Abstract
During fetal testis development, fetal Leydig cells (FLCs) are found to be originated from multiple progenitor cells. FLC specification and function are under tight regulation of specific genes and signaling proteins. Furthermore, Sertoli cells play a crucial role to regulate FLC differentiation during fetal testis development. FLC progenitor- and FLC-produced biomolecules are also involved in the differentiation and activity of rodent FLCs. The main function of FLCs is to produce androgens to masculinize XY embryos. However, FLCs are capable of producing androstenedione but not testosterone due to the lack of 17β-HSD (17β-hydroxysteroid dehydrogenase), but fetal Sertoli cells express 17β-HSD which thus transforms androstenedione to testosterone in the fetal testis. On the other hand, FLCs produce activin A to regulate Sertoli cell proliferation, and Sertoli cells in turn modulate testis cord expansion. It is now generally accepted that adult Leydig cells (ALCs) gradually replace FLCs during postnatal development to produce testosterone to support spermatogenesis as FLCs undergo degeneration in neonatal and pre-pubertal testes. However, based on studies using genetic tracing mouse models, FLCs are found to persist in adult testes, making up ~20% of total Leydig cells. In this review, we evaluate the latest findings regarding the development, function and fate of FLCs during fetal and adult testis development.
Keywords: Testis, Spermatogenesis, Fetal Leydig cells, Adult Leydig cells, Steroidogenesis
1. Introduction
During embryonic development, Sry gene (Sex-determining region of the Y chromosome) expressed exclusively in pre-Sertoli cells determines the sex of mammalian species [1]. Male hormones produced by fetal Leydig cells (FLCs) and fetal Sertoli cells masculinize the male embryos (for reviews, see [2–4]) (Fig. 1). Leydig cells were first identified in 1850 by Franz Leydig (for a review, see [5]) and the name “Leydig” was coined after him. Subsequent studies have identified two distinct Leydig cell populations namely FLCs and adult Leydig cells (ALCs) which are found in fetal and adult testes, respectively, during testis development in most species (for a review, see [6]). FLCs differentiate in the fetal testes by embryonic day 12.5 (E12.5) to E13.5 in rodents; after birth, FLCs undergo gradual atrophy, also known as involution or degeneration, and being replaced by ALCs in postnatal 2–3 weeks [7,8]. However, FLC atrophy is not an apoptotic process [9], and ALCs do not originate from FLCs [10,11], thus, the fate of FLCs remains controversial for years based on morphological analysis (for a review, see [7]). Recent studies using FLC specific lineage tracing methods have shown that FLCs persist in adult mouse testes as a subpopulation together with ALCs, constituted about ~20% of the total Leydig cell population [10,11]. However, FLCs found in adult testes are HSD17B3 and HSD3B6 negative, and also androgen-independent [11], indicating ALCs are still the only steroidogenic cells capable of producing testosterone in adult testes. FLC differentiation coincides with testis cord formation, and Sertoli cells serve as the command center in organizing testis cord formation during testis-specific architectural comparmentalization (for a review, see [3]). In short, Sertoli cells mediate the specification of other somatic cell types in the developing testis including FLCs. For instance, Sertoli cell-derived secreted proteins (e.g., DHH), mitogens (e.g., PDGFRα), and transcription factors (e.g., WT1) as well as microRNAs (e.g., miR-140-3p/5p) are known to regulate the migration, differentiation, proliferation, growth and/or function of FLC progenitors and FLCs. FLC progenitor- and FLC-derived molecules also self-support the differentiation and activity of FLCs during fetal testis development. One of the main functions of Leydig cells is to produce male sex hormones. In fetal testes, FLCs co-operate with fetal Sertoli cells to produce testosterone via steroidogenesis to support male embryo development [12] (Fig. 1). Herein, we provide a brief review based on in vitro, in vivo studies and also ex vivo rodent models concerning the cytogenesis, function and fate of FLCs. Based on these findings, we also attempt to better understand the function of FLCs by comparing the status of Sertoli cells, germ cells and steroidogenic pattern in fetal, neonatal, pubertal and adult testes in a spatiotemporal manner.
Fig. 1.
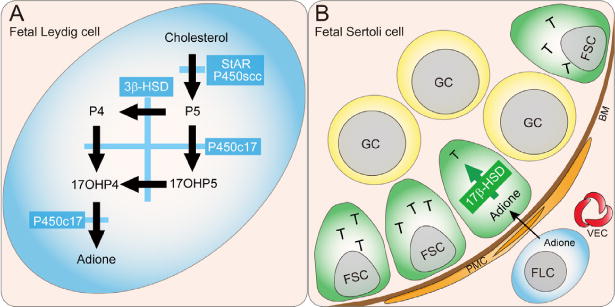
Steroidogenesis in murine fetal testes. (A) A schematic drawing that illustrates the synthetic pathway from cholesterol to androstenedione (adione) in murine fetal Leydig cells (FLCs). Steroidogenic acute regulatory protein (StAR) transfers cholesterol from the outer to the inner mitochondrial membrane, where the enzyme P450 side-chain cleavage (P450scc) resides. Thereafter, pregnenolone (P5) is transferred to smooth endoplasmic reticulum, where androstenedione is synthesized. Reaction 1 mediated by P450scc; reaction 2, 3β-hydroxysteroid dehydrogenase (3β-HSD); and reaction 3, cytochrome P450 17β-hydroxylase (P450c17). (B) The synthetic pathway from androstenedione to testosterone (T) in murine fetal Sertoli cells. Reaction 4, mediated by 17β-hydroxysteroid dehydrogenase (17β-HSD). Since 17β-HSD is not expressed in mouse FLCs but fetal Sertoli cells (FSCs), reaction 4 takes place only in FSCs to produce T in immature mice. P5, pregnenolone; P4, progesterone; 17OHP4, 17β-hydroxyprogesterone; 17OHP5, 17β-hydroxypregnenolone; adione, androstenedione; T, testosterone. GC, germ cell; FSC, fetal Sertoli cell; FLC, fetal Leydig cell; PMC, peritubular myoid cell; AR, androgen receptor; BM, basal membrane; VEC, vascular endothelial cell.
2. FLC cytogenesis
2.1. The origin of FLCs
Due to the lack of specific FLC progenitor markers, the origin of FLCs remains unknown. Nonetheless, steroidogenic cells of the adrenal gland and the gonad are thought to be derived from a common primordium during embryogenesis [13]. Hence early adreno-gonadal precursor cells are proposed to contribute to FLC progenitor population when the genital ridge is formed [13,14]. After sex determination, the male genital ridge begins to differentiate into the testis, which is further compartmentalized into the testis cords and interstitium. The testis interstitium harbors the progenitor pools of steroidogenic FLC lineage and other interstitial somatic cell types such as peritubular myoid cells and fibroblasts. Thus, FLCs originate from the testis interstitial compartment which contains MAFB (v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B) positive cells migrated from the coelomic epithelia [15,16], and MAFB and/or VCAM1 (vascular cell adhesion molecule 1) positive cells from the gonad–mesonephros border [15,17–19]. Other studies also suggest that cells derived from neural crest [20–22], and pericytes (for a review, see [23]) that invade developing testes with coelomic vessel are also the source of FLC progenitors. However, there is no direct evidence supporting the pericyte origination hypothesis, and lineage tracing experiments using mouse models have shown no evidence of neural crest cell contribution to FLC population [24]. Studies also suggest that vasculature invasion in the interstitial space regulates the migration and expansion of interstitial progenitor cells in the developing testis [15,25]. Thus, the vasculature-associated cells likely contribute to the interstitial progenitor pool including FLC progenitors. Additionally, it is possible that in the neural crest cell tracing mouse models, only a portion of the neural crest cells have been marked, and these marked neural crest cells did not contribute to FLC population, or the FLC progenitor cell population which originated from neural crest had already migrated toward genital ridge before the marking and tracing [26,27]. Moreover, using RNA-sequencing bioinformatics approach for analysis, a number of genes involved in neurogenesis/neurotransmission have been shown to be enriched in FLCs [28], supporting the notion that FLCs and neural cells could be originated from the same progenitor cell type. Thus, FLCs are derived from multiple and distinctive progenitor cells from adreno-gonadal primordium, coelomic epithelium, mesonephros, neural crest and early microvessels (for reviews, see [6,7]).
2.2. Differentiation of FLCs
FLC differentiation is known to be regulated strictly by Sertoli cells during fetal testis development (for a review, see [7]) (Fig. 2). Table 1 summarizes Sertoli cell-derived factors that regulate FLC progenitor formation, FLC differentiation and function. Emerging evidence has supported the notion that differentiation and activity of rodent FLCs are also regulated by FLC progenitor-and FLC-produced biomolecules during fetal testis development (Fig. 2). Table 2 summarizes FLC progenitor derived factors that are known to modulate FLC progenitor formation and FLC differentiation. Table 3 summarizes FLC derived factors that modulate FLC function. In a recent study, some FLC lineage markers are found in highly enriched-FLCs of fetal testes by RNA-sequencing analysis which include Tacr3 (Tachykinin receptor 3/neuromedin K receptor, Nk3r), Tac2 (Tachykinin 2), Clca1 (Chloride channel calcium activated 1), Robo2 (Roundabout homolog 2), Prlr (Prolactin receptor), Sox18 [SRY (Sex determining region Y)−box 18], Mc2r (Melanocortin 2 receptor) and Adcy7 (Adenylate cyclase 7) [28]. These genes are also candidates that can modulate the specification and differentiation of FLCs. The hepatocyte growth factor (HGF) is a pleiotropic cytokine known to regulate mouse embryonic organogenesis [29]. HGF is localized in the connective tissue of the interstitial compartment in E18.5 fetal testes, but it is not expressed by FLCs, while its receptor met proto-oncogene (MET, also known as c-MET, a glycoprotein with tyrosine kinase activity) is expressed in FLCs during late fetal testis development. HGF is known to promote FLC terminal development and protect FLCs from apoptosis in fetal testis development [30] (Fig. 2). In this context, it is of interest to note that in studies using organ cultures or mutant mouse models in which fetal testes display the phenotypes that illustrate a lack of FLC differentiation and/or function are also found to have an impairment of testis cord assembly, degeneration of germ cells, disrupted differentiation and/or proliferation of Sertoli cells or peritubular myoid cells, failure of mesonephric cell migration, or defects in vasculature establishment (Table 4). In short, such lack of FLC differentiation and/or function could be the result secondary to the defects described above. In summary, these findings illustrate the pivotal roles of Sertoli cell vs. FLC derived molecules in FLC differentiation. Furthermore, the development of FLC is not an independent process during fetal testis architectural assembly since testis cord formation, Sertoli cell and peritubular myoid cell differentiation and proliferation, germ cell status and male specific-vasculature development also play a role in modulating FLC differentiation.
Fig. 2.
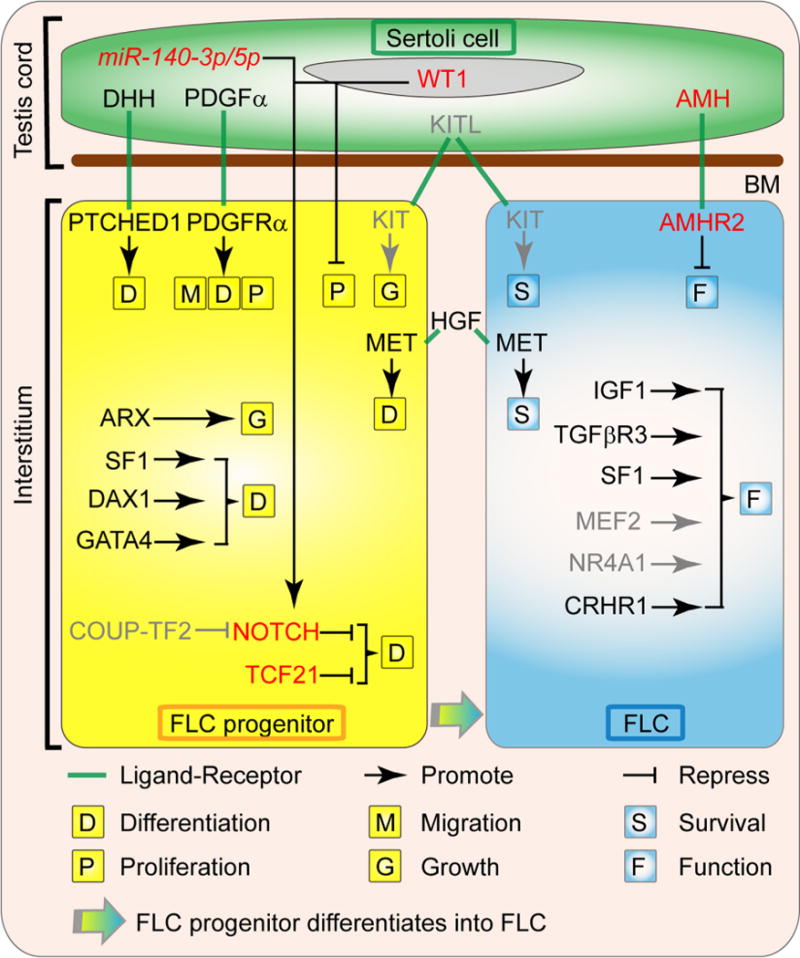
Factors involved in the ontogeny of fetal Leydig cells. The differentiation and steroidogenesis of FLC are regulated by diverse factors derived from Sertoli cells, FLC progenitors, FLCs and other interstitial components. The DHH (Desert hedgehog) and PDGFα (Platelet-derived growth factor A) are proteins secreted by the fetal Sertoli cells that are necessary for FLC ontogeny by inducing the commitment of the FLC progenitors through their receptor PTCH1 (Patched1) and PDGFRα (Platelet-derived growth factor receptor A), respectively. PDGFα also induces the migration and proliferation of FLC progenitors. The KITL (KIT ligand)/KIT (KIT oncogene) signaling is likely to be involved in FLC precursor growth and FLC survival. The HGF (Hepatocyte growth factor), which is localized in the connective tissue of the interstitial compartment, promotes FLC terminal development and protect FLCs from apoptosis. The transcription factor ARX (Aristaless-related homeobox) is essential for the establishment of FLC progenitors. SF1 (Steroidogenic factor 1, also called NR5A1 or AD4BP), DAX1 (dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene 1, also known as NR0B1 or AHCH) and GATA4 (GATA binding protein 4) also promote the differentiation of FLCs, while TCF21 (transcription factor 21, also known as POD1) and NOTCH signal pathway maintain the FLC progenitor pool. Sertoli cell transcription factor WT1 (Wilms’ tumor 1) and mircoRNA miR-140-3p/5p repress FLC differentiation via activating NOTCH signal pathway, while COUP-TF2 (Chicken Ovalbumin Upstream Promoter-Transcription Factor 2, also known as NR2F2) likely promotes FLC differentiation by repressing NOTCH signal pathway. After the establishment of FLC population during fetal testis development, IGF1 (insulin-like growth factor 1), TGFβR3 (Transforming growth factor, beta receptor III, also called betaglycan), SF1, MEF2 (Myocyte enhancer factor 2), NR4A1 (nuclear receptor subfamily 4, group A, member 1, also known as Nur77, NGFI-B, and TR3) and CRHR1 (Corticotropin-releasing hormone receptor 1) regulate the function of FLC, while Sertoli cell factor AMH (anti-Müllerian hormone) is a negative regulator of FLC function. Gray color indicates the regulations are inferred from ALCs. Abbreviations used: FLC, fetal Leydig cell; BM, basal membrane; DHH, Desert hedgehog; PTCH1, Patched1; PDGFα, Platelet-derived growth factor A; PDGFRα, Platelet-derived growth factor receptor A; KITL, KIT ligand; KIT, KIT oncogene; HGF, Hepatocyte growth factor; MET, met proto-oncogene; ARX, Aristaless-related homeobox; SF1, Steroidogenic factor 1; DAX1, dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene 1; GATA4, GATA binding protein 4; TCF21, transcription factor 21; WT1, Wilms’ tumor 1; COUP-TF2, Chicken Ovalbumin Upstream Promoter-Transcription Factor 2; IGF1, insulin-like growth factor 1; TGFβR3, Transforming growth factor, beta receptor III; MEF2, Myocyte enhancer factor 2; NR4A1, nuclear receptor subfamily 4, group A, member 1; CRHR1, Corticotropin-releasing hormone receptor 1; AMH, anti-Müllerian hormone; AMHR2, anti-Mullerian hormone type 2 receptor; miR-140-3p/5p, mircoRNA miR-140-3p/5p.
Table 1.
Sertoli cell-derived factors that regulate FLC progenitor formation and FLC differentiation.
| Factor symbol | Full name of factor | Function |
|---|---|---|
| Promoting factors KITL | KIT ligand, also known as stem cell factor, SCF | Blockade of KITL/KIT (KIT oncogene, also known as c-KIT, a protein tyrosine kinase) pathway in ethylene dimethane sulfonate (EDS)-induced ALC depletion in rat testes accelerates ALC apoptosis and inhibits ALC progenitor proliferation, suggesting that KITL/KIT signaling is involved in ALC survival and ALC progenitor growth [57]. Since KIT is also expressed by FLCs [58], it is likely that the KITL/KIT signaling is crucial to FLC development. |
| DHH | Desert Hedgehog | In Dhh knockout mouse, FLC number is down-regulated without changes in FLC progenitor migration and survival, while ectopically activation of Hedgehog pathway in fetal ovaries results in the transformation of ovarian somatic cells to functional FLCs, indicating DHH/PTCH1 (Patched 1, the receptor of DHH) signal pathway triggers FLC differentiation [59,60]. |
| PDGFα | Platelet-derived growth factor A | Pdgfrα (Platelet-derived growth factor receptor A, the receptor of PDGFα) deletion in fetal mouse leads to defects in mesonephric cell migration and down-regulation of FLC marker expression, indicating that PDGFα/PDGFRα signal pathway is involved in FLC progenitor migration and FLC differentiation [24]. |
| Inhibiting factors | ||
| AMH | Ati-Müllerian hormone, also known as Müllerian inhibiting substance (MIS) or factor (MIF) | AMH inhibits androgen production by dispersed rat FLCs in culture in a dose-dependent manner without affecting FLC number [61], suggesting that AMH serves as a negative modulator of FLC function. |
| WT1 | Wilms’ tumor 1 | Specific inactivation of Wt1 in Sertoli cells in fetal mouse testes using Wt1−/flox; Amh-Cre mice has shown that ALCs fail to replace FLCs in these mutant testes in adult mice, and FLCs remain mitotically active from postnatal day 1 to day 56 [8] through the NOTCH-mediated signaling pathway [62]. Thus, the WT1-NOTCH signaling plays an important role in modulating FLC differentiation during fetal, neonatal and postnatal testis development. |
| miR-140-3p/5p | microRNA miR-140-3p/5p | Analysis of the miR-140-3p/5p-null mouse revealed a significant increase in the number of FLCs in fetal testes and the predicted targeted genes regulated by canonical miR-140-3p/5p are associated with NOTCH signal pathway, suggesting an important role for Sertoli cell miR-140-3p/5p in FLC differentiation via NOTCH signal pathway [63]. |
Table 2.
FLC progenitor-derived factors that regulate FLC progenitor formation and FLC differentiation.
| Factor symbol | Full name of factor | Function |
|---|---|---|
| Promoting factors ARX | X-linked aristaless-related homeobox | In Arx mutant testis, FLC marker expression is severely diminished and FLC numbers largely decrease throughout the fetal life indicating ARX is essential for establishment of FLC progenitor cells [64,65] |
| SF1 | Steroidogenic factor 1, also called NR5A1 or AD4BP | Nr5a1 knockout mice exhibited gonadal agenesis [66]. Rescue of Nr5a1 knockout mice via transgene harboring rat Nr5a1 gene indicates SF1 is needed for FLC differentiation [67]. |
| DAX1 | Dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, also known as NR0B1 or AHCH | FLC differentiation is arrested in Dax1-deficient fetal male mice, suggesting that DAX1 regulates FLC differentiation [68]. |
| GATA4 | GATA binding protein 4 | Gata4-null embryonic stem cells can not differentiate into FLCs [69], and Gata4 mutant mouse testis (at E10.5) diminish FLC markers expression at E15.5 [70]. These phenotypes indicate GATA4 plays a role in modulating FLC differentiation. |
| COUP-TF2 | Chicken Ovalbumin Upstream Promoter-Transcription Factor 2, also known as NR2F2 | Ablation of Nr2f2 at E18.5 or prepubertal mouse testis results in failure of ALC progenitor formation or ALC differentiation via repressing NOTCH signal pathway [71,72]. Since COUP-TF2 is also expressed in fetal rat testis interstitium [73], COUP-TF2 likely regulated the FLC differentiation. |
| Inhibiting factors | ||
| NOTCH | Notch | Loss of Jag1 (Jagged1, ligand of NOTCH) or Hes1 (Hairy/Enhancer-of-split 1, downstream target gene of NOTCH) in fetal testes leads to FLC differentiation, whereas gain of the NOTCH function maintains interstitial mesenchymal progenitor cells and restricts FLC differentiation. Thus, JAG1/NOTCH signaling balance the population of progenitors and FLCs by repressing FLC differentiation [74,75]. |
| TCF21 | Transcription factor 21, also known as POD1 | In Tcf21 deficient fetal mouse testes, FLC number is up-regulated and SF1 expression is also elevated [76], implying TCF21 is involved in maintaining the FLC progenitor pool. |
Table 3.
FLC derived factors that regulate FLC function.
| Factor symbol | Full name of factor | Function |
|---|---|---|
| SF1 | Steroidogenic factor 1, also called NR5A1 or AD4BP | SF1 increases the expression of testosterone biosynthesis associated genes Cytochrome P450 steroid hydroxylases (including P450scc and P450c17) and Steroidogenic acute regulatory protein (StAR) in different cell lines [77,78] indicating SF1 is needed for FLC function. |
| IGF1 | Insulin-like growth factor 1 | Targeted mutation of the Igf1 gene leads to infertile males caused by failure of androgenization [79]. Addition of IGF1 to cultured dispersed fetal testicular cells increases FLC number via mesenchymal cell differentiation, and testosterone production [80,81]. These results suggest that IGF1 is involved in the regulation of FLC differentiation and function. |
| TGFβR3 | Transforming growth factor, beta receptor III, also called betaglycan | Loss of Tgfbr3 expression in fetal testes leads tothedownregulationof FLC markergenes associated with steroidogenesis, implicating TGFβ superfamily members are regulators of FLC [82,83]. |
| MEF2 | Myocyte enhancer factor 2 | MEF2 factors are expressed in Leydig cells including FLCs throughout development well into the adulthood. MEF2 is reported to regulate steroidogenesis in mouse MA-10 Leydig cells [84–86]. Since the FLCs also express MEF2 that MEF2 likely also modulates the function of FLCs. |
| NR4A1 | Nuclear receptor subfamily 4, group A, member 1, also known as Nur77, NGFI-B and TR3 | NR4A1 is found to play a crucial role in the regulation of genes involved in steroidogenesis in different Leydig cell lines [87–89]. Since NR4A1 is also found to express in fetal testis that NR4A1 likely regulates the steroidogenesis of FLCs. |
| CRHR1 | Corticotropin-releasing hormone receptor 1 | Using comparative analysis of microarray datasets from rodent fetal testes, CRHR1 activation is found to enhance fetal testis steroidogenesis in rat and mouse with ex vivo cultures, and in MA-10 Leydig cells. These results suggest that CRHR1 activation stimulates rodent FLC steroidogenesis [90]. |
Table 4.
Summary of testicular factors that modulate fetal testis development besides FLC development and function based on studies in genetic mouse models and rodent fetal testis cultures.
| Other phenotypes | Associated proteins |
|---|---|
| Genital ridge agenesis | SF1 [66], WT1 [91], GATA4 [92] |
| Impairment of mesonephric cell migration | PDGFα [24] |
| Failure or impairment of testis cord assembly | DHH [93], PDGFα [24], WT1 [94,95], DAX1 [68], GATA4 [70], HGF [96,97], TGFβR3 [83] |
| Sertoli cell trans-differentiation | WT1 [94,98] |
| Impairment of peritubular myoid cell development | DAX1 [68] |
| Germ cell loss | WT1 [94], DAX1 [68] |
| Impairment of vasculature formation | PDGFα [24], TCF21 [76] |
2.3. Function of FLCs
2.3.1. Testis cord formation is supported by cross-talks between FLCs and fetal Sertoli cells—FLCs regulate fetal Sertoli cell proliferation
Sertoli cells are the predominant somatic cell type that supports testis cord assembly during fetal testis development (for a review, see [3]). Sertoli cells also interact with peritubular myoid cells to deposit extracellular matrix components co-operatively to form the basement membrane that defines the testis cords and interstitium [31]. Additionally, Sertoli cells also produce cell adhesion molecules to maintain testis cord integrity between Sertoli cells as well as between Sertoli and germ cells [32,33]. Thus, Sertoli cells serve as the command center to orchestrate testis cord formation and elongation. It is also generally accepted that FLCs do not participate in testis cord development. This dogma, however, has been challenged in studies which illustrate that FLCs do play an active and essential role in Sertoli cell proliferation [34]. Activin A, also a member of TGFβ protein superfamily, is known to regulate epithelial patterning and tubulogenesis in multiple tissues (for a review, see [35]). Studies have shown that genetic disruption of activin βA (the gene encoding activin A) specifically in FLCs leads to failure in fetal testis cord elongation and expansion due to reduced Sertoli cell proliferation, illustrating the role of FLCs in modulating Sertoli cell function during fetal testis development. On the other hand, conditional inactivation of Smad4, the central component of TGFβ signaling, in Sertoli cells is found to cause testis cord dysgenesis and defects in Sertoli cell proliferation, displaying the phenotypes analogous to those of Leydig cell-specific activin βA knockout mouse testes. In short, these findings thus support the notion that FLCs are involved in testis cord elongation and expansion [34].
2.3.2. Production of androgens by FLCs via steroidogenesis
The main biological function of fetal Leydig cells (FLCs) is to produce androgens (Fig. 1). Androgens from FLCs and testosterone generated by FLCs and fetal Sertoli cells (Fig. 1) are required to masculinize the fetal mouse embryo, including differentiation of Wolffian duct, development and morphogenesis of the male genital tract and external genitalia, gonadogenesis, formation of ALC precursors, and potentially sexual dimorphism of the brain. Wolffian duct will differentiate into the epididymis, vas deferens, and seminal vesicles [36]. Androgens produced by FLCs and fetal Sertoli cells also influence brain development in males to confer the subtle differences in brain patterning and subsequent sex-specific behaviors in adult life (for a review, see [37]). ALCs produce testosterone to initiate, maintain and regulate spermatogenesis via its effects on blood testis barrier (BTB), germ cell meiosis, Sertoli-spermatid adhesion and sperm release in adult testes (for a review, see [36]). In addition, androgens and insulin-like factor 3 from FLCs also play a role in the regulation of transabdominal and inguinoscrotal testicular decent [2,3,37]. Androgen and insulin-like factor 3 deficiency during embryonic development lead to the retainment of testes in the body cavity, causing cryptorchidism (for a review, see [38]). Cryptorchidism leads to infertility later in adult life since the higher temperature of the body cavity at 37 °C is detrimental to spermatogenesis which requires an optimal temperature of 35 °C by residing in the scrotum. Furthermore, cryptorchidism is also associated with germ cell and/or Leydig cell tumors (for a review, see [37]). Thus, normal FLC development in the testis during embryonic development dictates the well-being of adult males in particular spermatogenesis, secondary sexual development, sexual characteristic maintenance and male sexual behaviors [39]. FLCs, however, are capable of synthesizing androstenedione only because of the lack of 17β-HSD in these cells (Fig. 1A). Fetal Sertoli cells, however, possess 17β-HSD (including HSD17B1 and HSD17B3), capable of converting androstenedione to testosterone (Fig. 1B). As such, FLCs and Sertoli cells in fetal and neonatal testes are working cooperatively to produce testosterone [12,40] (Fig. 1). However, ALCs also express high level of HSD17B3 besides other steroidogenic enzymes so that ALCs synthesize testosterone independently in adult testes without the need and support of adult Sertoli cells when 17ß-HSD ceases to express [12,40]. It is likely that in fetal testes, testosterone that produced by FLCs and fetal Sertoli cells does not target fetal Sertoli cells, FLCs, and germ cells via the pathway(s) involving androgen receptor (AR) since fetal Sertoli cells, FLCs and germ cells do not express AR in fetal testes [36,39]. Although PMCs expressed high levels of AR in fetal testes, mice lacking Ar in PMCs exhibit no detectable differences in the testes up to postnatal day 12 [41], indicating that AR signaling in PMCs is dispensable during fetal testis development. A recent study has shown that FLCs and ALCs share the same source of progenitor cells in the fetal testes [42], thus after FLC specification, progenitor ALCs are likely kept in dormant state similar to the undifferentiated mesenchymal cells in the fetal testis interstitium (Fig. 3). Although AR is not expressed in FLCs, the mesenchymal cells between and around FLCs strongly expressed AR in the interstitium of fetal testes [10]. These findings thus suggest that testosterone produced by FLCs and fetal SCs in fetal testes target progenitor ALCs through AR, and FLCs regulate the formation of progenitor ALCs via androgen/AR signal pathway. But most of the androgens produced in fetal testes are being used to target cells outside the fetal testis through microvessels in the interstitium (Fig. 1B), such as male genital tract, external genitalia and brain.
Fig. 3.
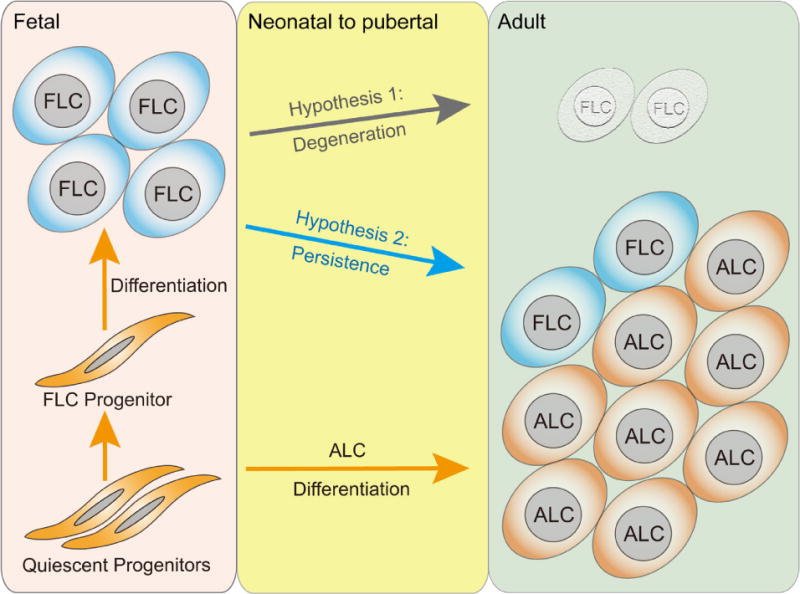
The fate of fetal Leydig cells. The FLC and adult Leydig cell (ALC) populations are established from the same precursor cells. Some of the precursor cells gave rise to FLCs in fetal testes and some stayed dormant throughout fetal development and differentiate to ALCs during postnatal testis development. ALCs do not originate from FLCs or degenerative FLCs. Based on the emerging evidence from morphology and cell tracing studies using genetic murine models, we propose 2 hypotheses herein: Hypothesis 1, FLC population undergoes involution or degeneration after birth and virtually replaced by ALC in adult testes. Hypothesis 2, FLC population survives and is a normal part of the Leydig cell population in adult testes. FLC, fetal Leydig cell; ALC, adult Leydig cell.
2.4. FLC fate
While androstenedione-producing FLC population is gradually substituted by testosterone-producing ALCs during postnatal development, the mechanism(s) that governs this process remains unclear, and the fate of FLCs has been controversial [3,7]. Based on histological, morphological, ultrastructural and quantitative analysis of testes in rodents and humans during embryonic and postnatal development, FLCs are known to undergo gradual atrophy after birth, and the population of FLCs gradually vanishes via degeneration in adult testes without involving apoptosis [9,43–45] (Fig. 3, Hypothesis 1). For instance, declining androgen levels found in the testis during the neonatal and pre-pubertal period indeed support FLC atrophy [46]. However, this dogma has been challenged in studies using Sprague-Dawley rat testes as a model by quantifying the population of FLCs from fetal to adult testes. In these studies, cells with characteristics of FLCs identified microscopically that contained large amounts of cytoplasmic lipid droplets, tubules and vesicles of smooth endoplasmic reticulum, mitochondria with tubular and lamellar cristae, and glycogen, were found in significant numbers in adult testes, and the absolute volume of these FLCs per testis were almost similar across all ages [47,48], suggesting FLCs persist in adult testes and do not undergo degeneration (Fig. 3, Hypothesis 2). Studies using genetic mouse assays to trace FLCs from fetal to adult testes specifically have yielded new insights on the FLC fate [10,11,49]. The adrenal 4 binding protein/steroidogenic factor 1 (Ad4BP/SF1), also known as NR5A1, is expressed in both FLCs and ALCs and it is crucial for their development and steroidogenesis [50]. A FLC-specific enhancer is identified in the upstream region of the mouse Ad4BP/SF-1 gene [49]. Based on this observation, a LacZ or enhanced green fluorescent protein (EGFP) has been inserted downstream of FLC-specific Ad4BP enhancer and promotor to generate a transgenic mouse line in which the LacZ or EGFP reporter is expressed in FLCs but not ALCs [49]. By using this transgenic mouse assay, the reporter genes are found to express in a small number of Ad4BP/SF-1 and 3β-hydroxysteroid dehydrogenase (3β-HSD) double positive FLCs, and are distributed throughout the interstitium in adult testes [11,49], confirming that FLCs, at least a small population, persist in adult testes. In another study in which a Cre/loxP recombination system was used to activate the expression of LacZ [51] or EGFP [52] reporter in FLCs or their precursors by the retinoic acid receptor isoform 2 gene-Cre transgene (Rarb-Cre) [53], FLCs identified by EGFP fluorescence, which marked the EGFP sites activated by the Rarb-Cre, were found to constitute ~20% of the total Leydig cell population in the interstitium of adult testes, illustrating FLCs and/or their precursors are part of the Leydig cell population in adult testis [10]. Collectively, these findings strongly suggest that FLCs indeed are found in adult testes and are integrated into the total Leydig cell population (Fig. 3, Hypothesis 2). Furthermore, almost all the FLCs in the adult testis do not express HSD3B6 and HSD17B3 [11], suggesting that they contribute relative little, perhaps none, to the pool of testosterone produced in the testis through steroidogenesis. As such, ALCs remain to be the major steroidogenic cells that produce testosterone in adult testes. It is also possible that some of the FLCs vanish postnatally via atrophy, but a few remaining FLCs proliferate to create a small population and integrated into the LC population in adult testes (Fig. 3, Hypothesis 1 and 2). Based on currently available data, we conclude that FLCs, at least a small population of FLCs, persist in adult testes. But due to the lack of HSD17B3 and HSD3B6, these FLCs do not produce testosterone independently in adult testis. These findings also support the notion that ALCs are not originated from FLCs (Fig. 4).
Fig. 4.
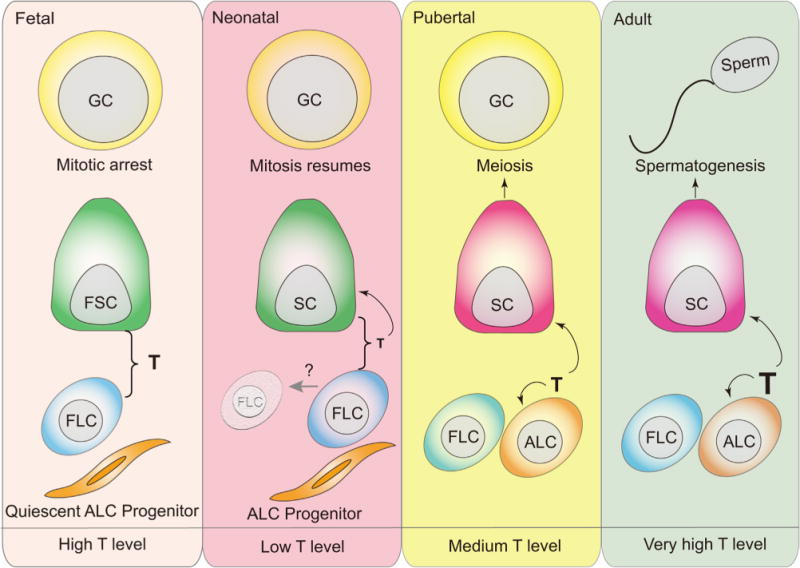
Spatial and temporal coordination of FLCs, fetal Sertoli cells and ALCs during fetal to adult testis development to support Sertoli cell maturation, germ cell mitosis and meiosis, and spermatogenesis. First panel from left: In mouse fetal testis, fetal Sertoli cells arise at around embryonic day 10.5 (E10.5) to initiate fetal testis organization. FLCs appear in fetal mouse testes at E12.5, working with Sertoli cells to supply the needed androgens to masculinize male embryos via steroidogenesis. Primordial germ cells (PGC) migrate into gonad at around E10.5 to colony as germ cells, then germ cells enter mitotic arrest between E13.5 and E15.5 in fetal testes. However, fetal Sertoli cells, FLCs and germ cells do not express nuclear or cytoplasmic androgen receptor (AR), therefore the androgens produced by FLCs and Sertoli cells do not target FLCs, Sertoli and germ cells in fetal testes. Second panel:Sertoli cells begin to express AR in postnatal 3–5 day mouse testes while some FLCs likely undergo degeneration (gray arrow) when androgen production by FLCs and Sertoli cells was reduced. Whereas germ cells resume mitosis as well as the ALC progenitors begin to differentiate. Third panel: ALC differentiation is initiated at around postnatal day 7–10 in mouse testes. Thus, testosterone production increases in pubertal mouse testes to support Sertoli cell maturation, germ cell meiosis and ALC development. Fourth panel: ALCs produce testosterone in adult mouse testes which serving as a paracrine factor to regulate spermatogenesis through its interaction with the androgen receptor expressed by mature Sertoli cells, and also as an autocrine factor to modulate ALC steroidogenesis. The small population of FLCs, about 20%, found in adult testes do not produce testosterone independently due to the lack of 17β-HSD. GC, germ cell; FSC, fetal Sertoli cell; FLC, fetal Leydig cell; ALC, adult Leydig cell; T, testosterone.
FLCs first appear in the mouse testis at E12.5 and increase their number during fetal life. On the other hand, ALC progenitors are first detected in postnatal testes by postnatal day 7 to 10 and gradually dominate the interstitial space after puberty [54]. Thus, mouse Leydig cell development exhibits a bi-phasic pattern. However, the physiological function of this bi-phasic pattern of FLC and ALC population during fetal and adult testis development in most species remains unclear. It is known that the trophic functions of androgens are mediated by their binding onto the androgen receptor (AR), a member of the nuclear receptor superfamily of ligand-activated transcription factors through canonical or non-canonical pathways [36]. Since the main function of Leydig cells is to produce testosterone, thus we compare the status of Sertoli cells, FLCs and germ cells, and steroidogenic pattern in fetal, neonatal, pubertal and adult testes spatiotemporally. During fetal testis development, FLCs are working with Sertoli cells to supply the testosterone to masculinize male embryos via steroidogenesis. However, fetal Sertoli cells, germ cells and FLCs do not express nuclear or cytoplasmic AR [10], and FLCs are one of the main somatic cell types in interstitial space, wherein germ cells have entered mitotic arrest as G0/G1 T-prospermatogonia between E13.5–E15.5 [55]. By day 3 to 5 after birth, Sertoli cells begin to express AR while the androgen production was reduced in fetal testes likely due to the atrophy of FLCs and down-regulation of 17β-HSD expression in Sertoli cells, whereas ALC progenitor cells begin to differentiate and germ cells resume mitosis [56]. At day 7–10 after birth, ALC differentiation initiates and germ cells enter meiosis at around postnatal day 8. At puberty, day 17–18 after birth in rodents, testosterone production increases again to support Sertoli cell maturation and germ cell meiosis. In adult testes, mature ALCs produce testosterone to regulate spermatogenesis. It is likely that the presence of FLCs and ALCs in fetal and adult testes, respectively, are working in concert to synchronize with Sertoli cell maturation and spermatogenesis, protecting the testis from precocious Sertoli cell maturation and the onset of spermatogenic development through the testosterone/AR pathway. ALCs are fully independent testosterone-producing cells in the interstitium in adult testes to support spermatogenesis. Since the lack of HSD17B3 in the small population of FLCs in adult testes, it is likely that these FLCs, if they can, produce androstenedione which is then transported to ALCs that express 17β-HSD to produce adequate testosterone to support spermatogenesis and the male secondary sexual characteristics. As such, FLCs found in the adult testis are working in concert with ALCs to produce testosterone. It is likely that the FLC pool in the adult testes also serve as a reserve to replace ALCs via differentiation following testis injury, such as following pathogenesis of the testis or environmental toxicant assault, to maintain the testosterone producing capability. Nonetheless, the physiological function of the bi-phase pattern of FLC and ALC, and the function of FLCs in adult testes require additional investigations in future studies.
3. Concluding remarks and future perspectives
Herein, we summary the in vivo, ex vivo and in vitro findings concerning the origination, differentiation, function and fate of FLCs in rodents. FLCs appear to be originated, at least in part, from the progenitor cells that also differentiate to Sertoli cells and peritubular myoid cells during fetal testis development (for a review, see [3]), but the precise origin of FLCs still require additional studies. Recent findings have supported the notion that Sertoli cells modulate the specification and function of FLCs, but whether peritubular myoid cells are also involved in these processes require additional studies. FLCs and ALCs are two distinct Leydig cell population that exert their functions in fetal and adult testes slightly differently, and the regulatory mechanisms identified in ALCs do not apply to FLCs in most circumstances. In order to better understand the development and function of FLCs, the regulatory mechanisms assigned to FLCs which are inferred from studies using ALCs should be carefully re-evaluated by FLC-specific studies, such as using the FLC-specific enhancer and promoter of Ad4BP/SF1 gene [11,49] or Rarb-Cre [10] mouse line as FLC-specific genetic editing tools, isolating highly enriched FLCs or developing 3D-fetal testis ex vivo cultures. It is commonly accepted that normal development of the testis during embryogenesis is critical for the function of the testis to support spermatogenesis during adulthood. However, the mechanism by which defects in fetal testis development that leads to reproductive disruption, such as infertility, in adult males remains poorly understood. Furthermore, the role of fetal testis dysfunction that impedes adult male fertility and reproductive function remains unknown. A better understanding on the ontogenesis, development, function and fate of FLCs should shed new insights in the treatment of some unexplained male infertilities among a growing population of infertile couples.
Acknowledgments
This study was supported by the Major Research Plan “973” Project (2011CB944302 and 2012CB944702), the National Technology Support Project (2012DAI131B08), and the National Nature Science Foundation of China (31471352, 31471400 and 31171380).
Footnotes
Disclosure statement
The authors have nothing to disclose.
References
- 1.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 2.Wainwright EN, Wilhelm D. The game plan: cellular and molecular mechanisms of mammalian testis development. Curr Top Dev Biol. 2010;90:231–262. doi: 10.1016/S0070-2153(10)90006-9. [DOI] [PubMed] [Google Scholar]
- 3.Svingen T, Koopman P. Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev. 2013;27:2409–2426. doi: 10.1101/gad.228080.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haider SG. Cell biology of Leydig cells in the testis. Int Rev Cytol. 2004;233:181–241. doi: 10.1016/S0074-7696(04)33005-6. [DOI] [PubMed] [Google Scholar]
- 5.Christensen AK. A History of Leydig Cell Research. In: Payne A, Hardy M, editors. The Leydig Cell in Health and Disease, Humana Press. 2007. pp. 3–30. [Google Scholar]
- 6.Yao HHC, Barsoum I. Fetal Leydig Cells. In: Payne AH, Hardy MP, editors. The Leydig Cell in Health and Disease. Humana Press; 2007. pp. 47–54. [Google Scholar]
- 7.Wen Q, Liu Y, Gao F. Fate determination of fetal Leydig cells. Front Biol. 2011;6:12–18. [Google Scholar]
- 8.Wen Q, Zheng QS, Li XX, Hu ZY, Gao F, Cheng CY, et al. Wt1 dictates the fate of fetal and adult Leydig cells during development in the mouse testis. Am J Physiol Endocrinol Metab. 2014;307:E1131–43. doi: 10.1152/ajpendo.00425.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faria MJ, Simoes ZL, Lunardi LO, Hartfelder K. Apoptosis process in mouse Leydig cells during postnatal development Microscopy and microanalysis: the official journal of Microscopy Society of America, Microbeam Analysis Society. Microsc Soc Can. 2003;9:68–73. doi: 10.1017/S1431927603030101. [DOI] [PubMed] [Google Scholar]
- 10.Kaftanovskaya EM, Lopez C, Ferguson L, Myhr C, Agoulnik AI. Genetic ablation of androgen receptor signaling in fetal Leydig cell lineage affects Leydig cell functions in adult testis. FASEB J. 2015;29:2327–2337. doi: 10.1096/fj.14-263632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shima Y, Matsuzaki S, Miyabayashi K, Otake H, Baba T, Kato S, et al. Fetal Leydig cells persist as an androgen-independent subpopulation in the postnatal testis. Mol Endocrinol. 2015;29:1581–1593. doi: 10.1210/me.2015-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shima Y, Miyabayashi K, Haraguchi S, Arakawa T, Otake H, Baba T, et al. Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Mol Endocrinol. 2013;27:63–73. doi: 10.1210/me.2012-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1:663–671. doi: 10.1046/j.1365-2443.1996.00254.x. [DOI] [PubMed] [Google Scholar]
- 14.Val P, Lefrancois-Martinez AM, Veyssiere G, Martinez A. SF-1 a key player in the development and differentiation of steroidogenic tissues. Nuclear Recep. 2003;1:8. doi: 10.1186/1478-1336-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFalco T, Takahashi S, Capel B. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol. 2011;352:14–26. doi: 10.1016/j.ydbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- 17.Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–281. doi: 10.1242/dev.117.1.273. [DOI] [PubMed] [Google Scholar]
- 18.Merchant-Larios H, Moreno-Mendoza N. Mesonephric stromal cells differentiate into Leydig cells in the mouse fetal testis. Exp Cell Res. 1998;244:230–238. doi: 10.1006/excr.1998.4215. [DOI] [PubMed] [Google Scholar]
- 19.Val P, Jeays-Ward K, Swain A. Identification of a novel population of adrenal-like cells in the mammalian testis. Dev Biol. 2006;299:250–256. doi: 10.1016/j.ydbio.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Angelova P, Davidoff M, Baleva K, Staykova M. Substance P- and neuron-specific enolase-like immunoreactivity of rodent Leydig cells in tissue section and cell culture. Acta Histochem. 1991;91:131–139. doi: 10.1016/S0065-1281(11)80266-7. [DOI] [PubMed] [Google Scholar]
- 21.Chiwakata C, Brackmann B, Hunt N, Davidoff M, Schulze W, Ivell R. Tachykinin (substance-P) gene expression in Leydig cells of the human and mouse testis. Endocrinology. 1991;128:2441–2448. doi: 10.1210/endo-128-5-2441. [DOI] [PubMed] [Google Scholar]
- 22.Mayerhofer A, Lahr G, Seidl K, Eusterschulte B, Christoph A, Gratzl M. The neural cell adhesion molecule (NCAM) provides clues to the development of testicular Leydig cells. J Androl. 1996;17:223–230. [PubMed] [Google Scholar]
- 23.Davidoff MS, Middendorff R, Müller D, Holstein AF, Müller D. The Neuroendocrine Leydig Cells and their Stem Cell Progenitors, the Pericytes. Springer Berlin; Heidelberg: 2009. Fetal and Adult Leydig Cells Are of Common Orig; pp. 89–103. [PubMed] [Google Scholar]
- 24.Brennan J, Tilmann C, Capel B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17:800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cool J, DeFalco TJ, Capel B. Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci U S A. 2011;108:167–172. doi: 10.1073/pnas.1010299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamauchi Y, Abe K, Mantani A, Hitoshi Y, Suzuki M, Osuzu F, et al. A Novel Transgenic Technique That Allows Specific Marking of the Neural Crest Cell Lineage in Mice. Dev Biol. 1999;212:191–203. doi: 10.1006/dbio.1999.9323. [DOI] [PubMed] [Google Scholar]
- 27.Chai Y, Jiang X, Ito Y, Bringas P, Han J, Rowitch DH, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 28.McClelland KS, Bell K, Larney C, Harley VR, Sinclair AH, Oshlack A, et al. Purification and Transcriptomic Analysis of Mouse Fetal Leydig Cells Reveals Candidate Genes for Specification of Gonadal Steroidogenic Cells. Biol Reprod. 2015 doi: 10.1095/biolreprod.115.128918. [DOI] [PubMed] [Google Scholar]
- 29.Zachow R, Uzumcu M. The hepatocyte growth factor system as a regulator of female and male gonadal function. J Endocrinol. 2007;195:359–371. doi: 10.1677/JOE-07-0466. [DOI] [PubMed] [Google Scholar]
- 30.Ricci G, Guglielmo MC, Caruso M, Ferranti F, Canipari R, Galdieri M, et al. Hepatocyte growth factor is a mouse fetal Leydig cell terminal differentiation factor. Biol Reprod. 2012;87:146. doi: 10.1095/biolreprod.112.104638. [DOI] [PubMed] [Google Scholar]
- 31.Skinner MK, Tung PS, Fritz IB. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol. 1985;100:1941–1947. doi: 10.1083/jcb.100.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrionuevo F, Georg I, Scherthan H, Lecureuil C, Guillou F, Wegner M, et al. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol. 2009;327:301–312. doi: 10.1016/j.ydbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Chang H, Gao F, Guillou F, Taketo MM, Huff V, Behringer RR. Wt1 negatively regulates beta-catenin signaling during testis development. Development. 2008;135:1875–1885. doi: 10.1242/dev.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Archambeault DR, Yao HHC. Activin A, a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion. Proc Natl Acad Sci. 2010;107:10526–10531. doi: 10.1073/pnas.1000318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ball EM, Risbridger GP. Activins as regulators of branching morphogenesis. Dev Biol. 2001;238:1–12. doi: 10.1006/dbio.2001.0399. [DOI] [PubMed] [Google Scholar]
- 36.Smith LB, Walker WH. The regulation of spermatogenesis by androgens, Semin. Cell Dev Biol. 2014;30C:2–13. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griswold SL, Behringer RR. Fetal Leydig cell origin and development. Sexual Development. 2009;3:1–15. doi: 10.1159/000200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anand-Ivell R, Ivell R. INSL3 as a monitor of endocrine disruption. Reproduction. 2013 doi: 10.1530/REP-13-0486. [DOI] [PubMed] [Google Scholar]
- 39.O’Hara L, McInnes K, Simitsidellis I, Morgan S, Atanassova N, Slowikowska-Hilczer J, et al. Autocrine androgen action is essential for Leydig cell maturation and function, and protects against late-onset Leydig cell apoptosis in both mice and men. FASEB J. 2015;29:894–910. doi: 10.1096/fj.14-255729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Shaughnessy PJ, Baker PJ, Heikkila M, Vainio S, McMahon AP. Localization of 17beta-hydroxysteroid dehydrogenase/17-ketosteroid reductase isoform expression in the developing mouse testis–androstenedione is the major androgen secreted by fetal/neonatal leydig cells. Endocrinology. 2000;141:2631–2637. doi: 10.1210/endo.141.7.7545. [DOI] [PubMed] [Google Scholar]
- 41.Welsh M, Saunders PT, Atanassova N, Sharpe RM, Smith LB. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J. 2009;23:4218–4230. doi: 10.1096/fj.09-138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barsoum IB, Kaur J, Ge RS, Cooke PS, Yao HHung-Chang. Dynamic changes in fetal Leydig cell populations influence adult Leydig cell populations in mice. FASEB J. 2013 doi: 10.1096/fj.12-225060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tapanainen J, Kuopio T, Pelliniemi LJ, Huhtaniemi I. Rat testicular endogenous steroids and number of Leydig-cells between the fetal period and sexual maturity. Biol Reprod. 1984;31:1027–1035. doi: 10.1095/biolreprod31.5.1027. [DOI] [PubMed] [Google Scholar]
- 44.Prince FP. Ultrastructural evidence of mature Leydig cells and Leydig cell regression in the neonatal human testis. Anat Rec. 1990;228:405–417. doi: 10.1002/ar.1092280406. [DOI] [PubMed] [Google Scholar]
- 45.Huhtaniemi I, Pelliniemi LJ. Fetal Leydig cells: cellular origin, morphology, life span, and special functional features. Proc Soc Exp Biol Med. 1992;201:125–140. doi: 10.3181/00379727-201-43493. [DOI] [PubMed] [Google Scholar]
- 46.O’Shaughnessy PJ, Baker PJ, Johnston H. The foetal Leydig cell? differentiation, function and regulation. Int J Androl. 2006;29:90–95. doi: 10.1111/j.1365-2605.2005.00555.x. discussion 105–8. [DOI] [PubMed] [Google Scholar]
- 47.Kerr JB, Knell CM. The fate of fetal Leydig cells during the development of the fetal and postnatal rat testis. Development. 1988;103:535–544. doi: 10.1242/dev.103.3.535. [DOI] [PubMed] [Google Scholar]
- 48.Ariyaratne HB, Mendis-Handagama S Chamindrani. Changes in the testis interstitium of Sprague Dawley rats from birth to sexual maturity. Biol Reprod. 2000;62:680–690. doi: 10.1095/biolreprod62.3.680. [DOI] [PubMed] [Google Scholar]
- 49.Shima Y, Miyabayashi K, Baba T, Otake H, Katsura Y, Oka S, et al. Identification of an enhancer in the Ad4BP/SF-1 gene specific for fetal Leydig cells. Endocrinology. 2012;153:417–425. doi: 10.1210/en.2011-1407. [DOI] [PubMed] [Google Scholar]
- 50.Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen AP, et al. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol. 2004;18:1610–1619. doi: 10.1210/me.2003-0404. [DOI] [PubMed] [Google Scholar]
- 51.Soriano P. Generalized lacZ expression with the ROSA26Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 52.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi A. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 2005;132:2809–2823. doi: 10.1242/dev.01858. [DOI] [PubMed] [Google Scholar]
- 54.Chen H, Ge RS, Zirkin BR. Leydig cells: from stem cells to aging. Mol Cell Endocrinol. 2009;306:9–16. doi: 10.1016/j.mce.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLaren A. Meiosis and differentiation of mouse germ cells. Symp, Soc Exp Biol. 1984;38:7–23. [PubMed] [Google Scholar]
- 56.McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 57.Yan W, Kero J, Huhtaniemi I, Toppari J. Stem cell factor functions as a survival factor for mature Leydig cells and a growth factor for precursor Leydig cells after ethylene dimethane sulfonate treatment: implication of a role of the stem cell factor/c-Kit system in Leydig cell development. Dev Biol. 2000;227:169–182. doi: 10.1006/dbio.2000.9885. [DOI] [PubMed] [Google Scholar]
- 58.Manova K, Nocka K, Besmer P, Bachvarova RF. Gonadal expression of c-kit encoded at the W locus of the mouse. Development. 1990;110:1057–1069. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- 59.Barsoum IB, Bingham NC, Parker KL, Jorgensen JS, Yao HH. Activation of the Hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal Leydig cells and female pseudohermaphroditism. Dev Biol. 2009;329:96–103. doi: 10.1016/j.ydbio.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rouiller-Fabre V, Carmona S, Merhi RA, Cate R, Habert R, Vigier B. Effect of anti-Mullerian hormone on Sertoli and Leydig cell functions in fetal and immature rats. Endocrinology. 1998;139:1213–1220. doi: 10.1210/endo.139.3.5785. [DOI] [PubMed] [Google Scholar]
- 62.Wen Q, Wang Y, Tang J, Cheng CY, Liu YX. Wt1 regulates fetal testis morphogenesis by modulating somatic cell differentiation and proliferation in the mouse testis. Reproduction. 2016 (submitted) [Google Scholar]
- 63.Rakoczy J, Fernandez-Valverde SL, Glazov EA, Wainwright EN, Sato T, Takada S, et al. MicroRNAs-140-5p/140-3p modulate Leydig cell numbers in the developing mouse testis. Biol Reprod. 2013;88:143. doi: 10.1095/biolreprod.113.107607. [DOI] [PubMed] [Google Scholar]
- 64.Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–369. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- 65.Miyabayashi K, Katoh-Fukui Y, Ogawa H, Baba T, Shima Y, Sugiyama N, et al. Aristaless related Homeobox Gene, Arx, is implicated in mouse fetal Leydig cell differentiation possibly through expressing in the progenitor cells. PLoS One. 2013;8:e68050. doi: 10.1371/journal.pone.0068050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 67.Karpova T, Ravichandiran K, Insisienmay L, Rice D, Agbor V, Heckert LL. Steroidogenic factor 1 differentially regulates fetal and adult Leydig cell development in male mice. Biol Reprod. 2015 doi: 10.1095/biolreprod.115.131193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meeks JJ, Crawford SE, Russell TA, Morohashi K, Weiss J, Jameson JL. Dax1 regulates testis cord organization during gonadal differentiation. Development. 2003;130:1029–1036. doi: 10.1242/dev.00316. [DOI] [PubMed] [Google Scholar]
- 69.Bielinska M, Seehra A, Toppari J, Heikinheimo M, Wilson DB. GATA-4 is required for sex steroidogenic cell development in the fetal mouse. Dev Dyn. 2007;236:203–213. doi: 10.1002/dvdy.21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manuylov NL, Zhou B, Ma Q, Fox SC, Pu WT, Tevosian SG. Conditional ablation of Gata4 and Fog2 genes in mice reveals their distinct roles in mammalian sexual differentiation. Dev Biol. 2011;353:229–241. doi: 10.1016/j.ydbio.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin FJ, Qin J, Tang K, Tsai SY, Tsai MJ. Coup d’Etat: an orphan takes control. Endocr Rev. 2011;32:404–421. doi: 10.1210/er.2010-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qin J, Tsai MJ, Tsai SY. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS One. 2008;3:e3285. doi: 10.1371/journal.pone.0003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van den Driesche S, Walker M, McKinnell C, Scott HM, Eddie SL, Mitchell RT, et al. Proposed role for coup-tfii in regulating fetal Leydig cell steroidogenesis, perturbation of which leads to masculinization disorders in rodents. PLoS One. 2012;7:e37064. doi: 10.1371/journal.pone.0037064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang H, Brennan J, Karl J, Hamada Y, Raetzman L, Capel B. Notch signaling maintains Leydig progenitor cells in the mouse testis. Development. 2008;135:3745–3753. doi: 10.1242/dev.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Defalco T, Saraswathula A, Briot A, Iruela-Arispe ML, Capel B. Testosterone levels influence mouse fetal Leydig cell progenitors through notch signaling. Biol Reprod. 2013;88:91. doi: 10.1095/biolreprod.112.106138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cui S, Ross A, Stallings N, Parker KL, Capel B, Quaggin SE. Disrupted gonadogenesis and male-to-female sex reversal in Pod1 knockout mice. Development. 2004;131:4095–4105. doi: 10.1242/dev.01266. [DOI] [PubMed] [Google Scholar]
- 77.Frigeri C, Tsao J, Czerwinski W, Schimmer BP. Impaired steroidogenic factor 1 (NR5A1) activity in mutant Y1 mouse adrenocortical tumor cells. Mol Endocrinol. 2000;14:535–544. doi: 10.1210/mend.14.4.0440. [DOI] [PubMed] [Google Scholar]
- 78.Morohashi K, Zanger UM, Honda S, Hara M, Waterman MR, Omura T. Activation of CYP11A and CYP11B gene promoters by the steroidogenic cell-specific transcription factor, Ad4BP. Mol Endocrinol. 1993;7:1196–1204. doi: 10.1210/mend.7.9.8247022. [DOI] [PubMed] [Google Scholar]
- 79.Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR, et al. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- 80.Rouiller-Fabre V, Lecref L, Gautier C, Saez JM, Habert R. Expression and effect of insulin-like growth factor I on rat fetal Leydig cell function and differentiation. Endocrinology. 1998;139:2926–2934. doi: 10.1210/endo.139.6.6035. [DOI] [PubMed] [Google Scholar]
- 81.Villalpando I, López-Olmos V. Insulin-like growth factor I (IGF-I) regulates endocrine activity of the embryonic testis in the mouse. J Steroid Biochem Mol Biol. 2003;86:151–158. doi: 10.1016/s0960-0760(03)00265-6. [DOI] [PubMed] [Google Scholar]
- 82.Sarraj MA, Chua HK, Umbers A, Loveland KL, Findlay JK, Stenvers KL. Differential expression of TGFBR3 (betaglycan) in mouse ovary and testis during gonadogenesis. Growth Factors. 2007;25:334–345. doi: 10.1080/08977190701833619. [DOI] [PubMed] [Google Scholar]
- 83.Sarraj MA, Escalona RM, Umbers A, Chua HK, Small C, Griswold M, et al. Fetal testis dysgenesis and compromised Leydig cell function in Tgfbr3 (beta glycan) knockout mice. Biol Reprod. 2010;82:153–162. doi: 10.1095/biolreprod.109.078766. [DOI] [PubMed] [Google Scholar]
- 84.Daems C, Martin LJ, Brousseau C, Tremblay JJ. MEF2 is restricted to the male gonad and regulates expression of the orphan nuclear receptor NR4A1. Mol Endocrinol. 2014;28:886–898. doi: 10.1210/me.2013-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Daems C, Di-Luoffo M, Paradis E, Tremblay JJ. MEF2Cooperates With Forskolin/cAMP and GATA4 to Regulate Star Gene Expression in Mouse MA-10 Leydig Cells. Endocrinology. 2015;156:2693–2703. doi: 10.1210/en.2014-1964. [DOI] [PubMed] [Google Scholar]
- 86.Di-Luoffo M, Brousseau C, Bergeron F, Tremblay JJ. The Transcription Factor MEF2 Is a Novel Regulator of Gsta Gene Class in Mouse MA-10 Leydig Cells. Endocrinology. 2015;156:4695–4706. doi: 10.1210/en.2015-1500. [DOI] [PubMed] [Google Scholar]
- 87.Song KH, Park JI, Lee MO, Soh J, Lee K, Choi HS. LH induces orphan nuclear receptor Nur77 gene expression in testicular Leydig cells. Endocrinology. 2001;142:5116–5123. doi: 10.1210/endo.142.12.8525. [DOI] [PubMed] [Google Scholar]
- 88.Martin LJ, Boucher N, Brousseau C, Tremblay JJ. The orphan nuclear receptor NUR77 regulates hormone-induced StAR transcription in Leydig cells through cooperation with Ca2+/calmodulin-dependent protein kinase I. Mol Endocrinol. 2008;22:2021–2037. doi: 10.1210/me.2007-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song CH, Gong EY, Park JS, Lee K. Testicular steroidogenesis is locally regulated by androgen via suppression of Nur77. Biochem Biophys Res Commun. 2012;422:327–332. doi: 10.1016/j.bbrc.2012.04.161. [DOI] [PubMed] [Google Scholar]
- 90.McDowell EN, Kisielewski AE, Pike JW, Franco HL, Yao HH, Johnson KJ. A transcriptome-wide screen for mRNAs enriched in fetal Leydig Cells: CRHR1 agonism stimulates rat and mouse fetal testis steroidogenesis. PLoS One. 2012;7:e47359. doi: 10.1371/journal.pone.0047359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, et al. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- 92.Hu YC, Okumura LM, Page DC. Gata4 is required for formation of the genital ridge in mice. PLoS Genet. 2013;9:e1003629. doi: 10.1371/journal.pgen.1003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pierucci-Alves F, Clark AM, Russell LD. A developmental study of the Desert hedgehog-null mouse testis. Biol Reprod. 2001;65:1392–1402. doi: 10.1095/biolreprod65.5.1392. [DOI] [PubMed] [Google Scholar]
- 94.Gao F, Maiti S, Alam N, Zhang Z, Deng JM, Behringer RR, et al. The Wilms tumor gene, Wt1, is required for Sox9 expression and maintenance of tubular architecture in the developing testis. Proc Natl Acad Sci USA. 2006;103:11987–11992. doi: 10.1073/pnas.0600994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen SR, Chen M, Wang XN, Zhang J, Wen Q, Ji SY, et al. The Wilms tumor gene Wt1, maintains testicular cord integrity by regulating the expression of Col4a1 and Col4a2. Biol Reprod. 2013;88:56. doi: 10.1095/biolreprod.112.105379. [DOI] [PubMed] [Google Scholar]
- 96.Ricci G, Catizone A, Innocenzi A, Galdieri M. Hepatocyte growth factor (HGF) receptor expression and role of HGF during embryonic mouse testis development. Dev Biol. 1999;216:340–347. doi: 10.1006/dbio.1999.9505. [DOI] [PubMed] [Google Scholar]
- 97.Ricci G, Catizone A, Galdieri M. Pleiotropic activity of hepatocyte growth factor during embryonic mouse testis development. Mech Dev. 2002;118:19–28. doi: 10.1016/s0925-4773(02)00247-2. [DOI] [PubMed] [Google Scholar]
- 98.Zhang L, Chen M, Wen Q, Li Y, Wang Y, Wang Y, et al. Reprogramming of Sertoli cells to fetal-like Leydig cells by Wt1 ablation. Proc Natl Acad Sci U S A. 2015;112:4003–4008. doi: 10.1073/pnas.1422371112. [DOI] [PMC free article] [PubMed] [Google Scholar]


