Abstract
Urinary tract infections caused by uropathogenic Escherichia coli (UPEC) pathovars belong to the most frequent infections in humans. In men, pathogens can also spread to the genital tract via the continuous ductal system, eliciting bacterial prostatitis and/or epididymo-orchitis. Antibiotic treatment usually clears pathogens in acute epididymitis; however, the fertility of patients can be permanently impaired. Because a premature acrosome reaction was observed in an UPEC epididymitis mouse model, and sialidases on the sperm surface are considered to be activated via proteases of the acrosome, we aimed to investigate whether alterations of the sialome of epididymal spermatozoa and surrounding epithelial cells occur during UPEC infection. In UPEC-elicited acute epididymitis in mice, a substantial loss of N-acetylneuraminic acid residues was detected in epididymal spermatozoa and epithelial cells using combined laser microdissection/HPLC-ESI-MS analysis. In support, a substantial reduction of sialic acid residues bound to the surface of spermatozoa was documented in men with a recent history of E. coli-associated epididymitis. In vitro, such an UPEC induced N-acetylneuraminic acid release from human spermatozoa was effectively counteracted by a sialidase inhibitor. These findings strongly suggest a substantial remodeling of the glycocalyx of spermatozoa and epididymal epithelial cells by endogenous sialidases after a premature acrosome reaction during acute epididymitis.
Keywords: bacterial pathogenesis, glycosylation, glycosylation inhibitor, reproduction, sialic acid, sialidase, sperm, spermatozoa
Introduction
Acute epididymitis or a combined epididymo-orchitis in men represents a relevant entity in urological practice. Epididymitis is usually the result of an infection starting in the urethra that ultimately ascends to the epididymis and testis (1). Uropathogenic Escherichia coli (UPEC)4 belongs to the most common microbes associated with the condition, particularly in men over the age of 35 (2). Although antibiotic treatment is usually successful in clearing the pathogens, about 40% of patients with UPEC epididymitis are subsequently diagnosed with impaired semen parameters causing sub- or infertility (3). Biopsies from patients with a past history of epididymitis showed drastic structural alterations such as epithelial cell damage, fibrosis, and absence of spermatozoa further distally (4). Rodent UPEC epididymitis models could replicate the damage seen in men and pointed to a role of the UPEC virulence factor α-hemolysin in an extensive premature acrosome reaction occurring in the epididymal duct, implying the untimely release of acrosomal enzymes in infected epididymis prior to reaching the female reproductive tract (5).
The acrosome contains various digestive enzymes, among them proteases as well as various glycosidases, such as hyaluronidases and sialidases, that are essential for penetration of the zona pellucida and glycan remodeling of the gametes as a prerequisite for spermatozoa-oocyte binding (6–11). Recent data revealed that, during capacitation, a biochemical process in the female reproductive tract in which spermatozoa accomplish full fertilizing “capacity,” two sialidases (the neuraminidases NEU1 and NEU3) are shed from the surface of spermatozoa, likely by the activity of proteases, which could be efficiently counteracted by protease inhibitors (10). This process led to the release of sialic acid residues from the surface of spermatozoa (10). The acrosome appears to contain a further reservoir of sialidases, which are released physiologically during the acrosome reaction prior to fertilization (8, 12). Because NEU1 and NEU3 together are able to desialylate several different glycoconjugates (α2,3-, α2,6-, and α2,8-sialylated), a significant restructuring of the glycocalyx can be expected (13–15).
Sialic acids are acidic nine-carbon backbone α-keto sugars and represent one of the most important molecules of life (16, 17). N-acetylneuraminic acid (Neu5Ac) as well as N-glycolylneuraminic acid (Neu5Gc) are the most abundant species in mammals. These sugar residues are commonly linked to the outermost position of the largest share of glycoproteins, which places them in an ideal position for their indispensable role in a diverse range of cellular processes such as intercellular adhesion and communication. However, sialic acids also play an important role during infection of many viruses, fungi, as well as bacteria (18–20). For instance, during invasion, pathogenic bacteria use sialic acid residues on the surface of epithelial cells for the initiation of infection by mediating bacterial adherence. On the other hand, detachment of sialic acid residues from host cells represents a further powerful means of bacteria to manipulate the immune response, e.g. by influencing the recognition of specific sugar moieties by sialic acid-binding immunoglobulin-type lectins (siglecs) on immune cells (21, 22). For example, several pathogenic bacteria secrete sialidases as a virulence factor, leading to sepsis as a consequence of an uncontrolled stimulation of the immune system. In this regard, bacterial sialidase inhibitors can efficiently counteract the dramatic progression of the disease in a mouse model, demonstrating their clinical potential during infection (23, 24). In consideration that UPEC infection of the epididymis results in a premature release of acrosomal content (5), we aimed to investigate whether this affects the sialome of spermatozoa and surrounding epididymal epithelial cells using a mouse epididymitis model and ejaculates of men with a recent history of the disease.
Results
Desialylation of Spermatozoa and Epithelial Cells Is a Consequence of UPEC Infection in the Epididymitis Mouse Model
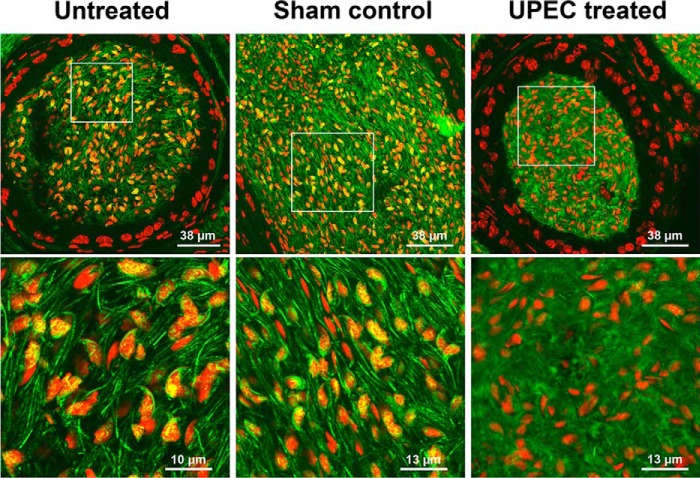
To elucidate whether the UPEC-induced acrosome reaction leads to an activation of endogenous sialidases, initiating a subsequent desialylation of spermatozoa, an established epididymitis mouse model was utilized (25). Histopathology in Masson-Goldner-stained epididymis demonstrated fibrotic remodeling and interstitial leukocytic infiltration as the most obvious consequences 3 days post-UPEC infection, as shown previously (Fig. 1A) (4, 26). Acrosomal staining with FITC-labeled peanut agglutinin lectin indicated a premature acrosome reaction in the majority of spermatozoa in the lumen of the epididymis following UPEC infection (Fig. 1B), similar to previous findings (5). In contrast, the acrosome was visible in most spermatozoa in untreated and sham controls.
FIGURE 1.
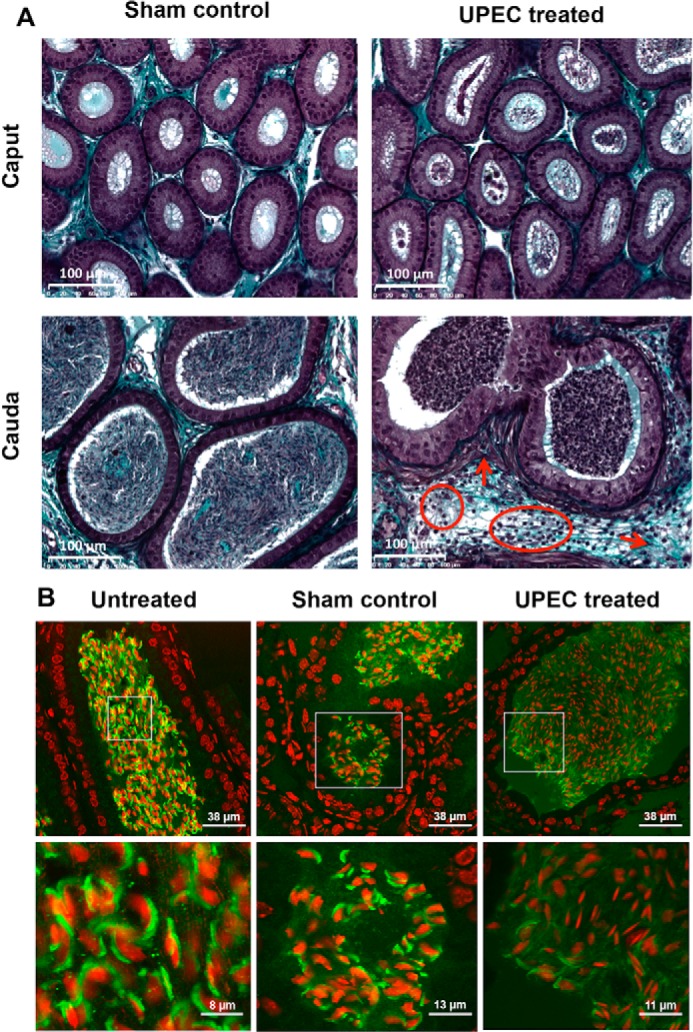
UPEC infection causes pathological alterations in the cauda epididymis and a premature acrosome reaction in epididymal spermatozoa. A, Masson-Goldner-stained sections of cauda epididymal tissues from mice 3 days post-UPEC infection and PBS sham controls were assessed by light microscopy. Arrows indicate fibrotic remodeling (thickening of the smooth muscle cell layer, collagen accumulation). Circles demonstrate interstitial infiltrating leukocytes. B, sperm acrosomes were fluorescently stained with peanut agglutinin-FITC lectin (green), and nuclei were counterstained using TO-PRO-3 (red) in the cauda epididymides from mice 3 days post-UPEC treatment, PBS sham controls, and untreated controls. The insets in the second row were magnified digitally.
To gain a first insight into the sialylation status, sialic acid residues were visualized on epididymal sections using FITC-conjugated SNA lectin (Fig. 2). SNA prevalently binds α2,6-linked sialic acid residues; however, α2,3-linked sialic acid residues will also be bound by this lectin but to a lesser degree. Fluorescence microscopy revealed diffusely distributed and weaker SNA labeling in spermatozoa of UPEC-infected mice, whereas in controls, prominent staining of sialic acid residues was evident in the acrosome and sperm tail (Fig. 2). The reduced SNA staining in UPEC-treated mice suggested that Neu5Ac residues were cleaved during infection.
FIGURE 2.
In UPEC epididymitis, sialic acid labeling is decreased in spermatozoa. α2,6-linked sialic acid residues were visualized by SNA-FITC lectin (green), and nuclei were counterstained with TO-PRO-3 (red) in sections from mouse epididymis 3 days post-UPEC and a PBS sham control as well as an untreated control. In contrast to untreated and sham controls, in UPEC infection, a more diffuse sialic acid labeling is evident. The images in the second row were magnified digitally.
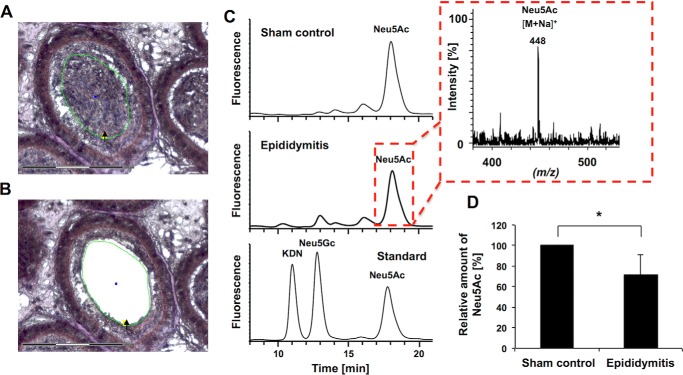
Because qualitative as well as quantitative lectin analyses of sialic acids are often misleading, and SNA lectin visualize only a proportion of all sialic acid residues on glycoconjugates (27), a recently developed combination of LCMD and DMB-HPLC-ESI-MS analyses was applied (Fig. 3) (28). Starting from murine paraffin-embedded tissue samples, epididymal spermatozoa were isolated via LCMD after nuclear staining using Mayer's hematoxylin (Fig. 3, A and B). Thereafter, sialic acids were released under acidic conditions, fluorescently labeled, and subjected to a HPLC-ESI-MS system. The obtained chromatograms showed smaller peaks of DMB-labeled Neu5Ac in infected material (Fig. 3C). The respective DMB-Neu5Ac mass at m/z 448 [M + Na]+ in the ESI-MS spectrum verified the presence of DMB-Neu5Ac during the corresponding retention time of an DMB-Neu5Ac standard (Fig. 3C). Calculation of corresponding peak areas demonstrated a significant reduction of Neu5Ac (∼19%) in spermatozoa in UPEC epididymitis (Fig. 3D).
FIGURE 3.
Epididymal spermatozoa are desialylated following UPEC infection. A, spermatozoa from the epididymal lumen were isolated by laser microdissection from hematoxylin-stained, paraffin-embedded tissue sections. The excised area is indicated by the black arrow and green circle. Scale bar = 150 μm. B, the same section after the excision of luminal content. C, the obtained spermatozoa were subsequently used for sialic acid quantification. After hydrolysis and fluorescent labeling, DMB-sialic acid residues were separated using reverse-phase HPLC. A set of sialic acid standards was used for the generation of a calibration line and to control the retention times. ESI-MS spectra were recorded during the HPLC runs to verify the presence of different sialic acid species, as shown exemplary for Neu5Ac in UPEC-treated and PBS sham control epididymides. D, the amounts of Neu5Ac and the respective standard deviations were calculated from four epididymides obtained from PBS sham control and UPEC-injected mice, respectively (n = 6 animals). Given mean values were set to 100% for the sham control. The statistical evaluation was performed using Student's t test (unequal variances, two-tailed). *, p ≤ 0.05.
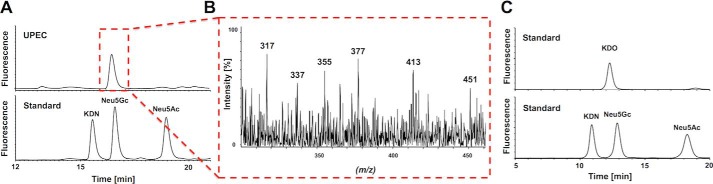
Although, in UPEC-treated samples, a peak for DMB-Neu5Gc was apparent in the HPLC chromatogram, in the sham control, hardly any signal was detectable. However, no mass corresponding to DMB-Neu5Gc was obtained by ESI-MS in untreated as well as infected mice (data not shown). Because of the fact that DMB reacts with all α-keto acids, possible contamination in infected mice by α-keto acids originating from the pathogen was examined by separate analysis of bacteria only. In UPEC, the presence of DMB-labeled KDO, a well known bacterial α-keto acid (16), was measurable as a prominent peak at the retention time of DMB-Neu5Gc (Fig. 4A). In addition, KDO-related monoisotopic pseudomolecular masses of DMB-KDO at m/z 337 ([M + H]+ − H2O), 355 ([M + H]+), and 377 ([M + Na]+) were monitored in the ESI-MS spectrum at this particular time (Fig. 4B). To verify this finding, KDO was applied to the DMB-HPLC analysis, demonstrating that Neu5Gc and KDO show overlapping peak areas (Fig. 4C). Thus, in UPEC-treated mice, bacterial KDO was obviously recorded at the retention time of DMB-Neu5Gc.
FIGURE 4.
Analysis of α-keto acids in UPEC. A, sialic acid standards and UPEC samples were hydrolyzed, and α-keto acids were fluorescently labeled with DMB for HPLC separation. B, the ESI-MS spectrum recorded during the elution time of the peak is highlighted by the red dotted frame. C, comparison of the retention time of KDO and Neu5Gc standards.
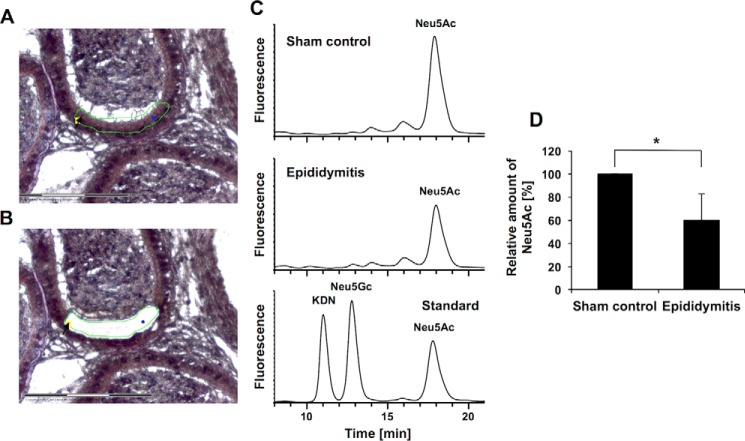
Release of sialic acids from the spermatozoa to the luminal fluid following UPEC infection could be enzymatically mediated by soluble sialidases, a hypothesis supported by an observation of Gagneux and co-workers (10), who described that the sialidases NEU1 and NEU3 are released from the cell surface of spermatozoa after proteolytic activation. Consequently, soluble neuraminidases would be also able to reach the cell surface of surrounding cells. To test this hypothesis, the sialylation status of epididymal epithelial cells (whose apical surface borders on the luminal fluid) was analyzed using the abovementioned combined LCMD/DMB-HPLC-ESI-MS strategy (Fig. 5). In support, epididymal epithelial cells showed a reduction of sialic acid residues (∼49%) 3 days post-infection with UPEC (Fig. 5, C and D). Reduction of Neu5Ac from epithelial cells is substantially stronger in comparison with spermatozoa (Fig. 3). Thus, the obtained results demonstrate that the sialylation status of epididymal spermatozoa in the lumen as well as that of the lumen lining epithelial cells decreases in the epididymis after bacterial infection.
FIGURE 5.
Reduction of sialic acid content in epithelial cells in UPEC epididymitis. A, epithelial cells were isolated from paraffin-embedded mouse epididymis by laser microdissection. The excised area is indicated by the black arrow and green line. B, the same section after excision of epithelium. Scale bar = 150 μm. C, DMB-labeled sialic acid residues of the isolated cells were separated using RP-HPLC. A set of sialic acid standards was used for quantification and to control the retention times. The ESI-MS spectrum was recorded during the HPLC runs (data not shown). D, the amounts of Neu5Ac and the respective standard deviations of six different controls and infected mice (one epididymis from six animals each). Given mean values were set to 100% for the sham control. The statistical evaluation was performed using Student's t test (unequal variances, two-tailed). *, p ≤ 0.05.
In Vitro Desialylation of Human Spermatozoa Can Be Counteracted with Sialidase Inhibitors
To investigate whether UPEC can also induce hyposialylation in human spermatozoa, motile spermatozoa of healthy donors collected after swim-up were subsequently infected with UPEC (Fig. 6). DMB-HPLC analysis pointed to a reduction of Neu5Ac to approximately half the amount measured in an untreated control (Fig. 6A). Correspondingly, PSA-FITC lectin staining detected a substantially higher number of acrosomally reacted spermatozoa compared with controls (Fig. 6B).
FIGURE 6.
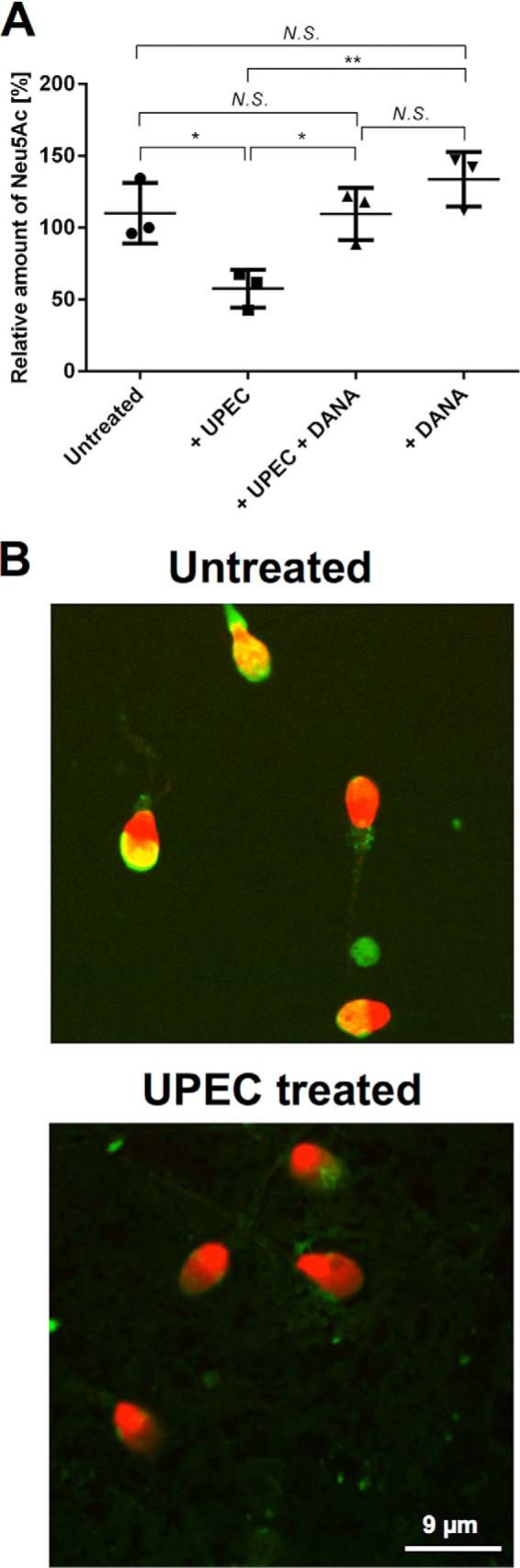
UPEC-dependent desialylation of human spermatozoa can be counteracted by sialidase inhibitor in vitro. A, spermatozoa from healthy donors prepared by swim-up were incubated with UPEC for 3 h, and the amount of Neu5Ac was compared with untreated samples using the DMB-HPLC approach as shown in Fig. 3. In parallel, UPEC-treated as well as untreated spermatozoa were co-incubated with the sialidase inhibitor DANA. One control was set as 100%, and the other controls were set in relation to it. Treatments were calculated relative to the mean of controls. Significance levels were calculated using one-way analysis of variance. *, p ≤ 0.05; **, p ≤ 0.01; N.S., not significant (n = 3). B, acrosomes were labeled by PSA-FITC lectin (green), and nuclei were counterstained with TO-PRO-3 (red). The presence of acrosomes was compared with the untreated control.
To examine whether the investigated hyposialylation of spermatozoa is mostly the consequence of a general loss of glycoconjugates, e.g. because of the degradation of the glycoprotein backbone or the activation of neuraminidases during the acrosome reaction, motile human spermatozoa were treated with UPEC in the presence of the sialidase inhibitor DANA (Fig. 6A). Here, co-incubation of DANA with UPEC completely prevented hyposialylation of human spermatozoa (Fig. 6A). Bacterial sialidase activity could be excluded by in silico analysis of the UPEC genome. Consequently, the decreased sialylation status in infected spermatozoa is likely based on the enzymatic activity of released sialidases.
Hyposialylation Is Detected in Spermatozoa of Men with a Recent History of Epididymitis
In addition, semen samples of patients suffering from E. coli epididymitis were included in our study. For this purpose, spermatozoa were obtained by swim-up from ejaculates of men 14 days after diagnosis and treatment of E. coli-based unilateral epididymitis. The analyzed samples showed malformation (sperm head, acrosome visualized by PSA-FITC staining, Fig. 7A) and much lower levels of Neu5Ac (∼75% reduction) compared with healthy control spermatozoa (Fig. 7B). Thus, in agreement with the determined loss of sialic acid residues in the epididymitis mouse model as well as in the in vitro experiments, spermatozoa of epididymitis patients are hyposialylated.
FIGURE 7.
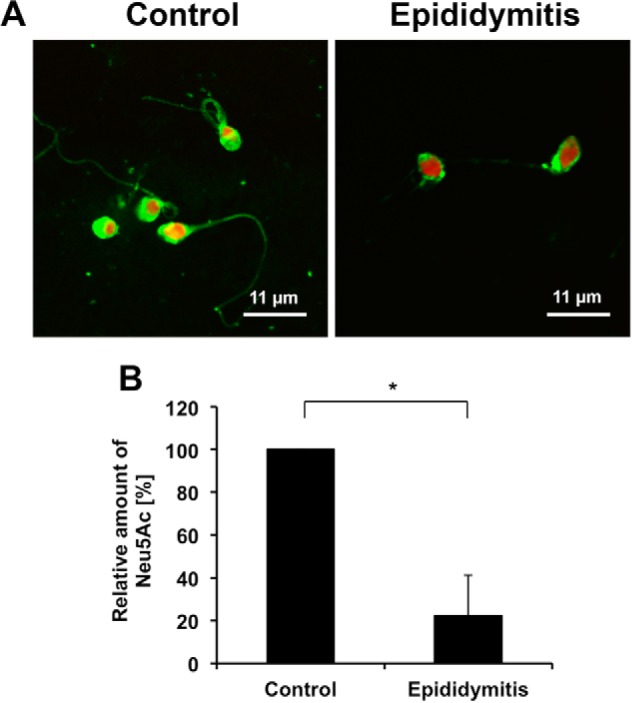
Reduced levels of Neu5Ac in patients with a history of acute epididymitis. A, acrosomes of swim-up spermatozoa obtained from healthy men (control) and patients 14 days after diagnosis and treatment of acute epididymitis were visualized by PSA-FITC lectin (green). Nuclei were counterstained with TO-PRO-3 (red). B, sialic acid residues of isolated spermatozoa from these patient groups (n = 3, respectively) were released and fluorescently labeled with DMB for quantification. The statistical evaluation was performed using Student's t test (unequal variances, two-tailed). Given mean values were set to 100% for all sham controls. *, p ≤ 0.05.
Discussion
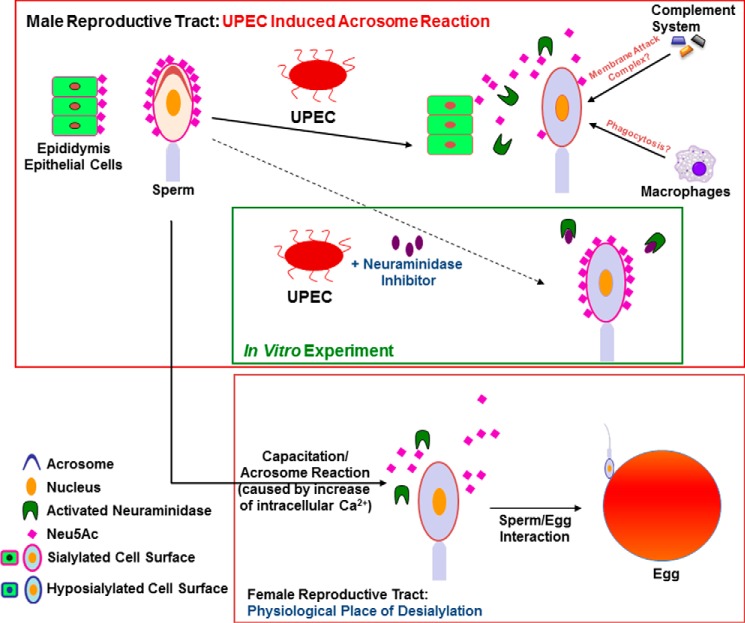
Glycans play an essential role in fertilization, particularly in mediating the species-specific interaction between spermatozoa and the zona pellucida of the egg (9, 11). Binding of spermatozoa to the zona pellucida is thereby strongly induced by terminal Neu5Ac residues of sialyl-LewisX oligosaccharides on the surface of the zona pellucida. In addition, successful interaction of both gametes requires previous desialylation of the spermatozoal surface (10) (Fig. 8). For this purpose, spermatozoa are equipped with sialidases (8, 10, 12, 29). Under normal conditions, neuraminidases are only activated in the female reproductive tract as a consequence of capacitation and/or acrosome reaction (8, 10, 12, 29). Premature release of neuraminidases prior ejaculation is usually avoided, as untimely desialylation could result in elimination of spermatozoa by phagocytic cells (30). Under pathophysiological conditions such as bacterial epididymitis, considerable premature exocytosis of the acrosome has been observed in vivo and in vitro, which contributes substantially to the loss of fertilizing capacity, as shown by subsequent in vitro fertilization experiments in mice (5). This prompted us to examine whether an undue acrosome reaction could affect the sialome of epididymal spermatozoa and surrounding epithelial cells by applying an established acute mouse bacterial infectious epididymitis model and ejaculates of men with a recent history of the disease.
FIGURE 8.
Summary and working model. Blue box, in the female reproductive tract, a capacitation/acrosome reaction leads to proteolytic activation and release of neuraminidases, resulting in desialylation of spermatozoa to allow sperm/egg interaction. Red box, the UPEC-induced acrosome reaction triggers premature desialylation of spermatozoa and the release of sialic acid residues from adjacent cells in the male reproductive tract. Consequently, pathological hyposialylation may negatively influence the protection of host cells against phagocytosis and the complement system. Green box, in vitro UPEC-induced hyposialylation of spermatozoa could be counteracted using neuraminidase inhibitors. Putatively, a further level of intervention could be upstream by blocking the proteolytic activation and release of neuraminidases using suitable protease inhibitors according to Ma et al. (10).
Both in murine epididymitis as well as in in vitro-treated human spermatozoa, UPEC stimulates a premature acrosome reaction, leading to a significant desialylation of spermatozoa as well as adjacent cells (illustrated in Fig. 8). Surprisingly, the investigated desialylation was much more pronounced in epididymitis patients. The desialylation could be efficiently blocked with a neuraminidase inhibitor in vitro, indicating that the loss of sialic acid residues is particularly a consequence of endogenous neuraminidases (Fig. 8).
A limitation in the analysis of human samples in this study is the relatively small number of semen samples from patients/controls suitable for glycoanalysis because of the need to clean ejaculates by the swim-up method. Because semen samples of patients with acute epididymitis are usually characterized by a largely reduced semen quality and leukocytospermia, the swim-up technique was employed to select the viable and motile sperm, thereby removing contaminating non-sperm cells (e.g. leukocytes) to avoid bias between patient and control samples.
Because sialic acids have multifactorial roles in the host response to infection, the massive release of sialic acid residues from the cell surface of epithelial cells and spermatozoa may contribute to the observed tissue damage in epididymitis (Fig. 8). This view is supported by the fact that bacterial lipopolysaccharide triggers complement activation and that hyposialylation promotes the perforation of host cell membranes by the membrane attack complex by counteracting a protective mechanism conveyed by complement factor H (31). Complement factor H exhibits sialic acid-specific binding domains that are necessary for efficient cell surface binding (32). Factor H on cell membranes is essential to remove activated complement factor 3b from cellular surfaces to prevent perforation of host cell membranes and limit tissue damage. As shown recently, complement factor H is an important component of seminal plasma, as it protects spermatozoa against such a complement attack in the female reproductive tract (33). Thus, hyposialylation of spermatozoa and other host cells may result in a deficit of sialic acid-dependent factor H binding and, thus, less protection against the activated complement system.
Moreover, many sialylated and polysialylated glycoproteins are integrated into the plasma membrane of spermatozoa during epididymal transit (34, 35). These glycoproteins inhibit, inter alia, in a sialic acid-dependent way the phagocytosis by leukocytes as seen in murine spermatozoa (30, 36). In this regard, the substantial loss of sialic acid residues during epididymitis could prompt phagocytosis of spermatozoa during transit through the epididymis and/or the female reproductive tract, as already indicated (30, 37).
The presence of leukocytes, mainly macrophages, is a frequent observation in the lumen of the epididymal duct of healthy men and rodents. Together with neutrophils and T cells, their numbers increase strongly in infection (25, 38, 39). It appears therefore likely that soluble neuraminidases may also detach sialic acid residues from the cell surface of immune cells during infection. However, their terminal sialic acids play an important role in counteracting excessive immune response via an interaction with siglecs in cis (40). As an example, the anti-inflammatory signaling of siglec-10 is activated by the interaction with sialic acid residues on CD24 in cis (24). This complex represses tissue damage-induced immune responses (41) similar to those seen in infected epididymis (4). Some bacteria, like Clostridium perfringens, secrete microbial sialidases targeting the described sialic acid mediated CD24·siglec-10 complex. As a consequence, mice have an increased risk of septicemia, as shown previously (22, 24). Interestingly, sialidase inhibitors suppress the inflammatory response and septic symptoms. Thus, in epididymitis patients, activated endogenous sialidases may act as an indirect virulence factor similar to microbial sialidases of C. perfringens, Vibrio cholerae, and Streptococcus pneumoniae (42).
Furthermore, the altered glycocalyx of epididymal epithelial cells could influence adhesion of UPEC. Because of the desialylation and the resulting loss of negatively charged sialic acid residues, type 1 fimbriae like FimH may better reach potential binding partners such as Mannoseα1–3Man or Manα1-3Manβ1–4GlcNAc (43). In contrast, sialic acid-dependent UPEC binding to epithelial cells was described (44). Accordingly, the adhesion of UPEC to epididymal epithelial cells would be inhibited by epithelial desialylation.
Taken together, UPEC infection causes hyposialylation in mouse epididymal spermatozoa and epithelial cells as well as in ejaculated spermatozoa of men with a history of epididymitis. Likely this happens mechanistically through activation of endogenous sialidases. Hyposialylation has the potential to negatively affect the course and magnitude of infection in patients suffering from UPEC-elicited epididymitis, providing a possible explanation for the characteristic long-term impairment of fertility associated with this pathovar (5, 45). However, the precise consequences of the examined hyposialylation and the potential of sialidase inhibitors as therapeutic agents during UPEC infection need to be further investigated. A better understanding is also needed in the comparative glycome analysis of human and mouse epididymal epithelial cells and spermatozoa to better judge how well the animal model reflects the situation in men.
Experimental Procedures
Materials
Neu5Ac, Neu5Gc, 2-keto-3-deoxynononic acid (KDN), and 3-deoxy-d-manno-octulosonic acid (KDO) were purchased from Sigma-Aldrich (Taufkirchen, Germany). All lectins were purchased from Vector Laboratories (Burlingame, CA). All reagents and chemicals used were of analytical grade.
Mouse and Human Ethics Statement
Adult inbred C57BL/6J mice (10–12 weeks old) were purchased from Charles River Laboratories (Sulzfeld, Germany). All studies were performed according to a protocol approved by the Animal Ethics Committee of the Regierungspräsidium Giessen (permit no. GI 18/17-No. 124/2012).
All patients and volunteers provided written informed consent for semen analysis. All samples were anonymized. As controls, three healthy men of reproductive age (median, 40 years; range, 19–54 years; ethics review board no. 32/11) were recruited during examination in the andrological/urological outpatient clinic. In addition, ejaculates of three patients (median, 40 years; range, 19–54 years) suffering from acute unilateral E. coli-related epididymitis were analyzed within the first 2 weeks after diagnosis. Patients were part of the Giessen Epididymitis Study (institutional review board no. 100/7, German Clinical Trials Register no. DRKS00003325) and treated according to current guideline recommendations (2). Of 22 patients providing semen, only the samples of three patients (aged 19, 54, and 47) had a suitable sperm concentration (>5 million/ml) to undergo further analysis. All patients underwent a comprehensive microbiological analysis for classical uropathogens by culture- and species-specific PCR analysis to detect Neisseria gonorrhoeae, Mycoplasma genitalium, Ureaplasma urealyticum, and Chlamydia trachomatis. Only patients suffering from E. coli epididymitis were part of the study. Empiric therapy was initiated with levofloxacin (500 mg once a day orally for 10 days). Sexually active men were asked to provide a semen sample 14 days after first presentation. Semen samples were collected by masturbation into a sterile container. Semen analysis was performed according to World Health Organization 2010 recommendations (46). To select motile sperm, the swim-up procedure was applied (47). Briefly, 1 ml of fresh semen was placed in a sterile centrifuge tube, and 1 ml of human tubal fluid (HTF) medium layered on top. Following 1 h of incubation at 37 °C, 1 ml of upper medium containing highly motile sperm was removed, diluted with 2 ml of HTF medium, and centrifuged at 500 × g for 5 min.
Bacterial Strains and Propagation
UPEC strain CFT073 was kindly provided by Prof. Chakraborty (Institute of Medical Microbiology, Justus Liebig University, Giessen, Germany). UPEC was propagated in Luria-Bertani medium as described previously (48). The concentration of bacteria was calculated using standard growth curves (48, 49). For in vivo and in vitro experiments, E. coli was diluted in PBS and RPMI 1640 medium (Invitrogen), respectively.
Experimental Mouse Epididymitis Model
5–10 μl of PBS suspension containing between 4–8 × 104 bacteria was injected bilaterally close to the epididymis into the vas deferens, which was previously ligated to prevent retrograde ascent of the bacteria (25). For sham controls, PBS alone was injected into the vas deferens of control animals. Three days post-infection, mice were sacrificed, and the epididymides were removed. Bacterial ascent from the injection site to the epididymis has been demonstrated previously (5). The epididymis from one side was snap-frozen in liquid nitrogen after cryo-embedding in optimum cutting temperature medium for lectin staining, whereas the contralateral organ was immediately fixed in Bouin's solution and embedded in paraffin. Subsequently, sections were used for histological staining and laser capture microdissection.
Masson-Goldner Staining
Deparaffinized and rehydrated sections of epididymides were stained by incubating sections consecutively in Weigert's hematoxylin (nuclei, 5 min), xylidine Ponceau (cytoplasm, 5 min), Orange G (erythrocytes, 20 s), and Light Green (connective tissue, 5 min). Dehydrated stained sections were subsequently embedded in Entellan® mounting medium (Merck, Darmstadt, Germany) and assessed with a Leica DM750 microscope.
Lectin Fluorescence Staining
After swim-up, freshly isolated human spermatozoa (∼50,000) were smeared and air-dried on SuperFrost glass slides (R. Langenbrinck). Serial cryosections (8 μm) were prepared from frozen mouse epididymides. Human spermatozoa and mouse epididymidis sections were fixed with 2% paraformaldehyde for 30 min, washed, and blocked with 0.1% BSA (Roth, Karlsruhe, Germany) for 1 h. FITC-conjugated peanut agglutinin (binds particularly galactosyl (β1,3) N-acetylgalactosamine glycan structure), Pisum sativum (PSA) (detects α-linked mannose-containing oligosaccharides with an N-acetylchitobiose-linked α-fucose), and Sambucus nigra (SNA) (binds prevalently sialic acid residues attached to terminal galactose in α2,6 and, to a lesser degree, α2,3 linkage) were used at a final concentration of 10 μg/ml in PBS containing 0.1% BSA and incubated for 30 min. All FITC-labeled lectins were purchased from Vector Laboratories (Burlingame, CA). Nuclei were visualized with TO-PRO®-3 (Life Technologies, dilution 1:1000, 1 min). Images were acquired using a confocal laser-scanning microscope (Leica Microsystems, Wetzlar, Germany). Excitation filters were set to 488 nm and 633 nm to detect FITC (lectin-bound glycostructures) and TO-PRO (nuclei), respectively.
Laser Capture Microdissection
Paraffin-embedded murine epididymides were sectioned (5 μm) and mounted on membrane slides for laser capture microdissection (LCMD) (Zeiss, Munich, Germany). As described previously, sections were stained with Mayer's hematoxylin, dehydrated, and air-dried (28). Tissue areas were marked using a ×20 objective and dissected in Robo-LPC-mode. The number of isolated cells was calculated by counting stained nuclei. Dissected tissue was collected in adhesive caps (Zeiss, Munich, Germany). The caps were cut and transferred into glass vials for sialic acid analysis (28).
Release and DMB Labeling of Sialic Acids
Washed spermatozoa collected after swim-up as well as collected tissue samples were subjected to sonication (10 min) and hydrolyzed in 500 μl of 2 N acetic acid for 90 min at 80 °C, transferred into glass vials, and dried. Fluorescent labeling of sialic acids was performed using 80 μl of 4,5-methylene dioxybenzene (DMB) reaction buffer (1 m β-mercaptoethanol, 9 mm sodium hydrosulfite, 20 mm TFA, and 2.7 mm DMB (Dojindo, Kumamoto, Japan)) for 2 h at 55 °C as already described (50–53). Labeling was terminated by adding 20 μl of 0.2 m NaOH. To calculate the amount of Neu5Ac, a four-point calibration line was generated.
HPLC and Online HPLC-ESI-MS
DMB-labeled sialic acids were separated for quantification by a reverse-phase column (Superspher 100 C-18e-RP, 250 × 4 mm, Merck-Hitachi, Darmstadt, Germany) at 40 °C using an Ultimate LC system that was directly coupled with an Esquire 3000 ESI-ion trap-MS (Bruker Daltonik) (28, 54). Fluorescently labeled sialic acids were separated by applying mobile phases (A and B) containing acetonitrile (Chem Solute, Th. Geyer, Renningen, Germany)/methanol (Merck)/water/TFA (Promochem, Wesel, Germany (4:4:92:0.1)) (A) and acetonitrile/methanol/water/TFA (45:45:10:0.1) (B). The LC method consisted of a linear gradient from 0% to 15% (B) over 30 min. The flow rate was 250 μl/min over 60 min. Fluorescence detection settings were λexcitation = 372 nm and λemission = 456 nm. Typical ESI source conditions were as follows: spray voltage, 1.4 kV; capillary temperature, 250 °C; end plate offset, −500 V; capillary exit, 140 V (28).
Infection of Human Spermatozoa with UPEC in Vitro
Isolated spermatozoa (∼7.5 × 106 were mixed in 1 ml HTF medium with approximately 5 × 105 bacteria (multiplicity of infection ∼0.07) for 3 h at 37 °C and 5% CO2. Subsequently, the pellet (700 × g, 10 min) was collected by centrifugation after two washing steps using PBS for DMB-HPLC analysis.
Inhibition of Spermatozoal Sialidase
As a control, UPEC-induced desialylation of isolated human spermatozoa was inhibited by adding 1 mm N-acetyl-2,3-dehydro-2-deoxyneuraminic acid (DANA, Sigma) to HTF medium for 3 h in 37 °C and 5% CO2. After treatment, spermatozoa were collected by centrifugation (700 × g, 10 min) after two washing steps using PBS.
Statistical Analysis
HPLC data were analyzed by utilizing two-tailed, paired Student's t test. One-way analysis of variance was used for sialidase inhibitor experiments (Fig. 6A). All indicated values are mean ± S.E. Sample size was estimated using a post hoc G*Power analysis.
Author Contributions
S. P. G. and A. M. designed the study and analyzed the data. F. K., S. P. G., and A. M. wrote the paper. V. M. performed LCMD as well as Masson-Goldner staining, and S. B. applied the mouse model. F. K. performed all lectin staining. F. K., P. C., and C. E. G. performed the DMB-HPLC analysis. C. E. G. and F. K. interpreted the MS spectra. H. C. S. and A. P. recruited and examined patients and provided andrological laboratory data. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We acknowledge Werner Mink and Siegfried Kühnhardt (Biochemistry, Justus Liebig University Giessen) for expert technical assistance and Negah Ahmadvand, Zhengguo Zhang, Ferial Aslani, and Elke Stoschek (Institute of Anatomy and Cell Biology, Justus Liebig University Giessen) for help.
This work was supported by Deutsche Forschungsgemeinschaft Grants GA 1755/1-2 (to S. P. G.) GRK 1871/1 (to A. M.), the von Behring-Roentgen-Stiftung, and the Faculty of Medicine of the Justus Liebig University of Giessen. The authors declare that they have no conflicts of interest with the contents of this article.
- UPEC
- uropathogenic Escherichia coli
- Neu5Ac
- N-acetylneuraminic acid
- Neu5Gc
- N-glycolylneuraminic acid
- siglec
- sialic acid-binding immunoglobulin-type lectin
- SNA
- Sambucus nigra
- LCMD
- laser capture microdissection
- KDO
- 3-deoxy-d-manno-oct-2-ulosonic acid
- DANA
- N-acetyl-2,3-dehydro-2-deoxyneuraminic acid
- KDN
- 2-keto-3-deoxynononic acid
- HTF
- human tubal fluid
- PSA
- Pisum sativum
- DMB
- 4,5-methylene dioxybenzene.
References
- 1. Berger R. E., Kessler D., and Holmes K. K. (1987) Etiology and manifestations of epididymitis in young men: correlations with sexual orientation. J. Infect. Dis. 155, 1341–1343 [DOI] [PubMed] [Google Scholar]
- 2. Pilatz A., Hossain H., Kaiser R., Mankertz A., Schüttler C. G., Domann E., Schuppe H. C., Chakraborty T., Weidner W., and Wagenlehner F. (2015) Acute epididymitis revisited: impact of molecular diagnostics on etiology and contemporary guideline recommendations. Eur. Urol. 68, 428–435 [DOI] [PubMed] [Google Scholar]
- 3. Rusz A., Pilatz A., Wagenlehner F., Linn T., Diemer T., Schuppe H. C., Lohmeyer J., Hossain H., and Weidner W. (2012) Influence of urogenital infections and inflammation on semen quality and male fertility. World J. Urol. 30, 23–30 [DOI] [PubMed] [Google Scholar]
- 4. Stammler A., Hau T., Bhushan S., Meinhardt A., Jonigk D., Lippmann T., Pilatz A., Schneider-Hüther I., and Middendorff R. (2015) Epididymitis: ascending infection restricted by segmental boundaries. Hum. Reprod. 30, 1557–1565 [DOI] [PubMed] [Google Scholar]
- 5. Lang T., Dechant M., Sanchez V., Wistuba J., Boiani M., Pilatz A., Stammler A., Middendorff R., Schuler G., Bhushan S., Tchatalbachev S., Wübbeling F., Burger M., Chakraborty T., Mallidis C., and Meinhardt A. (2013) Structural and functional integrity of spermatozoa is compromised as a consequence of acute uropathogenic E. coli-associated epididymitis. Biol. Reprod. 89, 59. [DOI] [PubMed] [Google Scholar]
- 6. Tambe A. S., Kaore S. B., Sawane M. V., and Gosavi G. B. (2001) Acrosome intactness and seminal hyaluronidase activity: relationship with conventional seminal parameters. Indian J. Med. Sci. 55, 125–132 [PubMed] [Google Scholar]
- 7. Talbot P., and Franklin L. E. (1974) The release of hyaluronidase from guinea-pig spermatozoa during the course of the normal acrosome reaction in vitro. J. Reprod. Fertil. 39, 429–432 [DOI] [PubMed] [Google Scholar]
- 8. Srivastava P. N., Zaneveld L. J., and Williams W. L. (1970) Mammalian sperm acrosomal neuraminidases. Biochem. Biophys. Res. Commun. 39, 575–582 [DOI] [PubMed] [Google Scholar]
- 9. Pang P. C., Chiu P. C., Lee C. L., Chang L. Y., Panico M., Morris H. R., Haslam S. M., Khoo K. H., Clark G. F., Yeung W. S., and Dell A. (2011) Human sperm binding is mediated by the sialyl-Lewisx oligosaccharide on the zona pellucida. Science 333, 1761–1764 [DOI] [PubMed] [Google Scholar]
- 10. Ma F., Wu D., Deng L., Secrest P., Zhao J., Varki N., Lindheim S., and Gagneux P. (2012) Sialidases on mammalian sperm mediate deciduous sialylation during capacitation. J. Biol. Chem. 287, 38073–38079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tecle E., and Gagneux P. (2015) Sugar-coated sperm: Unraveling the functions of the mammalian sperm glycocalyx. Mol. Reprod. Dev. 82, 635–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Srivastava P. N., and Abou-Issa H. (1977) Purification and properties of rabbit spermatozoal acrosomal neuraminidase. Biochem. J. 161, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sajo M., Sugiyama H., Yamamoto H., Tanii T., Matsuki N., Ikegaya Y., and Koyama R. (2016) Neuraminidase-dependent degradation of polysialic acid is required for the lamination of newly generated neurons. PLoS ONE 11, e0146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takahashi K., Mitoma J., Hosono M., Shiozaki K., Sato C., Yamaguchi K., Kitajima K., Higashi H., Nitta K., Shima H., and Miyagi T. (2012) Sialidase NEU4 hydrolyzes polysialic acids of neural cell adhesion molecules and negatively regulates neurite formation by hippocampal neurons. J. Biol. Chem. 287, 14816–14826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cairo C. W. (2014) Inhibitors of the human neuraminidase enzymes. MedChemComm. 5, 1067–1075 [Google Scholar]
- 16. Angata T., and Varki A. (2002) Chemical diversity in the sialic acids and related α-keto acids: an evolutionary perspective. Chem. Rev. 102, 439–469 [DOI] [PubMed] [Google Scholar]
- 17. Varki A., and Schauer R. (2009) in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Freeze H. H., Freeze P., Bertozzi C. R., Hart G. W., Etzler M. E., eds) Chapter 14, pp. 199–218, Cold Spring Harbor Laboratory Press, New York: [PubMed] [Google Scholar]
- 18. Schauer R. (2004) Sialic acids: fascinating sugars in higher animals and man. Zoology 107, 49–64 [DOI] [PubMed] [Google Scholar]
- 19. Schauer R. (2009) Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 19, 507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varki A., and Gagneux P. (2012) Multifarious roles of sialic acids in immunity. Ann. N.Y. Acad. Sci. 1253, 16–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewis A. L., and Lewis W. G. (2012) Host sialoglycans and bacterial sialidases: a mucosal perspective. Cell Microbiol. 14, 1174–1182 [DOI] [PubMed] [Google Scholar]
- 22. Macauley M. S., Crocker P. R., and Paulson J. C. (2014) Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14, 653–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paulson J. C., and Kawasaki N. (2011) Sialidase inhibitors DAMPen sepsis. Nat. Biotechnol. 29, 406–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen G. Y., Chen X., King S., Cavassani K. A., Cheng J., Zheng X., Cao H., Yu H., Qu J., Fang D., Wu W., Bai X. F., Liu J. Q., Woodiga S. A., Chen C., et al. (2011) Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat. Biotechnol. 29, 428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang T., Hudemann C., Tchatalbachev S., Stammler A., Michel V., Aslani F., Bhushan S., Chakraborty T., Renz H., and Meinhardt A. (2014) Uropathogenic Escherichia coli modulates innate immunity to suppress Th1-mediated inflammatory responses during infectious epididymitis. Infect. Immun. 82, 1104–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michel V., Duan Y., Stoschek E., Bhushan S., Middendorff R., Young J. M., Loveland K. A., Kretser D. M., Hedger M. P., and Meinhardt A. (2016) Uropathogenic Escherichia coli cause fibrotic remodelling of the epididymis. J. Pathol. [DOI] [PubMed] [Google Scholar]
- 27. Galuska S. P. (2013) in Sialobiology: Structure, Biosynthesis, and Function (Tiralongo J., and Martinez-Duncker I. eds.) pp. 448–475, Betham eBooks [Google Scholar]
- 28. Bartel J., Feuerstacke C., Galuska C. E., Weinhold B., Gerardy-Schahn R., Geyer R., Münster-Kühnel A., Middendorff R., and Galuska S. P. (2014) Laser microdissection of paraffin embedded tissue as a tool to estimate the sialylation status of selected cell populations. Anal. Chem. 86, 2326–2331 [DOI] [PubMed] [Google Scholar]
- 29. Srivastava P. N., Kumar V. M., and Arbtan K. D. (1988) Neuraminidase induces capacitation and acrosome reaction in mammalian spermatozoa. J. Exp. Zool. 245, 106–110 [DOI] [PubMed] [Google Scholar]
- 30. Toshimori K., Araki S., Oura C., and Eddy E. M. (1991) Loss of sperm surface sialic acid induces phagocytosis: an assay with a monoclonal antibody T21, which recognizes a 54K sialoglycoprotein. Arch. Androl. 27, 79–86 [DOI] [PubMed] [Google Scholar]
- 31. Pangburn M. K., and Müller-Eberhard H. J. (1978) Complement C3 convertase: cell surface restriction of β1H control and generation of restriction on neuraminidase-treated cells. Proc. Natl. Acad. Sci. U.S.A. 75, 2416–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blaum B. S., Hannan J. P., Herbert A. P., Kavanagh D., Uhrín D., and Stehle T. (2015) Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat. Chem. Biol. 11, 77–82 [DOI] [PubMed] [Google Scholar]
- 33. Sakaue T., Takeuchi K., Maeda T., Yamamoto Y., Nishi K., and Ohkubo I. (2010) Factor H in porcine seminal plasma protects sperm against complement attack in genital tracts. J. Biol. Chem. 285, 2184–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simon P., Bäumner S., Busch O., Röhrich R., Kaese M., Richterich P., Wehrend A., Müller K., Gerardy-Schahn R., Mühlenhoff M., Geyer H., Geyer R., Middendorff R., and Galuska S. P. (2013) Polysialic acid is present in mammalian semen as a post-translational modification of the neural cell adhesion molecule NCAM and the polysialyltransferase ST8SiaII. J. Biol. Chem. 288, 18825–18833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sullivan R., Frenette G., and Girouard J. (2007) Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 9, 483–491 [DOI] [PubMed] [Google Scholar]
- 36. Shahraz A., Kopatz J., Mathy R., Kappler J., Winter D., Kapoor S., Schütza V., Scheper T., Gieselmann V., and Neumann H. (2015) Anti-inflammatory activity of low molecular weight polysialic acid on human macrophages. Sci. Rep. 5, 16800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma X., Pan Q., Feng Y., Choudhury B. P., Ma Q., Gagneux P., and Ma F. (2016) Sialylation facilitates the maturation of mammalian sperm and affects its survival in female uterus. Biol. Reprod. 94, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kang T. W., Lee K. H., Piao C. Z., Yun K. J., Joo H. J., Park K. S., Choi Y. D., Juhng S. W., and Choi C. (2007) Three cases of xanthogranulomatous epididymitis caused by E. coli. J. Infect. 54, e69–73 [DOI] [PubMed] [Google Scholar]
- 39. Jantos C., Baumgärtner W., Durchfeld B., and Schiefer H. G. (1992) Experimental epididymitis due to Chlamydia trachomatis in rats. Infect. Immun. 60, 2324–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crocker P. R., Paulson J. C., and Varki A. (2007) Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 [DOI] [PubMed] [Google Scholar]
- 41. Chen G. Y., Tang J., Zheng P., and Liu Y. (2009) CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 323, 1722–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim S., Oh D. B., Kang H. A., and Kwon O. (2011) Features and applications of bacterial sialidases. Appl. Microbiol. Biotechnol. 91, 1–15 [DOI] [PubMed] [Google Scholar]
- 43. Bouckaert J., Mackenzie J., de Paz J. L., Chipwaza B., Choudhury D., Zavialov A., Mannerstedt K., Anderson J., Piérard D., Wyns L., Seeberger P. H., Oscarson S., De Greve H., and Knight S. D. (2006) The affinity of the FimH fimbrial adhesin is receptor-driven and quasi-independent of Escherichia coli pathotypes. Mol. Microbiol. 61, 1556–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sakarya S., Ertem G. T., Oncu S., Kocak I., Erol N., and Oncu S. (2003) Escherichia coli bind to urinary bladder epithelium through nonspecific sialic acid mediated adherence. FEMS Immunol. Med. Microbiol. 39, 45–50 [DOI] [PubMed] [Google Scholar]
- 45. Michel V., Pilatz A., Hedger M. P., and Meinhardt A. (2015) Epididymitis: revelations at the convergence of clinical and basic sciences. Asian J. Androl. 17, 756–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. WHO. (2010) WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed., WHO Press, Geneva, Switzerland [Google Scholar]
- 47. Younglai E. V., Holt D., Brown P., Jurisicova A., and Casper R. F. (2001) Sperm swim-up techniques and DNA fragmentation. Hum. Reprod. 16, 1950–1953 [DOI] [PubMed] [Google Scholar]
- 48. Bhushan S., Hossain H., Lu Y., Geisler A., Tchatalbachev S., Mikulski Z., Schuler G., Klug J., Pilatz A., Wagenlehner F., Chakraborty T., and Meinhardt A. (2011) Uropathogenic E. coli induce different immune response in testicular and peritoneal macrophages: implications for testicular immune privilege. PLoS ONE 6, e28452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bhushan S., Tchatalbachev S., Klug J., Fijak M., Pineau C., Chakraborty T., and Meinhardt A. (2008) Uropathogenic Escherichia coli block MyD88-dependent and activate MyD88-independent signaling pathways in rat testicular cells. J. Immunol. 180, 5537–5547 [DOI] [PubMed] [Google Scholar]
- 50. Hara S., Takemori Y., Yamaguchi M., Nakamura M., and Ohkura Y. (1987) Fluorometric high-performance liquid chromatography of N-acetyl- and N-glycolylneuraminic acids and its application to their microdetermination in human and animal sera, glycoproteins, and glycolipids. Anal. Biochem. 164, 138–145 [DOI] [PubMed] [Google Scholar]
- 51. Hara S., Yamaguchi M., Takemori Y., Furuhata K., Ogura H., and Nakamura M. (1989) Determination of mono-O-acetylated N-acetylneuraminic acids in human and rat sera by fluorometric high-performance liquid chromatography. Anal. Biochem. 179, 162–166 [DOI] [PubMed] [Google Scholar]
- 52. Bayer N. B., Schubert U., Sentürk Z., Rudloff S., Frank S., Hausmann H., Geyer H., Geyer R., Preissner K. T., and Galuska S. P. (2013) Artificial and natural sialic acid precursors influence the angiogenic capacity of human umbilical vein endothelial cells. Molecules 18, 2571–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Galuska S. P., Geyer H., Weinhold B., Kontou M., Röhrich R. C., Bernard U., Gerardy-Schahn R., Reutter W., Münster-Kühnel A., and Geyer R. (2010) Quantification of nucleotide-activated sialic acids by a combination of reduction and fluorescent labeling. Anal. Chem. 82, 4591–4598 [DOI] [PubMed] [Google Scholar]
- 54. Galuska S. P., Geyer H., Mink W., Kaese P., Kühnhardt S., Schäfer B., Mühlenhoff M., Freiberger F., Gerardy-Schahn R., and Geyer R. (2012) Glycomic strategy for efficient linkage analysis of di-, oligo- and polysialic acids. J. Proteomics 75, 5266–5278 [DOI] [PubMed] [Google Scholar]