Abstract
Monoclonal antibodies (mAbs) are the fastest growing class of therapeutic drugs, because of their high specificities to target cells. Facile analysis of therapeutic mAbs and their post-translational modifications (PTMs) is essential for quality control, and mass spectrometry (MS) is the most powerful tool for antibody characterization. This study uses pepsin-containing nylon membranes as controlled proteolysis reactors for mAb digestion prior to ultrahigh-resolution Orbitrap MS analysis. Variation of the residence times (from 3 ms to 3 s) of antibody solutions in the membranes yields “bottom-up” (1–2 kDa) to “middle-down” (5–15 kDa) peptide sizes within less than 10 min. These peptides cover the entire sequences of Trastuzumab and a Waters antibody, and a proteolytic peptide comprised of 140 amino acids from the Waters antibody contains all three complementarity determining regions on the light chain. This work compares the performance of “bottom-up” (in-solution tryptic digestion), “top-down” (intact protein fragmentation), and “middle-down” (in-membrane digestion) analysis of an antibody light chain. Data from tandem MS show 99%, 55%, and 99% bond cleavage for “bottom-up”, “top-down”, and “middle-down” analyses, respectively. In-membrane digestion also facilitates detection of PTMs such as oxidation, deamidation, N-terminal pyroglutamic acid formation, and glycosylation. Compared to “bottom-up” and “top-down” approaches for antibody characterization, in-membrane digestion uses minimal sample preparation time, and this technique also yields high peptide and sequence coverage for the identification of PTMs.
Graphical Abstract
Monoclonal antibodies (mAbs) have emerged as an important class of biotherapeutic drugs with high selectivity and specificity,1–3 and the U.S. Food and Drug Administration (FDA) has approved more than 35 antibodies4 for treatment of diseases such as breast cancer,5 non-Hodgkin lymphoma,6 and colorectal cancer.7 According to the FDA’s “quality by design” policy, biotherapeutic materials such as mAbs must adhere to a consistent, predefined quality during manufacturing.8 Facile mAb characterization, especially in the complementarity determining regions (CDRs), is vital for quality control, not only because these proteins are vulnerable to chemical modifications during expression, purification, and long-term storage, but also because they have natural heterogeneities.9–11 Common PTMs on mAbs include methionine oxidation, asparagine deamidation, asparagine glycosylation in the heavy-chain constant region 2 (CH2), and heavy chain C-terminal processing.12–21
MS is the most powerful tool for antibody characterization, because of its high resolution and mass accuracy within a wide dynamic range. Current MS-based strategies for antibody characterization employ “top-down”,22–25 “bottom-up”,26–35 and “middle-down”36–40 approaches with ultrahigh-resolution time-of-flight (TOF),24,27,29,30,33–35,39 Fourier transform ion cyclotron resonance,25,41 and Orbitrap22,23,26,36–38,40 mass spectrometers. “Top-down” methods introduce the intact antibody into the mass spectrometer through liquid chromatography (LC) or direct infusion. Determination of the intact protein mass and subsequent gas-phase fragmentation via collision-induced dissociation (CID),22,23 higher-energy collision dissociation (HCD), electron capture dissociation (ECD),25 or/and electron transfer dissociation (ETD)24 give an overview of the major PTMs with minimal sample manipulation time. Unfortunately, PTMs with small mass changes (e.g., deamidation (+1 Da)) cannot be detected, and sequence coverage for “top-down” analysis typically reaches only ~35%.25,42 The incomplete fragmentation likely results from highly structured and disulfide bond-protected areas.41
The “bottom-up” method uses enzymatic antibody digestion followed by LC and tandem mass spectrometry (MS/MS) analysis to provide accurate mass values and product ions that imply the sequences of individual peptides. However, protein digestion typically requires several time-consuming steps during which antibody modification may occur.43,44 Trypsin digestion, for instance, generally includes antibody denaturation and reduction, followed by alkylation of thiol groups to prevent reforming of disulfide bonds. Moreover, proteolysis usually takes place at 37 °C overnight, and basic digestion conditions favor deamidation of asparagine.45 Peptide coverage is frequently incomplete, because of weak ionization efficiencies for some peptides, along with the loss of a few peptides during LC.46 Nevertheless, the analysis of several digests catalyzed by different proteases often yields 100% sequence coverage.47 Recently, Srzentić et al. reported that digestion using the enzyme Sap 9 yields relatively large peptides (compared to tryptic digestion) and enables extended “bottom-up” LC-MS/MS analysis with almost 100% peptide coverage for both light chains and heavy chains.26 Importantly, this enzyme functions under acidic conditions that limit deamidation and avoid the need for alkylation of cysteine. However, Sap9 must be recombinantly expressed and purified.
Analysis of larger peptides (3–20 kDa) obtained from limited digestion is usually termed a “middle-down” approach.48 Ultrahigh-resolution mass spectrometers can resolve the isotopic distributions of these peptides, and their relatively large size enhances the total peptide coverage (relative to tryptic digestion), which increases the sequence coverage when fragmentation is complete. In addition, compared to “bottom-up” methods, the large size of “middle-down” peptides increases the probability that two or more PTMs will occur on the same peptide to enable correlation of these PTMs.49 In one “middle-down” strategy that yields peptides with masses of ~25 kDa, papain cleaves antibodies at the hinge region and forms subunits such as Fab or F(ab′)2, but the digestion is sometimes difficult to control.11 Fornelli and co-workers employed the immunoglobulin-degrading enzyme of Streptococcus (IdeS) to fragment Adalimumab into F(ab′)2 and Fc portions at the G–G bond below the hinge region.38 After reduction and denaturation, they obtained Fd, Lc, and Fc/2 fragments and analyzed these large peptides with LC-ETD MS/MS. Sequence coverage reached 70%. The large size of these peptides may still make detection of deamidation difficult, and incomplete fragmentation limits sequence coverage.
In addition to varying the digestion enzyme, limiting the digestion time may yield the large peptides required for “middle-down” protein characterization. Recently, Tan and coworkers adsorbed pepsin in the pores of nylon membranes and found that the lengths of proteolytic peptides from myoglobin and bovine serum albumin vary with the residence time of the protein solution in the membrane (i.e., shorter residence times generate longer peptides).50 This method can generate both “bottom-up” (long residence times) and “middle-down” (short residence times) peptides using a single enzyme, so control over digestion yields peptides with overlapping sequences. Small peptides give detailed sequence information, whereas larger peptides lead to higher coverage. The large peptides may also contain many basic residues that lead to higher charge states, which is beneficial for ETD fragmentation.51
This research employs antibody proteolysis in pepsin-containing porous membranes to decrease the time and cost of digestion, increase both the peptide and sequence coverages in MS analysis, and limit antibody modification during digestion. Figure 1 shows the work flow for digestion and analysis. Porous nylon membranes and pepsin are inexpensive and readily available, and the high enzyme concentration in membrane pores allows digestion within a few minutes. Moreover, acidic digestion conditions limit deamidation and do not require protection of the thiol groups of cysteine. Other recent substrates for pepsin immobilization include aldehyde-modified polymethacrylate monoliths52 and fused-silica capillaries,53 but such supports do not readily afford the millisecond residence times available with in-membrane digestion. This study investigates in-membrane digestion of the entire antibody without separation of the light and heavy chains, as well as digestion of separated chains. Remarkably, digestion of 35 pmol of a reduced Waters antibody (WIgG1) occurs within less than 1 min with 100% peptide coverage of the light and heavy chains. We further demonstrate the benefits of this digestion strategy by comparing MS analyses of a mAb light chain after digestion in a pepsin-containing membrane, after traditional insolution digestion and using a “top-down” method.
Figure 1.
Workflow for controlled digestion and analysis of antibodies. [Acronyms: TCEP, tris(2-carboxyethyl) phosphine; VH, variable region of the heavy chain; CH1, CH2, and CH3, different constant regions of the heavy chain; CL, constant region of the light chain; VL, variable region of the light chain; Lc, light chain; Hc, heavy chain.]
EXPERIMENTAL SECTION
Materials
A monoclonal immunoglobulin G was purchased from Waters (WIgG1, Intact mAb Mass Check Standard, No. 186006552), and Trastuzumab (Herceptin, Genentech) was dissolved in a phosphate buffer (KH2PO4 144 mg/L, NaCl 9000 mg/L, and Na2HPO4·7H2O 795 mg/L, pH 7.4, Thermo Fisher) at a concentration of 21 mg/mL. Nylon membranes (LoProdyne LP, pore size 1.2 µm, 110 µm thickness) were acquired from Pall Corporation. The holder for membrane digestion (flangeless fitting system, Upchurch Scientific, No. A-424) was connected to 1/16 in. outer diameter (OD) tubing via ferrules.50 Pepsin from porcine gastric mucosa (lyophilized powder, 3200–4500 units/mg protein), iodoacetamide (IAM, ≥ 99%), polystyrene sulfonate (PSS, average molecular weight ~70 000), and acetonitrile (ACN, HPLC grade, ≥ 99.9%) were obtained from Sigma–Aldrich. Isopropyl alcohol (IPA, MACRON), sequencing-grade modified trypsin (Promega), and trifluoroacetic acid (TFA, purchased from EMD) were used as received. Important chemicals for reduction and digestion include tris(2-carboxyethyl) phosphine hydrochloride (TCEP-HCl, >98%, Fluka), acetic acid (HOAc, Mallinckrodt, ACS), formic acid (>96%, Spectrum), and ammonium bicarbonate (Columbus Chemical).
Modification of Membranes with Pepsin
We previously described pepsin-containing nylon membranes,50 but this work uses membranes with a nominal pore size of 1.2 µm, instead of 0.45 µm. The modification procedure includes sequential adsorption of PSS and pepsin, and the amount of pepsin adsorbed to the nylon membrane was estimated by determining the pepsin concentration in the loading solution before and after circulation through the membrane. A Nanodrop UV-vis spectrometer (NanoDrop 2000, Thermo) measured the pepsin UV absorbance at 280 nm.
mAb Reduction and Characterization
Antibodies were dissolved (WIgG1) or diluted (Trastuzumab) in deionized water to prepare 1 mg/mL stock solutions. The solution was stored at 4 °C until use. For antibody reduction, 1 µL of 0.1 M HOAc and 1 µL of 0.1 M TCEP-HCl were added to 10 µL of antibody stock solution, and this reaction mixture was incubated at 75 °C for 15 min and finally diluted with 88 µL of 5% FA. Alkylation of antibodies after reduction was conducted only prior to in-solution, tryptic digestion. In that case, 20 µg of WIgG1 was dried and reconstituted in 7 µL of 2 mM TCEP-HCl solution prepared in 0.1% HOAc containing 8 M urea. This mixture was incubated at 50 °C for 10 min, and 7 µL of 20 mM IAM in a 2 M NH4HCO3 solution containing 8 M urea was added. After incubation in darkness for 30 min, 6 µL of 30 mM dithiothreitol in 100 mM NH4HCO3 solution containing 8 M urea was added. The reaction was incubated in darkness for 20 min to quench the IAM. Ultra-performance liquid chromatography, coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-QTOF-MS), confirmed separation of the light and heavy chains (see Figure S1 in the Supporting Information). Offline HPLC isolation of light and heavy chains (prior to digestion of these chains) was performed with a Shimadzu Model LC-20AB instrument equipped with a SPD-20AV ultraviolet detector (see the Supporting Information for details).
In-Membrane Digestion of Intact Antibody
After reduction by TCEP, the nonalkylated mixture was passed through a pepsin-containing membrane in an Upchurch holder at flow rates of 0.13 or 130 mL/h using a syringe pump. The residence time was estimated assuming a nylon membrane porosity of 50% and an exposed membrane area of 0.02 cm2 (see eq 1 in the Results and Discussion). Hence, residence times were 3 s and 3 ms for 0.13 and 130 mL/h flow rates, respectively, and 100 µL of effluent was collected for direct infusion MS analysis.
Digestion of the mAb Light and Heavy Chains
In-Membrane Digestion
Ten µg of nonalkylated light chain was dissolved in 100 µL of 2 mM TCEP in 5% FA solution. The mixture was heated at 80 °C for 10 min, allowed to cool, and the light chain solution was passed through the membrane at flow rates of 0.13, 13, and 130 mL/h. Effluent was collected, dried with a Speed Vac and saved for MS analysis. The heavy chain was digested in a similar manner.
In-Solution Digestion
Four µg of alkylated light chain was dissolved in 10 µL of 2 mM TCEP solution containing 10 mM NH4HCO3. The mixture was heated at 80 °C for 10 min and then cooled to room temperature. Two microliters (2 µL) of 0.1 µg/µL trypsin solution was added to the mixture prior to incubation at 37 °C for 16 h. The reaction was quenched via the addition of 5 µL of acetic acid, immediately frozen with liquid nitrogen, and dried with a Speed Vac before reconstitution for MS.
“Top-Down” Analysis of a mAb Light Chain
An Agilent Technologies (Palo Alto, CA) 1100 Series binary HPLC system was interfaced with a Thermo Fisher Scientific Orbitrap Elite Hybrid Ion Trap-Orbitrap Mass Spectrometer (San Jose, CA) for online separation of TCEP-reduced WIgG1. About 100 fmol of reduced WIgG1 was pressure-loaded onto a fused-silica capillary column (75 µm I.D. × 360 µm O.D.) packed with 10 cm of Agilent POROSHELL 300SB-C18 particles (5 µm diameter, 300 Å pore size). The back end of the column was equipped with a laser-pulled nanoelectrospray emitter tip,54 and the column was initially rinsed for 10 min with 0.3% formic acid in water to remove salts. Protein sample was eluted at 60 °C at a flow rate of 100 nL/min using the following gradient: 0%–30% B for 5 min, 30%–50% B for 20 min, 50%–100% B for 5 min. Solution A contained 0.3% FA in H2O, whereas solution B consisted of 0.3% FA, 72% ACN, 18% IPA, and 9.7% H2O.
Mass Spectrometry and Data Analysis
In-membrane and in-solution digests were reconstituted in 1% acetic acid, 49% H2O, and 50% methanol, loaded into a Whatman multichem 96-well plate (Sigma–Aldrich), and sealed with Teflon Ultrathin Sealing Tape (Analytical Sales and Services, Prompton Plains, NJ). An Advion Triversa Nanomate nanoelectrospray ionization (nESI) source (Advion, Ithaca, NY) was used to introduce the sample into a high-resolution accurate mass Thermo Fisher Scientific LTQ Orbitrap Velos mass spectrometer (San Jose, CA) that was equipped with a dual-pressure ion trap, HCD cell, and ETD. The spray voltage and gas pressure were set to 1.4 kV and 1.0 psi, respectively. The ion source interface had an inlet temperature of 200 °C with an S-Lens value of 65%. High-resolution mass spectra were acquired in positive ionization mode across the m/z range of 300–2000, using the FT analyzer operating at a mass resolving power of 100 000. Spectra were the average of 100 scans. Mass spectra were deconvoluted using the Xtract function of the XCalibur software. Proteolytic peptide identification and CID/HCD/ETD MS/MS data analysis were performed manually (for isotopic distributions with a signal-to-noise ratio of >5) by matching MS and MS/MS product ions with data generated in silico using ProteinProspector (v 5.14.1, University of California, San Francisco, CA). Mass tolerance was set for 5 ppm.
“Top-down” MS analyses of the WIgG1 light chain include a full MS scan at m/z 300–2000 in the Orbitrap at 240 000 mass resolving power, and three MS/MS scans (5 ms ETD, 15 ms ETD, and CID targeted on the +28 charge-state Lc ion at m/z 865.2 with a 3 m/z isolation window) in the Orbitrap at a mass resolving power of 120 000 (5 microscans per MS/MS scan). The Lc-targeted ETD (13 scans) or CID (9 scans) MS/MS spectra were merged and extracted from the raw file using Xcalibur 2.1 (Thermo Scientific). Each extracted ETD or CID spectrum was then searched against the sequence of WIgG1 (provided by Waters), using ProSightPC 3.0. Search parameters included the following: 5 Da precursor tolerance (monoisotopic), 15 ppm fragment tolerance (monoisotopic), Δm mode on, and disulfide off. The c-, z-, b-, and y-type fragment ions assigned by ProSightPC 3.0 were manually verified before acceptance.
RESULTS AND DISCUSSION
Protease-Containing Membranes
Figure 1 shows the workflow that we employ to analyze mAbs using in-membrane digestion. The procedure exploits enzyme-containing membranes that we prepare using sequential adsorption of PSS and pepsin in nylon membranes at pH 2.3. PSS adsorption provides a negatively charged surface that captures pepsin, which is positively charged at low pH. Similar to other membrane modifications through electrostatic adsorption, protease immobilization should occur throughout the membrane.55 These reactors are very active, because of the high local enzyme concentration in membrane pores.50 The extent of digestion varies with the solution residence time in the membrane (tres), which is a function of the membrane thickness (l), the volumetric flow rate (Q), the exposed area at the faces of the membrane (A), and the membrane porosity (ε) (eq 1).
| (1) |
Our prior study used a membrane with nominal 0.45 µm pores and a thickness of 170 µm,50 whereas this work employs both a larger pore size (1.2 µm) and a lower thickness (110 µm) to further limit digestion and provide longer peptides. The lower thickness decreases the residence time for a given flow rate, and the larger pore size should give longer radial diffusion distances to immobilized enzymes. Analysis of the pepsin loading solution before and after circulating through the membrane suggests an immobilized pepsin concentration of ~70 mg per mL of membrane.
mAb Reduction and Characterization
Effective antibody digestion requires reduction of disulfide bonds to give the protease access to cleavage sites. We employ TCEP as a reducing agent, because it can function under acidic conditions that both prevent reformation of disulfide bonds and partially denature the antibody. Thus, acidic conditions will avoid the need for urea denaturation and alkylation. Because pepsin is enzymatically active under acidic conditions, peptic digests are compatible with ESI-MS without further purification. UPLC-ESI-QTOF-MS analysis verified the separation of light and heavy chains after TCEP reduction (Figure S1). Based on deconvolution using MasEnt1 software, the average mass of the antibody light chain is 24 198.0, which agrees with the theoretical mass of 24 197.7, which was provided by the manufacturer. The deconvoluted heavy-chain mass spectrum shows three glycoforms with mass differences of 162 Da (see Figure S1 for the mass spectrum and Figure 9 (presented later in this work) for glycoform assignments).
Figure 9.
Part of the ESI-Orbitrap mass spectrum of a reduced WIgG1 heavy chain after digestion with a residence time of 3 s in a pepsin-containing membrane. The labeled isotopic envelopes result from the peptide H274–301 (+4 charge state), which contains 3 N292 glycoforms separated by 162 Da intervals due to galactose units. The inset shows the spectrum that result from deconvolution of the mass range in the spectrum. Unlabeled peaks stem from other peptides. Drawings represent the different glycoforms (legend: Gal, galactose; GlcNac, N-acetylglucosamine; Man, mannose; and Fuc, fucose).
mAb Digestion in Membranes
In-membrane digestion occurs during the passage of a reduced-antibody solution through a pepsin-containing membrane. At a flow rate of 130 mL/h, which corresponds to a residence time of 3 ms, the digestion of a 100 µL solution requires <1 min. As Figure 2 shows, the infusion mass spectrum of digested WIgG1 contains signals from two large peptides, L1–140 (light-chain amino acids 1–140) and L141–219, which cover the entire light-chain sequence. L1–140 shows consecutive charge distribution isotopic envelopes from +8 to +14, and this large peptide (15 kDa) covers the CDR-L1, CDR-L2 and CDR-L3 regions of the antibody. Similarly, the other large peptide with light-chain amino acids (L141–219) shows multiple charge states from +6 to +13. These charge states correspond well with the number of basic residues in the peptides. L1–140, for instance, includes 13 basic residues—6 K, 5 R, and 2 H—which explains the highest charge state of +14, considering that the N-terminus also can capture one proton. L141–219 contains 12 basic residues—7 K, 3 R, and 2 H—and the highest charge state is +13. Some of the other abundant signals in the mass spectrum of the reduced antibody (see Figure S2 in the Supporting Information) result from peptides whose sequences overlap with these two large peptides, such as amino acids L1–51, L1–75, L1–90, L76–140, L91–140, L141–165, and L166–219. Combinations of these peptides also cover the entire light-chain sequence.
Figure 2.
Part of the mass spectrum of an in-membrane digest (residence time = 3 ms) of reduced WIgG1 antibody. The labeled signals show the charge-state distributions of peptides containing the amino acids 1–140 (triangles), and 141–219 (circles) of the light chain. These two large peptides cover the entire light-chain sequence.
The mass spectrum of the reduced-antibody digest (Figure S2) also shows many peptides from the heavy chain, such as H1–104, H105–113, H114–179, H180–235, H236–273, H274–363, H364–399, and H400–441. H1–104 covers the CDR-H1 and CDR-H2 regions and shows N-terminal pyroglutamic acid formation (−17 Da, compared to the mass of the original sequence). The heavy-chain peptide coverage (percentage of amino acids comprised by the detected peptides) for the 3 ms digestion is 100%. Replicate 3 ms, in-membrane digestions with three different membrane pieces show similar signal intensities (see Figure S3 in the Supporting Information). In contrast to millisecond digestion, 3 s in-membrane digestion (slower flow through the membrane) shows shorter peptides from the Lc and Hc (for lists of the peptides, see Tables S1 and S2 in the Supporting Information).
As a second example, we digested Trastuzumab (Herceptin), which is a commercial humanized mAb for breast cancer treatment. Detailed characterization of Trastuzumab is crucial for quality control, and several groups recently presented MS-based characterization of this antibody.3,37,56–60 Remarkably, a 3 ms digestion of reduced Trastuzumab, followed by MS analysis, yields 100% peptide coverage for both the light and heavy chains. (See Figure S4 in the Supporting Information for the mass spectrum.) A large peptide, L1–83, covers the CDRL1 and CDR-L2 regions. Moreover, a peptide containing amino acids 1–115 on the heavy chain covers all of the CDR-H regions. These data demonstrate that rapid digestion (total digestion time of <1 min) with a pepsin-containing membrane can yield essentially complete peptide coverage. Moreover, the simple acidic reduction procedure requires only 15 min, and the MS data collection takes 5 min, when using direct infusion.
mAb Light-Chain Analysis Using In-Membrane or Tryptic In-Solution Digestion
To further investigate antibody sequence information and compare “middle-down” and “bottom-up” methods, we analyzed the WIgG1 light chain after its isolation using HPLC. SDS-PAGE showed the separation of light and heavy chains, and HPLC retention times guided offline collection of the desired fractions. We reconstituted the antibody light chain in a low-concentration TCEP solution to prevent the reformation of disulfide bonds.
Figure 3 shows the deconvoluted mass spectra of the WIgG1 light chain digested with residence times of 3 ms and 3 s in the pepsin-containing membrane. Upon increasing the digestion residence time, large peptides undergo additional cleavages to give smaller peptides. Dominant signals from the 3 ms digestion stem from large peptides, such as L52–90, L91–140, L1–51, L166–219, L76–140, L4–75, L1–75, and L141–219. In contrast, 3 s of digestion yields smaller peptides such as L41–51, L52–75, and L76–90, and, in several cases, combinations of the small peptides give the larger peptides from the 3 ms of digestion. The 3 ms digestion enables 100% peptide coverage of the light chain, whereas the 3 s of digestion shows only 95% coverage, because of the absence of amino acids 1–11. The lack of basic amino acids in the first 11 residues (see Figure 5 (presented later in this work) for the light-chain sequence) may explain the absence of peptide coverage in this region.
Figure 3.
Deconvoluted ESI-Orbitrap mass spectra of the WIgG1 light chain digested with residence times of (A) 3 ms and (B) 3 s in a pepsin-containing membrane. Other small peptides appear at lower m/z values. Deconvoluted peaks (generated by Xtract) represent m/z values of peptides with a +1 charge.
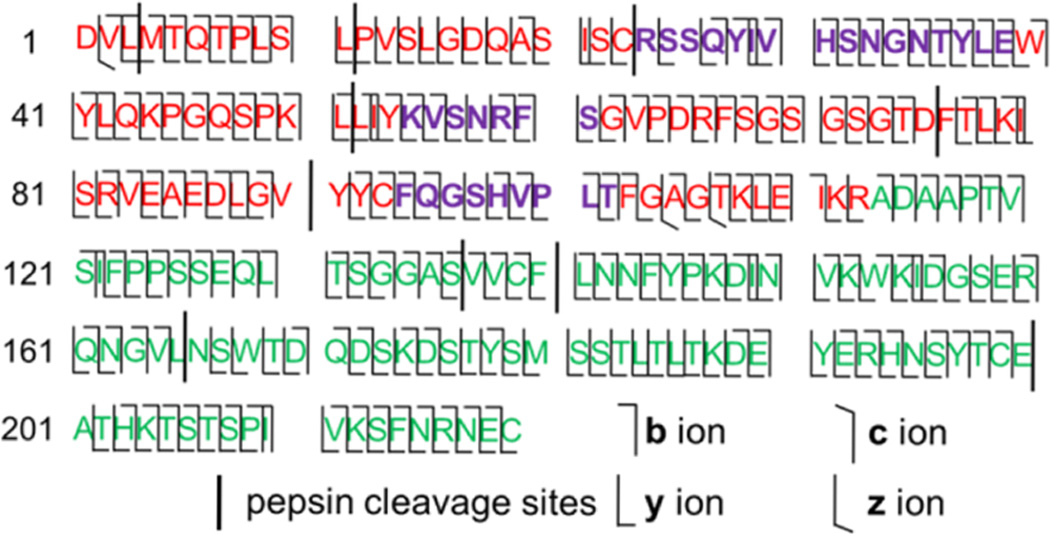
Figure 5.
Summary of bond cleavage sites from CID, HCD, and ETD-MS/MS of WIgG1 light-chain peptides obtained from 30 ms of digestion in pepsin-containing membranes. Red and purple letters cover the light-chain variable region (VL), with purple letters denoting complementarity determining regions (CDRs). Green letters represent the light-chain constant region (CL). The figure does not show redundant cleavages sites from c- and z-ions.
We also performed an in-solution, tryptic digestion of the alkylated light chain for comparison. Eighteen (18) tryptic peptides cover the entire Lc sequence. These peptides show an average length of 12 amino acids, which agrees with the theoretical tryptic peptide length, 8–25 residues. Table S3 in the Supporting Information gives details of the tryptic peptide m/z values.
Comparison of Light-Chain Sequence Coverage Using “Middle-Down”, “Bottom-Up”, and “Top-Down” Methods
Specific amino acid sequence information requires MS/MS data with extensive fragmentation. While larger peptides may contain multiple CDRs and give high peptide coverage, small peptides are easier to fragment in some MS/MS methods and may provide higher sequence coverage. CID is widely used in “bottom up” LC-MS/MS proteomics and gives the peptide sequence information via a series of b- and y-ions. One of the peptides, L52–75, from a 30 ms, in-membrane digestion of the WIgG1 light chain covers the entire CDR-L2 region. CID-MS/MS of this peptide shows 100% sequence coverage, i.e., cleavage of all amide bonds (Figure 4). However, not all peptides show such complete fragmentation. One limitation of quadrupole ion-trap CID is the loss of fragment ions in the low m/z range due to the low-mass cutoff determined by the radiofrequency amplitude. In contrast, HCD in the LTQ Orbitrap Velos mass spectrometer does not have this limitation and facilitates identification of N and C terminal fragment ions.61 MS/MS analyses of 12 proteolytic peptides L1–51, L4–51, L12–51, L24–51, L52–75, L76–90, L91–136, L110–136, L91–140, L141–165, L166–200, and L201–219 were conducted using both CID and HCD. Combining CID and HCD gives higher sequence coverage than CID alone. For instance, CID of L141–165 does not break the Pro-Lys bond, while HCD cleaves this bond and gives 100% sequence coverage of this peptide (Figure S5 in the Supporting Information).
Figure 4.
CID-MS/MS spectrum of the WIgG1 light-chain peptide L52–75, which covers the entire CDR-L2 region. The sequence at the top of the figure denotes the formation of b- and y-ions (only some of the b- and y-ions are labeled in the spectrum).
CID and HCD of L52–75 and L76–90 give 100% sequence coverage of L52–90. Relatively large peptides produced by 3 ms of light-chain digestion, L1–51 and L91–140, were fragmented by ETD. In contrast to CID, which cleaves the labile bonds on the peptide chain, ETD induces fragmentation in a sequence-independent manner. A low residue/charge ratio results in effective ETD fragmentation, so we chose the highest charge states of each peptide for ETD analysis, (+5 for L1–51 and for L91–140). The c- and z-ions produced by ETD (of L1–51 and L91–140) along with the b- and y-ions from CID and HCD of the 12 peptides mentioned previously give 99% bond cleavages in the light chain. Only three amide bond linkages did not dissociate: 25S–26S, 100P–101L, and 113R–114A. Figure 5 gives a summary of the cleavage sites.
For comparison, we fragmented the 18 alkylated light-chain tryptic peptides (mentioned in the last section), using CID. The MS/MS spectra reveal the cleavage of 205 out of 218 bonds via either enzyme cleavage or CID-MS/MS, which gives 94% sequence coverage. We also conducted HCD on these peptides to obtain 9 more fragmentation sites. The combination of CID, HCD, and tryptic cleavage sites yields 99% sequence coverage (Figure 6). Notably, with in-solution tryptic digestion, the CDR-L2 region spans three peptides—51–55, 56–59, and 60–66—making direct characterization of this region difficult.
Figure 6.
Summary of the bond cleavage sites from CID and HCD-MS/MS of peptides obtained from tryptic, in-solution digestion of the WIgG1 Lc.
We also performed ETD and CID for the entire reduced WIgG1 light chain. Figure S6 shows Orbitrap MS/MS spectra from online LC-MS analysis with 5 ms of ETD, 15 ms of ETD, and CID with default energy, respectively. Figure S7 gives an example of “top-down” ETD MS/MS showing partial sequence coverage. Direct ETD (combining the results from 5 ms and 15 ms ETD) of the light chain generated 53 and 50 detectable c- and z-type ions, respectively; and CID yielded 9 and 25 detectable b- and y-type ions, respectively. These c-, z-, b-, and y-type fragment ions collectively give 120 unique gas-phase backbone cleavages (Figure 7), which corresponds to 55% sequence coverage of the WIgG1 light chain.
Figure 7.
Cleavage sites in “top-down” analysis of the antibody light chain. The sequence coverage is 55% after combining CID, 5 ms of ETD, and 15 ms of ETD.
Detecting PTMs on the Light and Heavy Chains
PTMs affect antibody activities in a variety of ways and are commonly introduced to the sequence during manufacture, purification, and storage. Hu et al. showed that oxidation on the light chain may induce a structural change and destabilize the protein.62 We compared two batches of WIgG1 by conducting 30 ms digestions of their light chains and performing MS analysis. Figure 8 compares the signals for peptide L166–219 (+5 charge state) for the two batches of antibody. For the second batch of antibody, the spectrum shows two strong isotopic envelopes, whose deconvoluted mass difference is 15.9980, suggesting a Met oxidation. Because the spectra for the two batches of antibody were obtained under the same analysis conditions, this result suggests that oxidation does not occur significantly during ESI. Further MS/MS analysis of these two peptides from the second batch of antibody confirms that oxidation occurs at M 180. (See Figure S8 in the Supporting Information for MS/MS spectra.) A similar strategy revealed M 393 oxidation (Figure S9 in the Supporting Information) and N 138 deamidation on the heavy chain (Figure S10 in the Supporting Information).
Figure 8.
Part of the ESI-Orbitrap mass spectra of reduced WIgG1 light chains after digestion for 30 ms in pepsin-containing membranes. The top and bottom spectra come from two batches of antibody, and the largest isotopic envelopes represent the +5 charge state of the peptide containing amino acids L166–219.
Another important PTM, glycosylation, plays an important role for binding to the Fc receptor and, thus, affects antibody-dependent cell-mediated cytotoxicity. A 3 s digestion of the heavy chain produced peptides with amino acids H274–301, and these peptides show a clear mass distribution characteristic of three glycoforms (see Figure 9). This result matches with characterization of the entire heavy chain by LC-ESI-Q-TOF MS (Figure S1C). Overall, these data show that rapid, in-membrane digestion enables facile characterization of mAb PTMs.
CONCLUSIONS
This work used pepsin-modified membranes as controlled reactors for antibody proteolysis prior to MS analysis. Pepsin is an inexpensive protease that enables membrane digestion under acidic conditions, which avoids the need for antibody alkylation and minimizes oxidation during digestion. Moreover, the high local enzyme concentration in membrane pores affords the digestion of 100 µL of antibody solution within less than 1 min. Variation of the residence times of reduced antibody solutions in the membranes yields “bottom-up” (1–2 kDa) to “middle-down” sized peptides (5–15 kDa) for the light and heavy chains, and these peptides cover the entire antibody sequence. As needed, digestion with different flow rates can enhance sequence coverage. Analysis of the light-chain and heavy-chain proteolytic peptides reveals sites for oxidation, deamidation, and N-terminal pyroglutamic acid formation, as well as glycosylation patterns. Furthermore, 30 ms in-membranes proteolysis of the light chain, followed by CID, HCD, and ETD MS/MS of peptic peptides cleaves 99% of the amino acid bonds in the light chain. Traditional in-solution tryptic digestion of the light chain combined with CID and HCD MS/MS also gives 99% sequence coverage, whereas “top-down” analysis of the entire light chain by CID, as well as 5 ms and 15 ms of ETD, shows a sequence coverage of 55%. With minimal sample preparation time, membrane digestion leads to high peptide and sequence coverages for identification of PTMs by MS.
Supplementary Material
Acknowledgments
We gratefully acknowledge the U.S. National Science Foundation (Nos. CHE-1152762 and CHE-1506315) for funding this work. We thank Dr. Mohammad Muhsin Chisti from Michigan State University for providing Trastuzumab. We also thank the Michigan State University Mass Spectrometry Facility and Dr. Todd Lydic (Molecular Metabolism and Disease Collaborative Mass Spectrometry Core) for helping to analyze the samples.
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Details on mAb reduction and characterization; isolation of light and heavy chains; demonstration of membrane reusability; the WIgG1 Hc sequence; mass spectra of 3 ms, in-membrane digests of WIgG1 and Trastuzumab; mass spectra showing the reproducibility of in-membrane digestion; comparison of CID and HCD-MS/MS spectra; “top-down” analysis of the WIgG1 Lc; deconvoluted “top-down” mass spectra; comparison of CID-MS/MS of an oxidized and nonoxidized Lc peptide; CID-MS/MS analysis of a peptide containing deamidation; tables of peptides identified from in-membrane WIgG1 digestions of 3 ms and 3 s, and a table of peptides identified from in-solution trypsin digestion of the WIgG1 Lc (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.Weiner GJ. Nat. Rev. Cancer. 2015;15:361–370. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiner LM, Surana R, Wang S. Nat. Rev. Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck A, Sanglier-Cianferani S, Van Dorsselaer A. Anal. Chem. 2012;84:4637–4646. doi: 10.1021/ac3002885. [DOI] [PubMed] [Google Scholar]
- 4.Reichert JM. MAbs. 2014;6:5–14. doi: 10.4161/mabs.27333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duong MN, Cleret A, Matera EL, Chettab K, Mathe D, Valsesia-Wittmann S, Clemenceau B, Dumontet C. Breast Cancer Res. 2015;17:57. doi: 10.1186/s13058-015-0569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motta G, Cea M, Moran E, Carbone F, Augusti V, Patrone F, Nencioni A. Clin. Dev. Immunol. 2010;2010:428253. doi: 10.1155/2010/428253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saltz L, Easley C, Kirkpatrick P. Nat. Rev. Drug Discovery. 2006;5:987–988. doi: 10.1038/nrd2204. [DOI] [PubMed] [Google Scholar]
- 8.Rathore AS, Winkle H. Nat. Biotechnol. 2009;27:26–34. doi: 10.1038/nbt0109-26. [DOI] [PubMed] [Google Scholar]
- 9.Beck A, Wagner-Rousset E, Ayoub D, Van Dorsselaer A, Sanglier-Cianferani S. Anal. Chem. 2013;85:715–736. doi: 10.1021/ac3032355. [DOI] [PubMed] [Google Scholar]
- 10.Rosati S, van den Bremer ET, Schuurman J, Parren PW, Kamerling JP, Heck AJ. MAbs. 2013;5:917–924. doi: 10.4161/mabs.26282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan B, Valliere-Douglass J, Brady L, Steen S, Han M, Pace D, Elliott S, Yates Z, Han Y, Balland A, Wang W, Pettit D. J. Chromatogr. A. 2007;1164:153–161. doi: 10.1016/j.chroma.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Pan H, Chen X. Mass Spectrom. Rev. 2009;28:147–176. doi: 10.1002/mas.20190. [DOI] [PubMed] [Google Scholar]
- 13.Rosati S, Yang Y, Barendregt A, Heck AJ. Nat. Protoc. 2014;9:967–976. doi: 10.1038/nprot.2014.057. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Cui W, Gross ML. FEBS Lett. 2014;588:308–317. doi: 10.1016/j.febslet.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song T, Ozcan S, Becker A, Lebrilla CB. Anal. Chem. 2014;86:5661–5666. doi: 10.1021/ac501102t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai B, Pan H, Flynn GC. Biotechnol. Bioeng. 2011;108:404–412. doi: 10.1002/bit.22933. [DOI] [PubMed] [Google Scholar]
- 17.Liu YD, van Enk JZ, Flynn GC. Biologicals. 2009;37:313–322. doi: 10.1016/j.biologicals.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Montesino R, Calvo L, Vallin A, Rudd PM, Harvey DJ, Cremata JA. Biologicals. 2012;40:288–298. doi: 10.1016/j.biologicals.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Maeda E, Kita S, Kinoshita M, Urakami K, Hayakawa T, Kakehi K. Anal. Chem. 2012;84:2373–2379. doi: 10.1021/ac300234a. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Lu J, Wroblewski VJ, Beals JM, Riggin RM. Anal. Chem. 2005;77:1432–1439. doi: 10.1021/ac0494174. [DOI] [PubMed] [Google Scholar]
- 21.Bailey MJ, Hooker AD, Adams CS, Zhang S, James DC. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2005;826:177–187. doi: 10.1016/j.jchromb.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Shah B. Anal. Chem. 2007;79:5723–5729. doi: 10.1021/ac070483q. [DOI] [PubMed] [Google Scholar]
- 23.Bondarenko PV, Second TP, Zabrouskov V, Makarov AA, Zhang Z. J. Am. Soc. Mass Spectrom. 2009;20:1415–1424. doi: 10.1016/j.jasms.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Tsybin YO, Fornelli L, Stoermer C, Luebeck M, Parra J, Nallet S, Wurm FM, Hartmer R. Anal. Chem. 2011;83:8919–8927. doi: 10.1021/ac201293m. [DOI] [PubMed] [Google Scholar]
- 25.Mao Y, Valeja SG, Rouse JC, Hendrickson CL, Marshall AG. Anal. Chem. 2013;85:4239–4246. doi: 10.1021/ac303525n. [DOI] [PubMed] [Google Scholar]
- 26.Srzentić K, Fornelli L, Laskay UA, Monod M, Beck A, Ayoub D, Tsybin YO. Anal. Chem. 2014;86:9945–9953. doi: 10.1021/ac502766n. [DOI] [PubMed] [Google Scholar]
- 27.Ayoub D, Bertaccini D, Diemer H, Wagner-Rousset E, Colas O, Cianferani S, Van Dorsselaer A, Beck A, Schaeffer-Reiss C. Anal. Chem. 2015;87:3784–3790. doi: 10.1021/ac504427k. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Ortiz R, Tran LT, Salimi-Moosavi H, Malella J, James CA, Lee JW. AAPS J. 2013;15:337–346. doi: 10.1208/s12248-012-9435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang T, Zhang J, Hewitt D, Tran B, Gao X, Qiu ZJ, Tejada M, Gazzano-Santoro H, Kao YH. Anal. Chem. 2012;84:7112–7123. doi: 10.1021/ac301426h. [DOI] [PubMed] [Google Scholar]
- 30.Du Y, Wang F, May K, Xu W, Liu H. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2012;907:87–93. doi: 10.1016/j.jchromb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Lu Q, Wu SL, Karger BL, Hancock WS. Anal. Chem. 2011;83:3133–3140. doi: 10.1021/ac200128d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesur A, Varesio E, Hopfgartner G. J. Chromatogr. A. 2010;1217:57–64. doi: 10.1016/j.chroma.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Xiang T, Chumsae C, Liu H. Anal. Chem. 2009;81:8101–8108. doi: 10.1021/ac901311y. [DOI] [PubMed] [Google Scholar]
- 34.Rehder DS, Dillon TM, Pipes GD, Bondarenko PV. J. Chromatogr. A. 2006;1102:164–175. doi: 10.1016/j.chroma.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Amphlett G, Lambert JM, Blattler W, Zhang W. Pharm. Res. 2005;22:1338–1349. doi: 10.1007/s11095-005-5267-7. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Liu H, Katta V. J. Mass Spectrom. 2009;45:112–120. doi: 10.1002/jms.1700. [DOI] [PubMed] [Google Scholar]
- 37.Wang B, Gucinski AC, Keire DA, Buhse LF, Boyne MT., II Analyst. 2013;138:3058–3065. doi: 10.1039/c3an36524g. [DOI] [PubMed] [Google Scholar]
- 38.Fornelli L, Ayoub D, Aizikov K, Beck A, Tsybin YO. Anal. Chem. 2014;86:3005–3012. doi: 10.1021/ac4036857. [DOI] [PubMed] [Google Scholar]
- 39.An Y, Zhang Y, Mueller HM, Shameem M, Chen X. MAbs. 2014;6:879–893. doi: 10.4161/mabs.28762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang D, Wynne C, Gu F, Becker C, Zhao J, Mueller HM, Li H, Shameem M, Liu YH. Anal. Chem. 2015;87:914–921. doi: 10.1021/ac503158g. [DOI] [PubMed] [Google Scholar]
- 41.Nicolardi S, Deelder AM, Palmblad M, van der Burgt YE. Anal. Chem. 2014;86:5376–5382. doi: 10.1021/ac500383c. [DOI] [PubMed] [Google Scholar]
- 42.Fornelli L, Damoc E, Thomas PM, Kelleher NL, Aizikov K, Denisov E, Makarov A, Tsybin YO. Mol. Cell. Proteomics. 2012;11:1758–1767. doi: 10.1074/mcp.M112.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zang L, Carlage T, Murphy D, Frenkel R, Bryngelson P, Madsen M, Lyubarskaya Y. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2012;895–896:71–76. doi: 10.1016/j.jchromb.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Dick LW, Jr, Mahon D, Qiu D, Cheng KC. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2009;877:230–236. doi: 10.1016/j.jchromb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Yang H, Zubarev RA. Electrophoresis. 2010;31:1764–1772. doi: 10.1002/elps.201000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hahne H, Pachl F, Ruprecht B, Maier SK, Klaeger S, Helm D, Medard G, Wilm M, Lemeer S, Kuster B. Nat. Methods. 2013;10:989–991. doi: 10.1038/nmeth.2610. [DOI] [PubMed] [Google Scholar]
- 47.Swaney DL, Wenger CD, Coon JJ. J. Proteome Res. 2010;9:1323–1329. doi: 10.1021/pr900863u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu C, Tran JC, Zamdborg L, Durbin KR, Li M, Ahlf DR, Early BP, Thomas PM, Sweedler JV, Kelleher NL. Nat. Methods. 2012;9:822–824. doi: 10.1038/nmeth.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan ZF, Arnaudo AM, Garcia BA. Annu. Rev. Anal. Chem. 2014;7:113–128. doi: 10.1146/annurev-anchem-071213-015959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan YJ, Wang WH, Zheng Y, Dong J, Stefano G, Brandizzi F, Garavito RM, Reid GE, Bruening ML. Anal. Chem. 2012;84:8357–8363. doi: 10.1021/ac3019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Good DM, Wirtala M, McAlister GC, Coon JJ. Mol. Cell. Proteomics. 2007;6:1942–1951. doi: 10.1074/mcp.M700073-MCP200. [DOI] [PubMed] [Google Scholar]
- 52.Han W, Yamauchi M, Hasegawa U, Noda M, Fukui K, van der Vlies AJ, Uchiyama S, Uyama H. J. Biosci. Bioeng. 2015;119:505–510. doi: 10.1016/j.jbiosc.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 53.Long Y, Wood TD. J. Am. Soc. Mass Spectrom. 2015;26:194–197. doi: 10.1007/s13361-014-1015-8. [DOI] [PubMed] [Google Scholar]
- 54.Udeshi ND, Compton PD, Shabanowitz J, Hunt DF, Rose KL. Nat. Protoc. 2008;3:1709–1717. doi: 10.1038/nprot.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan YJ, Sui D, Wang WH, Kuo MH, Reid GE, Bruening ML. Anal. Chem. 2013;85:5699–5706. doi: 10.1021/ac400198n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beck A, Debaene F, Diemer H, Wagner-Rousset E, Colas O, Van Dorsselaer A, Cianferani S. J. Mass Spectrom. 2015;50:285–297. doi: 10.1002/jms.3554. [DOI] [PubMed] [Google Scholar]
- 57.Marcoux J, Champion T, Colas O, Wagner-Rousset E, Corvaia N, Van Dorsselaer A, Beck A, Cianferani S. Protein Sci. 2015;24:1210–1223. doi: 10.1002/pro.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lew C, Gallegos-Perez JL, Fonslow B, Lies M, Guttman A. J. Chromatogr. Sci. 2015;53:443–449. doi: 10.1093/chromsci/bmu229. [DOI] [PubMed] [Google Scholar]
- 59.Gahoual R, Burr A, Busnel JM, Kuhn L, Hammann P, Beck A, Francois YN, Leize-Wagner E. MAbs. 2013;5:479–490. doi: 10.4161/mabs.23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie H, Chakraborty A, Ahn J, Yu YQ, Dakshinamoorthy DP, Gilar M, Chen W, Skilton SJ, Mazzeo JR. MAbs. 2010;2:379–394. doi: 10.4161/mabs.2.4.11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olsen JV, Macek B, Lange O, Makarov A, Horning S, Mann M. Nat. Methods. 2007;4:709–712. doi: 10.1038/nmeth1060. [DOI] [PubMed] [Google Scholar]
- 62.Hu D, Qin Z, Xue B, Fink AL, Uversky VN. Biochemistry. 2008;47:8665–8677. doi: 10.1021/bi800806d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.