Abstract
Aminoacyl-tRNA synthetases (aaRSs) are housekeeping enzymes essential for protein synthesis. Apart from their parent aminoacylation activity, several aaRSs perform non-canonical functions in diverse biological processes. The present study explores the twin attributes of Leishmania tyrosyl-tRNA synthetase (LdTyrRS) namely, aminoacylation, and as a mimic of host CXC chemokine. Leishmania donovani is a protozoan parasite. Its genome encodes a single copy of tyrosyl-tRNA synthetase. We first tested the canonical aminoacylation role of LdTyrRS. The recombinant protein was expressed, and its kinetic parameters were determined by aminoacylation assay. To study the physiological role of LdTyrRS in Leishmania, gene deletion mutations were attempted via targeted gene replacement. The heterozygous mutants showed slower growth kinetics and exhibited attenuated virulence. LdTyrRS appears to be an essential gene as the chromosomal null mutants did not survive. Our data also highlights the non-canonical function of L. donovani tyrosyl-tRNA synthetase. We show that LdTyrRS protein is present in the cytoplasm and exits from the parasite cytoplasm into the extracellular medium. The released LdTyrRS functions as a neutrophil chemoattractant. We further show that LdTyrRS specifically binds to host macrophages with its ELR (Glu-Leu-Arg) peptide motif. The ELR-CXCR2 receptor interaction mediates this binding. This interaction triggers enhanced secretion of the proinflammatory cytokines TNF-α and IL-6 by host macrophages. Our data indicates a possible immunomodulating role of LdTyrRS in Leishmania infection. This study provides a platform to explore LdTyrRS as a potential target for drug development
Keywords: aminoacyl tRNA synthetase, chemokine, cytokine, drug action, Leishmania, ELR peptide motif, fisetin, moonlighting
Introduction
Aminoacyl-tRNA synthetases (aaRSs)3 are the central. enzymes in protein translation, providing charged tRNAs for the appropriate construction of peptide chains. The canonical function of aaRSs is to charge specific tRNAs with their cognate amino acids and thereby contribute to accurate mRNA translation during protein synthesis. Thus, aaRSs are essential components of protein synthesis in every living species.
Apart from their basic function of charging tRNA molecules for protein synthesis, non-canonical functions like ribosomal RNA biogenesis, angiogenesis, apoptosis, transcriptional regulation, and cell signaling have also been reported for several aaRSs (1, 2). Novel functions of this group of enzymes depend on the addition of one or more new domains or motifs during the course of evolution (3).
Tyrosyl-tRNA synthetase (TyrRS) is one such aaRS, which belong to a family of class I synthetases, characterized by a structurally well conserved amino-terminal Rossmann-fold domain, which contains the signature sequences “HIGH” and “KMSKS.” The mammalian TyrRS contains a closely homologous endothelial monocyte activating polypeptide II (EMAPII) domain at the C terminus (4). Under specific conditions, human TyrRS is processed by an elastase enzyme into a free carboxyl-terminal EMAPII-like domain and a second amino-terminal part known as mini-TyrRS. Both released proteins are active in distinct immune signaling pathways (4, 5). The carboxyl-terminal domain of human TyrRS mimics the cytokine function of EMAPII. On the other hand, human mini-TyrRS, via its ELR (Glu-Leu-Arg) motif, interacts with the CXC-chemokine receptor (CXCR1/2), and like IL-8, functions as a chemoattractant for polymorphonuclear leukocytes (4, 5). The ELR motif is a signature motif of CXC chemokines such as IL-8 that are active as polymorphonuclear leukocyte chemoattractants (6). This motif is essential for receptor binding and the chemotactic activity of CXC chemokines (7–9). The ELR motif in mini-TyrRS is also important for its chemokine-like activity (4, 5). This data on human TyrRS suggests that the twin attributes of chemokine trigger and aminoacylation coexist in TyrRS.
Not much is known about aaRSs in Leishmania sp. Leishmania genus is the causative agent of leishmaniasis, a group of neglected diseases. The clinical symptoms of the disease depend on the species involved. Leishmania has a digenetic life cycle. Infected sand flies inoculate the mammalian host with promastigotes. Within the mammalian host, promastigotes differentiate into amastigotes that replicate in phagolysosomes. Leishmania parasites have the capability of subverting host function, thereby allowing the parasite to thrive within the organism (10, 11). The development of resistance to currently available anti-leishmanial drugs has led to an urgent need to discover novel drug targets (12). In this regard, aaRSs constitute ideal targets for drug development.
The crystal structure of Leishmania major TyrRS has been recently solved, and it is known to exist as an asymmetric pseudodimer (13). In the present study, we report the catalytic promiscuity and moonlighting function of L. donovani TyrRS (LdTyrRS). The twin attributes of L. donovani tyrosyl-tRNA synthetase, namely, aminoacylation and chemokine trigger have been determined. Our earlier comprehensive bioinformatic analysis led to the identification of a total of 26 aaRSs in Leishmania (14). The Leishmania genome encodes a single copy of TyrRS (tritrypDB ID LdBPK_141460.1). The present study characterizes the aminoacylation activity of LdTyrRS. To elucidate the physiological role of LdTyrRS, gene deletion mutations were attempted via targeted gene replacement. Heterozygous knock-out mutants of LdTyrRS showed reduced growth and were attenuated in their infectivity, indicating the essentiality of this protein. Several attempts to generate homozygous null mutants of LdTyrRS were unsuccessful due to the presence of a single copy of the TyrRS gene. Fisetin, a natural flavonoid compound, was found to inhibit parasite growth by inhibiting the aminoacylation activity of LdTyrRS. Apart from its role in translation, we also report the non-canonical function of LdTyrRS. The most notable and intriguing feature of LdTyrRS is the presence of an “ELR” motif, which is the signature motif conserved among IL-8 chemokines (15) and indicates a possible immunomodulating role of this protein. We explored the significance of this ELR motif in the parasite housekeeping enzyme LdTyrRS. Our comprehensive study involving the characterization, localization, and immunological attributes of LdTyrRS provides a platform to explore LdTyrRS as a potential target for drug development.
Results
Characterization of Leishmania Tyrosyl-tRNA Synthetase (LdTyrRS)
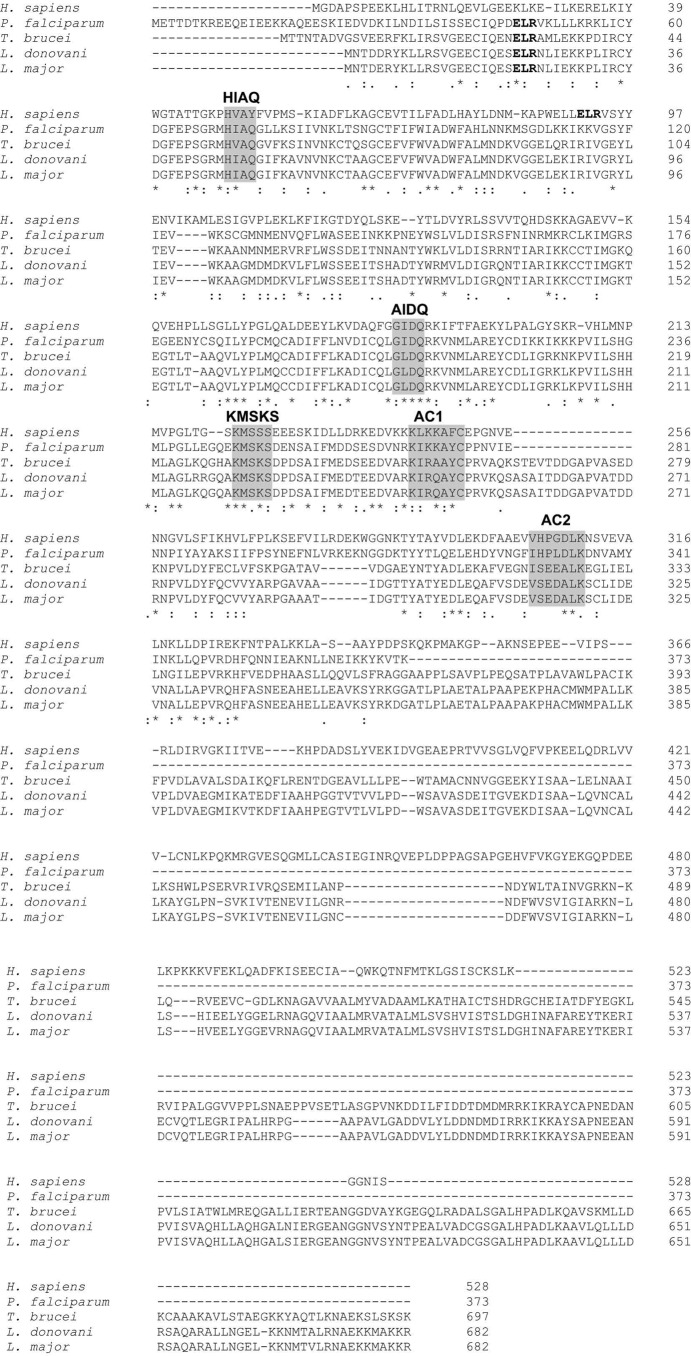
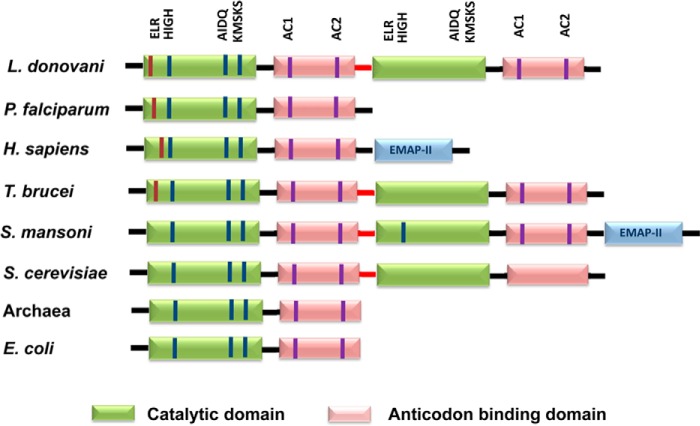
Multiple sequence alignment of LdTyrRS (Uniprot ID A4HW83; tritrypDB ID LdBPK_141460.1) with representative sequences from other eukaryotes such as human (Uniprot ID P54577), Plasmodium (UniProt ID Q8IAR7), Trypanosoma brucei (Uniprot ID Q57WH7; tritrypDB ID Tb927.7.3620), and L. major (Uniprot ID Q4QFJ7; tritrypDB ID LMJF_14_1370) was generated using CLUSTALW. This multiple sequence alignment showed that LdTyrRS belongs to a family of class I synthetases, characterized by a structurally well conserved amino-terminal Rossmann-fold domain that contains the signature sequences HIGH and KMSKS. Our earlier bioinformatics analysis has revealed the presence of an “ELR (Glu-Leu-Arg) motif,” which is the signature motif conserved among CXC chemokines (14). The ELR motif in LdTyrRS was found to be present at the 22nd amino acid position and indicated a possible immunomodulating role of this enzyme (Fig. 1). The alignment also suggested complete conservation of the ELR motif in parasitic tyrosyl-tRNA synthetases (Plasmodium, T. brucei, and L. major). Interestingly, the ELR motif was found to be evolutionarily absent in TyrRSs from lower eukaryotes and prokaryotes (Fig. 2).
FIGURE 1.
Multiple sequence alignment of representative TyrRS sequences from kinetoplastids, human, and Plasmodium species generated using CLUSTALW. The key residues present in the aminoacylation and catalytic domains are highlighted in a gray background. The ELR motif is highlighted in bold font.
FIGURE 2.
Domain organization of TyrRS from eukaryotes and prokaryotes. The catalytic and anticodon binding domains are indicated. The ELR motif is present at the N terminus and is shown in red. The HIGH and KMSKS active site motifs are common to all class I catalytic domains. The AIDQ motif is characteristic of the ATP binding site in TyrRS. The AC1 motif corresponds to the anticodon-binding domain that interacts with the anticodon stem of tRNATyr. The AC2 motif specifically recognizes the anticodon bases G34 and U/ψ35.
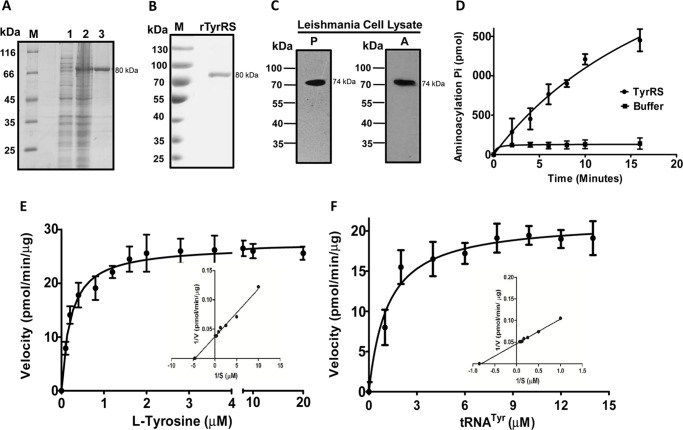
To characterize the recombinant LdTyrRS protein, the full-length gene was cloned into a bacterial expression vector pET30a. A histidine-tagged fusion protein with an estimated molecular mass of ∼80 kDa was induced. This size correlated with the amino acid composition of the LdTyrRS protein (∼74 kDa) with a His6 tag (∼6 kDa) at the N terminus (Fig. 3A). Recombinant LdTyrRS was purified to homogeneity by metal affinity chromatography (Fig. 3A). Purification yielded ∼2 mg of purified protein/liter of bacterial culture. The recombinant protein (rLdTyrRS) was recognized by an anti-His tag monoclonal antibody (Fig. 3B). To further characterize rLdTyrRS, the purified protein was analyzed by MALDI-TOF/TOF mass spectroscopy (data not shown). The spectrum of the protein analyzed by BioTools version 2.2 showed an intensity coverage of 46.6% for tyrosyl-tRNA synthetase (Leishmania infantum). The expression of the full-length LdTyrRS enzyme was confirmed in Leishmania cell lysates by immunoblot analysis (Fig. 3C). The anti-LdTyrRS antibody detected a ∼74 kDa band in the cell extracts of both the promastigotes and the amastigotes (Fig. 3C).
FIGURE 3.
Purification and enzymatic characterization of recombinant LdTyrRS. A, purification of recombinant LdTyrRS protein on nickel-nitrilotriacetic acid affinity resin. M, molecular weight marker; Lane 1, uninduced cell lysate; lane 2, induced cell lysate; lane 3, rLdTyrRS. B, Western blotting analysis of the recombinant LdTyrRS protein using anti-His tag mouse antibody (1:3000). rTyrRS, purified rLdTyrRS. C, immunoblotting analysis of the Leishmania promastigote (P) and amastigote (A) cell lysate (∼40 μg) with the anti-LdTyrRS antibody. D, time course of tRNATyr aminoacylation by recombinant LdTyrRS. Reactions were performed with l-tyrosine and tRNATyr as the substrates. The data shows an average of three experiments performed in duplicate ± S.D. E and F, aminoacylation kinetics of LdTyrRS as a function of l-tyrosine concentration (E) or tRNATyr concentration (F). The results represent mean ± S.D. with n = 3.
Enzymatic Activity and Kinetic Parameters for LdTyrRS
To assess the aminoacylation activity of rLdTyrRS, a coupled enzyme assay was performed. The aminoacylation reaction was carried out with rLdTyrRS in the presence of inorganic pyrophosphatase, and the Pi produced was measured with a malachite green solution. Recombinant LdTyrRS acylated tRNATyr in a time-dependent manner, demonstrating that the L. donovani TyrRS gene encodes a functional enzyme (Fig. 3D). The kinetic parameters and specificity of rLdTyrRS were determined with l-tyrosine and tRNATyr as substrates in vitro (Fig. 3, E and F). Enzyme kinetics was performed with varying concentrations of l-tyrosine (from 0.1 to 20 μm), whereas other components were kept constant (Fig. 3E). The results showed that the enzyme reaction was dependent on l-tyrosine concentration (Fig. 3E). The Km value of rLdTyrRS for l-tyrosine was 0.21 ± 0.0245 μm, which is closer to that reported in the case of human TyrRS (0.3 μm) (16). Because tRNATyr is another essential substrate of the aminoacylation reaction, therefore, we also performed tRNATyr-dependent enzyme kinetics studies (Fig. 3F). The estimated Km of LdTyrRS, for tRNATyr (1.177 ± 0.2271 μm), was closer to that of humans (0.9 μm) (16) but higher than that of Saccharomyces cerevisiae (0.2 μm) (17).
Subcellular Localization of LdTyrRS
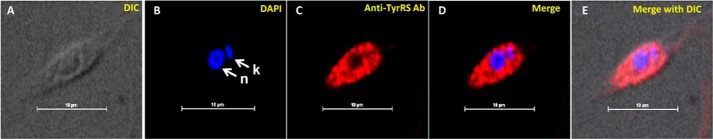
The amino acid sequence analysis of LdTyrRS by web-based programs like signalP and pSORT II predicted that LdTyrRS does not contain any detectable signal peptide or cleavage site. Moreover, predictions with MARSPred and LocTree3 also indicated a preferentially cytosolic localization. To ascertain the localization of TyrRS in L. donovani, immunofluorescence analysis of log phase promastigotes stained with anti-LdTyrRS antibody and DAPI was conducted. The kinetoplast and nuclear DNA in these cells were readily identified by their bright staining with DAPI (Fig. 4B). LdTyrRS was found to be localized only in the cytoplasm of the parasite (Fig. 4, C and D). Earlier data from mass spectrometry has demonstrated a predominantly cytoplasmic localization of TyrRS in T. brucei (18). Controls performed with mouse preimmune sera, non-permeabilized cells, and secondary antibody alone showed no detectable signal (data not shown).
FIGURE 4.
Subcellular localization of LdTyrRS in L. donovani. Immunofluorescence analysis by confocal micrograph of wild-type log phase promastigotes. Panel A, phase-contrast image. Panel B, promastigotes stained with DAPI. Panel C, anti-LdTyrRS antibody detected using Alexa 542 (red)-conjugated secondary antibody. Panels D and E, merged micrographs. k and n indicate kinetoplastid and nuclear DNA, respectively. The scale bar represents 10 μm.
Gene Deletion Studies of Tyrosyl-tRNA Synthetase
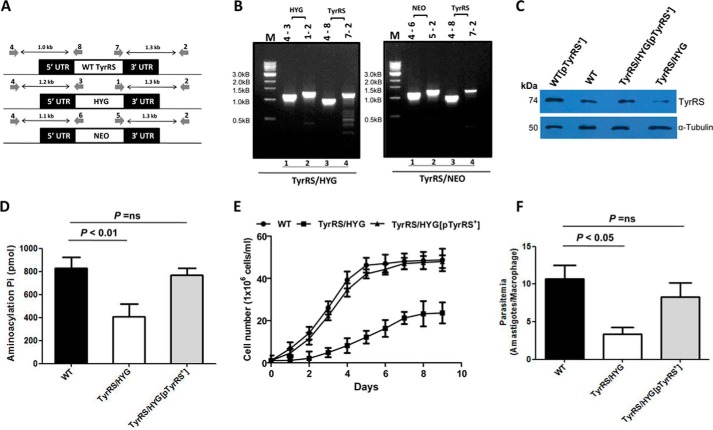
Because TyrRS is an important component of protein translation, we explored whether its depletion from the cell would affect aminoacylation and impact parasite growth and infection. The essentiality of LdTyrRS was assessed by classical gene replacement experiments where attempts were made to replace both the alleles of LdTyrRS by drug-resistance genes. This was achieved by the generation of inactivation cassettes with hygromycin phosphotransferase (HYG) or neomycin phosphotransferase (NEO) as selection markers along with 5′- and 3′-UTRs of the TyrRS gene, as described under “Experimental Procedures.” Linear replacement cassettes made by fusion PCR were electrotransfected into wild-type (WT) L. donovani promastigotes leading to the generation of heterozygous parasites (TyrRS/HYG or TyrRS/NEO) in which one allele of the LdTyrRS gene was replaced with either the hygromycin or neomycin drug resistance gene. The replacement of a single allele of the LdTyrRS gene by the drug resistance gene cassette was confirmed by a PCR-based analysis. After 3–4 passages, DNA from heterozygous mutant parasites (TyrRS/HYG or TyrRS/NEO) was isolated and subjected to a PCR-based analysis using primers external to the inactivation cassette of the LdTyrRS gene (Fig. 5A). The PCR analysis demonstrated the correct integration of HYG and NEO replacement cassettes at the TyrRS locus in heterozygotes (TyrRS/HYG or TyrRS/NEO), as indicated by the appearance of 1.2- (Fig. 5B, lane 1) and 1.3-kb (Fig. 5B, lane 2) bands in the case of HYG cassette and 1.1- (Fig. 5B, lane 1) and 1.3-kb (Fig. 5B, lane 2) bands in the case of NEO cassette, along with the 1.0- (Fig. 5B, lane 3) and 1.3-kb (Fig. 5B, lane 4) bands corresponding to the WT LdTyrRS gene. This data confirmed that a single allele of the LdTyrRS gene had been replaced in heterozygous mutant parasites (TyrRS/HYG or TyrRS/NEO). Several attempts to replace both alleles of the LdTyrRS gene to generate homozygous gene deletion mutants failed. Although few clones resistant to both drugs were obtained, PCR analyses demonstrated that the LdTyrRS gene was still present in the genome of these parasite lines (data not shown), thus indicating that LdTyrRS is an essential gene.
FIGURE 5.
Generation and characterization of heterozygous knock-out mutants of LdTyrRS. A, map of LdTyrRS genomic locus and location of the primers used for confirmation by PCR-based analysis along with the expected band sizes. Primer 4 was designed as a forward primer to match the upstream region of the LdTyrRS gene, and primers 8, 3, and 6 were designed internal to TyrRS, HYG, and NEO coding regions, respectively. Primer 2 was designed as a reverse primer to match the downstream region of LdTyrRS gene, and primers 7, 1, and 5 were designed as forward primers, internal to TyrRS, HYG, and NEO coding regions, respectively. B, genomic DNA from heterozygous TyrRS/HYG and TyrRS/NEO mutant parasites was used as a template for PCR analysis. The specific integration of the replacement cassette was checked with HYG, NEO, and LdTyrRS (WT) gene-specific primers. M indicates the molecular size marker in kb. C, Western blotting analysis of equal protein quantities (∼30 μg) from whole cell lysates prepared from WT, TyrRS overexpressors (WT[pTyrRS+]), add-back (TyrRS/HYG[pTyrRS+]), and heterozygous mutant (TyrRS/HYG) parasites. The loading was normalized with α-tubulin (50 kDa) antibody. D, comparison of the aminoacylation activity of TyrRS in cell lysate of WT, heterozygous (TyrRS/HYG) mutant, and add-back (TyrRS/HYG[pTyrRS+]) parasites. E, growth curve of L. donovani WT, add-back (TyrRS/HYG[pTyrRS+]), and heterozygous mutant (TyrRS/HYG) promastigotes in M199 media. The results represent mean ± S.D. with n = 3. F, comparison of infectivity of L. donovani WT, heterozygous mutant (TyrRS/HYG), and add-back (TyrRS/HYG[pTyrRS+]) parasites in J774A.1 murine macrophage cell line. The murine macrophage cell line J774A.1 was infected with the stationary-phase promastigotes at an multiplicity of infection of 20:1. The cells were stained after 24 h and amastigotes enumerated visually. The results represent mean ± S.D. with n = 3. Student's t test was performed, and the p values are indicated.
The effect of disruption of a single allele of the TyrRS gene at the protein level was studied by Western blotting analysis. Densitometric analysis was performed to evaluate the levels of TyrRS protein across different parasite lines. Comparative densitometry of the bands revealed a ∼1.8-fold decreased expression of TyrRS protein in heterozygous mutants (TyrRS/HYG) (Fig. 5C, lane 4) as compared with that in WT parasites. Complementation of the heterozygous mutant parasites (TyrRS/HYG) with an episomal copy of the TyrRS gene (TyrRS/HYG[pTyrRS+]) restored protein expression to levels comparable with that of WT parasites (Fig. 5C, lane 3). The overexpression of LdTyrRS protein in overexpressing mutants (WT[pTyrRS+]) was also confirmed by Western blotting analysis. A ∼1.5-fold increase in the TyrRS protein level was observed in TyrRS overexpressors (WT[pTyrRS+]) (Fig. 5C, lane 1) as compared with that in WT parasites.
To further establish that the elimination of a single allele of the TyrRS gene in L. donovani conferred TyrRS enzyme deficiency, the aminoacylation activity of TyrRS was determined in genetically manipulated parasites and compared with that in WT L. donovani parasites. A ∼2-fold decrease in the aminoacylation activity of TyrRS was observed in heterozygous parasites (TyrRS/HYG) as compared with that in WT parasites (Fig. 5D). Similar results were obtained with TyrRS/NEO parasites (data not shown). The WT and the “add-back” lines (TyrRS/HYG[pTyrRS+]) exhibited comparable TyrRS activity levels (Fig. 5D).
To assess if the reduced expression of TyrRS compromised the cellular growth of heterozygous mutant parasites, growth kinetic studies were undertaken. Heterozygous parasites (TyrRS/HYG) consistently showed growth delay as compared with WT parasites (Fig. 5E). The complementation of TyrRS/HYG mutants with an episomal copy (pTyrRS+) rescued the growth of these parasites similar to that of the WT control. Thus, it is reasonable to assume that a gene dosage effect resulted in the production of lesser TyrRS protein, and such a conjecture would suggest that TyrRS is involved in optimal cell proliferation.
We also examined the survival of heterozygous mutant parasites (TyrRS/HYG) inside murine macrophages in vitro. Virulence studies in a mouse macrophage cell line were carried out to determine the effects of genetic deficiency of TyrRS and to characterize the LdTyrRS enzyme further as a potential therapeutic target. To this end, a murine macrophage cell line was infected with WT, TyrRS heterozygous mutant (TyrRS/HYG), and add-back (TyrRS/HYG[pTyrRS+]) parasites at an multiplicity of infection of 20:1. WT parasites were capable of infecting and sustaining robust infection in murine macrophages, whereas the parasitemia of the heterozygous mutants was reduced by ∼50% relative to WT parasites 24 h post-infection (Fig. 5F). Similar results were obtained with TyrRS/NEO parasites (data not shown). Complementation with an episomal copy of the TyrRS gene restored the infectivity of the heterozygous (TyrRS/HYG) mutants similar to that of the WT parasites. Taken together, our data suggests that the LdTyrRS gene has a significant role in the growth and intramacrophage survival of amastigotes.
Leishmanicidal Activity of TyrRS Inhibitors
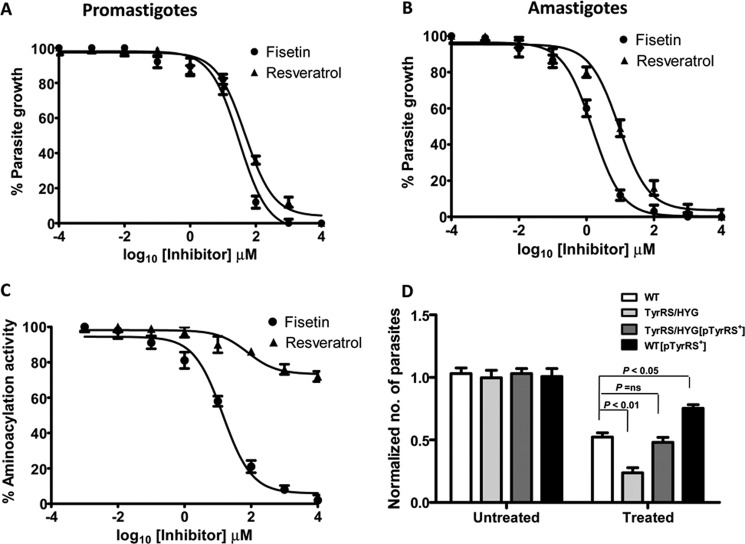
Resveratrol is a natural phenolic compound that has recently been shown to inhibit the activity of human TyrRS (19). A co-crystal structure of resveratrol bound to the active site of human TyrRS has been reported (19). Another flavanoid compound fisetin has also been identified to bind TyrRS of L. major (13). To test the efficacy of these compounds on L. donovani, log phase promastigotes were cultured in the presence of increasing concentrations of fisetin and resveratrol. Both compounds were found to inhibit the growth of promastigotes in a dose-dependent manner (Fig. 6A). The effective concentration that caused 50% inhibition of growth (IC50) after 72 h of drug addition was 31.07 μm for fisetin and 47.21 μm for resveratrol (Fig. 6A). The sensitivities of amastigotes were also tested in an intracellular amastigote-macrophage model. The IC50 of fisetin and resveratrol for amastigotes after 3 days of drug treatment was 1.53 and 9.3 μm, respectively (Fig. 6B). At these concentrations, both fisetin and resveratrol did not affect the viability of the macrophage cell line J774A.1, with the IC50 being >400 μm after 48 h of drug treatment.
FIGURE 6.
Effect of TyrRS inhibitors on parasite growth and enzyme activity. A, inhibition of promastigote growth in the presence of resveratrol and fisetin. The assay was done in 96-well plates and growth was estimated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Percentage growth of parasite was plotted against different concentrations of inhibitors. B, effect of resveratrol and fisetin on amastigote growth progression was studied by observing Giemsa-stained infected macrophages under a microscope. C, inhibition of rLdTyrRS aminoacylation activity by resveratrol and fisetin. D, comparison of the effect of fisetin activity on WT and genetically manipulated parasites. WT, overexpressors (WT[pTyrRS+]), heterozygous mutants (TyrRS/HYG), and add-back (TyrRS/HYG[TyrRS+]) parasites were treated with fisetin at a concentration of 35 μm. The cell growth was determined after 72 h. In the absence of drug treatment (untreated), the growth of each parasitic line was normalized to 1.0. After treatment with fisetin (treated), growth was calculated relative to the corresponding untreated control. The bar graph represents the mean ± S.D. with n = 3. Student's t test was performed, and p values are indicated.
The effect of these compounds on the aminoacylation activity of LdTyrRS (Fig. 6C) was also tested. Fisetin inhibited the enzymatic activity of recombinant LdTyrRS with an IC50 of ∼14.23 μm (Fig. 6C), whereas a concentration of resveratrol as high as 1 mm failed to inhibit the enzymatic activity of LdTyrRS (Fig. 6C). Thus, our results suggest that the anti-leishmanial effect of resveratrol is not due to the inhibition of LdTyrRS.
To ascertain whether the anti-leishmanial activity of fisetin is mediated through the inhibition of LdTyrRS, we also evaluated the effect of fisetin on the growth of genetically manipulated parasites (Fig. 6D). WT, overexpressors (WT[pTyrRS+]), heterozygous mutants (TyrRS/HYG), and add-back (TyrRS/HYG[pTyrRS+]) parasites were treated with fisetin at a concentration of 35 μm. In the absence of drug treatment (untreated), the growth of each parasitic line was normalized to a value of 1.0. After 72 h of treatment with fisetin, the growth rate of each parasitic line was calculated relative to the untreated control. Parasites overexpressing LdTyrRS (WT[pTyrRS+]) were found to be more resistant to growth inhibition by fisetin as compared with WT parasites (Fig. 6D). In contrast, heterozygous mutant parasites (TyrRS/HYG) were found to be more susceptible to inhibition by fisetin when compared with WT parasites (Fig. 6D). Complementation of TyrRS/HYG parasites with an episomal TyrRS gene (TyrRS/HYG[pTyrRS+]) decreased the sensitivity of heterozygous mutant parasites (Fig. 6D). The increased susceptibility of the heterozygous mutants to fisetin may be explained by the reduced levels of LdTyrRS expression in heterozygous knock-out parasites. The above data further indicates that fisetin exerts its antileishmanial effect through the inhibition of LdTyrRS.
Localization of TyrRS during Macrophage Infection with Leishmania
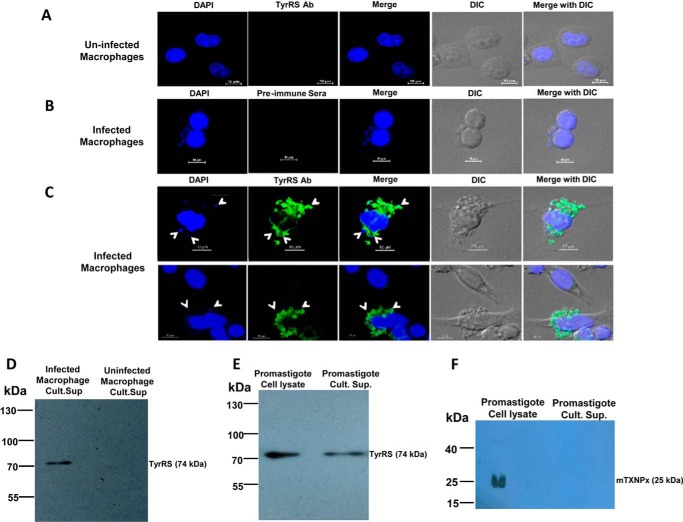
To determine the localization of LdTyrRS during macrophage infection, confocal microscopy of infected macrophages was performed. Infected and uninfected macrophages were fixed, permeabilized, and processed for immunofluorescence using either the anti-LdTyrRS antibody or preimmune sera (Fig. 7, B and C). DAPI staining was utilized for visualizing both the host and parasite nuclei. The anti-LdTyrRS-specific fluorescence observed in infected macrophages was not only associated with parasite nuclei staining but was also present throughout the cytosol of the infected macrophages (Fig. 7C). Thus, the confocal data demonstrates the presence of LdTyrRS in the cytosol of infected macrophages. Uninfected macrophages served as a negative control (Fig. 7A). No signal could be detected with the preimmune sera in infected macrophages (Fig. 7B). The immunofluorescence data thus indicates the possible secretion of TyrRS from Leishmania amastigotes (Fig. 7, A–C).
FIGURE 7.
Localization of the LdTyrRS in infected macrophages. A–C, confocal microscopy of the uninfected (A) and L. donovani-infected macrophages (B and C) showing the secretion of LdTyrRS protein in the macrophage cytoplasm. White arrows indicate intracellular amastigotes. Parasite and macrophage nuclei were visualized with DAPI (blue). Anti-LdTyrRS antibody (green) was used to visualize the parasitic LdTyrRS. Staining of infected macrophages with rabbit preimmune sera was used as a negative control (B). Scale bar corresponds to a size of 10 μm. D, Western blotting analysis of the parasite culture supernatants using antibodies against LdTyrRS. The culture supernatant of the infected (lane 1) and uninfected (lane 2) macrophages was electroblotted onto nitrocellulose membrane and probed with the anti-LdTyrRS antibody and HRP-conjugated anti-rabbit antibody. The blot was then developed with commercially available ECL reagent. Western blotting analysis of the promastigote culture supernatants was used to check for the presence of LdTyrRS (E) and mitochondrial tryparedoxin peroxidase (mTXNPx) (F). Lane 1, L. donovani promastigote cell lysate (20 μg); lane 2, promastigote culture supernatant. The parasites were suspended at a concentration of ∼108 parasites/ml in RPMI 1640 media without FBS and incubated for 8 h under normal culture conditions. The LdTyrRS protein was immunoprecipitated from a minimum of ∼80 ml of culture supernatants containing a total of ∼8 × 109 promastigotes and detected by a Western blotting analysis as described under “Experimental Procedures.”
The presence of LdTyrRS in the host cytosol also hinted toward its possible secretion into the extracellular milieu. Therefore, culture supernatants from uninfected or L. donovani-infected macrophages were analyzed for the presence of LdTyrRS by immunoprecipitation and immunoblotting analysis (Fig. 7D). As expected, no TyrRS was detected in the culture supernatant of uninfected macrophages (Fig. 7D). In contrast, a single band corresponding to the wild-type LdTyrRS (∼74 kDa) was observed in the culture supernatant of infected macrophages (Fig. 7D).
We also analyzed the secretion of TyrRS by Leishmania promastigotes (Fig. 7E). A ∼74-kDa band corresponding to wild-type LdTyrRS was observed in the parasite culture supernatants (Fig. 7E). To exclude the possibility that the apparent secretion of LdTyrRS was due to cell lysis, we measured the amount of the cytosolic marker glucose-6-phosphate dehydrogenase in the parasite (promastigote) culture supernatants by an enzymatic assay (20). The glucose-6-phosphate dehydrogenase activity in culture supernatants was found to be negligible (data not shown). As a control, parasite culture supernatants were also analyzed for the presence of mitochondrial tryparedoxin peroxidase, which has been reported to be a parasitic non-secretory protein (21) (Fig. 7F). No secretion of mitochondrial tryparedoxin peroxidase was observed in promastigote culture supernatants (Fig. 7F). Taken together, our immunofluorescence, immunoprecipitation, and Western blotting data provide compelling evidence indicating the secretion of LdTyrRS from both promastigotes and amastigotes.
Tyrosyl-tRNA Synthetase of Leishmania Possesses an Immunologically Active ELR Motif
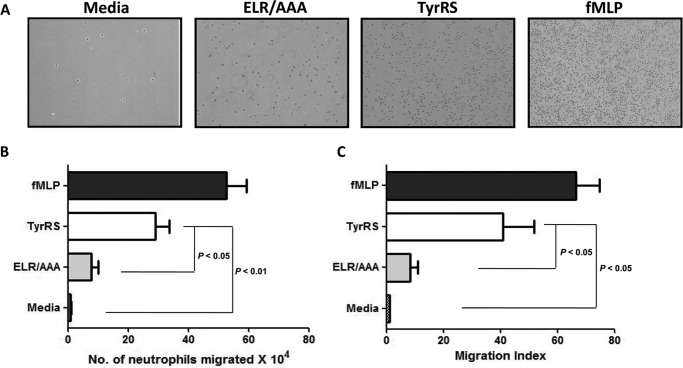
Previously, human “mini”-TyrRS has been shown to display potent neutrophil chemotaxis activity because of the presence of an immunologically active ELR motif (4). A transwell migration assay was performed to evaluate the chemotactic activity of LdTyrRS toward mouse neutrophils (Fig. 8A). The neutrophils that migrated into the lower chamber were quantitated by measuring their myeloperoxidase activity (Fig. 8B) as described under “Experimental Procedures.” N-Formyl-Met-Leu-Phe (fMLP) is a chemotactic peptide that was used as a positive control. The addition of both fMLP and rLdTyrRS stimulated the chemotactic migration of neutrophils (Fig. 8, B and C). Recombinant LdTyrRS stimulated the chemotactic activity of neutrophils at a concentration of 0.1 nm (6 ng), which is equivalent to the physiological amount of protein present in ∼2 × 105 parasites. Because the ELR motif is critical for chemotactic activity, we also prepared LdTyrRS mutant in which the ELR motif was mutated to “AAA.” The mutant LdTyrRS (ELR/AAA) did not show any chemotactic activity toward neutrophils (Fig. 8, B and C). Also, under these experimental conditions, spontaneous migration of neutrophils was negligible as indicated by the media control (Fig. 8, B and C).
FIGURE 8.
The effect of LdTyrRS on neutrophil chemotaxis was studied by a transwell assay. Wild-type rLdTyrRS, mutated rLdTyrRS (ELR/AAA), media (negative control), and fMLP (positive control) were used as chemoattractants. A shows a representative photograph of the cell migration in each condition. B, the number of cells that migrated due to the effect of different chemoattractants were quantitated using a myeloperoxidase assay. C, migration is also plotted as migration index (the number of cells migrating in each condition/number of cells migrating in basal medium). Data (i.e. the number of neutrophils migrated and the migration index) are represented as mean ± S.D. with n = 4. Student's t test was performed, and p values are indicated.
Overall, these results suggest that because of its ELR motif, LdTyrRS functions as a chemoattractant for neutrophils. It is also known that the Leishmania parasite exploits neutrophils as Trojan horses before they enter their definitive host cells, i.e. macrophages (22). In light of the role of neutrophils as host cells for Leishmania, it may be hypothesized that secreted LdTyrRS functions as a virulence factor to induce an inflammatory recruitment of neutrophils at the site of infection.
LdTyrRS Interaction with Host Macrophages
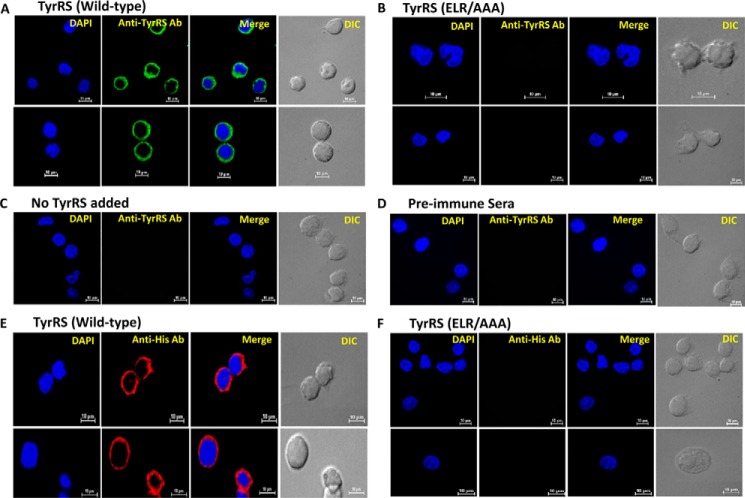
In addition to attracting neutrophils, another important function of the ELR motif is to mediate the interaction of CXC chemokines with host immune cells like macrophages. This interaction further triggers the release of proinflammatory cytokines from host immune cells (23–25). Direct binding of rLdTyrRS to host macrophages was visualized by immunofluorescence analysis. The specific interaction of rLdTyrRS with mouse macrophages was observed by using anti-LdTyrRS (Fig. 9A) and anti-His antibodies (Fig. 9E). On the other hand, the mutant LdTyrRS (ELR/AAA) did not show any binding to host macrophages (Fig. 9, B and F). Also, no binding could be detected in macrophages that were not incubated with any protein and preimmune sera controls (Fig. 9, C and D). This data indicated an ELR motif-mediated interaction of LdTyrRS with host macrophages.
FIGURE 9.
LdTyrRS interaction with macrophages in vitro. A and B, confocal images representing the binding of rLdTyrRS (green) (A) and mutant LdTyrRS (ELR/AAA) to mouse macrophages (B). C and D, represent controls for primary/secondary antibodies alone (that is, no added LdTyrRS) (C) and preimmune sera (D). For nucleus visualization, DAPI (blue) localization is shown. The interaction of LdTyrRS with the mouse macrophage cell line was visualized by staining with the anti-LdTyrRS antibody (A–D). E and F, the binding of rLdTyrRS (E) and ELR/AAA mutant LdTyrRS (F) to mouse macrophages was also detected using anti-His monoclonal antibody. The scale bar corresponds to a size of 10 μm.
Triggering of Cytokine Secretion by LdTyrRS
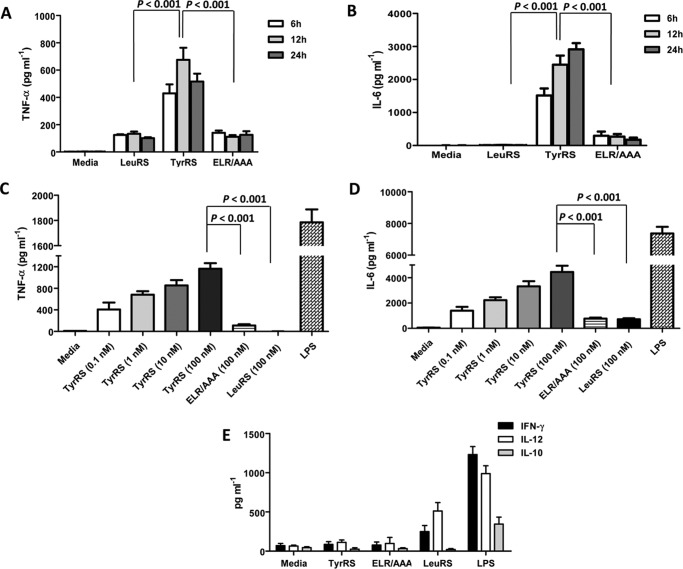
To check if LdTyrRS triggers cytokine activity, a murine macrophage cell line was incubated with rLdTyrRS, mutant ELR/AAA-LdTyrRS, lipopolysaccharide (LPS) (as a positive control), and rLdLeuRS (as a negative control). The culture supernatants were analyzed for the presence of proinflammatory cytokines such as TNF-α, IL-6, IL-12, and IFN-γ. Time kinetic analysis by ELISA revealed maximal production of the inflammatory cytokine TNF-α (Fig. 10A) during 12 h of culture and IL-6 within 24 h of culture (Fig. 10B). Interestingly, the mutant ELR/AAA-LdTyrRS did not trigger cytokine release from macrophages and behaved similarly to the control protein rLdLeuRS (Fig. 10, A and B). Moreover, the release of TNF-α and IL-6 by rLdTyrRS was mediated in a concentration-dependent manner (Fig. 10, C and D). Other proinflammatory cytokines like IL-12 and IFN-γ were not induced by rLdTyrRS (Fig. 10E). We also checked the induction of an important anti-inflammatory cytokine, IL-10, which is involved in disease progression in leishmaniasis (26). Recombinant LdTyrRS failed to trigger IL-10 secretion (Fig. 10E) by host macrophages. This data indicates that LdTyrRS specifically triggers the release of IL-6 and TNF-α in a time- and dose-dependent manner.
FIGURE 10.
Effect of rLdTyrRS on mouse macrophages. A and B, the secretion profile of TNF-α (A) and IL-6 (B) was measured at different time points using ELISA. C and D, dose-dependent increase in the secretion of cytokines like TNF-α (C) and IL-6 (D) from mouse macrophages was also measured. Media alone, rLdLeuRS enzyme, and ELR-AAA mutant of LdTyrRS were used as negative controls. LPS was used as a positive control in all the experiments. E, the secretion profile of other cytokines like IFN-γ, IL-10, and IL-12 that are secreted from mouse macrophages on exposure to rLdTyrRS was also analyzed. The results represent mean ± S.D. with n = 3. Tukey's test was performed, and p values are indicated.
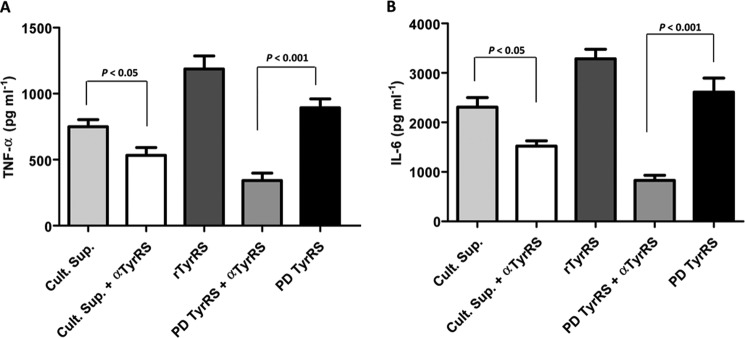
We also corroborated our findings with the native LdTyrRS that was immunoprecipitated from promastigote culture supernatants (PDTyrRS). Native LdTyrRS was equally capable of triggering TNF-α and IL-6 release from murine macrophages (Fig. 11, A and B). It was also observed that preincubation of native LdTyrRS (PDTyrRS) with anti-LdTyrRS antibodies (PDTyrRS + αTyrRS) substantially blocked TNF-α (p < 0.001) and IL-6 production (p < 0.001) from macrophages (Fig. 11, A and B), thereby suggesting a specific motif-based LdTyrRS interaction with host macrophages.
FIGURE 11.
Effect of native LdTyrRS on the cytokine secretion profile of mouse macrophages. A and B, secretion profile of TNF-α (A) and IL-6 (B) using the L. donovani promastigote culture supernatants alone (Cult. Sup.), promastigote culture supernatants neutralized with the anti-TyrRS antibody (Sup + α-TyrRS Ab), native LdTyrRS immunoprecipitated from promastigote culture supernatant (PD-TyrRS), and finally native LdTyrRS (PD-TyrRS) neutralized with its own antibody (PD-TyrRS + α-TyrRS Ab). Recombinant LdTyrRS was used as a positive control. TNF-α and IL-6 secretion are clearly enhanced significantly when immunoprecipitated native LdTyrRS (PD-TyrRS) is used. The triggering activity of native LdTyrRS (PD-TyrRS + α-TyrRS Ab) is reduced when preincubated with anti-LdTyrRS antibodies (1:3000), indicating specific activation of macrophages with native LdTyrRS (PD-TyrRS). The results represent mean ± S.D. with n = 3. Tukey's test was performed, and p values are indicated.
Potential Receptor(s) of LdTyrRS on Host Macrophages
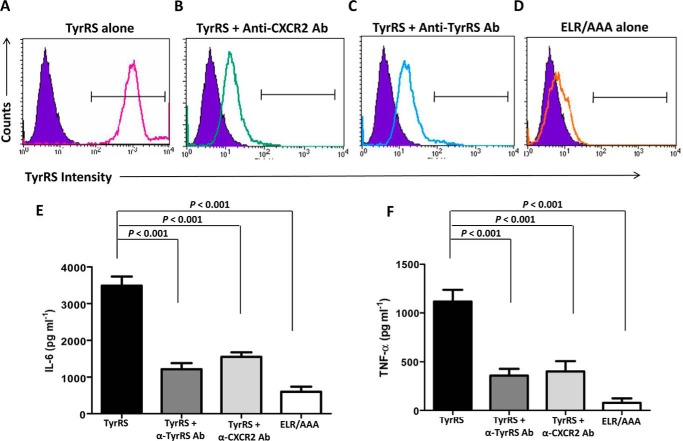
ELR motif-based binding of CXC chemokines to a CXCR2 receptor on immune cells has been shown to activate NF-κB and induce proinflammatory cytokine expression (23). To determine whether the binding of LdTyrRS is also mediated through ELR-CXCR2 receptor interaction, we preincubated human macrophages with anti-CXCR2 and anti-LdTyrRS antibodies and tested whether antibody-mediated receptor or protein blockades inhibited rLdTyrRS-macrophage interaction. The interaction was analyzed by FACS analysis. Recombinant LdTyrRS bound to mouse macrophages was detected by an anti-LdTyrRS antibody (Fig. 12A). On the other hand, specific antibody-mediated blocking of the CXCR2 receptor on murine macrophages reduced rLdTyrRS binding (Fig. 12B). The blocking of rLdTyrRS protein with anti-LdTyrRS antibodies also reduced binding of the protein to macrophages (Fig. 12C). As expected, mutant LdTyrRS (ELR/AAA) protein did not show any binding to host macrophages (Fig. 12D). Furthermore, blockage of rLdTyrRS-macrophage interactions using either anti-CXCR2 or anti-LdTyrRS antibodies significantly reduced the release of TNF-α (p < 0.001) and IL-6 (p < 0.001) from murine macrophages (Fig. 12, E and F). Our data strongly highlights the role of ELR motif in mediating LdTyrRS interactions with host macrophages. We, therefore, conclude that ELR motif-based LdTyrRS binding in vitro likely occurs using specific receptors on host macrophages and that this binding might lead to very specific host immune system activation.
FIGURE 12.
Potential LdTyrRS receptor on mouse macrophages. A–D, FACS analysis to assess the binding of rLdTyrRS to mouse macrophages. rLdTyrRS bound to mouse macrophages was analyzed by indirect staining with the anti-TyrRS antibody. Overlay histograms show binding of rLdTyrRS to the surface of mice macrophages (solid line) with unstained controls (purple solid) as background. Histogram (solid line) shows positive binding of rLdTyrRS to mouse macrophages (A). rLdTyrRS binding to macrophages is reduced when macrophages are preincubated anti-CXCR2 (B) or when rLdTyrRS is neutralized with anti-LdTyrRS antibodies (C). The binding is also significantly reduced with the mutant LdTyrRS (ELR/AAA) protein (D). E and F, cytokine secretion assays for IL-6 (E) and TNF-α (F) using mice macrophages and anti-CXCR2 and anti-LdTyrRS antibodies. The secretion of these proinflammatory cytokines is reduced when macrophages and LdTyrRS protein were preincubated with the anti-CXCR2 (TyrRS + α-CXCR2 Ab) and anti-LdTyrRS (TyrRS + α-TyrRS Ab) antibodies, respectively. The results represent mean ± S.D. with n = 3. Tukey's test was performed, and p values are indicated.
Discussion
aaRSs are key enzymes that drive the protein translational machinery. Apart from their translational functions, aaRSs are implicated in various non-canonical functions such as gene transcription, mRNA translation, inflammation, and immune response (1). Therefore, aaRSs constitute a significant subset of proteins, and inhibition of their enzymatic activity can be deleterious to the organism. Hence, experimental dissection of critical translation components like aaRSs is on high priority as one of the avenues of novel target discovery in pathogen biology.
TyrRS are yet to be experimentally investigated as drug targets in parasites. The few parasite-specific studies available on TyrRS have majorly focused on the structural aspects of the enzyme. A recent comprehensive study highlighted the structural and functional aspect of TyrRS from Plasmodium falciparum (24). The structural analysis of the TyrRS orthologue from L. major reveals several crucial differences between the host and pathogen tyrosyl-tRNA synthetase active sites (13). These differences could potentially be exploited for the design of structure-based inhibitors of parasite TyrRSs. The present study highlights the functional attributes of tyrosyl-tRNA synthetase of Leishmania as a housekeeping protein translation enzyme and also as a mimic of host CXC chemokine.
In the present study, we, for the first time, report the characterization of tyrosyl-tRNA synthetase from L. donovani (LdTyrRS). The overall Km values deduced by kinetic analysis for LdTyrRS appear to be closer to the Km values reported for other mammalian TyrRS. TyrRS appears to be an essential gene as attempts to delete both copies of the TyrRS gene from the parasite genome failed. Heterozygous parasites (TyrRS/HYG) were found to have slower growth kinetics and exhibited attenuated virulence. Because L. donovani TyrRS appears to be an essential gene for parasite survival, we also analyzed the efficacy of known tyrosyl-tRNA synthetase inhibitors, resveratrol and fisetin, on parasite survival and the aminoacylation activity of LdTyrRS. Fisetin was found to inhibit the parasite growth by inhibiting the LdTyrRS aminoacylation activity. This data are in agreement with a previous report that suggests binding of fisetin to the active site of trypanosomal TyrRS (13). Several subtle yet crucial differences between Leishmania and human TyrRS (13) could potentially be exploited for the design of structure-based inhibitors for LdTyrRS.
Leishmania has evolved sophisticated mechanisms to evade or subvert host immune responses and establish chronic infection. Leishmania parasites have the capacity to subvert phagocytosis (27) and modulate cytokine secretion (11, 28), thus allowing the parasite to thrive within phagocytic cells and within the host organism as a whole. From previous studies, it has become clear that a range of effector molecules secreted by the Leishmania parasite play a vital role in this process (20, 29). In this context, the expression of a mimic of a human cytokine could contribute to the modulation of host signaling pathways to the advantage of the parasite. This report highlights the moonlighting activity of a parasitic tyrosyl-tRNA synthetase (LdTyrRS) to function as a mimic of host CXC chemokine. Aminoacyl-tRNA synthetases have a vital role in translating the genetic code. Numerous studies have shown that members of this enzyme family are quite adept at “moonlighting” (1, 3, 30). Non-canonical roles have been suggested for several parasite tRNA synthetases. Studies have indicated that human tyrosyl-, tryptophanyl-, and lysyl-tRNA synthetases can be secreted extracellularly and can mimic cytokines (4, 31, 32). TyrRS of Plasmodium and LysRS of Entamoeba also mimic the functions of human cytokines (24, 33).
Our immunofluorescence and immunoblotting data reveal that both promastigotes and amastigotes secrete LdTyrRS. Interestingly, the secreted tyrosyl-tRNA synthetase from L. donovani was found to lack a classical N-terminal secretion signal peptide. Previous reports have identified secretion by exosomes as a major mechanism by which Leishmania exports secreted virulence factors that help in communication with the host (29, 34). Due to the absence of any defined secretory signal, it may be hypothesized that the release of LdTyrRS also occurs by a non-classical secretion mechanism. Understanding the exact mechanism by which the secretion of LdTyrRS occurs remains to be explored and is the focus of ongoing work.
We further investigated the capacity of LdTyrRS to modulate neutrophil recruitment. Our data shows that by using its ELR motif, secreted LdTyrRS can induce direct chemotaxis of neutrophils. A recent study has highlighted the role of neutrophils as host cells for Leishmania (35–37). Although parasite culture supernatants have been found to have chemotactic activity toward neutrophils (35), parasite-secreted protein(s) that specifically trigger the initial recruitment of neutrophils are poorly understood. The present study identifies LdTyrRS present in the parasite culture supernatant, as a parasite secretory protein having the capacity to modulate neutrophil recruitment. Thus, it may be hypothesized that secretion of LdTyrRS is a pro-parasitic response that is likely to bring about the recruitment of neutrophils to the site of infection.
Because ELR motif is also involved in a ligand-based interaction with CXCR2 receptors on immune cells, we also analyzed the binding of LdTyrRS to host macrophages. Our confocal and FACS data show that via its ELR motif, released LdTyrRS interacts with a specific receptor on host macrophages. Our data also indicates that the LdTyrRS-macrophage interaction triggers the release of the proinflammatory cytokines TNF-α and IL-6. Previous studies have shown that Leishmania infection triggers the release of TNF-α and IL-6 (38–41). A central function of these two proinflammatory cytokines is to help in the recruitment of phagocytes like neutrophils and monocytes (42–44). Both cell types are infection targets and essential for the establishment of Leishmania infection (45–47). In the case of Leishmania infections, the release of TNF-α and IL-6 by the parasite secretory protein GP63 has been directly associated with inflammatory phagocyte (neutrophil and monocyte) recruitment (40). Thus, it may be hypothesized that the secretion of TNF-α and IL-6 by LdTyrRS also contributes toward the accrual of neutrophils at the site of Leishmania infection. These results clearly indicate that the secreted LdTyrRS enables Leishmania to elicit proinflammatory cytokine release and neutrophil recruitment, both of which contribute to the establishment of infection. In our present set of experiments, we observed that 0.1 nm (6 ng) LdTyrRS was sufficient to initiate neutrophil chemotaxis and cytokine release from host cells. This concentration was found to correlate with the amount of protein present in ∼2 × 105 parasites.
During Leishmania infection, most sand flies transmit 103-105 parasites per blood meal (48). Earlier reports indicate that tissue damage to the skin caused by the infected sand fly bites is important to induce neutrophil recruitment within the first 90 min, further indicating that the initial neutrophil recruitment in the case of a natural infection is parasite-independent (46). However, in the case of an experimental infection in mice, a needle injection of 105 Leishmania parasites is required to observe parasite-dependent neutrophil recruitment at later time points (6–24 h post-infection) (49, 50). Thus, in a natural Leishmania infection, apart from parasitic proteins other factors derived from the sand fly also contribute to neutrophil chemotaxis in the infected dermis. Because all these chemotactic factors (both parasite and sandfly derived) work in a synergistic manner, it would be interesting to observe the cooperation between the LdTyrRS and other chemoattractants. Although in the present study, the amount of LdTyrRS used was within the physiological range, the exact amount of protein secreted during natural infection, and the minimal effective concentration required for its chemotactic activity needs to be evaluated by further experimentation.
In summary, our data indicates a possible immunomodulating role of LdTyrRS in Leishmania infection. LdTyrRS functions as a direct chemoattractant for neutrophils. LdTyrRS was also found to induce TNF-α and IL-6 release, which is known to be associated with inflammatory phagocyte recruitment in Leishmania infections. Improved knowledge of Leishmania-induced inflammation will further our understanding of how the parasite establishes infection, modulates the immune response, metastasizes, and causes pathology.
Considering that drug resistance is a major concern in anti-parasitic chemotherapy, there is continuous pressure to identify new drug targets. Our comprehensive analyses on the twin abilities of Leishmania parasite tyrosyl-tRNA synthetase provide a platform to explore LdTyrRS as a potential target for drug development.
Experimental Procedures
Materials
All restriction enzymes and DNA modifying enzymes were obtained from New England Biolabs. Paromomycin and hygromycin were obtained from Sigma. Plasmid pET-30a was obtained from Novagen. Escherichia coli DH10β and Rosetta were used as hosts for plasmid cloning and protein expression, respectively. Nickel-nitrilotriacetic acid-agarose was purchased from Qiagen. DNA and protein markers were acquired from New England Biolabs. Resveratrol and fisetin were obtained from Sigma. ELISA kits and antibodies were obtained from BD Bioscience. The rabbit anti-tubulin-α antibody was obtained from Neomarker (Fremont, CA). Other materials used in this study were of analytical grade and were commercially available.
Strains and Culture Conditions
L. donovani Bob (LdBob/strain/MHOM/SD/62/1SCL2D) was obtained from Dr. Stephen Beverley (Washington University, St. Louis, MO). Wild-type (WT) promastigotes were cultured at 22 °C in M199 medium (Sigma) supplemented with 100 units/ml of penicillin (Sigma), 100 μg/ml of streptomycin (Sigma), and 5% heat-inactivated fetal bovine serum (Gibco). WT parasites were routinely cultured in media with no drug supplementation, whereas genetically manipulated TyrRS heterozygotes (TyrRS/HYG, TyrRS/NEO) were maintained in either 200 μg/ml of hygromycin or 300 μg/ml of paromomycin, respectively. The TyrRS overexpressing (WT[pTyrRS+]) parasites were maintained in 800 μg/ml of zeocin. The add-back line TyrRS/HYG[pTyrRS+], was grown in 800 μg/ml of zeocin and 150 μg/ml of hygromycin. For characterization of mutant parasites phenotypically, the cells were subcultured without selection markers prior to experiments.
Axenic amastigotes were generated by using the standard protocol as described earlier (51, 52). Briefly, late-log phase promastigote cultures (∼2 × 106/ml) were adapted to grow at 26 °C in an acidic amastigote media (RPMI 1640, 25 mm MES, pH 5.5). Once established, these parasites were subsequently grown in acidic medium (RPMI 1640/MES, pH 5.5) at 37 °C in a humidified atmosphere containing 5% CO2. Throughout this in vitro adaptation process, the cellular morphology of the parasites was examined by both phase-contrast light microscopy and Giemsa-stained preparations. The mouse macrophage-like cell line J774.A1 obtained from ATCC was cultured in RPMI 1640 media (Sigma) supplemented with 10% FBS and antibiotics (100 units/ml of penicillin and 100 μg/ml of streptomycin) at 37 °C with 5% CO2.
Ethics Statement
All animal experiments were performed according to the guidelines approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India. The protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Jawaharlal Nehru University (JNU), New Delhi (IAEC code number 11/2013). All mice used for the experiments were ethically sacrificed by asphyxiation with carbon dioxide according to the institutional and the CPCSEA (Government of India) regulations.
Cloning, Expression, and Purification of Recombinant LdTyrRS Protein
The gene for LdTyrRS (tritrypDB ID LdBPK_141460.1) was amplified by PCR using a forward primer with a flanking BamHI site (5′-TTTTGGATCCATGAACACGGACGACCGCTAC-3′) and reverse primer with a flanking HindIII site (5′-TTTTAAGCTTTACCTCTTCTTTGCCATCTTCTTC-3′) from L. donovani genomic DNA. The 2049-bp amplification product encompassing the LdTyrRS open reading frame (ORF) was cloned into a pET30a vector (Novagen) using BamHI and HindIII restriction sites. This construct (LdTyrRS-pET30a) containing a His6 tag at the N terminus was transformed into the E. coli Rosetta strain (Novagen). The expression of recombinant LdTyrRS (rLdTyrRS) was induced with 0.5 mm isopropyl β-d-thiogalactopyranoside at 18 °C for 16 h. The protein was purified by a Ni2+-nitrilotriacetic acid-agarose resin (Qiagen) by eluting with increasing concentrations of imidazole. The purified protein was found to be >95% pure as judged by SDS-PAGE.
Site-directed Mutagenesis of the ELR Peptide Motif of LdTyrRS
The ELR motif of LdTyrRS was replaced with AAA by site-directed mutagenesis. The mutation in the LdTyrRS gene was performed using the QuikChange II site-directed mutagenesis kit (Agilent Technologies, USA) following the manufacturer's instructions. Subsequently, a DNA sequence analysis was performed to confirm the mutations. The mutated recombinant protein (ELR/AAA) was expressed and purified using the same protocol as specified above for the recombinant WT LdTyrRS.
Aminoacylation Spectrophotometric Assay
The aminoacylation assays were performed as previously described (53). Briefly, the substrate L. donovani tRNATyr was synthesized by in vitro transcription from a PCR product template that contained a T7 promoter followed by the L. donovani tRNATyr sequence (tritrypDB ID LinJ.34.tRNA7) and the terminal CCA sequence. The in vitro transcription reaction was performed with the MEGAScript T7 polymerase kit (Ambion; Life Technologies) according to the manufacturer's instructions. The reaction mixtures were then extracted with phenol/chloroform/isoamyl alcohol (25:24:1 (v/v), Sigma), and the tRNAs were precipitated with isopropyl alcohol (Sigma). The tRNA was refolded by heating at 70 °C for 10 min, followed by the addition of 10 mm MgCl2 and slow cooling to room temperature. The aminoacylation reaction was performed as described earlier (53) in 30 mm Hepes (pH 7.5), 150 mm NaCl, 30 mm KCl, 50 mm MgCl2, 1 mm DTT, 200 μm ATP, 10 mm l-tyrosine, 8 μm tRNATyr, 2 units/ml of inorganic pyrophosphatase (Sigma), and 0.2 μm rLdTyrRS protein at 37 °C. The reaction was stopped at different time points by the addition of 10 mm EDTA and developed by malachite green (Echelon Bioscience). The absorbance was then measured at 620 nm by a Spectramax M2 reader (Molecular Devices). The determination of Km and Vmax for l-tyrosine and tRNATyr was achieved by varying the concentration of l-tyrosine or tRNATyr in the reaction mixture while maintaining the other components in excess. To determine the effect of the inhibitors on the aminoacylation activity of rLdTyrRS, reactions were performed in the presence of inhibitors as described previously (53). Briefly, a reaction mixture containing rLdTyrRS (0.2 μm) was incubated with different concentrations of fisetin/resveratrol (0.1 nm to 1000 μm) for 30 min at 37 °C. The reactions were stopped and quantitated as described above. To determine the 50% inhibitory concentration (IC50), the dose-response data were fitted to the log (inhibitor) versus response equation using GraphPad Prism.
Molecular Constructs for Replacement of TyrRS Alleles
For inactivation of the LdTyrRS gene, a targeted gene replacement strategy based on PCR fusion was employed (54). Briefly, the flanking regions of the TyrRS gene (5′-UTR and 3′-UTR) were amplified and fused by PCR to the hygromycin phosphotransferase gene (HYG) or neomycin phosphotransferase gene (NEO). The 5′-UTR (599 bp) of the LdTyrRS gene was obtained from WT L. donovani genomic DNA by PCR amplification with either primer A and BHyg or primers A and BNeo (Table 1). The NEO gene was amplified from pX63-NEO with primers CNeo and DNeo. The HYG gene was amplified from pX63-HYG with primers CHyg and DHyg (Table 1). The 3′-UTR (580 bp) of the LdTyrRS gene was obtained from L. donovani WT genomic DNA by PCR amplification using primers EHyg/ENeo and reverse primer F (Table 1). The 5′-UTR of L. donovani TyrRS gene was then ligated to either of the antibiotic resistance marker genes (HYG/NEO) by PCR using primers A and DHyg or A and DNeo. This fragment (5′-UTR marker gene) was then fused with the 3′-UTR using primers A and F, yielding the linear replacement cassette, 5′UTR-Hyg-3′UTR or 5′UTR-Neo-3′UTR.
TABLE 1.
Primers used for generation of the Hyg and Neo specific linear replacement cassette fragments
| S. No. | L. donovani primers | Sequences |
|---|---|---|
| 1 | A | 5′-AACATGACGCAGTGGTGTCTCCGTT-3′ |
| 2 | BHyg | 5′-GGTGAGTTCAGGCTTTTTCATATGCGGCGTGCTCAGCGAACGAAA-3′ |
| 3 | CHyg | 5′-CGTTTCGTTCGCTGAGCACGCCGCATATGAAAAAGCCTGAACTCA-3′ |
| 4 | DHyg | 5′-GCCGCCTCTTCACCTATACCCTATTCCTTTGCCCTCGGACGA-3′ |
| 5 | EHyg | 5′-CTCGTCCGAGGGCAAAGGAATAGGGTATAGGTGAAGAGGCGGC-3′ |
| 6. | BNeo | 5′-CAATCCATCTTGTTCAATCATATGCGGCGTGCTCAGCGAACGAAA-3′ |
| 7 | CNeo | 5′-CGTTTCGTTCGCTGAGCACGCCGCATATGATTGAACAAGATGGATT-3′ |
| 8 | DNeo | 5′-GCCGCCTCTTCACCTATACCTCAGAAGAACTCGTCAAGAA-3′ |
| 9 | ENeo | 5′-CTTCTTGACGAGTTCTTCTGAGGTATAGGTGAAGAGGCGGC-3′ |
| 10 | F | 5′-GTGGAAGCAAGGGCAACACA-3′ |
| 11 | G | 5′-TTTTTCTAGAATGAACACGGACGACCGCTAC-3′ |
| 12 | H | 5′-TTTTAAGCTTTACCTCTTCTTTGCCATCTTCTTC-3′ |
To generate the episomal construct, the full-length LdTyRS coding sequence was amplified with a forward primer harboring the XbaI site (primer G) and a reverse primer with the HindIII site (primer H) (Table 1). The amplified LdTyRS gene was then cloned into the pSP72α-zeo-α vector to get the pSP72α-zeo-α-TyRS episomal construct. All the fragments and constructs were sequenced for confirmation.
Generation of Genetically Manipulated Parasites
After PCR amplification and purification, ∼2 μg of the linear replacement cassette (5′UTR-Hyg-3′UTR or 5′-UTR-Neo-3′UTR) was individually transfected by electroporation in WT L. donovani promastigotes (55). The transfectants were subjected to antibiotic selection depending on the marker gene. The cells resistant to antibiotic selection were further subjected to PCR-based analysis to check for the correct integration of replacement cassettes using primers as shown in Table 2. Thereafter, the second round of transfection was initiated to knockout the other allele of the TyrRS gene.
TABLE 2.
Primers used for the molecular characterization of the genetically manipulated parasites by PCR-based analysis
| S. No. | L. donovani primers | Sequences |
|---|---|---|
| 1 | Primer 1 | 5′-TGTAGAAGTACTCGCCGATAGTGG-3′ |
| 2 | Primer 2 | 5′-AGATCGCATTGCAGCACAGC-3′ |
| 3 | Primer 3 | 5′-CGCAGCTATTTACCCGCAGGACAT-3′ |
| 4 | Primer 4 | 5′-CGTCGTCATTTCCGCCTTACGC-3′ |
| 5 | Primer 5 | 5′-ATAGCGTTGG CTACCCGTGATATTGC-3′ |
| 6 | Primer 6 | 5′-AACACGGCGGCATCAGAGCAGCCGATTG-3′ |
| 7 | Primer 7 | 5′-GAAGAAGATGGCAAAGAAGAGGTAA-3′ |
| 8 | Primer 8 | 5′-GCGGTCGTCCGTGTTCAT-3′ |
To generate the episomal complementation mutants, the episomal construct (pSP72α-zeo-α-TyrRS) was transfected into the heterozygous TyrRS/HYG parasites to get the add-back line (TyrRS/HYG[pTyrRS+]). The wild-type promastigotes were also transfected with the episomal construct (pSP72α-zeo-α-TyrRS) to generate the overexpressing (WT[pTyrRS+]) mutant parasites. The correct integration was confirmed by PCR (data not shown) and Western blotting analysis.
Growth and Infectivity Assays
Growth rate experiments were conducted by inoculating stationary phase parasites at a density of 1 × 106 cells/ml in M199 medium with 5% FBS in 25-cm2 flasks without respective selection drug at 22 °C. The growth rate of each culture was determined at 24-h intervals with a Neubauer hemocytometer. Growth studies with individual cell lines were done at least three times, and similar results were consistently obtained.
For the infectivity assay, the J774.A1 murine macrophage cell line was plated at a cell density of 5 × 105 cell/well in a 6-well flat bottom plate. The adherent cells were infected with stationary phase promastigotes, at a ratio of 20:1 for 6 h. Excess non-adherent promastigotes were then removed by incubating the cells for 30 s in phosphate-buffered saline (PBS). These were then subsequently maintained in RPMI 1640 media containing 10% FBS at 37 °C with 5% CO2. Giemsa staining was performed to visualize the intracellular parasite load.
Drug Sensitivity Assay
To determine the effect of inhibitors (resveratrol and fisetin) on the viability of L. donovani promastigote cells a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed as described previously (56). Briefly, log phase promastigotes (5 × 104 cells/well) were seeded in a 96-well flat-bottomed plate (Nunc) and incubated with different inhibitor concentrations at 22 °C. Because the inhibitors were dissolved in DMSO, a sample without inhibitors but with an equal volume of DMSO served as an additional control. After 72 h of incubation, 10 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (5 mg/ml) was added to each well and the plates were further incubated at 37 °C for 4 h. The reaction was stopped by the addition of 50 μl of 50% isopropyl alcohol and 20% SDS followed by gentle shaking at 37 °C for 30 min to 1 h. The absorbance was measured at 570 nm in a microplate reader (SpectraMax M2 from Molecular Devices). The percentage of parasite growth at different inhibitor concentrations was determined relative to untreated control cells and 50% inhibitory concentration (IC50) was calculated.
The sensitivity of amastigotes to fisetin and resveratrol was tested in an intracellular amastigote-macrophage model as described earlier (57). Briefly, the J774A.1 cell line (1 × 105 cells/well) was cultured in eight-chamber Lab-Tek tissue culture slides (Nunc, USA) and infected with stationary phase promastigotes at an multiplicity of infection of 20:1 as described above. The infected macrophages were then incubated for 72 h with different concentrations of inhibitors (0.1 nm to 10,000 μm). The slides were fixed and stained with Giemsa. The number of amastigotes per cell was counted in 100 macrophages at different drug concentrations. The 50% inhibitory concentration (IC50) was obtained by determining the reduction in parasite burden relative to the untreated infected controls.
Antibody Generation and Immunofluorescence Microscopy
Antibodies against the rLdTyrRS were raised commercially in rabbits (Merck). Briefly, the purified rLdTyrRS protein (50 μg) was subcutaneously injected in rabbit using Freund's complete adjuvant (Sigma), followed by three booster doses of the recombinant protein (20 μg) in Freund's incomplete adjuvant (Sigma), at a 2-week interval. The sera were then collected after the last booster. Protein A-purified IgG fractions (anti-LdTyrRS antibody) were utilized for further immunological assays.
For the intracellular localization of LdTyrRS, L. donovani promastigotes were immobilized on poly-l-lysine-coated coverslips. The cells were fixed and permeabilized followed by incubation with the anti-LdTyrRS antibody (1:1000) for 1 h at room temperature. Subsequently, the cells were washed and then incubated for 45 min with the Alexa 546-conjugated goat anti-rabbit IgG antibody (Thermo Fisher Scientific catalogue number A-11071). The nuclear and the kinetoplastid DNA was stained with DAPI (Invitrogen). The fluorescence of the parasites stained with the anti-LdTyrRS antibody was visualized by a confocal laser scanning microscope (Olympus FluoViewTM FV1000 with objective lenses PLAPON ×60 O, NA-1.42) at an excitation wavelength of 556 nm. The cellular DNA stained with DAPI was visualized at an excitation wavelength of 405 nm.
Immunofluorescence analysis of the infected macrophages was performed as previously described (58). Briefly, J774 cells (1 × 105) were grown on coverslips in RPMI 1640 supplemented with 10% FBS. The adherent cells were then infected with L. donovani stationary-phase promastigotes at an multiplicity of infection of 20:1 or left uninfected. After 24 h of infection, the cells were fixed and permeabilized followed by incubation with the primary (anti-LdTyrRS and secondary (Alexa 488-conjugated goat anti-rabbit IgG) antibodies. All antibody incubations were of 50 min duration and were followed by six to eight washes in PBS. The images were then visualized by the confocal laser scanning microscope (Olympus FluoViewTM FV1000 with objective lenses PLAPON ×60 O, NA-1.42) at an excitation wavelength of 495 nm. DAPI was used to stain the nuclei of both the parasite and host macrophages.
Immunoprecipitation and Western Blotting Analysis
Immunoprecipitation of the LdTyrRS was performed using the culture supernatants as previously described (58). Briefly, the culture supernatants from uninfected macrophages, infected macrophages, and promastigote cultures were collected by centrifugation. The culture supernatants were neutralized with 5 n NaOH and concentrated using an Amicon Ultra-15 centrifugal filter unit (Millipore). The concentrated culture supernatants were incubated overnight at 4 °C with anti-LdTyrRS antibodies. Protein A-Sepharose beads (Sigma) were then added to each sample for 30 min, following which the beads were washed with Leishmania lysis buffer (50 mm Tris, pH 8, 137 mm NaCl, complete protease inhibitor mixture (Roche)). The immunoprecipitated proteins were eluted from the beads using 0.2 m glycine buffer. SDS-PAGE was used to separate the eluted immunoprecipitated proteins from the culture supernatant, which were then detected by Western blotting analysis.
Protein samples for Western blotting analysis were fractionated on a 10% SDS-PAGE gel and blotted onto a nitrocellulose membrane using electrophoretic transfer cell (Bio-Rad). After blocking with 5% skimmed milk, the membrane was incubated for 2 h at room temperature with the anti-LdTyrRS antibody (1:1000). The membrane was then washed with PBS containing 0.05% Tween 20 (PBS-T) and incubated with the horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (Cell Signaling Technology, catalogue number 7076S; 1:5000). The blot was developed by the enhanced chemiluminescence (ECL®) kit (Amersham Biosciences) according to the manufacturer's protocol.
The quantification of LdTyrRS in the immunoblot was carried out as described earlier (59, 60). Briefly, different concentrations of rLdTyrRS were analyzed using anti-LdTyrRS antibody by Western blotting analysis. Band intensities for rLdTyrRS were measured densitometrically. A standard curve of protein load versus band intensity was then produced (data not shown). The band intensity of LdTyrRS from the parasite lysate was calibrated against the standard curve generated for the recombinant protein. 30 ng of LdTyrRS was estimated to be present in ∼106 parasites. In our present set of experiments with mammalian cells, the minimum quantity of LdTyrRS used was 6 ng, which is equivalent to the amount of protein present in ∼2 × 105 Leishmania parasites. Because the experimental infections in mice are done using 105-107 Leishmania parasites (49, 61), it would appear that the amount of LdTyrRS (6 ng) used is within the physiological range.
Chemotaxis Assays
For the neutrophil chemotactic assay, mice neutrophils were isolated with a Ficoll-Paque (Sigma) centrifugation method, as previously described (62). The final preparation consisted of >98% neutrophils, as determined by Wright-Giemsa staining. The cells were resuspended in chemotaxis media (RPMI 1640 containing 10% FBS) at a concentration of 5 × 106 cells/ml. 200 μl of this cell suspension (1 × 106 cells) was plated in the upper well of a 24-well transwell Boyden chemotaxis plate (Costar). Recombinant LdTyrRS (0.1 nm or 6 ng), fMLP (10−8 m), and mutant LdTyrRS protein (ELR/AAA) (0.1 nm or 6 ng) were diluted in 600 μl of the chemotaxis medium and placed in the lower well. The filled chemotaxis transwell plates were then incubated at 37 °C in a humidified CO2 incubator for 4 h. After the incubation, non-migratory cells on the upper surface of the filter were removed by wiping with a cotton swab. The number of neutrophils that migrated into the lower chamber was determined. For quantification, the myeloperoxidase activity of neutrophils was measured as previously described (63). Briefly, neutrophils sedimented by light centrifugation were solubilized in 0.1% Triton X-100. To this lysate, 300 μl of the o-dianisidine solution containing 0.003% sodium perborate was added. The color of the reaction was developed for 30 min and terminated by the addition of 50 μl of 0.5 n HCl. The absorbance of the reaction was measured at 405 nm. The number of neutrophils that migrated to the lower chamber was calculated from a standard curve obtained with the lysates of a known number of neutrophils. Migration was plotted either as the mean number of neutrophils migrated in the lower chamber or as migration index (MI = number of migrating cells in each condition/number of migrating cells in basal medium).
Cell Binding Assays
In vitro binding of the rLdTyrRS to mouse macrophages was performed by immunofluorescence analysis using standard protocols. The murine macrophage cell line J774 was cultured on coverslips in 6-well plates and maintained in RPMI 1640 medium containing 10% FBS. Recombinant LdTyrRS (0.1 nm or 6 ng) and mutant LdTyrRS protein (0.1 nm or 6 ng) (ELR/AAA) were incubated with the cells for 2 h at 4 °C. The cells were then washed and incubated with 2% paraformaldehyde (Sigma) for 15 min. Following this, the primary (anti-LdTyrRS, 1:1000) and fluorescently conjugated secondary (Thermo Fisher Scientific catalogue number A-11071, 1:3000) antibodies were added. Fluorescence microscopy was performed with confocal laser scanning microscopy (Olympus FluoViewTM FV1000 with objective lenses PLAPON ×60 O, NA-1.42).
In vitro binding of the rLdTyrRS to J774 cells was also analyzed by fluorescence-activated cell sorting (FACS) using standard protocols. Analytical flow cytometry was performed by indirect staining of the macrophages with the anti-LdTyrRS antibody (1:1000) and the fluorescently conjugated secondary antibodies (Thermo Fisher Scientific, catalogue number A-11071; 1:3000). The data obtained on BD FACS Calibur (BD Biosciences) were analyzed using CellQuest software.
Cytokine Secretion Assays
The concentrations of IL-6, TNF-α, IL-12, IFN-γ, and IL-10 in culture supernatants were determined by ELISA. Briefly, J774 murine macrophages (5 × 105) were cultured in RPMI 1640 medium with 10% FBS in 24-well plates. LPS, rLdTyrRS, and control proteins (rLdTyrRS mutant protein (ELR/AAA) and rLdLeuRS) were added to the cells at different concentrations. After 6, 12, and 24 h, the culture supernatants were collected, serially diluted, and the cytokine concentrations were quantified by ELISA. The assay was performed using a BD Pharmingen Opt EIA kit according to the manufacturer's instructions. To remove endotoxin contamination, recombinant proteins (rLdTyrRS, rLdTyrRS mutant protein (ELR/AAA), and rLdLeuRS) were passed through polymyxin beads (Sigma) before the assay. To confirm that the proteins were endotoxin-free, a LAL (limulus amebocyte lysate) assay (Thermo Fisher Scientific catalogue number 88282) was performed.
LdTyrRS Interaction with Murine Macrophages
J774 murine macrophages (5 × 105) were cultured with rLdTyrRS in the absence or presence of different antibodies (anti-LdTyrRS or anti-CXCR2). Binding of rLdTyrRS was then tested by using a standard FACS protocol as described above. In related experiments, the cytokine profiling of these murine macrophages was also done. The cytokines released in the culture supernatants were detected by ELISA using commercially available kits according to the manufacturer's protocol (BD Pharmingen Opt EIA).
Statistical Analysis
Results for the aminoacylation activity, parasitemia, and the neutrophil migration index were entered as column data in GraphPad Prism and analyzed using Student's t test. Results for cytokine analysis were entered as grouped data in GraphPad Prism 5 and analyzed by two-way analysis of variance followed by Tukey's multiple comparison post test. The data are represented as mean ± S.D. A p value of < 0.05 was accepted as an indication of statistical significance.
Author Contributions
S. A. conducted the experiments. R. M. B. designed the study, supervised the experiments, and edited the manuscript with contributions from S. A. Both the authors reviewed the manuscript.
Acknowledgments
We thank the Central Instrumentation Facility at the School of Life Sciences, Jawaharlal Nehru University, for MALDI-TOF analysis and for providing the imaging facility. We are grateful to Dr. Chandrima Saha for providing the Leishmania mitochondrial tryparedoxin peroxidise (mTXNPX) antibody.
This work was supported in part by Department of Biotechnology, Government of India Grant 102/IFD/SAN/3321/2014–15 (to R. M.). The authors declare that they have no conflicts of interest with the contents of this article.
- aaRS
- aminoacyl-tRNA synthetases
- TyrRS
- tyrosyl-tRNA synthetase
- LdTyrRS
- L. donovani TyrRS
- tRNATyr
- tyrosyl-tRNATyr
- EMAPII
- endothelial monocyte activating polypeptide II domain
- fMLP
- formylmethionyl-leucyl-phenylalanine
- ELR/AAA
- mutant LdTyrRS protein in which ELR motif has been mutated to AAA
- rLdLeuRS
- recombinant L. donovani leucyl-tRNA synthetase.
References
- 1. Guo M., and Schimmel P. (2013) Essential nontranslational functions of tRNA synthetases. Nat. Chem. Biol. 9, 145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park S. G., Ewalt K. L., and Kim S. (2005) Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers. Trends Biochem. Sci. 30, 569–574 [DOI] [PubMed] [Google Scholar]
- 3. Guo M., Yang X. L., and Schimmel P. (2010) New functions of aminoacyl-tRNA synthetases beyond translation. Nat. Rev. Mol. Cell Biol. 11, 668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wakasugi K., and Schimmel P. (1999) Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 284, 147–151 [DOI] [PubMed] [Google Scholar]
- 5. Wakasugi K., and Schimmel P. (1999) Highly differentiated motifs responsible for two cytokine activities of a split human tRNA synthetase. J. Biol. Chem. 274, 23155–23159 [DOI] [PubMed] [Google Scholar]
- 6. Strieter R. M., Polverini P. J., Kunkel S. L., Arenberg D. A., Burdick M. D., Kasper J., Dzuiba J., Van Damme J., Walz A., and Marriott D. (1995) The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 270, 27348–27357 [DOI] [PubMed] [Google Scholar]
- 7. Addison C. L., Daniel T. O., Burdick M. D., Liu H., Ehlert J. E., Xue Y. Y., Buechi L., Walz A., Richmond A., and Strieter R. M. (2000) The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J. Immunol. 165, 5269–5277 [DOI] [PubMed] [Google Scholar]
- 8. Stillie R., Farooq S. M., Gordon J. R., and Stadnyk A. W. (2009) The functional significance behind expressing two IL-8 receptor types on PMN. J. Leukoc. Biol. 86, 529–543 [DOI] [PubMed] [Google Scholar]
- 9. Graves D. T., and Jiang Y. (1995) Chemokines, a family of chemotactic cytokines. Crit. Rev. Oral Biol. Med. 6, 109–118 [DOI] [PubMed] [Google Scholar]
- 10. Kaye P., and Scott P. (2011) Leishmaniasis: complexity at the host-pathogen interface. Nat. Rev. Microbiol. 9, 604–615 [DOI] [PubMed] [Google Scholar]
- 11. Cecílio P., Pérez-Cabezas B., Santarém N., Maciel J., Rodrigues V., and Cordeiro da Silva A. (2014) Deception and manipulation: the arms of Leishmania, a successful parasite. Front. Immunol. 5, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Croft S. L., Sundar S., and Fairlamb A. H. (2006) Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19, 111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larson E. T., Kim J. E., Castaneda L. J., Napuli A. J., Zhang Z., Fan E., Zucker F. H., Verlinde C. L., Buckner F. S., Van Voorhis W. C., Hol W. G., and Merritt E. A. (2011) The double-length tyrosyl-tRNA synthetase from the eukaryote Leishmania major forms an intrinsically asymmetric pseudo-dimer. J. Mol. Biol. 409, 159–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gowri V. S., Ghosh I., Sharma A., and Madhubala R. (2012) Unusual domain architecture of aminoacyl tRNA synthetases and their paralogs from Leishmania major. BMC Genomics 13, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baggiolini M., and Clark-Lewis I. (1992) Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 307, 97–101 [DOI] [PubMed] [Google Scholar]
- 16. Jia J., Li B., Jin Y., and Wang D. (2003) Expression, purification, and characterization of human tyrosyl-tRNA synthetase. Protein Expr. Purif. 27, 104–108 [DOI] [PubMed] [Google Scholar]
- 17. Kijima S., Ohta T., and Imahori K. (1968) Purification and characterization of tyrosyl-RNA synthetase from baker's yeast. J. Biochem. 63, 434–445 [DOI] [PubMed] [Google Scholar]
- 18. Cestari I., Kalidas S., Monnerat S., Anupama A., Phillips M. A., and Stuart K. (2013) A multiple aminoacyl-tRNA synthetase complex that enhances tRNA-aminoacylation in African trypanosomes. Mol. Cell. Biol. 33, 4872–4888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sajish M., and Schimmel P. (2015) A human tRNA synthetase is a potent PARP1-activating effector target for resveratrol. Nature 519, 370–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silverman J. M., Chan S. K., Robinson D. P., Dwyer D. M., Nandan D., Foster L. J., and Reiner N. E. (2008) Proteomic analysis of the secretome of Leishmania donovani. Genome Biol. 9, R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gadelha F. R., Gonçalves C. C., Mattos E. C., Alves M. J., Piñeyro M. D., Robello C., and Peloso E. F. (2013) Release of the cytosolic tryparedoxin peroxidase into the incubation medium and a different profile of cytosolic and mitochondrial peroxiredoxin expression in H2O2-treated Trypanosoma cruzi tissue culture-derived trypomastigotes. Exp. Parasitol. 133, 287–293 [DOI] [PubMed] [Google Scholar]
- 22. Laskay T., van Zandbergen G., and Solbach W. (2003) Neutrophil granulocytes: trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 11, 210–214 [DOI] [PubMed] [Google Scholar]
- 23. Chandrasekar B., Melby P. C., Sarau H. M., Raveendran M., Perla R. P., Marelli-Berg F. M., Dulin N. O., and Singh I. S. (2003) Chemokine-cytokine cross-talk. The ELR+ CXC chemokine LIX (CXCL5) amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase-NF-κB pathway. J. Biol. Chem. 278, 4675–4686 [DOI] [PubMed] [Google Scholar]
- 24. Bhatt T. K., Khan S., Dwivedi V. P., Banday M. M., Sharma A., Chandele A., Camacho N., Ribas de Pouplana L., Wu Y., Craig A. G., Mikkonen A. T., Maier A. G., Yogavel M., and Sharma A. (2011) Malaria parasite tyrosyl-tRNA synthetase secretion triggers pro-inflammatory responses. Nat. Commun. 2, 530. [DOI] [PubMed] [Google Scholar]
- 25. Gupta G., Bhattacharjee S., Bhattacharyya S., Bhattacharya P., Adhikari A., Mukherjee A., Bhattacharyya Majumdar S., and Majumdar S. (2009) CXC chemokine-mediated protection against visceral leishmaniasis: involvement of the proinflammatory response. J. Infect. Dis. 200, 1300–1310 [DOI] [PubMed] [Google Scholar]
- 26. Kane M. M., and Mosser D. M. (2001) The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166, 1141–1147 [DOI] [PubMed] [Google Scholar]
- 27. Gupta G., Oghumu S., and Satoskar A. R. (2013) Mechanisms of immune evasion in leishmaniasis. Adv. Appl. Microbiol. 82, 155–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matte C., and Olivier M. (2002) Leishmania-induced cellular recruitment during the early inflammatory response: modulation of proinflammatory mediators. J. Infect. Dis. 185, 673–681 [DOI] [PubMed] [Google Scholar]
- 29. Silverman J. M., Clos J., de'Oliveira C. C., Shirvani O., Fang Y., Wang C., Foster L. J., and Reiner N. E. (2010) An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell Sci. 123, 842–852 [DOI] [PubMed] [Google Scholar]
- 30. Brown M. V., Reader J. S., and Tzima E. (2010) Mammalian aminoacyl-tRNA synthetases: cell signaling functions of the protein translation machinery. Vascul. Pharmacol. 52, 21–26 [DOI] [PubMed] [Google Scholar]
- 31. Wakasugi K., Slike B. M., Hood J., Otani A., Ewalt K. L., Friedlander M., Cheresh D. A., and Schimmel P. (2002) A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 99, 173–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park S. G., Kim H. J., Min Y. H., Choi E. C., Shin Y. K., Park B. J., Lee S. W., and Kim S. (2005) Human lysyl-tRNA synthetase is secreted to trigger proinflammatory response. Proc. Natl. Acad. Sci. U.S.A. 102, 6356–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castro de Moura M., Miro F., Han J. M., Kim S., Celada A., and Ribas de Pouplana L. (2011) Entamoeba lysyl-tRNA synthetase contains a cytokine-like domain with chemokine activity towards human endothelial cells. PLoS Negl. Trop. Dis. 5, e1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lambertz U., Silverman J. M., Nandan D., McMaster W. R., Clos J., Foster L. J., and Reiner N. E. (2012) Secreted virulence factors and immune evasion in visceral leishmaniasis. J. Leukoc. Biol. 91, 887–899 [DOI] [PubMed] [Google Scholar]
- 35. van Zandbergen G., Hermann N., Laufs H., Solbach W., and Laskay T. (2002) Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit γ interferon-inducible protein 10 production by neutrophil granulocytes. Infect. Immun. 70, 4177–4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laufs H., Müller K., Fleischer J., Reiling N., Jahnke N., Jensenius J. C., Solbach W., and Laskay T. (2002) Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infect. Immun. 70, 826–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aga E., Katschinski D. M., van Zandbergen G., Laufs H., Hansen B., Müller K., Solbach W., and Laskay T. (2002) Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 169, 898–905 [DOI] [PubMed] [Google Scholar]
- 38. Arena A., Capozza A. B., Delfino D., and Iannello D. (1997) Production of TNF α and interleukin 6 by differentiated U937 cells infected with Leishmania major. New Microbiol. 20, 233–240 [PubMed] [Google Scholar]
- 39. Wenzel U. A., Bank E., Florian C., Förster S., Zimara N., Steinacker J., Klinger M., Reiling N., Ritter U., and van Zandbergen G. (2012) Leishmania major parasite stage-dependent host cell invasion and immune evasion. FASEB J. 26, 29–39 [DOI] [PubMed] [Google Scholar]
- 40. Arango Duque G., Fukuda M., Turco S. J., Stäger S., and Descoteaux A. (2014) Leishmania promastigotes induce cytokine secretion in macrophages through the degradation of synaptotagmin XI. J. Immunol. 193, 2363–2372 [DOI] [PubMed] [Google Scholar]
- 41. Karam M. C., Hamdan H. G., Abi Chedid N. A., Bodman-Smith K. B., Eales-Reynolds L. J., and Baroody G. M. (2006) Leishmania major: low infection dose causes short-lived hyperalgesia and cytokines upregulation in mice. Exp. Parasitol. 113, 168–173 [DOI] [PubMed] [Google Scholar]
- 42. Griffin G. K., Newton G., Tarrio M. L., Bu D. X., Maganto-Garcia E., Azcutia V., Alcaide P., Grabie N., Luscinskas F. W., Croce K. J., and Lichtman A. H. (2012) IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J. Immunol. 188, 6287–6299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fielding C. A., McLoughlin R. M., McLeod L., Colmont C. S., Najdovska M., Grail D., Ernst M., Jones S. A., Topley N., and Jenkins B. J. (2008) IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J. Immunol. 181, 2189–2195 [DOI] [PubMed] [Google Scholar]
- 44. Vieira S. M., Lemos H. P., Grespan R., Napimoga M. H., Dal-Secco D., Freitas A., Cunha T. M., Verri W. A. Jr., Souza-Junior D. A., Jamur M. C., Fernandes K. S., Oliver C., Silva J. S., Teixeira M. M., and Cunha F. Q. (2009) A crucial role for TNF-α in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5. Br. J. Pharmacol. 158, 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ribeiro-Gomes F. L., and Sacks D. (2012) The influence of early neutrophil-Leishmania interactions on the host immune response to infection. Front. Cell Infect. Microbiol. 2, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peters N. C., Egen J. G., Secundino N., Debrabant A., Kimblin N., Kamhawi S., Lawyer P., Fay M. P., Germain R. N., and Sacks D. (2008) In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321, 970–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ribeiro-Gomes F. L., Roma E. H., Carneiro M. B., Doria N. A., Sacks D. L., and Peters N. C. (2014) Site-dependent recruitment of inflammatory cells determines the effective dose of Leishmania major. Infect. Immun. 82, 2713–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hurrell B. P., Regli I. B., and Tacchini-Cottier F. (2016) Different Leishmania species drive distinct neutrophil functions. Trends Parasitol. 32, 392–401 [DOI] [PubMed] [Google Scholar]
- 49. Hurrell B. P., Schuster S., Grün E., Coutaz M., Williams R. A., Held W., Malissen B., Malissen M., Yousefi S., Simon H. U., Müller A. J., and Tacchini-Cottier F. (2015) Rapid Sequestration of Leishmania mexicana by neutrophils contributes to the development of chronic lesion. PLoS Pathog. 11, e1004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thalhofer C. J., Chen Y., Sudan B., Love-Homan L., and Wilson M. E. (2011)) Leukocytes infiltrate the skin and draining lymph nodes in response to the protozoan Leishmania infantum chagasi. Infect. Immun. 79, 108–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mittra B., Cortez M., Haydock A., Ramasamy G., Myler P. J., and Andrews N. W. (2013) Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. J. Exp. Med. 210, 401–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Debrabant A., Joshi M. B., Pimenta P. F., and Dwyer D. M. (2004) Generation of Leishmania donovani axenic amastigotes: their growth and biological characteristics. Int. J. Parasitol. 34, 205–217 [DOI] [PubMed] [Google Scholar]
- 53. Cestari I., and Stuart K. (2013) A spectrophotometric assay for quantitative measurement of aminoacyl-tRNA synthetase activity. J. Biomol. Screen. 18, 490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Darveau A., Pelletier A., and Perreault J. (1995) PCR-mediated synthesis of chimeric molecules. Methods Neurosci. 26, 77–85 [Google Scholar]
- 55. Kapler G. M., Coburn C. M., and Beverley S. M. (1990) Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol. Cell. Biol. 10, 1084–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mosmann T. (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 [DOI] [PubMed] [Google Scholar]
- 57. Kansal S., Tandon R., Dwivedi P., Misra P., Verma P. R., Dube A., and Mishra P. R. (2012) Development of nanocapsules bearing doxorubicin for macrophage targeting through the phosphatidylserine ligand: a system for intervention in visceral leishmaniasis. J. Antimicrob. Chemother. 67, 2650–2660 [DOI] [PubMed] [Google Scholar]
- 58. McCall L. I., and Matlashewski G. (2010) Localization and induction of the A2 virulence factor in Leishmania: evidence that A2 is a stress response protein. Mol. Microbiol. 77, 518–530 [DOI] [PubMed] [Google Scholar]
- 59. Krobitsch S., Brandau S., Hoyer C., Schmetz C., Hübel A., and Clos J. (1998) Leishmania donovani heat shock protein 100: characterization and function in amastigote stage differentiation. J. Biol. Chem. 273, 6488–6494 [DOI] [PubMed] [Google Scholar]
- 60. Taylor S. C., and Posch A. (2014) The design of a quantitative Western blot experiment. Biomed. Res. Int. 2014, 361590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rolão N., Melo C., and Campino L. (2004) Influence of the inoculation route in BALB/c mice infected by Leishmania infantum. Acta Trop. 90, 123–126 [DOI] [PubMed] [Google Scholar]
- 62. Luo Y., and Dorf M. E. (2001) Isolation of mouse neutrophils. Curr. Protoc. Immunol. Chapter 3, Unit 3 20 [DOI] [PubMed] [Google Scholar]
- 63. Kawa S., Kimura S., Hakomori S., and Igarashi Y. (1997) Inhibition of chemotactic motility and trans-endothelial migration of human neutrophils by sphingosine 1-phosphate. FEBS Lett. 420, 196–200 [DOI] [PubMed] [Google Scholar]