Abstract
Importance
Physicians and investigators have sought to determine the relationship between body mass index (BMI) and colorectal cancer (CRC) outcomes, but methodologic limitations including sampling selection bias, reverse causality, and “collider” bias have prevented the ability to draw definitive conclusions.
Objective
We evaluated the impact of BMI at the time of, and following colorectal cancer (CRC) diagnosis, on mortality in a complete population using causal diagrams.
Design
Retrospective observational study with prospectively collected data
Setting
Kaiser Permanente Northern California
Participants
3,408 men and women diagnosed 2006-2011 with stages I-III colorectal cancer who had surgery
Exposures
BMI at diagnosis, and 15 months following diagnosis
Main Outcomes and Measures
Hazard ratios for all-cause and CRC-specific mortality, relative to normal-weight patients, adjusted for sociodemographics, disease severity, treatment, and pre-diagnosis BMI.
Results
At-diagnosis BMI was associated with all-cause mortality in a nonlinear fashion, with underweight (BMI<18.5 kg/m2, hazard ratio [HR]=2.65, 95% confidence interval [CI]:1.63-4.31) and class II/III obese (BMI≥35 kg/m2, HR=1.33, 95% CI: 0.89-1.98) patients exhibiting elevated mortality risks, compared with low normal-weight (BMI 18.5-<23 kg/m2) patients. In contrast, high-normal-weight (BMI 23-<25 kg/m2, HR=0.77, 95% CI: 0.56-1.06), low-overweight (BMI 25-<28 kg/m2, HR=0.75, 95% CI:0.55-1.04), and high-overweight (BMI 28-<30 kg/m2, HR=0.52, 95% CI: 0.35-0.77) patients had lower mortality risks, and class I obese (BMI 30-<35 kg/m2) patients showed no difference in risk. Spline analysis confirmed a U-shaped relationship in participants (p-value, test for nonlinearity<0.001) with lowest mortality at BMI=28 kg/m2. Associations with CRC-specific mortality were similar. Associations of post-diagnosis BMI and mortality were also similar, but class I obese had significantly lower all-cause and cancer-specific mortality risks.
Conclusions and Relevance
Overweight patients consistently had the lowest mortality after a CRC diagnosis. Though strong scientific evidence shows that exercise in cancer patients should be encouraged, findings suggest, among overweight CRC patients, that recommendations for weight loss in the immediate post-diagnosis period may be unwarranted.
Keywords: Body mass index, weight, survival, mortality, colorectal cancer
Introduction
Body mass index (BMI) is positively associated with the risk of colorectal cancer1-9. For that reason, investigators have hypothesized that overweight/obesity leads to worse colorectal cancer (CRC) prognosis. However, though previous studies have shown an apparently elevated mortality risk in class II/III obese (BMI≥35 kg/m2) patients10-12, associations of class I obesity (BMI 30-<35 kg/m2) and survival are mixed; some studies show a higher mortality risk6, 10, 13-16 and others show no higher17 or a possible lower18 mortality risk, depending in part on when BMI is measured relative to diagnosis. Overweight and obese CRC patients have shown lower mortality risks11, 18 compared with normal- or underweight patients when BMI is measured following diagnosis but concerns are that this “obesity paradox” could reflect sampling selection bias, reverse causality, and/or collider bias19-21. We sought to employ methods to overcome these concerns in our evaluation of the impact of BMI on post-diagnosis mortality.
First, many studies of weight and CRC survival are conducted in samples recruited after diagnosis causing concerns that only the healthiest may enroll. Such sampling selection bias could produce an obesity paradox if the sickest overweight patients are less likely to enroll than the sickest normal-weight patients or they die prior to enrollment. Second, underweight or normal-weight patients may have higher mortality, compared with overweight patients because they may include cachectic patients nearing death, i.e., reverse causality22. A third concern is collider bias20; in the presence of an unmeasured risk factor for CRC diagnosis (see eFigure 1 in the supplement), selecting a population based on a CRC diagnosis could introduce a spurious association between an exposure (e.g., post-diagnosis BMI) and an outcome (e.g., death) that could reverse the direction of association, making a harmful exposure appear protective21. I.e., collider bias may occur if overweight/obesity leads to higher disease incidence, but unmeasured risk factors occurring disproportionately in normal-weight patients are more strongly related to mortality than is overweight/obesity. In this case, spuriousness can be avoided with adjustment for pre-diagnosis BMI (eFigure 1).
Using electronic medical record (EMR) data collected as a part of routine clinical care within the Kaiser Permanente Northern California (KPNC) population, we examined the effect of BMI at, and following, diagnosis on all-cause and CRC-specific mortality in KPNC CRC patients diagnosed from 2006-2011, using several strategies to overcome bias. These included the use of data from a complete population of CRC patients with longitudinally-collected data, examination of associations in stage I patients not expected to be at imminent risk of death, stratification by weight loss status, and the use of causal diagrams to identify sufficient adjustment sets23 of covariates. Related to this, we were able to adjust for prospectively collected data on pre-diagnosis BMI, a unique feature of our data.
We hypothesized higher mortality risks in underweight and class II/III obese CRC patients, and a lower risk in overweight, vs. normal-weight, patients.
Methods
Study Population
The study population consisted of all patients ages 18-80 from KPNC diagnosed from 2006-2011 with stage I-III invasive CRC whose cancer was confirmed by computed tomography, who received surgery, and for whom an electronic weight and height were available at diagnosis. Case ascertainment began in 2006, one year after weights routinely became available in the EMR, to enable inclusion of pre-diagnosis weight in analyses. A third of the California population are KP members; members represent the underlying population except at socioeconomic extremes24. Approximately 5% of patients were missing at-diagnosis BMI data in a year. Loss to follow-up was <3%. 49.7% of study participants were female and 50.3% male. A waiver of written informed consent was obtained and the study was approved by the KPNC institutional review board.
Data Collection
Body mass index
Height and weight were measured by a medical assistant at each medical visit. BMI was computed in kilograms per height in meter squared. Patients were included in analyses if they had a recorded BMI <6 months of a CRC diagnosis prior to surgery. BMI closest to the diagnosis date (range -5.3 to 6.0 months, median=0.0 mo) was used in analyses of at-diagnosis BMI. BMI measured ≥9 months before CRC diagnosis (range 9.0-68.9 months prior, median=-12.5 mo) was used to assess pre-diagnosis BMI, and BMI measured approximately fifteen months following diagnosis (range 9.0-27.0 months, median=14.7 mo), post-treatment, was used to assess post-diagnosis BMI.
We initially categorized BMI using World Health Organization (WHO) categories25. However, the optimal weight for CRC patients is unknown and large BMI categories may obscure risk in the case of U- or J-shaped relationships, so we categorized BMI into finer categories (<18.5, 18.5-<23, 23-<25, 25-<28, 28-<30, 30-<35, and 35+) consistent with those used in a recent meta-analysis of weight and mortality26, and to distinguish risks for lower (18.5-<23 kg/m2) and upper (23-<25 kg/m2) normal-weight, as well as lower (25-<28 kg/m2) and upper (28-<30 kg/m2) overweight.
Clinical variables and endpoints
KPNC Cancer Registry data and the EMR were reviewed for information on prognostic factors, including disease stage, tumor characteristics, surgical procedures, and treatment (chemotherapy, radiation therapy). Data on overall and CRC-specific mortality were obtained from the KPNC computerized mortality file, which is comprised of data from the California State Department of Vital Statistics, U.S. Social Security Administration, and KPNC utilization data sources. Colorectal cancer death was attributed to persons if CRC was listed as a cause of death on the death certificate.
Other covariate data
EMR data were accessed for information on numerous potential confounders including demographics (self-reported race is included in the EMR) and smoking status. The Charlson index was used to measure (any vs. no) comorbidity. Physical activity data (minutes per week of moderate or vigorous activity) were available in 22% of the population.
Statistical analysis
We used analysis of covariance to examine linear covariates by categories of at-diagnosis BMI adjusted for age. For categorical variables, we examined covariate distributions by categories of at-diagnosis BMI.
We used Cox proportional hazards regression models to examine associations between BMI at the time of, and following diagnosis, and all-cause and CRC-specific mortality. For analyses of at-diagnosis BMI, time was computed from time of diagnosis. In analyses of post-diagnosis BMI, time was computed from the time of the follow-up BMI measure to time of event or study end.
To address possible reverse causality, we evaluated associations in those with stage I cancer. Typically, researchers eliminate deaths occurring early after measurement of a risk factor. Though this can lead to collider bias20, this strategy is commonly used so we nonetheless conducted sensitivity analyses eliminating deaths occurring within the first six months and the first year to facilitate comparison to other studies.
Potential confounding variables in models were selected based on subject matter expertise encoded in directed acyclic graphs (DAGs)27, diagrams that help elucidate the causal structure relating variables under study. We compared models controlling for age, race, and sex, to those adjusted additionally for stage, grade, cancer site (colon; distal or proximal, and rectal), smoking, and physical activity. To address concerns about the potential for collider bias because of the restriction of analyses to diagnosed CRC patients (Supplementary eFigure 1), we adjusted for pre-diagnosis BMI when evaluating the effects of both at- and post-diagnosis BMI. Adjustment for chemotherapy and radiation were not suggested by the DAG in the analysis of at-diagnosis BMI based on the time order of covariates. We nonetheless included these variables in models based on convention; adjustment had no substantive effect on associations. We considered adjustment for comorbidity but sought to avoid overadjustment since CRC and comorbidities have mechanisms in common related to BMI.
We used both standard WHO25, and expanded, BMI categories. We also examined possible nonlinear relationships between BMI and survival, nonparametrically, and by sex, with restricted cubic splines28, a technique enabling specification of a relationship between two variables when the function is nonlinear. Tests for nonlinearity used the likelihood ratio test, comparing the model with the linear term to one with linear and cubic spline terms.
Finally, we conducted analyses of at-diagnosis BMI and outcomes, stratified in separate analyses by sex, age (<65 vs. ≥65 years), race, stage, comorbidity, treatment status, CRC site, and weight loss status between diagnosis and post-diagnosis. Heterogeneity in associations in stratified analyses were examined via introduction of cross-product terms for BMI categories and stratification variables in regression models and evaluation of significance with likelihood ratio χ2 tests. We conducted sensitivity analyses restricting to the population with complete information on pre-diagnosis BMI. We also conducted tests of proportionality with variable by time interactions. Tests of statistical significance were two-sided. Significant results denote p-values≤0.05.
Results
Of the 3,408 CRC patients, 617 died (411 from CRC), with follow-up ranging from 0-8.7 years, with a median 4.5 years follow-up. For analyses of post-diagnosis BMI and mortality (N=3,157), 482 died (317 from CRC), and follow-up ranged from 0-7.9 years, with a median 3.5 years follow-up.
Baseline characteristics
Examining covariates, age was inversely associated with at-diagnosis BMI. Women were more likely to be underweight, normal weight, and class II/III obese, compared with men. Asians were more likely to be underweight or normal-weight; Whites, Blacks, and Hispanics were more likely to be class II/III obese. Those with stages II and III cancers were more likely to be underweight or normal weight compared with those with stage I cancer. However, those with stage III cancer were more likely to be obese (BMI≥30 kg/m2) than those with earlier stage cancer. Predictably, levels of smoking and physical activity were higher in underweight and normal-weight patients. Distal cancers were more common among obese patients; those with BMI<25 kg/m2 were more likely to have proximal colon or rectal cancers. Underweight and class II/III obese patients were less likely to receive chemotherapy (Table 1).
Table 1. Selected characteristics according to categories of BMI at diagnosis in the Kaiser Permanente Northern California population (N=3,408).
| BMI at diagnosis (kg/m2) – WHO categories | |||||
|---|---|---|---|---|---|
|
| |||||
| <18.5 | 18.5-<25 | 25-<30 | 30-<35 | >=35 | |
| N | 67 | 1,050 | 1,214 | 671 | 408 |
| N, pre-diagnosis BMI | 45 | 729 | 823 | 461 | 287 |
| N, post-diagnosis BMI | 50 | 974 | 1,141 | 623 | 369 |
| Age at diagnosis | 65 | 64 | 63 | 62 | 60 |
| Mean pre-diagnosis BMI | 18.5 | 23.6 | 28.1 | 32.8 | 40.7 |
| Mean BMI at diagnosis | 17.4 | 22.6 | 27.4 | 32.1 | 40.4 |
| Female (%) | 77.3 | 58.3 | 40.4 | 44.6 | 58.7 |
| Race | |||||
| White | 57.4 | 62.5 | 65.1 | 69.0 | 70.0 |
| Black | 8.8 | 5.8 | 6.4 | 8.5 | 10.6 |
| Hispanic | 2.9 | 6.8 | 12.4 | 15.3 | 13.0 |
| Asian/Pacific Islander | 30.9 | 24.6 | 15.5 | 6.7 | 4.7 |
| Stage | |||||
| I | 26.5 | 28.4 | 30.7 | 31.3 | 31.4 |
| II | 38.2 | 33.5 | 33.2 | 27.3 | 28.7 |
| III | 35.3 | 38.2 | 36.1 | 41.3 | 40.0 |
| Grade | |||||
| I | 2.9 | 7.0 | 7.6 | 7.2 | 7.4 |
| II | 75.5 | 75.4 | 75.9 | 74.5 | 75.0 |
| III | 16.2 | 11.1 | 10.2 | 11.0 | 11.5 |
| IV | 1.5 | 1.8 | 1.2 | 1.6 | 1.0 |
| Unknown | 5.9 | 4.7 | 5.1 | 5.7 | 5.2 |
| Site | |||||
| Proximal | 52.9 | 46.3 | 43.2 | 42.8 | 47.1 |
| Distal | 17.7 | 24.4 | 27.2 | 28.7 | 29.4 |
| Rectal | 29.4 | 29.3 | 29.6 | 28.5 | 23.5 |
| Treatment | |||||
| Chemotherapy | 38.5 | 45.6 | 44.0 | 45.7 | 40.5 |
| Radiation | 15.9 | 16.0 | 16.1 | 14.6 | 10.2 |
| Physical activity (min/wk) | |||||
| 0 | 33.3 | 24.0 | 24.4 | 34.2 | 46.2 |
| >0-<120 | 22.2 | 31.3 | 34.6 | 34.2 | 36.9 |
| ≥120 | 44.4 | 44.6 | 41.0 | 31.5 | 17.9 |
| Smoking status | |||||
| Never | 37.3 | 48.4 | 47.0 | 43.8 | 46.6 |
| Past | 43.3 | 37.3 | 42.3 | 45.0 | 42.1 |
| Current | 19.4 | 14.3 | 10.8 | 11.0 | 10.8 |
BMI and all-cause mortality
Using WHO criteria, in models adjusted for age, sex, and race, at-diagnosis BMI was associated with all-cause and CRC-specific mortality in a nonlinear fashion, with underweight (BMI<18.5 kg/m2) and class II/III obese (BMI≥35 kg/m2) patients exhibiting elevated, and overweight (BMI 25-<30 kg/m2) patients showing reduced, mortality risks, compared with normal-weight (BMI 18.5-<25 kg/m2) patients. Mortality risks of class I obese and normal-weight patients were similar. In multivariable-adjusted analyses, associations were similar though the association for overall mortality in overweight patients was somewhat attenuated (Table 2). After comorbidity adjustment, the association between class II/III obesity and all-cause mortality was no longer significant (HR=1.27, 95% CI: 0.88-1.84).
Table 2. Hazard ratios (HR) of at-diagnosis body mass index and mortality* (N=3,408).
|
|
||||||
|---|---|---|---|---|---|---|
| N | Event | Age, race, sex adjusted HR | 95% CI | MV-adjusted HR | 95% CI | |
|
|
|
|||||
| All-cause mortality | ||||||
|
|
|
|||||
| BMI (kg/m2)—WHO categories** | ||||||
|
|
|
|||||
| <18.5 | 67 | 32 | 3.55 | (2.44, 5.17) | 3.01 | (1.88, 4.83) |
| 18.5-<25 | 1,050 | 198 | ref | ref | ||
| 25-<30 | 1,214 | 179 | 0.75 | (0.61, 0.92) | 0.81 | (0.64, 1.03) |
| 30-<35 | 671 | 119 | 0.94 | (0.74, 1.19) | 1.03 | (0.77, 1.38) |
| 35+ | 406 | 89 | 1.36 | (1.06, 1.76) | 1.63 | (1.13, 2.33) |
| BMI (kg/m2)—expanded categories | ||||||
| <18.5 | 67 | 32 | 3.06 | (2.07, 4.53) | 2.65 | (1.63, 4.31) |
| 18.5-<23 | 553 | 118 | Ref | ref | ||
| 23-<25 | 497 | 80 | 0.71 | (0.54, 0.95) | 0.77 | (0.56, 1.06) |
| 25-<28 | 787 | 125 | 0.70 | (0.54, 0.91) | 0.75 | (0.55, 1.04) |
| 28-<30 | 427 | 54 | 0.54 | (0.39, 0.74) | 0.52 | (0.35, 0.77) |
| 30-<35 | 671 | 119 | 0.80 | (0.62, 1.04) | 0.81 | (0.57, 1.15) |
| 35+ | 406 | 89 | 1.17 | (0.88, 1.55) | 1.33 | (0.89, 1.98) |
| CRC-specific mortality | ||||||
|
|
|
|||||
| BMI (kg/m2)—WHO categories | ||||||
|
|
|
|||||
| <18.5 | 67 | 20 | 3.29 | (2.05, 5.28) | 3.35 | (1.92, 5.87) |
| 18.5-<25 | 1,050 | 130 | ref | Ref | ||
| 25-<30 | 1,214 | 116 | 0.74 | (0.57, 0.95) | 0.77 | (0.57, 1.03) |
| 30-<35 | 671 | 92 | 1.07 | (0.81, 1.40) | 1.06 | (0.75, 1.50) |
| 35+ | 406 | 53 | 1.14 | (0.82, 1.58) | 1.47 | (0.96, 2.27) |
| BMI (kg/m2)—expanded categories | ||||||
| <18.5 | 67 | 20 | 3.02 | (1.84, 4.96) | 3.18 | (1.78, 5.69) |
| 18.5-<23 | 553 | 73 | ref | Ref | ||
| 23-<25 | 497 | 57 | 0.83 | (0.58, 1.17) | 0.89 | (0.60, 1.31) |
| 25-<28 | 787 | 84 | 0.76 | (0.55, 1.05) | 0.81 | (0.55, 1.19) |
| 28-<30 | 427 | 32 | 0.51 | (0.34, 0.78) | 0.45 | (0.28, 0.74) |
| 30-<35 | 671 | 92 | 0.97 | (0.71, 1.33) | 0.88 | (0.58, 1.32) |
| 35+ | 406 | 53 | 1.04 | (0.73, 1.49) | 1.12 | (0.61, 2.06) |
Age-adjusted HR adjusted for age (continuous), sex, and race (White (ref), Black, Hispanic, Asian/Pacific Islander, Other). MV-adjusted analysis adjusted additionally for stage (I (ref), II, III), grade (1, 2 (ref), 3, 4), chemotherapy (no (ref), yes), radiation (no (ref), yes), disease site (proximal (ref), distal, rectal), smoking (never (ref), past, current), physical activity (0, >0 to <120, 120+ minutes/wk), and pre-diagnosis BMI (<18.5, 18.5-<25 (ref), 25-<30, 30-<35, 35+ kg/m2).
Note: Adjustment for comorbidity attenuated the association of those with BMI 35+ for all-cause mortality to HR=1.27, 95% CI: 0.88-1.84 and for other cause mortality in analyses (data not shown). Adjustment for comorbidity had minimal influence on associations with colorectal cancer-specific mortality.
Using expanded BMI categories, in models adjusted for age, sex, and race, at-diagnosis BMI was also associated with all-cause and CRC-specific mortality in a nonlinear fashion. Multivariable-adjusted results were qualitatively similar (Table 2). Underweight (BMI<18.5 kg/m2, hazard ratio [HR]=2.65, 95% confidence interval [CI]:1.63-4.31) and class II/III obese (BMI≥35 kg/m2, HR=1.33, 95% CI:0.89-1.98) patients exhibited elevated mortality risks; high-normal-weight (BMI 23-<25 kg/m2, HR=0.77, 95% CI:0.56-1.06), low-overweight (BMI 25-<28 kg/m2, HR=0.75, 95% CI:0.55-1.04), and high-overweight (BMI 28-<30 kg/m2, HR=0.52, 95% CI:0.35-0.77) patients showed reduced mortality risks; and class I obese (BMI 30-<35 kg/m2) patients showed no difference in risk, compared with low-normal-weight (BMI 18.5-<23 kg/m2) patients. Mortality risk did not differ comparing high-normal-weight and low-overweight patients (Wald χ2=0.03, p=0.87).
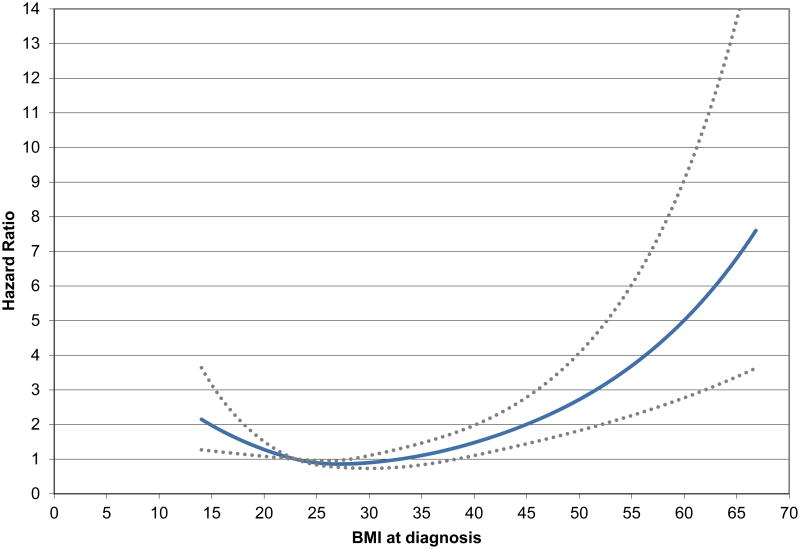
In spline analyses of all-cause mortality, risk was lowest among CRC patients with a BMI=28 kg/m2 (Figure 1), evident in men (eFigure 2) and women (eFigure 3). Tests for linear trend suggested linear relationships of BMI with mortality hazard overall (p=0.01) and women (p=0.01) but not in men (p=0.21). In all cases, spline analyses provided evidence of a nonlinear component (p<0.001 for nonlinear association [men, p<0.001; women, p=0.02].
Figure 1.
Spline curve of BMI at diagnosis and overall mortality, p-value, test for nonlinearity <0.0001. Adjusted for age, sex, race, stage, site, grade, chemotherapy, radiation, pre-diagnosis BMI, smoking, and physical activity.
Patterns of associations of post-diagnosis (Table 3) BMI and all-cause mortality were similar to results for at-diagnosis BMI and all-cause mortality. However, class I obese patients had a lower mortality risk, and class II/III obese patients showed no higher mortality risk compared with low normal-weight patients.
Table 3. Post-diagnosis body mass index (BMI) and mortality outcomes (N=3,157).
|
|
||||||
|---|---|---|---|---|---|---|
| N | Event | Age, race, sex adjusted HR | 95% CI | MV-adjusted HR | 95% CI | |
|
|
|
|||||
| All-cause mortality | ||||||
|
|
|
|||||
| BMI (kg/m2) | ||||||
|
|
|
|||||
| <18.5 | 78 | 38 | 3.71 | (2.52, 5.45) | 3.38 | (2.19, 5.20) |
| 18.5-<23 | 539 | 108 | Ref | Ref | ||
| 23-<25 | 411 | 60 | 0.70 | (0.51, 0.96) | 0.72 | (0.52, 1.02) |
| 25-<28 | 721 | 90 | 0.56 | (0.42, 0.75) | 0.56 | (0.41, 0.77) |
| 28-<30 | 396 | 44 | 0.46 | (0.32, 0.66) | 0.39 | (0.26, 0.58) |
| 30-<35 | 643 | 78 | 0.57 | (0.42, 0.77) | 0.51 | (0.35, 0.73) |
| 35+ | 400 | 72 | 0.96 | (0.70, 1.30) | 0.85 | (0.56, 1.30) |
| CRC-specific mortality | ||||||
|
|
|
|||||
| <18.5 | 78 | 25 | 3.25 | (2.00, 5.27) | 3.21 | (1.88, 5.47) |
| 18.5-<23 | 539 | 70 | Ref | Ref | ||
| 23-<25 | 411 | 38 | 0.70 | (0.47, 1.04) | 0.69 | (0.46, 1.05) |
| 25-<28 | 721 | 53 | 0.53 | (0.37, 0.76) | 0.50 | (0.34, 0.75) |
| 28-<30 | 396 | 30 | 0.53 | (0.34, 0.82) | 0.42 | (0.26, 0.67) |
| 30-<35 | 643 | 60 | 0.67 | (0.47, 0.96) | 0.56 | (0.36, 0.85) |
| 35+ | 400 | 45 | 0.86 | (0.59, 1.27) | 0.84 | (0.51, 1.37) |
Age-adjusted HR adjusted for age (continuous), sex, and race (White (ref), Black, Hispanic, Asian/Pacific Islander, Other). MV-adjusted analysis adjusted additionally for stage (I (ref), II, III), grade (1, 2 (ref), 3, 4), chemotherapy (no (ref), yes), radiation (no (ref), yes), disease site (proximal (ref), distal, rectal), smoking (never (ref), past, current), physical activity (0, >0 to <120, 120+ minutes/wk), and pre-diagnosis BMI (<18.5, 18.5-<23 (ref), 23-<25, 25-<28, 28-<30, 30-<35, 35+ kg/m2).
BMI and CRC-specific mortality
Associations of both at-diagnosis and post-diagnosis BMI with CRC-specific mortality were similar to those for analyses of all-cause mortality. However, in post-diagnosis analyses, class I obese patients had a significantly lower mortality risk and class II/III obese patients showed no higher and a possibly lower mortality risk compared with the reference (Tables 2-3).
Stratified analyses
In stratified analyses, there were few differences by age, race, sex, stage, comorbidity, treatment, or weight loss status (Table 4, eTable 1). However, there was strong evidence of statistical interaction by primary tumor site. The nonlinear pattern of BMI and mortality was apparent in all sites, but elevated risks for underweight and class II/III obesity were most evident in proximal cases whereas reduced risks for overweight and class I obese were most evident in distal and rectal cancers cases (p-interaction=0.005) (Table 4).
Table 4. Stratified analyses of weight and all-cause mortality (N=3,408)*.
| BMI at diagnosis (kg/m2) | ||||||||
|---|---|---|---|---|---|---|---|---|
| <18.5 | 18.5-<23 | 23-<25 | 25-<28 | 28-<30 | 30-<35 | ≥35 | p** | |
| Site | ||||||||
| Proximal, N | 36 | 264 | 223 | 333 | 191 | 288 | 191 | 0.005 |
| Events | 22 | 57 | 42 | 61 | 30 | 56 | 51 | |
| HR | 4.82 | 1.00 | 1.02 | 1.02 | 0.77 | 1.07 | 1.76 | |
| 95% CI | (2.57, 9.02) | (0.65, 1.61) | (0.64, 1.61) | (0.44, 1.33) | (0.64, 1.76) | (0.99, 3.13) | ||
| Distal, N | 11 | 132 | 124 | 205 | 125 | 192 | 120 | |
| Events | 2 | 28 | 13 | 21 | 11 | 35 | 16 | |
| HR | 0.86 | 1.00 | 0.40 | 0.38 | 0.32 | 0.86 | 0.49 | |
| 95% CI | (0.19, 3.76) | (0.19, 0.85) | (0.19, 0.79) | (0.13, 0.75) | (0.41, 1.83) | (0.20, 1.24) | ||
| Rectal, N | 20 | 157 | 150 | 249 | 111 | 191 | 95 | |
| Events | 8 | 33 | 25 | 43 | 13 | 28 | 22 | |
| HR | 1.73 | 1.00 | 0.75 | 0.65 | 0.37 | 0.47 | 1.39 | |
| 95% CI | (0.61, 4.86) | (0.42, 1.35) | (0.36, 1.18) | (0.16, 0.82) | (0.24, 0.93) | (0.67, 2.91) | ||
|
| ||||||||
| Stage | ||||||||
| I, N | 18 | 137 | 161 | 232 | 140 | 210 | 128 | 0.58 |
| Events | 5 | 18 | 13 | 19 | 9 | 13 | 10 | |
| HR | 4.04 | 1.00 | 0.57 | 0.48 | 0.39 | 0.56 | 1.25 | |
| 95% CI | (1.12, 14.6) | (0.24, 1.34) | (0.20, 1.18) | (0.14, 1.10) | (0.20, 1.54) | (0.39, 4.00) | ||
| II, N | 25 | 192 | 160 | 277 | 127 | 183 | 117 | |
| Events | 15 | 27 | 21 | 42 | 17 | 26 | 27 | |
| HR | 2.81 | 1.00 | 0.85 | 1.00 | 0.95 | 1.41 | 2.24 | |
| 95% CI | (1.31, 6.04) | (0.45, 1.63) | (0.53, 1.90) | (0.45, 2.01) | (0.68, 2.92) | (1.02, 4.93) | ||
| III, N | 24 | 224 | 176 | 278 | 160 | 278 | 161 | |
| Events | 12 | 73 | 46 | 64 | 28 | 80 | 52 | |
| HR | 1.87 | 1.00 | 0.76 | 0.69 | 0.37 | 0.66 | 1.01 | |
| 95% CI | (0.81, 4.31) | (0.50, 1.15) | (0.45, 1.04) | (0.22, 0.62) | (0.43, 1.03) | (0.61, 1.68) | ||
Models adjusted for age (continuous), sex, and race (White (ref), Black, Hispanic, Asian/Pacific Islander, Other), stage (I (ref), II, III), grade (1, 2 (ref), 3, 4), chemotherapy (no (ref), yes), radiation (no (ref), yes), and disease site (proximal (ref), distal, rectal), smoking (never (ref), past, current), physical activity (0, >0 to <120, 120+ minutes/wk), and pre-diagnosis BMI (<18.5, 18.5-<23 (ref), 23-<25, 25-<28, 28-<30, 30-<35, 35+ kg/m2) though models were not adjusted for the stratification variable.
p-value, Likelihood Ratio χ2 test for interaction
There were no significant differences in analyses stratified by age (< vs. ≥65 years) (p-value, test for interaction=0.83), race (p=0.18), comorbidity status (p=0.64), chemotherapy (p=0.41) or radiotherapy (p=0.63).
In sensitivity analyses excluding early deaths, we noted little qualitative change or attenuation in associations for at-diagnosis weight and mortality. Associations for underweight and mortality in post-diagnosis analyses were somewhat attenuated when we excluded first year deaths, but other associations were similar (data not shown). Results in sensitivity analyses were also similar (data not shown). With stratification on grade, proportional hazards assumptions were met.
Discussion
Consistent with hypotheses, CRC patients who were underweight or class II/III obese at diagnosis had worse overall prognosis than normal-weight patients. By contrast, neither all-cause nor disease-specific mortality were elevated for class II/III obese CRC patients >1 year after diagnosis. Overweight patients consistently had the best prognosis, with the lowest risks of all-cause and CRC-specific mortality, though the mortality risks of low-overweight and high-normal-weight CRC patients did not differ. We were able to overcome limitations of previous studies with the availability of a complete CRC patient population, prospectively measured pre-diagnosis weight data, and methods which may improve causal claims regarding the effects of BMI on post-CRC-diagnosis mortality. These findings, in the largest CRC cohort to date with data on weight prior to, at the time of, and following diagnosis, provide support that overweight does not confer an increased mortality risk, and may support an obesity paradox, in CRC patients.
Previous investigators have generally shown that underweight and class II/III obese CRC patients have a higher mortality risk when BMI is assessed prior to13, 29, 30 or near the time of10-12, 14, 31 diagnosis. Risks for underweight are higher than for other patients. Our findings are generally consistent with these, though we found elevated mortality risks for underweight patients at all timepoints, in contrast with previous findings showing diminished effects in post-diagnosis analyses. Consistent with our findings, several though not all previous studies have shown lower overall and/or disease-specific mortality risks in overweight18, 30, 32, 33, and even mildly obese33, CRC patients when BMI has been evaluated after diagnosis. However, results from these analyses have been a source of concern among researchers interested in causal methods who have argued that inverse associations are due to collider bias21, 34.
With sufficient covariate adjustment, including pre-diagnosis BMI, we found robust evidence that prognosis is best in CRC patients who are overweight at, and after, the time of diagnosis. In this study, overweight CRC patients had a lower mortality risk among stage I patients, whether or not we eliminated early mortality, and among those with or without weight loss, suggesting results were not attributable to reverse causality. Our strong inverse findings for overweight and both all-cause and disease-specific mortality are novel, obtained using improved methodologies.
A lack of association of overweight around diagnosis and outcomes in previous studies may be due to small sample sizes11, 17, 32 and to broad categorization of weight groups; most studies have defined normal-weight CRC patients with BMI 18.5-<25 kg/m2, though some omit lower normal-weight patients in this category10, 11, 14, 15. Our findings, including a nonsignificant inverse association in overweight patients with similar categorization, but strong inverse findings in all other analyses, suggest that traditional BMI categories are insufficiently granular to understand mortality risk in CRC patients since the nature of the relationship appears convincingly nonlinear. Intriguingly, some studies with long (median≥8 years) follow-up have shown weak, significant or borderline significant positive findings of overweight and mortality10, 12. However, hazard ratios of approximately 1.0 suggest that these estimates are influenced by the tail at the high end of the BMI distribution or that they might represent time-averaged effects of being overweight and/or obese on patients outcomes with increasing risks further from diagnosis. Long-term studies of BMI and CRC survival, with regularly updated BMI measures, are needed.
By examining associations in a population unaffected by sample selection bias and using causal methods to address confounding, we provide support that results are biologically meaningful. In the context of disease, overweight may confer survival benefits35-48, attributed to better nutritional status36, more optimal medical treatment49, greater endothelial progenitor cells50, lower thromboxane production51, higher ghrelin sensitivity52, and lower concentrations of tumor necrosis factor-α53, 54. CRC patients with extra weight may have greater muscle and fat mass enabling them to cope with the metabolic demands of tumor progression and treatment49-54. However, though mortality was lowest in the overweight, risk was similarly low in high-normal-weight patients, suggesting that a BMI in this range may minimize the risks entailed in managing disease.
A study strength was the ability to examine prospectively measured weight at, and following diagnosis, with outcomes, adjusted for pre-diagnosis BMI. Other study strengths include a large sample size, data on treatment and comorbidities, weights and height measured by a medical assistant, and follow-up to 8.7 years. Study limitations include lack of physical activity data for the full cohort. However, adjustment for this variable did not affect results in the subset with these data (data not shown). As with all observational studies, residual confounding is possible. We were unable to control for detailed treatment information. However, Glymour found that the level of unmeasured confounding would have to be very large to explain the obesity paradox55 and covariate adjustment in our study had little influence on associations.
In summary, extremes in weight at diagnosis were associated with elevated all-cause and CRC-specific mortality in a large population of stage I-III CRC patients. By contrast, overweight CRC patients had the best prognosis. Studies are needed to understand the mechanisms underlying these results, particularly studies of body composition. Though strong scientific evidence shows that exercise in cancer patients should be encouraged, findings suggest, among overweight CRC patients, that recommendations for weight loss in the immediate post-diagnosis period may be unwarranted.
Supplementary Material
Supplementary Figure 1 (eFigure 1): Directed acyclic graph (DAG) representing possible causal relationships between variables including pre-diagnosis BMI, post-diagnosis BMI, colorectal cancer, mortality, and unmeasured risk factors.
Supplementary Figure 2 (eFigure 2): Spline curve of BMI at diagnosis and overall mortality in men, p-value, test for nonlinear association<0.0001. Adjusted for age, race, stage, site, grade, chemotherapy, radiation, pre-diagnosis BMI, smoking, and physical activity.
Supplementary Figure 3 (eFigure 3): Spline curve of BMI at diagnosis and overall mortality in women, p-value, test for nonlinear association=0.02. Adjusted for age, race, stage, site, grade, chemotherapy, radiation, pre-diagnosis BMI, smoking, and physical activity.
Supplementary Table 1 (eTable 1): Stratified analyses of weight and all-cause mortality (N=3,408)*
Acknowledgments
This work was supported by the National Cancer Institute at the National Institutes of Health (grant 5R01CA175011). NCI had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Dr. Kroenke and Ms. Weltzien from Kaiser Permanente, who each contributed to data analyses, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
CH Kroenke contributed to conceptualization and design of the study, analysis, writing and interpretation. R Neugebauer contributed to design, interpretation, analysis, and editing. J Meyerhardt contributed to design, interpretation, and editing. C Prado contributed to interpretation and editing. M Kwan contributed to interpretation and editing. E Weltzien contributed to analysis. B Caan contributed to study design, analysis, and editing. All authors of this research paper have approved the final version submitted.
The authors report no conflicts of interest.
References
- 1.Aleksandrova K, Pischon T, Buijsse B, et al. Adult weight change and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition. Eur J Cancer. 2013 Nov;49(16):3526–3536. doi: 10.1016/j.ejca.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Keimling M, Renehan AG, Behrens G, et al. Comparison of associations of body mass index, abdominal adiposity, and risk of colorectal cancer in a large prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2013 Aug;22(8):1383–1394. doi: 10.1158/1055-9965.EPI-13-0353. [DOI] [PubMed] [Google Scholar]
- 3.Renehan AG, Flood A, Adams KF, et al. Body mass index at different adult ages, weight change, and colorectal cancer risk in the National Institutes of Health-AARP Cohort. Am J Epidemiol. 2012 Dec 15;176(12):1130–1140. doi: 10.1093/aje/kws192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Yang G, Xiang YB, et al. Body weight, fat distribution and colorectal cancer risk: a report from cohort studies of 134 255 Chinese men and women. Int J Obes (Lond) 2012 Sep 18; doi: 10.1038/ijo.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odegaard AO, Koh WP, Yu MC, Yuan JM. Body mass index and risk of colorectal cancer in Chinese Singaporeans: the Singapore Chinese Health Study. Cancer. 2011 Aug 15;117(16):3841–3849. doi: 10.1002/cncr.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laake I, Thune I, Selmer R, Tretli S, Slattery ML, Veierod MB. A prospective study of body mass index, weight change, and risk of cancer in the proximal and distal colon. Cancer Epidemiol Biomarkers Prev. 2010 Jun;19(6):1511–1522. doi: 10.1158/1055-9965.EPI-09-0813. [DOI] [PubMed] [Google Scholar]
- 7.Campbell PT, Cotterchio M, Dicks E, Parfrey P, Gallinger S, McLaughlin JR. Excess body weight and colorectal cancer risk in Canada: associations in subgroups of clinically defined familial risk of cancer. Cancer Epidemiol Biomarkers Prev. 2007 Sep;16(9):1735–1744. doi: 10.1158/1055-9965.EPI-06-1059. [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Zhang SM, Cook NR, Rexrode KM, Lee IM, Buring JE. Body mass index and risk of colorectal cancer in women (United States) Cancer Causes Control. 2004 Aug;15(6):581–589. doi: 10.1023/B:CACO.0000036168.23351.f1. [DOI] [PubMed] [Google Scholar]
- 9.Caan BJ, Coates AO, Slattery ML, Potter JD, Quesenberry CP, Jr, Edwards SM. Body size and the risk of colon cancer in a large case-control study. Int J Obes Relat Metab Disord. 1998 Feb;22(2):178–184. doi: 10.1038/sj.ijo.0800561. [DOI] [PubMed] [Google Scholar]
- 10.Sinicrope FA, Foster NR, Yothers G, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013 Apr 15;119(8):1528–1536. doi: 10.1002/cncr.27938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008 Sep 1;26(25):4109–4115. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006 Nov 15;98(22):1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 13.Prizment AE, Flood A, Anderson KE, Folsom AR. Survival of women with colon cancer in relation to precancer anthropometric characteristics: the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 2010 Sep;19(9):2229–2237. doi: 10.1158/1055-9965.EPI-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinicrope FA, Foster NR, Sargent DJ, O'Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010 Mar 15;16(6):1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doria-Rose VP, Newcomb PA, Morimoto LM, Hampton JM, Trentham-Dietz A. Body mass index and the risk of death following the diagnosis of colorectal cancer in postmenopausal women (United States) Cancer Causes Control. 2006 Feb;17(1):63–70. doi: 10.1007/s10552-005-0360-0. [DOI] [PubMed] [Google Scholar]
- 16.Murphy TK, Calle EE, Rodriguez C, Kahn HS, Thun MJ. Body mass index and colon cancer mortality in a large prospective study. Am J Epidemiol. 2000 Nov 1;152(9):847–854. doi: 10.1093/aje/152.9.847. [DOI] [PubMed] [Google Scholar]
- 17.Alipour S, Kennecke HF, Woods R, et al. Body mass index and body surface area and their associations with outcomes in stage II and III colon cancer. J Gastrointest Cancer. 2013 Jun;44(2):203–210. doi: 10.1007/s12029-012-9472-4. [DOI] [PubMed] [Google Scholar]
- 18.Schlesinger S, Siegert S, Koch M, et al. Postdiagnosis body mass index and risk of mortality in colorectal cancer survivors: a prospective study and meta-analysis. Cancer Causes Control. 2014 Jul 19; doi: 10.1007/s10552-014-0435-x. [DOI] [PubMed] [Google Scholar]
- 19.Pearl J. Causal diagrams for empirical research. Biometrika. 1995;82:669–710. [Google Scholar]
- 20.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004 Sep;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 21.Banack HR, Kaufman JS. The obesity paradox: understanding the effect of obesity on mortality among individuals with cardiovascular disease. Prev Med. 2014 May;62:96–102. doi: 10.1016/j.ypmed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Flegal KM, Graubard BI, Williamson DF, Cooper RS. Reverse causation and illness-related weight loss in observational studies of body weight and mortality. Am J Epidemiol. 2011 Jan 1;173(1):1–9. doi: 10.1093/aje/kwq341. [DOI] [PubMed] [Google Scholar]
- 23.Pearl J. An introduction to causal inference. Int J Biostat. 2010;6(2) doi: 10.2202/1557-4679.1203. Article 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon NP. Characteristics of Adult Health Plan Members in Kaiser Permanente's Northern California Region, as Estimated from the 2011 Member Health Survey. Oakland, CA: Division of Research, Kaiser Permanente Medical Care Program; 2013. [Google Scholar]
- 25.BMI Classification. World Health Organization. [Accessed April 7, 2014];2014 http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Updated Last Updated Date.
- 26.Winter JE, Macinnis RJ, Wattanapenpaiboon N, Nowson CA. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014 Apr;99(4):875–890. doi: 10.3945/ajcn.113.068122. [DOI] [PubMed] [Google Scholar]
- 27.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999 Jan;10(1):37–48. [PubMed] [Google Scholar]
- 28.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989 May;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 29.Pelser C, Arem H, Pfeiffer RM, et al. Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer. 2014 May 15;120(10):1540–1547. doi: 10.1002/cncr.28573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell PT, Newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012 Jan 1;30(1):42–52. doi: 10.1200/JCO.2011.38.0287. [DOI] [PubMed] [Google Scholar]
- 31.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004 Feb 15;22(4):648–657. doi: 10.1200/JCO.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 32.Kuiper JG, Phipps AI, Neuhouser ML, et al. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control. 2012 Dec;23(12):1939–1948. doi: 10.1007/s10552-012-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev. 2011 Jul;20(7):1410–1420. doi: 10.1158/1055-9965.EPI-11-0079. [DOI] [PubMed] [Google Scholar]
- 34.Lajous M, Bijon A, Fagherazzi G, et al. Body mass index, diabetes, and mortality in French women: explaining away a “paradox”. Epidemiology. 2014 Jan;25(1):10–14. doi: 10.1097/EDE.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012 Aug 8;308(6):581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casas-Vara A, Santolaria F, Fernandez-Bereciartua A, Gonzalez-Reimers E, Garcia-Ochoa A, Martinez-Riera A. The obesity paradox in elderly patients with heart failure: analysis of nutritional status. Nutrition. 2012 Jun;28(6):616–622. doi: 10.1016/j.nut.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Doehner W, Erdmann E, Cairns R, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol. 2012 Dec 15;162(1):20–26. doi: 10.1016/j.ijcard.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 38.Kim BJ, Lee SH, Jung KH, Yu KH, Lee BC, Roh JK. Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Neurology. 2012 Aug 28;79(9):856–863. doi: 10.1212/WNL.0b013e318266fad1. [DOI] [PubMed] [Google Scholar]
- 39.Lancefield T, Clark DJ, Andrianopoulos N, et al. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter Australian registry. JACC Cardiovasc Interv. 2010 Jun;3(6):660–668. doi: 10.1016/j.jcin.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Lavie CJ, De Schutter A, Patel D, Artham SM, Milani RV. Body composition and coronary heart disease mortality--an obesity or a lean paradox? Mayo Clin Proc. 2011 Sep;86(9):857–864. doi: 10.4065/mcp.2011.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng CH. Obesity paradox: differential effects on cancer and noncancer mortality in patients with type 2 diabetes mellitus. Atherosclerosis. 2013 Jan;226(1):186–192. doi: 10.1016/j.atherosclerosis.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007 Oct;120(10):863–870. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Angeras O, Albertsson P, Karason K, et al. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J. 2013 Feb;34(5):345–353. doi: 10.1093/eurheartj/ehs217. [DOI] [PubMed] [Google Scholar]
- 44.Blum A, Simsolo C, Sirchan R, Haiek S. “Obesity paradox” in chronic obstructive pulmonary disease. Isr Med Assoc J. 2011 Nov;13(11):672–675. [PubMed] [Google Scholar]
- 45.Jackson RS, Black JH, 3rd, Lum YW, et al. Class I obesity is paradoxically associated with decreased risk of postoperative stroke after carotid endarterectomy. J Vasc Surg. 2012 May;55(5):1306–1312. doi: 10.1016/j.jvs.2011.11.135. [DOI] [PubMed] [Google Scholar]
- 46.Jialin W, Yi Z, Weijie Y. Relationship between body mass index and mortality in hemodialysis patients: a meta-analysis. Nephron Clin Pract. 2012;121(3-4):c102–111. doi: 10.1159/000345159. [DOI] [PubMed] [Google Scholar]
- 47.Komukai K, Minai K, Arase S, et al. Impact of body mass index on clinical outcome in patients hospitalized with congestive heart failure. Circ J. 2012;76(1):145–151. doi: 10.1253/circj.cj-11-0727. [DOI] [PubMed] [Google Scholar]
- 48.Stein PD, Matta F, Goldman J. Obesity and pulmonary embolism: the mounting evidence of risk and the mortality paradox. Thromb Res. 2011 Dec;128(6):518–523. doi: 10.1016/j.thromres.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 49.Schenkeveld L, Magro M, Oemrawsingh RM, et al. The influence of optimal medical treatment on the ‘obesity paradox’, body mass index and long-term mortality in patients treated with percutaneous coronary intervention: a prospective cohort study. BMJ Open. 2012;2:e000535. doi: 10.1136/bmjopen-2011-000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biasucci LM, Graziani F, Rizzello V, et al. Paradoxical preservation of vascular function in severe obesity. Am J Med. 2010 Aug;123(8):727–734. doi: 10.1016/j.amjmed.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 51.Graziani F, Biasucci LM, Cialdella P, et al. Thromboxane production in morbidly obese subjects. Am J Cardiol. 2011 Jun 1;107(11):1656–1661. doi: 10.1016/j.amjcard.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 52.Lund LH, Williams JJ, Freda P, LaManca JJ, LeJemtel TH, Mancini DM. Ghrelin resistance occurs in severe heart failure and resolves after heart transplantation. Eur J Heart Fail. 2009 Aug;11(8):789–794. doi: 10.1093/eurjhf/hfp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldman AM, Combes A, Wagner D, et al. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. 2000 Mar 1;35(3):537–544. doi: 10.1016/s0735-1097(99)00600-2. [DOI] [PubMed] [Google Scholar]
- 54.McTiernan CF, Feldman AM. The role of tumor necrosis factor alpha in the pathophysiology of congestive heart failure. Curr Cardiol Rep. 2000 May;2(3):189–197. doi: 10.1007/s11886-000-0068-4. [DOI] [PubMed] [Google Scholar]
- 55.Glymour MM, Vittinghoff E. Commentary: selection bias as an explanation for the obesity paradox: just because it's possible doesn't mean it's plausible. Epidemiology. 2014 Jan;25(1):4–6. doi: 10.1097/EDE.0000000000000013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 (eFigure 1): Directed acyclic graph (DAG) representing possible causal relationships between variables including pre-diagnosis BMI, post-diagnosis BMI, colorectal cancer, mortality, and unmeasured risk factors.
Supplementary Figure 2 (eFigure 2): Spline curve of BMI at diagnosis and overall mortality in men, p-value, test for nonlinear association<0.0001. Adjusted for age, race, stage, site, grade, chemotherapy, radiation, pre-diagnosis BMI, smoking, and physical activity.
Supplementary Figure 3 (eFigure 3): Spline curve of BMI at diagnosis and overall mortality in women, p-value, test for nonlinear association=0.02. Adjusted for age, race, stage, site, grade, chemotherapy, radiation, pre-diagnosis BMI, smoking, and physical activity.
Supplementary Table 1 (eTable 1): Stratified analyses of weight and all-cause mortality (N=3,408)*