Abstract
Reactive oxygen species (ROS) serve as a prime signal in the commitment to hematopoiesis in both mammals and Drosophila. In this study, the potential function of ROS during hematopoiesis in the crayfish Pacifastacus leniusculus was examined. The antioxidant N-acetylcysteine (NAC) was used to decrease ROS in both in vivo and in vitro experiments. An increase in ROS was observed in the anterior proliferation center (APC) after LPS injection. In the absence of NAC, the LPS-induced increase in ROS levels resulted in the rapid restoration of the circulating hemocyte number. In the presence of NAC, a delay in the recovery rate of the hemocyte number was observed. NAC treatment also blocked the spread of APC and other hematopoietic tissue (HPT) cells, maintaining these cells at an undifferentiated stage. Extracellular transglutaminase (TGase) has been shown previously to play a role in maintaining HPT cells in an undifferentiated form. In this study, we show that extracellular TGase activity increased when the ROS level in HPT or APC cells was reduced after NAC treatment. In addition, collagen, a major component of the extracellular matrix and a TGase substrate were co-localized on the HPT cell surface. Taken together, the results of this study show that ROS are involved in crayfish hematopoiesis, in which a low ROS level is required to maintain hematopoietic progenitor cells in the tissue and to reduce hemocyte release. The potential roles of TGase in this process are investigated and discussed.
Keywords: extracellular matrix, hematopoiesis, invertebrate, reactive oxygen species (ROS), transglutaminase
Introduction
Hematopoiesis, the process by which new blood cells are formed, occurs in different organs in different animal phyla, and several regulatory pathways of this process are evolutionary conserved from invertebrates to vertebrates (1–3). However, few invertebrate species have been investigated, and therefore knowledge of invertebrate hematopoiesis is lacking. One of the more well established invertebrate models in the field of blood cell (hemocyte) production, except for Drosophila melanogaster, is the freshwater crayfish Pacifastacus leniusculus, in which proliferation of hematopoietic cells and their differentiation into mature hemocytes have been characterized in detail (1, 4). In this species, a technique for the culture of hematopoietic tissue and stem cells was successfully established, resulting in the isolation of important cytokines; namely, astakines (5). The hemocyte production in crayfish occurs in hematopoietic tissue (HPT),2 a separate organ located on the dorsal part of the stomach. The most anterior part of the HPT is a specific region called the anterior proliferation center (APC), where actively proliferating cells are localized (Fig. 1) (6). The APC can be easily separated from the other part of the HPT, and the cells isolated from the APC rapidly divide in culture, forming spherical clusters, in contrast to cells from the posterior HPT, which slowly proliferate and form monolayers (6). It is not known whether the APC area plays a similar role as the posterior signaling center in Drosophila, which functions as a hematopoietic niche by providing signals to progenitor cells (7, 8).
FIGURE 1.
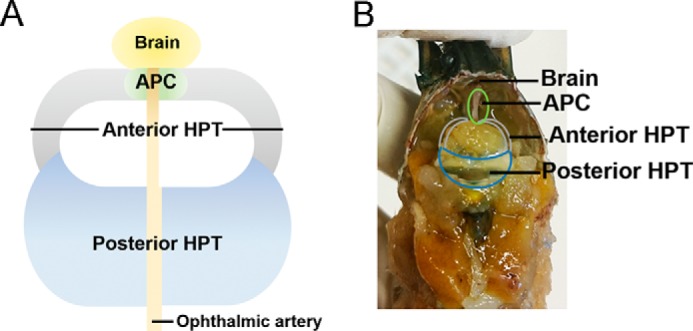
Location and structure of the crayfish APC and different parts of hematopoietic tissue. Shown is the location of the APC and HPT in crayfish underneath the carapace. The HPT covers the dorsal part of the stomach. The HPT is divided into two parts, the posterior HPT (blue in A and B) and anterior HPT (gray in A and B). The APC (green in A and B) is a special small area in the anterior part of the HPT.
The remaining HPT (reHPT) contains five different morphological cell types characterized by morphological studies based on electron microscopy, and these cells serve as progenitors of crayfish mature hemocytes (9). Within the reHPT, these cells are arranged in lobule-like structures surrounded by connective tissue. This connective tissue serves as a structural support for the cells inside the tissue and provides a specific microenvironment for the regulation of the differentiating cells. Previously, we demonstrated the importance of extracellular transglutaminase (TGase) activity to control hematopoiesis through the interaction of hematopoietic cells and the extracellular matrix (ECM) to maintain the immature HPT cells inside the hematopoietic tissue (10). The importance of ECM proteins for hematopoiesis has been shown in Drosophila, where a Perlecan homolog, the heparin sulfate proteoglycan Terribly Reduced Optic (Trol), was identified as part of the ECM in the lymph gland (11). Interestingly, the loss of Trol caused a dramatic conformational change of the ECM and induced the differentiation of blood cell progenitors. In addition, Trol regulates PDGF signaling in plasmatocytes (12). PDGF-like factors and their receptors, PDGF/VEGF receptors, are involved in hemocyte production in the Drosophila embryo (13–15). The interaction between Trol and the PDGF/VEGF receptor signaling pathway participates in hemocyte homeostasis in Drosophila (11, 12). The balance between the differentiation and proliferation of hematopoietic cells can be regulated through both internal and external factors (16). A new group of cytokines, astakines (Ast1 and Ast2), were isolated and characterized from crayfish (5, 17). Ast1 contains a prokineticin domain that regulates the development of the nervous system, immunity, and hematopoiesis in vertebrates (18, 19). In crayfish, Ast1 is a hematopoietic growth factor important for the regulation of the balance between proliferation/differentiation and apoptosis (4). Furthermore, Ast1 manipulates the ECM structure through the regulation of extracellular TGase activity (10).
Cell respiration leads to the production of reactive oxygen species (ROS), and all cells have to maintain ROS at a harmless level to reduce the risk of oxidative stress, as high ROS levels are dangerous for cells (20). However, recent studies have shown that low/medium ROS levels can be of great importance for the regulation of hematopoiesis. In mammals, the hematopoietic stem cell niche retains a certain level of ROS to maintain hematopoietic stem cell functions (21). In addition, increased levels of ROS have induced the differentiation of hematopoietic stem cells in Drosophila and mammals (21, 22). In Drosophila, high ROS levels were detected in hematopoietic progenitor cells, and increased ROS levels were detected in the posterior signaling center of the lymph gland after a wasp infestation (23). In the fly, ROS serve as an important signal for progenitor cell differentiation into lamellocytes, a hemocyte type that encapsulates wasp eggs (24, 25). In crayfish, a high level of ROS was detected in the APC, where actively proliferating cells are located (6). The ROS level in the APC is controlled through circadian rhythms and thereby regulates the circadian variation in the number of circulating hemocytes (6). Furthermore, LPS injection dramatically decreased the number of circulating hemocytes, which was followed by a high ROS level in the APC area and the subsequent differentiation and release of new hemocytes (6), indicating a conserved role for ROS signaling in the regulation of hematopoiesis.
In this study, we focused on the potential roles of ROS in controlling hematopoiesis in the crayfish P. leniusculus. The antioxidant N-acetylcysteine (NAC) was used to decrease ROS levels in both in vivo and in vitro experiments. NAC is a precursor that provides cysteine and glutamine for glutathione production to reduce ROS levels. Here we showed that the NAC-induced low ROS levels hindered the recovery of circulating hemocytes in hemolymph after LPS injection. In contrast, LPS injection induced high ROS levels, likely enhancing the restoration of hemocyte number. We also showed that the levels of ROS regulate hematopoiesis through the regulation of extracellular TGase activity and significant up-regulation of extracellular TGase activity after reducing the ROS level through NAC treatment.
Results
The Effects of NAC on ROS Production
To investigate the effect of decreased ROS production on hematopoiesis, the antioxidant NAC was used as a scavenger for ROS. First, we examined whether NAC could reduce ROS levels in APC or reHPT tissues in live animals (in vivo) and cultured cells (in vitro). NAC (75 μg/g fresh weight of the animal) was injected into the crayfish. 1 h after injection, significantly reduced ROS levels were observed both in APC (p < 0.05) and reHPT (p < 0.001) tissues compared with PBS-injected animals (Fig. 2A). 24 h after NAC injection, the ROS level in APC tissue was continuously decreased but not significantly different compared with control PBS-injected animals. In reHPT tissue, there was no difference in ROS level between NAC-injected animals and the PBS control at 24 h (data not shown). Furthermore, in vitro experiments showed that the ROS levels in APC cells and cells from reHPT treated with NAC for 30 min was significantly reduced compared with the control (Fig. 2, B (p < 0.001 and 0.01, respectively) and C). In contrast, the ROS level in cells incubated with 1 μm H2O2 as a positive control significantly increased both in the APC cells (p < 0.05) and reHPT cells (p < 0.01) (Fig. 2, B and C). After 24 h, the ROS level in 30-min NAC-treated reHPT cells was slightly lower but not significantly different compared with the control group (data not shown). APC is a small tissue situated in the anterior part of the HPT and contains a high percentage of actively proliferating cells (Fig. 1, A and B, green). High ROS levels have been reported in this area (6). The results of this study suggest that the ROS production in the APC, which contains less differentiated cells, could be most efficiently reduced after NAC treatment.
FIGURE 2.
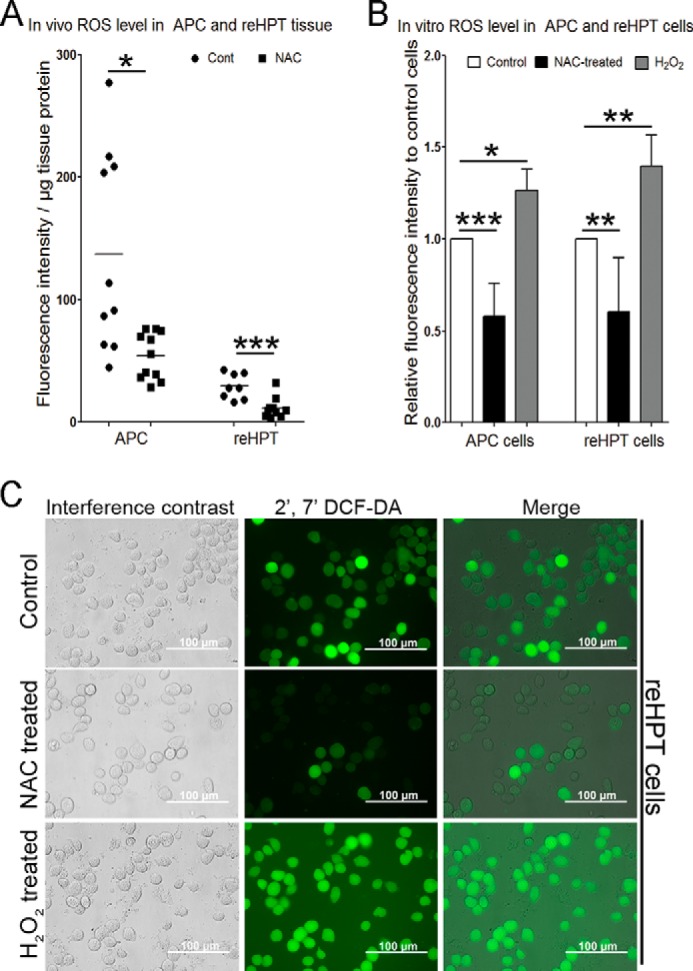
The effect of NAC on ROS production in APC and reHPT tissues and cells. ROS production was determined as fluorescence intensity using a plate reader with an excitation wavelength of 485 nm and an emission wavelength of 535 nm. A, 1 h after injection of NAC (75 μg/g (■) or PBS (●), the ROS level in the APC and reHPT tissues was detected. ●, the ROS level per microgram of tissue protein in APC or reHPT tissues of each individual PBS injected crayfish; ■, the ROS level per microgram of tissue protein in APC or reHPT tissues of each individual NAC injected crayfish; −, the mean ROS level of 8–12 crayfish. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with the control. B, the levels of ROS production in APC and reHPT cells were also examined at 30 min after in vitro treatment with NAC (10 μm) or H2O2 (1 μm) (a positive control). The columns represent the mean of three to five crayfish from three to five separate experiments, and the error bars represent the S.D. value. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with the control. C, ROS signal in reHPT cells 30 min after NAC and H2O2 treatments. The ROS level was observed under a fluorescence microscope. The pictures were captured immediately after staining. DCF-DA, 2′,7′-dichlorofluorescin diacetate.
ROS Signaling Is Involved in New Hemocyte Synthesis
The ROS level in the APC increased after the LPS-induced loss of circulating hemocytes, and the high ROS level was followed by an increase in the number of circulating hemocytes (6). This finding suggests the involvement of ROS in hematopoiesis. Therefore, to confirm the importance of ROS signaling in hemocyte production, we investigated the total hemocyte number in NAC-treated animals compared with crayfish PBS (CPBS)-injected animals. After a reduction in ROS production by NAC injection, the hemocyte number was decreased at 16 h (p < 0.05) and 24 h (p < 0.01) compared with CPBS-injected crayfish (Fig. 3A) but restored to a normal level at 48 h and later. Moreover, to reveal whether NAC could affect the process of circulating hemocyte replenishment after LPS injection, LPS and NAC were injected together. The results showed that, 30 min after LPS injection, the total hemocyte count dramatically decreased (p < 0.001) and recovered after 24 h (Fig. 3B). Expectedly, in animals injected with NAC and LPS, the recovery rate of circulating hemocytes was significantly slower than in animals injected with LPS alone. These results indicate that ROS provide essential signals to control proliferation and differentiation and promotes new hemocyte release into the hemolymph.
FIGURE 3.
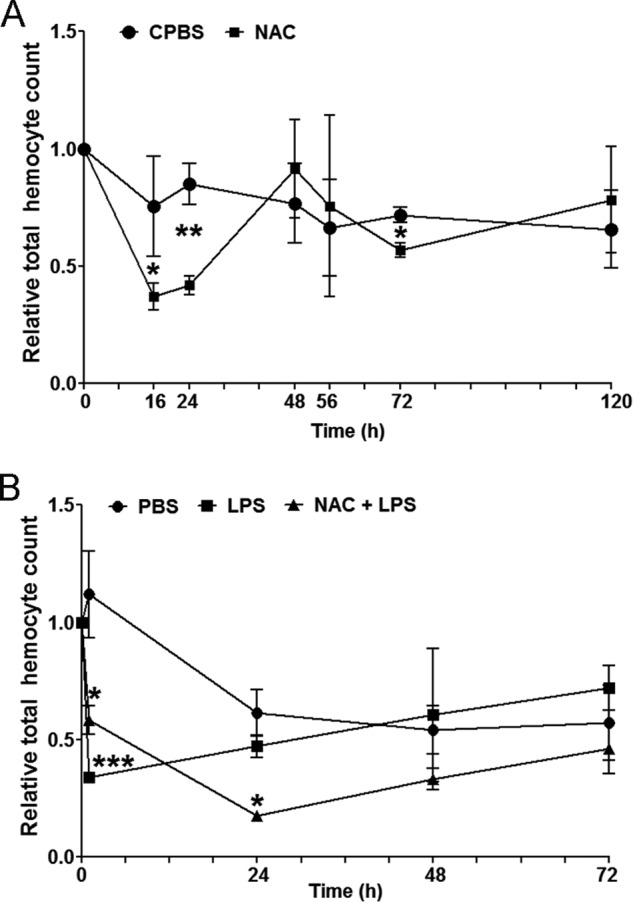
The effect of NAC on the total circulating hemocyte number. A, the total hemocyte count (THC) 16, 24, 48, 56, 72 and 120 h post-injection of NAC or CPBS (control) was examined. B, total hemocyte count 24, 48, and 72 h post-injection with PBS (control), LPS, or NAC together with LPS. Three to six crayfish were used in each experimental group. The relative hemocyte number was calculated by dividing the hemocyte number after injection with the number prior to injection. The columns represent the mean of three to six crayfish, and the error bars represent the S.D. value. The statistical analysis was performed using two-way analysis of variance. *, p < 0.05; **, p < 0.01; ***, p < 0.001 indicate a significant difference compared with the control.
Low Levels of ROS Inhibit Cell Spreading
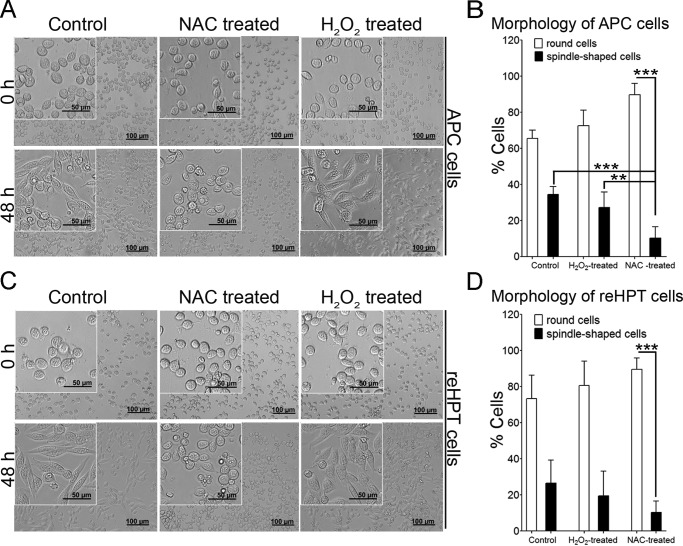
To clarify the mechanism by which hematopoietic cells respond to ROS signals, in vitro experiments were conducted. When crude Ast1 from plasma is added to cultured cells, proliferation and cell spreading (migration) are stimulated (5, 10). When the ROS level was decreased by incubating the cells for 30 min with 10 μm NAC, the APC and reHPT cells did not show any signs of spreading (spindle-shaped morphology) at 48 h compared with control cells and H2O2-treated cells (Fig. 4, A and C). In NAC-treated cells, the numbers of spindle-shaped cells were significantly lower than the number of round cells (p < 0.001) in both APC and reHPT tissues (Fig. 4, B and D). The significant effect of ROS on cell morphology was clearly observed in APC cells, as shown in Fig. 4B. The number of spindle-shaped cells among the NAC-treated APC cells was significantly lower than that in control and H2O2-treated cells (p < 0.001 and p < 0.01, respectively). According to these results, blocking ROS signaling results in cells maintained in a round stage in the tissue, whereas the induction of ROS production, for example through LPS injection, leads to cell spreading and release from hematopoietic tissue.
FIGURE 4.
The effect of NAC treatment on cell spreading. A, APC cell morphology 48 h after reduced ROS production through NAC treatment. B, percent of round or spindle-like APC cells (spreading cells) relative to the total APC cell number. The columns represent the mean of three to five crayfish from three to five separate experiments, and the error bars represent the S.D. value. **, p < 0.01; ***, p < 0.001. C, reHPT cell morphology 48 h after reduced ROS production through NAC treatment. D, percent of round or spindle-like reHPT cells relative to the total reHPT cell number. The columns represent the mean of three to five separate experiments, and the error bars represent the S.D. value. ***, p < 0.001.
ROS Function through TGase
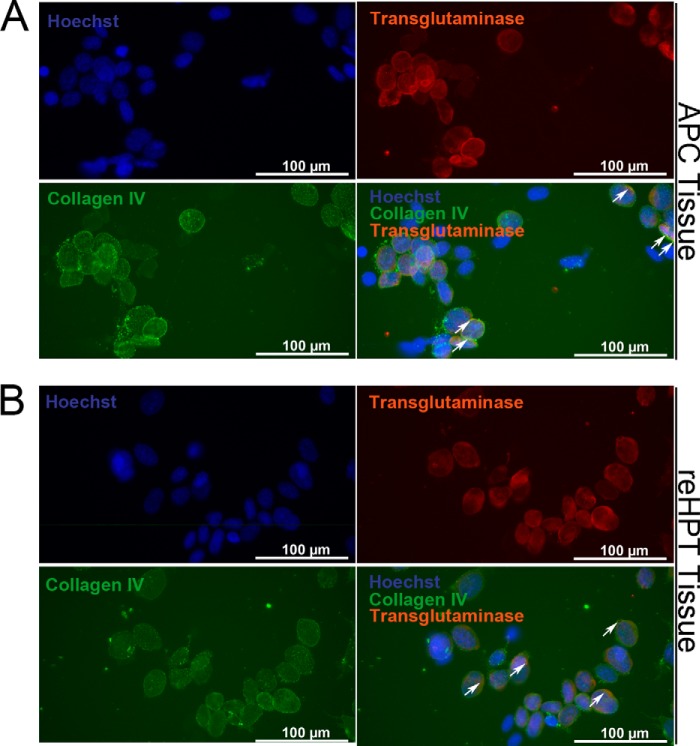
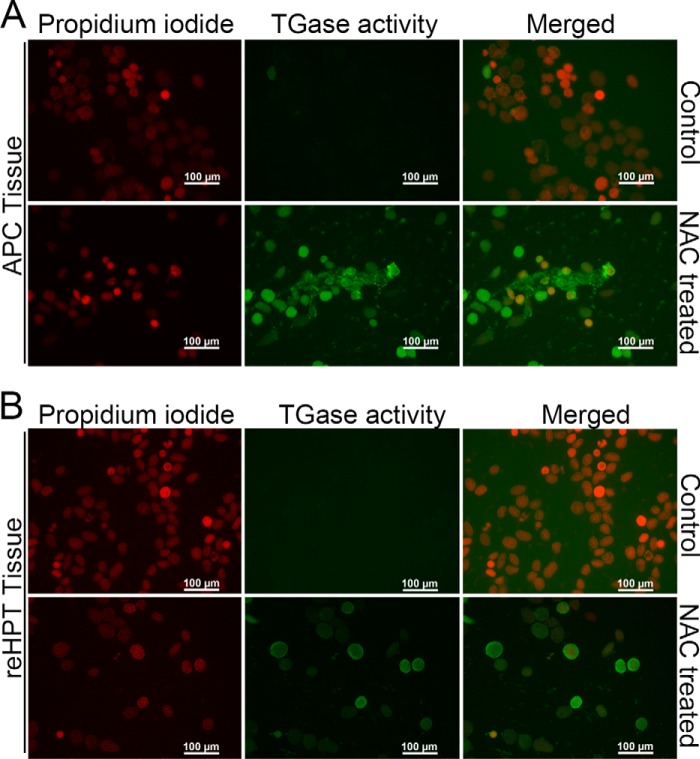
TGase is one of the most abundant proteins in crayfish reHPT, and high extracellular TGase activity has been shown to maintain reHPT cells in the tissue (10). Here we show that extracellular TGase colocalizes with collagen type IV, a component of the ECM in reHPT, on the surface of some APC and reHPT cells (Fig. 5, A and B). Because ROS affect Ast1-induced cell spreading, we examined the connection between ROS signaling and TGase activity. First, we examined the transcription level of TGase after reducing the ROS level through NAC treatment. In both reHPT and APC cells, the expression of TGase mRNA appeared slightly higher although not statistically significant (data not shown). Next, extracellular TGase activity was examined in partially digested APC and reHPT tissue cultures after NAC treatment. In both APC and reHPT tissue cultures, high extracellular TGase activity was present as a result of NAC treatment compared with controls (Fig. 6, A and B). In addition, TGase activity in the APC was higher than the activity in reHPT. This result was consistent with the results of previous studies showing that extracellular TGase played a role in blocking cell migration out of the HPT (10). Moreover, using an antibody against the ϵ-(γ-glutamyl)-lysine isopeptide, we detected some degree of protein cross-linking activity of TGase in the APC and reHPT tissue lysates by Western blots (Fig. 7) and in the HPT sections (data not shown). This cross-linking was slightly decreased in tissue cultures in which TGase was knocked down, and cell spreading was observed, although the difference was not statistically significant (data not shown), suggesting that extracellular TGase in crayfish HPT and APC possesses a cross-linking function to a minor degree, but the non-cross-linking functions of TGase are likely as important for the regulation of hemocyte release. Taken together, the inhibition of ROS signaling affected the extracellular TGase activity in hematopoietic precursor cells, slowing the release of new hemocytes into hemolymph.
FIGURE 5.
Co-localization of extracellular TGase and collagen IV on the surface of APC and reHPT tissues. A, double staining for extracellular TGase and collagen IV, a component of the extracellular matrix and a substrate of TGase, was performed on partially digested APC tissue. B, double staining for extracellular TGase and collagen IV was performed on partially digested reHPT tissue. Hoechst 33258 (blue) was used as a nuclear stain. Arrows indicate co-localization of TGase and collagen IV on the surface of APC and reHPT cells.
FIGURE 6.
The effect of NAC on extracellular TGase activity in APC and reHPT tissue cultures. The effect of NAC on extracellular TGase activity was determined after incubating the tissues with the TGase substrate 5-(biotinamindo)-pentylamine for 16 h. Subsequently, the cross-linking of the substrate was visualized after streptavidin-FITC addition. A, extracellular TGase activity in the partially digested APC tissue culture. B, extracellular TGase activity in the partially digested reHPT culture.
FIGURE 7.
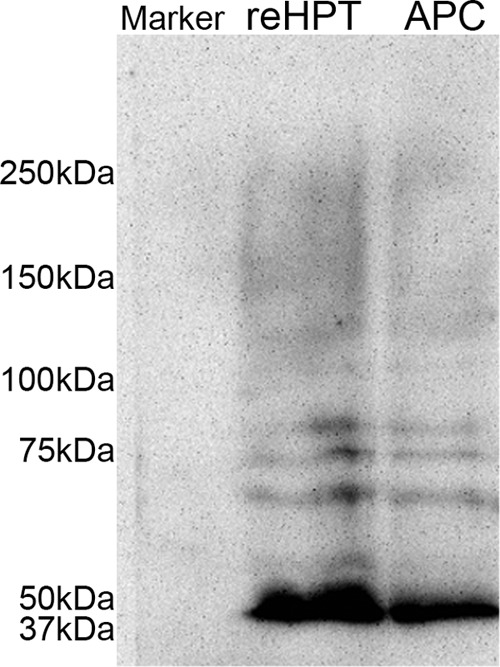
Western blot showing the presence of ϵ-(γ-glutamyl)-lysine cross-links in the HPT. Transglutaminase-mediated protein polymerization in whole APC and reHPT tissues lysates was detected through Western blotting analysis using anti-ϵ-(γ-glutamyl)-lysine antibody (1:500).
Discussion
Hematopoiesis or blood cell production in crayfish is tightly regulated and controlled. When, for example, an infection occurs, the total hemocyte count dramatically declines, and there is a need for new hemocyte production. Stem cells have self-renewal capacity and can differentiate into multiple cell lineages. In most animals, stem cells are situated in an environment surrounded with ECM, which plays a key role in providing structural support and binding different growth factors and cytokines (11). The balance between self-renewal and the commitment of stem cells is controlled through a combination of cell-intrinsic and external regulatory factors (26). Recent studies have indicated that the mitochondrial production of ROS is involved in this tightly controlled process and plays an important role in cellular signaling (27). In mammals, cellular ROS levels affect the differentiation of stem cells, and low levels of ROS are required to maintain quiescence and stem cell maintenance, whereas high ROS levels induce proliferation and differentiation programs (28). In addition, studies of the Drosophila lymph gland demonstrated that blood progenitor cells in the medullary zone exhibit relatively high levels of ROS, and these ROS establish a critical signal threshold for proper progenitor maintenance and differentiation (25). In crayfish, high ROS levels have been detected in the APC. Moreover, increased ROS levels in this area upon LPS injection are correlated with a rapid decrease of hemocytes, thereby increasing the subsequent synthesis and release of hemocytes (6). Therefore, we hypothesized that ROS play an important role in regulating hematopoiesis in crustaceans.
We first investigated the role of ROS in hematopoiesis by manipulating the ROS level using the antioxidant NAC. NAC treatment diminished the ROS level in hematopoietic tissue in both APC and reHPT in vivo and in vitro. Previous studies have shown that the ROS levels in the APC rapidly increased (30 min) after LPS or laminarin injection (6). Here we showed that ROS production can be inhibited 1 h after NAC injection in vivo and 30 min after NAC addition to cultured cells. In Drosophila, the posterior signaling center, a Drosophila hematopoietic niche, similarly responded with a high ROS pulse after wasp infection and subsequently induced the production and release of lamellocytes into the hemolymph (24). These results showed that NAC treatment had a more severe effect on the APC compared with reHPT. In APC cells, where most ROS are produced, this effect might reflect the fact that the cells in this area are highly proliferative and exhibit a high metabolic rate, resulting in mitochondrial ROS production (29). However, to balance the ROS level is important for cell survival as well as for regulating differentiation. In crayfish, it is likely that the APC might serve as a niche for hematopoietic cells to facilitate physiological communication between stem cells and posterior parts of the HPT to moderate the number of circulating hemocytes. We therefore analyzed the total number of hemocytes after reducing the ROS level through NAC injection. Decreased ROS levels suppressed hemocyte recovery in the circulatory system after LPS injection, again demonstrating the importance of ROS signals for hemocyte release.
The innate immune response serves as a first line of defense against infections. ROS are emerging as important regulators of some of these pathways. An infection might activate the production of ROS and initiate anti-infection responses (30). Moreover, ROS production is also detected in viral diseases. For example, HSV induces the early production of ROS, and ROS play a critical role in the activation of innate immune responses to this virus (31). In crayfish, the rapid increase of ROS levels in the APC after an infection might serve as a first signal to activate the hematopoietic process and release hemocytes to combat invaders. These results confirm this view, as the combined injection of NAC and LPS decreased new hemocyte synthesis compared with CPBS- or LPS-injected animals. This result confirms the importance of ROS signals from the APC to the reHPT to control cell proliferation and differentiation and promote the release of new hemocytes into the circulatory system.
We also examine the mechanism by which a ROS signal affects hemocyte release. In crustaceans, Ast1 serves as a hematopoietic growth factor and induces the proliferation of reHPT cells and the release of semigranular hemocytes. This process was mimicked in an in vitro culture system and can be visualized as cultured cells spread and migrate. In this study, we observed that cell spreading was clearly decreased in NAC-treated cultures when Ast1 was added. The causal link between increased ROS and a premature differentiation phenotype has been reported previously for the stem cell-like progenitors in Drosophila lymph glands (25) and in neurogenesis in mice (32), although the reason remains unknown. Because we showed previously that the cross-linking enzyme TGase plays an important role in maintaining stem cells in an undifferentiated state and that the knockdown of TGase increases the migration of hemocytes from the HPT, we examined whether ROS affect TGase mRNA expression and the activity of this enzyme in the reHPT and APC.
Interestingly, initial experiments showed a clear increase in extracellular TGase activity in NAC-treated tissues, indicating a link between TGase and ROS production, and it is well known that TGase has extracellular functions. Non-enzymatic and enzymatic functions have been reported for tissue transglutaminase (TG2) in vertebrates (33). Extracellular activities include not only the cross-linking of ECM proteins but also non-enzymatic modulations of the ECM, and cross-linking with cell surface proteins has been described previously (34). To reveal these roles in crayfish hematopoiesis, we first showed some colocalization of TGase with collagen IV, one of the ECM proteins in the HPT and a known substrate of TGase (35). However, colocalization is not evidence of the cross-linking activity of TGase. Therefore, we examined the amount of ϵ-(γ-glutamyl)-lysine cross-linking after TGase knockdown and in control reHPT tissues. The results showed some extracellular cross-links in the ECM, and, after TGase knockdown, these cross-links were not significantly affected. Based on these results, we hypothesize that one function of extracellular TGase in the crayfish HPT is to cross-link to ECM proteins, and the other non-enzymatic functions of TGase are likely also important.
These results are consistent with the hypothesis that the main target for ROS signaling is TGase. In mammals, extracellular TG2 promotes interactions between cells and the ECM and is important for cell spreading, migration, and differentiation (34). Moreover, the down-regulation of TG2 inhibited angiogenesis in cell culture (36). In addition, osteoblast differentiation is regulated by the presence of collagen type I in the ECM, and TGase is required for collagen secretion and extracellular deposition, which controls osteoblast differentiation (37). TG2 can also associate with several ECM proteins and mediate interactions between the ECM and soluble growth factors through the regulation of integrins and growth factor receptors (38, 39). TG2 was further reported as an early response gene up-regulated in response to inflammation in the presence of cytokines or stress signals, such as ROS in cancer cells (40). The results of this study provide new information and show that ROS are important for regulating extracellular TGase activity in the stem cell environment.
In conclusion, although high ROS levels damage cells, it is important to maintain a certain ROS level in tissues with stem cells. In this study, we show an important role for ROS signals in controlling the extracellular milieu of the hematopoietic tissue in crayfish. Low ROS levels might be required to maintain cells in a quiescent state through interactions with the ECM, whereas a short ROS signal decreases extracellular TGase activity in hematopoietic tissues and thereby provides a suitable environment for the differentiation of hematopoietic precursor cells.
Experimental Procedures
Animals
Freshwater crayfish, P. leniusculus, were acquired from Lake Erken (Sweden). The animals were maintained in an aquarium with aeration at 10 °C. Healthy and intermolt male crayfish were used for the experiments.
Cell Culture and NAC Treatment
The APC (Fig. 1A, green area) was separated from the remaining HPT (Fig. 1A, gray and blue areas). Subsequently, the cells were isolated from the APC or the reHPT and cultured as described previously (41). Briefly, after dissection, the APC and the reHPT were incubated for 20 min in 800 μl of 0.1% collagenase type I and type IV (Sigma) in CPBS (10 mm Na2HPO4, 10 mm KH2PO4, 150 mm NaCl, 10 mm CaCl2, and 10 mm MnCl2 (pH 6.8)) at room temperature. Subsequently the collagenase solution was removed after centrifugation at 800 × g for 5 min. The resulting cell pellet was washed twice with 1 ml of CPBS and suspended in L-15 medium (Sigma-Aldrich) supplemented with 1 mm phenylthiourea, 60 mg/ml penicillin, 50 mg/ml streptomycin, 50 mg/ml gentamicin (Sigma-Aldrich), and 2 mm l-glutamine. The cells were cultured in 96-well plates at a density of 2.5 × 104 cells/well at 16 °C. The remaining undigested APC tissue and the reHPT tissue after collagenase treatments were cultured in 24-well plates containing 300 μl of L-15s medium (L-15 medium supplemented as above). Crayfish plasma (crude Ast1) was prepared as described previously (41) and added to each well at a concentration of 2%. One-third of the medium was changed and supplemented with crude Ast1 every second day. After culture for 1 day, the culture medium was removed, and the cells or partially digested tissues were treated for 30 min with 10 μm NAC or 1 μm H2O2 (a positive control) in L-15s medium, and then the ROS level was examined. To reveal cell morphology, the medium containing NAC or H2O2 was removed, and the cells were maintained in new medium supplemented with crude Ast1. Next, the cell morphology was microscopically observed at 1 h, 1 day, and 2 days after NAC or H2O2 treatment. To quantify the cells with different morphologies, the number of cells with round shapes and the number of cells with spindle-like shapes in each treatment group were counted by two independent persons who were not involved in this study (blind test). The result was calculated and reported as the percentage of cells with different morphologies.
ROS Detection
To detect the ROS levels in the tissues, the reHPT and APC were dissected and washed twice with PBS (137 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, and 2 mm KH2PO4 (pH 7.4)). For cultured cells, the APC and the reHPT were cultured at 2.5 × 104 cells/well. Subsequently, the culture medium was removed, and the cells were washed twice with PBS at room temperature. A stock solution of 5 mg/ml 2′,7′-dichlorofluorescin diacetate (Sigma) was freshly diluted with PBS (1:1000) and added to the tissues or cells and incubated for 15 min in darkness. After washing three times with PBS, the ROS level was observed under a fluorescence microscope, and the fluorescence intensity was determined using a microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The fluorescence intensity was calculated as the fluorescence intensity of the sample minus the fluorescence intensity of PBS without tissue (serving as a baseline value). The results were reported as fluorescence intensity per microgram of tissue protein or relative fluorescence intensity to control cells.
Circulating Hemocyte Count after NAC and LPS Injection
The experiments were performed using three to six crayfish (35–40 g) for each experimental group. Prior to the injection of LPS or NAC, one drop of hemolymph was collected and immediately fixed in 10% formalin. The hemocyte number prior to injection was counted and served as the baseline value. After baseline bleeding, the animals were rested for 24 h. Subsequently, the animals were injected in the base of the fourth walking leg with NAC alone (75 μg/g crayfish), LPS alone (15 μg/crayfish), NAC together with LPS, or PBS. At different time points after injection, hemolymph was collected and fixed in 10% formalin. The total hemocyte number after injection was subsequently counted and reported as relative total hemocyte count (hemocyte number after injection divided by the hemocyte number prior to injection).
Immunocytochemistry of Collagen IV and TGase
To examine the co-localization of collagen IV and TGase, partially digested APC and reHPT tissues were cultured and maintained in 24-well plates for 24 h. Subsequently, the cultured tissues were fixed with 4% paraformaldehyde in PBS for 1 h, washed three times with PBS, and blocked with horse serum (Sigma) for 2 h. Next, the tissues were incubated for 1 h with mouse anti-collagen type IV antibody (Sigma, 1:100) and rabbit anti-giant freshwater prawn transglutaminase (MrTGII-N) (1:1000) kindly provided by Dr. Kallaya Sritunyalucksana (National Science and Technology Development Agency, Thailand). Subsequently, the primary antibodies were removed, and the tissues were washed five times with PBST (0.5% Tween 20 in PBS buffer) and incubated for 1 h with FITC-conjugated anti-mouse IgG (1:300) and Alexa Fluor® 594-conjugated anti-rabbit IgG (Life Technologies, 1:300). After washing five times with PBST, the tissues were incubated with Hoechst 33258 dye at a concentration of 1 μg/ml to stain the nuclei. The localization of collagen IV and TGase was observed under a fluorescence microscope.
Detection of TGase Activity
Partially digested APC and reHPT tissues were cultured and treated with NAC as described above. 1–2 h after treatment, 1 mm of 5-(biotinamido)-pentylamine (Pierce), a substrate for TGase, was added to the tissue culture and incubated for 18 h. Subsequently, the tissues were fixed according to Lin et al. (10). Briefly, the medium was removed, and the tissues were fixed with 4% paraformaldehyde in PBS for 1 h at room temperature, followed by 100% methanol for 1 h at −20 °C. Next, 25 mm glycine in PBS was added to the wells, and the cells were incubated for 30 min. The cells were subsequently washed five times with PBST and blocked with 10% BSA in PBST for 1 h. For detection, the tissues were incubated for 1 h with streptavidin-FITC conjugate (GE Healthcare) diluted with 1% BSA in PBST (1:200). After washing five times with PBST, propidium iodide was added at a concentration of 1 mg/ml to stain the nuclei. The TGase activity was observed under a fluorescence microscope.
Cross-linking through TGase and Detection through Western Blotting Analysis
APC and reHPT tissues were collected and homogenized in protein lysis buffer (PBS (pH 7.4), 0.1% Triton X-100, and 1× proteinase inhibitor mixture (Roche)). A total of 45 μg of the protein samples was dissolved in Laemmli sample buffer (62.5 mm Tris-HCl, 2% SDS, 10% (v/v) glycerol, 0.1 m DTT, and 0.01% bromphenol blue (pH 6.8)) and separated using 8% SDS-PAGE. Subsequently, the proteins were electrotransferred onto a PVDF membrane (GE Healthcare Life Sciences) for 2 h at room temperature. The blot was blocked in 10% skim milk in PBST (0.1% Tween 20 in PBS buffer) for 1 h. The membrane was incubated with anti-ϵ-(γ-glutamyl)-lysine (1:500) for 1 h at room temperature to detect cross-linked ϵ-(γ-glutamyl)-lysine. After extensive washing, the membrane was incubated with secondary antibody conjugated with horseradish peroxidase (Sigma), and the detection was performed using an ECL Western blotting reagent kit (Amersham Biosciences) according to the instructions of the manufacturer.
Statistical Analysis
The ROS levels and total hemocyte counts are shown as the mean ± S.D., and the statistical analysis was performed using one-way analysis of variance followed by Duncan's new multiple range test and Tukey's test. For comparisons between two groups, a t test was used, and statistical significance was considered at p < 0.05. The number of animals in each experiment was 3–6 crayfish/group.
Author Contributions
K. J. performed experiments, analyzed results, prepared figures, and wrote the paper. C. N. performed an experiment and analyzed the result shown in Fig. 2B. K. J., K. S., I. S., and C. N. contributed to the study design, interpreted the data, and participated in writing the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Dr. Xionghui Lin for optimizing co-immunostaining of collagenase and extracellular TGase.
This work was supported by Swedish Science Research Council, VR 2011-4797 (to I. S.) and VR 621-2012-2418 (to K. S.). The authors declare that they have no conflicts of interest with the contents of this article.
- HPT
- hematopoietic tissue
- APC
- anterior proliferation center
- reHPT
- remaining hematopoietic tissue
- TGase
- transglutaminase
- ECM
- extracellular matrix
- ROS
- reactive oxygen species
- NAC
- N-acetylcysteine
- CPBS
- crayfish PBS.
References
- 1. Söderhäll I. (2016) Crustacean hematopoiesis. Dev. Comp. Immunol. 58, 129–141 [DOI] [PubMed] [Google Scholar]
- 2. Grigorian M., and Hartenstein V. (2013) Hematopoiesis and hematopoietic organs in arthropods. Dev. Genes Evol. 223, 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gold K. S., and Brückner K. (2014) Drosophila as a model for the two myeloid blood cell systems in vertebrates. Exp. Hematol. 42, 717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin X., and Söderhäll I. (2011) Crustacean hematopoiesis and the astakine cytokines. Blood 117, 6417–6424 [DOI] [PubMed] [Google Scholar]
- 5. Söderhäll I., Kim Y.-A., Jiravanichpaisal P., Lee S.-Y., and Söderhäll K. (2005) An ancient role for a prokineticin domain in invertebrate hematopoiesis. J. Immunol. 174, 6153–6160 [DOI] [PubMed] [Google Scholar]
- 6. Noonin C., Lin X., Jiravanichpaisal P., Söderhäll K., and Söderhäll I. (2012) Invertebrate hematopoiesis: an anterior proliferation center as a link between the hematopoietic tissue and the brain. Stem Cells Dev. 21, 3173–3186 [DOI] [PubMed] [Google Scholar]
- 7. Krzemień J., Dubois L., Makki R., Meister M., Vincent A., and Crozatier M. (2007) Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature 446, 325–328 [DOI] [PubMed] [Google Scholar]
- 8. Mandal L., Martinez-Agosto J. A., Evans C. J., Hartenstein V., and Banerjee U. (2007) A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature 446, 320–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaga O., Lignell M., and Söderhäll K. (1995) The haemopoietic cells of the freshwater crayfish, Pacifastacus leniusculus. Anim. Biol. 4, 59–70 [Google Scholar]
- 10. Lin X., Söderhäll K., and Söderhäll I. (2008) Transglutaminase activity in the hematopoietic tissue of a crustacean, Pacifastacus leniusculus, importance in hemocyte homeostasis. BMC Immunol. 9, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grigorian M., Liu T., Banerjee U., and Hartenstein V. (2013) The proteoglycan Trol controls the architecture of the extracellular matrix and balances proliferation and differentiation of blood progenitors in the Drosophila lymph gland. Dev. Biol. 384, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lindner J. R., Hillman P. R., Barrett A. L., Jackson M. C., Perry T. L., Park Y., and Datta S. (2007) The Drosophila Perlecan gene trol regulates multiple signaling pathways in different developmental contexts. BMC Dev. Biol. 7, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andrae J., Gallini R., and Betsholtz C. (2008) Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 22, 1276–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Munier A.-I., Doucet D., Perrodou E., Zachary D., Meister M., Hoffmann J. A., Janeway C. A. Jr., and Lagueux M. (2002) PVF2, a PDGF/VEGF-like growth factor, induces hemocyte proliferation in Drosophila larvae. EMBO Rep. 3, 1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mondal B. C., Mukherjee T., Mandal L., Evans C. J., Sinenko S. A., Martinez-Agosto J. A., and Banerjee U. (2011) Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell 147, 1589–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang C. C., and Lodish H. F. (2008) Cytokines regulating hematopoietic stem cell function. Curr. Opin. Hematol. 15, 307–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin X., Novotny M., Söderhäll K., and Söderhäll I. (2010) Ancient cytokines, the role of astakines as hematopoietic growth factors. J. Biol. Chem. 285, 28577–28586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monnier J., and Samson M. (2010) Prokineticins in angiogenesis and cancer. Cancer Lett. 296, 144–149 [DOI] [PubMed] [Google Scholar]
- 19. Negri L., Lattanzi R., Giannini E., Canestrelli M., Nicotra A., and Melchiorri P. (2009) Bv8/Prokineticins and their receptors: a new pronociceptive system. Int. Rev. Neurobiol. 85, 145–157 [DOI] [PubMed] [Google Scholar]
- 20. Finkel T., and Holbrook N. J. (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 [DOI] [PubMed] [Google Scholar]
- 21. Simon M. C., and Keith B. (2008) The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 9, 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao H., Wu X., Simon L., and Fossett N. (2014) Antioxidants maintain E-cadherin levels to limit Drosophila prohemocyte differentiation. PLoS ONE 9, e107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Small C., Ramroop J., Otazo M., Huang L. H., Saleque S., and Govind S. (2014) An unexpected link between Notch signaling and ROS in restricting the differentiation of hematopoietic progenitors in Drosophila. Genetics 197, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sinenko S. A., Shim J., and Banerjee U. (2012) Oxidative stress in the haematopoietic niche regulates the cellular immune response in Drosophila. EMBO Rep. 13, 83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Owusu-Ansah E., and Banerjee U. (2009) Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461, 537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suda T., Arai F., and Hirao A. (2005) Hematopoietic stem cells and their niche. Trends Immunol. 26, 426–433 [DOI] [PubMed] [Google Scholar]
- 27. Hamanaka R. B., and Chandel N. S. (2010) Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 35, 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsatmali M., Walcott E. C., and Crossin K. L. (2005) Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res. 1040, 137–150 [DOI] [PubMed] [Google Scholar]
- 29. Nemoto S., Takeda K., Yu Z. X., Ferrans V. J., and Finkel T. (2000) Role for mitochondrial oxidants as regulators of cellular metabolism. Mol. Cell. Biol. 20, 7311–7318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghaffari S. (2008) Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid. Redox Signal. 10, 1923–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gonzalez-Dosal R., Horan K. A., Rahbek S. H., Ichijo H., Chen Z. J., Mieyal J. J., Hartmann R., and Paludan S. R. (2011) HSV infection induces production of ROS, which potentiate signaling from pattern recognition receptors: role for S-glutathionylation of TRAF3 and 6. PLoS Pathog. 7, e1002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le Belle J. E., Orozco N. M., Paucar A. A., Saxe J. P., Mottahedeh J., Pyle A. D., Wu H., and Kornblum H. I. (2011) Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependent manner. Cell Stem Cell 8, 59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nurminskaya M. V., and Belkin A. M. (2012) Cellular functions of tissue transglutaminase. Int. Rev. Cell Mol. Biol. 294, 1–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Belkin A. M. (2011) Extracellular TG2: emerging functions and regulation. FEBS J. 278, 4704–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dieterich W., Esslinger B., Trapp D., Hahn E., Huff T., Seilmeier W., Wieser H., and Schuppan D. (2006) Cross linking to tissue transglutaminase and collagen favours gliadin toxicity in coeliac disease. Gut 55, 478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Z., Perez M., Caja S., Melino G., Johnson T. S., Lindfors K., and Griffin M. (2013) A novel extracellular role for tissue transglutaminase in matrix-bound VEGF-mediated angiogenesis. Cell Death Dis. 4, e808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Piercy-Kotb S. A., Mousa A., Al-Jallad H. F., Myneni V. D., Chicatun F., Nazhat S. N., and Kaartinen M. T. (2012) Factor XIIIA transglutaminase expression and secretion by osteoblasts is regulated by extracellular matrix collagen and the MAP kinase signaling pathway. J. Cell. Physiol. 227, 2936–2946 [DOI] [PubMed] [Google Scholar]
- 38. Akimov S. S., and Belkin A. M. (2001) Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fibronectin. Blood 98, 1567–1576 [DOI] [PubMed] [Google Scholar]
- 39. Song S.-W., Chang W., Song B.-W., Song H., Lim S., Kim H.-J., Cha M.-J., Choi E., Im S.-H., Chang B.-C., Chung N., Jang Y., and Hwang K.-C. (2009) Integrin-linked kinase is required in hypoxic mesenchymal stem cells for strengthening cell adhesion to ischemic myocardium. Stem Cells. 27, 1358–1365 [DOI] [PubMed] [Google Scholar]
- 40. Chhabra A., Verma A., and Mehta K. (2009) Tissue transglutaminase promotes or suppresses tumors depending on cell context. Anticancer Res. 29, 1909–1919 [PubMed] [Google Scholar]
- 41. Liu H., and Söderhäll I. (2007) Histone H2A as a transfection agent in crayfish hematopoietic tissue cells. Dev. Comp. Immunol. 31, 340–346 [DOI] [PubMed] [Google Scholar]