Abstract
Importance
Marine ω-3 polyunsaturated fatty acids (PUFAs; including eicosapentaenoic acid, docosahexaenoic acid, and docosapentaenoic acid) possess potent immunomodulatory activity and may protect against cancer development. However, evidence relating marine ω-3 PUFAs to colorectal cancer risk remains inconclusive.
Objective
To test the hypothesis that marine ω-3 PUFA intake may be associated with lower risk of colorectal cancer subsets characterized by immune infiltrate.
Design
Prospective cohort study
Setting
Nurses' Health Study (1984-2010) and Health Professionals Follow-up Study (1986-2010)
Participants
Among 173,229 predominantly white participants, 125,172 provided data about marine ω-3 PUFA intake every 4 years through a validated food frequency questionnaire and followed up for incident colorectal cancer. We documented 614 colorectal cancer cases from which we could assess T-cell infiltration in the tumor microenvironment. Cause-specific Cox proportional hazards regression was used to estimate hazard ratios for risks of colorectal cancer subtypes.
Exposure
Intake of marine ω-3 PUFAs
Main Outcome and Measures
Incidence of colorectal cancer according to CD3+, CD8+, CD45RO (PTPRC)+, or FOXP3+ T-cell densities in tumor tissue, measured by immunohistochemistry and computer-assisted image analysis.
Results
The inverse association of marine ω-3 PUFAs with colorectal cancer risk differed according to FOXP3+ T-cell infiltration (P for heterogeneity = 0.006). Compared to intake of <0.15 g/day of marine ω-3 PUFAs, intake of ≥0.35 g/day was associated with a multivariable hazard ratio of 0.57 (95% confidence interval, 0.40 to 0.81) (P for trend < 0.001) for FOXP3+ T-cell-high tumors. In contrast, the corresponding hazard ratio was 1.14 (95% confidence interval, 0.81 to 1.60) (P for trend = 0.77) for FOXP3+ T-cell-low tumors. No statistically significant differential association was found according to CD3+, CD8+, or CD45RO+ cell density (P for heterogeneity ≥ 0.34). In functional assays, the suppressive activity of regulatory T cells was approximately two-fold lower when pre-incubated with docosahexaenoic acid at 50, 100 and 200 μM than without docosahexaenoic acid (P<0.0001).
Conclusion and Relevance
High marine ω-3 PUFA intake was associated with lower risk of colorectal cancer with high-level, but not low-level, FOXP3+ T-cell density, suggesting a potential role of ω-3 PUFAs in cancer immunoprevention through modulation of regulatory T cells.
Introduction
Substantial experimental evidence demonstrates that omega-3 polyunsaturated fatty acids (ω-3 PUFAs), namely eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA), exert potent anti-inflammatory effects and protect against the development of colorectal cancer (CRC).1 However, human data relating ω-3 PUFA intake to CRC risk remain inconclusive, with a null association reported in all2-10 but one11 prospective study. Recently, we found that the inverse association between intake of marine ω-3 PUFAs and CRC was confined to the 10-15% of tumors with high microsatellite instability (MSI)12 caused by loss of DNA mismatch repair activity.13 These data suggest that the anticancer effects of ω-3 PUFAs may vary by tumor subtypes, and require further characterization. Understanding the mechanisms underlying such differential benefits may lead to more effective precision medicine-based chemopreventive strategies.
The multifaceted roles of marine ω-3 PUFAs in immune regulation have long been recognized, including suppression of T-cell proliferation and promotion of CD4+ T helper type 1 (Th1) cell differentiation.14 Likewise, growing evidence indicates that immune cells in the tumor microenvironment play an active role in cancer evolution.15 A greater lymphocytic reaction to CRC has been associated with better prognosis,16,17 whereas regulatory T (Treg) cells with prerequisite expression of the transcription factor FOXP3 may be supportive to cancer development by reducing the host antitumor immune responses.18-20 Moreover, the immune microenvironment of cancer is uniquely associated with tumor molecular features.15 For example, MSI-high CRC is characterized by high infiltration of activated Th1 cells and altered expression of immunomodulatory and immune checkpoint genes.21-24
Taken together, these data suggest that marine ω-3 PUFAs may exert an antitumor effect through their immunomodulatory activity, and that our previous observations demonstrating a predominant benefit of high marine ω-3 PUFA intake on MSI-high tumor development may be due to differences in tumor-infiltrating immune cells associated with MSI status. Hence, we hypothesized that the inverse association of marine ω-3 PUFA intake with CRC might be stronger for certain immune-subtypes of tumors. To test this hypothesis, we classified CRC cases according to the density of T cells in the tumor microenvironment, and investigated whether the association of prediagnostic marine ω-3 PUFA intake with CRC differed by these subtypes. We then corroborated our findings with experimental studies to examine the mechanisms by which marine ω-3 PUFAs influence T-cell function.
Methods
Study population
Details about the Nurses' Health Study (NHS) (started in 1976, n=121,700 women aged 30-55 years) and Health Professionals Follow-up Study (HPFS) (initiated in 1986, n=51,529 men aged 40-75 years) cohorts have been described elsewhere.25,26 Briefly, participants completed a detailed questionnaire about their medical history and lifestyle at baseline, and every two years thereafter. The response rates have been 95.4% in the NHS and 95.9% in the HPFS for each of the questionnaires though 2010. Dietary data were collected and updated using the food frequency questionnaires (FFQs) every four years. In the present analysis, we used 1984 for the NHS and 1986 for the HPFS as baseline, when we first collected detailed data on ω-3 PUFA intake. Among 81,761 women and 49,938 men who returned the baseline FFQ, we excluded 4,454 women and 1,997 men who had a history of cancer at baseline and 59 women and 17 women who left more than 70 blank responses on the baseline FFQ, had missing information about marine ω-3 PUFA intake, or reported implausible energy intake (<600 or >3500 kcal/day for women, <800 or >4200 kcal/day for men). After exclusions, 77,248 women and 47,924 men were eligible for analysis. We obtained informed consent from all participants. This study was approved by the Institutional Review Board at Brigham and Women's Hospital and Harvard T.H. Chan School of Public Health.
Assessment of marine ω-3 PUFA intake
Detailed description of ω-3 PUFA assessment is provided in the eSupplement.10,27 In each FFQ, we asked participants how often, on average, they consumed each food of a standard portion size during the previous year. We calculated the average daily intake for each nutrient by multiplying the reported frequency of consumption of each item by its nutrient content and then summing across from all foods. We adjusted nutrient intake for total caloric intake using the nutrient residual method.28 Marine ω-3 PUFA intake was calculated by summing EPA, DHA and DPA consumption. Use of fish oil supplement was also assessed and included in the estimation of marine ω-3 PUFA intake. To estimate long-term habitual consumption, we calculated the cumulative average of marine ω-3 PUFA intake during follow-up using all of the preceding dietary measures. FFQs have demonstrated good reproducibility and validity in assessing marine ω-3 PUFA intake,29,30 as described in the eSupplement.
Ascertainment of CRC cases
In both cohorts, cancer diagnoses were reported by participants on the biennial questionnaires. Deaths were reported by family members or the postal system, or identified through a search of the National Death Index. With consent from participants or next of kin, medical records were obtained and reviewed by study physicians, who were blinded to exposure information, to confirm CRC diagnosis and extract information on anatomic location, stage, and histologic type. We retrieved formalin-fixed paraffin-embedded tissue blocks for immunity assessment from hospitals throughout the U.S. where participants had undergone surgical resection. Through 2010, we documented 1,488 CRC cases in the NHS and 1,016 cases in the HPFS. Tissue specimens were successfully collected and assayed for T-cell density measurements from 362 patients in the NHS and 252 patients in the HPFS.
Tumor tissue analyses
Tissue microarrays were constructed to assess the density of CD3+, CD8+, CD45RO (PTPRC)+, and FOXP3+ T cells in tumor tissue, as previously described.31-33 We used immunohistochemistry and an automated scanning microscope and Ariol image analysis system (Genetix, San Jose, CA) to calculate the average density (cells/mm2) of each T-cell marker in tissue microarray cores. We dichotomized cases based on the median density of each T-cell marker among all cases. MSI assessment methods34 are provided in the eSupplement.
In vitro T cell suppression assay
Colonic lamina propria Foxp3+ Treg cells were isolated from C57BL/6J wild-type mice bred and housed in microisolator cages in a barrier facility as previously described35 and pre-incubated for 4h with different concentrations of DHA (cis-4,7,10,13,16,19-docosahexaenoic acid) (Sigma-Aldrich, St. Louis, MO) in complete RPMI 1640 medium containing 0.5% BSA. DHA was removed by washing cells with RPMI medium containing 1% FBS. We employed Cell-Trace™ (Cell-Trace Violet Cell Proliferation kit, Molecular Probes) to monitor distinct generation of proliferating cells by dye dilution in flow cytometry. Isolated CD4+IL2RA- T effector cells were incubated with Cell-Trace™ (5microM/mL) at 37°C for 20 min and washed with pre-warmed RPMI 1640 containing 5% FBS. DHA-treated Treg cells were cultured with Cell-Trace™ stained CD4+IL2RA- T effector (Teff) cells (Treg:Teff=1:4) in the presence of anti-CD3 antibody (5ug/ml) and anti-CD28 antibody (BioLegend, San Diego, CA) (5ug/ml) in 96 well round plates for 48h at 37°C. The proliferation percentage of Teff cells was analyzed by flow cytometry.36 More details about flow cytometry and cell sorting are provided in the eSupplement. Experiments employing mice were approved and carried out in accordance with Harvard Medical School's Standing Committee on Animals and the National Institutes of Health guidelines for animal use.
Statistical analysis
Our primary hypothesis testing was the heterogeneity test between the association of marine ω-3 PUFA intake with lymphocyte-rich CRC compared to that with lymphocyte-poor CRC. All other assessments, including evaluation of individual hazard ratio (HR) estimates, represented secondary analyses. More details about statistical analysis are provided in the eSupplement.
Participants were followed from the age at which the baseline questionnaire was returned until the age at the date of death, CRC diagnosis, loss to follow-up, or end of follow-up (June 1, 2010 for the NHS, January 31, 2010 for the HPFS), whichever came first. For overall CRC risk, we used a time-varying Cox proportional hazards regression model to estimate HR and 95% confidence interval (CI) associated with marine ω-3 PUFA intake. We first performed the analyses in each cohort separately. Because no appreciable difference was detected by cohort (P for heterogeneity=0.13), we then conducted a pooled analysis in the combined cohort.
For subtype-specific CRC risk, we fitted a cause-specific Cox proportional hazards regression model with a duplication method for competing risk data to compute HR and 95% CI.37,38 A heterogeneity test was performed using a likelihood ratio test, by comparing the model in which the association with marine ω-3 PUFAs was allowed to vary by tumor subtypes to a model in which all the associations were held constant. We stratified all analyses by age, sex, and year of questionnaire return. We conducted the multivariable analysis by adjusting for several risk factors of CRC (see footnote of Table 2). Details about covariate assessment are provided in the eSupplement.
Table 2.
Risk of colorectal cancer, overall and by tumor-infiltrating T-cell subset density, according to intake of marine ω-3 polyunsaturated fatty acids in the combined cohorts of the Nurses' Health Study (1984-2010) and Health Professionals Follow-up Study (1986-2010)
| <0.15 g/day | 0.15-0.24 g/day | 0.25-0.34 g/day | ≥0.35 g/day | P for trend* | P for heterogeneity† | ||
|---|---|---|---|---|---|---|---|
| Overall‡ | Person-years | 931,900 | 798,692 | 539,046 | 629,860 | ||
| No. of cases (n=614) | 205 | 188 | 93 | 128 | |||
| Age-adjusted HR (95% CI)§ | 1 (referent) | 1.02 (0.84-1.24) | 0.74 (0.58-0.95) | 0.81 (0.64-1.01) | 0.02 | ||
| Multivariable HR (95% CI)‖ | 1 (referent) | 1.00 (0.82-1.23) | 0.74 (0.57-0.95) | 0.85 (0.67-1.09) | 0.07 | ||
| CD3+ cells | Low | ||||||
| No. of cases (n=301) | 104 | 92 | 49 | 56 | |||
| Age-adjusted HR (95% CI)§ | 1 (referent) | 0.99 (0.75-1.32) | 0.79 (0.56-1.11) | 0.70 (0.50-0.98) | 0.02 | 0.34 | |
| Multivariable HR (95% CI)‖ | 1 (referent) | 0.98 (0.73-1.30) | 0.78 (0.55-1.10) | 0.73 (0.52-1.03) | 0.05 | 0.34 | |
| High | |||||||
| No. of cases (n=302) | 100 | 88 | 43 | 71 | |||
| Age-adjusted HR (95% CI)§ | 1 (referent) | 0.97 (0.73-1.30) | 0.69 (0.48-0.99) | 0.91 (0.66-1.25) | 0.31 | ||
| Multivariable HR (95% CI)‖ | 1 (referent) | 0.95 (0.71-1.28) | 0.68 (0.47-0.98) | 0.95 (0.69-1.32) | 0.49 | ||
| CD8+ cells | Low | ||||||
| No. of cases (n=290) | 99 | 92 | 40 | 59 | |||
| Age-adjusted HR (95% CI)§ | 1 (referent) | 1.02 (0.76-1.35) | 0.69 (0.47-0.99) | 0.82 (0.59-1.15) | 0.10 | 0.89 | |
| Multivariable HR (95% CI)‖ | 1 (referent) | 0.99 (0.75-1.32) | 0.67 (0.46-0.97) | 0.86 (0.61-1.20) | 0.17 | 0.90 | |
| High | |||||||
| No. of cases (n=302) | 101 | 90 | 50 | 61 | |||
| Age-adjusted HR (95% CI)§ | 1 (referent) | 1.00 (0.75-1.33) | 0.80 (0.57-1.12) | 0.75 (0.54-1.04) | 0.05 | ||
| Multivariable HR (95% CI)‖ | 1 (referent) | 0.98 (0.73-1.30) | 0.79 (0.56-1.11) | 0.78 (0.56-1.09) | 0.11 | ||
| CD45RO+ cells | Low | ||||||
| No. of cases (n=310) | 92 | 107 | 42 | 69 | |||
| Age-adjusted HR (95% CI)§ | 1 (referent) | 1.27 (0.96-1.69) | 0.72 (0.50-1.04) | 0.88 (0.64-1.22) | 0.13 | 0.72 | |
| Multivariable HR (95% CI)‖ | 1 (referent) | 1.26 (0.95-1.67) | 0.71 (0.49-1.03) | 0.94 (0.67-1.30) | 0.26 | 0.73 | |
| High | |||||||
| No. of cases (n=304) | 113 | 81 | 51 | 59 | |||
| Age-adjusted HR (95% CI)§ | 1 (referent) | 0.81 (0.61-1.08) | 0.77 (0.55-1.07) | 0.75 (0.54-1.03) | 0.06 | ||
| Multivariable HR (95% CI)‖ | 1 (referent) | 0.80 (0.60-1.06) | 0.77 (0.55-1.08) | 0.79 (0.57-1.10) | 0.13 | ||
| FOXP3+ cells | Low | ||||||
| No. of cases (n=285) | 82 | 92 | 42 | 69 | |||
| Age-adjusted HR (95% CI)§ | 1 (referent) | 1.25 (0.93-1.69) | 0.85 (0.58-1.23) | 1.11 (0.80-1.54) | 0.95 | 0.005 | |
| Multivariable HR (95% CI)‖ | 1 (referent) | 1.21 (0.90-1.64) | 0.82 (0.56-1.20) | 1.14 (0.81-1.60) | 0.77 | 0.006 | |
| High | |||||||
| No. of cases (n=299) | 122 | 80 | 46 | 51 | |||
| Age-adjusted HR (95% CI)§ | 1 (referent) | 0.72 (0.55-0.96) | 0.62 (0.44-0.87) | 0.55 (0.39-0.77) | <0.001 | ||
| Multivariable HR (95% CI)‖ | 1 (referent) | 0.71 (0.53-0.94) | 0.61 (0.43-0.87) | 0.57 (0.40-0.81) | <0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Trend test was performed using the median intake of each category.
Likelihood ratio test was used to compare the model that allows for separate associations for tumors with different subtypes to the model that assumes a common association across subtypes.
Cases without tumor-infiltrating T-cell data were excluded. A few cases with missing data on specific T-cell subsets were excluded for analyses of individual T cells: 11 for CD3+ cells; 22 for CD8+ cells; and 30 for FOXP3+ cells.
Cox proportional hazards model was used with stratification by age, sex (i.e., cohort) and calendar year of current questionnaire cycle.
Additionally adjusted for family history of colorectal cancer, history of endoscopy, pack-years of smoking before age 30 (in women: 0, 0 to <5, and ≥5; in men: 0, >0 to <10, and ≥10), current smoking status, body mass index (continuous), physical activity (in women: 0 to <5, 5 to <11.5, 11.5 to <22, and ≥22 METs/week; in men: 0 to <10, 10 to <22.5, 22.5 to <41.5, and ≥41.5 METs/week), multivitamin use, regular use of aspirin or non-steroidal anti-inflammatory drugs (≥2 tablets/week), alcohol consumption (in women: 0 to <0.15, 0.15 to <2.0, 2.0 to <7.5, and ≥7.5 g/d; in men: 0 to <5, 5 to <10, 10 to <15, 15 to <30, and ≥30 g/d), calcium intake (in quartiles), and Alternative Healthy Eating Index (in quartiles).
Results
Characteristics of study participants
Among 125,172 participants with 2,895,704 person-years of follow-up, we documented 2,504 CRC cases, of which 614 cases had available tissue and data of T-cell density in tumor tissue. As shown in Table 1, participants with higher marine ω-3 PUFA intake were less likely to be current smokers and were more likely to engage in physical activity, undergo endoscopy, use multivitamins, and consumed a healthy diet.
Table 1.
Age-standardized characteristics according to intake of marine ω-3 PUFAs in the Nurses' Health Study (women, 1984-2010) and Health Professionals Follow-up Study (men, 1986-2010)*
| Characteristic | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|
| <0.15 g/day | 0.15-0.24 g/day | 0.25-0.34 g/day | ≥0.35 g/day | <0.15 g/day | 0.15-0.24 g/day | 0.25-0.34 g/day | ≥0.35 g/day | |
| No. of participants | 35,176 | 17,461 | 12,895 | 11,716 | 14,691 | 10,444 | 9,288 | 13,501 |
| Intake of marine ω-3 PUFAs, g/d | 0.08 | 0.19 | 0.29 | 0.62 | 0.08 | 0.20 | 0.29 | 0.68 |
| Age, years | 62.1 | 60.3 | 60.7 | 62.2 | 61.8 | 61.8 | 62.2 | 63.8 |
| Body mass index, kg/m2 | 26.2 | 26.1 | 26.0 | 26.2 | 25.8 | 25.7 | 25.7 | 25.6 |
| Physical activity, MET-hours/wk† | 15.1 | 17.7 | 19.0 | 20.9 | 31.7 | 33.4 | 35.0 | 37.3 |
| Pack-years of smoking before age 30 | 6.9 | 6.9 | 7.0 | 7.1 | 11.3 | 11.0 | 10.8 | 10.9 |
| Current smoking, % | 14 | 13 | 12 | 11 | 7 | 6 | 5 | 4 |
| Colorectal cancer in a parent or sibling, % | 17 | 18 | 17 | 17 | 14 | 14 | 14 | 14 |
| History of previous endoscopy, % | 25 | 26 | 28 | 30 | 23 | 25 | 27 | 29 |
| Current multivitamin use, % | 52 | 54 | 55 | 60 | 48 | 49 | 51 | 56 |
| Regular aspirin or NSAID use, %‡ | 52 | 52 | 51 | 53 | 50 | 50 | 51 | 52 |
| Postmenopausal, % | 81 | 81 | 82 | 82 | ||||
| Current hormone use, %§ | 51 | 52 | 51 | 46 | ||||
| Dietary intake | ||||||||
| ω-3 PUFAs, g/d | 1.08 | 1.21 | 1.32 | 1.72 | 1.22 | 1.35 | 1.46 | 1.92 |
| 18:3ω-3 (ALA), g/d | 0.96 | 0.97 | 0.98 | 1.07 | 1.13 | 1.13 | 1.14 | 1.20 |
| 20:5ω-3 (EPA), mg/d | 21 | 58 | 92 | 235 | 21 | 59 | 94 | 257 |
| 22:5ω-3 (DPA), mg/d | 12 | 18 | 23 | 33 | 13 | 20 | 25 | 40 |
| 22:6ω-3 (DHA), mg/d | 49 | 117 | 175 | 349 | 49 | 118 | 174 | 381 |
| ω-6 PUFAs, g/d | 9.03 | 8.94 | 8.83 | 9.07 | 12.2 | 12.0 | 11.8 | 11.8 |
| Fish, servings/week | 0.8 | 1.8 | 2.4 | 3.7 | 0.7 | 1.6 | 2.1 | 3.9 |
| Total red meat, servings/week | 5.4 | 5.3 | 4.8 | 4.0 | 7.1 | 7.1 | 6.2 | 4.9 |
| Alcohol, g/d | 4.8 | 6.3 | 6.6 | 6.1 | 10.4 | 12.4 | 12.5 | 11.3 |
| Folate, μg/d | 526 | 532 | 558 | 631 | 580 | 588 | 616 | 713 |
| Calcium, mg/d | 1,157 | 1,163 | 1,202 | 1,303 | 983 | 968 | 973 | 1,039 |
| Vitamin D, IU/d | 378 | 408 | 457 | 566 | 370 | 404 | 444 | 580 |
| Total fiber, g/d | 18.1 | 18.8 | 19.5 | 20.4 | 21.7 | 22.2 | 23.0 | 24.7 |
| AHEI score | 44.6 | 46.5 | 48.5 | 51.6 | 38.7 | 41.5 | 43.1 | 46.8 |
Abbreviations: AHEI, Alternative Healthy Eating Index; ALA, α-linolenic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; NSAID, non-steroidal anti-inflammatory drug; PUFA, polyunsaturated fatty acid.
Updated information throughout follow-up was used to calculate the mean for continuous variables and percentage for categorical variables. All variables are age-standardized except age.
Physical activity is represented by the product sum of the metabolic equivalent (MET) of each specific recreational activity and hours spent on that activity per week.
Regular users are defined as ≥2 standard (325-mg) tablets of aspirin or ≥ 2 tablets of NSAIDs per week.
Proportion of current menopausal hormone use is calculated among postmenopausal women only.
Association of marine ω-3 PUFAs with CRC according to tumor-infiltrating T-cell density
Consistent with our previous report,10 marine ω-3 PUFA intake was not statistically significantly associated with CRC risk after adjusting for other risk factors (P for trend=0.07, Table 2), with a HR (95% CI) of 0.85 (0.67 to 1.09) comparing participants with ≥0.35-g/day intake to those with <0.15-g/day intake. Similar results were observed among cases without tumor immunity data (eTable 1). We then classified CRC cases into subtypes with low-level versus high-level infiltrate of each of the four T-cell markers and tested our primary hypothesis that the association between marine ω-3 PUFAs and CRC risk differed according to T-cell densities in tumor tissues. To account for multiple-hypothesis testing, we adjusted the statistical significance level to α=0.012 (≈0.05/4). We found that the beneficial association of high marine ω-3 PUFA intake appeared confined to cancers with high infiltration of FOXP3+ T cells (P for heterogeneity = 0.006). Compared to intake of <0.15 g/day of marine ω-3 PUFAs, intake of ≥0.35 g/day was associated with a multivariable HR of 0.57 (95% CI, 0.40 to 0.81) (P for trend<0.001) for FOXP3+ T-cell-high tumors. In contrast, the corresponding HR was 1.14 (95% CI, 0.81 to 1.60) (P for trend=0.77) for FOXP3+ T-cell-low tumors. Similar heterogeneity was observed for each individual PUFA (EPA, DHA and DPA, eTable 2). We did not observe statistically significant heterogeneity for tumors classified by CD3+, CD8+, or CD45RO+ cells (P for heterogeneity=0.34, 0.90 and 0.73, respectively). We obtained similar results from analyses conducted within each cohort separately (eTables 3-4). We assessed the dose-response relationship using spline analysis and found that marine ω-3 PUFA intake appeared linearly associated with lower risk of FOXP3+ T-cell-high cancer (P=0.001), but not with FOXP3+ T-cell-low cancer (P=0.58). (eFigure 1)
In secondary analyses, given our previous finding that marine ω-3 PUFA intake was inversely associated with MSI-high CRC but not MSS cancer,12 we further classified tumors into four subtypes jointly according to FOXP3+ T-cell infiltration and MSI status. As shown in Table 3, high marine ω-3 PUFA intake appeared to be associated with lower risk of FOXP3+ T-cell-high tumors regardless of MSI status, although the number of MSI-high cases was limited. By comparing T-cell density between MSS and MSI-high tumors, we found that CD45RO+ and FOXP3+ T cells were more enriched in MSI-high than MSS tumors31 (eTable 5).
Table 3.
Risk of colorectal cancer, jointly classified by microsatellite instability and tumor-infiltrating FOXP3+ T-cell density, according to intake of marine ω-3 polyunsaturated fatty acids in the combined cohorts of the Nurses' Health Study (1984-2010) and Health Professionals Follow-up Study (1986-2010)
| Microsatellite instability status | FOXP3+ T-cell density | <0.15 g/day | 0.15-0.24 g/day | 0.25-0.34 g/day | ≥0.35 g/day | P for trend* | P for heterogeneity† |
|---|---|---|---|---|---|---|---|
| Microsatellite stable | FOXP3+ T-cell-low CRC | ||||||
| No. of cases (n=251)§ | 70 | 85 | 37 | 59 | |||
| Age-adjusted HR (95% CI) § | 1 (referent) | 1.36 (0.99-1.87) | 0.87 (0.58-1.30) | 1.10 (0.77-1.58) | 0.95 | 0.01 | |
| Multivariable HR (95% CI) ‖ | 1 (referent) | 1.32 (0.96-1.82) | 0.85 (0.57-1.27) | 1.14 (0.79-1.64) | 0.89 | 0.02 | |
| FOXP3+ T-cell-high CRC | |||||||
| No. of cases (n=230)§ | 91 | 64 | 34 | 41 | |||
| Age-adjusted HR (95% CI) § | 1 (referent) | 0.77 (0.56-1.07) | 0.59 (0.39-0.87) | 0.56 (0.38-0.82) | <0.001 | ||
| Multivariable HR (95% CI) ‖ | 1 (referent) | 0.76 (0.55-1.05) | 0.58 (0.39-0.87) | 0.58 (0.39-0.86) | 0.003 | ||
| Microsatellite instable-high | FOXP3+ T-cell-low CRC | ||||||
| No. of cases (n=30)§ | 12 | 6 | 4 | 8 | |||
| Age-adjusted HR (95% CI) § | 1 (referent) | 0.52 (0.19-1.38) | 0.52 (0.17-1.64) | 0.89 (0.35-2.25) | 0.86 | 0.34 | |
| Multivariable HR (95% CI) ‖ | 1 (referent) | 0.49 (0.18-1.32) | 0.49 (0.16-1.55) | 0.92 (0.36-2.36) | 0.94 | 0.35 | |
| FOXP3+ T-cell-high CRC | |||||||
| No. of cases (n=64)§ | 30 | 13 | 12 | 9 | |||
| Age-adjusted HR (95% CI) § | 1 (referent) | 0.49 (0.25-0.93) | 0.74 (0.38-1.45) | 0.47 (0.22-1.00) | 0.07 | ||
| Multivariable HR (95% CI) ‖ | 1 (referent) | 0.48 (0.25-0.91) | 0.74 (0.37-1.45) | 0.50 (0.23-1.07) | 0.10 |
Abbreviations: CI, confidence interval; CRC, colorectal cancer; HR, hazard ratio.
Trend test was performed using the median intake of each category.
Likelihood ratio test with one degree of freedom was used to compare the model that allows for separate associations for tumors with the same microsatellite instability status but infiltrated with different densities of FOXP3+ T-cells to the model that assumes a common association.
Cases without data on either microsatellite instability or tumor-infiltrating FOXP3+ T-cell density were excluded.
Cox proportional hazards model was used with stratification by age, sex (i.e., cohort) and calendar year of current questionnaire cycle.
Additionally adjusted for family history of colorectal cancer, history of endoscopy, pack-years of smoking before age 30 (in women: 0, 0 to <5, and ≥5; in men: 0, >0 to <10, and ≥10), current smoking status, body mass index (continuous), physical activity (in women: 0 to <5, 5 to <11.5, 11.5 to <22, and ≥22 METs/week; in men: 0 to <10, 10 to <22.5, 22.5 to <41.5, and ≥41.5 METs/week), multivitamin use, regular use of aspirin or non-steroidal anti-inflammatory drugs (≥2 tablets/week), alcohol consumption (in women: 0 to <0.15, 0.15 to <2.0, 2.0 to <7.5, and ≥7.5 g/d; in men: 0 to <5, 5 to <10, 10 to <15, 15 to <30, and ≥30 g/d), calcium intake (in quartiles), and Alternative Healthy Eating Index (in quartiles).
Marine ω-3 PUFAs decrease the T-cell suppressive activity of Treg cells
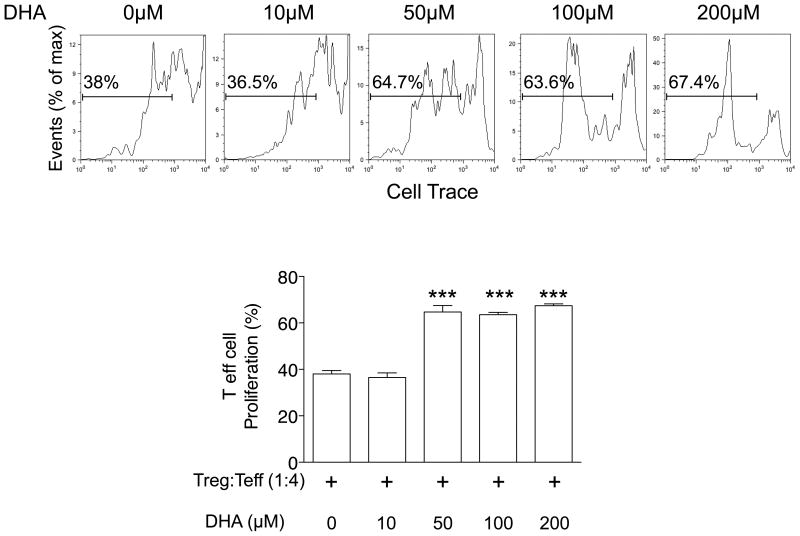
Finally, to investigate the effects of marine ω-3 PUFAs on the T-cell suppressive activity of Foxp3+ Treg cells, we assessed T-cell proliferation by using colonic Treg cells pre-incubated with different concentrations of DHA. We observed that DHA decreased the suppressive capacity of colonic Treg cells on naïve CD4+ Teff-cell proliferation in a dose-dependent manner (Figure 1). The suppressive activity of Treg cells pre-incubated with DHA at 50, 100 and 200 μM was approximately two-fold lower than Treg cells pre-incubated without DHA (P<0.0001).
Figure 1.
Docosahexaenoic acid (DHA) reduces the suppressive activity of colonic Treg cells. Colonic Treg cells were isolated and pre-incubated with different concentration of DHA for 4h. Cell-Trace ™ stained CD4+IL2RA- Teff cells were coincubated for 48h with DHA-treated Treg cells. Representative flow cytometric analyses of Teff cell proliferation are shown in the upper panel. Bar graph in the lower panel shows mean ± SEM of two independent experiments. ***P<0.0001 (unpaired, two-tailed student's t test)
Discussion
In two large prospective cohorts, we found that high intake of marine ω-3 PUFAs was associated with lower risk of FOXP3+ T-cell-high CRC, but not associated with FOXP3+ T-cell-low CRC. This differential association appeared to be independent of MSI status. Consistent with these epidemiologic findings, our in vitro experiment showed that marine ω-3 PUFA DHA reduced the T-cell suppressive activity of colonic Treg cells. Taken together, these data support an antitumor effect of marine ω-3 PUFAs on CRC that is, at least in part, mediated through immune modulation of Treg cells.
Despite compelling experimental evidence supporting an anti-inflammatory and anti-neoplastic effect of marine ω-3 PUFAs, human studies have generally not shown a strong association between high intake of marine ω-3 PUFAs and lower CRC risk.2-10 If marine ω-3 PUFA intake indeed lowers CRC risk, identifying a specific tumor subtype that is prevented by marine ω-3 PUFAs can yield more precise risk reduction, and may lead to an effective prevention strategy against such a tumor subtype.39 Recent studies suggest that marine ω-3 PUFAs are predominantly associated with risk of proximal colon cancer.8,10 Our recent molecular pathological epidemiology study39 showed that high marine ω-3 PUFA intake was primarily associated with lower risk of MSI-high tumors, which predominantly arise from the proximal colon, and thus provided a potential explanation for the previously reported subsite heterogeneity.12 However, a mechanistic explanation for this differential association with MSI-high CRC was unclear. Therefore, our current results substantially extend upon previous findings and provide novel evidence by demonstrating that immune modulation may be a potential mechanism linking marine ω-3 PUFAs to lower CRC risk.
The colon is constantly exposed to a variety of environmental antigens from foods, resident microbiota and pathogens, underscoring the critical importance of the intestinal immune system in maintaining a delicate balance between immunity and tolerance.40 Perturbations of this immune homeostasis can result in chronic inflammation that can in turn increase cancer risk.40 Gut Treg cells tune Teff immune responses, modulate the basal inflammatory tone of the intestine and reset appropriate immune responses for the critical maintenance of intestinal homeostasis.41 Although FOXP3+ Treg cells can dampen aberrant immune responses against the resident gut microbiota or harmless dietary antigens, they may also suppress antitumor immune surveillance and facilitate cancer evasion.19,42 Indeed, a high ratio of FOXP3+ Treg cells and CD3+ T cells has been shown to predict CRC risk.43 The number of FOXP3+ Treg cells is higher in human CRC than in surrounding unaffected mucosa,44-46 and an enhanced infiltration of FOXP3+ Treg cells has been detected in MSI-high CRCs compared to MSS cancers.31,47 Moreover, in mouse models transient ablation of Foxp3+ Treg cells has been shown to decrease colorectal tumor burden through expansion of CD8+ T cells.18 Consistent with these data, a low density of FOXP3+ T cells with a high density of CD8+ T cells in CRC tissue has been associated with a favorable outcome in patients with CRC,48,49 although the independent effect of FOXP3+ Treg cells on CRC prognosis remains inconclusive.17
Marine ω-3 PUFAs exert immunomodulatory effects on T-cell proliferation and apoptosis.50 In this study, we found that DHA decreased the suppressive functions of colonic Treg cells on Teff-cell proliferation. These results are consistent with previous reports that a DHA-enriched diet curtails the suppressive activity of murine splenic Treg cells.36 Collectively, these data support the hypothesis that marine ω-3 PUFAs protect against CRC through inhibition of the T-cell-suppressive activity of Treg cells and therefore suggest the possibility of a strategy in which marine ω-3 PUFAs are used to blunt immunosuppressive Treg cells for the purpose of CRC prevention.51
Our study has several strengths, including the prospective design, repeated dietary assessment, long-term follow-up, and collection of detailed data on a variety of lifestyle risk factors for confounding control. Uniquely, our characterization of immune cells in tumor tissue enabled us to identify the specific cancer subtype that can be potentially prevented by intervention. Finally, our in vitro experiments provide a mechanistic correlate for our human data to support a causal basis for our observations.
The limitations of our study should also be noted. First, dietary assessment using FFQ is subject to measurement error. However, given the prospective design, any measurement error in ω-3 PUFA intake would have likely biased the observed associations toward the null, and would be unlikely to explain the differential associations observed between subtypes of CRC. Second, despite our best efforts, we did not obtain tumor specimens from many patients for immunity evaluation. This is owing to the fact that we began retrieving tumor specimens in 1997 in the HPFS and in 2001 in the NHS. For many cases diagnosed earlier in follow-up, sufficient archived tumor tissue was not available for analysis. However, for this to lead to any bias in our main results, tissue availability would need to be associated with both cancer subtype and marine ω-3 PUFA intake-related CRC risk, which is unlikely. Indeed, as shown in eTable 1, we observed similar associations between marine ω-3 PUFA intake and CRC risk regardless of tissue availability, suggesting that lack of tissue data in some participants is unlikely to cause substantial bias. Third, we are aware of multiple-hypothesis testing which is inherent in tumor subgroup analyses. Hence, we set subtype heterogeneity assessment (rather than evaluation of each subtype stratum) as our primary hypothesis testing, and adjusted statistical significance level by Bonferroni correction. Finally, due to sparse data, some of the HRs were estimated with limited precision and should be interpreted cautiously.
In summary, we have shown that marine ω-3 PUFA intake is associated with lower risk of CRC that arises in the immune microenvironment with high, but not low, infiltration of FOXP3+ T cells. High DHA reduces the T-cell-suppressive activity of Treg cells in the in vitro model. Our data provide evidence that the anticancer effect of marine ω-3 PUFAs may be mediated through enhancing the immune response against cancer via modulation of FOXP3+ Treg cells. Further studies are needed to determine the potential utility of marine ω-3 PUFAs as an immunomodulatory agent for CRC prevention.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by U.S. National Institutes of Health (NIH) grants [P01 CA87969 to M.J. Stampfer; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett; UM1 CA167552 to W.C. Willett; P50 CA127003 to C.S.F.; K24 DK098311, R01 CA137178 to A.T.C.; R01 CA151993 to S.O.; R35 CA197735 to S.O.; and K07 CA190673 to R.N.]; and by grants from The Paula and Russell Agrusa Fund for Colorectal Cancer Research, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. K.M. is supported by a fellowship grant from Uehara Memorial Foundation and a grant from Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers from Japanese Society for the Promotion of Science. Y.M. is supported by a fellowship grant of the Keio Gijuku Fukuzawa Memorial Fund for the Advancement of Education and Research.
We would like to thank the participants and staff of the Nurses' Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Abbreviations
- CI
confidence interval
- CRC
colorectal cancer
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EPA
eicosapentaenoic acid
- FFQ
food frequency questionnaire
- HPFS
Health Professionals Follow-up Study
- HR
hazard ratio
- MSI
microsatellite instability
- MSS
microsatellite stable
- NHS
Nurses' Health Study
- PUFAs
polyunsaturated fatty acids
- Teff
effector T
- Th1
T helper type 1
- Treg
regulatory T
Footnotes
M.S., R.N., Y.C. and E.C. contributed equally. W.S.G., C.S.F., S.O. and A.T.C. contributed equally.
Use of standardized official symbols: We use HUGO (Human Genome Organisation)-approved official symbols for genes and gene products, including CD28, CD4, FOXP3, IL2RA, and PTPRC, all of which are described at www.genenames.org. Gene names are italicized, and gene product names are non-italicized.
Author Contributions: Drs Song and Chan had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: M.S., E. G., W.S.G., C.S.F., S.O., A.T.C.
Acquisition of data: M.S., R.N., Y.C., E.C., K.M., Z.R.Q., K.I., Y.M., J.A.N., K.N., K.W., M.W., E. G., W.S.G., C.S.F., S.O., A.T.C.
Analysis and interpretation of data: M.S., R.N., Y.C., E.C., M.W., E. G., W.S.G., C.S.F., S.O., A.T.C.
Drafting of the manuscript: M.S., E.C.
Critical revision of the manuscript for important intellectual content: R.N., Y.C., E.C., K.M., Z.R.Q., K.I., Y.M., J.A.N., K.N., K.W., M.W., E. G., W.S.G., C.S.F., S.O., A.T.C.
Statistical analysis: M.S., E.C.
Study supervision: S.O., A.T.C.
Role of Funder/Sponsor Statement: The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding acquisition: R.N., C.S.F., S.O., A.T.C.
Administrative, technical, or material support: K.W., E. G., W.S.G., C.S.F., S.O., A.T.C.
Conflict of Interest Disclosures: Andrew T. Chan previously served as a consultant for Bayer Healthcare, Pozen Inc, and Pfizer Inc. for work unrelated to the topic of this manuscript. This study was not funded by Bayer Healthcare, Pozen Inc, or Pfizer Inc. No other conflict of interest exists.
References
- 1.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012 Jan;61(1):135–149. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 2.Terry P, Bergkvist L, Holmberg L, Wolk A. No association between fat and fatty acids intake and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001 Aug;10(8):913–914. [PubMed] [Google Scholar]
- 3.Lin J, Zhang SM, Cook NR, Lee IM, Buring JE. Dietary fat and fatty acids and risk of colorectal cancer in women. Am J Epidemiol. 2004 Nov 15;160(10):1011–1022. doi: 10.1093/aje/kwh319. [DOI] [PubMed] [Google Scholar]
- 4.Oba S, Shimizu N, Nagata C, et al. The relationship between the consumption of meat, fat, and coffee and the risk of colon cancer: a prospective study in Japan. Cancer Lett. 2006 Dec 8;244(2):260–267. doi: 10.1016/j.canlet.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Butler LM, Wang R, Koh WP, Stern MC, Yuan JM, Yu MC. Marine n-3 and saturated fatty acids in relation to risk of colorectal cancer in Singapore Chinese: a prospective study. Int J Cancer. 2009 Feb 1;124(3):678–686. doi: 10.1002/ijc.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murff HJ, Shu XO, Li H, et al. A prospective study of dietary polyunsaturated fatty acids and colorectal cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2009 Aug;18(8):2283–2291. doi: 10.1158/1055-9965.EPI-08-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel CR, McCullough ML, Patel RC, et al. Dietary intake of omega-6 and omega-3 fatty acids and risk of colorectal cancer in a prospective cohort of U.S. men and women. Cancer Epidemiol Biomarkers Prev. 2009 Feb;18(2):516–525. doi: 10.1158/1055-9965.EPI-08-0750. [DOI] [PubMed] [Google Scholar]
- 8.Sasazuki S, Inoue M, Iwasaki M, et al. Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int J Cancer. 2011 Oct 1;129(7):1718–1729. doi: 10.1002/ijc.25802. [DOI] [PubMed] [Google Scholar]
- 9.Kantor ED, Lampe JW, Peters U, Vaughan TL, White E. Long-chain omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer. Nutr Cancer. 2014;66(4):716–727. doi: 10.1080/01635581.2013.804101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song M, Chan AT, Fuchs CS, et al. Dietary intake of fish, omega-3 and omega-6 fatty acids and risk of colorectal cancer: A prospective study in U.S. men and women. Int J Cancer. 2014 Nov 15;135(10):2413–2423. doi: 10.1002/ijc.28878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall MN, Chavarro JE, Lee IM, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2008 May;17(5):1136–1143. doi: 10.1158/1055-9965.EPI-07-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song M, Nishihara R, Wu K, et al. Marine omega-3 polyunsaturated fatty acids and risk of colorectal cancer according to microsatellite instability. J Natl Cancer Inst. 2015 Apr;107(4) doi: 10.1093/jnci/djv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010 Mar;7(3):153–162. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015 Apr;1851(4):469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012 Apr;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 16.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006 Sep 29;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 17.Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014 Mar 18;110(6):1595–1605. doi: 10.1038/bjc.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pastille E, Bardini K, Fleissner D, et al. Transient ablation of regulatory T cells improves antitumor immunity in colitis-associated colon cancer. Cancer Res. 2014 Aug 15;74(16):4258–4269. doi: 10.1158/0008-5472.CAN-13-3065. [DOI] [PubMed] [Google Scholar]
- 19.Betts G, Jones E, Junaid S, et al. Suppression of tumour-specific CD4(+) T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut. 2012 Aug;61(8):1163–1171. doi: 10.1136/gutjnl-2011-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaqub S, Henjum K, Mahic M, et al. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008 Jun;57(6):813–821. doi: 10.1007/s00262-007-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015 Jan;5(1):43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjea A, Ahmed S, Hands RE, et al. Colorectal cancers with microsatellite instability display mRNA expression signatures characteristic of increased immunogenicity. Mol Cancer. 2004 Aug 6;3:21. doi: 10.1186/1476-4598-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Gouvello S, Bastuji-Garin S, Aloulou N, et al. High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut. 2008 Jun;57(6):772–779. doi: 10.1136/gut.2007.123794. [DOI] [PubMed] [Google Scholar]
- 24.di Pietro M, Sabates Bellver J, Menigatti M, et al. Defective DNA mismatch repair determines a characteristic transcriptional profile in proximal colon cancers. Gastroenterology. 2005 Sep;129(3):1047–1059. doi: 10.1053/j.gastro.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991 Aug 24;338(8765):464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 26.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997 Feb;6(1):49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 27.Iso H, Rexrode KM, Stampfer MJ, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. Jama. 2001 Jan 17;285(3):304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 28.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. The American journal of clinical nutrition. 1997 Apr;65(4 Suppl):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S-1231S. [DOI] [PubMed] [Google Scholar]
- 29.Garland M, Sacks FM, Colditz GA, et al. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr. 1998 Jan;67(1):25–30. doi: 10.1093/ajcn/67.1.25. [DOI] [PubMed] [Google Scholar]
- 30.Hunter DJ, Rimm EB, Sacks FM, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol. 1992 Feb 15;135(4):418–427. doi: 10.1093/oxfordjournals.aje.a116302. [DOI] [PubMed] [Google Scholar]
- 31.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010 Dec;222(4):350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007 May 24;356(21):2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 33.Song M, Nishihara R, Wang M, et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut. 2016 Feb;65(2):296–304. doi: 10.1136/gutjnl-2014-308852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009 Jan;58(1):90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013 Aug 2;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yessoufou A, Ple A, Moutairou K, Hichami A, Khan NA. Docosahexaenoic acid reduces suppressive and migratory functions of CD4+CD25+ regulatory T-cells. J Lipid Res. 2009 Dec;50(12):2377–2388. doi: 10.1194/jlr.M900101-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. Journal of the American Statistical Association. 1989;84(408):1065–1073. [Google Scholar]
- 38.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995 Jun;51(2):524–532. [PubMed] [Google Scholar]
- 39.Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011 Mar;60(3):397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013 Nov;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 41.Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014 Mar;14(3):154–165. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 42.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012 May 1;72(9):2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barth SD, Schulze JJ, Kuhn T, et al. Treg-Mediated Immune Tolerance and the Risk of Solid Cancers: Findings From EPIC-Heidelberg. J Natl Cancer Inst. 2015 Nov;107(11) doi: 10.1093/jnci/djv224. [DOI] [PubMed] [Google Scholar]
- 44.Ling KL, Pratap SE, Bates GJ, et al. Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immun. 2007;7:7. [PMC free article] [PubMed] [Google Scholar]
- 45.Girardin A, McCall J, Black MA, et al. Inflammatory and regulatory T cells contribute to a unique immune microenvironment in tumor tissue of colorectal cancer patients. Int J Cancer. 2013 Apr 15;132(8):1842–1850. doi: 10.1002/ijc.27855. [DOI] [PubMed] [Google Scholar]
- 46.Lin YC, Mahalingam J, Chiang JM, et al. Activated but not resting regulatory T cells accumulated in tumor microenvironment and correlated with tumor progression in patients with colorectal cancer. Int J Cancer. 2013 Mar 15;132(6):1341–1350. doi: 10.1002/ijc.27784. [DOI] [PubMed] [Google Scholar]
- 47.Michel S, Benner A, Tariverdian M, et al. High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer. 2008 Dec 2;99(11):1867–1873. doi: 10.1038/sj.bjc.6604756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon HH, Orrock JM, Foster NR, Sargent DJ, Smyrk TC, Sinicrope FA. Prognostic impact of FoxP3+ regulatory T cells in relation to CD8+ T lymphocyte density in human colon carcinomas. PLoS One. 2012;7(8):e42274. doi: 10.1371/journal.pone.0042274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki H, Chikazawa N, Tasaka T, et al. Intratumoral CD8(+) T/FOXP3 (+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother. 2010 May;59(5):653–661. doi: 10.1007/s00262-009-0781-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim W, Khan NA, McMurray DN, Prior IA, Wang N, Chapkin RS. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog Lipid Res. 2010 Jul;49(3):250–261. doi: 10.1016/j.plipres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Philpott M, Ferguson LR. Immunonutrition and cancer. Mutat Res. 2004 Jul 13;551(1-2):29–42. doi: 10.1016/j.mrfmmm.2004.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.