Abstract
Phosphodiesterase 5 (PDE5) inhibitors limit myocardial injury caused by stresses, including doxorubicin chemotherapy. cGMP binding to PKG Iα attenuates oxidant-induced disulfide formation. Because PDE5 inhibition elevates cGMP and protects from doxorubicin-induced injury, we reasoned that this may be because it limits PKG Iα disulfide formation. To investigate the role of PKG Iα disulfide dimerization in the development of apoptosis, doxorubicin-induced cardiomyopathy was compared in male wild type (WT) or disulfide-resistant C42S PKG Iα knock-in (KI) mice. Echocardiography showed that doxorubicin treatment caused loss of myocardial tissue and depressed left ventricular function in WT mice. Doxorubicin also reduced pro-survival signaling and increased apoptosis in WT hearts. In contrast, KI mice were markedly resistant to the dysfunction induced by doxorubicin in WTs. In follow-on experiments the influence of the PDE5 inhibitor tadalafil on the development of doxorubicin-induced cardiomyopathy in WT and KI mice was investigated. In WT mice, co-administration of tadalafil with doxorubicin reduced PKG Iα oxidation caused by doxorubicin and also protected against cardiac injury and loss of function. KI mice were again innately resistant to doxorubicin-induced cardiotoxicity, and therefore tadalafil afforded no additional protection. Doxorubicin decreased phosphorylation of RhoA (Ser-188), stimulating its GTPase activity to activate Rho-associated protein kinase (ROCK) in WTs. These pro-apoptotic events were absent in KI mice and were attenuated in WTs co-administered tadalafil. PKG Iα disulfide formation triggers cardiac injury, and this initiation of maladaptive signaling can be blocked by pharmacological therapies that elevate cGMP, which binds kinase to limit its oxidation.
Keywords: apoptosis, cardiomyopathy, heart failure, oxidative stress, phosphodiesterases, protein kinase G (PKG)
Introduction
The anthracycline drug doxorubicin is an effective and frequently used chemotherapeutic in the treatment of cancer. A major side effect of doxorubicin is cardiotoxicity, leading to heart failure. This cardiotoxicity occurs acutely within days of treatment in ∼11% of cases but also after chronic exposure in ∼2% of patients (1). Mechanisms proposed to contribute to doxorubicin cardiotoxicity include oxidative stress, down-regulation of genes expressing contractile proteins, and apoptosis (2). Doxorubicin-induced cardiomyopathy has poor prognosis and is frequently fatal; consequently, there is a need for defining therapies that combat this common side effect of chemotherapy.
Several animal and human studies showed cGMP analogues or phosphodiesterase (PDE)2 5 inhibitors, which prevent hydrolysis of cGMP, are protective against doxorubicin-induced cardiomyopathy without interfering with the efficacy of the treatment (3, 4), although the mechanism of this protection remains incompletely understood. cGMP binds and allosterically activates cGMP-dependent protein kinase, otherwise known as protein kinase G (PKG). PKG Iα comprises a homodimer with two parallel-aligned monomers held together by a leucine zipper (5). PKG Iα forms a homo interprotein disulfide between Cys-42 on each of its two chains in response to oxidative stress, activating the kinase independently of cGMP (6). This disulfide activation of PKG Iα in blood vessels induces vasodilation and blood pressure lowering. Thus, C42S PKG Iα knock-in (KI) mice engineered so that they cannot form this disulfide are resistant to oxidant-induced vasodilation and are basally hypertensive in vivo. Despite living with chronic hypertension, it was notable that the KI mice did not develop cardiac hypertrophy or progress to heart failure (7). This may suggest that expression of the mutant C42S PKG Iα in the heart is able to limit the maladaptive changes that normally occur because of the workload stress imposed on the myocardium by hypertension.
When cGMP binds PKG Iα, it attenuates oxidant-induced disulfide formation (8, 9). Because inhibition of PDE5 elevates cGMP and also provides protection from doxorubicin- or pressure overload-induced injury, we reasoned that this may be because it limits oxidation of the kinase to the disulfide. Oxidation of PKG Iα is anticipated during doxorubicin treatment because it induces oxidative stress through mitochondrial redox cycling reactions (10). In this scenario disulfide activation of myocardial PKG Iα would be rationalized to be maladaptive, explaining why strategies like cGMP elevation are cardioprotective. Furthermore, the C42S PKG Iα KI would likely be protected from doxorubicin-induced injury, in the same way as they are from hypertension-induced cardiac hypertrophy (7). Indeed, in this study, we provide evidence that disulfide activation of PKG Iα in the heart couples to apoptosis and explains why therapies that limit oxidation are cardioprotective. Previous studies have primarily investigated the redox control of vascular PKG Iα in terms of blood pressure regulation, whereas here the focus is the heart, where this kinase is also abundantly expressed.
Results
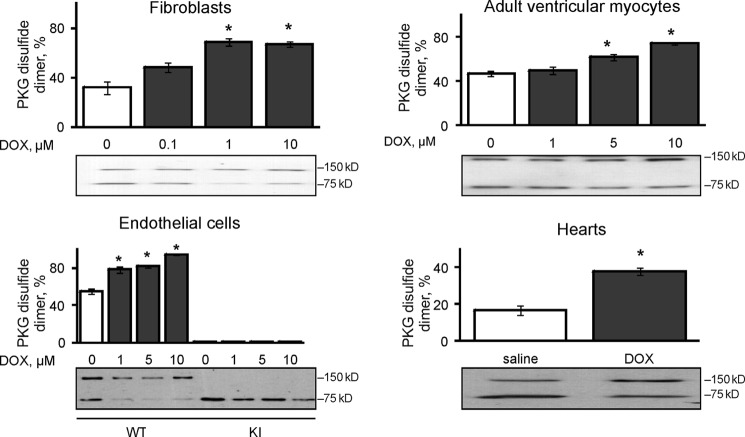
Doxorubicin caused dose-dependent PKG Iα disulfide dimerization in a primary culture of mouse fibroblasts, freshly isolated adult rat ventricular myocytes and bovine aorta endothelial cells transfected with WT, but not a C42S PKG Iα construct. Intraperitoneal injection of doxorubicin into WT mice induced the acute oxidation of cardiac PKG Iα measured on day 5 after treatment (Fig. 1).
FIGURE 1.
Doxorubicin induced PKG Iα disulfide dimerization in cells and in the heart. The treatment of primary cultures of mouse fibroblasts (n = 3) or adult rat ventricular myocytes (n = 3) with 0–10 μm doxorubicin (DOX) induced dose-dependent disulfide dimerization of endogenous WT but not C42S kinase. Bovine aortic endothelial cells, which were constitutively deficient in PKG Iα, were transfected with WT or a C42S kinase (n = 3) and showed similar results. Intraperitoneal injection of doxorubicin into mice induced cardiac PKG Iα disulfide dimerization (n = 12). *, p < 0.05 between vehicle and DOX groups.
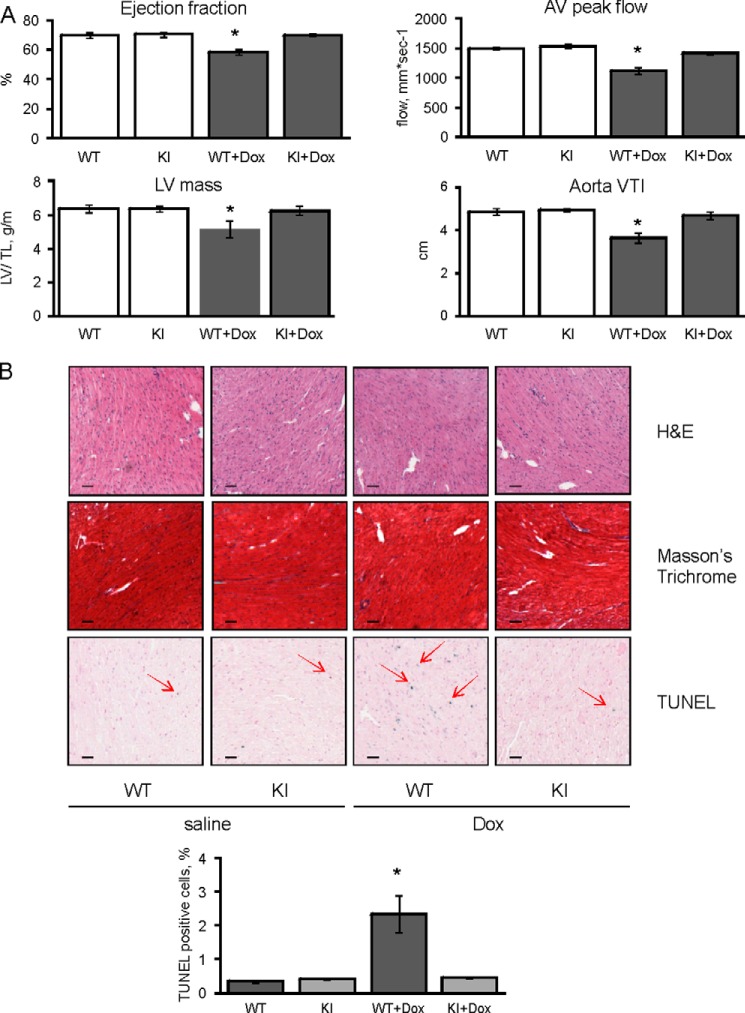
Doxorubicin-induced cardiomyopathy was compared in WT or C42S PKG Iα KI mice. Echocardiographic analysis of the heart showed that treatment of WT mice with doxorubicin has adverse effects on myocardial structure and function. The drug decreased ejection fraction by 17.1%, reduced left ventricular mass by 19.1%, lowered aortic valve peak flow by 25.3%, and attenuated the aortic velocity time integral by 24.7%. In marked contrast KI mice were resistant to this dysfunction induced by doxorubicin. For example, the ejection fraction was only rescued by 0.3%, left ventricular mass by 1.9%, aortic valve peak flow by 7.2%, and aortic velocity time integral by 5.8% in the KI mice expressing PKG that cannot be disulfide-activated (Fig. 2A).
FIGURE 2.
Doxorubicin induces apoptosis and reduces heart function in WT but not KI myocardium. A, echocardiographic examination of cardiac function was performed on WT or KI mice 5 days after doxorubicin (Dox) or vehicle injection. The ejection fraction, left ventricular mass (normalized by tibia length), aortic valve peak flow, and aortic velocity time integral decreased in WT, but not KI mice, treated with doxorubicin (n = 4). B, histological assessment of hearts from WT and KI mice treated with doxorubicin or vehicle (n = 4). Hematoxylin and eosin did not show any structural changes. Masson's Trichrome did not provide any evidence for collagen formation. TUNEL staining demonstrated a significant increase in the percentage of apoptotic nuclei (red arrows) after doxorubicin treatment in WT, but not KI hearts (representative image and quantification). Scale bar, 50 μm. *, p < 0.05 between vehicle and doxorubicin groups.
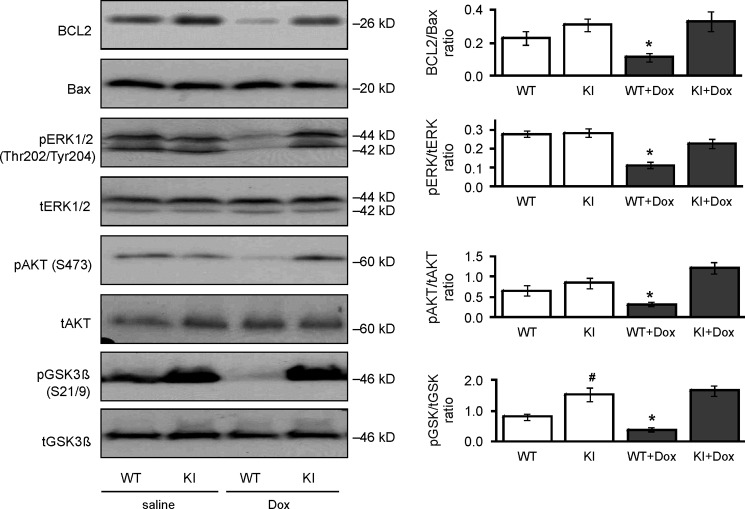
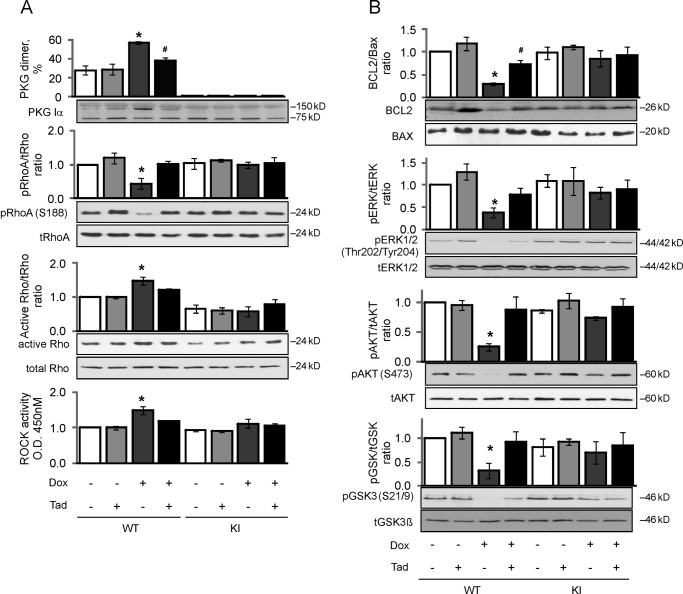
This cardioprotection afforded to the KI mouse was associated with reduction in myocardial tissue apoptosis compared with WT. Doxorubicin treatment increased myocardial tissue TUNEL-positive nuclear staining in WT heart from 0.34 ± 0.04% to 2.35 ± 0.54%. In contrast, doxorubicin did not increase the number of apoptotic nuclei in KI hearts, which was 0.41 ± 0.02% basally and remained similar at 0.44 ± 0.02% following chemotherapy treatment (Fig. 2B). Consistent with protection in the KI, the BCL2/Bax ratio, phospho-ERK, phospho-AKT, and phospho-GSK3β were each significantly decreased in doxorubicin-treated WT but not C42S PKG transgenic hearts subjected to the chemotherapy (Fig. 3).
FIGURE 3.
Doxorubicin decreased anti-apoptotic signaling in WT but not KI hearts. The expression and phosphorylation of the pro-survival, anti-apoptotic signaling proteins BCL2, Bax, ERK, AKT, and GSK3β were compared in hearts from WT or KI mice treated with doxorubicin (Dox) or vehicle. Doxorubicin caused a general loss in pro-survival signaling in WT cardiac tissue, but the hearts from KI were resistant to this pro-apoptotic event (n = 9–12). *, p < 0.05 between vehicle and doxorubicin groups; #, p < 0.05 between WT and KI groups.
In a subsequent, separate series of experiments the influence of PDE3, PDE5, or PDE9 inhibitors on the development of doxorubicin-induced cardiomyopathy and levels of cyclic nucleotides (cGMP or cAMP) in WT or KI mice was investigated. The PDE3 inhibitor milrinone did not protect WT mice from doxorubicin-induced cardiomyopathy and did not alter cGMP or cAMP levels. The PDE5 long half-life inhibitor tadalafil significantly increased cGMP, but not cAMP, in both WT or KI hearts and protected the WT myocardium from loss of function. Administration of the PDE9 inhibitor BAY 73-6691 resulted in partial protection of contractile function and a trend toward increased cardiac cGMP levels (supplemental Table S1).
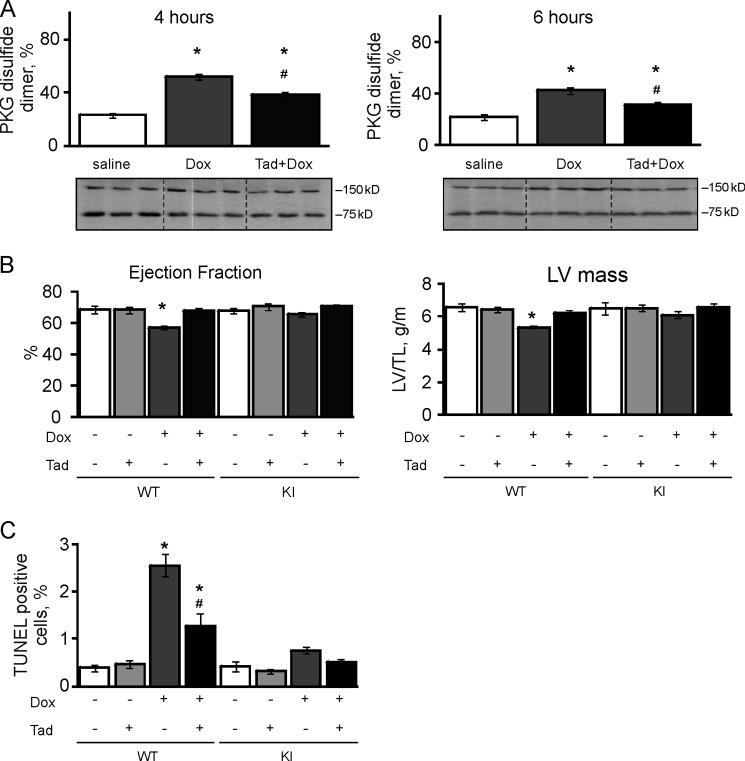
Based on the findings noted above, the PDE5 inhibitor tadalafil was used in subsequent experiments. Co-administration of tadalafil with doxorubicin reduced the oxidation induced by acute 4- or 6-h treatment with the chemotherapeutic alone (Fig. 4A). Again doxorubicin induced cardiac dysfunction in WT, lowering ejection fraction and left ventricular mass by 16.5 and 18.2%, respectively. Tadalafil afforded protection against doxorubicin, with only 1.3 and 5.5% losses in ejection fraction and left ventricular mass, respectively (Fig. 4B). Tadalafil treatment did not provide protection against doxorubicin-mediated injury in KI (Fig. 4B), although this was probably because the chemotherapy alone had little adverse effect on the myocardium of these mice. Similarly, the WT hearts exposed to doxorubicin once again showed enhanced levels of apoptosis as indexed by nuclear TUNEL staining (2.56 ± 0.24%). Treatment of WTs with tadalafil protected their hearts as evidenced by a lower level of TUNEL staining (1.26 ± 0.29%). The KIs were again resistant to doxorubicin-induced apoptosis (0.75 ± 0.07%) compared with WTs; this innate protection was not further improved by the co-administration of tadalafil (0.52 ± 0.04%; Fig. 4C).
FIGURE 4.
Tadalafil protects WT hearts from doxorubicin-induced injury and dysfunction. A, intraperitoneal injection of doxorubicin (Dox) caused oxidation of PKG Iα in mouse hearts 4 or 6 h after treatment (n = 3). Tadalafil (Tad) co-treatment significantly decreased the disulfide dimerization induced by the chemotherapy. B, echocardiographic examination of cardiac function was performed on WT or KI mice 5 days after doxorubicin or vehicle injection with or without Tadalafil treatment (n = 4). The ejection fraction and left ventricular mass (normalized by tibia length) decreased in WT, but not KI mice, treated with doxorubicin. Co-treatment with tadalafil prevented these maladaptive changes induced by the chemotherapy. C, a histological assessment was carried out on hearts from WT or KI mice treated with doxorubicin or vehicle in the presence or absence of tadalafil (n = 4). TUNEL staining demonstrated increased apoptotic nuclei after doxorubicin treatment in WT, but not KI hearts. Tadalafil treatment prevented the induction of apoptosis in WT but had little effect on the KI, which was basally resistant. *, p < 0.05 between vehicle and DOX groups; #, p < 0.05 between DOX alone and DOX + tadalafil groups.
Recurrent daily co-treatment with tadalafil limited disulfide dimerization in WT mouse hearts induced by 5 days of continuous doxorubicin exposure (Fig. 5A). Doxorubicin once again induced a significant reduction in the BCL2/Bax ratio, phospho-ERK, phospho-AKT, and phospho-GSK3β in WT mouse hearts. This doxorubicin-induced apoptosis (BCL2/Bax ratio) and attenuation of pro-survival kinase (phospho-ERK, phospho-AKT, and phospho-GSK3β) signaling in WT hearts was blocked by co-treatment with tadalafil (Fig. 5B). Tadalafil had little impact on these indices of cell health measured in KI hearts exposed to doxorubicin, albeit the C42S PKG transgenic mice had innate resistance to injury caused by doxorubicin (Figs. 4 and 5). Doxorubicin decreased phospho-RhoA (Ser-188) levels in WT hearts, which was abrogated by co-treatment with tadalafil (Fig. 5A). Mirroring these changes, doxorubicin also increased Rho-associated protein kinase (ROCK) activity in hearts from WTs exposed to doxorubicin. The phospho-RhoA Ser-188 levels or ROCK activity was the same in both genotypes basally, and was not modulated in KI following doxorubicin treatment either with or without tadalafil co-administration (Fig. 5A).
FIGURE 5.
Tadalafil limits doxorubicin-induced PKG Iα oxidation and the associated apoptotic signaling in WT, whereas KI are basally protected. A, PKG Iα disulfide dimerization, RhoA Ser-188 phosphorylation, RhoA, and ROCK activity in WT or KI hearts treated with doxorubicin (Dox) or vehicle, in the presence or absence of tadalafil (Tad), was indexed (n = 4). This demonstrated that doxorubicin triggered a loss of pro-survival phospho-RhoA (Ser-188) signaling in WT, and this was attenuated by PDE5 inhibition with tadalafil. B, BCL2/Bax ratio, ERK, AKT, and GSK3β phosphorylation in hearts from WT or KI mice treated with doxorubicin or vehicle, in the presence or absence of tadalafil, was indexed. Consistent with the loss in anti-apoptotic phospho-RhoA induced by doxorubicin in WT hearts, there was a concomitant loss of pro-survival signaling in terms of BCL2/Bax ratio, ERK, AKT, and GSK3β. *, p < 0.05 between vehicle and DOX groups; #, p < 0.05 between DOX alone and DOX + tadalafil groups.
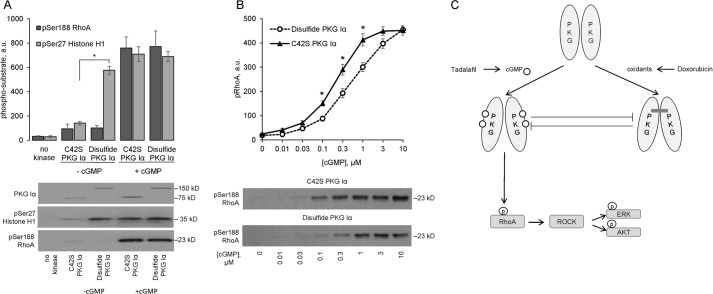
In vitro kinase assays showed disulfide-activated WT PKG Iα, as well as cGMP-activated C42S PKG Iα that cannot undergo oxidant-induced activation, phosphorylated histone H1. Similarly, cGMP-activated C42S PKG Iα also induced phosphorylation of RhoA, whereas the disulfide-activated kinase failed to induce phosphorylation of this target protein (Fig. 6A). Because oxidant-induced PKG Iα interprotein disulfide formation modulates cGMP-dependent substrate phosphorylation (9), the impact of the disulfide bond on the ability of PKG Iα to phosphorylate Ser-188 in RhoA was investigated using an in vitro kinase activity assay. Both oxidized or C42S mutant PKG Iα showed dose-dependent activation to cGMP as assessed by RhoA phosphorylation. However, the disulfide form of PKG Iα was less sensitive than the C42S mutant to cGMP-dependent activation. Thus, the EC50 for cGMP was 0.22 ± 0.07 μm for C42S PKG Iα, whereas the presence of the disulfide significantly (p < 0.05) desensitized the kinase ∼2.5-fold to 0.56 ± 0.06 μm cGMP (Fig. 6B).
FIGURE 6.
Oxidation of PKG Iα to the interprotein disulfide state attenuates cGMP-stimulated phosphorylation of Ser-188 RhoA. A, comparison of phosphorylation of Ser-188 RhoA with that of Ser-27 histone H1 by recombinant interprotein disulfide dimer or C42S PKG Iα (n = 3). Disulfide dimerization of PKG Iα caused its activation and resulted in phosphorylation of histone H1 but not of RhoA. In contrast, cGMP-dependent activation of the kinase resulted in phosphorylation of both substrates. B, impact of interprotein disulfide formation on the dose-dependent activation and phosphorylation of RhoA by PKG Iα (n = 4). Oxidation of PKG Iα attenuated cGMP-dependent activation of the kinase, such that the EC50 for cGMP was 0.22 ± 0.07 μm for C42S PKG Iα, whereas the presence of the disulfide significantly desensitized the kinase ∼2.5-fold to 0.56 ± 0.06 μm cGMP. *, p < 0.05 between disulfide dimer and C42S PKG Iα groups. C, schematic overview of the proposed mechanism by which tadalafil limits cardiac injury otherwise caused by chemotherapy with doxorubicin. Oxidation of PKG Iα to its disulfide state triggered by doxorubicin limits its classical activation and interaction with RhoA. This abolishes classical cGMP-PKG Iα pro-survival signaling that Rho phosphorylation would otherwise stimulate in the absence of oxidized kinase; this potentiates apoptosis with a consequential loss in cardiac tissue and function. These observations provide a molecular level rationalization for the cardioprotection PDE5 inhibitors can offer in the setting of failure, including that commonly triggered by doxorubicin chemotherapy.
Discussion
The widely expressed PKG Iα is stimulated by binding of cGMP, a second messenger that is elevated in cells in response to NO or natriuretic peptides. PKG Iα can also be activated independently of the NO-cGMP pathway by oxidants that induce interprotein disulfide bond formation (6). cGMP- or disulfide-activated PKG likely induce different conformations in the N terminus of PKG Iα. Because the N-terminal leucine zipper of the kinase mediates substrate binding, it is probable that differential targeting occurs depending on the amount of cGMP or oxidant in the cell. There is likely additional regulatory complexity because cGMP or endogenous oxidants can also be generated at different locations within the cell. Consequently, it is possible that scenario-specific localization of PKG Iα occurs depending on the subcellular source of cGMP or oxidant, as well as the amounts of each generated. Indeed, it is notable that the ability of PKG Iα to form a disulfide dimer impacts on its subcellular localization in cardiac myocytes in response to exogenous hydrogen peroxide or those from transaortic constriction (TAC)-stressed hearts (11).
The disulfide activation of PKG Iα is an integral mechanism of physiological blood pressure regulation under basal conditions. This PKG disulfide-induced vasodilation mechanism also in part mediates nitroglycerin- or hydrogen peroxide disulfide-induced blood pressure lowering, as well as contributing to sepsis-induced hypotension (7, 12–14). In contrast to the vasculature, the role of oxidant-induced disulfide activation of PKG in the heart is less explored and was the focus of this work.
Although doxorubicin treatment doubles the amount of PKG Iα disulfide to modulate downstream signaling, it is notable that there is also a significant level of oxidation under basal conditions. If PKG Iα oxidation limits cell survival, we have to reconcile this with the basal level present in viable tissue. There are many pro-survival or pro-apoptotic pathways, with a balance between them that has the net effect of limiting cell death in healthy myocardium. We reason that there is probably a threshold effect, in this case PKG Iα oxidation, which, when exceeded, tips the net balance in favor of apoptosis. Such threshold-mediated regulatory mechanisms are common in biological systems.
The role of different PDE isoforms in the development of cardiomyopathy and apoptosis has been widely explored. PDE3 has high affinity for both cAMP and cGMP. However, because PDE3 only slowly hydrolyzes the cGMP, this competitively blocks degradation of cAMP; thus, it has been called a “cGMP-inhibited PDE” (15). Acute PDE3 inhibition increases inotropy and cardiac output, as well as vasodilation. Thus, PDE3 inhibitors, including milrinone, have been used to treat congestive heart failure but failed because they increased mortality with long term therapy (16). One of the potential explanations of the detrimental effects of chronic PDE3 inhibition is enhanced cardiomyocyte apoptosis (15). In our experiments milrinone did not attenuate dysfunction induced by doxorubicin, but it only increased cAMP levels slightly and insignificantly. The lack of an effect by milrinone may have differed if an alternant dosing regimen had been used, especially because it has a half-life of only ∼2.3 h. PDE9, which is also expressed in the heart, hydrolyzes natriuretic-peptide-stimulated cGMP in cardiomyocytes. Its genetic depletion or selective pharmacological inhibition protects against pressure overload-induced pathological remodeling and reverses hypertrophy (17). BAY 73-6691, a selective and potent PDE9 inhibitor, efficiently inhibited this phosphodiesterase in cells (18). It improved learning and memory in rats (19) and increased corpus cavernosum relaxation (20). BAY 73-3391 co-treatment partially protected WT hearts from doxorubicin-induced loss of contractile function. However, although the inhibitor increased the cGMP levels by ∼50%, this did not reach statistical significance. The short 2-h half-life of BAY 73-3391 adds complexity when trying to correlate cGMP levels with cardioprotection from doxorubicin-induced toxicity.
Previously cGMP-elevating strategies achieved with pharmacological inhibitors of PDE5 limited cardiac injury caused by treatment with the anti-cancer drug doxorubicin, which induces oxidative stress through redox cycling reactions (3, 21–23). Indeed, cells or mice exposed to doxorubicin showed elevated PKG disulfide levels, which was attenuated by the presence of tadalafil. Because tadalafil increases cGMP, we reasoned that the protective effect of this long half-life PDE5 inhibitor involved it blocking PKG oxidation to the activated disulfide state. This was plausible because two independent previous studies showed that cGMP blocks PKG oxidation (8, 9). Indeed, this turned out to be the case, with tadalafil limiting increases in disulfide PKG Iα occurring during either acute (several hours) or chronic (5 days) exposure to doxorubicin. This led to the hypothesis that disulfide bond formation in myocardial PKG Iα may be maladaptive, because it would provide a rational mechanistic explanation for the reported ability of tadalafil, and also sildenafil, to limit doxorubicin-induced injury (3, 21–23).
To further investigate the potential causative role for PKG oxidation in myocardial injury, a C42S PKG Iα KI mouse that is fully resistant to interprotein disulfide formation was compared with littermate WT controls. This involved monitoring their basal status and subsequent responses to doxorubicin with or without co-treatment with tadalafil in both genotypes. The most significant finding was that the KI mouse was highly resistant to myocardial injury induced by doxorubicin compared with the WT. This protection in the KI was evident from echocardiographic analysis of heart mass and cardiac function, as well as histological assessment of apoptosis. Clearly genetic engineering strategies that prevent, or pharmacological interventions that limit, PKG Iα oxidation are cardioprotective. The protective effect of PDE5 inhibitors, including sildenafil or tadalafil, has been widely observed in different models of heart failure (4, 21, 24–26). This protection afforded by cGMP is not mediated by classical activation of PKG, because the PDE5 inhibitors were not protective in the C42S PKG Iα KI, which is fully responsive to the NO-cGMP pathway (7).These findings were consistent with oxidative activation of the kinase coupling to injury, with cGMP elevating strategies being protective significantly because they limit PKG Iα oxidation.
The NO-cGMP-PKG pathway is known to regulate apoptosis (27, 28), with cGMP limiting activation of pro-apoptotic pathways to promote cell survival (29). Consequently, a logical hypothesis was that the KI was innately protected from doxorubicin toxicity because it was resistant to induction of apoptosis, and this could be recapitulated in WT by tadalafil because it represses the maladaptive pathway triggered by PKG Iα oxidation. Several lines of evidence suggested that that the myocardium of KI mice was highly resistant to apoptosis (TUNEL positive nuclei) or loss of flux through the ERK, AKT, and GSK3β pro-survival signaling pathways that occurred in WT following exposure to doxorubicin. This loss or pro-survival kinase signaling and elevated levels of apoptosis induced by doxorubicin was efficiently blocked by co-administration of tadalafil, further supporting a causative role for disulfide PKG in myocardial cell death during oxidative stress.
We reasoned that there was likely a molecular link between disulfide PKG and loss of pro-survival signaling and induction of apoptosis. One of the well established targets of PKG Iα is RhoA, a member of the small GTPase family, members of which are known for their ability to regulate growth and survival (30). Indeed, RhoA itself mediates many cell processes including survival (31, 32), transmitting signals to end effectors by binding the C terminus of ROCK, which activates it (33). Unfortunately, we failed to develop a co-immunoprecipitation protocol that would allow the interaction of PKG Iα with RhoA to be assessed in tissue under the various experimental conditions. However, we did demonstrate that disulfide-activated PKG Iα does not phosphorylate RhoA in vitro. This was not because the disulfide-activated kinase lacked catalytic competency, because this oxidized form efficiently phosphorylated histone protein. Furthermore, when this disulfide form of PKG Iα was subsequently incubated with cGMP, it then also induced phosphorylation of RhoA. Although we found that the interprotein disulfide decreased the sensitivity of the kinase to cGMP-dependent activation. Thus, a higher concentration of cGMP is required to induce the same degree of RhoA phosphorylation achieved by C42S PKG Iα, which cannot form the disulfide. Thus, disulfide-PKG alters its substrate preference, an event that is further modulated by the cGMP concentration. In WT heart there was a basal level of phospho-RhoA (Ser-188), which was enhanced by tadalafil treatment, consistent with an elevation in cGMP levels that classically activate PKG. In contrast, doxorubicin treatment that induced disulfide PKG Iα caused a robust decrease in phospho-RhoA levels. Thus, it seems that oxidation of PKG Iα limits the phosphorylation of RhoA stimulated by the NO-cGMP pathway that can be potentiated by tadalafil, which enhances cGMP levels by inhibiting its hydrolysis by PDE5. This concept is robustly supported by the in vitro studies that show that interprotein disulfide formation activates the kinase, although this form of the kinase does not phosphorylate RhoA unless cGMP is also present. Even when cGMP is present, the interdisulfide desensitizes the kinase to this classical mode of activation by the second messenger. These observations are in line with another study also showing that PKG Iα interprotein disulfide desensitizes the kinase to cGMP-dependent phosphorylation of VASP (vasodilator-stimulated phosphoprotein) protein (9). Because cGMP abrogates disulfide formation in PKG Iα (8, 9), we conclude that each mode of activation reciprocally negatively regulates the alternate mechanism as illustrated in Fig. 6C.
The phosphorylation of RhoA at Ser-188 negatively regulates its activity through an enhanced Rho guanine-dissociation inhibitor interaction (34), and so the reduced phospho-RhoA levels induced by doxorubicin in WT hearts are anticipated to stimulate RhoA activity, which it did. This enhanced RhoA activity should elevate ROCK activity, which it did, an event tightly associated with myocardial apoptotic injury (35). This series of events provides an explanation for why doxorubicin triggers apoptosis in WT but not KI mouse heart and why tadalafil limits the development of injury. Further credence for this proposed order of events, which are summarized in Fig. 6C, comes from studies showing that RhoA binds the N-terminal leucine zipper of PKG Iα (36). The importance of the leucine zipper in the cGMP-dependent binding to RhoA with PKG Iα is demonstrated by subtle alterations to the amphipathic helix preventing their binding (36). Therefore phosphorylation and inactivation of RhoA requires cGMP-activated PKG Iα with an intact leucine zipper (36), which if perturbed in vivo results in loss of competent kinase function and hypertension (37). These PKG Iα zipper mutant mice also have potentiated pathologic cardiac hypertrophy responses to TAC-induced pressure overload compared with WT mice. Furthermore, the zipper mutant mice lacked the sildenafil-mediated protection from TAC afforded to WT controls (24). Thus, the zipper in PKG Iα is integral to the protection that elevation in cGMP by PDE5 inhibitors can provide, and this is because it enables the kinase to bind and phospho-activate RhoA, which ultimately limits apoptosis. The C42S PKG Iα KI mice are also innately resistant to TAC-induced cardiac dysfunction (11). The disulfide in PKG Iα occurs between Cys-42 residues directly within the leucine zipper domain required for targeting of the kinase to RhoA. This Cys-42 is ideally located to modulate the interaction with the RhoA GTPase depending on the redox state of the kinase, with disulfide formation limiting the phosphoactivation and anti-apoptotic signaling the GTPase would otherwise afford in the absence of Cys-42 oxidation. Pharmacological inhibition of PDE5 also protected hair cells from noise-induced cell death and hearing loss (38), perhaps consistent with disulfide activation of PKG Iα being a generic mechanism of cell death in different cell types.
In summary, oxidation of PKG Iα to its disulfide state triggered by doxorubicin limits its interaction with RhoA. This abolishes classical cGMP-PKG Iα pro-survival signaling, resulting in potentiated apoptosis and a consequential loss in cardiac mass and function. These observations provide a molecular level rationalization for the broad cardioprotection PDE5 inhibitors can offer, including in the setting of doxorubicin chemotherapy. Previous studies have primarily focused on the role of PKG Iα redox state in the control of vascular tone and blood pressure, whereas this work highlights an important role for the oxidation state of this kinase in regulating myocardial tissue viability during stress.
Experimental Procedures
Animals
All procedures were performed in accordance with the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act 1986 in the United Kingdom and were approved by the King's College London Animal Welfare and Ethical Review Body. Mice constitutively expressing PKG Iα C42S were generated on a pure C57BL/6 background by Taconic as described elsewhere (7) with male mice solely being used in this study. For the assessment of doxorubicin-induced cardiomyopathy, male WT or C42S PKG Iα KI age- and weight-matched littermate mice were randomly assigned to intraperitoneal saline (0.2 ml) or doxorubicin (Sigma) (15 mg/kg) injection.
In a separate set of experiments, the PDE3 inhibitor milrinone (sc-201193, Santa Cruz Biotechnology, 0.5 mg/kg in 10% DMSO), the PDE5 inhibitor tadalafil (14024, Cayman, 1 mg/kg in 10% DMSO), the PDE9 inhibitor BAY 73-6691 (sc-252407, Santa Cruz Biotechnology, 1 mg/kg in 10% DMSO), or vehicle (0.2 ml 10% DMSO in saline) was injected 30 min before doxorubicin on day 0. Thereafter PDE inhibitors were administered daily, until day 5 when the experiment was terminated. The mice were culled by intraperitoneal injection of 6.6% sodium pentobarbitone (200 mg/kg) premixed with heparin (500 USP units), and tissues were collected for biochemical or histological analyses.
Echocardiography
The mice were anesthetized and examined by echocardiography on day 5 using a high resolution Vevo 770 echocardiography system (VisualSonics) with a RMV-707B transducer running at 30 MHz. High resolution, two-dimensional B-mode and M-mode images at the level of the papillary muscles were obtained for offline measurements with Vevo Software (VisualSonics).
cGMP and cAMP Measurements
Assessment of cGMP or cAMP content was made with Amersham Biosciences cAMP (RPN225, GE Healthcare) or cGMP (RPN226, GE Healthcare) Biotrak Enzyme immunoassay kits following the manufacturer's instructions. Briefly hearts were snap frozen and homogenized in cold 6% trichloroacetic acid at 2–8 °C to provide a 10% (w/v) homogenate. Aliquots were centrifuged at 2000 × g for 15 min at 4 °C, and the pellet was discarded. The supernatant was washed 4 times with 5 volumes of water-saturated diethyl ether. The remaining aqueous extract was lyophilized and then dissolved in the manufacturer's assay buffer prior to analysis.
Western Immunoblotting and Rho-ROCK Activity Assays
Immunoblotting was carried out as before (39), utilizing maleimide (100 mm) in preparation buffers to alkylate thiols and limit thiol disulfide exchange. Antibodies used in these studies included Enzo Life Science PKG (ADI-KAP-PK005), BD Transduction BCL2 (610538), Sigma phospho-histone H1.4 (Ser-27) (H7664), Cell Signaling Bax (2774), phospho-AKT (Ser-473) (9271), AKT (9272), phospho-p44/42 MAPK (Erk1/2) (Thr-202/Tyr-204) (9101), p44/42 MAPK (Erk1/2) (9102), phospho-GSK-3α/β (Ser-21/9) (9331), GSK-3β (27C10) (9315), Santa Cruz Biotechnology phospho-Rho A (Ser-188) (sc-32954), HRP-linked secondary antibody (Cell Signaling), and ECL reagent (GE Healthcare) were used. Digitized immunoblots were analyzed quantitatively with Gel-Pro Analyzer 3.1 software.
Rho activity was assessed using a Cell Signaling kit (8820) according to the manufacturer's instructions. Measurement of GTPase activity was based on the ability of GTP-bound (active) form to bind Rhotekin-RBD fusion protein, which can then be immunoprecipitated with glutathione resin. The level of activation was then determined using Western blot analysis with a Rho antibody. ROCK activity was estimated using the ELISA-based ROCK Assay from Cell Biolabs, following the manufacturer's protocol.
Generation of Recombinant WT or C42S PKG Iα
Expression and purification of WT or mutant PKG Iα encoded in a pCDNA3 expression vector was essentially as before (40), but with some minor protocol alterations. Suspension FreeStyle 293-F cells (R79007, ThermoFisher Scientific) were transfected with the appropriate PKG Iα construct using polyethyleneimine as a transfection reagent. The cells were harvested by centrifugation (400 × g at room temperature for 15 min) after ∼72 h. They were then resuspended in ice-cold lysis buffer comprising 25 mm sodium phosphate buffer, pH 6.8, 10 mm EDTA, 100 mm NaCl, 10 mm benzamidine hydrochloride, and 10 mm dithiothreitol and then frozen in liquid nitrogen. The cells were lysed by three freeze-thaw (liquid nitrogen/37 °C) cycles. The resulting lysate was centrifuged at 140,000 × g and 4 °C for 30 min, and the soluble supernatant was loaded onto a pre-equilibrated 8-(2-aminoethylamino)adenosine-3′,5′-cyclic monophosphate column (8-AEA-cAMP agarose; BioLog, Germany), after which the column was washed with 20 column volumes of lysis buffer and then 5 column volumes of lysis buffer supplemented with 3 m NaCl. PKG Iα was then eluted with lysis buffer supplemented with 150 mm NaCl and 500 μm cAMP, with subsequent extensive dialysis against 25 mm sodium phosphate buffer, pH 6.8, 2 mm EDTA, and 100 mm NaCl to remove the cAMP. In some protocols, to generate interprotein disulfide WT PKG Iα, it was incubated with 10 mm lipoic acid for 2 h on ice to obtain ∼100% oxidized kinase, as confirmed by SDS-PAGE. Disulfide WT or C42S PKG Iα was then underwent size exclusion chromatography on a HiLoad 16/600 Superdex 200-pg column (GE Healthcare) conditioned with 50 mm sodium phosphate buffer, pH 7.4, and 100 mm NaCl. Proteins were subsequently concentrated with an Amicon Ultra centrifugal filter (Merck Millipore).
In Vitro Kinase Activity Assay
Stoichiometrically oxidized (to the interprotein disulfide homodimer) or C42S recombinant PKG Iα (200 ng) was incubated with 800 ng of recombinant RhoA (SRP5127, Sigma) or histone H1 (sc-221729, Santa Cruz) in 20 μl of Omnia Tyr kinase reaction buffer (10× stock, KB002A, Invitrogen) comprising 20 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 1 mm EGTA, 5 mm β-glycerophosphate, 5% glycerol, 1 mm ATP, and 0–10 μm cGMP (B1381, Sigma). The reactions were incubated at 30 °C for 15 min and terminated by addition of 2× non-reducing SDS sample buffer supplemented with 100 mm maleimide. Proteins were separated by 4–20% SDS-PAGE, and phosphorylation of substrates was visualized by Western immunoblot analysis.
Histology
Myocardium was fixed with 4% formaldehyde, paraffin-embedded, and sectioned into 5-μm slices. Sections were stained with hematoxylin and eosin. Masson's trichrome staining was used to visualize collagen. Apoptosis in the left ventricle was detected by TUNEL staining using the CardioTacs staining kit (Trevigen) to generate a dark blue precipitate in sections. Image capture was performed using Leica DMRB microscope tiling. Nuclei count was obtained in a blinded fashion with an automated algorithm using Image Pro Plus (Media Cybernetics).
Statistics
The results are presented as means ± S.E. Differences between groups were assessed using one-way analysis of variance followed by a t test. Differences were considered significant at the 95% confidence level.
Author Contributions
O. P., J. B., and P. E. conceived and designed the study and wrote the paper. O. P., J. B., and J. S. performed and analyzed experiments. D. K. conceived the study and contributed to analysis and interpretation of results. S. G. provided technical assistance and contributed to the preparation of Fig. 2. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Material
This work was supported by the European Research Council (Advanced Award), the British Heart Foundation, the Medical Research Council and Fondation Leducq, and the Department of Health via the National Institute for Health Research Comprehensive Biomedical Research Centre award to Guy's & St Thomas' National Health Service Foundation Trust. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Table S1.
- PDE
- phosphodiesterase
- KI
- knock-in
- PKG
- protein kinase G
- TAC
- transaortic constriction
- DOX
- doxorubicin
- ROCK
- rho-associated protein kinase.
References
- 1. Chatterjee K., Zhang J., Honbo N., and Karliner J. S. (2010) Doxorubicin cardiomyopathy. Cardiology 115, 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi Y., Moon M., Dawood S., McManus B., and Liu P. P. (2011) Mechanisms and management of doxorubicin cardiotoxicity. Herz 36, 296–305 [DOI] [PubMed] [Google Scholar]
- 3. Koka S., Das A., Zhu S. G., Durrant D., Xi L., and Kukreja R. C. (2010) Long-acting phosphodiesterase-5 inhibitor tadalafil attenuates doxorubicin-induced cardiomyopathy without interfering with chemotherapeutic effect. J. Pharmacol. Exp. Ther. 334, 1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guazzi M., Vicenzi M., Arena R., and Guazzi M. D. (2011) PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ. Heart Fail. 4, 8–17 [DOI] [PubMed] [Google Scholar]
- 5. Francis S. H., Busch J. L., Corbin J. D., and Sibley D. (2010) cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 62, 525–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burgoyne J. R., Madhani M., Cuello F., Charles R. L., Brennan J. P., Schröder E., Browning D. D., and Eaton P. (2007) Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 317, 1393–1397 [DOI] [PubMed] [Google Scholar]
- 7. Prysyazhna O., Rudyk O., and Eaton P. (2012) Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat. Med. 18, 286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burgoyne J. R., Prysyazhna O., Rudyk O., and Eaton P. (2012) cGMP-dependent activation of protein kinase G precludes disulfide activation: implications for blood pressure control. Hypertension 60, 1301–1308 [DOI] [PubMed] [Google Scholar]
- 9. Müller P. M., Gnügge R., Dhayade S., Thunemann M., Krippeit-Drews P., Drews G., and Feil R. (2012) H2O2 lowers the cytosolic Ca2+ concentration via activation of cGMP-dependent protein kinase Iα. Free Radic Biol. Med. 53, 1574–1583 [DOI] [PubMed] [Google Scholar]
- 10. Davies K. J., and Doroshow J. H. (1986) Redox cycling of anthracyclines by cardiac mitochondria: I. Anthracycline radical formation by NADH dehydrogenase. J. Biol. Chem. 261, 3060–3067 [PubMed] [Google Scholar]
- 11. Nakamura T., Ranek M. J., Lee D. I., Shalkey Hahn V., Kim C., Eaton P., and Kass D. A. (2015) Prevention of PKG1α oxidation augments cardioprotection in the stressed heart. J. Clin. Invest. 125, 2468–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rudyk O., Phinikaridou A., Prysyazhna O., Burgoyne J. R., Botnar R. M., and Eaton P. (2013) Protein kinase G oxidation is a major cause of injury during sepsis. Proc. Natl. Acad. Sci. U.S.A. 110, 9909–9913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rudyk O., Prysyazhna O., Burgoyne J. R., and Eaton P. (2012) Nitroglycerin fails to lower blood pressure in redox-dead Cys42Ser PKG1α knock-in mouse. Circulation 126, 287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stubbert D., Prysyazhna O., Rudyk O., Scotcher J., Burgoyne J. R., and Eaton P. (2014) Protein kinase G Iα oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension 64, 1344–1351 [DOI] [PubMed] [Google Scholar]
- 15. Yan C., Miller C. L., and Abe J. (2007) Regulation of phosphodiesterase 3 and inducible cAMP early repressor in the heart. Circ. Res. 100, 489–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baim D. S., McDowell A. V., Cherniles J., Monrad E. S., Parker J. A., Edelson J., Braunwald E., and Grossman W. (1983) Evaluation of a new bipyridine inotropic agent–milrinone–in patients with severe congestive heart failure. N. Engl. J. Med. 309, 748–756 [DOI] [PubMed] [Google Scholar]
- 17. Lee D. I., Zhu G., Sasaki T., Cho G. S., Hamdani N., Holewinski R., Jo S. H., Danner T., Zhang M., Rainer P. P., Bedja D., Kirk J. A., Ranek M. J., Dostmann W. R., Kwon C., et al. (2015) Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature 519, 472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wunder F., Tersteegen A., Rebmann A., Erb C., Fahrig T., and Hendrix M. (2005) Characterization of the first potent and selective PDE9 inhibitor using a cGMP reporter cell line. Mol. Pharmacol. 68, 1775–1781 [DOI] [PubMed] [Google Scholar]
- 19. van der Staay F. J., Rutten K., Bärfacker L., Devry J., Erb C., Heckroth H., Karthaus D., Tersteegen A., van Kampen M., Blokland A., Prickaerts J., Reymann K. G., Schröder U. H., and Hendrix M. (2008) The novel selective PDE9 inhibitor BAY 73-6691 improves learning and memory in rodents. Neuropharmacology 55, 908–918 [DOI] [PubMed] [Google Scholar]
- 20. da Silva F. H., Pereira M. N., Franco-Penteado C. F., De Nucci G., Antunes E., and Claudino M. A. (2013) Phosphodiesterase-9 (PDE9) inhibition with BAY 73-6691 increases corpus cavernosum relaxations mediated by nitric oxide-cyclic GMP pathway in mice. Int. J. Impot. Res. 25, 69–73 [DOI] [PubMed] [Google Scholar]
- 21. Fisher P. W., Salloum F., Das A., Hyder H., and Kukreja R. C. (2005) Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation 111, 1601–1610 [DOI] [PubMed] [Google Scholar]
- 22. Das A., Durrant D., Mitchell C., Mayton E., Hoke N. N., Salloum F. N., Park M. A., Qureshi I., Lee R., Dent P., and Kukreja R. C. (2010) Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc. Natl. Acad. Sci. U.S.A. 107, 18202–18207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jin Z., Zhang J., Zhi H., Hong B., Zhang S., Guo H., and Li L. (2013) Beneficial effects of tadalafil on left ventricular dysfunction in doxorubicin-induced cardiomyopathy. J. Cardiol. 62, 110–116 [DOI] [PubMed] [Google Scholar]
- 24. Blanton R. M., Takimoto E., Lane A. M., Aronovitz M., Piotrowski R., Karas R. H., Kass D. A., and Mendelsohn M. E. (2012) Protein kinase Giα inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J. Am. Heart Assoc. 1, e003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagayama T., Hsu S., Zhang M., Koitabashi N., Bedja D., Gabrielson K. L., Takimoto E., and Kass D. A. (2009) Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J. Am. Coll Cardiol 53, 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takimoto E., Champion H. C., Li M., Belardi D., Ren S., Rodriguez E. R., Bedja D., Gabrielson K. L., Wang Y., and Kass D. A. (2005) Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat. Med. 11, 214–222 [DOI] [PubMed] [Google Scholar]
- 27. Fiscus R. R. (2002) Involvement of cyclic GMP and protein kinase G in the regulation of apoptosis and survival in neural cells. Neurosignals 11, 175–190 [DOI] [PubMed] [Google Scholar]
- 28. Kim Y. M., Bombeck C. A., and Billiar T. R. (1999) Nitric oxide as a bifunctional regulator of apoptosis. Circ. Res. 84, 253–256 [DOI] [PubMed] [Google Scholar]
- 29. Fiscus R. R., Yuen J. P., Chan S. L., Kwong J. H., and Chew S. B. (2002) Nitric oxide and cyclic GMP as pro- and anti-apoptotic agents. J. Card. Surg. 17, 336–339 [DOI] [PubMed] [Google Scholar]
- 30. Li F., Jiang Q., Shi K. J., Luo H., Yang Y., and Xu C. M. (2013) RhoA modulates functional and physical interaction between ROCK1 and Erk1/2 in selenite-induced apoptosis of leukaemia cells. Cell Death Dis. 4, e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown J. H., Del Re D. P., and Sussman M. A. (2006) The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ. Res. 98, 730–742 [DOI] [PubMed] [Google Scholar]
- 32. Lauriol J., Keith K., Jaffré F., Couvillon A., Saci A., Goonasekera S. A., McCarthy J. R., Kessinger C. W., Wang J., Ke Q., Kang P. M., Molkentin J. D., Carpenter C., and Kontaridis M. I. (2014) RhoA signaling in cardiomyocytes protects against stress-induced heart failure but facilitates cardiac fibrosis. Sci. Signal. 7, ra100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jacobs M., Hayakawa K., Swenson L., Bellon S., Fleming M., Taslimi P., and Doran J. (2006) The structure of dimeric ROCK I reveals the mechanism for ligand selectivity. J. Biol. Chem. 281, 260–268 [DOI] [PubMed] [Google Scholar]
- 34. Ellerbroek S. M., Wennerberg K., and Burridge K. (2003) Serine phosphorylation negatively regulates RhoA in vivo. J. Biol. Chem. 278, 19023–19031 [DOI] [PubMed] [Google Scholar]
- 35. Loirand G., Guérin P., and Pacaud P. (2006) Rho kinases in cardiovascular physiology and pathophysiology. Circ. Res. 98, 322–334 [DOI] [PubMed] [Google Scholar]
- 36. Kato M., Blanton R., Wang G. R., Judson T. J., Abe Y., Myoishi M., Karas R. H., and Mendelsohn M. E. (2012) Direct binding and regulation of RhoA protein by cyclic GMP-dependent protein kinase Iα. J. Biol. Chem. 287, 41342–41351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Michael S. K., Surks H. K., Wang Y., Zhu Y., Blanton R., Jamnongjit M., Aronovitz M., Baur W., Ohtani K., Wilkerson M. K., Bonev A. D., Nelson M. T., Karas R. H., and Mendelsohn M. E. (2008) High blood pressure arising from a defect in vascular function. Proc. Natl. Acad. Sci. U.S.A. 105, 6702–6707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jaumann M., Dettling J., Gubelt M., Zimmermann U., Gerling A., Paquet-Durand F., Feil S., Wolpert S., Franz C., Varakina K., Xiong H., Brandt N., Kuhn S., Geisler H. S., Rohbock K., et al. (2012) cGMP-Prkg1 signaling and Pde5 inhibition shelter cochlear hair cells and hearing function. Nat. Med. 18, 252–259 [DOI] [PubMed] [Google Scholar]
- 39. Brennan J. P., Bardswell S. C., Burgoyne J. R., Fuller W., Schröder E., Wait R., Begum S., Kentish J. C., and Eaton P. (2006) Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J. Biol. Chem. 281, 21827–21836 [DOI] [PubMed] [Google Scholar]
- 40. Alverdi V., Mazon H., Versluis C., Hemrika W., Esposito G., van den Heuvel R., Scholten A., and Heck A. J. (2008) cGMP-binding prepares PKG for substrate binding by disclosing the C-terminal domain. J. Mol. Biol. 375, 1380–1393 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.