Abstract
Purpose
We examined plasma oxytocin concentration and postpartum depression (PPD) symptom severity in women who were not depressed during pregnancy, and whether this differed by major depressive disorder (MDD) history.
Methods
We assessed psychiatric history and plasma oxytocin in 66 healthy pregnant women in the third trimester (M = 35 ± 3 weeks) and depressive symptoms at 6 weeks postpartum (M = 5.9 ± 0.8 weeks). Linear regression analysis was used to examine oxytocin and PPD symptom severity, and moderation of oxytocin and PPD by past MDD.
Results
Women with (n = 13) and without (n = 53) past MDD differed in third trimester depressive symptom severity, but not oxytocin level, demographic factors or birth outcomes. Controlling for third trimester depressive symptoms, oxytocin level was unrelated to PPD symptom severity [B(SE) = −.019(.084); β = −.025; t = −.227; p = .821]. However, oxytocin level interacted with past MDD to predict PPD symptom severity [(B(SE) = 7.489(2.429); β = .328; t = 3.084; p = .003]. Higher oxytocin predicted greater PPD symptom severity in women with past MDD (p = .019), but not in women without (p = .216).
Conclusions
Replication in a larger sample and methodologic challenges are discussed.
Keywords: postpartum depression, mood, oxytocin, biomarker, hormone, oxytocin receptor gene
Introduction
Major depressive disorder (MDD) occurring after birth, referred to as postpartum depression (PPD), is a common complication of childbearing, affecting some one in five mothers in the first postpartum year (Wisner et al. 2013). PPD has been associated with far-reaching adverse consequences to maternal and child health, including impaired maternal-infant bonding (McFarland et al. 2011), increased risk of recurrence of maternal depression (Yonkers et al. 2009), and the development of mental disorders and medical problems in children later in life (Wisner et al. 2006). A number of psychosocial predictors of PPD have been identified through systematic meta-analysis; these are a prior history of depression, depression during pregnancy, anxiety during pregnancy, general life stress, low social support, and low marital satisfaction (Robertson et al. 2004). As such, routine screening for PPD at the postpartum obstetric follow up visit is recommended by the American College of Obstetrics and Gynecology (ACOG 2015).
However, time constraints related to caring for a new infant, and social stigma associated with depression and mental health problems more broadly, still represent significant barriers to seeking professional help even among women who are identified with PPD (Goodman 2009). Many mothers with PPD feel that they are failing because they expect that they should be happy, which decreases the likelihood that they will seek or accept help (Dennis & Chung-Lee 2006). If women who are destined to develop PPD could be accurately identified during pregnancy, prevention could implemented (Zlotnick et al. 2006). Obstetricians routinely screen for non-psychiatric complications of child bearing with similar prevalence to PPD, such as gestational diabetes, using readily available biomarkers (Wen et al. 2000). The prevalence and far-reaching adverse effects of PPD justify the search for biomarker screening as well.
Oxytocin, a neuropeptide hormone produced primarily in the hypothalamus, and released from the pituitary into the peripheral circulation, long known for its role in delivery and lactation (Carter 1998, Carter et al. 2001) has also received substantial attention for its role in social affiliation and behavior, stress management (Carter & Altemus 1997; Feldman 2012), and increasingly, its potential for therapeutic benefit across a broad range of disorders (Meyer-Lindenberg et al. 2011), including PPD (Kim et al. 2014). There is also early evidence that measures related to oxytocin functioning, including plasma oxytocin concentration (Skrundz et al. 2011) and indices of oxytocin receptor availability (Bell et al. 2015), may represent promising biomarkers of elevated risk for PPD. Clarification of these processes could support the future use of oxytocin as a screening biomarker for PPD during pregnancy.
However, a substantial challenge to the study of peripheral oxytocin concerns its accurate measurement. Recent findings using mass spectrometry suggest that following release, oxytocin is quickly bound or sequestered on components of blood. Only a small fraction of the oxytocin in peripheral circulation is detectable by conventional immunoassays (Martin-Protean 2015), although the oxytocin measured by immunoassay may represent a biologically relevant, or free component. Moreover, immunoassays—either enzyme immunoassays (EIA) or radioimmunoassays (RIA)—indicate that free oxytocin levels vary inter-individually, and that transient increases can be measured in response to social interactions and also nonsocial stressors (Feldman et al. 2011).
Further complicating matters, the relationship between plasma oxytocin concentration and PPD may not be linear. Seay and colleagues (2014) found that very high and very low plasma oxytocin concentrations predicted an increase in depressive symptoms 10 weeks later. Similarly, in a detailed study involving multiple serial measurements of plasma oxytocin by RIA before, during, and after affiliative and stressful tasks, Cyranowski and colleagues (2008) showed that depressed women exhibited greater increases in oxytocin concentrations following a one-hour affiliation task compared to non-depressed women. Thus, it is possible that prior to the manifestation of clinically detectable depressive symptoms, individuals might experience dysregulation in oxytocin function. This could be reflected by abnormal variation in measured plasma oxytocin levels, in some cases as the oxytocin system attempts to compensate for reduced receptor availability or rapid changes in the hormone. This early dysregulation of the oxytocin system may be detectable as increases in concentrations of the oxytocin peptide in response to affiliative interactions as this system attempts to compensate for lower levels of receptor availability.
Finally, the relationship between oxytocin and depressive symptoms may vary by past exposure to MDD via stable dynamic transcriptional alterations to genes, or epigenetic mechanisms (Sun et al. 2013; Klengel et al. 2014). For example, methylation of the oxytocin receptor gene (OXTR) has been linked to past exposure to acute psychological stress exposure that predates the onset of MDD (Unternmaehrer et al. 2012). Recently, methylation of OXTR at key regulatory sites, indicative of reduced receptor expression, was found to be associated with a greater risk for PPD among a subset of women who had not experienced depression during pregnancy (Bell et al. 2015). Taken together, these findings suggest that MDD prior to pregnancy may render certain women more vulnerable to PPD, possibly mediated by methylation of OXTR, and subsequent dysregulation of oxytocinergic systems. These systems may in turn regulate affect and resilience in the face of stress (Tops et al. 2013) which leads to increased vulnerability to PPD.
The first goal of this study was to determine the relationship between third trimester plasma oxytocin and PPD symptom severity. Based on findings by Skrundz et al., (2009) showing that women with lower third trimester oxytocin were at greater risk for developing PPD at 2 weeks postpartum, we expected to find an inverse relationship between third trimester plasma oxytocin and depressive symptoms at 6 weeks postpartum. Next, we secondarily explored how the relationship between oxytocin and PPD symptom severity might differ by lifetime history of MDD. Based on limited literature, we hypothesized that among women with a history of depression prior to pregnancy, higher oxytocin levels would predict greater PPD symptom severity.
Materials and Methods
Sample and Recruitment
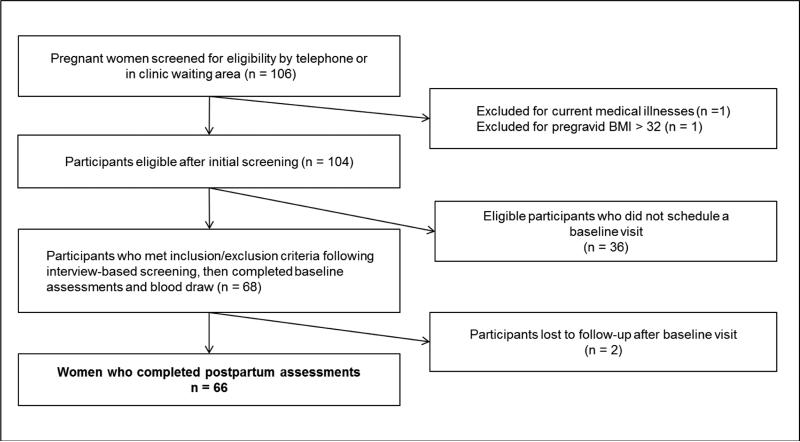
Participants were 66 pregnant women recruited during their third trimester of gestation (M = 34.9 weeks, SD = 3.3 weeks, range = 29 – 40 weeks) using online advertisements placed on websites of a large urban university medical center in the Midwest region of the United States. Flyers and brochures were also placed throughout the medical center campus, affiliated clinics, and in nearby local businesses. In addition to advertisements, a research assistant recruited participants from the waiting area of the academic faculty obstetric clinic by assessing interest in “a study about oxytocin and mood.” Informed consent was obtained from all individual participants included in the study. Potential participants were screened for the following preliminary inclusion criteria: age ≥ 18 years, English-literacy, and third trimester singleton pregnancy. Exclusion criteria were: (a) current mood disorder; (b) medical illness prior to or during pregnancy including diabetes, hypertension, sleep apnea, or thyroid disease; (c) current treatment for depression or anxiety; (d) fetal malformation or multiple gestation; (e) cigarette smoking beyond the 10th week of gestation; and (f) pregravid body mass index (BMI) > 32 kg/m2. Derivation of the final analytic sample is illustrated in Figure 1. Eligible women identified through initial screening who did not participate (n = 36) and participants lost to follow-up (n = 2) did not differ in any measured characteristics from those included in the analytic sample (n = 66).
Figure 1.
Flow chart of potential participants to the analytic sample
Study procedures
Women who met initial inclusion and exclusion criteria were invited for baseline assessments. At the baseline visit, after completing written informed consent procedures, demographic information, parity, breast feeding intention, and estimated pre-gravid weight were obtained by a combination of patient report and medical records. Women were evaluated for a current mood disorder using a modified Mini International Neuropsychiatric Interview (MINI) Version 5.0.0 (Sheehan et al. 1998), the Mood Disorders Questionnaire (Hirschfield et al. 2000), and study assessments of depressive symptoms severity described below. None of the women who met initial inclusion and exclusion criteria through screening were found to have MDD or bipolar disorder at the baseline visit.
Following these assessments, women were escorted to the clinical research unit by the research assistant for blood draws. Prior to the blood draw, height, weight, and vital signs were measured by a research nurse. Participants were scheduled for follow up visits approximately 6 weeks following their projected due date (M = 5.9 weeks, SD = 6 days). The study coordinator checked medical records for actual delivery dates, and adjusted the timing of postpartum assessments such that they occurred as close to 6 weeks following delivery as possible. Women were offered the option to complete postpartum assessments either in person or by telephone. All but one women (98.5 %, n = 65 out of 66) opted for the telephone assessment. Participants were asked about birth outcomes and complications, and assessed again for depressive symptom severity. Participants were given a $50 gift card following completion of the postpartum assessments. Data collection began in March 2014 and ended in June 2015. All participants were offered referrals for treatment at the end of the study. To ensure participant safety, all participants were urged to contact the study coordinator or PI if they developed worsening or new depressive symptoms from the baseline visit until the postpartum follow-up. Any participant requesting support or treatment, regardless of their depressive symptom severity, were invited to obtain it from the study center. All procedures were approved by the Institutional Review Board of Northwestern University prior to conduct and were performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Measures
Depressive symptoms
Depressive symptoms during the third trimester and postpartum visits were assessed using the Inventory for Depressive Symptoms – Self Report 30 (IDS-SR30) (Rush et al., 2000). This 30-item questionnaire was designed to assess the severity of depressive symptoms in nine symptom domains using well-defined anchors. The IDS-SR30 was chosen for use in this sample due to its sensitivity to change in the lower range of scores relative to other depression scales (Rush et al., 2005). The IDS-SR30 contains the following cut points of symptom severity: 0 – 13 = no depression; 14 – 25 = mild depression; 26 – 38 = moderate depression; 39 – 48 = severe depression; 49 – 84 = very severe depression. In addition to the IDS-SR30, the 10-item Edinburgh Postnatal Depression Scale (EPDS) was also administered during the postpartum follow-up. A score of 10 or greater of the EPDS was considered a positive screen for depression (Cox et al. 1996).
Past Major Depressive Disorder
To evaluate a past history of major depressive disorder, Module A4 from the MINI, which assesses history of any past major depressive episodes (Sheehan et al. 1998), was administered. For participants meeting criteria for a past depressive episode, the length of time from resolution of the episode to the present time was recorded.
Blood sample preparation
To minimize diurnal fluctuations and variability due to social context, visits were conducted in the aforementioned order (assessments, followed by blood draw); all participants had blood samples drawn between 11:00 am and 1:00 pm. A 3 mL blood sample was drawn from a peripheral upper extremity vein into chilled glass dipotassium EDTA tubes and placed immediately on ice. After collection, the sample was centrifuged at 3500 rpm for 10 minutes at 4° C. The plasma (supernatant) was collected as 400 μL aliquots into 1.5 ml Eppendorf tubes and immediately stored at −70° C until shipment. All samples were sent in one shipment packed in dry ice to the lab of Dr. Sue Carter at The Kinsey Institute of Indiana University for enzyme-linked immunosorbent assay (EIA)
Measurement of oxytocin
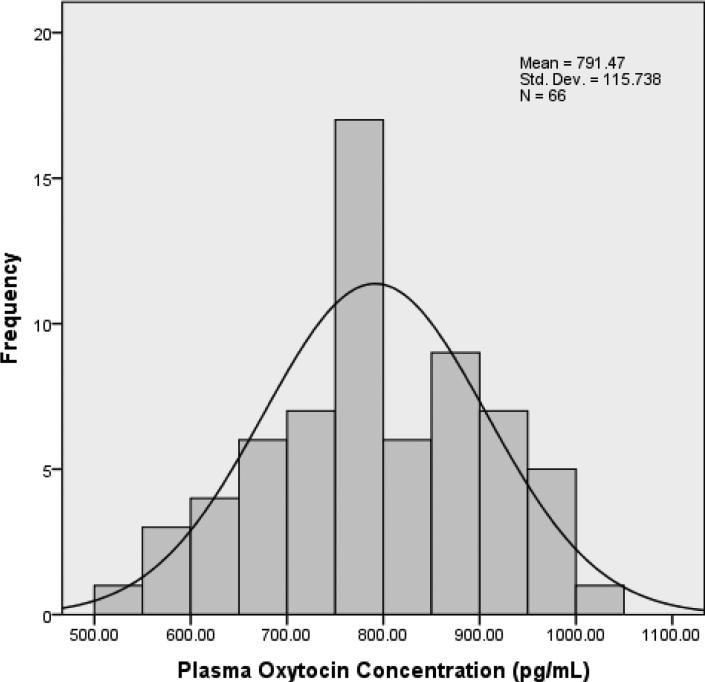
The enzyme-immunoassay was based on kit ADI 901 153A from Enzo Life Sciences, Farmingdale, NY. Direct measurement of plasma was done following the manufacturers‘ protocols and samples were diluted 1:8 to obtain values on the most sensitive part of the standard curve. Samples were assayed without extraction in duplicate. The minimum detection limit for oxytocin was 15.6 pg/ml, with inter- and intra-assay coefficients of less than 10%. The distribution of oxytocin concentrations in this sample are shown in Figure 2.
Figure 2.
Distribution of Third Trimester Plasma Oxytocin Levels (N = 66)
It is worthwhile to note that previous studies assaying unextracted plasma samples from our laboratory used EIA kits ADI 901 153 from the same company (Enzo, previously Assay Designs), but using an older antibody that has since been depleted. The previous kits yielded reliable and consistent, albeit lower oxytocin values averaging around 250 - 350 pg/ml in samples from thousands of healthy adults (Goldman et al. 2008; Gouin et al. 2010; Rubin et al. 2010; Feldman et al. 2012; Hammock et al. 2012), including pregnant women (MacKinnon et al. 2014; Zelkowitz et al. 2014). We and other laboratories found that the newer ENZO kit (ADI 901 153A) gave values that were approximately 2.5 to 3 fold higher than those obtained with kit ADI 901 153. The precise origins of this shift in peptide values are unknown. However, the inter- and intra-assay coefficients of variation with the new kit remain small. Because of the consistency across laboratories, we assume that the change in basal values reflects differences in the antibody used in the new kits.
Data analysis
All variables were checked for normal distribution using both visual inspection and the Jarque-Bera goodness-of-fit test. Significantly skewed independent variables were transformed by natural logarithm prior to entry into the regression model. We examined data for outliers, defined as more than two standard deviations from the mean (none found). Participants were divided into those with a past MDD history and those without. Demographic and clinical characteristics were compared between these groups using t-tests and χ2 tests for continuous and binary variables, respectively. Variables were also examined for multi-co-linearity (defined a priori as r = .60) using bivariate correlation analysis (none found). The relationship between third trimester plasma oxytocin concentration and PPD symptom severity was examined using linear regression analysis, with third trimester depressive symptom severity as a covariate. Next, the relationship between oxytocin and PPD symptom severity was tested using past history of MDD as a moderator. In this second model, characteristics that differed between past MDD and no past MDD groups were entered as covariates.
Results
Characteristics for the whole sample, and by past MDD history are shown in Table 1. Racial and ethnic breakdown was as follows: non-Hispanic Caucasian 72.7 % (n = 48); African-American 10.6% (n = 7); Asian 9.1 % (n = 6); Hispanic 7.6% (n = 5). The distribution of pre-gravid BMI in this sample was: Underweight (BMI < 18.5) = 4.5% (n = 3); normal weight (BMI 18.5 – 24.9) = 68.2% (n = 45); overweight (BMI 25 – 29.9) = 16.7% (n = 11); obese (BMI ≥ 30 - 32) = 10.6% (n = 7). Birth complications included preeclampsia (n = 2); infections (n = 2); prolonged labor/failure to progress n = 6; postpartum hemorrhage (n = 1); meconium stained amniotic fluid (n = 1), and breach presentation (n = 1).
Table 1.
Descriptive characteristics of participants (N = 66)
| Mean (SD) or % |
|||
|---|---|---|---|
| Third trimester measures | Total sample N = 66 | Past MDD n = 13 | No Past MDD n = 53 |
| Age (years) | 33.1 (4.9) | 33.1 (5.4) | 33.1 (4.8) |
| Gestational age (weeks) | 35.0 (3.2) | 34.2 (3.1) | 35.2 (3.3) |
| Unmarried | 11.8% | 0.0% | 14.5% |
| Multiparous | 57.4% | 61.5 | 56.4% |
| Racial or ethnic minority | 29.4% | 30.8% | 29.1% |
| Pregravid BMI, (kg/m2) | 23.3. | 22.2 (3.3) | 23.5 (3.9) |
| Gestational weight gain (lbs.) | 30.0 (10.0) | 31.2 (11.8) | 29.7 (9.7) |
| Intention to breastfeed | 98.5% | 100% | 98.2% |
| History of past MDDa | 19.1% | 100%* | 0%* |
| Any current psychiatric diagnosisa | 4.4% | 7.7% | 3.6% |
| Any past psychiatric diagnosisa | 27.9% | 100%* | 10.9%* |
| Family history of bipolar disorderb | 13.2% | 15.4% | 12.7% |
| IDS-SR30 scored | 13.4 (9.3) | 15.7 (8.7) | 12.9 (9.5) |
| Oxytocin concentration (pg/mL) | 791.5 (115.7) | 835.3 (82.1) | 780.7 (120.8) |
| Birth Outcomes | |||
| Gestational age at delivery (weeks) | 39.8 (1.1) | 39.9 (1.4) | 39.8 (1.1) |
| Timing of assessment since delivery (weeks) | 5.9 (0.8) | 5.8 (0.5) | 5.9 (0.9) |
| Birth weight in pounds | 7.6 (1.0) | 7.8 (0.9) | 7.6 (1.0) |
| Low birth weight (< 5lbs 5 oz.) | 1.5% | 0.0% | 1.9% |
| Prematurity | 1.5% | 7.7% | 0.0% |
| Gender of baby | |||
| Boy | 51.5% | 61.5% | 49.1% |
| Girl | 48.5% | 38.5% | 50.9% |
| Caesarian delivery | 24.2% | 23.1% | 24.5% |
| Any birth complications | 19.4% | 7.7% | 22.2% |
| Postpartum Measures | |||
| Current breast feeding | 89.4% | 100% | 86.8% |
| Estimated feedings per day | 8.6 (3.3) | 9.3 (1.8) | 8.4 (3.6) |
| Return of menses? | 14.3 % | 0.0% | 18.0% |
| IDS-SR30 scorec | 8.8 (7.6) | 14.1 (9.9) | 7.5 (6.4) |
| EPDS scored | 2.8 (3.4) | 3.8 (3.4) | 2.5 (3.4) |
Modified Mini International Neuropsychiatric Interview Version 5.0.0
Mood Disorders Questionnaire
Inventory of Depressive Symptoms – Self Report 30
Edinburgh Postnatal Depression Scale
p < .05
Approximately one in five participants (n = 13) had a previous major depressive episode; the length of time between the most recent depressive episode and the current pregnancy ranged from 2.5 to 20 years (M = 10.3 years, SD = 6.8 years). Other past psychiatric diagnoses detected by the MINI were: panic disorder (n = 2); post-traumatic stress disorder (n = 1); obsessive-compulsive disorder (n = 1); generalized anxiety disorder (n = 1); alcohol abuse (n = 1); alcohol dependence (n = 1), marijuana abuse (n = 1), antisocial personality disorder (n = 1). Women in both past and no past MDD groups scored in the “no depression” to “mild depression” range on the IDS-SR30 in the third trimester and at 6 weeks postpartum. Mean EPDS scores were below the positive screening threshold of 10 in both groups. With the exception of past MDD and any past psychiatric history, no significant differences in third trimester measures, birth outcomes, or postpartum measures were found between past MDD and no past MDD groups. As shown in Figure 2, plasma oxytocin concentrations were normally distributed [skewness S(SE) = −.094 (.295); kurtosis C(SE) = −.664(.582)]. Scores from the third trimester IDS-SR30 assessment were right-skewed [S(SE) = 3.623(.295)] and therefore natural log-transformed prior to entry into the regression model.
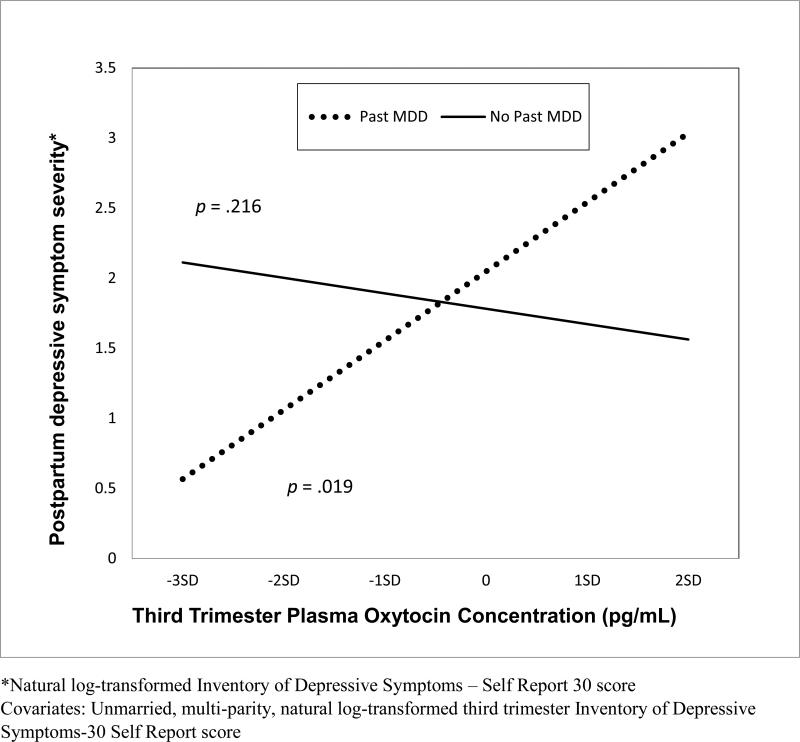
Plasma oxytocin levels were unrelated to PPD symptom severity in univariate (r = .086; p = .493) and multivariate [B(SE) = −.019(.084); β = −.025; t = −.227; p = .821] analyses. For the test of moderation of oxytocin and PPD symptom severity by past MDD history, regression coefficients are shown in Table 2 (F = 14.296 p < .001; df = 4; adjusted R2 = .450). Third trimester depressive symptom severity predicted PPD symptom severity [(B(SE) = 4.219(.718); β = .554; t = 5.874; p < .001]. Third trimester plasma oxytocin concentration interacted with a history of past MDD to predict postpartum depressive symptom severity [(B(SE) = 7.489(2.429); β = .328; t = 3.084; p = .003]. Specifically, as illustrated in Figure 3, higher plasma oxytocin concentration measured in the third trimester predicted greater PPD symptom severity in women with a history of MDD (p = .019), but not in women without (p = .216).
Table 2.
Factors associated depressive symptom severitya at 6 weeks postpartum (N = 66)
| B | SE | β | t | Sig. | 95% C.I. | |
|---|---|---|---|---|---|---|
| Third trimester depressive symptom severitya | 4.219 | .718 | .554 | 5.874 | .000 | 2.783 – 5.655 |
| History of MDDb | 2.304 | 1.956 | .121 | 1.178 | .244 | −1.608 - 6.215 |
| Third trimester plasma oxytocin (pg/mL) | −.906 | .754 | −.119 | −1.202 | .234 | −2.413 - .602 |
| Oxytocin level × History of MDD | 7.489 | 2.429 | .328 | 3.084 | .003 | 2.633 - 12.346 |
Inventory of Depressive Symptoms –Self Report 30 (IDS-SR30)
Modified Mini Neuropsychiatric Interview Version 5.0.0
Figure 3.
Interaction of third trimester plasma oxytocin concentration with past major depressive disorder to predict postpartum depressive symptom severity (N = 66).
Discussion
In contrast to two similar studies to our knowledge (Skrundz et al. 2011; Eapen et al. 2014), we did not find an inverse relationship between third trimester plasma oxytocin level and PPD as hypothesized. Rather, we found a direct relationship between oxytocin and PPD symptom severity, though only among women with a lifetime history of depression. We carefully considered a number of potential explanations for our discrepant findings.
First, as transient increases in oxytocin levels have been shown following affiliative tasks (Cyranowski et al. 2008), it is important to note that in the current study, oxytocin was measured directly following approximately one hour of study assessments with the research assistant and study psychiatrist, followed by assessment of weight and vital signs by a study nurse. While not intended as such, these interactions among participants and research staff, could have simulated the effects of an affiliative task. Indeed the range of oxytocin levels in the current study were fairly narrowly, and normally distributed (Figure 2) in comparison to the very wide ranges and skewed distributions reported by others (Weisman et al. 2013; Lancaster et al. 2015). As very high or very low oxytocin concentrations have been predictive of depressive symptom severity 10 weeks later (Seay et al. 2014), and depressed individuals may experience greater oxytocin increases in response to affiliation relative to non-depressed individuals (Cyranowski et al., 2008), it is possible that measurement of oxytocin at the beginning of the visit (prior to ‘affiliation’) instead of at the end (following ‘affiliation’), could actually have resulted in finding an inverse relationship between oxytocin and PPD symptom severity.
Next, earlier investigations were drawn from different populations, with different individual histories of both adversity and social support across their lifespans. PPD symptoms were assessed at two weeks postpartum in the Skrundz study (2011) compared to assessment at six weeks postpartum in the current study. Women in the Eapen study (2014) were recruited for a larger study on separation anxiety whereas we specifically recruited healthy women not receiving any treatment for a psychiatric condition. In the Zelkowitz study (2014), while an inverse relationship between oxytocin and depressive symptoms was found in their high stress group, a direct relationship (high oxytocin—greater depressive symptom severity) was observed in the low stress group, which roughly mirrors the direct relationship found in our sample. In summary, our present findings are tentative, and support the value of studying the predictive value of oxytocin in this context of past MDD history.
Next, interpretations of the functional effects of oxytocin must take into account cross-reactivity of oxytocin with other peptide receptors, including those for vasopressin (Albers 2015). At high levels oxytocin might stimulate vasopressin receptors, with effects that are contradictory to those typically attributed to oxytocin (Carter 2014). We have previously found that high levels of oxytocin in the third trimester could be associated with a long or painful labor, possibly through actions on the vasopressin receptor, which may slow labor (Prevost et al. 2014). Thus, the relationship of higher third trimester oxytocin levels to greater PPD symptom severity among women with prior MDD history could have been mediated by the stimulation of vasopressin receptors and associated depression symptoms (Frank & Landgraf 2008).
Variation in methods, including extraction of samples and different antibodies used for measuring oxytocin are another possible source of differences across studies. For this reason, direct comparisons of hormone values across studies are challenging. Of note, mass spectrometry methods to determine the true values for oxytocin in human plasma have recently led to the discovery that oxytocin is in fact available in concentrations as high as 1 ng/ml in human blood (Martin-Protean 2015) and that oxytocin is sequestered/bound to a variety of other molecules in plasma and in whole blood. Thus, assay methods that involve extraction, as well as separation of plasma from other components of blood, although recommended by some (Szeto et al. 2011; McCullough et al. 2013), greatly underestimate the levels of oxytocin in human blood. This putative carrier system might influence the presentation of the epitope (antibody binding site on the peptide), and explain differences among the values obtained with different antibodies.
A final factor that could contribute to discrepancies among studies is that different antibodies may recognize different forms of oxytocin. For example, in addition to the classical 9 amino acid form, these antibodies may recognize the 6 amino acid ring of oxytocin (tocinoic acid) or the precursor molecule, which contains 10-12 amino acids (Gainer 1982). As more reliable methods become available, individual differences in the oxytocin peptide and measures of receptor availability may offer new insights and more reliable methods for the prediction of vulnerability to postpartum depression.
Limitations
While pregnancy, postpartum, and birth outcomes were not different between women with past MDD and women without, providing some support of the validity of findings, replication in a larger sample would be important. Next, depressive symptom severity was in the mild range, so it is unknown whether findings also apply to a broader range of depressive symptom severity. Finally, measures of the oxytocin receptor or the gene for this receptor were not available for the present study; these might have provided additional clues to individual genetic or epigenetic differences in the vulnerability to PPD (Bell et al. 2015).
Conclusions
Confirmation of our findings in larger more representative samples can contribute to a more in-depth understanding of the etiology of perinatal depression, and may lead to improved prediction of PPD. In the future, availability of an “objective” blood test could help to challenge misperceptions or stigma associated with depression by providing biological evidence about risk for this condition. In light of the far-reaching consequences of untreated PPD to women and their children, the ability to predict which individuals are at greatest risk for developing PPD before it manifests yields the exciting possibility for prevention.
Acknowledgements
We are deeply appreciative of the women who volunteered their time and efforts to participate in this study, and Rebecca L. Newmark, BA for editorial assistance in the preparation of this manuscript for publication.
Funding:
This work was funded by an Evergreen Invitational Women`s Health Grant from the Northwestern Memorial Foundation to Dr. Massey (9/12/13 agreement date), and grant K23 DA037913 from the National Institute on Drug Abuse (NIDA) to Dr. Massey. The Evergreen Invitational, Northwestern Memorial Foundation, and NIDA had no role in the study design, collection, analysis or interpretation of data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Previous presentation: None
Location of work:
1 Department of Psychiatry & Behavioral Sciences, Northwestern University Feinberg School of Medicine, Chicago, IL
2 The Kinsey Institute at Indiana University, Bloomington, IN
Conflicts of Interest:
The Department of Psychiatry at Northwestern University received contractual fees for Dr. Wisner's consultation to Quinn Emanuel Urquhart & Sullivan, LLP (New York City), who represent Pfizer Pharmaceutical Company.
Conflict of Interest Statement
None declared.
References
- 1.Albers HE. Species, sex and individual differences in the vasotocin/vasopressin system: Relationship to neurochemical signaling in the social behavior neural network. Front. Neuroendocrinol. 2015;36:49–71. doi: 10.1016/j.yfrne.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists (ACOG) Screening for perinatal depression. Committee Opinion No. 630. Obstet. Gynecol. 2015;125:1268–71. doi: 10.1097/01.AOG.0000465192.34779.dc. [DOI] [PubMed] [Google Scholar]
- 3.Bell AF, Carter CS, Steer CD, Golding J, Davis JM, Steffen AD, Rubin LH, Lillard TS, Gregory SP, Harris JC, Connelly JJ. Interaction between oxytocin receptor DNA methylation and genotype is associated with risk of postpartum depression in women without depression in pregnancy. Front. Genet. 2015;6:243. doi: 10.3389/fgene.2015.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter CS, Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Ann. N.Y. Acad. Sci. 1997;807:164–174. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- 5.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocr. 1998;23(8):779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 6.Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the post-partum period. Prog. Brain Res. 2001;133:241–249. doi: 10.1016/s0079-6123(01)33018-2. [DOI] [PubMed] [Google Scholar]
- 7.Carter CS. Oxytocin pathways and the evolution of human behavior. Annu. Rev. Psychol. 2014;65:17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- 8.Cox JL, Chapman G, Murray D, Jones P. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. J. Affect. Disord. 1996;39(3):185–189. doi: 10.1016/0165-0327(96)00008-0. [DOI] [PubMed] [Google Scholar]
- 9.Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom. Med. 2008;70(9):967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment preferences: A qualitative systematic review. Birth. 2006;33(4):323–331. doi: 10.1111/j.1523-536X.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- 11.Eapen V, Dadds M, Barnett B, Kohlhoff J, Khan F, Radom N, Silove DM. Separation anxiety, attachment and inter-personal representations: Disentangling the role of oxytocin in the perinatal period. PLoS One. 2014;9:e107745. doi: 10.1371/journal.pone.0107745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: considering stress and affiliation components of human bonding. Dev. Sci. 2011;14(4):752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- 13.Feldman R. Oxytocin and social affiliation in humans. Horm. Behav. 2012;61(3):380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, Shalev I, Ebstein RP. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol. Psychiatry. 2012;72(3):175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Frank E, Landgraf R. The vasopressin system—from antidiuresis to psychopathology. Eur. J. Pharmacol. 2008;583(2):226–242. doi: 10.1016/j.ejphar.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 16.Gainer H. Precursors of vasopressin and oxytocin. Prog. Brain Res. 1982;60:205–215. doi: 10.1016/S0079-6123(08)64388-5. [DOI] [PubMed] [Google Scholar]
- 17.Goldman MB, Marlow-O'Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr. Res. 2008;98:247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman JH. Women‘s attitudes, preferences, and perceived barriers to treatment for perinatal depression. Birth. 2009;36(1):60–69. doi: 10.1111/j.1523-536X.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- 19.Gouin JP, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, Stowell J, Kiecolt-Glaser JK. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocr. 2010;35(7):1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammock E, Veenstra-VanderWeele J, Yan Z, Kerr TM, Morris M, Anderson G, Carter CS, Cook EH, Jacob S. Examining autism spectrum disorders by biomarkers: example from the oxytocin and serotonin systems. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:712–721. doi: 10.1016/j.jaac.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschfeld RM, Williams JB, Spitzer RL, Calabrese JR, Flynn L, Keck PE, Jr., Lewis L, McElroy SL, Post RM, Rapport DJ, Russell JM, Sachs GS, Zajecka J. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am. J. Psychiatry. 2000;157(11):1873–1875. doi: 10.1176/appi.ajp.157.11.1873. [DOI] [PubMed] [Google Scholar]
- 22.Klengel T, Pape J, Binder EB, Mehta D. The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacol. 2014;80:115–132. doi: 10.1016/j.neuropharm.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Soeken TA, Cromer SJ, Martinez SR, Hardy LR, Strathearn L. Oxytocin and postpartum depression: Delivering on what's known and what's not. Brain Res. 2014;1580:219–232. doi: 10.1016/j.brainres.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lancaster K, Carter CS, Pournajafi-Nazarloo H, Karaoli T, Lillard TS, Jack A, Davis JM, Morris JP, Connelly JJ. Plasma oxytocin explains individual differences in neural substrates of social perception. Front. Hum. Neurosci. 2015;9:132. doi: 10.3389/fnhum.2015.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacKinnon AL, Gold I, Feeley N, Hayton B, Carter CS, Zelkowitz P. The role of oxytocin in mothers‘ theory of mind and interactive behavior during the perinatal period. Psychoneuroendocr. 2014;48:52–63. doi: 10.1016/j.psyneuen.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Martin-Protean, LLC. [September 17, 2015];Oxytocin quantification products. 2015 http://martin-protean.com/oxytocin.html.
- 27.McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 2013;37(8):1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 28.McFarland J, Salisbury AL, Battle CL, Hawes K, Halloran K, Lester BM. Major depressive disorder during pregnancy and emotional attachment to the fetus. Arch. Womens Ment. Health. 2011;14(5):425–34. doi: 10.1007/s00737-011-0237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 30.Prevost M, Zelkowitz P, Tulandi T, Hayton B, Feeley N, Carter CS, Joseph L, Pournajafi-Nazarloo H, Yong EY, Abenhaim H, Gold I. Oxytocin in pregnancy and the postpartum: relations to labor and its management. Front. Public Health. 2014;27(2):1. doi: 10.3389/fpubh.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen. Hosp. Psychiatry. 2004;26(4):289–295. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr. Res. 2010;124:13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rush AJ, Carmody T, Reimitz PE. The Inventory of Depressive Symptomatology (IDS): Clinician (IDS-C) and self-report (IDS-SR) ratings of depressive symptoms. Int. J. Methods Psychiatric Res. 2000;9:45–59. [Google Scholar]
- 34.Rush AJ, Trivedi MH, Carmody TJ, Ibrahim HM, Markowitz JC, Keitner GI, Kornstein SG, Arnow B, Klein DN, Manber R, Dunner DL, Gelenberg AJ, Kocsis JH, Nemeroff CB, Fawcett J, Thase ME, Russell JM, Jody DN, Borlan FE, Keller MB. Self-reported depressive symptom measures: Sensitivity to detecting change in a randomized, controlled trial of chronically depressed, nonpsychotic outpatients. Neuropsychopharmacol. 2005;30:405–416. doi: 10.1038/sj.npp.1300614. [DOI] [PubMed] [Google Scholar]
- 35.Seay JS, Lattie E, Schneiderman N, Antoni MH, Fekete EM, Mendez AJ, Szeto A, Fletcher MA. Linear and quadratic associations of plasma oxytocin with depressive symptoms in ethnic minority women living with HIV. J. Appl. Biobehav. Res. 2014;19(1):70–78. [Google Scholar]
- 36.Sheehan DV, Lecubrier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Herqueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 37.Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacol. 2011;36(9):1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun H, Kennedy PJ, Nestler EJ. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacol. 2013;38(1):124–137. doi: 10.1038/npp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, Schneiderman N, Mendez AJ. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom. Med. 2011;73(5):393. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tops M, Buisman-Pijlman FTA, Carter CS. Attachment and oxytocin as modulators of stress and resilience. In: Kent M, Davis MC, Reich JW, editors. Handbook of Resilience: Approaches to Stress and Trauma. Routledge, New York: 2013. pp. 115–130. [Google Scholar]
- 41.Unternaehrer E, Luers P, Mill J, Dempster E, Meyer AH, Staehli S, Lieb R, Hellhammer DH, Meinlschmidt G. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF) after acute psychosocial stress. Transl. Psychiatry. 2012;2(8):e150. doi: 10.1038/tp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, Feldman R. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocr. 2013;38(5):694–701. doi: 10.1016/j.psyneuen.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Wen SW, Liu S, Kramer MS, Joseph KS, Levitt C, Marcoux S, Liston RM. Impact of prenatal glucose screening on the diagnosis of gestational diabetes and on pregnancy outcomes. Am. J. Epidemiol. 2000;152(11):1009–1014. doi: 10.1093/aje/152.11.1009. [DOI] [PubMed] [Google Scholar]
- 44.Wisner KL, Chambers C, Sit DKY. Postpartum depression: A major public health problem. J. Am. Med. Assoc. 2006;296(21):2616–2618. doi: 10.1001/jama.296.21.2616. [DOI] [PubMed] [Google Scholar]
- 45.Wisner KL, Sit DKY, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, Eng HF, Luther JF, Wisniewski SR, Costantino ML, Confer AL, Moses-Kolko EL, Famy CS, Hanusa BH. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70(5):490–498. doi: 10.1001/jamapsychiatry.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen. Hosp. Psychiatry. 2009;31(5):403–413. doi: 10.1016/j.genhosppsych.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zelkowitz P, Gold I, Feeley N, Hayton B, Carter CS, Tulandi T, Abenhaim HA, Levin P. Psychosocial stress moderates the relationships between oxytocin, perinatal depression, and maternal behavior. Horm. Behav. 2014;66:351–360. doi: 10.1016/j.yhbeh.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Zlotnick C, Miller IW, Pearlstein T, Howard M, Sweeney P. A preventive intervention for pregnant women on public assistance at risk for postpartum depression. Am. J. Psychiatry. 2006;163(8):1443–1445. doi: 10.1176/appi.ajp.163.8.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]