Abstract
The error-free segregation of chromosomes, which requires the precisely timed search and capture of chromosomes by spindles during early mitotic and meiotic cell division, is responsible for genomic stability and is achieved by the spindle assembly checkpoint in the metaphase-anaphase transition. Mitotic kinases orchestrate M phase events, such as the reorganization of cell architecture and kinetochore (KT) composition with the exquisite phosphorylation of mitotic regulators, to ensure timely and temporal progression. However, the molecular mechanisms underlying the changes of KT composition for stable spindle attachment during mitosis are poorly understood. Here, we show that the sequential action of the kinase Cdk1 and the phosphatase Cdc14A control spindle attachment to KTs. During prophase, the mitotic spindle protein Spag5/Astrin is transported into centrosomes by Kinastrin and phosphorylated at Ser-135 and Ser-249 by Cdk1, which, in prometaphase, is loaded onto the spindle and targeted to KTs. We also demonstrate that Cdc14A dephosphorylates Astrin, and therefore the overexpression of Cdc14A sequesters Astrin in the centrosome and results in aberrant chromosome alignment. Mechanistically, Plk1 acts as an upstream kinase for Astrin phosphorylation by Cdk1 and targeting phospho-Astrin to KTs, leading to the recruitment of outer KT components, such as Cenp-E, and the stable attachment of spindles to KTs. These comprehensive findings reveal a regulatory circuit for protein targeting to KTs that controls the KT composition change of stable spindle attachment and chromosome integrity.
Keywords: cell cycle, cell division, cyclin-dependent kinase (Cdk), kinetochore, phosphorylation, mitosis
Introduction
The maintenance of genome integrity during mitosis is crucial for cell survival and organismal development. To generate two daughter cells with identical genetic information, each sister chromatid must be captured by spindle microtubules (MTs),4 aligned at the mitotic equator, and segregated toward the spindle poles. Cells build a proteinaceous structure, known as the KT, at the centromere and form a bipolar spindle with an amphitelic spindle attachment to achieve accurate chromosome segregation (1). The KT is made up of protein complexes that control its localization on the chromosome, assembly, attachment to spindle MTs, and chromosome movements, such as congression and segregation (2). KTs lacking essential proteins for KT-MT attachment, such as Knl1, Mis12, and Ndc80 protein subcomplexes, are unable to attach to spindle MTs (3), which in turn results in chromosome loss and concurrent cell death (4).
Entry into mitosis is controlled by the concerted action of several mitotic kinases, such as Cyclin-dependent kinase 1 (Cdk1), Polo-like kinase 1 (Plk1), and Aurora A, whose activities are regulated indirectly in a positive feedback loop (5). Plk1 activates Cdk1 by phosphorylating and activating Cdc25, which then removes inhibitory phosphorylation at the Thr-14 and Tyr-15 sites of Cdk1. Aurora A phosphorylates Thr-210 in the active loop of Plk1 with the aid of Bora (6). Cdk1 also activates Plk1 via Bora phosphorylation to promote mitotic entry in Caenorhabditis elegans (7). Although mitotic kinases also govern metaphase-anaphase transition and faithful chromosome segregation by ensuring that spindles are properly assembled, their roles in the prometaphase-metaphase transition remain one of the least understood facets of the mitotic process. Interestingly, prometaphase arrest, which involves a chromosome ring with a monopolar spindle, is triggered by a Plk1 inhibitor (8, 9). Although Plk1 may be involved in the process of centrosome maturation by sensing initial spindle attachment, its physiological substrates in prometaphase have not yet been identified. Furthermore, the altered interaction mode between KTs and MTs requires changes in KT composition and structure (10–12). Intriguingly, a slight increase in KT-MT stability in early mitosis causes chromosome segregation defects in normal untransformed human cells that resemble those in cancer cells with chromosomal instability. Although stable KT-MT attachment is clearly important for chromosome integrity, the mechanistic details underlying how cells recruit outer KT components to achieve stable spindle attachment remain unclear.
The centrosome not only nucleates spindle MTs in prophase to ensure proper mitotic spindle orientation and chromosome segregation (13) but also functions as a reaction center for the activation of mitotic kinases, including Cdk1 and Plk1, that trigger the G2/M transition (14). In prophase, Cdk1 is recruited to centrosomes by Cep63 and activated by forming a complex with phosphorylated Cyclin B1 (15, 16). The most intriguing process in prometaphase is the appropriately timed search and capture of chromosomes by spindles (17). For efficient KT capture, laterally attached chromosomes align around an equatorial ring with the polar ejection force necessary to facilitate KT binding with highly dense MTs and the formation of stable end-on attachments (18). The change of interaction mode between MTs and KTs (11, 12, 19), which enables error-free chromosome segregation by repairing syntelic or merotelic attachment and restoring amphitelic attachment (20, 21) and thus prevents aneuploid human tumors (22), requires changes in KT composition and structure (23, 24).
Recently, microtubule-associated proteins, such as Astrin and Ska1, have been implicated in stable MT-KT attachment (25–27). Astrin, which contains two coiled coil domains in its C terminus, is associated with spindle MTs as early as prophase and functions in centrosome integrity, spindle formation prior to metaphase chromosome alignment, and chromosome segregation (28, 29). Mitotic proteins, including Kinastrin/Skap (27), hNinein (30), cytoplasmic linker-associated protein-1α (Clasp1α) (31), and dynein light chain 8 (32), interact with Astrin and target it to spindle poles or KTs. Several kinases also regulate the function of Astrin. Glycogen synthase kinase 3β (Gsk3β) phosphorylates Astrin at Thr-111, Thr-937, Ser-974, and Thr-978 to regulate its spindle-forming ability but has no effect on localization (33). Aurora A regulates separase activity and the interaction of Astrin with Kinastrin/Skap and Clasp1α by phosphorylating Astrin at Ser-115 to promote mitotic progression (34).
Although it is known that Astrin recruits the outer KT resident motor protein Cenp-E and its partner Cenp-F for stable MT-KT interactions (26), precisely how it is targeted to KTs is unclear. Here, we show that Astrin phosphorylation at Ser-135 and Ser-249 by Cdk1 is essential not only for bipolar spindle formation but also for targeting it to KTs. Furthermore, we demonstrate that the phosphorylation of Astrin by Cdk1 is mediated by Plk1 during the prometaphase-metaphase transition and is fine-tuned by Cdc14A, a phosphatase. Overall, our results suggest that Astrin is a substrate of a Plk1-Cdk1 activation loop and that it coordinates stable KT-MT attachment to ensure chromosome integrity.
Results
Astrin Is Phosphorylated in the N-terminal Region during Mitosis
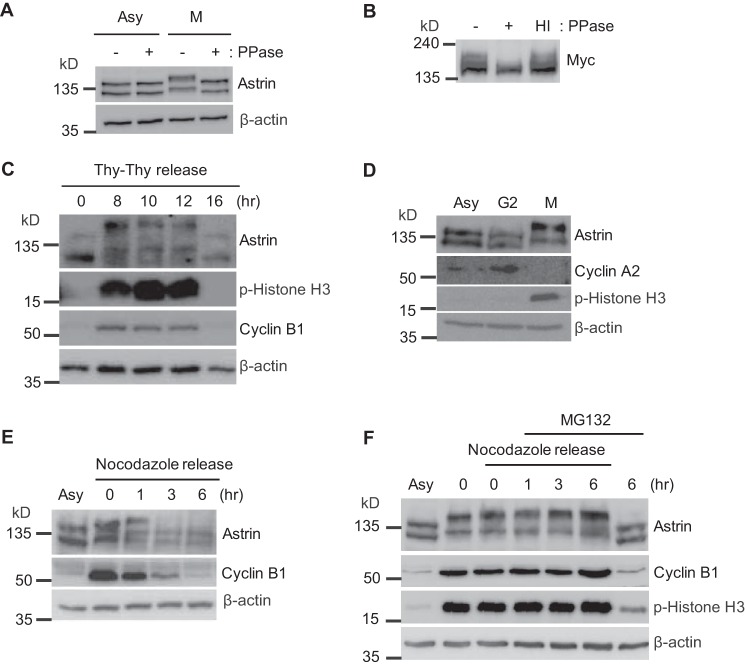
Although Astrin is known to be phosphorylated at multiple sites (33, 34), it is unclear whether Astrin phosphorylation by mitotic kinases regulates its KT targeting. This uncertainty prompted us to research other phosphorylation sites, the mitotic kinase(s) responsible for Astrin targeting to KTs, and the resulting KT structural ensemble. As shown in Fig. 1, A and B, the slower migrating band of endogenous or overexpressed Astrin disappeared in response to the addition of γ-phosphatase, which suggested that the shifted Astrin corresponded to the phosphorylated protein. A phosphorylated band appeared in early mitotic cells (compared with asynchronous or G2 cells) (Fig. 1, C and D) and began diminishing 3 h after the release from a nocodazole block (Fig. 1E). Furthermore, phospho-Astrin accumulated in mitotic cells in response to MG132 treatment (Fig. 1F), which suggests that Astrin is phosphorylated in all stages from mitotic entry to metaphase.
FIGURE 1.
Astrin is phosphorylated during early mitosis. A, 20 μg of proteins from asynchronous (Asy) or mitotic (M) HeLa cell lysates were incubated with or without γ-phosphatase (PPase) and subjected to Western blotting analysis with anti-Astrin antibody. B, Myc-Astrin expression plasmid was transfected into HeLa cells. The transfected HeLa cells were synchronized by treatment with nocodazole for 16 h, which was followed by a washout. Twenty micrograms of mitotic HeLa cell lysates were incubated with or without γ-phosphatase (PPase) and subjected to Western blotting analysis with anti-Myc antibody. HI indicates heat-inactivated γ-phosphatase treatment. C, cell cycle distributions were analyzed by Western blotting using 20 μg of HeLa cell lysates loaded with the indicated antibodies after a double thymidine (Thy) block and release. D, HeLa cells were synchronized at the G1/S boundary via 24-h treatment with thymidine, then washed, and allowed to progress through the cell cycle for 12 h in the presence of nocodazole. Mitotic (M) round cells were collected by shake-off, and the remaining attached cells (G2 phase) were also harvested. Twenty micrograms of cell lysates were subjected to immunoblotting with the indicated antibodies. Cell cycle distributions were assessed by Western blotting analysis with the indicated antibodies. E, cell cycle distributions were analyzed by Western blotting analysis using 20 μg of HeLa cell lysates loaded with the indicated antibodies after release from nocodazole treatment. F, HeLa cells were synchronized with nocodazole to arrest mitosis and then released from arrest with MG132. The released cells were harvested at the indicated time points, and 20 μg of cell lysates were subjected to immunoblotting with the indicated antibodies. Cell cycle distributions were analyzed by Western blotting with the indicated antibodies. p-Histone, phospho-Histone.
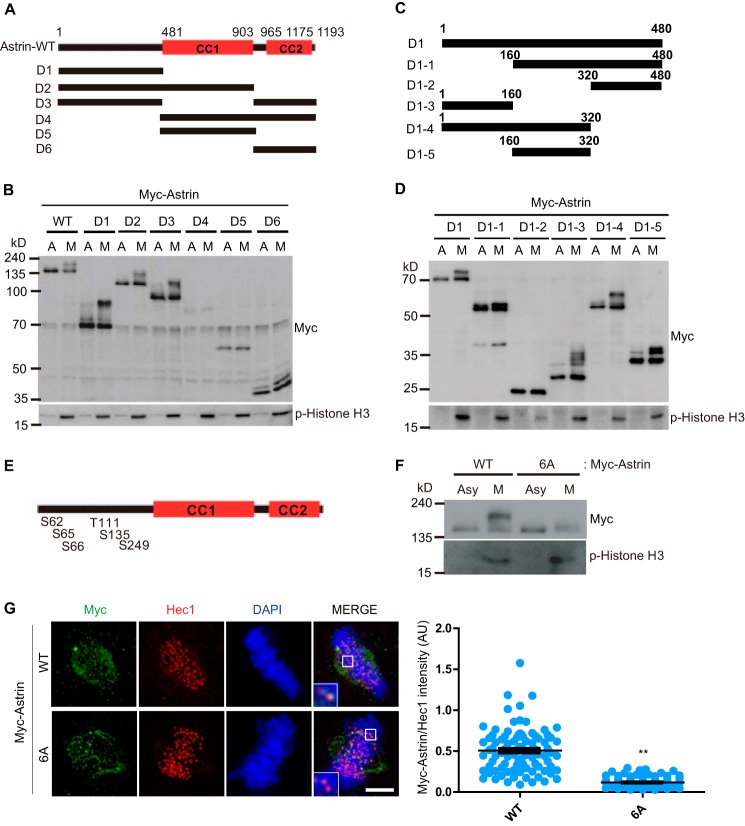
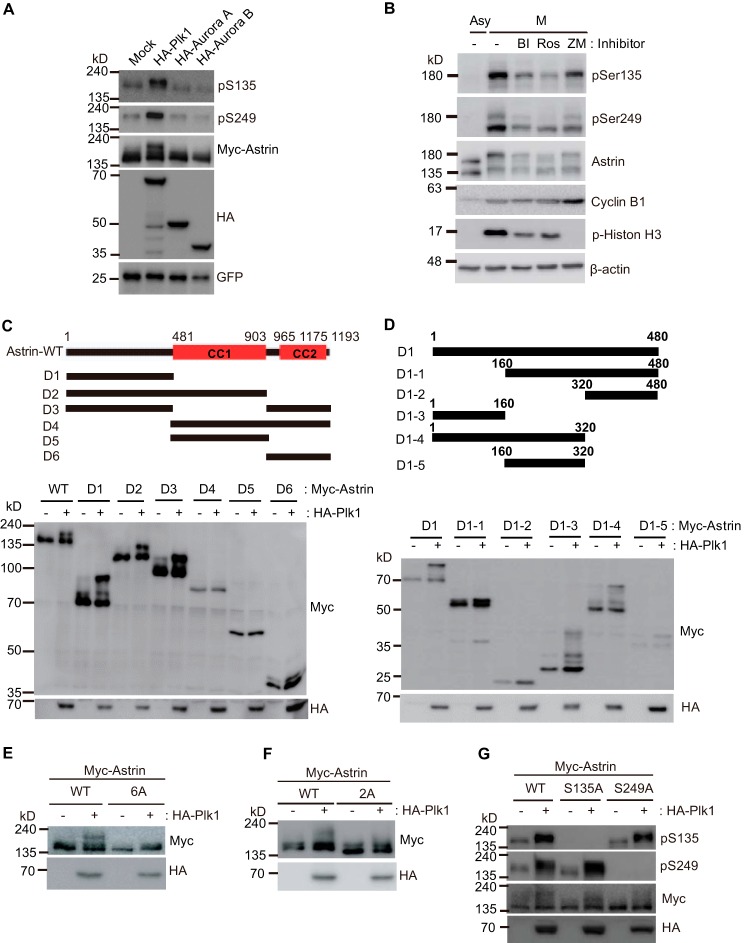
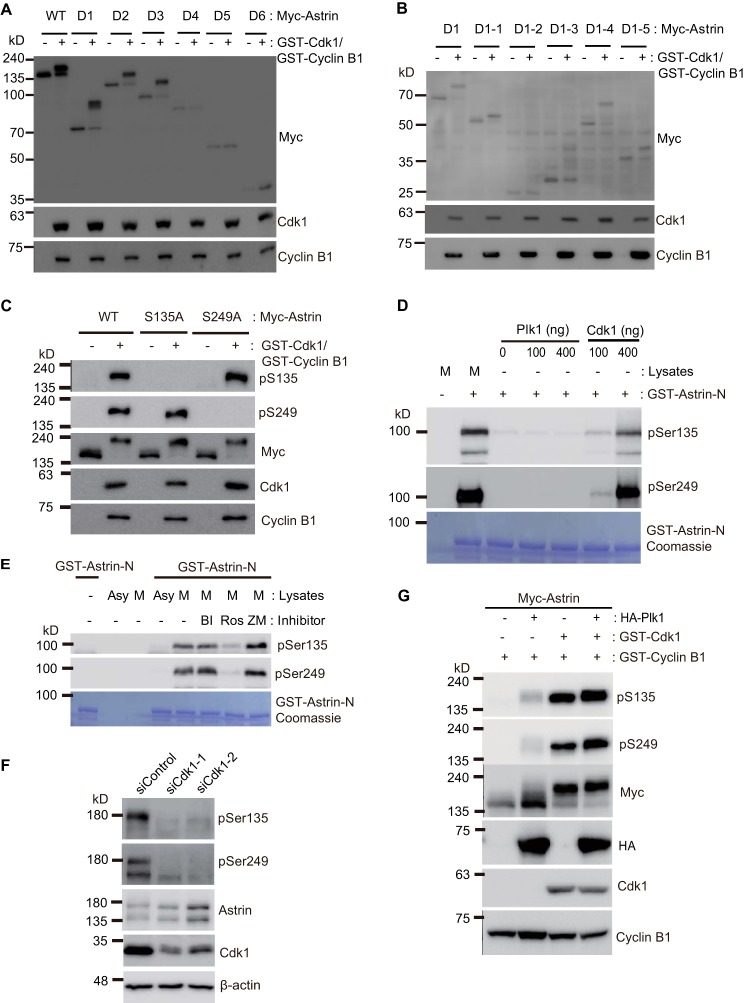
Next, we examined the Astrin phosphorylation sites during mitosis. To evaluate the phosphorylation sites of Astrin during mitosis, the six generated Astrin deletion mutants (Fig. 2A) were transfected into 293T cells and treated with/without nocodazole. The cell lysates were assessed for Astrin phosphorylation. WT Astrin and the deletion mutants D1, D2, and D3 were phosphorylated during mitosis, but the Astrin mutants D4, D5, and D6 were not phosphorylated (Fig. 2B). This result indicated that the Astrin sites phosphorylated during mitosis were present in the N terminus of the Astrin D1 mutant (amino acids 1–480). To narrow down the relevant phosphorylation sites, Astrin D1 was divided into five regions: D1-1, -2, -3, -4, and -5 (Fig. 2C). Transfection experiments using these mutants revealed that only Astrin D1-2 was not phosphorylated during mitosis (Fig. 2D).
FIGURE 2.
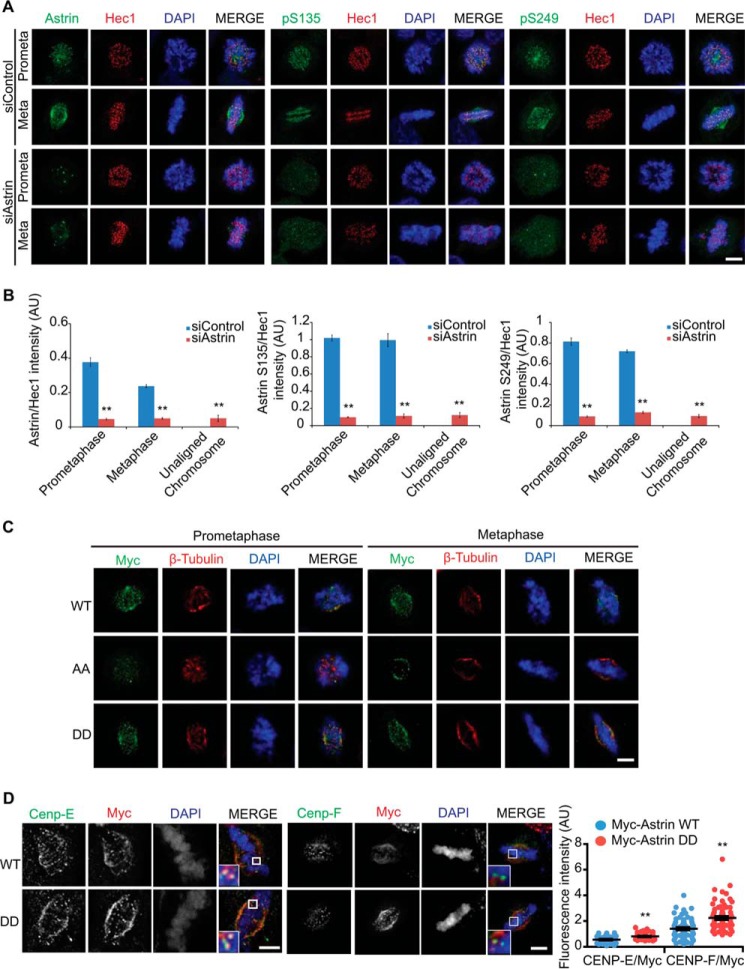
N-terminal region of Astrin is phosphorylated during mitosis. A and B, the phosphorylated region of Astrin. WT, D1, D2, D3, D4, D5, or D6 deletion constructs of the Myc-Astrin expression plasmid were transfected into HEK 293T cells. The transfected cells were treated with/without nocodazole for 16 h. Mitotic (M) round cells were collected by shake-off; asynchronous (A) cells were also harvested. Ten micrograms of cell lysates were subjected to immunoblotting with the indicated antibodies. C and D, HEK 293T cells were transfected with Astrin D1 or its deletion mutants and treated with/without nocodazole for 16 h. Mitotic (M) round cells were collected by shake-off; asynchronous (A) cells were also harvested. Ten micrograms of cell lysates were immunoblotted using the indicated antibodies. E, diagrams of N-terminal Astrin phosphorylation sites. F, HEK 293T cells were transfected with WT or 6A Myc-Astrin and treated with/without nocodazole for 16 h. Mitotic (M) round cells were collected by shake-off; asynchronous (Asy) cells were also harvested. Ten micrograms of cell lysates were subjected to immunoblotting with the indicated antibodies. G, HeLa cells were transfected with WT or 6A Myc-Astrin. Images are maximum projections from z stacks of representative transfected cells stained for Myc (green), Hec1 (red), and DNA (blue). Ninety KTs were quantified and plotted from three independent experiments. Scale bar, 5 μm. CC, coiled coil domain; p-Histone, phospho-Histone; AU, arbitrary units.
To better identify Astrin phosphorylation sites, we used deletion mutants. We identified the phosphorylation sites of Astrin during mitosis using the PhosphoSite program. We selected 6 serine/threonine sites with more than five references (Fig. 2E). Next, we mutated all 6 serine/threonine residues located in the N-terminal region of Astrin to alanine to determine the physiological phosphorylation site and to assess the effect of the N-terminal phosphorylation of Astrin. This mutant (Astrin 6A) was not phosphorylated during mitosis (Fig. 2F) and displayed normal spindle localization but functional defects in translocation to the KT (Fig. 2G).
Astrin Phosphorylation at Ser-135 and Ser-249 Is Essential for Mitotic Function
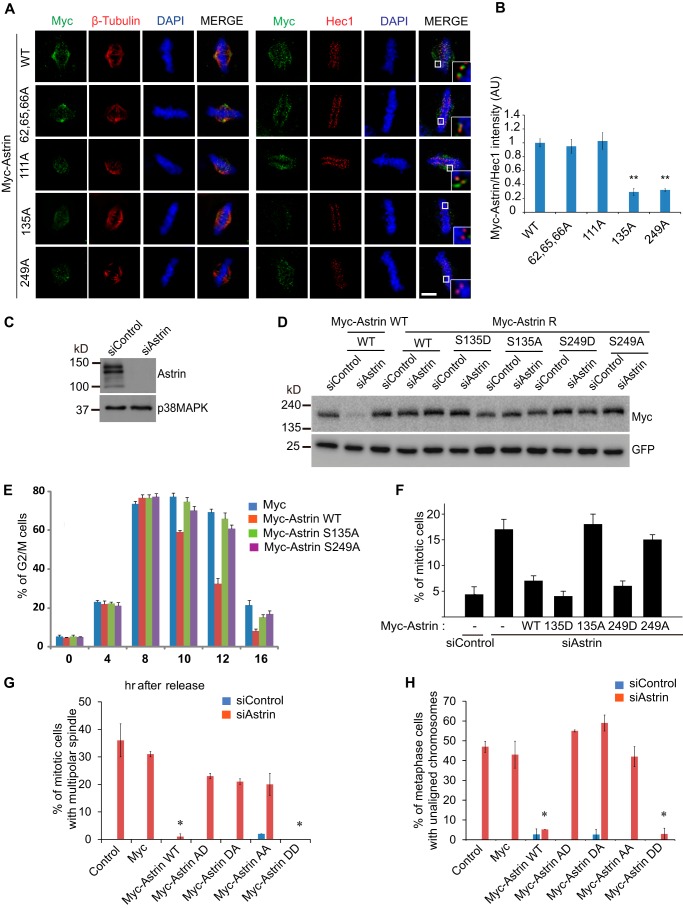
To confirm which residues were responsible for the KT localization of Astrin, we introduced point mutations into Astrin and observed the localization of these mutants in mitotic cells. Strikingly, the S135A and S249A Astrin mutants could not be targeted to KTs (Fig. 3, A and B), which suggested that the phosphorylation of at least one of these residues was responsible for the KT localization of Astrin. To investigate whether Astrin phosphorylation at Ser-135 and/or Ser-249 is associated with mitotic function, we generated siRNA-resistant constructs (Fig. 3, C and D). As expected, the overexpression of the siRNA-resistant mutants S135A or S249A in Astrin knockdown HeLa cells did not rescue G2/M arrest (Fig. 3E). Consistent with this finding, the overexpression of Astrin WT and phosphomimetic S135D and S249D mutants overcame Astrin depletion-mediated mitotic arrest and multipolar spindle formation, but phospho-dead S135A and S249A mutants did not (Fig. 3F). To further analyze the functional relevance of these phosphorylation sites, we tested the ability of a phospho-dead mutant to rescue mitotic defects, such as multipolar spindles and misaligned chromosomes, caused by Astrin depletion. Interestingly, WT and S135D/S249D (DD) mutants rescued the mitotic defects (Fig. 3, G and H), suggesting that the phosphorylation of Ser-135 and Ser-249 is essential for the mitotic function of Astrin in spindle biorientation and chromosome congression.
FIGURE 3.
Astrin phosphorylation at Ser-135 and Ser-249 is essential for its KT localization and mitotic progression. A and B, HeLa cells were transfected with WT or mutants of Myc-Astrin and stained with the indicated antibodies. Approximately 30 cells were quantified and plotted (B) from three independent experiments. Scale bar, 5 μm. C, HeLa cells were transfected with siAstrin, and 20 μg of cell lysates were analyzed by Western blotting. p38MAPK served as a loading control. D, construction of Myc-tagged Astrin expression plasmids with siRNA resistance (Myc-Astrin R). After depletion of endogenous Astrin with Astrin siRNA, WT Astrin or siRNA-resistant mutants were transfected into HEK 293T cells. Ten micrograms of transfected cell lysates were subjected to immunoblotting with the indicated antibodies. GFP was used to demonstrate the specific depletion of Astrin. E, after depletion of endogenous Astrin with Astrin siRNA, HeLa cells were transfected with siRNA-resistant WT or mutant Myc-Astrin expression plasmids. The transfected cells were synchronized using a double thymidine block followed by removal of the block. The cells were harvested for FACS analysis at a specific time point after release from the cell cycle block. The results represent the average of three independent experiments. F, after depletion of endogenous Astrin with Astrin siRNA, HeLa cells were transiently transfected with siRNA-resistant versions of Myc-Astrin WT, S135D, S135A, S249D, or S249A. The mitotic index was evaluated (n = 200 cells) by phospho-Histone H3 staining in three independent experiments. Error bars, S.E. G and H, after depletion of endogenous Astrin with Astrin siRNA, HeLa cells were transfected with WT or mutant Myc-Astrin S135A/S249A (AA), S135A/S249D (AD), S235D/S249A (DA), or DD and fixed with MeOH 28 h after transfection. The percentages of mitotic cells with multipolar spindles (G) or metaphase cells with unaligned chromosomes (H) in the total number of Myc-positive cells were quantified and plotted (n = 300 cells from three independent experiments). *, p < 0.05 (two-tailed t test relative to control cells). Error bars, S.E. AU, arbitrary units.
Astrin Phosphorylation at Ser-135 and Ser-249 Is Required for KT Localization and KT Formation
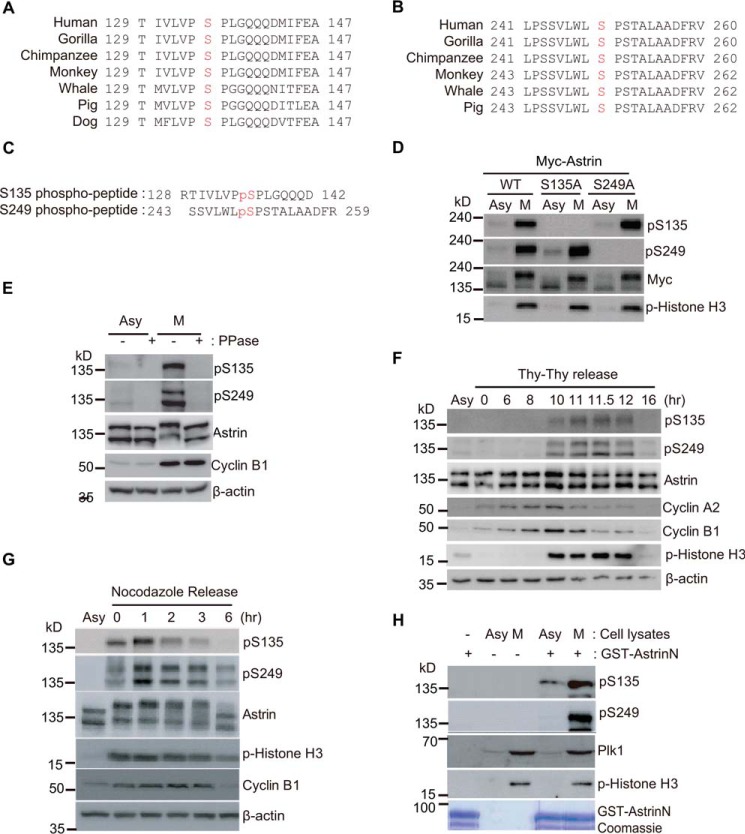
Given that the Ser-135 and Ser-249 sites of Astrin are conserved in many vertebrate species (Fig. 4, A and B), we generated specific antibodies against the phosphopeptide sequences surrounding the Ser-135 and Ser-249 residues (Fig. 4C). The phospho-Astrin antibody showed high specificity because it did not recognize the phospho-dead mutants (Fig. 4D). Endogenous Astrin proteins in mitotic cell lysates were also recognized by the phospho-specific antibodies, but phosphatase-treated endogenous Astrin proteins were not (Fig. 4E). The levels of Astrin phosphorylation at the Ser-135 and Ser-249 residues were monitored during mitosis (Fig. 4F) and were highest 1 h after release from the nocodazole block (Fig. 4G). To further confirm the phosphorylation of Astrin at Ser-135 and Ser-249, we used a cell-free system derived from asynchronous or mitotic HeLa cells. The N-terminal region of Astrin fused with GST (GST-Astrin-N) was phosphorylated at Ser-135 or Ser-249 in nocodazole-treated mitotic cell extracts; asynchronous cell extracts were used as a control (Fig. 4H). Immunofluorescence microscopy revealed that Astrin phosphorylated at Ser-135 or Ser-249 was mainly localized to KTs; however, small amounts were observed at the centrosome and spindle (Fig. 5, A and B). To further test the specificity of antibodies against phospho-Astrin, we confirmed that the knockdown of Astrin completely abolished the localization of phospho-Astrin at the KTs (Fig. 5, A and B). Consistent with this observation, the phospho-mimetic DD mutant localized to KTs (Fig. 5C) and recruited outer KT components such as Cenp-E and Cenp-F more efficiently compared with WT Astrin (Fig. 5D). Taken together, we conclude that Astrin is targeted to KTs via its phosphorylation at both Ser-135 and Ser-249.
FIGURE 4.
Ser-135 and Ser-249 of Astrin are phosphorylated during mitosis. A and B, sequence alignment of the Astrin region containing the Ser-135 and Ser-249 residues in mammalian species. C, the amino acid sequences of the phosphopeptide. D, Mitotic phosphorylation of overexpressed Astrin at S135 or S249. The indicated expression plasmids were transfected into HEK 293T cells. The transfected cells were treated with/without nocodazole for 16 h. Mitotic (M) round cells were collected by shake-off; asynchronous (Asy) cells were also harvested. Ten micrograms of cell lysates were subjected to immunoblotting with the indicated antibodies. E, asynchronous (Asy) or mitotic (M) HeLa cell lysates were incubated with or without γ-phosphatase (PPase), and 20 μg of proteins for each sample were subjected to Western blotting analysis using the indicated antibodies. F, HeLa cells were synchronized with a double thymidine (Thy) block followed by release of the block. The released cells were harvested at the indicated time points, and 20 μg of cell lysates were subjected to immunoblotting with the indicated antibodies. Cell cycle distributions were analyzed by Western blotting using the indicated antibodies. G, HeLa cells were synchronized by treatment with nocodazole for 16 h followed by washout and harvested at the indicated time points. Twenty micrograms of cell lysates were subjected to immunoblotting with the indicated antibodies. H, GST-Astrin-N was incubated with 10 μg of proteins from asynchronous cell extracts (Asy) or mitotic cell extracts (M) obtained from cells treated with nocodazole for 1 h at 30 °C. The reaction mixture was separated by SDS-PAGE, and Astrin phosphorylation was measured with the (phospho-Ser-135 (pS135) or phospho-Ser-249 (pS249) Astrin antibodies. A Coomassie stain (bottom panel) shows the amount of GST fusion proteins. p-Histone, phospho-Histone.
FIGURE 5.
Phospho-Astrin localizes at KTs and recruits Cenp-E. A and B, images are maximum projections from z stacks of representative cells stained for the indicated antibodies. Astrin intensity at KT in control or Astrin-depleted cells was quantified and plotted (B; n = 150 KTs from three independent experiments). C, 28 h after transfection of the indicated plasmid, HeLa cells were fixed and stained with the indicated antibodies. D, 28 h after transfection of the indicated plasmid, HeLa cells were fixed and stained with the indicated antibodies. Images, which were acquired under constant exposure in each channel for all cells, are maximum projections from z stacks of representative cells stained for Cenp-E or Cenp-F (green), Myc (red), and DNA (blue). Fluorescence intensity of Cenp-E or Cenp-F was quantified and plotted (D; n = 100 KTs from three independent experiments). **, p < 0.01 (two-tailed t test relative to control cells). Error bars, S.E. Scale bars, 5 μm. AU, arbitrary units; AA, S135A/S249A.
Cdk1 Phosphorylates Astrin at Ser-135 and Ser-249 in a Plk1-dependent Manner
To determine which mitotic kinase is responsible for Astrin phosphorylation at Ser-135 and Ser-249, we tested Plk1, Aurora A, Aurora B, and Cdk1. As shown in Fig. 6A, the ectopic expression of Plk1 increased Astrin phosphorylation at Ser-135 and Ser-249. Furthermore, Astrin phosphorylation during mitosis was inhibited by treatment with both a Plk1 and a Cdk1 inhibitor (Fig. 6B). The phosphorylation of Astrin by Plk1 is similar to its phosphorylation pattern during mitosis (Fig. 6, C and D). As expected, Plk1 overexpression was unable to phosphorylate the 6A and S135A/S249A Astrin mutants (Fig. 6, E and F), but it also did not phosphorylate the individual S135A and S249A mutants (Fig. 6G), suggesting that Plk1 is directly or indirectly involved in Astrin phosphorylation.
FIGURE 6.
Plk1 is involved in Astrin phosphorylation. A, the Myc-Astrin expression plasmid was transfected with Plk1, Aurora A, or Aurora B kinase expression plasmids in HEK 293T cells. After 24 h, 10 μg of transfected cell lysates were subjected to immunoblotting with the indicated antibodies. B, Western blotting for phospho-Astrin using 20 μg of HeLa cell extracts prepared from cells treated with the indicated kinase inhibitors. C, WT, D1, D2, D3, D4, D5, or D6 deletion mutants of the Myc-Astrin expression plasmid were transfected with/without the Plk1 kinase expression plasmid into HEK 293T cells. After 24 h, 10 μg of transfected cell lysates were subjected to immunoblotting with the indicated antibodies. D, D1, D1-1, D1-2, D1-3, D1-4, or D1-5 deletion mutants of the Myc-Astrin expression plasmid were transfected with/without the Plk1 kinase expression plasmid into HEK 293T cells. After 24 h, 10 μg of transfected cell lysates were subjected to immunoblotting with the indicated antibodies. E–G, 48 h after transfecting the indicated plasmids into HEK 293T cells, 10 μg of lysates were subjected to immunoblotting using the indicated antibodies. CC, coiled coil domain; Asy, asynchronous; M, mitotic; BI, BI 2536; Ros, RO3306; ZM, ZM447439.
A similar phosphorylation pattern of Astrin deletion mutants was detected in Cdk1-overexpressing cells, Plk1-overexpressing cells, and mitotic cells (Fig. 7, A and B). Consistent with this finding, the ectopic expression of Cdk1 induced Astrin phosphorylation at Ser-135 and Ser-249 (Fig. 7C). To determine the physiological mitotic kinase for Astrin phosphorylation at Ser-135 and Ser-249, we developed an in vitro kinase assay with purified kinases and the recombinant N-terminal region of Astrin. As shown in Fig. 7, D and E, only Cdk1 phosphorylated Astrin at Ser-135 and Ser-249, and the phosphorylation of recombinant Astrin in mitotic cell extracts was only blocked by a Cdk1 inhibitor. Furthermore, Cdk1 siRNA abolished the phosphorylation of endogenous Astrin during mitosis (Fig. 7F), and both Ser-135 and Ser-249 are the minimal Cdk1 consensus sites ((S/T)P) (Fig. 4, A and B), indicating that Cdk1 is the mitotic kinase responsible for Astrin phosphorylation at Ser-135 and Ser-249. Astrin phosphorylation was reduced by a Plk1 inhibitor (Fig. 6B) and induced by the ectopic expression of Plk1 (Fig. 6A), suggesting that Cdk1 might be activated by a positive feedback loop involving Plk1. To test this hypothesis, we transfected Myc-Astrin with Cdk1 and Plk1. Ectopic Plk1 alone weakly phosphorylated Myc-Astrin, and it increased the phosphorylation of Astrin by ectopic Cdk1 (Fig. 7G), suggesting that Plk1 activates Cdk1 and thereby phosphorylates Astrin at Ser-135 and Ser-249.
FIGURE 7.
Cdk1 phosphorylates Astrin during mitosis. A, WT, D1, D2, D3, D4, D5, or D6 deletion mutants of the Myc-Astrin expression plasmid were transfected with/without the Cdk1 expression plasmid into HEK 293T cells. After 24 h, 10 μg of transfected cell lysates were subjected to immunoblotting with the indicated antibodies. B, D1, D1-1, D1-2, D1-3, D1-4, or D1-5 deletion mutants of the Myc-Astrin expression plasmid were transfected with/without the Cdk1 expression plasmid into HEK 293T cells. After 24 h, 10 μg of transfected cell lysates were subjected to immunoblotting with the indicated antibodies. C, 48 h after transfection of the indicated plasmids, 10 μg of lysates from HEK 293T cells were subjected to immunoblotting with the indicated antibodies. D, GST-Astrin-N was incubated with 10 μg of lysates from mitotic cell extracts (M) obtained from nocodazole-treated cells with the active form of purified recombinant Plk1 or with a Cdk1-Cyclin B complex for 1 h at 30 °C. The reaction mixture was separated by SDS-PAGE, and Astrin phosphorylation was detected using Ser-135 (pS135) or Ser-249 (pS249) anti-Astrin antibodies. The Coomassie-stained gel in the bottom panel shows the amounts of GST fusion protein. E, GST-Astrin-N was incubated with 10 μg of lysates from mitotic cell extracts (M) obtained from nocodazole-treated cells with/without mitotic kinase inhibitors for 1 h at 30 °C. The reaction mixture was separated by SDS-PAGE, and Astrin phosphorylation was detected using Ser-135 or Ser-249 anti-Astrin antibodies. The Coomassie-stained gel in the bottom panel shows the amount of GST fusion protein. F, HeLa cells were transfected with siCdk1, treated with nocodazole for 16 h, and analyzed by Western blotting. Actin served as a loading control. G, mitotic phosphorylation of overexpressed Astrin at Ser-135 or Ser-249. The indicated expression plasmids were transfected into HEK 293T cells. Ten micrograms of transfected cell lysates were separated by SDS-PAGE, and Astrin phosphorylation was detected using the Ser-135 or Ser-249 Astrin antibody. Asy, asynchronous; BI, BI 2536; Ros, RO3306; ZM, ZM447439.
Phospho-Astrin Targeting to KTs Is Critical for Prometaphase-to-metaphase Transition
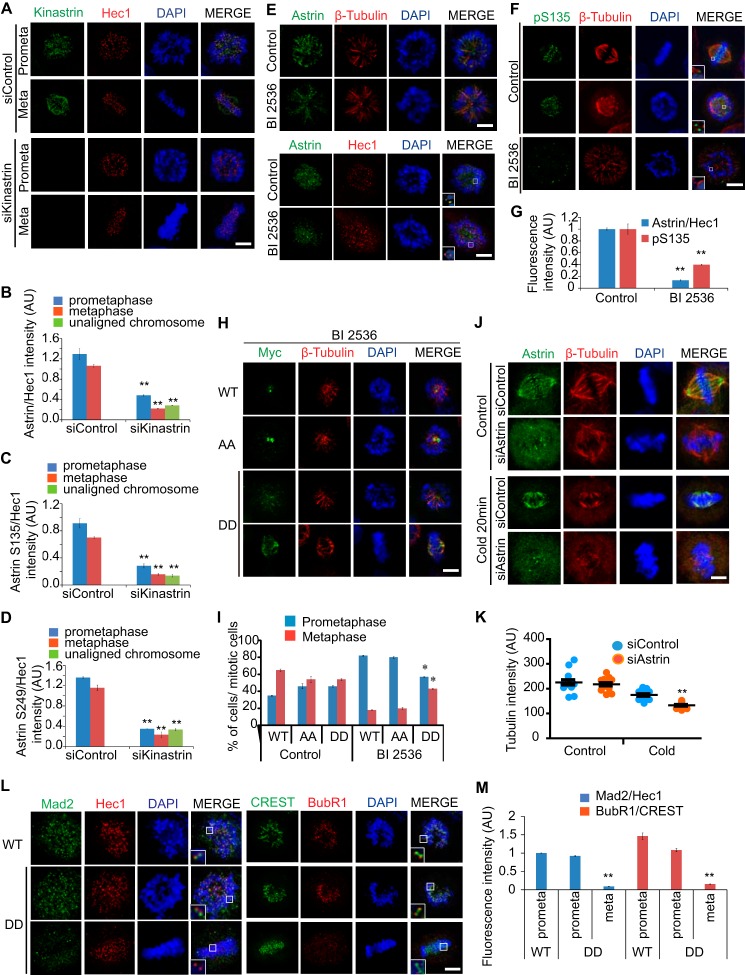
To elucidate the molecular basis of the relationship between the phosphorylation and the KT targeting of Astrin, Kinastrin-depleted cells were analyzed in detail. Consistent with previous reports (27), the levels of Astrin and phospho-Astrin decreased in KTs in Kinastrin-depleted cells (Fig. 8, A–D), indicating that Kinastrin transports phospho-Astrin to KTs. We were further interested in delineating the physiological consequences of phospho-Astrin in relation to KT composition and KT-MT attachment. We therefore examined the localization of Astrin after treatment with a Plk1 inhibitor that arrests cells in prometaphase where there is a monopolar spindle (Polo spindle) encircled by a chromosome ring with a lateral KT attachment (8, 9) (Fig. 8E). We took advantage of the S135A/S249A phospho-dead and the DD phospho-mimetic double mutants. As expected, the Plk1 inhibitor reduced the targeting of Astrin to KTs concurrent with a reduction in Astrin phosphorylation (Fig. 8, E–G). Strikingly, the number of Polo spindle-containing prometaphase cells decreased substantially with the expression of the DD mutant along with treatment with the Plk1 inhibitor (Fig. 8, H and I), indicating that the phosphorylation of Astrin at Ser-135 and Ser-249 mediated by Plk1 is crucial for spindle attachment to KTs and the prometaphase-to-metaphase transition. Consistent with this finding, the spindle MTs in Astrin-depleted cells were less stable under cold stress (Fig. 8, J and K). Furthermore, the expression of the DD mutant increased metaphase cells with stable KT-MT attachment because the DD mutant diminished the spindle assembly checkpoint, including Mad2 and BubR1 at KTs, in the presence of the Plk1 inhibitor (Fig. 8, L and M). Therefore, we conclude that Astrin is a substrate of the Plk1-Cdk1 positive feedback loop and is responsible for outer KT formation, stable spindle attachment to KTs, and the prometaphase-to-metaphase transition.
FIGURE 8.
Astrin phosphorylation and KT localization in prometaphase-to-metaphase transition. A–D, HeLa cells were transfected with control or Kinastrin-specific siRNA for 48 h and stained with the indicated antibodies. Total Astrin (B) or phospho-Astrin (C and D) intensities were quantified and plotted (n = 300 KTs from three independent experiments). E–G, 2 h after Plk1 inhibitor (BI 2536) treatment, HeLa cells were fixed and stained with the indicated antibodies. Images were acquired under a constant exposure in each channel. Total Astrin or phospho-Astrin intensities at KTs in prometaphase cells were quantified and plotted (G; n = 150 KTs from three independent experiments). H and I, 28 h after transfection, cells were treated with Plk1 inhibitor (BI 2536) for 2 h. The cells were fixed and stained with the indicated antibodies. The ratio of metaphase or prometaphase cells to the total number of mitotic cells in green-positive cells was quantified and plotted (n = 100 cells from three independent experiments). J and K, 48 h after transfection, cells were incubated at 4 °C for 20 min. The cells were fixed and stained with the indicated antibodies. The spindle intensity was quantified and plotted (K; n = 14 cells from three independent experiments). L and M, 28 h after transfection, cells were treated with Plk1 inhibitor (BI 2536) for 2 h. The cells were fixed and stained with the indicated antibodies. Mad2 and BubR1 intensities were quantified and plotted (M; n = 150 KTs from three independent experiments). *, p < 0.05; **, p < 0.01 (two-tailed t test relative to control cells). Error bars, S.E. Scale bars, 5 μm. AU, arbitrary units; AA, S135A/S249A.
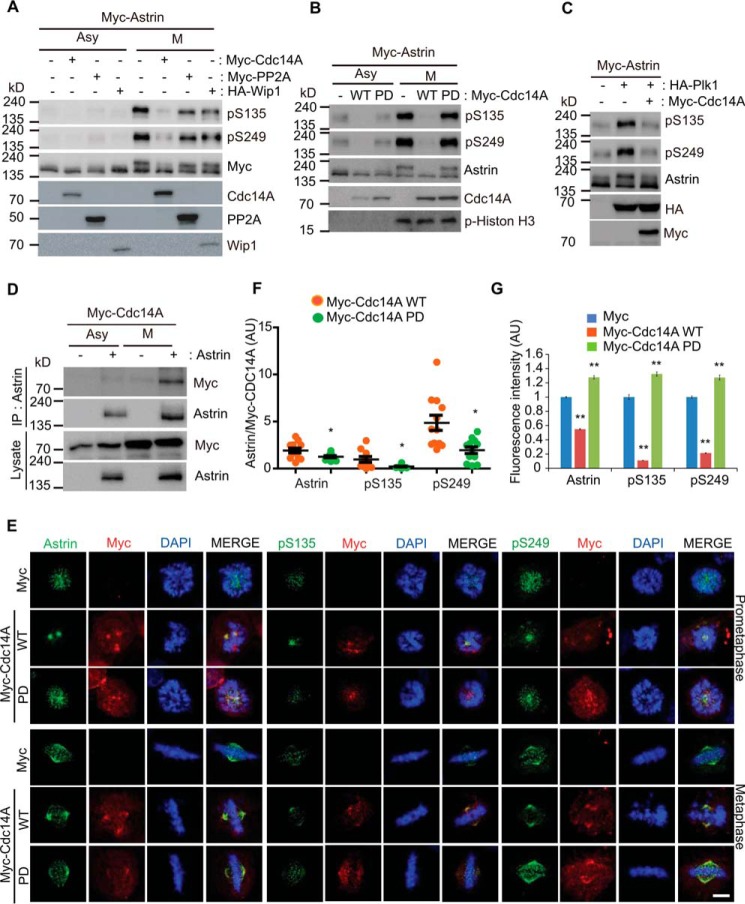
Cdc14A Dephosphorylates Astrin and Controls Its KT Targeting
Although Plk1-mediated Astrin phosphorylation by Cdk1 is pivotal for the KT targeting of Astrin and stable spindle attachment, unphosphorylated Astrin still localizes to the spindle (Figs. 2G and 5A). To explore whether Astrin dephosphorylation is required for the fine-tuning of Astrin phosphorylation and KT targeting, we tested three mitotic phosphatases, Cdc14A, PP2A, and Wip1. Notably, only Cdc14A significantly dephosphorylated Astrin at Ser-135 and Ser-249 (Fig. 9A). Furthermore, the C278S mutant, the phosphatase-dead form of Cdc14A, did not dephosphorylate Astrin (Fig. 9B), suggesting that Cdc14A acts as the phosphatase for Astrin Ser-135 and Ser-249 during mitosis. Additionally, WT Cdc14A dephosphorylated Astrin at Ser-135 and Ser-249, which was mediated by Plk1 (Fig. 9C). We found that the interaction of Astrin with Cdc14A was specific to mitosis (Fig. 9D). To further investigate whether Cdc14A controls Astrin targeting to KTs, we analyzed the translocation of phospho-Astrin following the expression of WT Cdc14A or the phosphatase-dead mutant. Consistent with the biochemical data, ectopically expressed Cdc14A localized to the centrosome and the spindle in prometaphase and metaphase cells (Fig. 9E). Interestingly, Astrin and phospho-Astrin were sequestered in centrosomes via the ectopic expression of WT Cdc14A (Fig. 9F). In contrast, the phosphatase-dead mutant increased the translocation of phospho-Astrin from the centrosome to KTs (Fig. 9G), indicating that Cdc14A negatively regulates Astrin targeting to KTs via Astrin dephosphorylation. We conclude that Cdc14A dephosphorylates phospho-Astrin and thus regulates the amount of phospho-Astrin in KTs.
FIGURE 9.
Cdc14A controls Astrin translocation to KTs by dephosphorylating Ser-135 and Ser-249. A and B, 24 h after transfection, HEK 293T cells were treated with/without nocodazole for 16 h, harvested, and subjected to Western blotting analysis with the indicated antibodies. C, 24 h after transfection, cells were harvested and subjected to Western blotting analysis using the indicated antibodies. D, 24 h after transfection, the cells were treated with/without nocodazole for 16 h, harvested, and subjected to immunoprecipitation with anti-Astrin and Western blotting analysis using the indicated antibodies. E–G, 28 h after transfection with Myc, Myc-Cdc14A, or Myc-Cdc14A phosphatase-dead (PD), cells were fixed and stained for the indicated antigens. Images were acquired under constant exposure in each channel for all cells and are maximum projections from z stacks of representative cells. Astrin and phospho-Astrin intensities at the centrosome in prometaphase cells were quantified and plotted (F; n = 12 centrosomes from three independent experiments). Total Astrin and phospho-Astrin intensities at KTs in metaphase cells were quantified and plotted (G; n = 150 KTs from three independent experiments). *, p < 0.05; **, p < 0.01 (two-tailed t test relative to control cells). Error bars, S.E. Scale bar, 5 μm. AU, arbitrary units; Asy, asynchronous; M, mitotic; IP, immunoprecipitation.
Discussion
A central goal of research in the field of mitosis has been to define the molecular mechanisms that govern the attachment of spindle MTs to KTs. Although modern computational models suggest it would take hours for a single MT to encounter each KT on 46 chromosomes (35), human cells complete mitosis in 30 min (36). Clearly, the regulatory mechanisms that explain mitotic spindle assembly, the microtubule search and capture model, have remained elusive. Recent work has demonstrated that the prepositioning of chromosomes near the spindle equator facilitates the KT “search” with highly dense MTs and the formation of stable amphitelic attachments (18). Here, we identify the requirements of a signal messenger that is vital in KT composition, phospho-Astrin, which acts downstream of Cdk1 and Plk1 to establish stable spindle attachments and promote the prometaphase-metaphase transition. Cdk1 generates phospho-Astrin in the centrosome, and Kinastrin transports it to the KTs. Phospho-Astrin lands on KTs when the spindle MT encounters the KT and induces a change in KT composition by recruiting Cenp-E to KTs. Furthermore, we identified Cdc14A as a phosphatase that regulates the phosphorylation status of Astrin on the mitotic spindle.
During early mitosis, MT nucleation from the centrosome and kinetochore formation in the centromere after nuclear envelope breakdown are precisely regulated. The dynamic plus ends of spindle MTs search for KTs by growing and shrinking and initially form weak bonds with KT components, including the Ndc80 complex and Knl1, which have low affinity binding site architecture in the case of abnormal MT attachment (3). Thus, MT binding affinity increases in response to the recruitment of outer KT components, such as motor proteins, including dynein and Cenp-E, or MT plus end-binding proteins, including EB1, Clip-170, Ska complex, and Cenp-F. Plk1 is known to be a KT regulator because it localizes to mitotic structures, such as the centrosome, spindle, and KTs, and controls the localization of several KT-associated proteins, such as Hec1, Cdc20, Mad2, and Cenp-E (37). Additionally, a recent report corroborated that Clip-170-mediated Plk1 targeting to KTs promotes K-fiber stability and chromosome alignment by recruiting Cenp-E in an MT-independent manner (38). However, the molecular mechanisms by which Plk1 recruits Cenp-E and the identity of the substrate in this process remain unknown. Plk1 is discriminative: Polo-box domain recognizes its mitotic substrates in which the consensus motif is phosphorylated by the priming kinase Cdk1 (39, 40). This very precise mechanism regulates the stability of KT-MT attachment and chromosome alignment via the priming phosphorylation of Ser-1234 in Clasp2 and the associated recruitment of Plk1 to phosphorylate the C terminus (41). Our data, in contrast, suggest that Astrin is the substrate of the Plk1-Cdc25-Cdk1 positive feedback loop involved in Cenp-E recruitment to KTs (Fig. 7, D and G). These data provide a straightforward explanation for the mechanism by which Plk1 controls stable spindle attachment and chromosome integrity: Astrin is phosphorylated by Cdk1 and converts labile spindle attachments to stable attachments via the recruitment of Cenp-E (Figs. 5D and 8L). In addition to identifying Cdk1 as a mitotic kinase essential in Astrin phosphorylation, we also demonstrated that the phosphatase Cdc14A is specific for phospho-Astrin (Fig. 9).
In summary, our work reveals a new agent involved in search and capture, the signal messenger phospho-Astrin, which is generated by Cdk1 and targeted to KTs by Kinastrin and is involved in stable KT attachment to the spindle. Communication between the centrosome and KT via phospho-Astrin ensures an efficient search and capture process and precisely timed bioriented spindle formation. By defining the key molecular events required for stable spindle attachment and the Plk1-Cdk1-Astrin-Cdc14A regulatory circuit, we elucidate a key mechanism in mitotic progression. Further analyses will provide additional information about both the mechanism underlying bioriented spindle formation and chromosome instability in cancer cells.
Experimental Procedures
Plasmids, Chemicals, and Antibodies
Rabbit anti-phospho-Ser-135 or Ser-249 antibodies were generated by immunizing rabbits with phosphopeptide 128KTIVLVP(pS)PLGQQQD142 or 243SSVLWL(pS)PSTALA255 where pS is phosphoserine. Anti-Astrin antibodies were from Proteintech (14726-1-AP) and Sigma (WH0010615M1). Anti-FLAG (F3165) and -β-actin (A5316) antibodies were purchased from Sigma. Anti-Myc (11814150001) and -HA (12013819001) antibodies were purchased from Roche Applied Science. Anti-Hsp90 (sc-69703), -Plk1 (sc-17783), -p38MAPK (sc-535), -Cenp-E (sc-22790), -Cyclin B1 (sc-245), and -Cdk1 (sc-954) antibodies were purchased from Santa Cruz Biotechnology. Anti-Hec1 (GTX70268) and -Cenp-F (GTX30232) were purchased from GeneTex, Inc. Anti-β-tubulin E7 monoclonal antibody (AB 2315513) was obtained from the Developmental Studies Hybridoma Bank. The following antibodies were obtained from commercial sources: anti-Mad2 (PA5-21594, Thermo Scientific Pierce Antibodies), anti-BubR1 (LS-C2771, LifeSpan BioSciences), anti-phosphorylated Histone H3 (9701, Cell Signaling Technology), anti-GFP (632389, Clontech), anti-Kinastrin (HPA042027, Atlas Antibodies), and anti-Cyclin A2 (ab7956, Abcam).
The Cdc14A gene and the Astrin gene were purchased from the Korean human gene bank and Addgene (Cambridge), respectively. Myc-tagged Cdc14A expression plasmids were cloned into a Myc-tagged mammalian expression vector. Astrin or Cdc14A deletion/point mutants were generated by mutagenesis. HA-Plk1, HA-Aurora A, and HA-Aurora B were cloned into an HA-tagged mammalian expression vector.
For kinase inhibition, cells were incubated with 200 nm Plk1 inhibitor BI 2536 (Selleckchem), 2 μm Aurora kinase inhibitor ZM447439 (Selleckchem), and 10 μm Cdk1 inhibitor RO3306 (Alexis). In addition, we used 5 μm MG132 to block metaphase using a mitotic shake-off method.
Cell Culture, siRNA, and Transfection
Human embryonic kidney 293T (HEK 293T) cells and HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM; WelGENE Inc.) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). The cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2. The control siRNA was described previously (42). The Astrin siRNA sequences were described previously (26). The Kinastrin siRNA sequences were described previously (27). siRNAs were transfected into HeLa cells using DharmaFect 1 (Dharmacon, Inc.). DNA transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
In Vitro Kinase Assay
Assays were performed with the active form of purified recombinant Plk1 (P41-10H, SignalChem) or Cdk1-Cyclin B complex (P6020, New England Biolabs). GST-Astrin-N was incubated with lysates from mitotic cell extracts obtained from nocodazole-treated cells with the active form of purified recombinant Plk1 or with Cdk1-Cyclin B complex and 200 μm ATP for 1 h at 30 °C. The reaction mixture was separated by SDS-PAGE, and Astrin phosphorylation was detected using phospho-Ser-135 or phospho-Ser-249 anti-Astrin antibodies.
Immunoprecipitation
For immunoprecipitation, cells were washed with ice-cold PBS and then lysed in NETN buffer (0.5% Nonidet P-40, 20 mm Tris (pH 8.0), 50 mm NaCl, 50 mm NaF, 100 μm Na3VO4, 1 mm DTT, and 50 μg/ml PMSF) at 4 °C for 10 min. Crude lysates were cleared by centrifugation at 14,000 rpm at 4 °C for 5 min, and the supernatants were incubated with protein A-agarose-conjugated primary antibodies. The resulting immunocomplexes were washed three times with NETN buffer and subjected to SDS-PAGE. Western blotting was performed using the antibodies indicated in the figure legends.
Purification of GST Fusion Proteins
GST fusion proteins were expressed in Escherichia coli and purified as described previously (43).
Immunofluorescence
HeLa cells plated on coverglass were fixed with methanol at −20 °C for 30 min. Alternatively, the cells were extracted with BRB80-T buffer (80 mm PIPES, pH 6.8, 1 mm MgCl2, 5 mm EGTA, and 0.5% Triton X-100) and then fixed with 4% paraformaldehyde for 15 min at room temperature. The fixed cells were then permeabilized and blocked with PBS-BT (1× PBS, 3% BSA, and 0.1% Triton X-100) for 30 min at room temperature. The coverslips were then incubated in primary and secondary antibodies diluted in PBS-BT. Images were acquired using AxioVision 4.8.2 (Carl Zeiss) under a Zeiss Axiovert 200M microscope with a plan-Apo 100× oil immersion lens and an HRm CCD camera. Deconvolved images were obtained using AutoQuant X3 (AutoQuant Imaging). All images are maximum projections from z stacks of representative cells stained with the indicated antigens. Images used for quantification were acquired under constant exposure in each channel for all cells.
Statistical Analysis
Student's t test was performed. Error bars represent the standard error of several independent experiments. A p value <0.05 (two-tailed) was considered statistically significant. *, p < 0.05; **, p < 0.01 (two-tailed t test relative to control cells).
Author Contributions
H. K. and C.-Y. J. were responsible for the experimental design, data interpretation, and writing of the manuscript. H. J. C. and N. S. L conducted most of the biochemical experiments. J. E. P. performed most of the imaging experiments.
This work was supported by Institute for Basic Science Grant IBS-R015-D1 and National Research Foundation of Korea grants NRF-2015R1A2A2A01003975 and 2011-0030074 funded by the Korea government. The authors declare that they have no conflicts of interest with the contents of this article.
- MT
- microtubule
- KT
- kinetochore
- Cdk1
- Cyclin-dependent kinase 1
- Plk1
- Polo-like kinase 1
- Clasp
- cytoplasmic linker-associated protein
- DD
- S135D/S249D.
References
- 1. Walczak C. E., Cai S., and Khodjakov A. (2010) Mechanisms of chromosome behaviour during mitosis. Nat. Rev. Mol. Cell Biol. 11, 91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheeseman I. M., and Desai A. (2008) Molecular architecture of the kinetochore-microtubule interface. Nat. Rev. Mol. Cell Biol. 9, 33–46 [DOI] [PubMed] [Google Scholar]
- 3. Cheeseman I. M., Chappie J. S., Wilson-Kubalek E. M., and Desai A. (2006) The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127, 983–997 [DOI] [PubMed] [Google Scholar]
- 4. DeLuca J. G., Moree B., Hickey J. M., Kilmartin J. V., and Salmon E. D. (2002) hNuf2 inhibition blocks stable kinetochore-microtubule attachment and induces mitotic cell death in HeLa cells. J. Cell Biol. 159, 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murray A. W. (2004) Recycling the cell cycle: cyclins revisited. Cell 116, 221–234 [DOI] [PubMed] [Google Scholar]
- 6. Seki A., Coppinger J. A., Jang C. Y., Yates J. R., and Fang G. (2008) Bora and the kinase Aurora A cooperatively activate the kinase Plk1 and control mitotic entry. Science 320, 1655–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tavernier N., Noatynska A., Panbianco C., Martino L., Van Hove L., Schwager F., Léger T., Gotta M., and Pintard L. (2015) Cdk1 phosphorylates SPAT-1/Bora to trigger PLK-1 activation and drive mitotic entry in C. elegans embryos. J. Cell Biol. 208, 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lénárt P., Petronczki M., Steegmaier M., Di Fiore B., Lipp J. J., Hoffmann M., Rettig W. J., Kraut N., and Peters J. M. (2007) The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 17, 304–315 [DOI] [PubMed] [Google Scholar]
- 9. Steegmaier M., Hoffmann M., Baum A., Lénárt P., Petronczki M., Krssák M., Gürtler U., Garin-Chesa P., Lieb S., Quant J., Grauert M., Adolf G. R., Kraut N., Peters J. M., and Rettig W. J. (2007) BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr. Biol. 17, 316–322 [DOI] [PubMed] [Google Scholar]
- 10. Tanaka K. (2012) Dynamic regulation of kinetochore-microtubule interaction during mitosis. J. Biochem. 152, 415–424 [DOI] [PubMed] [Google Scholar]
- 11. Dong Y., Vanden Beldt K. J., Meng X., Khodjakov A., and McEwen B. F. (2007) The outer plate in vertebrate kinetochores is a flexible network with multiple microtubule interactions. Nat. Cell Biol. 9, 516–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maiato H., DeLuca J., Salmon E. D., and Earnshaw W. C. (2004) The dynamic kinetochore-microtubule interface. J. Cell Sci. 117, 5461–5477 [DOI] [PubMed] [Google Scholar]
- 13. Bornens M. (2012) The centrosome in cells and organisms. Science 335, 422–426 [DOI] [PubMed] [Google Scholar]
- 14. Doxsey S., Zimmerman W., and Mikule K. (2005) Centrosome control of the cell cycle. Trends Cell Biol. 15, 303–311 [DOI] [PubMed] [Google Scholar]
- 15. Löffler H., Fechter A., Matuszewska M., Saffrich R., Mistrik M., Marhold J., Hornung C., Westermann F., Bartek J., and Krämer A. (2011) Cep63 recruits Cdk1 to the centrosome: implications for regulation of mitotic entry, centrosome amplification, and genome maintenance. Cancer Res. 71, 2129–2139 [DOI] [PubMed] [Google Scholar]
- 16. Jackman M., Lindon C., Nigg E. A., and Pines J. (2003) Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 5, 143–148 [DOI] [PubMed] [Google Scholar]
- 17. Tanaka K., Mukae N., Dewar H., van Breugel M., James E. K., Prescott A. R., Antony C., and Tanaka T. U. (2005) Molecular mechanisms of kinetochore capture by spindle microtubules. Nature 434, 987–994 [DOI] [PubMed] [Google Scholar]
- 18. Magidson V., O'Connell C. B., Lončarek J., Paul R., Mogilner A., and Khodjakov A. (2011) The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell 146, 555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akiyoshi B., Sarangapani K. K., Powers A. F., Nelson C. R., Reichow S. L., Arellano-Santoyo H., Gonen T., Ranish J. A., Asbury C. L., and Biggins S. (2010) Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 468, 576–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanaka T. U., Stark M. J., and Tanaka K. (2005) Kinetochore capture and bi-orientation on the mitotic spindle. Nat. Rev. Mol. Cell Biol. 6, 929–942 [DOI] [PubMed] [Google Scholar]
- 21. Bakhoum S. F., Thompson S. L., Manning A. L., and Compton D. A. (2009) Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat. Cell Biol. 11, 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson S. L., and Compton D. A. (2011) Chromosomes and cancer cells. Chromosome Res. 19, 433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McEwen B. F., Hsieh C. E., Mattheyses A. L., and Rieder C. L. (1998) A new look at kinetochore structure in vertebrate somatic cells using high-pressure freezing and freeze substitution. Chromosoma 107, 366–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suzuki A., Hori T., Nishino T., Usukura J., Miyagi A., Morikawa K., and Fukagawa T. (2011) Spindle microtubules generate tension-dependent changes in the distribution of inner kinetochore proteins. J. Cell Biol. 193, 125–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Santaguida S., and Musacchio A. (2009) The life and miracles of kinetochores. EMBO J. 28, 2511–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thein K. H., Kleylein-Sohn J., Nigg E. A., and Gruneberg U. (2007) Astrin is required for the maintenance of sister chromatid cohesion and centrosome integrity. J. Cell Biol. 178, 345–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dunsch A. K., Linnane E., Barr F. A., and Gruneberg U. (2011) The astrin-kinastrin/SKAP complex localizes to microtubule plus ends and facilitates chromosome alignment. J. Cell Biol. 192, 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mack G. J., and Compton D. A. (2001) Analysis of mitotic microtubule-associated proteins using mass spectrometry identifies astrin, a spindle-associated protein. Proc. Natl. Acad. Sci. U.S.A. 98, 14434–14439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gruber J., Harborth J., Schnabel J., Weber K., and Hatzfeld M. (2002) The mitotic-spindle-associated protein astrin is essential for progression through mitosis. J. Cell Sci. 115, 4053–4059 [DOI] [PubMed] [Google Scholar]
- 30. Cheng T. S., Hsiao Y. L., Lin C. C., Hsu C. M., Chang M. S., Lee C. I., Yu R. C., Huang C. Y., Howng S. L., and Hong Y. R. (2007) hNinein is required for targeting spindle-associated protein Astrin to the centrosome during the S and G2 phases. Exp. Cell Res. 313, 1710–1721 [DOI] [PubMed] [Google Scholar]
- 31. Manning A. L., Bakhoum S. F., Maffini S., Correia-Melo C., Maiato H., and Compton D. A. (2010) CLASP1, astrin and Kif2b form a molecular switch that regulates kinetochore-microtubule dynamics to promote mitotic progression and fidelity. EMBO J. 29, 3531–3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmidt J. C., Kiyomitsu T., Hori T., Backer C. B., Fukagawa T., and Cheeseman I. M. (2010) Aurora B kinase controls the targeting of the Astrin-SKAP complex to bioriented kinetochores. J. Cell Biol. 191, 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng T. S., Hsiao Y. L., Lin C. C., Yu C. T., Hsu C. M., Chang M. S., Lee C. I., Huang C. Y., Howng S. L., and Hong Y. R. (2008) Glycogen synthase kinase 3β interacts with and phosphorylates the spindle-associated protein astrin. J. Biol. Chem. 283, 2454–2464 [DOI] [PubMed] [Google Scholar]
- 34. Chiu S. C., Chen J. M., Wei T. Y., Cheng T. S., Wang Y. H., Ku C. F., Lian C. H., Liu C. C., Kuo Y. C., and Yu C. T. (2014) The mitosis-regulating and protein-protein interaction activities of astrin are controlled by aurora-A-induced phosphorylation. Am. J. Physiol. Cell Physiol. 307, C466–C478 [DOI] [PubMed] [Google Scholar]
- 35. Wollman R., Cytrynbaum E. N., Jones J. T., Meyer T., Scholey J. M., and Mogilner A. (2005) Efficient chromosome capture requires a bias in the 'search-and-capture' process during mitotic-spindle assembly. Curr. Biol. 15, 828–832 [DOI] [PubMed] [Google Scholar]
- 36. Yang Z., Loncarek J., Khodjakov A., and Rieder C. L. (2008) Extra centrosomes and/or chromosomes prolong mitosis in human cells. Nat. Cell Biol. 10, 748–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahonen L. J., Kallio M. J., Daum J. R., Bolton M., Manke I. A., Yaffe M. B., Stukenberg P. T., and Gorbsky G. J. (2005) Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr. Biol. 15, 1078–1089 [DOI] [PubMed] [Google Scholar]
- 38. Amin M. A., Itoh G., Iemura K., Ikeda M., and Tanaka K. (2014) CLIP-170 recruits PLK1 to kinetochores during early mitosis for chromosome alignment. J. Cell Sci. 127, 2818–2824 [DOI] [PubMed] [Google Scholar]
- 39. Cheng K. Y., Lowe E. D., Sinclair J., Nigg E. A., and Johnson L. N. (2003) The crystal structure of the human polo-like kinase-1 polo box domain and its phospho-peptide complex. EMBO J. 22, 5757–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elia A. E., Rellos P., Haire L. F., Chao J. W., Ivins F. J., Hoepker K., Mohammad D., Cantley L. C., Smerdon S. J., and Yaffe M. B. (2003) The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell 115, 83–95 [DOI] [PubMed] [Google Scholar]
- 41. Maia A. R., Garcia Z., Kabeche L., Barisic M., Maffini S., Macedo-Ribeiro S., Cheeseman I. M., Compton D. A., Kaverina I., and Maiato H. (2012) Cdk1 and Plk1 mediate a CLASP2 phospho-switch that stabilizes kinetochore-microtubule attachments. J. Cell Biol. 199, 285–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim H., Chen J., and Yu X. (2007) Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science 316, 1202–1205 [DOI] [PubMed] [Google Scholar]
- 43. Cho H. J., Oh Y. J., Kwon J., Kwon J. Y., Kim K. S., and Kim H. (2010) c-Myc stimulates cell invasion by inhibiting FBX8 function. Mol. Cells 30, 355–362 [DOI] [PubMed] [Google Scholar]