Abstract
The ubiquitous use of engineered nanomaterials – particulate materials measuring approximately 1–100 nanometers (nm) on their smallest axis, intentionally engineered to express novel properties – in semiconductor fabrication poses unique issues for protecting worker health and safety. Use of new substances or substances in a new form may present hazards that have yet to be characterized for their acute or chronic health effects. Uncharacterized or emerging occupational health hazards may exist when there is insufficient validated hazard data available to make a decision on potential hazard and risk to exposed workers under condition of use. To advance the knowledge of potential worker exposure to engineered nanomaterials, the National Institute for Occupational Safety and Health Nanotechnology Field Studies Team conducted an on-site field evaluation in collaboration with on-site researchers at a semiconductor research and development facility on April 18–21, 2011. The Nanomaterial Exposure Assessment Technique (2.0) was used to perform a complete exposure assessment. A combination of filter-based sampling and direct-reading instruments was used to identify, characterize, and quantify the potential for worker inhalation exposure to airborne alumina and amorphous silica nanoparticles associated with the chemical mechanical planarization wafer polishing process. Engineering controls and work practices were evaluated to characterize tasks that might contribute to potential exposures and to assess existing engineering controls. Metal oxide structures were identified in all sampling areas, as individual nanoparticles and agglomerates ranging in size from 60nm to >1,000nm, with varying structure morphology, from long and narrow to compact. Filter-based samples indicated very little aerosolized material in task areas or worker breathing zone. Direct-reading instrument data indicated increased particle counts relative to background in the wastewater treatment area; however, particle counts were very low overall, indicating a well-controlled working environment. Recommendations for employees handling or potentially exposed to engineered nanomaterials include hazard communication, standard operating procedures, conservative ventilation systems, and prevention through design in locations where engineered nanomaterials are used or stored, and routine air sampling for occupational exposure assessment and analysis.
Keywords: Occupational exposure assessment, engineered nanomaterials, uncharacterized occupational health hazard, semiconductor fabrication, chemical mechanical planarization, NEAT 2.0
INTRODUCTION
The nanotechnology workforce is projected to hit 6 million workers by 2020, 2 million of whom are expected to work in the U.S.(1) and will potentially handle or come into contact with engineered nanomaterials (ENMs). ENMs are particulate materials intentionally engineered to express novel or new properties that measure approximately 1–100nm on their smallest axis. Due to their size and the unique physicochemical properties that can emerge at the nanoscale for some materials, ENMs may be more toxic and present a different risk profile than their larger macroscale material counterparts. This reality gives rise to the concern that existing occupational exposure limits (OELs) for the larger macroscale material may not be sufficient to protect workers against exposure to ENMs. The National Institute for Occupational Safety and Health (NIOSH) has published several guidance documents addressing occupational exposure to ENMs. These guidance documents provide mass-based recommended exposure limits (RELs) for controlling worker exposure to carbon nanotubes (CNTs), carbon nanofibers (CNFs), and ultrafine engineered titanium dioxide (TiO2) nanoparticles.(2,3)
Following exposure assessment methodology and guidelines described by NIOSH,(4,5) this study focuses on detailed characterization of potential worker inhalation exposures to metal oxide ENMs associated with a wafer polishing process called chemical mechanical planarization (CMP) in a semiconductor research and development cleanroom facility, and in spaces associated with the CMP process, including the subfab level below the cleanroom, and the wastewater treatment (WWT) area.
Background
The semiconductor industry has responded to consumer demand for faster and smaller devices, such as memory chips, computers, and smartphones, by utilizing materials and processes refined at the nanoscale. Realizing that handling ENMs may pose unique issues for worker health and safety, researchers at the SUNY Polytechnic Institute Colleges of Nanoscale Science and Engineering (CNSE) in Albany, New York are combining proactive research on workplace exposure assessment with semiconductor research and development. The goal is development of ENM exposure sampling and analytical protocols that will assist the semiconductor industry in designing and following effective worker precautions. The CMP wafer polishing process has been identified for exposure assessment. CMP occurs approximately 20 times during fabrication of a single silicon complementary metal-oxide-semiconductor (CMOS) wafer. The CMP process flattens dielectric films by removing metallic overlayers through both chemical reactions and mechanical abrasion. ENMs contained in aqueous chemical slurry are either dispersed on a porous rotating pad or affixed to the pad surface itself. The CMP tool and planarization process is conducted in a ventilated enclosure within a cleanroom. This process is described in detail and illustrated in Brenner et al.(6)
Most commonly used CMP slurries contain macroscale active chemicals and abrasive nanoparticles (NPs), such as amorphous silicon dioxide (silica; SiO2) nanoparticles, cerium (IV) oxide (ceria; CeO2) nanoparticles, or aluminum oxide (alumina; Al2O3) nanoparticles generally ranging in size from 10nm–80nm with a spherical morphology. However, during this study, only CMP slurries containing amorphous silica (likely a mixture of colloidal and fumed) and alumina nanoparticles were in use. Nanoparticles used in slurries have been analyzed via transmission electron microscopy (TEM) and energy-dispersive x-ray spectroscopy (EDS) for comparison to TEM images of airborne particulate captured in the field from air monitoring (Figure 1S).
Potential Health Effects of the Nanomaterials of Interest
OELs for larger macroscale amorphous silica and alumina have been established. The NIOSH REL for silica is a time-weighted average (TWA) for up to a 10-hour workday of 6mg/m3 and the Occupational Safety and Health Administration (OSHA) permissible exposure limit (PEL) is an 8-hour TWA of 20 million particles per cubic foot (mppcf) or 80mg/m3/percent SiO2.(7) The NIOSH REL is TWA 10mg/m3 (total dust) and 5mg/m3 (respirable fraction); and the OSHA PEL for alumina is TWA 15mg/m3 (total dust) and 5mg/m3 (respirable fraction). The American Conference of Governmental Industrial Hygienists (ACGIH) threshold limit value (TLV) is TWA 10mg/m3 (total dust).(8–10) While there are established OELs for the macroscale materials, no exposure limits exist yet specifically for the nanoscale forms. Based on experience with other ENMs, it is important to review the biological and toxicological health effects reported for the nanoscale form of the different CMP slurry components.
Silica
In vivo murine models of amorphous silica NP exposure have demonstrated inflammatory responses with increased reactive oxygen species (ROS) production and decreased antioxidant enzyme levels(11) as well as increased pulmonary injury and neutrophilic infiltration.(12) In vitro studies similarly implicate amorphous silica in inducing pro-inflammatory responses as well as in mitochondrial toxicity, cytotoxicity, and endothelial cell dysfunction.(11,13–16) Note that in all studies referenced here, the silica used was specified as amorphous, except for Kim et al.(12) and Liu et al.,(14) which did not specify between amorphous or crystalline.
Alumina
Alumina NPs have been shown to significantly decrease cell viability at a concentration of 1,025 micrograms (μg)/milliliter (mL) and 25μg/mL in human lung epithelial cells in vitro.(17) In another study, cellular interaction of alumina NPs (30nm and 40nm) on rat alveolar macrophages was evaluated: a marginal effect on macrophage viability was seen after exposure for 24 hours (hr) to 100μg/mL.(18) Although Al2O3 does exhibit some adverse health effects, it seems to be less toxic than other nanoparticles (such as CeO2, TiO2, and ZnO) on human lung epithelial cells, A549 carcinoma cells and L-132 normal cells in vitro, even after long (72hr) exposure.(19)
METHODS
Facility Description
Three locations associated with the CMP process were evaluated in this study: the cleanroom, the subfab level below the cleanroom, and wastewater treatment (WWT) area. The cleanroom is an ISO class 5 (<100 particles/cubic foot (ft3) size 0.5μm and larger and <300 particles/ft3 size 0.3μm and larger). Cleanroom entry requires specific apparel: full gown with hood, gloves, boot covers, hairnets, beard nets (when applicable), latex or nitrile gloves, and safety glasses. The CMP process tool is located within a ventilated enclosure and under negative pressure with one exception, the entry port, which is under positive pressure to prevent component contamination. When CMP tools are in use, the process engineer and the technicians remain in close proximity to them. Two CMP tools were investigated for inhalation exposure potential in this study. Each tool is used to run different slurry chemistries in tandem, and the tools may operate simultaneously.
Directly below the cleanroom is the subfab level, which is an average of ISO class 6 with intermittent episodes of over ISO class 7 due to being part of the air return and its location directly below the ISO class 5 cleanroom. Holes in the floor of the cleanroom allow laminar airflow from the cleanroom into the subfab level. The subfab houses 55-gallon drums containing the master batch CMP slurry as well as equipment to agitate the slurry and pump it into the CMP tools during operation. The subfab is not considered a cleanroom; however, some cleanroom procedures are required. Personal protective equipment (PPE) must be worn in the subfab and includes: gown, latex or nitrile gloves, hairnets, beard nets (when applicable), boot covers, safety glasses, and a hard hat.
The WWT area is located on the same floor as the subfab level. Engineering controls in the WWT general space include a 5,600 ft3/minute (min) exhaust fan and continuous flow heating. The ventilating and air conditioning (HVAC) system is a continuous flow system performing approximately 7.3 complete air changes per hour, which does not vary in volume or velocity.(8) No cleanroom procedures are required for entry into this space; however, specific job tasks require different types of recommended PPE. Waste handlers perform tasks associated with the treatment system for CMP acid and base effluent.(20)
Process Area and Task Descriptions
Several CMP process tasks were identified for worker exposure evaluation, particularly regarding the potential for inhalation of the ENMs from slurry if materials became aerosolized. This could occur during a specific task such as agitation or mixing or if the slurry is splashed or spilled and the particles are manually re-aerosolized after drying (e.g., during cleaning).
Wastewater Treatment (WWT)
Pre-filter bags on the acid and base effluent handling systems must be changed periodically, which takes approximately 15 minutes (min) per tank. The NIOSH Field Studies Team (NFST) observed and evaluated the filter change procedure twice, on April 19, 2011 and April 20, 2011. For this task, required PPE was a face shield, waterproof apron, and chemical resistant gloves. The system was shut down and residual wastewater was drained into an empty bucket located approximately 6–12 inches (in) below the pre-filter housing, with the sides of the bucket shielding against splashing. There was no local exhaust during this task. The tank was disconnected and a new tank was put in place, the transfer lines were reconnected, and the system was returned to working order. All wastewater that had been drained into the bucket was poured back into the system for treatment; this occurred above the worker’s waist level, bringing potential aerosols closer to the worker’s personal breathing zone (PBZ). Additional details on the WWT area, including engineering controls and other tasks that occur in this space that have been monitored for potential inhalation exposure, have been published previously.(6)
Subfab
A slurry drum change was observed on April 20, 2011. This task occurs approximately every two days and takes approximately 5min. Required PPE included a chemical resistant apron and face shield, in addition to the standard PPE for this location. To change the drum, all hoses were disconnected from the old drum, a new drum was moved into place, and a slurry-stirring rod was inserted to continuously stir the product to prevent sedimentation and ENM agglomeration.
The slurry system for a particular CMP tool must be changed daily, which takes approximately 20min. The NFST observed and evaluated this process twice, on April 20 and 21, 2011. The cabinet containing the pumps was opened and the system was purged of the old slurry. Then, the system was replenished with the requested slurry and hydrogen peroxide, and the cabinet closed. When closed, the system operates under negative pressure with exhaust air, as verified by the magnehelic gauge. This ventilation system is breached while the system is open.
Cleanroom
The CMP tool consumables must be changed before the start of “tool time” per the specifications of each tool user. This task occurs daily and takes approximately 45min. No additional PPE is required beyond the standard cleanroom apparel. This process was observed and evaluated on the first day of sampling. The CMP tool interlocks were opened and the technician opened the tool enclosure door(s) and placed his or her upper torso inside the tool to change the polishing pad. The magnehelic gauges indicated that this system operated with exhaust ventilation and under negative pressure when closed. This ventilation system is breached while the system is open.
Sampling Approach
Filter-Based Samples
Three types of filter-based samples were collected during this study. PBZ samples were collected as close as possible to the employee’s breathing zone (e.g., their lapel) and only collected while the worker was performing a specific task. Area samples were collected on a cart near the worker while the task was being performed to measure potential airborne ENM migration from the specific task into the area that might be most affected and would be considered an exposure zone. Background samples for airborne particulates were collected at least 10ft from the task to characterize the presence of incidental particles, which was the maximum feasible distance given physical space constraints (e.g., walls, other processes, or process containment structures) in these workspaces. Background samples varying in time length were started prior to the start of the task, and sampled for at least 2hr after the completion of the task. This approach allowed for the assessment of fluctuations in particulate counts over time, and, in particular, the effects of particulate migration throughout the sampling space and beyond the task area both prior to and after the task evaluated.
All filter-based air samples were collected using Leland Legacy personal pumps (SKC Inc., Eighty Four, PA). The pump flow rate was calibrated between 5.0–6.5L/min. Flow rate was set as high as possible in an attempt to obtain a detectable concentration in spite of the short sampling duration, taking the pressure differential, filter resistance, and cassette size into consideration. Air samples to determine the airborne mass concentration of amorphous silica NPs were collected on 25 millimeter (mm) diameter, 5 micrometer (μm) pore size, closed-face polyvinyl chloride membrane filters (PVC) and analyzed according to the NIOSH Manual of Analytical Methods (NMAM) 7501(21) for amorphous silica. Air samples used to determine the airborne mass concentration of aluminum oxide NPs were collected on 25mm diameter, 0.8μm pore size, open-faced, mixed cellulose ester (MCE) filters. Samples were analyzed for aluminum oxide by inductively coupled plasma optical emission spectrometry (ICP-OES).(22) The results reported for aluminum oxide are calculated from the aluminum result, and assumes all aluminum detected was in the form of aluminum oxide.
Alongside each mass-based air sample, an additional air sample was collected on a 37mm diameter, 0.8μm pore size, open-face MCE filter attached to a Leland Legacy personal pump and analyzed for the nanoparticle of interest using TEM with EDS in a manner similar to NMAM 7402.(23) TEM provides an indication of the relative abundance of nanostructures per volume of air, as well as other characteristics such as size, shape, and degree of agglomeration. EDS provides confirmation of the presence of the material of interest based on chemical composition. The microscopist also analyzed the nanoparticles used in slurries for visual verification and identification through comparison with field air samples collected.
Real-Time Direct-Reading Instruments (DRIs)
Three real-time, field-portable direct-reading instruments (DRIs) [TSI model 3007 condensation particle counter (CPC), ART Instruments Model HHPC-6 optical particle counter (OPC), and TSI DustTrak DRX aerosol monitor] were used together to characterize the process emissions by determining the number concentration (CPC and OPC) or mass concentration (DustTrak) and approximate size range of airborne particles. Air samples were captured and recorded once every 22 seconds by the OPC and once every second by both the CPC and the DustTrak. The instruments continuously recorded normal fluctuations in particle counts and mass, attributable to the process or task. Although DRIs are not specific to the ENMs of interest, the combined data from the CPC and OPC assists in identifying the presence of particles in the 10nm–15,000nm size range.(24) Instrument details can be found in Supplementary Materials.
RESULTS
The NFST and CNSE researchers performed a complete exposure assessment of potential sources of nanoparticle emissions using NEAT 2.0.(25)
Morphology, Mass Concentration, and Elemental Composition
A total of 31 filter-based air samples (12 for amorphous silica, 6 for aluminum oxide, and 12 for TEM analysis) were collected (Tables I, II and III). Two media blanks were also analyzed. Sampling times ranged from 19min-333min (volume of air sampled ranged from 102L–2,028L), dependent on the time necessary to complete the task and/or the process being evaluated.
TABLE I.
Filter-based air samples: concentration of amorphous silica (PVC filters)
| Sample Type (mg/m3) |
Sampling Location Average Flow Rate (L/min) |
Process/Task Air Volume (L) |
Total Time Sampled (min) Total ConcentrationA |
||||
|---|---|---|---|---|---|---|---|
| Task Area | WWT | 3ft from filter change task | 36 | 6.03 | 217 | NDB | |
| Background | WWT | on cart, 20ft from task area | 333 | 5.99 | 1,995 | ND | |
| PBZ | WWT | filter changes | 39 | 5.97 | 233 | ND | |
| PBZ | Subfab | tank change; mock-up replenish batch | 22 | 5.99 | 132 | ND | |
| Task Area | Subfab ND | changing tanks and replenishing batch | 29 | 6.11 | 177 | ||
| Background | Subfab | 15ft from tanks | 189 | 5.40 | 1,021 | ND | |
| PBZ | WWT | filter and pre-filter change of acid tank | 33 | 6.11 | 200 | ND | |
| Task Area | WWT | filter and pre-filter change of acid tank | 37 | 6.04 | 225 | ND | |
| PBZ | Cleanroom | set-up of CMP tool | 25 | 6.04 | 151 | ND | |
| Task Area | Cleanroom ND | on workstation operator’s table near CMP tool | 45 | 6.04 | 273 | ||
| Background | Cleanroom | 5ft from CMP tool | 209 | 5.64 | 1,167 | ND | |
| Task Area | Subfab | 2ft from slurry system | 19 | 6.02 | 115 | 0.497C | |
No sample results yielded sufficient particulate to be sent for analysis of amorphous silica content.
ND = Non-detectable.
Value is between the limit of detection (50μg/sample) and the limit of quantification (180μg/sample); estimated result.
TABLE II.
Filter-based air samples: concentration of aluminum oxide (MCE filters)
| Sample Type (mg/m3)A |
Sampling Location Average Flow Rate (L/min) |
Process/Task Air Volume (L) |
Total Time Sampled (min) Total Concentration |
||||
|---|---|---|---|---|---|---|---|
| Task Area | WWT | 3ft from filter change task | 36 | 5.52 | 199 | ND | |
| Task Area | Subfab | changing tanks and replenishing batch | 28 | 5.51 | 154 | ND | |
| Background | Subfab | 15ft from tanks | 189 | 5.50 | 1,040 | ND | |
| Task Area | WWT | filter and pre-filter change of acid tank | 37 | 5.60 | 206 | ND | |
| Task Area | Cleanroom ND |
on workstation operator’s table near CMP tool | 45 | 5.60 | 250 | ||
| Task Area | Subfab | 2ft from slurry system | 19 | 5.78 | 109 | ND | |
Limit of detection (2μg/sample).
TABLE III.
Filter-based air samples: differential particle filter concentrations as determined by electron microscopy and energy-dispersive x-ray spectroscopy (MCE filters)
| Type | Sampling Location | Sample Process/Task | Total Time Sampled (min) Average | |||||
|---|---|---|---|---|---|---|---|---|
| Flow Rate (L/min) | Air Volume (L) | Si p/cm3 | Al p/cm3 | Mixed Al and Si p/cm3 | ||||
| Task Area | WWT 0.011 |
3ft from filter change task | 37 | 5.55 | 205 | 0.559 | 0.232 | |
| Background | WWT 0.002 |
on cart, 20ft from task area | 349 | 5.81 | 2,028 | 0.084 | 0.023 | |
| PBZ | WWT | filter changes | 39A | 5.97 | 233A | 0.328 | 0.599 | ND |
| PBZ | Subfab 0.156 |
tank change; mock-up replenish batch ND |
22 | 5.5 | 121 | 0.068 | ||
| Task Area | Subfab ND |
changing tanks and replenishing batch 0.002 |
28 | 5.82 | 163 | 0.014 | ||
| Background | Subfab | 15ft from tanks | 189 | 5.59 | 1,057 | 0.225 | 0.149 | ND |
| PBZ | WWT ND |
filter and pre-filter change of acid tank ND |
33 | 5.51 | 183 | 0.113 | ||
| Task Area | WWT ND |
filter and pre-filter change of acid tank ND |
37 | 5.69 | 213 | 0.078 | ||
| PBZ | Cleanroom ND |
set-up of CMP tool | 25 | 5.81 | 142 | 0.023 | 0.010 | |
| Task Area | Cleanroom | on workstation operator’s table near CMP tool | 45 | 5.69 | 259 | |||
| 0.240 | 0.074 | ND | ||||||
| Background | Cleanroom | 5ft from CMP tool | 209 | 5.64 | 1,167 | 0.206 | 0.124 | ND |
| Task Area | Subfab ND |
2ft from slurry system | 19 | 5.07 | 101 | 0.536 | 0.170 | |
Pump failed during sampling; time estimated.
Of the 12 samples analyzed for total particulates prior to amorphous silica analysis, only one sample yielded results (57μg silica/sample or 0.497 mg/m3) above the analytical limit of detection (50μg/sample) (Table I). It was determined that there was insufficient analyte collected on filters to be analyzed for amorphous silica. All results were below the limit of quantification (180μg/sample). The results were blank corrected with the average of the laboratory media blanks. The task area sample containing 0.497 mg/m3 (57μg silica/sample) was taken in the subfab 2ft from the slurry system, on the second day of sampling in this location. The slurry that was being used during sampling on this day contained alumina ENMs, not amorphous silica.
Of the 6 samples analyzed for aluminum oxide, none of the samples yielded results above the analytical limit of detection (2μg/sample) (Table II). The results were blank corrected with the average of the media blanks. Control spikes were prepared and analyzed using aluminum oxide powder.
Twelve filter samples were analyzed for amorphous silica (Si) and aluminum (Al) oxide NPs by TEM. Table III shows the silica, aluminum, and combination of silica and aluminum particle counts/cm3. Of the 12 samples, all were positive for silica, 9 were positive for aluminum oxide, and 3 were positive for silica-aluminum agglomerates. The agglomerates ranged in shape from long and narrow (Figure 1) to compact. Sizes ranged from about 60nm–800nm across for the compact agglomerates, while the long, narrow particles were typically about 100nm across with lengths of approximately 400nm to >1,000nm. The aluminum oxide particles were typically found in clusters of individual particles that had roughly spherical or oblong shapes (Figure 1). They ranged from 50nm–100nm across, on average. The widest cluster dimension ranged from 150nm–400nm. Based on TEM and EDS analysis, some agglomerates contained both silica and aluminum nanoparticles. These were usually larger, with a maximum cluster dimension width of >1,000nm.
FIGURE 1.
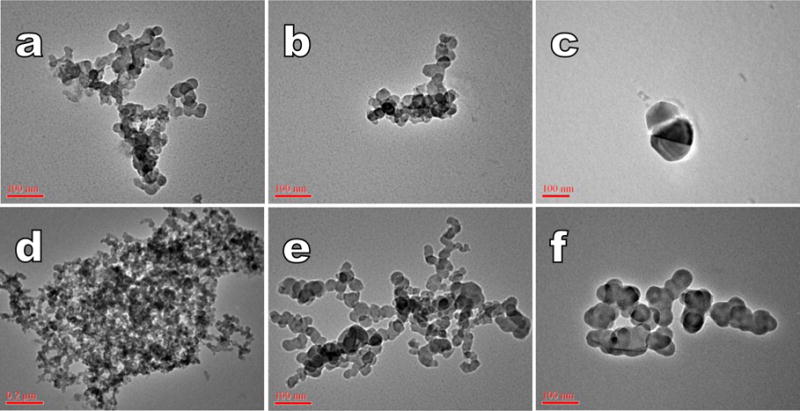
TEM images of air samples obtained in the cleanroom, subfab, and WWT. a – b) Agglomerates of amorphous Si from WWT task area. Scale bars = 100nm. c) Alumina from worker PBZ in WWT. Scale bar = 100nm. d) Mixed Al-Si agglomerate from WWT background. Scale bar = 0.2μm. e) Agglomerate of amorphous Si from subfab task area. Scale bar = 100nm. f) Agglomerate of alumina from the cleanroom task area. Scale bar = 100nm.
The three highest number counts for silica NPs were found in the WWT area 3ft from the task (0.559 particles/cm3), in the subfab 2ft from the slurry system (0.536 particles/cm3), and in the PBZ of the WWT operator during filter changes (0.328 particles/cm3). The highest aluminum NP concentrations were noted in the WWT area. The highest concentration was in the worker PBZ sample that was responsible for changing tank filters and pre-filters (0.599 particles/cm3) while the second highest was sampled on the cart within 3ft of the filter change task (0.232 particles/cm3). The combined Al-Si agglomerates were predominantly seen in WWT. However, the two field blanks both yielded detectable particles per filter area with silica (35 and 78 Si particles/cm3) and aluminum (8 and 35 Al structures/cm3) structure counts. In addition, the media blanks also yielded detectable particles per filter area with silica (10 and 7.5 structures/cm3).
Particle Concentration
The NFST evaluated changing the pre-filters on the acid and base wastewater tanks on two days. On WWT day 1 (Figure 2S) and day 2 (Figure 3S), the CPC indicated particles between 10nm–1,000nm were released when removing and draining the pre-filter canister. Two peaks over background occurred at 11:18 and 11:24 on day 1, and one at 11:10 on day 2. The same peaks occurred on the OPC (300–1,000nm size range). At each of these timepoints, valves were open, allowing for water to drain out of the system. The average background concentration in the WWT area was approximately 2,842p/cm3 and the highest peak was 3,676p/cm3 (using the CPC). Due to the small magnitude of the peak, it is unclear if the peak was actually caused by a release of nanomaterial or another source. However, during this operation, we observed that when the canisters were opened, a large amount of pressure was released and water droplets were observed falling off the filter and into the bucket below, which could potentially create an aerosol. In addition, the DustTrak™ detected a peak in dust mass concentration of the respirable fraction when the drum containing used filters was opened. Furthermore, the waste drum appeared to have dried slurry inside of it.
The subfab was also evaluated twice. On day 1 (Figure 4S), a large peak occurred in the background after the task was completed and was detected by all three DRIs. The magnitude of this peak with a short time duration was almost 4,182p/cm3 while the average background was 3.7p/cm3 (on the CPC, excluding the peak). It is unknown whether this peak was a result of an unknown incidental particulate occurrence or a result of the task, with a delay due to particle migration within the sampling space over time. The only peaks over background that were detected occurred on day 2 (Figure 5S) of sampling. The CPC indicated four peaks in the task area during the task, around 7:14, 7:22, 7:26, and 7:27. The highest peak (43p/cm3) occurred at 7:27 after the task was complete. The 7:14 peak occurred during the purge of the old slurry, the 7:22 peak occurred while the system was replenishing, and the 7:26 peak occurred when the cabinet was closed. It is possible that these peaks could be attributed to particles shed from worker/team apparel while passing in front of the instruments or other incidental sources rather than from the nanomaterials of interest. The DustTrak™ data indicated that the highest PBZ exposure peak (0.06mg/m3) occurred prior to the start of the task (Figure 6S).
The NFST evaluated the CMP process in the cleanroom once (Figure 7S). All background particle counts in the cleanroom were very low. Although a peak in the background particle count was noted, this was not specific to the task evaluated. The OPC indicated a spike at 1:27 when it was held inside the CMP by the tool close to the worker when the pad was being changed. An unexpected finding based on the CPC results was that the background concentration in the cleanroom was 30.8p/cm3 and the average background particle count in the subfab was 9.0p/cm3. Without performing a ventilation evaluation, it is difficult to interpret the differences in average background particle count results with the current data available.
DISCUSSION
TEM/EDS results from filter-based air samples indicate very little particulate in any of the PBZ or area samples; however, all observed tasks were wet processes that reduce the potential for particulate to become airborne. The CMP process in the cleanroom was carried out in an enclosure, further reducing the potential for inhalation of aerosols containing the nanomaterials of interest. The magnehelic gauges on the CMP enclosure exhaust in the cleanroom and on the slurry pumping system in the subfab indicated operation under negative pressure, further containing any possible nanoparticulate emissions.
The only filter-based air sample that contained a detectable concentration of total particulate (0.497 mg/m3 or 57μg silica/sample) was collected in the subfab, 2ft from the task. However, this filter did not contain sufficient material to confirm identification of amorphous silica. It should be noted that, while appropriate for larger materials, mass concentration may not be appropriate for nanoscale materials.
Filter-based data collected in the WWT area indicated the highest overall particulate count in comparison with the cleanroom and the subfab, which was expected (Figures 2S–7S).(6,26) Particle concentration variation with respect to distance from the worker’s PBZ was likely due to differences in agglomeration of aerosolized silica versus alumina particles as indicated by TEM results [the gradient observed for silica NPs was from less to more (PBZ to background), while the gradient observed for alumina NPs was from more to less (PBZ to background)]. The TEM samples collected indicated Si and Al NPs present on the sample filters; however, the field blanks that were collected away from any processing also showed the presence of both Si and Al NPs consistent with the morphology of the NPs in slurry.
Field blanks are collected to examine bias in sample collection, while media blanks are collected to examine laboratory errors. The media blanks, which were never opened in the field, contained 10 and 7.5 structures/mm2 of silica. This indicates silica NPs were present on the filters prior to sampling. The average ash content of MCE membrane filters indicate that <2.0ppm Al and <20.0ppm Si are a common component of MCE filters. The field blanks collected for silica revealed 35 and 78 Si structures/cm3 while the blanks for aluminum contained 8 and 35 Al structures/cm3. The presence of materials of interest on the media blanks indicates that this filter type is likely inappropriate for metal oxide NP exposure assessment and other filter types should be considered and tested for this sampling environment. Subsequent filter-based sampling for metal oxide nanomaterials in this sampling environment utilized polycarbonate (PC) filters due to the contamination observed with the MCE filters.(6) Since the bias indicated from the field blank was larger than that of the media blank, the field blanks were used to adjust the particulate counts. This indicates the only location that incurred a NP count above that of the background was in the WWT area. In addition, this location was the only one to have mixed Si and Al NP agglomerates.
On examining the DRI data obtained during the tasks evaluated (Figures 2S–7S), the particle count peaks did not correspond directly to specific tasks and were not significantly higher than the background; the peaks were frequently less than one order of magnitude greater than background. Therefore, we are unable to determine if the few observed peaks were a result of the task or employee movement, work environment (e.g., room air distribution), or external ambient environment.(27) Assessment of real-time concentration peaks when compared to background readings may be suggestive of emission sources but cannot be confirmed without investigation of other possible sources of particle releases in the sample area that may have contributed to the particle load in the size range of the instrument. Additionally, interpretation of these peaks must be conducted in conjunction with an assessment of the mass-based or TEM samples. Confirmation of positive elemental results and/or identification of the nanomaterial with TEM/EDS can be used to provide stronger confidence that the observed real-time concentration peaks are indeed the result of nanomaterials emitted during the assessed activity. DRI data may also indicate the efficacy of engineering controls and worker practices. All DRI data indicate a well-controlled environment with a very low particulate count.
Limitations
Known limitations of current instrumentation and sampling approaches for ENMs have been reviewed in the literature.(6,28–30) The investigators attempted to minimize any variances that occurred due to the nature of field-based sampling wherever possible. Due to the silica and aluminum content that was discovered in the blank MCE filters in this study, it is not advisable to perform MCE filter-based sampling for any occupational exposure assessment where these materials represent the ENMs of interest. Possible alternatives include testing media blanks before sampling to ensure absence of contaminants, or selecting other filter media (e.g., polycarbonate). Another alternative is to sample directly onto the TEM grid using an electrostatic precipitator (ESP) or thermophoretic sampler (TPS).(31–33)
CONCLUSIONS
Results of this study indicate low potential for worker inhalation exposure to nanoparticles used and/or generated during the CMP-related tasks during which samples were collected. Currently, there are no OELs specific to metal oxide ENMs in use by the semiconductor industry. Although samples collected during this study indicated that elemental concentrations were below the NIOSH REL for both silica and alumina, it is not yet known if either of these RELs is protective for the nanosized material. For example, in order to be protective and take into account the special characteristics of the nanoscale material, the NIOSH REL for ultrafine (nanoscale) TiO2 is approximately 10% of the REL for fine TiO2. As with many nanomaterials, size and surface area of amorphous silica and alumina may be a critical factor to the biological effects.(34,35)
This study provides a sampling approach strategy that may be used or modified to assess worker exposure to various nanoparticles in semiconductor or other industries. It is prudent practice to maintain exposures to uncharacterized or emerging materials as low as possible. Recommendations for employees handling or potentially exposed to ENMs include general hazard communication, standard operating practices, conservative ventilation systems and prevention through design where ENMs are used or stored, and routine sampling for occupational exposure assessment and analysis.
Supplementary Material
Acknowledgments
FUNDING
This research effort was financially supported by NIOSH and the New York State NanoHealth and Safety Center (NSC). The authors thank the management and employees of the participating companies and study site. We would like to extend special thanks to Michele Shepard, Kenneth Martinez, Laura Hodson, and Terri Pearce for their consultative expertise. In addition, the authors are grateful to Matthew Hull of Virginia Tech Institute and Keith Swain of DuPont for their review of this manuscript.
Abbreviations
- ACGIH
American Conference of Governmental Industrial Hygienists
- CMOS
complementary metal oxide semiconductor
- CMP
chemical mechanical planarization
- CNF
carbon nanofiber
- CNT
carbon nanotube
- CPC
condensation particle counter
- DRI
direct-reading instrument
- EDS
energy-dispersive x-ray spectroscopy
- ENM
engineered nanomaterial
- ESP
electrostatic precipitator
- HVAC
heating, ventilating, air conditioning
- ICP-OES
inductively coupled plasma optical emission spectrometry
- MCE
mixed cellulose ester
- NEAT 2.0
Nanomaterial Exposure Assessment Technique
- NFST
NIOSH Field Studies Team
- NIOSH
National Institute for Occupational Safety and Health
- NMAM
NIOSH Manual of Analytical Methods
- NP
nanoparticle
- OEL
occupational exposure limit
- OPC
optical particle counter
- OSHA
Occupational Safety and Health Administration
- PBZ
personal breathing zone
- PC
polycarbonate
- PEL
permissible exposure limit
- PPE
personal protective equipment
- PVC
polyvinyl chloride
- REL
recommended exposure limit
- ROS
reactive oxygen species
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
- TLV
threshold limit value
- TPS
thermophoretic sampler
- TWA
time-weighted average
- WPS
wide-range particle spectrometer
- WWT
wastewater treatment
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- 1.Roco MC, Mirkin CA, Hersam MC. WTEC panel report on nanotechnology research directions for societal needs in 2020: retrospective and outlook. WTEC. 2010 [Google Scholar]
- 2.National Institute for Occupational Safety and Health (NIOSH) U.S. Centers for Disease Control. Current Intelligence Bulletin 65: Occupational exposure to carbon nanotubes and nanofibers. 2013 [Google Scholar]
- 3.National Institute for Occupational Safety and Health (NIOSH) U.S. Centers for Disease Control. Current Intelligence Bulletin 63: Occupational exposure to titanium dioxide. 2011 [Google Scholar]
- 4.Methner M, Hodson L, Geraci C. Nanoparticle Emission Assessment Technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials – Part A. J Occ Environ Hyg. 2009;7:127–132. doi: 10.1080/15459620903476355. [DOI] [PubMed] [Google Scholar]
- 5.Methner M, Hodson L, Dames A, Geraci C. Nanoparticle Emission Assessment Technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials – Part B: results from 12 field studies. J Occ Environ Hyg. 2010;7:163–176. doi: 10.1080/15459620903508066. [DOI] [PubMed] [Google Scholar]
- 6.Brenner SA, Neu-Baker NM, Caglayan C, Zurbenko IG. Occupational exposure to airborne nanomaterials: an assessment of worker exposure to aerosolized metal oxide nanoparticles in semiconductor wastewater treatment. J Occ Environ Hyg. 2015;12(7):469–481. doi: 10.1080/15459624.2015.1018515. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Occupational Safety and Health (NIOSH) U.S. Centers for Disease Control. Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs). Silica, amorphous. 1994 Available online at: http://www.cdc.gov/niosh/idlh/7631869.html. Accessed September 5, 2014.
- 8.New Jersey Department of Health. Right to Know: Hazardous Substance Fact Sheet. Aluminum Oxide. 2001 Available online at: http://nj.gov/health/eoh/rtkweb/documents/fs/2891.pdf. Accessed September 7, 2014.
- 9.United States Department of Labor. Occupational Safety & Health Administration (OSHA). Chemical Sampling Information. Aluminum (as Al), Metal (Respirable Fraction) 2012 Available online at: https://www.osha.gov/dts/chemicalsampling/data/CH_217975.html. Accessed September 7, 2014.
- 10.United States Department of Labor. Occupational Safety & Health Administration (OSHA). Chemical Sampling Information. Aluminum (as Al), Metal (Total Dust) 2012 Available online at: https://www.osha.gov/dts/chemicalsampling/data/CH_217980.html. Accessed September 5, 2014.
- 11.Park EJ, Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol Lett. 2009;184(1):18–25. doi: 10.1016/j.toxlet.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Kim HW, Ahn EK, Jee BK, Yoon HK, Lee KH, Lim Y. Nanoparticulate-induced toxicity and related mechanism in vitro and in vivo. J Nanopart Res. 2009;11(1):55–65. [Google Scholar]
- 13.Sun L, Li Y, Liu X, Jin M, Zhang L, Du Z, Guo C, Huang P, Sun Z. Cytotoxicity and mitochondrial damage caused by silica nanoparticles. Toxicol in Vitro. 2011;25:1619–1629. doi: 10.1016/j.tiv.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Sun J. Endothelial cells dysfunction induced by silica nanoparticles through oxidative stress via JNK/P53 and NF-kB pathways. Biomaterials. 2010;31:8198–8209. doi: 10.1016/j.biomaterials.2010.07.069. [DOI] [PubMed] [Google Scholar]
- 15.Yu K, Grabinski C, Schrand A, Murdock R, Wang W, Gu B, et al. Toxicity of amorphous silica nanoparticles in mouse keratinocytes. J Nanopart Res. 2009;11(1):15–24. [Google Scholar]
- 16.Napierska D, Thomassen LC, Rabolli V, Lison D, Gonzalez L, Kirsch-Volders M, et al. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small. 2009;5(7):846–853. doi: 10.1002/smll.200800461. [DOI] [PubMed] [Google Scholar]
- 17.Lin W, Stayton I, Huang Y, Zhou XD. Cytotoxicity and cell membrane depolarization induced by aluminum oxide nanoparticles in human lung epithelial cells A549. Toxicol Environ Chem. 2007;90(5–6):983–996. [Google Scholar]
- 18.Wagner AJ, Bleckmann CA, Murdock RC, Schrand AM, Schlager JJ, Hussain SM. Cellular interaction of different forms of aluminum nanoparticles in rat alveolar macrophages. J Phy Chem B. 2007;111(25):7353–7359. doi: 10.1021/jp068938n. [DOI] [PubMed] [Google Scholar]
- 19.Kim IS, Baek M, Choi SJ. Comparative cytotoxicity of Al2O3, CeO2, TiO2 and ZnO nanoparticles to human lung cells. J Nanosci Nanotechnol. 2010;10(5):3453–3458. doi: 10.1166/jnn.2010.2340. [DOI] [PubMed] [Google Scholar]
- 20.Roth GA, Neu-Baker NM, Brenner SA. SEM analysis of particle size during conventional treatment of CMP process wastewater. Sci Tot Env. 2015;508:1–6. doi: 10.1016/j.scitotenv.2014.11.075. [DOI] [PubMed] [Google Scholar]
- 21.Schlecht PC, O’Connor PF, editors. National Institute for Occupational Safety and Health (NIOSH) U.S. Centers for Disease Control. NIOSH Manual of Analytical Methods (NMAM®) 4th. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2014. (DHHS (NIOSH) Publication 94–113(August 1994); 1st supplemental publication 96–135, 2nd supplemental publication 98–119, 3rd supplemental publication 2003-154). Available online at: http://www.cdc.gov/niosh/docs/2003-154/pdfs/7501.pdf. Accessed September 3, 2014. [Google Scholar]
- 22.Lomer M, Thompson R, Commisso J, Keen C, Powell J. Determination of titanium dioxide in foods using inductively coupled plasma optical emission spectrometry. The Analyst. 2000;125:2339–2343. doi: 10.1039/b006285p. [DOI] [PubMed] [Google Scholar]
- 23.Schlecht PC, O’Connor PF. National Institute for Occupational Safety and Health (NIOSH) U.S. Centers for Disease Control. NIOSH Manual of Analytical Methods (NMAM®) 4th. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2014. (DHHS (NIOSH) Publication 94–113 (August 1994); 1st supplemental publication 96–135, 2nd supplemental publication 98–119, 3rd supplemental publication 2003-154). Available online at: http://www.cdc.gov/niosh/docs/2003-154/pdfs/7402.pdf. Accessed September 3, 2014. [Google Scholar]
- 24.Pashby C, Barcus R, Sloan M. Guidelines for selecting an optical particle counter. http://www.iprocessmart.com/particlecounters/guidelines_for_selecting_an_opti.htm. Accessed September 8, 2014.
- 25.Eastlake A, Beaucham C, Martinez K, Dahm M, Sparks C, Hodson L, Geraci C. Refinement of the Nanoparticle Emission Assessment Technique into the Nanomaterial Exposure Assessment Technique (NEAT 2.0) Accepted to J Occup Environ Hyg. 2016 Mar; doi: 10.1080/15459624.2016.1167278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shepard MN, Brenner S. An occupational exposure assessment for engineered nanoparticles used in semiconductor fabrication. Ann Occup Hyg. 2014;58(2):251–265. doi: 10.1093/annhyg/met064. [DOI] [PubMed] [Google Scholar]
- 27.Yeganeh B, Kull CM, Hull MS, Marr LC. Characterization of airborne particles during productions of carbonaceous nanomaterials. Environ Sci Technol. 2008;42(12):4600–4606. doi: 10.1021/es703043c. [DOI] [PubMed] [Google Scholar]
- 28.Brouwer D, Berges M, Virji MA, Fransman W, Bello D, Hodson L, et al. Harmonization of measurement strategies for exposure to manufactured nano-objects; report of a workshop. Ann Occup Hyg. 2012;56:1–9. doi: 10.1093/annhyg/mer099. [DOI] [PubMed] [Google Scholar]
- 29.Abbott LC, Maynard AD. Exposure assessment approaches for engineered nanomaterials. Risk Anal. 2010;30:1634–44. doi: 10.1111/j.1539-6924.2010.01446.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuhlbusch TA, Asbach C, Fissan H, Göhler D, Stintz M. Nanoparticle exposure at nanotechnology workplaces: a review. Part Fibre Toxicol. 2011;8:22. doi: 10.1186/1743-8977-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller A, Frey C, King G, Sunderman C. A handheld electrostatic precipitator for sampling airborne particles and nanoparticles. NIOSH Spokane Research Lab; Washington, USA: 2010. [Google Scholar]
- 32.Thayer D, Koehler KA, Marchese A, Volckens J. A personal, thermophoretic sampler for airborne nanoparticles. Aerosol Sci Technol. 2011;45(6):744–750. [Google Scholar]
- 33.Leith D, Miller-Lionberg D, Casuccio G, Lersch T, Lentz H, Marchese A, Volckens J. Development of a transfer function for a personal, thermophoretic nanoparticle sampler. Aerosol Sci Technol. 2014;48(1) [Google Scholar]
- 34.Oberdörster G, Oberdörster E, Oberdörster J Nanotoxicology: an emerging discipline involving studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulte PA, Trout D, Zumwalde RD, Kuempel E, Geraci CL, Castranova V, Mundt DJ, Mundt KA, Halperin WE. Options for occupational health surveillance of workers potentially exposed to engineered nanoparticles: state of the science. J Occ Environ Med. 2008;50(5):517–526. doi: 10.1097/JOM.0b013e31816515f7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


