenes (12, 13), streptococcal β protein of Streptococcus agalactiae (14), and SdrE and Sbi of Streptococcus aureus (15, 16). In another example, some pathogens that cause invasive disease exploit the central role of C3b in all complement activation pathways by triggering futile consumption of C3b by binding it in host blood. This is demonstrated by the Sbi protein of S. aureus, which forms a ternary complex with FH and C3b, acting as a potent inhibitor of the alternative pathway (16). Also in S. aureus, secretion of C3b-binding proteins (Ecb and Efb) can result in the blocking of C3 convertases of the alternative pathway, which enhances complement evasion (17, 18). In our previous study (19), FH binding to the surface of S. suis 2 was reported to involve Fhb, a surface protein with a proline-rich repeat domain and LPXTG motif. Moreover, activation of human complement by S. suis mainly via the alternative pathway and FH bound to the Fhb protein inhibits complement activation in human blood (19).
Interestingly, in the present study, Fhb was frequently found to bind to both C3b/C3d and FH directly, forming a ternary Fhb-C3-FH complex. This interaction was different from that in S. aureus, in which C3b/C3d-mediated FH binding to Sbi and C3 influenced the stability of the Sbi-C3-FH complex (16). Because Fhb displayed low similarity to other function-known proteins, the binding domain of Fhb was determined in this study. We found that the formation of immune complexes was mediated by domain II of Fhb and seemed to depend on both electrostatic and hydrophobic interactions; to our knowledge, this is a new type of interaction. Strikingly, Fhb was also present in the culture supernatant, where secreted Fhb could inhibit complement activation via domain II, thereby enhancing antiphagocytic clearance by PMNs. Thus, the functional interaction between human C3/FH and the Fhb protein reported here represents an additional mechanism used by S. suis 2 to escape PMN-mediated innate immunity in human blood.
Results
Interactions between Fhb and FH/C3b/C3d Were Determined by GST Pull-down Assays
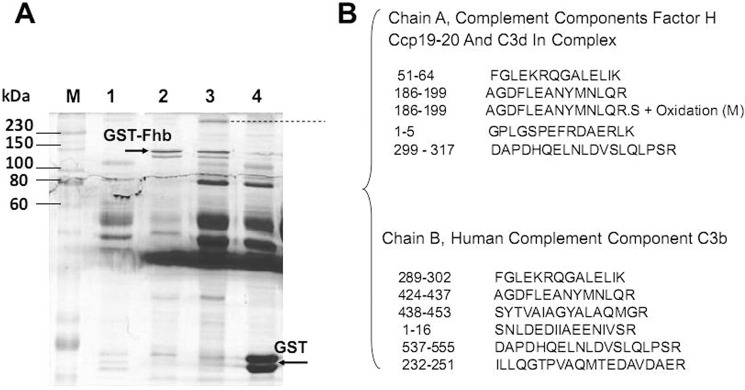
To identify the additional serum factor that interacts with Fhb (as well as FH), a GST pull-down assay was performed to capture human proteins by bait protein GST-Fhb. SDS-PAGE analysis of the serum proteins contained in the samples captured by GST-Fhb is shown in Fig. 1A. Some serum proteins interacted with GST-Fhb (lane 3) but did not interact with GST (lane 4). Based on the SDS-PAGE profile, the origin of the peptides in the gel fractions was identified by LC-MS (Fig. 1B), a large molecular mass protein, labeled with a dotted line in Fig. 1A (that appeared in four independent experiments) was identified as complement components FH, Ccp19-20, and C3d in complex and the human complement component C3b. It appeared that GST-Fhb might interact with FH, C3b, and C3d (a cleavage product of C3b) to form an immune complex.
FIGURE 1.
Interaction between FH/C3b/C3d and Fhb determined by GST pull-down assays. A, in vitro GST pull-down assays for interactions between purified GST-Fhb fusion proteins and 5% human serum. SDS-PAGE analysis of the human serum protein component eluted from glutathione-Sepharose 4B beads with immobilized recombinant GST-Fhb as bait protein. M, marker; lane 1, human serum alone with beads; lane 2, GST-Fhb alone with beads; lane 3, the binding of serum proteins by recombinant GST-Fhb as bait protein; lane 4, the binding of serum proteins by GST as a negative control. B, specific pull-down proteins were identified by LC-MS as FH, C3b, and C3d.
Epitope Expression of Recombinant Fhb-associated Proteins
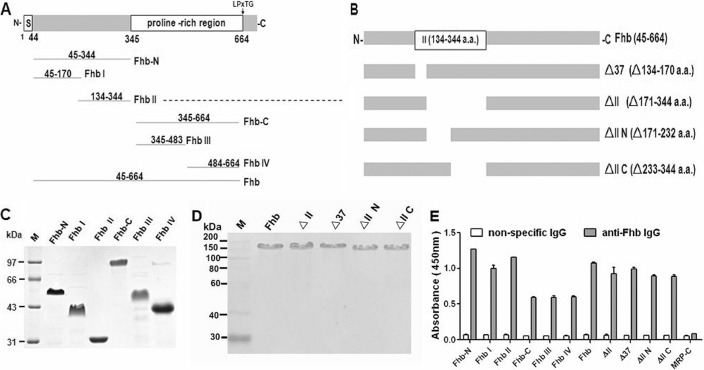
It is possible that the deletion of domain II has an impact on the expression of Fhb II deletion-associated proteins. Thus, epitope expressions of Fhb II deletion proteins, as well as Fhb truncates, were identified by ELISA. The anti-Fhb IgG was analyzed for reactivity with these Fhb-associated proteins. In this analysis, Fhb II deletion proteins exhibited high reactivity as Fhb and Fhb truncates, whereas the irrelevant control protein MRP-C (C terminus of MRP) did not react with the anti-Fhb IgG (Fig. 2E). Neither Fhb truncates nor Fhb II deletion proteins reacted with nonspecific IgG (as a negative control of anti-Fhb IgG). The rabbit anti-Fhb IgG used for ELISA had been used to identify Fhb expression on the bacterial surface in the present study (see Fig. 5B). These data indicated that Fhb II deletion proteins expressed with proper epitope as Fhb and Fhb truncates.
FIGURE 2.
Recombinant Fhb, truncated Fhb, and Fhb II deletion mutants. A and B, schematic representation of the Fhb constructs used in these experiments. The Fhb-N, Fhb I, Fhb II, Fhb-C, Fhb III, and Fhb IV constructs were engineered based on the predicted secondary structure. C, recombinant truncated Fhb. D, Fhb II deletion constructs expressed in E. coli were purified by nickel-chelating chromatography, separated by SDS-PAGE, and identified by Coomassie staining. M, molecular weight marker. E, epitope expression of Fhb truncates and Fhb II deletion proteins. The anti-Fhb IgG (purified from immune serum) was analyzed for reactivity with Fhb truncates and Fhb II deletion proteins, as indicated. The Fhb truncates and Fhb II deletion proteins exhibited high reactivity, whereas the irrelevant control protein MRP-C (C terminus of MRP) did not react with the anti-Fhb IgG. The nonspecific IgG (purified from preimmune serum) was used as a background control. Error bars, S.D.
FIGURE 5.
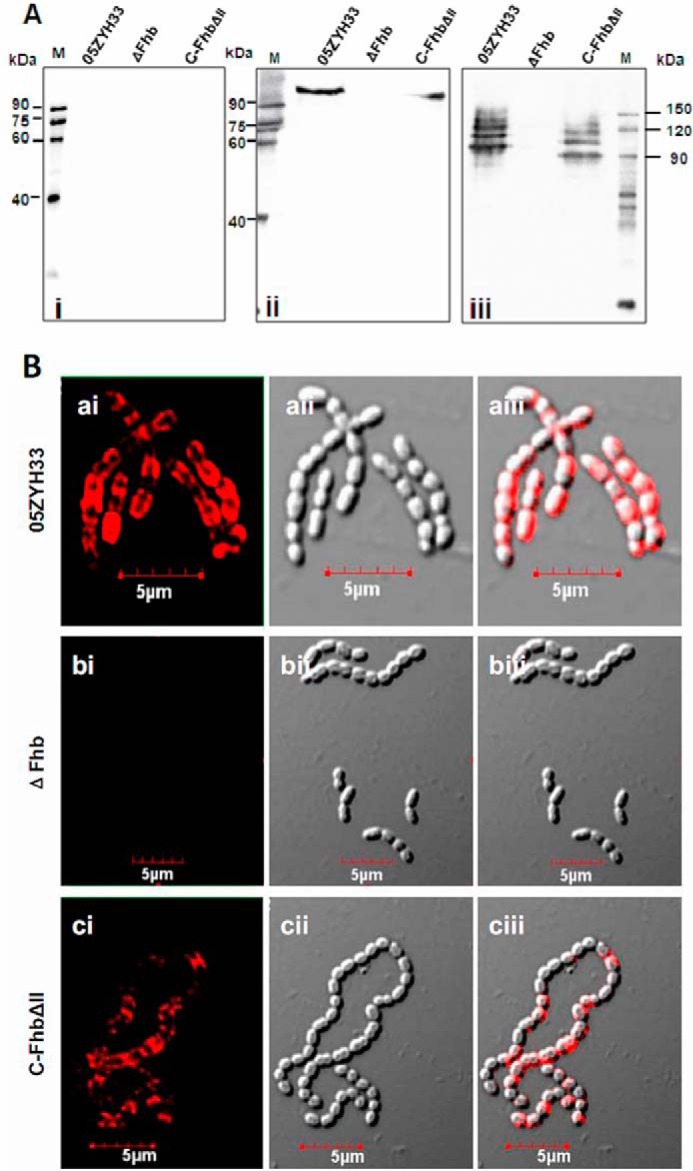
Presence of Fhb in the culture supernatant and on the bacterial surface. A, cell-bound Fhb and released Fhb were detected by Western blotting. Samples of concentrated culture supernatant (25:1, A600 = 1.0) and cell wall proteins (mutanolysin extracts, A600 = 1.0) were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were probed with rabbit anti-Fhb sera (1:400) followed by HRP-conjugated goat anti-rabbit IgG (1:8000). Fhb could be specifically detected in both the supernatant (ii) and cell wall proteins (iii) of the WT and C-FhbΔII strains but not those of the mutant ΔFhb strain. Fhb was not detected in the supernatant from any of the strains following treatment with preimmune sera. B, visualizing Fhb protein on the bacterial surface. Cells from S. suis WT strain 05ZYH33 (a), mutant strain ΔFhb (b), and complementary strain C-FhbΔII (c) were washed and stained for Fhb using rabbit anti-Fhb IgG and TRITC-conjugated goat anti-rabbit IgG. For each sample, merging of the two images is shown in aiii, biii, and ciii.
The FH-binding Site Is Located in Domain II of the Fhb Protein
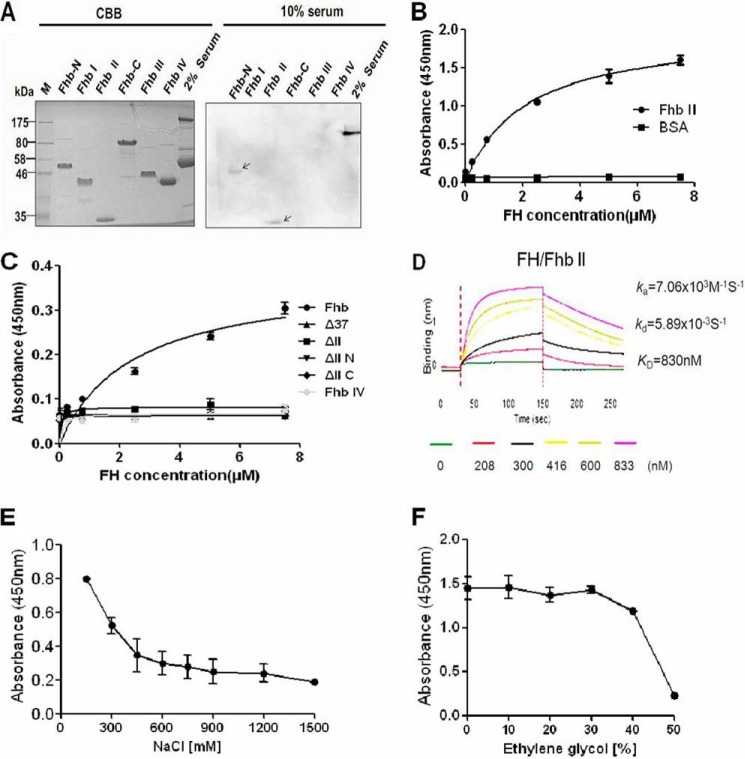
Purified FH bound to domains III and IV of Sbi mediated by C3b/C3d and formed a ternary Sbi-C3-FH complex (16). Through GST pull-down assays (Fig. 1), we found that Fhb also interacted with both FH and host C3b/C3d, but the molecular mechanisms of these interactions remained unclear. We expected similar behavior to be observed for the Fhb-C3-FH complex with Sbi. Fhb-associated proteins were engineered according to the predicted secondary structure as follows: Fhb (amino acids 45–664); Fhb-N (amino acids 45–344) covering Fhb I (amino acids 45–170, α-coil-rich region) and Fhb II (amino acids 134–344, mainly formed by β-sheet); Fhb-C (amino acids 345–664, proline-rich region) covering Fhb III (amino acids 345–483) and Fhb IV (amino acids 484–664), as shown in Fig. 2A. Recombinant Fhb-associated proteins were purified using immobilized metal chelate affinity chromatography (Fig. 2C) and were identified with proper epitope expression (Fig. 2E). First, to determine which domain of Fhb interacts with FH, ligand blotting analysis was performed. Recombinant Fhb-associated proteins were subjected to SDS-PAGE and then transferred to a PVDF membrane, followed by incubation with 10% human serum. As shown in Fig. 3A, only Fhb II and Fhb-N (N terminus of Fhb that includes domain II) reacted with FH in serum. Positive signals were not detected in the ligand-negative control, in which TBST was used instead of 10% human serum, or the primary antibody negative control, in which TBST was used instead of goat anti-human FH polyclonal antibody (data not shown). Second, this result was verified by an ELISA-type binding assay. Different from Sbi, the direct interaction between immobilized Fhb II (1 μg/well) and FH was determined, which increased with the FH protein concentration (Fig. 3B). To further confirm the above results, we constructed four Fhb proteins with a short deletion in Fhb II (amino acids 134–344), the N terminus of Fhb II (amino acids 134–232), the C terminus of Fhb II (amino acids 233–344), and 37 amino acids (amino acids 134–170) that overlap with Fhb I (Fig. 2, B and D). Pure preparations of intact Fhb and its domain deletion proteins (6.5 μg/well) were immobilized in microtiter wells and analyzed for their ability to bind FH. None of the four deletion proteins bound FH, supporting the conclusion that FH binds solely to the II region of Fhb (Fig. 3C). The binding constants of Fhb II were further evaluated by bio-layer interferometry (BLI). The KD value for the binding of Fhb II to FH was 830 nm (Fig. 3D). KD values for other FH/microbially produced Fhb interactions have been also reported to be in the nanomolar range (20, 21).
FIGURE 3.
Fhb II (amino acids 134–344) binds to FH. A, ligand blotting analysis in which 20 μg of truncated Fhb constructs were run on SDS-polyacrylamide gels and stained with Coomassie Brilliant Blue (CBB), transferred to PVDF membranes, and probed with 10% serum. Bound FH was detected using goat anti-human FH IgG (1:5000) followed by HRP-conjugated anti-goat IgG (1:20,000). M, molecular mass of protein standards in kDa. 2% serum was used as a positive control for goat anti-FH IgG. B, increasing amounts of biotin-labeled FH were incubated with immobilized Fhb II or BSA (1 μg/well) by ELISA analysis. As expected, FH specifically bound to Fhb II in a dose-dependent manner. C, increasing amounts of biotin-labeled FH were incubated with immobilized Fhb and II deletion proteins or Fhb IV (6.5 μg/well). Fhb IV was negative control. Deficiency of domain II resulted in a loss of Fhb binding to FH. D, the interaction of Fhb II and FH was analyzed by BLI. Streptavidin-coated biosensors with immobilized biotinylated Fhb II were exposed to different concentrations of FH. Association and dissociation rate constants (ka and kd, respectively) for the interactions were derived from curve fitting and used to calculate the dissociation constant KD (KD = kd/ka, where ka = 7.06 × 103 m−1 s−1, kd = 5.895 × 10−3 s−1, and KD = 830 nm). E and F, binding of FH (5 μg/ml) to immobilized Fhb II (1 μg/well) was analyzed under increasing amounts of either NaCl (E) or ethylene glycol (F), revealing ionic and hydrophobic interactions. Results from three independent experiments performed in duplicate are shown in B, C, E, and F. Error bars, S.D.
The binding of FH to fHbp of Neisseria meningitidis involves charged carbohydrate chemistry (21); we therefore wanted to explore whether similar behavior is observed for Fhb and FH. Interestingly, we found that increasing the ionic strength reduced the binding of FH to Fhb. We detected a decrease in binding of 75% when the NaCl concentration was increased from 100 mm to 1.5 m (Fig. 3E). To estimate the influence of hydrophobic interactions between FH and Fhb, we incubated FH with immobilized Fhb II in the presence of increasing amounts of ethylene glycol. We found that a concentration of 50% ethylene glycol reduced 85% of the binding between the two proteins (Fig. 3F). Taken together, the interaction between FH and Fhb seems to depend on both electrostatic and hydrophobic interactions.
C3b/C3d-binding Site in the Fhb Protein
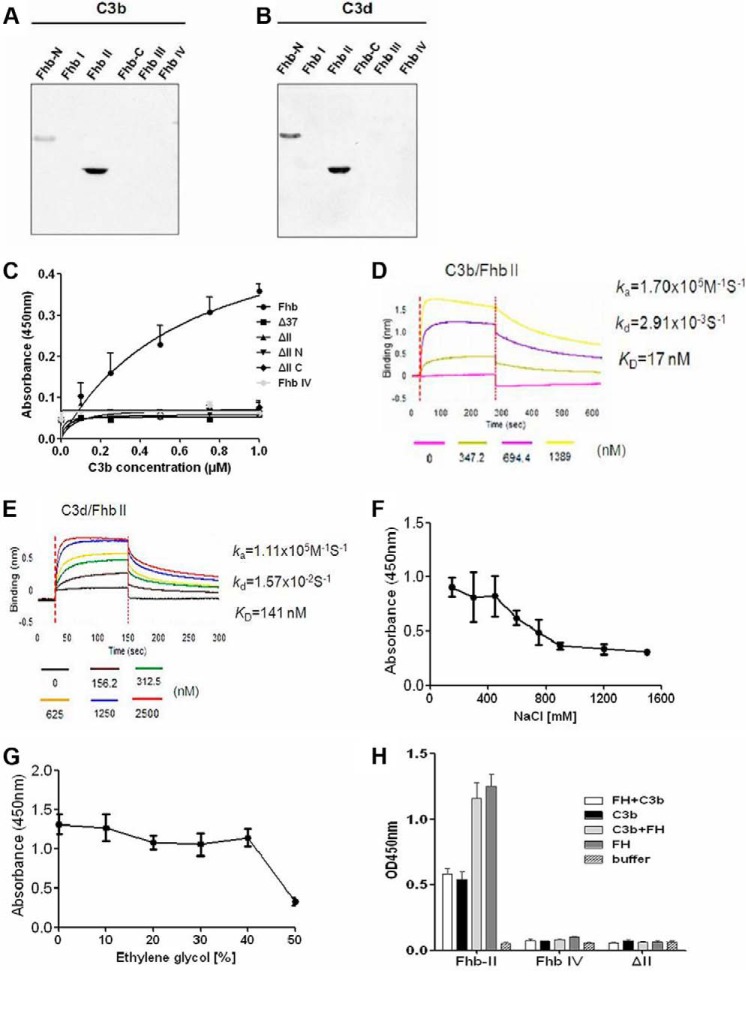
Fhb displayed low similarity to other function-known proteins (19), so determining the region of Fhb responsible for binding to C3 may help elucidate its role in the pathogenesis of S. suis. By ligand blotting, we found that domain II of Fhb is responsible for the interaction with C3b/C3d as FH (Fig. 4, A and B). Moreover, Fhb lost its ability to bind C3b when the II region was deleted, and the N terminus and C terminus of Fhb II and the 37 amino acids (amino acids 134–170) seemed to be involved in the Fhb-C3b interaction (Fig. 4C). Similar to FH, the interaction between C3b/C3d and Fhb II appeared to depend on both electrostatic and hydrophobic interactions (Fig. 4, F and G). However, the affinity for the C3b/C3d-Fhb II interaction was greater than for the FH-Fhb II interaction, and the KD values for the Fhb II-C3b and Fhb II-C3d interactions were 17 nm (Fig. 3D) and 141 nm (Fig. 3E), respectively. As described above, Fhb II is a FH binding domain within the N-terminal recognition region, which also forms a major point of contact with C3b/C3d. Because both C3 and FH are present in high concentration in human blood, we asked whether Fhb directly interacts with FH and C3 and there is competition for Fhb binding between FH and C3. We found that preincubation of C3 with Fhb II did not affect FH binding to Fhb II. Interestingly, preincubation of FH with Fhb II had no effect on C3 binding to Fhb II (Fig. 4H). Taken together, these results indicated that although the binding region (II, amino acids 233–344) of Fhb to C3 and FH overlap, both components can bind to Fhb directly without stereospecific blockade.
FIGURE 4.
Binding region of Fhb to C3b/C3d. A and B, ligand blotting analysis in which 20 μg of truncated Fhb constructs were run on SDS-polyacrylamide gels, transferred to PVDF membranes, and incubated with 5 μg/ml biotinylated C3b/C3d, and then HRP-conjugated streptavidin (1:8000) was used to detect binding. Both C3b and C3d specifically bound to Fhb II. M, molecular mass of protein standards in kDa. C, increasing amounts of biotin-labeled C3b were incubated with immobilized Fhb and II deletion proteins or Fhb IV (6.5 μg/well). Fhb IV was a negative control. Deficiency of domain II resulted in loss of Fhb binding to C3b. D and E, the interactions of Fhb II with C3b (D) or C3d (E) were analyzed by BLI. Streptavidin-coated biosensors with immobilized biotinylated Fhb II were exposed to different concentrations of C3b or C3d. Association and dissociation rate constants (ka and kd, respectively) for the interactions were derived from curve fitting and used to calculate the dissociation constant KD (KD = kd/ka). For the Fhb II-C3b interaction, ka = 1.70 × 105 m−1 s−1, kd = 2.91 × 10−3 s−1, and KD = 17 nm; for the Fhb II-C3d interaction, ka = 1.11 × 105 m−1 s−1, kd = 1.57 × 10−2 s−1, and KD = 141 nm. F and G, binding of C3b (5 μg/ml) to immobilized Fhb II (1 μg/well) was analyzed under increasing amounts of either NaCl (F) or ethylene glycol (G), which indicated ionic and hydrophobic interactions. H, ELISA was employed to analyze whether competition for binding to Fhb II occurred between C3b and FH. Preincubation of C3b or FH (5 μg/ml) with immobilized Fhb II (1 μg/well), followed by incubation with biotinylated FH or C3b (5 μg/ml) and HRP-conjugated streptavidin (1:8000), was performed to detect binding. Results from three independent experiments performed in duplicate are shown in C and F–H. Error bars, S.D.
Presence of Fhb in the Culture Supernatant and on the Bacterial Surface
Fhb has been demonstrated to be a surface protein with an LPXTG motif (19, 22). However, the protein is possibly also present in culture supernatants because some surface proteins are released from the bacterial cell wall into the culture supernatant (23). Therefore, the location of Fhb was investigated. Western blotting indicated that as for cell wall proteins (Fig. 5A, iii), Fhb was detected in the culture supernatant in the 05ZYH33 strain and was not detected in the mutant strain (Fig. 5A, ii) using specific antiserum against Fhb-N; however, they were not detected using preimmune serum (Fig. 5A, i). The surface location of Fhb was also visualized by confocal laser scanning microscopy on the WT strain 05ZYH33 (Fig. 5B, a) but not on the mutant (Fig. 5B, b). To further elucidate the role of the Fhb binding region (II, amino acids 233–344) in immune evasion, a complementary C-FhbΔII strain was constructed. As expected, C-FhbΔII was detected both in the cell wall proteins (Fig. 5A, iii) and the culture supernatant (Fig. 5A, ii). Moreover, the surface location of C-FhbΔII was observed by confocal laser scanning microscopy (Fig. 5B, c). These data indicated that Fhb is released into the culture supernatant and verified that the complementary strain (C-FhbΔII), which expresses Fhb with deletion of domain II, had been successfully constructed.
Immune Evasion by S. suis 2 Mediated by Domain II of the Secreted (Released) Fhb
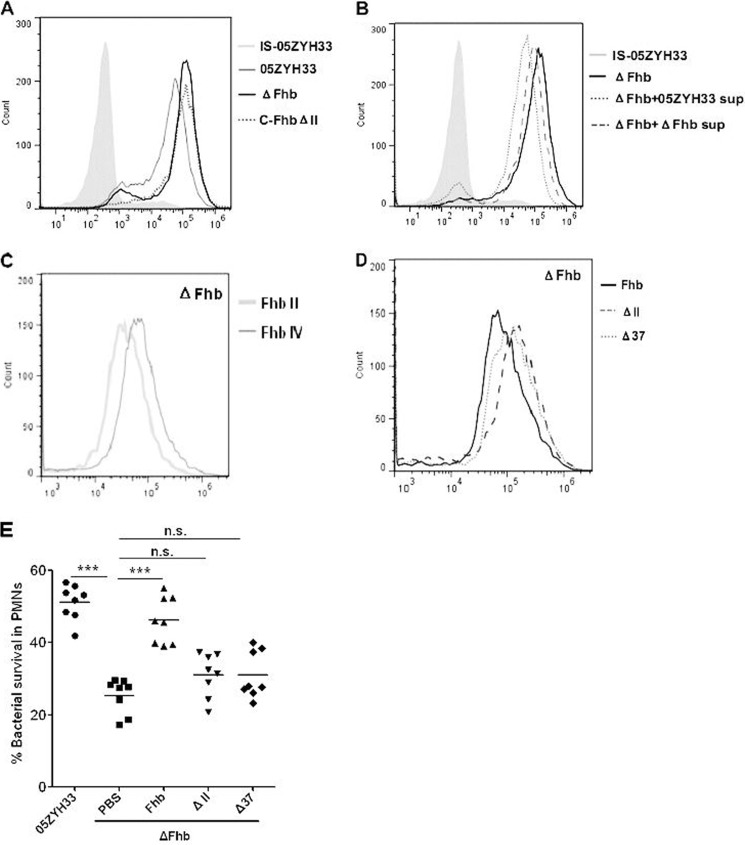
To evaluate the role of the Fhb binding region in immune evasion, the amount of C3b/iC3b bound to the surface of S. suis incubated in fresh human serum was determined by FACS analysis. Complement activated by S. suis in heat-inactivated serum was used as a negative control. The data showed that there was an increase in the amount of C3b/iC3b bound to the mutant ΔFhb relative to WT 05ZYH33, as shown in our previous study (19). In addition, the levels of C3b/iC3b deposited on C-FhbΔII were not rescued because of deletion of Fhb II (Fig. 6A). These data suggested that Fhb plays a role in the inhibition of C3b/iC3b deposition on S. suis via its FH/C3b/C3d binding region (Fhb II).
FIGURE 6.
Role of the Fhb binding region in complement inhibition and immune evasion by S. suis. A, representative histograms for the FI of C3b/iC3b deposition on WT strain 05ZYH33, mutant strain ΔFhb, and complementary strain C-FhbΔII. B, representative histograms for the FI of C3b/iC3b deposition on mutant strain ΔFhb by preincubation of the serum with the supernatants from WT strain 05ZYH33 and mutant strain ΔFhb. C, comparing the FI of C3b/iC3b deposition on mutant strain ΔFhb by preincubation of the serum with recombinant Fhb II or Fhb IV (80 nm). D, comparing the FI of C3b/iC3b deposition on the mutant strain ΔFhb by preincubation of the serum with the recombinant Fhb full-length protein or Fhb II deletion proteins ΔII and Δ37 (80 nm). E, survival of the mutant strain ΔFhb in PMN-mediated killing assays by preincubation of serum with Fhb-associated proteins (80 nm). Data are averages of three independent experiments, and unpaired Student's t tests were used for statistical analysis. ***, p < 0.001; n.s., no significance.
Secretion of C3b-binding proteins, such as Ecb and Efb, by S. aureus (17, 18) usually results in enhanced complement evasion by consumption of C3b, a key factor in activation of the alternative pathway in blood. It may be that released Fhb in the supernatant is involved in the antiphagocytic ability of S. suis 2 by binding of C3b. To evaluate this possibility, the amount of C3b/iC3b bound to the surface of the mutant strain ΔFhb when incubated in fresh human serum was determined by FACS analysis. The serum was preincubated with the supernatants from either WT strain 05ZYH33 or mutant strain ΔFhb. Interestingly, lower C3b/iC3b deposition was detected on S. suis after treatment with the WT supernatant than with the ΔFhb supernatant (Fig. 6B). Moreover, lower C3b/iC3b deposition on the mutant ΔFhb was found by pretreatment of serum with Fhb II compared with pretreatment of serum with Fhb IV (Fig. 6C). Furthermore, increased C3b/iC3b deposition was detected on mutant strain ΔFhb when domain II or the 37-amino acid region was deleted in Fhb (Fig. 6D). These results indicated that secreted (released) Fhb inhibits complement activation via its FH/C3b/C3d binding region (Fhb II, amino acids 233–344).
To investigate the role of secreted Fhb in evasion of PMN-mediated innate immunity, the survival of fhb-deficient mutants (ΔFhb) was evaluated after the serum was pretreated with recombinant Fhb and its FH/C3b/C3d binding region (Fhb II) deletion mutants. Interestingly, the viability of strain ΔFhb increased when human serum was preincubated with Fhb but not when it was preincubated with the ΔII or Δ37 mutants in PMN killing assays (Fig. 6E). This finding further confirmed that secreted Fhb mediates S. suis evasion of host complement attack via its FH/C3b/C3d binding domain.
Identification of Other Fhb Proteins in S. suis Serotypes 1, 7, and 9
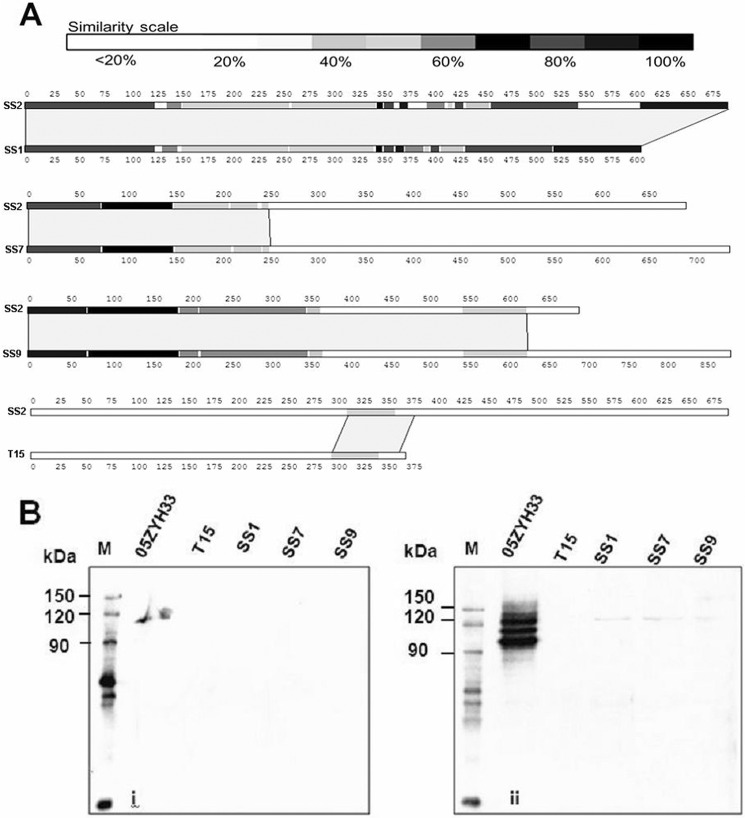
Our earlier sequence analysis revealed that the fhb gene sequence varies among S. suis strains of different serotypes (19). When the amino acid sequences of Fhb from S. suis strains were analyzed in the current study, sequence variations were evident in the Fhb II region (Fig. 7A), potentially indicating that these Fhb II regions have evolved independently to have different functions. To further analyze the function of other Fhb variants in different serotypes, we first confirmed expression of Fhb-N in different S. suis serotype strains. To our surprise, Fhb-N was not detected among the cell wall proteins (Fig. 7B, ii) or culture supernatants (Fig. 7B, i) of S. suis serotype 1, 7, and 9 strains (SS1, SS7, and SS9). These results suggested that the Fhb protein and the Fhb-FH-C3 immune complex might be unique to S. suis 2 and may represent a specific mechanism by which S. suis 2 evades host innate immunity.
FIGURE 7.
Identification of other Fhb proteins in S. suis serotype 1, 7, and 9 strains. A, sequence alignment of Fhb in S. suis serotype 1, 2, 7, and 9 strains (SS1,SS2, SS7, and SS9, respectively). B, cell wall Fhb and released Fhb in the supernatant from S. suis serotypes 1, 2, 7, and 9 was detected by Western blotting. Samples of the concentrated culture supernatant (25:1, A600 = 1.0) and cell wall proteins (mutanolysin extracts, A600 = 1.0) were separated by SDS-PAGE and transferred to PVDF membrane that was probed with rabbit anti-Fhb sera (1:400) followed by HRP-conjugated goat anti-rabbit IgG (1:8000). Fhb could be specifically detected in both the supernatant (i) and cell wall proteins (ii) from S. suis 2 strain 05ZYH33 but not in the S. suis serotype 1, 7, and 9 strains or the T15 strain (negative control).
Discussion
For S. suis to cause bacteremia in the blood of non-immune hosts, the bacterium must first survive by evading host complement attack. Increasing numbers of microbes have been shown to acquire FH on their surface to protect themselves from complement attack (10). The surface of S. suis is armed with molecules that allow it to evade the host's immune defenses through interactions with FH by Fhb (19). Alternatively, some pathogens causing invasive disease inhibit complement activation by targeting C3b, a key molecule in all complement activation pathways, through C3b-binding proteins (18).
Interestingly, in the present study, we determined that Fhb, expressed by S. suis 2, possesses the capacity to bind C3b/C3d as well as FH. Using GST pull-down assays with immobilized recombinant GST-Fhb as the bait protein, we co-eluted a human serum protein complex with a high molecular mass. Mass spectrometry analyses of the in-gel trypsin-digested proteins identified peptides with sequence identity to FH and C3b/C3d. To our knowledge, no other previously reported S. suis protein product has been shown to bind both FH and C3b/C3d. In S. aureus, two secreted proteins, Sbi (16) and Ecb (24), have been reported to bind FH and C3b/C3d. However, Fhb displayed low similarity to these proteins by amino acid sequence analysis and exhibited no known binding motifs for Sbi or Ecb. Here, we demonstrated a novel mechanism for FH/C3 binding by Fhb. Direct binding of Fhb to FH and C3 was, however, distinct from Sbi binding to FH that occurs indirectly via C3b/C3d. The region responsible for binding of Fhb to FH and C3 (amino acids 134–244 in N-terminal domain II) is overlapping. In our recent study, the structure of Fhb-N was determined using the single-wavelength anomalous dispersion method, and the core structure of domain II was found to be a β-sandwich displayed on the Fhb-N surface (25). However, streptococcal M proteins are mostly α-helical, coiled-coil, homodimeric fibrillar molecules (26). FH binding sites on M proteins have been mapped within the N-terminal hypervariable region (27) and the C-repeats (28). Our recent surface charge distribution analysis indicated that the Fhb II surface is rich in amino acids with both negative and positive charges (25), which may contribute to the electrostatic interactions between Fhb and FH/C3. Previous studies have shown that FhbB and Fhbp bound to human FH mainly through surface-exposed negatively charged amino acids (21, 29). However, Efb bound to C3 through positively charged amino acids on the Efb protein (24).
In the present study, we detected Fhb in both the cell wall and the culture supernatant. Moreover, both the WT culture supernatant and recombinant Fhb/Fhb II inhibited complement deposition on fhb-deficient mutants (ΔFhb). In contrast, neither the complementary strain (C-FhbΔII) nor ΔII/Δ37 protein displayed this inhibitory effect. These results suggested that, similar to some secreted C3b-binding proteins of S. aureus, the secreted (released) Fhb evaded host complement attack by binding to the central complement component C3b via its domain II. Although the fhb gene exhibited molecular polymorphisms in other non-serotype 2 strains, the Fhb protein was not detected in either the cell wall or culture supernatant of serotype 1, 7, and 9 strains by Western blotting, suggesting that Fhb might be unique to S. suis 2. However, both Scl (an Fhb of S. pyogenes) and its phylogenetically linked variants in different serotype strains can bind FH (13).
In summary, we found that Fhb is present in the culture supernatant and is also associated with the cell envelope. Fhb expressed by S. suis 2 strains could bind to both FH and C3b/C3d directly via its domain II (amino acids 134–244), which is rich in charged amino acids potentially contributing to the electrostatic interactions between Fhb and FH or C3b/C3d. Moreover, both secreted and cell envelope-associated forms of Fhb contribute to immune evasion by S. suis 2.
Experimental Procedures
Bacterial Strains and Growth Conditions
The highly virulent S. suis 2 strain 05ZYH33, originally isolated from a deceased streptococcal toxic shock syndrome patient in a 2005 outbreak in Sichuan, China, was used in this study. S. suis 2 strains were cultured as described previously (4, 19). Bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains, primers, and plasmids used in this study
| Strain or plasmid | Descriptiona or sequenceb (5′–3′) | Source/reference, PCR products |
|---|---|---|
| Strains | ||
| E. coli DH5α | Host for cloning vector | This laboratory |
| 05ZYH33 | Virulent Chinese S. suis serotype 2 isolate | This laboratory |
| ΔFhb | Gene fhb knockout mutant strain; CmR | This laboratory (19) |
| CΔFhb-ΔII | Complemented strain of ΔMRP; CmREmR | This laboratory |
| Plasmids | ||
| pET-28a | Expression vector, lacZ, KanR | TaKaRa |
| pAT18 | The complemented expression vector, EmR | This laboratory |
| pAT18::Fhb-ΔII | pAT18 containing the intact fhb gene with its upstream promoter but with deletion of II domain; CmREmR | This study |
| Primers | PCR products | |
| Fhb-N-F | CGCGGATCCGAATCGCTAGAACCAGAT | ORF of Fhb-N |
| Fhb-N-R | CCGCTCGAGCTATAGAGTTTTTTCTTTCTC | |
| Fhb I-F | CGCGGATCCGAATCGCTAGAACCAGAT | ORF of Fhb I |
| Fhb I-R | CCGCTCGAGCTACAACAGATTTATAGATAC | |
| Fhb II-F | CGCGGATCCAAGGCGGAAGAAGGTCAG | ORF of Fhb II |
| Fhb II-R | CCGCTCGAGCTATAGAGTTTTTTCTTTCTC | |
| Fhb-C-F | CGCGGATCCCCACCTAAGGAGAATCCA | ORF of Fhb C |
| Fhb-C-R | CCGCTCGAGCTACGGTTGAACATCTTCTTG | |
| Fhb III-F | CGCGGATCCCCACCTAAGGAGAATCCA | ORF of Fhb III |
| Fhb III-R | CCGCTCGAGCTACGGTTGAACATCTTCTTG | |
| Fhb IV-F | CGCGGATCCAAACAAGAAGATGTTCAAC | ORF of Fhb IV |
| Fhb IV-R | CCGCTCGAGCTAAGTTGCTTTTGGTGCCTC | |
| ΔII-1 | CGCGGATCCGAATCGCTAGAACCAGAT | ORF of Fhb |
| ΔII-4 | CCGCTCGAGCTAAGTTGCTTTTGGTGCCTC | |
| ΔII-2 | GTTTTGGATTCTCCTTAGGTGGCAACAGATTTATAGATACTGATG | 3′-Flanking regions of ΔII for overlay PCR |
| ΔII-3 | CATCAGTATCTATAAATCTGTTGCCACCTAAGGAGAATCCAAAAC | 5′-Flanking regions of ΔII for overlay PCR |
| ΔII N-2 | CTAAAGAAACGATATTTTTCTTGACCCAACAGATTTATAGATACTG | 3′-Flanking regions of ΔII N for overlay PCR |
| ΔII N-3 | CAGTATCTATAAATCTGTTGGGTCAAGAAAAATATCGTTTCTTTAG | 5′-Flanking regions of ΔII N for overlay PCR |
| ΔII C-2 | GTTTTGGATTCTCCTTAGGTGGAGAATTTCTGTAATCATTAATATC | 3′-Flanking regions of ΔII C for overlay PCR |
| ΔII C-3 | GATATTAATGATTACAGAAATTCTCCACCTAAGGAGAATCCAAAAC | 5′-Flanking regions of ΔII C for overlay PCR |
| Δ37-2 | GAATCTGACCTTCTTCCGCCTTCGTATTATCTGAAGGTTCATC | 3′-Flanking regions of Δ37 for overlay PCR |
| Δ37-3 | GATGAACCTTCAGATAATACGAAGGCGGAAGAAGGTCAGATTC | 5′-Flanking regions of Δ37 for overlay PCR |
| CΔFhb-ΔII-1 | CGGAATTCAGGAAAAACCAGCCTTTGCG | ORF of Fhb and its upstream promoter (19) |
| CΔFhb-ΔII-4 | CGGGATCCAAATTGATATTATTGTTTTAGCG | |
a AmpR, ampicillin-resistant; CmR, chloramphenicol-resistant; EmR, erythromycin-resistant; KanR, kanamycin-resistant.
b The underlined sequences are the restriction sites.
GST Pull-down to Capture the Serum Proteins Interacting with Fhb
An in vitro GST pull-down assay was performed to identify the serum proteins interacting with Fhb. The pET28a-Fhb plasmid (Table 1) was digested with BamHI and XhoI to release the fhb ORF, which was subcloned into pGEX4T-1 vector (Amersham Biosciences) to generate the fhb ORF expression plasmid pGEX4T-1-Fhb. Overexpression of GST-Fhb was induced in E. coli BL21 (DE3) strain by 1 mm isopropyl-β-d-thiogalactopyranoside when the cell density reached an A600 nm of 0.4. After growth for 6 h at 30 °C, the cells were collected and lysed by sonication. GST-Fhb-soluble expression was identified by Western blotting (data not shown). Finally, the GST-Fhb fusion proteins were purified in E. coli BL21 supernatant using GSTrap FF (GE Healthcare) following the manufacturer's instructions. GST pull-down was further performed. Human serum was diluted with PBS and mixed with 200 μl of NETN buffer (20 mm Tris-HCl (pH 7.2), 0.1 m NaCl, 1 mm EDTA, 0.5% Nonidet P-40, and protease inhibitor mixture (1:20, v/v))-equilibrated GST beads. The mixture was incubated at 4 °C overnight and then washed with NETN buffer five times. The binding of serum proteins by bait protein GST-Fhb was analyzed by SDS-PAGE (Fig. 1A), using GST as a negative control. Trypsinized protein fragments from excised gel slices were analyzed by LC-MS.
Cloning, Expression, and Purification of Recombinant Fhb Constructs
To determine the binding domain of Fhb, seven recombinant fragments of Fhb were engineered as follows: Fhb-N (amino acids 45–344), Fhb I (amino acids 45–170, α-coil-rich region), Fhb II (amino acids 134–344, mainly formed by β-sheet), Fhb-C (amino acids 345–664, proline-rich region), Fhb III (amino acids 345–483), and Fhb IV (amino acids 484–664), as shown in Fig. 2, A and B. The oligonucleotide primers and expression vectors used for Fhb-N, Fhb I, Fhb II, Fhb-C, Fhb III, and Fhb IV in this study are listed in Table 1. The ORFs encoding Fhb proteins and Fhb protein fragments were amplified by PCR using S. suis strain 05ZYH33 genomic DNA (accession number YP_001198119) as a template. Fhb I contains 37 overlapping amino acids (amino acids 134–170) with Fhb II that are involved in the interaction with FH/C3b/C3d. For the preparation of Fhb mutant protein with a deletion corresponding to amino acid residues 134–344 (ΔII), 134–232 (ΔII N), 233–344 (ΔII C), and 134–170 (Δ37), overlay extension PCR was performed and the oligonucleotide primers used to construct the Fhb mutants (ΔII, ΔII N, ΔII C, and Δ37) are also listed in Table 1. All of the amplified ORFs were cloned into pET-28a vector. Then the direct DNA sequencing analysis of the ORFs in plasmid and Western blotting analysis of the above Fhb-associated protein expressions were performed (data not shown) before purification. Finally, the recombinant Fhb-associated proteins were purified by nickel-chelating chromatography (GE Healthcare) according to the manufacturer's recommendations.
ELISA to Analyze the Epitope Expressions of Fhb II Deletion Proteins
It is possible that the deletion of domain II has an impact on the epitope expressions of Fhb II deletion-associated proteins. Thus, epitope expressions of Fhb II deletion proteins were identified by ELISA. 96-well plates were coated overnight at 4 °C with 100 μl of 10 μg/ml Fhb-associated proteins. After blocking with 5% BSA for 2 h at 37 °C and washing, the anti-Fhb IgG/nonspecific IgG (1:15,000) was added and incubated for 1 h at room temperature. After washing, the above Fhb-associated proteins were detected by HRP-labeled goat anti-rabbit IgG (1:20,000). Fhb and Fhb truncates were used as positive controls, whereas MRP-C (C terminus of MRP) was used as irrelevant control of Fhb II deletion proteins. The nonspecific IgG (purified from preimmune serum) was used to as background control.
ELISA-type Binding Assay
To detect the binding of FH/C3b/C3d to immobilized Fhb, 96-well plates were coated overnight at 4 °C with 100 μl of 10 μg/ml Fhb-associated proteins. After blocking with 0.2% gelatin in Dulbecco's PBS for 6 h at 4 °C and washing, 5 μg/ml biotinylated FH/C3b/C3d (Merck) was added and incubated for 1 h at room temperature. After washing, the bound FH/C3b/C3d was detected by incubation with HRP-conjugated streptavidin (1:8000; GE Healthcare). Background values obtained for the control (incubated with PBST containing 1% BSA, without biotinylated FH/C3b/C3d) were subtracted. FH/C3b/C3d (Merck) was biotinylated according to the Amersham Biosciences ECL protein biotinylation module assay (GE Healthcare).
Ligand Blotting
Ligand blotting analysis was performed as described previously (19). Briefly, for the FH binding assay, Fhb-associated proteins were separated by electrophoresis on 10% SDS-polyacrylamide gels and then transferred to PVDF membranes (Millipore). The membranes were blocked with 5% (w/v) nonfat dry milk at 4 °C for 12 h and incubated with 10% human serum. After three washes with Tris-buffered saline with 0.05% Tween 20 (TBST), membranes were incubated with goat anti-human FH polyclonal antibody (Merck; 1:5000) and HRP-conjugated rabbit anti-goat IgG (1:20,000). Bands were detected using ECL Western blotting detection reagents (Thermo Scientific) and exposure to x-ray film (Fuji) at room temperature for 120 s. For the C3b/C3d binding assay, the PVDF membranes were probed with 10 μg/ml biotinylated C3b/C3d (Merck) for 1 h at room temperature, washed three times with TBST, and incubated with HRP-conjugated streptavidin (1:8000; GE Healthcare). Bands were detected as described above.
BLI Analysis of the Fhb-FH/C3b/C3d Interactions
All BLI experiments were performed in PBST on a BLItz system (ForteBio Inc.) as described previously (30). Streptavidin-coated biosensors with immobilized biotinylated Fhb-associated proteins were exposed to different concentrations of FH/C3b/C3d. Association and dissociation rate constants (ka and kd, respectively) for the interactions were derived from curve fitting and used to calculate the dissociation constant KD (KD = kd/ka).
Generation of the Complementary C-FhbΔII Strains
To evaluate the effect of the Fhb II binding domain (amino acids 134–344) on complement activation and immune evasion, a complementary strain with a deletion in Fhb II (amino acids 134–344) was constructed. Two PCR fragments were first generated, one with the primer pair CΔFhb-ΔII-1/ΔII-2 that generates the upstream promoter and a DNA fragment encoding Fhb (amino acids 1–134) and the other with the primer pair ΔII-3/CΔFhb-ΔII-4 that generates a DNA fragment encoding Fhb (amino acids 345–675). A PCR fragment encoding FhbΔII was generated by overlap extension PCR, using the two first round PCR fragments and primers CΔFhb-ΔII-1/CΔFhb-ΔII-4. This long amplicon was subsequently cloned into the E. coli-S. suis shuttle vector pAT18; the recombinant plasmid pAT18::Fhb-ΔII was transformed into the ΔFhb strain; and the complementary strain CΔFhb-ΔII was screened on THB agar with selective pressure from erythromycin. RT-PCR, Western blotting, and confocal laser scanning microscopy analysis were used to confirm the transcription and expression of Fhb-ΔII (Fhb with deletion of domain II) in CΔFhb-ΔII and Fhb in the WT and ΔFhb mutant.
Confocal Laser Scanning Microscopy Analysis
To visualize Fhb proteins expressed by the WT, ΔFhb mutant, and the complementary stain CΔFhb-ΔII on the bacterial surface, confocal laser scanning microscopy analysis was performed as described previously (31). Briefly, an aliquot of streptococci (6 × 106 cfu/ml) was added to rabbit anti-Fhb IgG (10 μg/ml), incubated for 45 min on ice, and then washed. Next, 300 μl of the resuspension were incubated with TRITC-goat anti-rabbit IgG (Santa Cruz Biotechnology, Inc.) (0.3 mg/ml) for 45 min on ice. Bacteria were then washed with PBS and spotted onto Citoglas slides (Shitai). The slides were then analyzed using a Olympus FV1000 confocal scanning microscope.
Flow Cytometry Analysis of Complement Deposition on S. suis
To evaluate the effect of the binding domain (Fhb II) on complement activation, C3b/iC3b deposition was assessed on the WT, the ΔFhb mutant, and the CΔFhb-ΔII complementary strain using the flow cytometry assays described previously (19). Briefly, S. suis was washed in PBS and diluted to ∼5 × 106 cfu/ml. An aliquot (300 μl) of streptococci was added to 300 μl of normal human serum and incubated for 30 min at 37 °C and then washed with PBS. C3b/iC3b deposition was detected using an FITC-conjugated polyclonal goat anti-human C3 antibody (MP Biomedicals, LLC) (1:5000). Samples were analyzed using an Accuri C6 flow cytometer and CellQuest software (BD Biosciences), using forward and side scatter parameters to gate at least 30,000 bacteria. The threshold was set using the negative control. To verify the hypothesis that the secreted (released) Fhb inhibits complement activation mediated by its binding domain Fhb II, the serum was preincubated with S. suis culture supernatant or recombinant proteins Fhb/Fhb II/ΔII/Δ37/Fhb IV for 20 min at room temperature to trigger futile consumption of C3b by binding to it.
PMN Killing Assays
PMNs are the main phagocytes for defense against bacterial pathogens in human blood. To verify the hypothesis that the secreted (released) Fhb improved immune evasion of S. suis 2, mediated by its binding domain Fhb II, a simplified PMN killing assay was performed. The viability of strain ΔFhb in the PMN killing assay was evaluated by the exogenous addition of recombinant Fhb, ΔII, and Δ37. In the PMN killing assay, the serum was incubated with Fhb/FhbΔII/FhbΔ37 for 20 min at room temperature to trigger futile consumption of C3b by binding to it. Frequently, PMNs were infected with S. suis 2 at a multiplicity of infection of 1:15 (PMN/bacterium) in 50% pretreated serum and centrifuged at 380 × g for 5 min at 4 °C. The plates were incubated at 37 °C under 5% CO2. Samples were taken at 60 min and analyzed immediately as described previously (19). Colonies were counted, and the percentage of surviving S. suis 2 was calculated as (cfuPMN+/cfuPMN−) × 100%.
Statistical Analysis
Unless otherwise specified, all data are expressed as the mean ± S.D. Differences between groups were analyzed using unpaired two-tailed Student's t test. For all tests, a value of p < 0.05 was considered as the threshold for significance. All statistical analyses were carried out using SPSS version 15.0 (SPSS Inc.).
Author Contributions
Y. Y. designed the study and wrote the paper. S. G. purified GST-Fhb and performed the GST pull-down assay. X. L. designed and constructed vectors for expression of Fhb-associated proteins and purified recombinant proteins. X. L. performed ELISA-type binding assays and ligand blotting experiments. X. L. and P. L. analyzed complement deposition on S. suis and performed PMN killing assays. Y. Y. and X. L. performed BLI analysis of the protein interactions. Y. Y., Y. J., and C. Z. analyzed the data. Y. Z. contributed reagents/materials. All authors approved the final version of the manuscript.
Acknowledgments
We thank Dr. Yaya Pian (Institute of Biophysics, Chinese Academy of Sciences) for constructing the mutant ΔFhb strain. We also thank Dr. Huaijie Hao (Institute of Microbiology, Chinese Academy of Sciences) for providing rabbit anti-Fhb serum and rabbit anti-Fhb IgG.
This work was supported by National Basic Research Program (973) of China Grant 2012CB518804 (to Y. J.), National Natural Science Foundation Grants 81171528 and 81371766 (to Y. J.), and State Key Laboratory of Pathogen and Biosecurity Foundation (Academy of Military Medical Science) Grant SKLPBS1414 (to Y. Y.). The authors declare that they have no conflicts of interest with the contents of this article.
- S. suis 2
- Streptococcus suis serotype 2
- PMN
- polymorphonuclear leukocyte
- TRITC
- tetramethylrhodamine isothiocyanate
- FH
- factor H
- Fhb
- FH-binding protein
- BLI
- bio-layer interferometry.
References
- 1. Wertheim H. F. L., Nghia H. D. T., Taylor W., and Schultsz C. (2009) Streptococcus suis: an emerging human pathogen. Clin. Infect. Dis. 48, 617–625 [DOI] [PubMed] [Google Scholar]
- 2. Huong V. T., Ha N., Huy N. T., Horby P., Nghia H. D., Thiem V. D., Zhu X., Hoa N. T., Hien T. T., Zamora J., Schultsz C., Wertheim H. F., and Hirayama K. (2014) Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg. Infect. Dis. 20, 1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sriskandan S., and Slater J. D. (2006) Invasive disease and toxic shock due to zoonotic Streptococcus suis: an emerging infection in the East? PLoS Med. 3, e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang J., Wang C., Feng Y., Yang W., Song H., Chen Z., Yu H., Pan X., Zhou X., Wang H., Wu B., Wang H., Zhao H., Lin Y., Yue J., et al. (2006) Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med. 3, e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wangkaew S., Chaiwarith R., Tharavichitkul P., and Supparatpinyo K. (2006) Streptococcus suis infection: a series of 41 cases from Chiang Mai University Hospital. J. Infect. 52, 455–460 [DOI] [PubMed] [Google Scholar]
- 6. Yu H., Jing H., Chen Z., Zheng H., Zhu X., Wang H., Wang S., Liu L., Zu R., Luo L., Xiang N., Liu H., Liu X., Shu Y., Lee S. S., et al. (2006) Human Streptococcus suis outbreak, Sichuan, China. Emerg. Infect. Dis. 12, 914–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rus H., Cudrici C., and Niculescu F. (2005) The role of the complement system in innate immunity. Immunol. Res. 33, 103–112 [DOI] [PubMed] [Google Scholar]
- 8. Schmidt C. Q., Herbert A. P., Hocking H. G., Uhrín D., and Barlow P. N. (2008) Translational mini-review series on complement factor H: structural and functional correlations for factor H. Clin. Exp. Immunol. 151, 14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zipfel P. F., and Skerka C. (2009) Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 9, 729–740 [DOI] [PubMed] [Google Scholar]
- 10. Lambris J. D., Ricklin D., and Geisbrecht B. V. (2008) Complement evasion by human pathogens. Nat. Rev. Microbiol. 6, 132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dave S., Brooks-Walter A., Pangburn M. K., and McDaniel L. S. (2001) PspC, a pneumococcal surface protein, binds human factor H. Infect. Immun. 69, 3435–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horstmann R. D., Sievertsen H. J., Knobloch J., and Fischetti V. A. (1988) Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. U.S.A. 85, 1657–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caswell C. C., Han R., Hovis K. M., Ciborowski P., Keene D. R., Marconi R. T., and Lukomski S. (2008) The Scl1 protein of M6-type group A Streptococcus binds the human complement regulatory protein, factor H, and inhibits the alternative pathway of complement. Mol. Microbiol. 67, 584–596 [DOI] [PubMed] [Google Scholar]
- 14. Areschoug T., Linse S., Stålhammar-Carlemalm M., Hedén L. O., and Lindahl G. (2002) A proline-rich region with a highly periodic sequence in Streptococcal β protein adopts the polyproline II structure and is exposed on the bacterial surface. J. Bacteriol. 184, 6376–6383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharp J. A., Echague C. G., Hair P. S., Ward M. D., Nyalwidhe J. O., Geoghegan J. A., Foster T. J., and Cunnion K. M. (2012) Staphylococcus aureus surface protein SdrE binds complement regulator factor H as an immune evasion tactic. PLoS One 7, e38407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haupt K., Reuter M., van den Elsen J., Burman J., Hälbich S., Richter J., Skerka C., and Zipfel P. F. (2008) The Staphylococcus aureus protein Sbi acts as a complement inhibitor and forms a ternary complex with host complement Factor H and C3b. PLoS Pathog. 4, e1000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jongerius I., Garcia B. L., Geisbrecht B. V., van Strijp J. A., and Rooijakkers S. H. (2010) Convertase inhibitory properties of Staphylococcal extracellular complement-binding protein. J. Biol. Chem. 285, 14973–14979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen H., Ricklin D., Hammel M., Garcia B. L., McWhorter W. J., Sfyroera G., Wu Y. Q., Tzekou A., Li S., Geisbrecht B. V., Woods V. L. Jr., and Lambris J. D. (2010) Allosteric inhibition of complement function by a staphylococcal immune evasion protein. Proc. Natl. Acad. Sci. U.S.A. 107, 17621–17626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pian Y., Gan S., Wang S., Guo J., Wang P., Zheng Y., Cai X., Jiang Y., and Yuan Y. (2012) Fhb, a novel factor H-binding surface protein, contributes to the antiphagocytic ability and virulence of Streptococcus suis. Infect. Immun. 80, 2402–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cordes F. S., Roversi P., Kraiczy P., Simon M. M., Brade V., Jahraus O., Wallis R., Skerka C., Zipfel P. F., Wallich R., and Lea S. M. (2005) A novel fold for the factor H-binding protein BbCRASP-1 of Borrelia burgdorferi. Nat. Struct. Mol. Biol. 12, 276–277 [DOI] [PubMed] [Google Scholar]
- 21. Schneider M. C., Prosser B. E., Caesar J. J., Kugelberg E., Li S., Zhang Q., Quoraishi S., Lovett J. E., Deane J. E., Sim R. B., Roversi P., Johnson S., Tang C. M., and Lea S. M. (2009) Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature 458, 890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yuan Z. Z., Yan X. J., Zhang A. D., Chen B., Shen Y. Q., and Jin M. L. (2013) Molecular mechanism by which surface antigen HP0197 mediates host cell attachment in the pathogenic bacteria Streptococcus suis. J. Biol. Chem. 288, 956–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vecht U., Wisselink H. J., Jellema M. L., and Smith H. E. (1991) Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect. Immun. 59, 3156–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amdahl H., Jongerius I., Meri T., Pasanen T., Hyvärinen S., Haapasalo K., van Strijp J. A., Rooijakkers S. H., and Jokiranta T. S. (2013) Staphylococcal Ecb protein and host complement regulator factor H enhance functions of each other in bacterial immune evasion. J. Immunol. 191, 1775–1784 [DOI] [PubMed] [Google Scholar]
- 25. Zhang C., Yu Y., Yang M., and Jiang Y. (2015) Expression, purification, crystallization and structure determination of the N terminal domain of Fhb, a factor H binding protein from Streptococcus suis. Biochem. Biophys. Res. Commun. 466, 413–417 [DOI] [PubMed] [Google Scholar]
- 26. Fischetti V. A. (1989) Streptococcal M protein: molecular design and biological behavior. Clin. Microbiol. Rev. 2, 285–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnsson E., Berggård K., Kotarsky H., Hellwage J., Zipfel P. F., Sjöbring U., and Lindahl G. (1998) Role of the hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J. Immunol. 161, 4894–4901 [PubMed] [Google Scholar]
- 28. Sharma A. K., and Pangburn M. K. (1997) Localization by site-directed mutagenesis of the site in human complement factor H that binds to Streptococcus pyogenes M protein. Infect. Immun. 65, 484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller D. P., Bell J. K., McDowell J. V., Conrad D. H., Burgner J. W., Héroux A., and Marconi R. T. (2012) Structure of factor H-binding protein B (FhbB) of the periopathogen, Treponema denticola: insights into progression of periodontal disease. J. Biol. Chem. 287, 12715–12722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Durdagi S., Vullo D., Pan P., Kahkonen N., Maatta J. A., Hytonen V. P., Scozzafava A., Parkkila S., and Supuran C. T. (2012) Protein-protein interactions: inhibition of mammalian carbonic anhydrases I-XV by the murine inhibitor of carbonic anhydrase and other members of the transferrin family. J. Med. Chem. 55, 5529–5535 [DOI] [PubMed] [Google Scholar]
- 31. Liu P., Pian Y., Li X., Liu R., Xie W., Zhang C., Zheng Y., Jiang Y., and Yuan Y. (2014) Streptococcus suis adenosine synthase functions as an effector in evasion of PMN-mediated innate immunit. J. Infect. Dis. 210, 35–45 [DOI] [PubMed] [Google Scholar]