Abstract
Larynx may alternatively serve as a target or organs at risk (OAR) in head and neck cancer (HNC) image‐guided radiotherapy (IGRT). The objective of this study was to estimate IGRT parameters required for larynx positional error independent of isocentric alignment and suggest population‐based compensatory margins. Ten HNC patients receiving radiotherapy (RT) with daily CT on‐rails imaging were assessed. Seven landmark points were placed on each daily scan. Taking the most superior‐anterior point of the C5 vertebra as a reference isocenter for each scan, residual displacement vectors to the other six points were calculated postisocentric alignment. Subsequently, using the first scan as a reference, the magnitude of vector differences for all six points for all scans over the course of treatment was calculated. Residual systematic and random error and the necessary compensatory CTV‐to‐PTV and OAR‐to‐PRV margins were calculated, using both observational cohort data and a bootstrap‐resampled population estimator. The grand mean displacements for all anatomical points was 5.07 mm, with mean systematic error of 1.1 mm and mean random setup error of 2.63 mm, while bootstrapped POIs grand mean displacement was 5.09 mm, with mean systematic error of 1.23 mm and mean random setup error of 2.61 mm. Required margin for CTV‐PTV expansion was 4.6 mm for all cohort points, while the bootstrap estimator of the equivalent margin was 4.9 mm. The calculated OAR‐to‐PRV expansion for the observed residual setup error was 2.7 mm and bootstrap estimated expansion of 2.9 mm. We conclude that the interfractional larynx setup error is a significant source of RT setup/delivery error in HNC, both when the larynx is considered as a CTV or OAR. We estimate the need for a uniform expansion of 5 mm to compensate for setup error if the larynx is a target, or 3 mm if the larynx is an OAR, when using a nonlaryngeal bony isocenter.
PACS numbers: 87.55.D‐, 87.55.Qr
Keywords: image‐guided radiotherapy, head and neck radiotherapy, interfractional larynx setup error, quality assurance, target volume and organs‐at‐risk margins
I. INTRODUCTION
While intensity‐modulated radiotherapy (IMRT) has led to the ability to deliver highly conformal radiotherapy (RT) doses, a major limitation in sparing normal tissues while delivering tumoricidal doses to target volumes, after target delineation, is setup error. 1 , 2 Conceptually, in ICRU62 (3) and ICRU 83, (4) the planning target volume (PTV) and planning organs‐at‐risk volume (PRV) account for this setup error and ensure that precision target delineation does not result in either a geometric miss nor inadvertent normal tissue overdose. 5 , 6 However, a significant limitation of most current image‐guided radiation therapy (IGRT) systems is their reliance on a single point reference for corrective setup translations. For example, use of a single isocenter for portal imaging or an index slice or contour for cone‐beam CT data (7) has consequences in the head and neck, where target structures or organs at risk (OARs) are not necessarily fixed to bony landmarks and experience translational motion during delivery of radiation. 8 , 9 , 10 Consequently, despite excellent setup, TV/OAR displacement from the isocenter may occur in a directionally distinct manner. (11) Recently, there has been increased interest in efforts to spare the carotid arteries from significant dose for early‐stage laryngeal cancer. 12 , 13 , 14 To this end, IMRT is therapeutically justified, owing to the fact that these cancers are, by and large, highly curable (15) and long‐term toxicity, therefore, becomes a significant consideration in patients with potential decades of survival. Additionally, our group and others have adopted strategies to minimize laryngeal doses for nonlaryngeal head and neck cancers when a low neck match cannot be utilized practically. (16) Such approaches are beneficial for organs like larynx where a defined planning organ at risk volume (PRV) margin might be of possible value for plan optimization as it is well documented that laryngeal overdose results in quantifiable toxicity. (17)
While masks can serve to reduce patient external motion during treatment, internal target/organ movement is an unavoidable reality that must be considered, as well, for treatment accuracy. However, it is imperative that the use of IMRT does not result in inadvertent geometric miss which may in aggregate reduce survival probability. For this reason, we sought to ascertain the relative geometric variation in the motion of the larynx relative to a single isocenter (defined as a bony landmark) in order to ensure that our current radiotherapy margins are within evidence‐based limits.
The specific aims of the current study are:
-
1)
estimation of the relative interfraction setup error of the laryngeal apparatus relative to a fixed isocenter, using both experimentally observed CT on‐rails data and robust estimators of population setup error using a bootstrap methodology;
-
2)
determination of the PTV expansions required for laryngeal‐targeting radiotherapy for larynx cancers; and
-
3)
estimation of PRV expansions required for laryngeal sparing‐radiotherapy for nonlaryngeal head and neck cancers
II. MATERIALS AND METHODS
Daily DICOM RT from a series of ten patients previously enrolled on an adaptive RT study 18 , 19 were de‐archived after IRB approval. Daily noncontrast CT on‐rails (350 mm FOV, voxel dimensions) 20 , 21 scans were acquired, as detailed previously, 18 , 19 and imported into a treatment planning system (Pinnacle; Philips Healthcare, Andover, MA). For each daily CT on‐rails DICOM 3D image, the C5 vertebra and thyroid cartilage were identified and seven reference points were manually placed as a point of interest (POI) at the superior‐most voxel of the anterior aspect of the C5 vertebrae, the superior‐most and inferior‐most voxels of the anterior aspect of thyroid cartilage, as well as the superior‐most and inferior‐most voxels of the most lateral aspect of the right and left thyroid cartilage cornua (see Fig. 1). The selection of larynx six POIs was based on the fact that thyroid cartilage is the largest laryngeal cartilage that forms the external framework of the larynx and houses its structural components with strong attachments. The selected landmark points shape a three‐dimensional framework of the upper‐ and lower‐most boundaries of the thyroid cartilage in the midline and bilaterally to best represent laryngeal motion.
Figure 1.
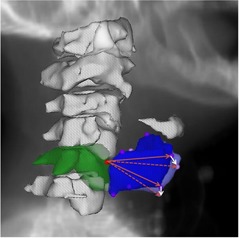
Visual depiction of the selected points of interest (POIs) with the red circle at the most superior aspect of the anterior C5 vertebra (3D reconstructed contour in green) representing the fixed isocenteric reference and the violet circles represent selected POIs of the thyroid cartilage on Day 1 (3D reconstructed contour in blue), while the pink circles represent the same POIs on Day 2 (not all POIs are visible because of the overlap) but in different spatial location caused by laryngeal inter‐fraction motion. White arrows show example of vector displacements of two POIs relative to their original position in relation to the fixed isocenter of Day 1 (solid red arrow) in Day two (dashed red arrow).
The most superior–anterior point of the C5 vertebra was defined a priori as a fixed origin for each daily scan. On each daily CT on‐rails scan, vector displacements were obtained to the other six reference POIs relative to this origin. All such vectors were brought to the same origin and, as such, the vectors represent the motion of the larynx relative to this origin. Conversely, the larynx represented by the six‐point structure, represents a three‐dimensional registered object moving relative to a fixed point. This isolates laryngeal motion changes relative to a point in the bony anatomy independent of errors in patient setup. The geometry is the same in every CT and any setup changes would result in only translational or rotational shifts, which would be negated by the use of vectors. Assuming each patient's initial verification (Day 1) scan was treated as a “gold standard” reference, vector differences between the initial verification scan and each daily CT on‐rails scan was calculated serially. The magnitudes of these vector differences were collected, representing the daily shifts of the entire larynx relative to a fixed point from a planned setup, and as a measure of the intrinsic movement of the larynx (i.e., the motion of the larynx despite immobilization and changes in day‐to‐day controlled setup) (see Fig. 1).
The mean magnitude of vector displacement over all days was calculated for each patient at each POI. From these mean values, a grand means was calculated at each point to characterize the cumulative displacement for each POI. The systematic error for the population was defined as the standard deviation of the grand mean. The random error for each individual was determined to be the standard deviation about an individual's mean value for each vector, while the population random error is given by the root mean square of individual random errors (see Fig. A1). The results from these calculations, the observational cohort systematic error () and the random error (), were used to calculate the necessary CTV‐to‐PTV correction sufficient for 90% of patients to receive 95% of the nominal dose for each POI, using Van Herk's formula: (22)
Figure Fig. A1.
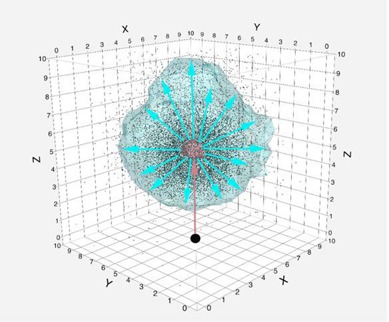
Three‐dimensional scatterplot illustrating the difference between the systemic errors of representative points of interest distribution from a starting point (black circle) presented as a red cloud and the random errors represented as a light blue cloud. The appropriate CTV‐PTV and OAR‐PRV margins accounts for both the systematic and random error component.
| (1) |
Additionally, using this data, the necessary PRV is calculated based on work by McKenzie et al. (23) and is calculated as:
| (2) |
To calculate robust nonparametric estimates for inference, bootstrap resampling was applied using Efron's bootstrap methodology. Using an iterative resampling/replacement method, a cumulative 1,000 random distributions for each POI, was drawn from each individual patient's distribution of daily shifts using the original 1854 individual experimentally derived daily POI displacement measures. The resultant resampled distributions (i.e., ) were then used to generate a robust systematic error () and random error () for robust probabilistic estimation of the population‐level magnitude of larynx interfractional motion at each POI. Likewise, a 95% tolerance interval (i.e., a 95% confidence interval of a range encompassing 95% of all displacements) was derived as an estimator of the internal target volume for both cohort and bootstrap distributions (95% and 95% ).
III. RESULTS
A total of 309 daily CT on‐rails DICOM images were utilized for all ten patients, an average of 31 scans per patient, with a minimum of 25 scans and a maximum of 35 daily scans. Mean observational cohort () displacement for each anatomic POI ranged from 4.77 to 5.30 mm, with a grand mean of 5.07 mm over‐and‐above the correction for bony landmark setup error among all points. Calculated systematic error for all POIs ranged between 1.01–1.38 mm, with a mean of 1.1 mm across all points, and random error of 2.48–2.87 mm with a mean of 2.63 mm across all points. For all sites, the bootstrapped POI mean displacement ranged from 4.85–5.35 mm, with a grand bootstrapped POI mean displacement of 5.09 mm. Figure 2 illustrates the difference in POI vector displacement distribution probability between the studied cohort and its bootstrap resampling.
Figure 2.
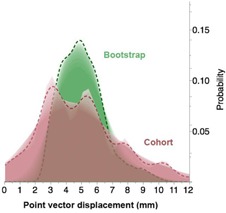
Shadowgram showing the difference in distribution probability of points of interest vector displacement over treatment time between the studied cohort and its bootstrap resampling.
Calculated for all POIs ranged between 1.06–1.46 mm, with a mean of 1.23 mm across all resampled points, and of 2.45–2.85 mm with a mean of 2.61 mm (Table 1).
Table 1.
Statistical data corresponding to individual POIs for the studied cohort and the bootstrap validation
| Point | Mean, Cohort Vector Displacement (mm) | Mean, Bootstrapped Vector Displacement (mm) | Σ, Cohort Systematic Error (mm) | σ, Cohort Random Error (mm) | Σ, Bootstrapped Systematic Error (mm) | σ, Bootstrapped Random Error (mm) |
|---|---|---|---|---|---|---|
| Most Superior Aspect of the Anterior Thyroid Cartilage | 4.77 | 4.85 | 1.17 | 2.59 | 1.24 | 2.57 |
| Most Inferior Aspect of Anterior Thyroid Cartilage | 4.97 | 5.01 | 1.01 | 2.65 | 1.06 | 2.62 |
| Most Superior Aspect of Left Superior Cornu of Left Thyroid Cartilage | 5.16 | 5.10 | 1.1 | 2.48 | 1.20 | 2.46 |
| Most Inferior Aspect of Left Inferior Cornu of Thyroid Cartilage | 5.09 | 5.10 | 1.23 | 2.48 | 1.30 | 2.45 |
| Most Superior Aspect of Right Superior Cornu of Thyroid Cartilage | 5.3 | 5.35 | 1.38 | 2.87 | 1.46 | 2.85 |
| Most Inferior Aspect of Right Inferior Cornu of Thyroid Cartilage | 5.08 | 5.13 | 1.07 | 2.73 | 1.13 | 2.69 |
For the observed patient cohort, the one‐sided upper limit ensuring 95% coverage of all residual displacements () was 10.18 mm, while the equivalent bootstrap‐estimated population limit () dropped to 7.52 mm (Fig. 3).
Figure 3.
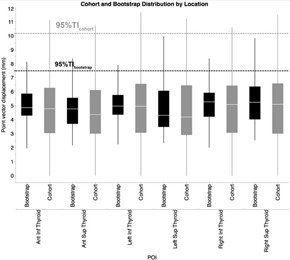
Distributional boxplot of geometric vector displacement of cohort POIs and its bootstrap validation. Pale line within the box indicates median value, while the box limits indicat the 25th and 75th percentiles. The lines represent the 10th and 90th percentiles, and the horizontal dotted lines represent the 95% TI.
Using van Herk's formula (22) (Eq. (1)) for the observed cohort, the margin required for CTV‐ to‐PTV expansion to ensure 90% population coverage with 95% of prescribed dose () was 4.6 mm over‐and‐above the correction for bony landmark setup error, while the calculated bootstrap estimator of the equivalent requisite coverage margin () was 4.9 mm. Moreover, using McKenzie formula (23) (Eq. (2)), the calculated OAR‐to‐PRV expansion for the observed residual setup error () was 2.7 mm, closely approximating the bootstrap estimated expansion over all POIs () of 2.9 mm.
IV. DISCUSSION
The goal of in‐room, image‐guided radiotherapy (IGRT) is to improve treatment delivery via a reduction of PTV volumes by imaging patients before or during treatment. Consequently, high‐frequency head and neck IGRT may be used as a feedback mechanism, ensuring accuracy of patient setup and providing an opportunity to adjust the PTV 24 , 25 or PRV (26) to account for institutionally dependent setup error. 27 , 28
In HNC, the accuracy of RT is of extreme importance owing to the close proximity of many OARs to the target volumes; the larynx, as either a target (PTV) or avoidance (PRV) structure is no exception. Our efforts herein define evidence‐based, institutional margins for cases when the laryngeal apparatus is a target (i.e., larynx cancer) 12 , 13 , 14 , 29 and define reasonable preplanning PRV margins to avoid beam‐path toxicities when laryngeal sparing is desired 30 , 31 (i.e., oropharynx/oral cavity cancers receiving elective neck radiation when a low‐neck match approach is not feasible). 16 , 32
In HNC treatment, immobilization masks are used to minimize interfractional variation in patient setup and motion during radiation delivery. The amount of error in daily setup in an immobilized patient has been studied previously 33 , 34 , 35 and CTV‐PTV corrections necessary have been suggested previously using a variety of IGRT devices. 7 , 36 Interestingly, while studies have been performed to determine the effect that setup error and the movement of a patient as a whole can have on RT accuracy in HNCs, there has been a lack of analysis of the effects of independent TV/OAR/ROI motion, although this data is beginning to emerge. 9 , 21 , 26 , 27 , 29
In head and neck cancers, the larynx provides an ideal model for nonisocentric treatment planning and patient setup as it potentially has large translational displacements relative to bony landmarks due to its flexible attachments. Additionally, the cartilaginous structures of the larynx are capable, on some level, of deformation throughout treatment, thus necessitating the use of multiple points in the assessment of this study. As an illustration, when serial daily CT scans are concatenated in movie format (see Appendix A) the concomitant effect of bony and laryngeal setup error is better appreciated. With traditional patient setup and immobilization, the large, dynamic changes in the laryngeal apparatus, as seen in Appendix A, are not appreciated or accounted for with daily isocentric alignment to rigid structures. Furthermore, as highly conformal approaches become the standard of practice, IGRT implementation can further improve outcomes through the direct assessment of interfractional laryngeal variability and laryngeal motion during radiation delivery. For example, at our institution, the entire larynx is routinely treated in cases with locally advanced disease; evaluation of kilovoltage portal radiography allows alignment of laryngeal structures directly rather than solely relying on bony landmarks — a feat that would have proven difficult in the megavoltage portal imaging era. Additionally, volumetric approaches, including CT on rails or cone‐beam CT, may be utilized with similar purpose. When using the larynx in toto for positional alignment, the proposed margins may be utilized directly for CTV‐PTV expansion, whereas with a nonlarynx‐based reference (e.g., bony landmark), the PTV margins require superimposition for isocentric alignment of the reference structure. Our data show that, utilization of IMRT for early stage larynx cancers (14) and emerging interest in even more aggressive and technical IGRT strategies, necessitate the need to mitigate intrafractional setup error 38 , 39 , 40 and thus require excellent geometric accuracy throughout the setup and delivery of treatment. For instance, CT on rails has impressive isocentric alignment performance characteristics (reported by Shiu et al. (41) with directional error and cumulative isocentric error when aligning to spinal structures).
In nonlaryngeal HNC, our data are equally important because OARs require dosimetric boundaries, as well, to ensure that overdosage does not occur. This is conceptually represented as a PRV. (42) At our institution, a low‐neck match is used whenever feasible; 16 , 32 , 43 however, alignment of the isocenter for the IMRT field must still account for potential laryngeal setup error during treatment in order to ensure that unanticipated dose overlap does not occur. Several authors have demonstrated that extraneous, but modifiable, laryngeal dose is associated with significant acute and long‐term toxicities. 44 , 45 Institutions using a full neck IMRT strategy would be prudent to consider PRV margination with magnitudes comparable to those listed, in order to ensure attempts at organ sparing are effectively realized.
Our data suggest that a population‐based CTV‐PTV margin of 5 mm reasonably accounts for larynx motion if the larynx is a target structure, or 3 mm if it is planned as a PRV before dose calculation (i.e., preoptimization), using established margination recipes. 22 , 23 These adjustments sufficiently account for geometric error associated of the laryngeal apparatus during setup and treatment execution, ensuring precise delivery of the prescribed dose for all measured values. Our study used a postisocentric alignment, suggesting that if a bony reference isocenter is used, an additional margin is required. However, if the laryngeal anatomy itself is used as alignment reference, the use of a bony (C5) isocenter may be obviated. Institutionally we now align directly to laryngeal structures.
There are several caveats inherent in our data. First, this series represents a limited number of patients from a single institution with serial imaging for predominately oropharyngeal cancers. Furthermore, our exclusive use of daily CT on rails does not allow for evaluation of intrafractional respiratory or swallowing motion associated with the larynx 40 , 46 , 47 which may require additional margination, and which we and other groups are currently investigating. The use of bootstrap resampling serves as a corrective for our limited sample size, as the large number of iterative daily measurements (, 1854 total measurements) and the large resampling run (, per POI) allows robust inferential estimation, at least for similar populations. The bootstrap distribution (and resultant systematic, random error, and confidence intervals) are designed to represent a large‐scale population from which the sampled experimental set is potentially drawn. Consequently, as expected some differences exist (as the resampled population central parameters will be of more utility, as compared to experimental cohort data), but on the whole, the magnitude of difference between bootstrap and experimental cohort for systematic and random error was for all measures (exceedingly small). This, in fact, suggests our presented experimental data largely would reflect any given head and neck cancer patient larynx motion distribution that might be seen in a significant patient cohort.
This is, however, to our knowledge, the first study to utilize diagnostic quality imaging via daily CT on rails to evaluate interfractional motion of the larynx. It serves as a model study to evaluate interfractional organ motion for delivery of IGRT, as well as a benchmark for institutional IGRT margination recipes.
V. CONCLUSIONS
In conclusion, interfractional larynx setup error is a source of significant potential geometric error, even after pristine isocentric alignment, in HNC treated with radiation therapy, both when the larynx is treated as a target (e.g., larynx primaries) or as a normal tissue avoidance structure (e.g., oropharyngeal cancer). We estimate the need for a uniform CTV‐to‐PTV expansion of approximately 5 mm to compensate for daily isocenter‐independent setup error if the larynx is a target, or an OAR‐to‐PRV margin of 3 mm if the larynx is an OAR, when using a nonlaryngeal isocenter with comparable immobilization platforms.
ACKNOWLEDGMENTS
Dr. Abdallah Mohamed acknowledges the support by a UICC American Cancer Society Beginning Investigators Fellowship funded by the American Cancer Society.
Dr. Fuller received/receives grant support from: the National Institutes of Health/National Cancer Institute's Paul Calabresi Clinical Oncology Award Program (K12 CA088084‐06) and Clinician Scientist Loan Repayment Program (L30 CA136381‐02); the National Institutes of Health Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center; the SWOG/Hope Foundation Dr. Charles A. Coltman, Jr., Fellowship in Clinical Trials; a General Electric Healthcare/MD Anderson Center for Advanced Biomedical Imaging In‐Kind Award: an Elekta AB/MD Anderson Department of Radiation Oncology Seed Grant; the Center for Radiation Oncology Research at MD Anderson Cancer Center. These listed funders/supporters played no role in the study design, collection, analysis, interpretation of data, manuscript writing, or decision to submit the report for publication.
APPENDICES
Appendix A: Dynamic daily changes in the laryngeal apparatus position after isocentric alignment
(view the movie, uploaded to www.jacmp.org as Supplementary Material).
Supporting information
Supplementary Material
REFERENCES
- 1. van Herk M, Remeijer P, Lebesque JV. Inclusion of geometric uncertainties in treatment plan evaluation. Int J Radiat Oncol Biol Phys. 2002;52(5):1407–22. [DOI] [PubMed] [Google Scholar]
- 2. Miszczyk L, Tarnawski R, Skladowski K. The impact of delivered dose errors on local control of irradiated advanced laryngeal cancer. Neoplasma. 2000;47(2):133–36. [PubMed] [Google Scholar]
- 3. Prescribing, recording, and reporting photon beam therapy (supplement to ICRU Report 50). ICRU Report 62. Bethesda, MD: ICRU; 1999. [Google Scholar]
- 4. Prescribing, recording, and reporting photon‐beam intensity‐modulated radiation therapy (IMRT): contents. J ICRU. 2010;10(1). [Google Scholar]
- 5. Purdy JA. Current ICRU definitions of volumes: limitations and future directions. Sem Radiat Oncol. 2004;14(1):27–40. [DOI] [PubMed] [Google Scholar]
- 6. van Herk M. Errors and margins in radiotherapy. Sem Radiat Oncol. 2004;14(1):52–64. [DOI] [PubMed] [Google Scholar]
- 7. Fuller CD, Scarbrough TJ, Sonke JJ, et al. Method comparison of automated matching software‐assisted cone‐beam CT and stereoscopic kilovoltage x‐ray positional verification image‐guided radiation therapy for head and neck cancer: a prospective analysis. Phys Med Biol. 2009;54(24):7401–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dionisi F, Palazzi MF, Bracco F, et al. Set‐up errors and planning target volume margins in head and neck cancer radiotherapy: a clinical study of image guidance with on‐line cone‐beam computed tomography. Int J Clin Oncol. 2013;18(3):418–27. [DOI] [PubMed] [Google Scholar]
- 9. van Kranen S, van Beek S, Rasch C, van Herk M, Sonke JJ. Setup uncertainties of anatomical sub‐regions in head‐and‐neck cancer patients after offline CBCT guidance. Int J Radiat Oncol Biol Phys. 2009;73(5):1566–73. [DOI] [PubMed] [Google Scholar]
- 10. Zhang L, Garden AS, Lo J, et al. Multiple regions‐of‐interest analysis of setup uncertainties for head‐and‐neck cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64(5):1559–69. [DOI] [PubMed] [Google Scholar]
- 11. Ove R, Cavalieri R, Noble D, Russo SM. Variation of neck position with image‐guided radiotherapy for head and neck cancer. Am J Clin Oncol. 2012;35(1):1–5. [DOI] [PubMed] [Google Scholar]
- 12. Camingue P, Christian R, Ng D, Williams P, Amin M, Roniger DL. Comparison of external beam treatment techniques for T1‐2, N0, M0 glottic cancers. Med Dosim. 2012;37(2):221–24. [DOI] [PubMed] [Google Scholar]
- 13. Chera BS, Amdur RJ, Morris CG, Mendenhall WM. Carotid‐sparing intensity‐modulated radiotherapy for early‐stage squamous cell carcinoma of the true vocal cord. Int J Radiat Oncol Biol Phys. 2010;77(5):1380–85. [DOI] [PubMed] [Google Scholar]
- 14. Rosenthal DI, Fuller CD, Barker JL Jr, et al. Simple carotid‐sparing intensity‐modulated radiotherapy technique and preliminary experience for T1‐2 glottic cancer. Int J Radiat Oncol Biol Phys. 2010;77(2):455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mendenhall WM, Werning JW, Hinerman RW, Amdur RJ, Villaret DB. Management of T1‐T2 glottic carcinomas. Cancer. 2004;100(9):1786–92. [DOI] [PubMed] [Google Scholar]
- 16. Dabaja B, Salehpour MR, Rosen I, et al. Intensity‐modulated radiation therapy (IMRT) of cancers of the head and neck: comparison of split‐field and whole‐field techniques. Int J Radiat Oncol Biol Phys. 2005;63(4):1000–05. [DOI] [PubMed] [Google Scholar]
- 17. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts for swallowing complications after intensity‐modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110–18. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz DL, Garden AS, Shah SJ, et al. Adaptive radiotherapy for head and neck cancer–dosimetric results from a prospective clinical trial. Radiother Oncol. 2013;106(1):80–84. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head‐and‐neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83(3):986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barker JL Jr, Garden AS, Ang KK, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head‐and‐neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59(4):960–70. [DOI] [PubMed] [Google Scholar]
- 21. Neubauer E, Dong L, Followill DS, et al. Assessment of shoulder position variation and its impact on IMRT and VMAT doses for head and neck cancer. Radiat Oncol. 2012;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: dose‐population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47(4):1121–35. [DOI] [PubMed] [Google Scholar]
- 23. McKenzie A, van Herk M, Mijnheer B. Margins for geometric uncertainty around organs at risk in radiotherapy. Radiother Oncol. 2002;62(3):299–307. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki M, Nishimura Y, Nakamatsu K, et al. Analysis of interfractional set‐up errors and intrafractional organ motions during IMRT for head and neck tumors to define an appropriate planning target volume (PTV)‐ and planning organs at risk volume (PRV)‐margins. Radiother Oncol. 2006;78(3):283–90. [DOI] [PubMed] [Google Scholar]
- 25. Astreinidou E, Bel A, Raaijmakers CP, Terhaard CH, Lagendijk JJ. Adequate margins for random setup uncertainties in head‐and‐neck IMRT. Int J Radiat Oncol Biol Phys. 2005;61(3):938–44. [DOI] [PubMed] [Google Scholar]
- 26. Delana A, Menegotti L, Bolner A, et al. Impact of residual setup error on parotid gland dose in intensity‐modulated radiation therapy with or without planning organ‐at‐risk margin. Strahlenther Onkol. 2009;185(7):453–59. [DOI] [PubMed] [Google Scholar]
- 27. Yang J, Garden AS, Zhang Y, Zhang L, Dong L. Variable planning margin approach to account for locoregional variations in setup uncertainties. Med Phys. 2012;39(8):5136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lozano EM, Perez LA, Torres J, et al. Correction of systematic set‐up error in breast and head and neck irradiation through a no‐action level (NAL) protocol. Clin Transl Oncol. 2011;13(1):34–42. [DOI] [PubMed] [Google Scholar]
- 29. Gomez D, Cahlon O, Mechalakos J, Lee N. An investigation of intensity‐modulated radiation therapy versus conventional two‐dimensional and 3D‐conformal radiation therapy for early stage larynx cancer. Radiat Oncol. 2010;5:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosenthal DI, Chambers MS, Fuller CD, et al. Beam path toxicities to non‐target structures during intensity‐modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;72(3):747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long‐term swallowing dysfunction after oropharyngeal intensity‐modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amdur RJ, Liu C, Li J, Mendenhall W, Hinerman R. Matching intensity‐modulated radiation therapy to an anterior low neck field. Int J Radiat Oncol Biol Phys. 2007;69(2 Suppl):S46–S48. [DOI] [PubMed] [Google Scholar]
- 33. Velec M, Waldron JN, O'Sullivan B, et al. Cone‐beam CT assessment of interfraction and intrafraction setup error of two head‐and‐neck cancer thermoplastic masks Int J Radiat Oncol Biol Phys. 2010;76(3):949–55. [DOI] [PubMed] [Google Scholar]
- 34. Rotondo RL, Sultanem K, Lavoie I, Skelly J, Raymond L. Comparison of repositioning accuracy of two commercially available immobilization systems for treatment of head‐and‐neck tumors using simulation computed tomography imaging. Int J Radiat Oncol Biol Phys. 2008;70(5):1389–96. [DOI] [PubMed] [Google Scholar]
- 35. Boda‐Heggemann J, Walter C, Rahn A, et al. Repositioning accuracy of two different mask systems‐3D revisited: comparison using true 3D/3D matching with cone‐beam CT. Int J Radiat Oncol Biol Phys. 2006;66(5):1568–75. [DOI] [PubMed] [Google Scholar]
- 36. Li H, Zhu XR, Zhang L, et al. Comparison of 2D radiographic images and 3D cone beam computed tomography for positioning head‐and‐neck radiotherapy patients. Int J Radiat Oncol Biol Phys. 2008;71(3):916–25. [DOI] [PubMed] [Google Scholar]
- 37. Hamming‐Vrieze O, van Kranen SR, van Beek S, et al. Evaluation of tumor shape variability in head‐and‐neck cancer patients over the course of radiation therapy using implanted gold markers. Int J Radiat Oncol Biol Phys. 2012;84(2):e201–07. [DOI] [PubMed] [Google Scholar]
- 38. Levendag PC, Teguh DN, Keskin‐Cambay F, et al. Single vocal cord irradiation: a competitive treatment strategy in early glottic cancer. Radiother Oncol. 2011;101(3):415–19. [DOI] [PubMed] [Google Scholar]
- 39. Osman SO, Astreinidou E, de Boer HC, et al. IMRT for image‐guided single vocal cord irradiation. Int J Radiat Oncol Biol Phys. 2012;82(2):989–97. [DOI] [PubMed] [Google Scholar]
- 40. Osman SO, de Boer HC, Astreinidou E, Gangsaas A, Heijmen BJ, Levendag PC. On‐line cone beam CT image guidance for vocal cord tumor targeting. Radiother Oncol. 2009;93(1):8–13. [DOI] [PubMed] [Google Scholar]
- 41. Shiu AS, Chang EL, Ye JS, et al. Near simultaneous computed tomography image‐guided stereotactic spinal radiotherapy: an emerging paradigm for achieving true stereotaxy. Int J Radiat Oncol Biol Phys. 2003;57(3):605–13. [DOI] [PubMed] [Google Scholar]
- 42. Manning MA, Wu Q, Cardinale RM, et al. The effect of setup uncertainty on normal tissue sparing with IMRT for head‐and‐neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(5):1400–09. [DOI] [PubMed] [Google Scholar]
- 43. Chen J, Shrieve DC, Hitchcock YJ. Comparison of cervical esophagus dose‐volumes for three radiotherapy techniques for head and neck cancer. Radiother Oncol. 2008;87(2):274–80. [DOI] [PubMed] [Google Scholar]
- 44. Amin N, Reddy K, Westerly D, Raben D, DeWitt P, Chen C. Sparing the larynx and esophageal inlet expedites feeding tube removal in patients with stage III‐IV oropharyngeal squamous cell carcinoma treated with intensity‐modulated radiotherapy. Laryngoscope. 2012;122(12):2736–42. [DOI] [PubMed] [Google Scholar]
- 45. Eisbruch A, Kim HM, Feng FY, et al. Chemo‐IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys. 2011;81(3):e93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Osman SO, de Boer HC, Heijmen BJ, Levendag PC. Four‐dimensional CT analysis of vocal cords mobility for highly focused single vocal cord irradiation. Radiother Oncol. 2008;89(1):19–27. [DOI] [PubMed] [Google Scholar]
- 47. Bradley JA, Paulson ES, Ahunbay E, Schultz C, Li XA, Wang D. Dynamic MRI analysis of tumor and organ motion during rest and deglutition and margin assessment for radiotherapy of head‐and‐neck cancer. Int J Radiat Oncol Biol Phys. 2011;81(5):e803–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


