Abstract
Sphingolipids are a series of cell membrane derived lipids which act as signaling molecules and play a critical role in cell death and survival, proliferation, recognition and migration. Sphingosine 1 phosphate acts as a key signaling molecule and regulates lymphocyte trafficking, glial cell activation, vasoconstriction, endothelial barrier function and neuronal death pathways which plays a critical role in numerous neurological conditions.
Stroke is a second leading cause of death all over the world and effective therapies are still in great demand, including ischemic stroke and hemorrhagic stroke as well as the post stroke repair. Significantly, sphingolipid activities change after stroke and correlate with stroke outcome, which has promoted efforts to testify whether sphingolipid pathway could be a novel therapeutic target in stroke.
Sphingolipid metabolic pathway, the connection between the pathway and stroke, as well as therapeutic interventions to manipulate the pathway to reduce stroke-induced brain injury are discussed in this review.
Keywords: Sphingolipids, sphingosine 1 phosphate, stroke, immune modulation
1. Introduction
The sphingolipids are a series of cell membrane derived lipids which act as signaling molecules and play a critical role in cell death and survival, proliferation, recognition and migration.(1–3) These essential lipids were first described by the Germany physician Ludwig Thudichum in his book “a treatise on the chemical constitution of the brain”.(1) The pro-apoptotic molecule ceramide (Cer) and the anti-apoptotic sphingosine-1-phosphate (S1P) act as the key signaling molecules in this pathway, whose balance is critical for sequelae after stressful stimulations. For example, S1P regulates lymphocyte trafficking,(4) glial cell activation,(2, 5) vasoconstriction,(6, 7) endothelial barrier function(8, 9) and neuronal death pathways,(10, 11) which act as important components in many neurological conditions.(1)
Stroke is the second leading cause of death in the world with an enormous public health burden. Effective therapies based on pathological mechanisms are still in great need.(12) Stroke causes prolonged major changes in sphingolipid signaling. This has promoted efforts examining whether the sphingolipid pathway is a novel therapeutic target in stroke.(1) This review mainly focuses on the sphingolipid metabolic pathway, the connection between the pathway and stroke, as well as tested and potential therapeutic interventions to manipulate that pathway to reduce stroke-induced brain injury.
2. The sphingolipid pathway
Sphingolipid de novo synthesis begins in the endoplasmic reticulum with the condensation of serine and palmitoyl coenzyme A (CoA) by serine palmitoyltransferase (SPT) to form 3-keto-dihydrosphingosine.(2) That compound is converted to dihydrosphingosine by 3-ketodihydrosphingosine reductase which is then N-acylated by ceramide synthases (CerS). The formed dihydroceramides are dehydrogenated to ceramide by desaturase. Ceramide can be transported from the endoplasmic reticulum to the Golgi by ceramide transfer protein (CERT) where it is used for sphingomyelin synthesis.(13) It can also be phosphorylated by ceramide kinase to form ceramide-1-phosphate (C1P).(2) Through sphingomyelin deacylase, it also can produce sphingosylphosphorylcholine (SPC) which is emerging as an important lipid mediator of cell regulation in multiple systems.(14, 15)
Although the sphingolipid family is derived from a common sphingosine backbone, the sphingosine molecule itself is not directly generated by de novo synthesis.(3) Sphingolipids have a rapid turnover and their levels are controlled by the balance between synthesis and degradation in multiple compartments, which forms a critical rheostat and their substrates are readily interconvertible.(1, 16) The production of ceramide, derived either from the membrane lipid sphingomyelin by sphingomyelinases or synthesized de novo, can be stimulated by many stressors.(1, 16) It acts as the central player of sphingolipid metabolism, being used to generate either complex sphingolipids, such as sphingomyelin and glycosphingolipid, or signaling molecules, such as C1P and S1P.(3) Ceramide is also degraded by ceramidase to sphingosine and this is the only known pathway to generate that molecule.(2) Additionally, the reutilization of sphingosine back to ceramide plays a critical role in sphingolipid homeostasis.(16) (Figure 1)
Figure 1. Sphingolipid metabolism pathway of S1P receptor signaling.
De novo sphingolipid metabolism occurs at the endoplasmic reticulum (ER) and starts with the condensation of palmitoyl coenzyme A (CoA) and serine by serine palmitoyltransferase (SPT), which can be negatively regulated by ORM1-like protein 3 (ORMDL3), followed by series of enzyme catalyzed reactions finally forming ceramide. Ceramide, generated either from the de novo pathway or from degradation of sphingomyelin (SM) or glycosphingolipids (GluCer) can be either phosphorylated by ceramide kinase (CerK) to ceramide-1-phosphate (C1P) or transferred into sphingosylphosphorylcholine (SPC) by SM deacylase or deacylated by ceramidase to sphingosine, which is then phosphorylated to sphingosine-1-phosphate (S1P) by sphingosine kinases (SphKs). S1P can be dephosphorylated to sphingosine by a sphingosine phosphate phosphatase (SPPase) or converted to phosphoethanolamine and hexadecenal by S1P lyase (SPL) which can then be further converted to glycerolipids.
S1P is exported from cells by a specific transporter, Spns2, and carried by proteins such as high density lipoprotein (HDL)-associated apoM and albumin to the circulation. Extracellular S1P also activates five S1P receptors, namely S1P1–5 and function in diverse cell signaling pathways.
CDase, ceramidase; CerS, ceramide synthase; GCS, glucosylceramide synthase; GCase, glucosylceramidase; ORMDL3, ORM1-like protein 3; PtdEtn, phosphatidylethanolamine; Pase, phosphatase; SMase, sphingomyelinases; SMS, sphingomyelin synthase.
Phosphorylation of sphingosine by two isoforms of sphingosine kinase (SphK1 and SphK2) produces S1P, a crucial metabolite and intermediate in the sphingolipid pathway.(17) S1P is an anti-inflammatory, pro-proliferative and anti-apoptotic signaling molecule. S1P can be transported out of cells by spinster 2 (Spns2)(18) where it signals through a G protein-coupled receptor family (GPCRs). While originally known as endothelial differentiation genes (EDG),(19) they were renamed by IUPHAR as S1P1–5.(20) S1P can then either be dephosphorylated by phosphatases, including two S1P-specific, endoplasmic reticulum-localized phosphatases (SPP1 and SPP2), or irreversibly degraded by S1P lyase (SPL) to phosphoethanolamine and hexadecenal that can be incorporated into glycerolipids.(2) (Figure 1)
Ceramide
Ceramide is a hydrophobic backbone of sphingolipids. It participates in multiple biological processes and all of its specific functions have not yet been clearly elucidated.(2) Mammals have six Cer synthases (CERS1–6).(21) Ceramide, as a second messenger, can regulate multiple processes such as cell growth, differentiation, necrosis, proliferation and apoptosis and also play a part in angiotensin II type 2 receptor induced growth inhibition and apoptosis in cardiac and vascular tissues.(22) PP1, PP2A and PP2C are major serine/threonine protein phosphatases activated by ceramide and are responsible for pro-apoptotic functions, mainly involving PP2A derived dephosphorylation of Akt.(23–25) Stimulation of Protein kinase C (PKC) by ceramide is also supposed to relate to its membrane recruitment, which also results in Akt inhibition. Additionally, ceramide-enriched membrane microdomains (or platforms) play a critical role in cell signaling. Changes in membrane domains can alter membrane structure and interactions of lipids and signaling proteins. A role of such microdomains has been shown in numerous signaling pathways involved in immune cell activation and apoptosis induction.(2, 26) Alterations and protective functions of ceramide after stroke and correlations with preconditioning as well as subarachnoid hemorrhage (SAH) have been reported in several studies. (27–29)
Ceramide-1-phosphate (C1P)
Ceramide is phosphorylated by ceramide kinase (CERK) to form C1P. That molecule can activate cytosolic phospholipase-A2α (cPLA2α) and, thus, eicosanoid production.(2, 30) It can also inhibit tumor necrosis factor (TNF)-converting enzyme (TACE also known as ADAM17) which cleaves pro-TNF to release the active inflammatory form.(31)
Sphingosylphosphorylcholine (SPC)
SPC is another ceramide metabolite. It is converted from ceramide by sphingomyelin deacylase and it is now emerging as a crucial lipid mediator. It has effects on cell proliferation, migration, differentiation, metabolism and cell death in many systems including the cardiovascular system, immune system, central nervous system and skin.(14, 15) It is elevated in some pathological conditions(32–37) and was recently discovered as a pro-inflammatory mediator in cerebral arteries.(38) This, combined with previous evidence on a correlation with post-SAH vasospasm,(32, 37) makes SPC a promising novel target in stroke worthy of further investigation.
Sphingosine-1-phosphate (S1P)
S1P is a major intercellular signaling molecules controlling a wide variety of essential cellular processes such as cell growth, survival, motility, invasion into parenchyma, angiogenesis, trafficking of immune cells, endothelial barrier integrity and vascular contractility.(17, 39, 40) These functions of S1P are mediated by S1P1-S1P5 receptors expressed on diverse cell types, including immune cells, endothelia and glia, which regulate multiple downstream signaling pathways.(5, 13)
S1P is present in blood and lymph in the submicromolar range but is present at much lower levels in tissues. This creates a sharp S1P gradient for crucial functions in immune cell trafficking regulation and maintenance of vascular integrity. The main source of S1P in blood is supposed to be erythrocytes and vascular endothelial cells(2, 41) while lymphatic endothelial cells mainly form lymph S1P.(2, 42) Transportation of S1P out of vascular and lymphatic endothelial cells is mainly through a specific transporter, Spns2.(18)
S1P receptors, expression and function
The multiple physiological functions of S1P are mediated by a series of specific, high-affinity GPCRs with five receptors, namely S1P1-S1P5. These were formerly termed endothelial differentiation genes EDG -1, EDG-5, EDG-3, EDG-6 and EDG-8, respectively.(5) Differential but overlapping expression patterns and intracellular signaling pathways for each receptor enable S1P to exert diverse functions. S1P receptors have differing expression in both the immune system and central nervous system (CNS), with versatile functions during growth and development as well as pathological processes (Table 1). Briefly, S1P1–3 receptors are widely expressed but especially rich in the cardiovascular and immune systems.(5) S1P4 is expressed in the lymphoid system(5, 43) and airway smooth muscle cells.(44, 45) S1P5 is present in the white matter tracts of the central nervous system.(46, 47) The expression levels of S1P4 and S1P5 are relatively low compared to S1P1–3. In the CNS, both in vivo and in vitro studies have shown that neurons principally express S1P1 and S1P3, microglia predominantly express S1P1, while astrocytes and oligodendrocytes mainly express S1P3 and S1P5, respectively.(5)
Table 1.
Expression and function of S1P receptors and corresponding agonist and antagonist
| Receptor | Coupling | mRNA distribution | Cellular expression |
Knock out phenotype | Agonist | Antagonist | ||
|---|---|---|---|---|---|---|---|---|
| CNS | Immune system | Other cells | ||||||
| S1P1 (EDG1) |
Gi/o | Brain, heart, spleen, liver, lung, thymus, kidney, skeletal muscle, lymphoid |
Neurons, astrocytes, oligodendrocyte, microglia |
Lymphocytes, mast cells, eosinophils |
Atrial myocytes, endothelial cells, smooth muscle cells, Schwann cells |
Embryonic lethal (E12.5-E14.5), lymphopenia, vascular maturation defects, reduced lymphocyte egress, impaired astrogenesis, impaired neurogenesis |
FTY720-P, KRP-203, AUY954, SEW2871, CYM-5442, Arylpropionic acids |
VPC-23019, W123 (R)-W146, VPC44116 |
| S1P2 (EDG5) |
Gi/o, Gq, and G12/13 |
Brain, heart, spleen, liver, lung, thymus, kidney, skeletal muscle |
Neurons, astrocytes, microglia |
NA | Smooth muscle cells, Schwann cells |
Spontaneous seizures, increased neuronal excitability, deafness, increased cerebrovascular permeability |
CYM-5520 (allosteric) | JTE-013 |
| S1P3 (EDG3) |
Gi/o, Gq, and G12/13 |
Brain, heart, spleen, liver, lung, thymus, kidney, testis, skeletal muscle |
Neurons, astrocytes, microglia |
NA | Atrial myocytes, endothelial cells, smooth muscle cells, lung, kidney, intestine, cartilage, Schwann cells |
Increased permeability of pulmonary epithelial barrier |
FTY720-P, KRP-203, CYM-5541 (allosteric) |
VPC-23019, SPM-202, SPM-354 |
| S1P4 (EDG6) |
Gi/o and G12/13 |
lymphoid, lung | NA | Leukocytes | Schwann cells | NA | FTY720-P, KRP-203, CYM-50308 (selective) |
CYM-50374 |
| S1P5 (EDG8) |
Gi/o and G12/13 |
Brain, skin, spleen | Oligodendrocyte, astrocytes, microglia |
NK cells | Schwann cells | Preserved myelination, aberrant NK cell homing |
FTY720-P, KRP-203 | NA |
CNS = Central nervous system; NK = Natural Killer cells; NA=Not applicable.
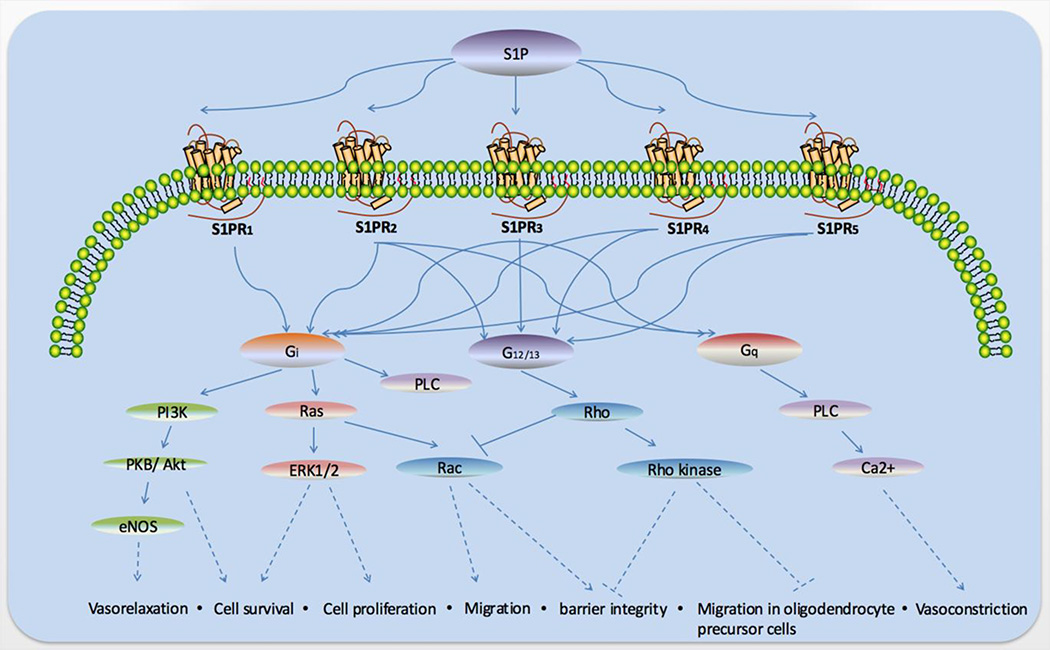
Generally, S1P1 couples with the Gi subunit of heterotrimeric G proteins, while S1P2 and S1P3 couple with Gi, Gq, and G12/13, and S1P4 and S1P5 with Gi and G12/13.(3) Signaling through Gi primarily leads to activation of the Ras/ERK pathway to promote proliferation, the PI3K/Akt pathway to prevent apoptosis, the PI3K/Rac pathway to promote cytoskeletal rearrangement and migration, and phospholipase C (PLC) activation to increase intracellular calcium and inhibit adenylyl cyclase to reduce Cyclic Adenosine monophosphate (cAMP) production. (Figure 2) Signaling through Gq could also activate the PLC pathway, while signaling through G12/13 promotes Rho activation to inhibit Rac and migration as well as barrier function. (3) (Figure 2)
Figure 2. Biological functions of S1P receptor signaling.
S1P can combine with five S1P receptors which is S1P1–5. These modulate diverse intracellular signals pathways dependent upon the coupled G proteins and expression of each receptor in specific cell types. S1P1–5 couples with the Gi subunit and can function in vasorelaxation and cell survival, proliferation and migration as well as barrier integrity, S1P2–5 can couple with G12/13 and play a role in cell migration barrier integrity and migration in oligodendrocyte precursor cells, and S1P2–3 couple with Gq which play a part in vasoconstriction.
PI3K, phosphatidylinositide 3 kinases; PKB, protein kinase B; PLC, phospholipase C.
The balance of signaling pathways downstream of each receptor plays a decisive role in the versatile function of S1P and that depends upon the expression pattern of S1P receptor subtypes in specific cell types. For example, S1P1 and S1P2 function competitively for cell migration. S1P1 promotes migration via Rac activation,(48) whereas S1P2 suppresses migration by Rho-mediated inhibition of Rac.(49, 50) Most of the S1P biological functions are considered to be mediated by S1P receptors mentioned above, but there are also several reports showing some intracellular S1P effects. Recent studies discovered that S1P binds to histone deacetylases (HDAC1 and HDAC2) inhibiting their enzymatic activity(51) and binds to TNF receptor associated factor 2 (TRAF2), a key component in Nuclear factor kappa B (NF-κB) signaling triggered by Tumor necrosis factor alpha (TNF-α), stimulating its E3 ubiquitin ligase activity.(52) The physiological relevance of these findings remains questioned and merit further investigation.
The development of specific gene deletion models has more clearly defined the function of S1P receptors and sphingolipid pathway enzymes. (Table 1) Deletion of S1P1 causes embryonic lethality between E12.5 and E14.5 due to excessive hemorrhage.(53) Additionally, a comparison between endothelial-specific deletion and global deletion indicates a critical role of S1P1 in endothelial cells for vascular stabilization.(54) Dysfunction in neurogenesis can also be caused by S1P1 depletion. Embryos at E12.5 have cell loss, increased apoptotic cells and decreased mitotic cells in different parts of the brain.(55) SphK1/SphK2 double knockout mice also show increased vascular leakage as well as neural cell death with similar outcomes to S1P1 knockout mice.(55, 56) These results clearly demonstrate a crucial role of SphK/S1P/S1P1 axis during neuronal/vascular development. The severest hemorrhage occurs in S1P1-S1P3 triple knockout mice during E10.5 and E11.5.(57) Taken as a whole, S1P1 acts as a crucial role in vascular maturation with/without support from S1P2 and S1P3. Double depletion of S1P2 and S1P3 can cause similar dysfunction and embryonic death around E13.5 due to hemorrhage, while single depletion did not cause embryonic lethality.(57–59)
S1P2 deficient mice are born without obvious deficits, but some will develop spontaneous, sporadic and occasionally lethal seizures between 3 to 7 weeks of age.(60) Simultaneously, a sharp increase in the excitability of neocortical pyramidal neurons was observed, indicating that S1P2 is essential for the development and mediation of neuronal excitability. Moreover, S1P2 is also crucial for auditory and vestibular system function.(61, 62) Several studies have also revealed that S1P2 plays a critical role in angiogenesis and atherogenesis.(63–66) A recent study demonstrated a role of S1P2 in vascular permeability and hemorrhagic complications after stroke.(67) Additionally, S1P2 inhibits macrophage recruitment during inflammation.(68)
S1P3 knockout mice also show no obvious phenotype.(59) However, the wide expression of S1P3 in the cardiovascular system as well described functions in cardiovascular function (heart rate, blood pressure), myocardial perfusion, cardiomyocyte protection after ischemia/reperfusion (I/R) injury,(69–71) vasoconstriction(40) as well as cardiac fibrosis,(72) indicate S1P3 is an important part of cardiovascular-related physiologic and pathologic processes. Additionally, S1P3 also functions to stimulate of endothelial progenitor cells(73) and several reports have unfolded that S1P3 participates in pro-inflammatory and coagulation related processes.(3) It has been reported that dendritic cell Protease activated receptor 1 (PAR1)-S1P3 crosstalk plays a critical role in the connection between coagulation and inflammation.(3)
S1P4 is expressed outside the CNS in lymphoid tissues.(5, 43) It has immunosuppressive effects by inhibiting T cell proliferation and secretion of cytokines. S1P5 is mainly expressed in oligodendrocytes in the brain, but S1P5 deficient mice do not show deficits in myelination.(47) In the immune system, S1P5 is expressed on natural killer cells and regulates their trafficking.(5, 74, 75)
Taken together, S1P exerts its effects through specific receptors in many organ systems, most notably cardiovascular, immune and central nervous systems. Each receptor has differential but partially overlapping tissue distributions and intracellular signaling pathways, and sometimes they work cooperatively and sometimes competitively with each other (Table 1, Figure 2).
Besides the S1P receptors, changes in the enzymes involved in the sphingolipid pathway also have important roles in neurological conditions. The SphKs catalyzing S1P formation, SphK1 with cytoplasmic localization and SphK2 as the predominant SphK isoform in the brain, with primarily nuclear localization(76) as well as the S1P degrading enzymes S1P lyase (S1PL),(77) S1P phosphatases (SPP1 and SPP2), and the lysophospholipid phosphatase 3 (LPP3)(78) have been found to be altered and contribute to several neurological diseases and also represent potential targets for neurological disease intervention.(1)
3. Stroke and the sphingolipid pathway
Stroke is the second most common cause of death in the world.(12) Due to either blood vessel occlusion (ischemia) or rupture (hemorrhage), stroke leads to transient or permanent neurological deficits, leaving a heavy burden for society. In developed countries, up to 75%–80% of strokes are caused by acute ischemic stroke (AIS), while 10–15% of strokes are characterized as primary intracerebral hemorrhage (ICH) and approximately 5–10% are subarachnoid hemorrhage (SAH).(79) There are either no or very limited therapeutic options for stroke and these are desperately needed. Mounting evidence has shown an intimate relationship between stroke and sphingolipid pathway. (Table 2, Figure 3)
Table 2.
Investigations on sphingolipid pathway in stroke
| Stroke subtype |
Target lysophospholipids |
Animal, stroke model |
Human specimens / Patients |
Intervention | Results | Reference | Year |
|---|---|---|---|---|---|---|---|
| AIS | GSLs | Male S-D rats, permanent MCAO |
- | One week after MCAO, L-PDMP 40mg/kg, i.p., B.i.d., for 14 consecutive days |
L-PDMP can improve re-acquisition of memory and learning function, indicated a neuronal protective effect of L-PDMP involving glucosylceramide biosynthesis |
Hisaki et al. |
2008 |
| AIS | S1P1, S1P3, S1P4, S1P5 |
Male C57BL/6 mice, tMCAO (90min) |
- | Onset of MCAO, FTY720 1mg/kg, i.p. |
FTY720 reduces lesion size and improve neurological outcome |
Czech et al. |
2009 |
| AIS | S1P1 | Male S-D rats, tMCAO (2h) |
- | Immediately after reperfusion, FTY720 0.25mg/kg and 1mg/kg, SEW2871 5mg/kg, VPC23019 0.5mg/kg, i.p. |
FTY720 can reduce infarct volume and improve neurological score and decreased apoptosis, the protective function may mainly act through S1P1 |
Hasegawa et al. |
2010 |
| AIS | S1P1, S1P3, S1P4, S1P5 |
Male C57BL/6 mice, tMCAO (90min), permanent MCAO |
- | 30min after reperfusion, FTY720 0.5mg/kg and 1mg/kg, i.p. |
FTY720 can reduce infarct volume and improve neurological outcome up to 15 days mainly through vasculo-protection rather than direct neuronal protection. |
Wei et al. | 2011 |
| AIS | S1P1, S1P3, S1P4, S1P5 |
Male C57BL/6 mice, permanent MCAO |
- | 48h before or 3h after occlusion, FTY720 1mg/kg, p.o. (single dose) / i.p. (Q.d.) |
FTY720 reduces CNS lymphocyte infiltration but no effect on infarct volume alleviation or neurobehavior promotion. |
Liesz et al. | 2011 |
| AIS | SphK2 | C57BL/6 mice, SphK1−/− mice, SphK2−/− mice, tMCAO (120min) |
- | Immediately after occlusion, FTY720 1mg/kg, i.p. |
SphK2−/− mice have larger ischemic lesions and more severe neurological deficit as well as abolished protective function of FTY720, indicated an endogenous protective mechanism of SphK2 in AIS |
Pfeilschifter et al. |
2011 |
| AIS | S1P1, S1P3, S1P4, S1P5 |
Male C57BL/6 mice and Rag1−/− mice, tMCAO (60min or 90min) |
- | Immediately before reperfusion, FTY720 1mg/kg, i.p. |
FTY720 reduces lesion size and improvse neurological outcome by induction of lymphopenia and microvascular thrombosis but not direct neuronal protection |
Kraft et al. | 2013 |
| AIS | SphK1, SphK2, S1P1 |
Male S-D rats, tMCAO (2h) |
- | Immediately after reperfusion, FTY720 0.25mg/kg, i.p. |
S1P1, SphK1, and SphK2 were downregulated in the infarct cortex, but preserved in the peri-infarct cortex after MCAO, activation of the sphingosine metabolic pathway may be neuroprotective in AIS, FTY720 reduced neuronal injury possibly via S1P1 activation. |
Hasegawa et al. |
2013 |
| AIS | S1P1, S1P3, S1P4, S1P5 |
- | 22 patients with anterior circulation ischemic stroke, 4.5–72h after onset (more than 4.5h) |
Beginning within 1h after baseline MRI and no later than72h after onset, FTY720 0.5mg, p.o., Q.d., for 3 consecutive days |
FTY720 can reduce secondary lesion enlargement, microvascular permeability, and improve clinical outcomes during the acute phase and 3-mo follow-up visit. |
Fu et al. | 2014 |
| AIS | SphK1 | Male CD-1 mice, tMCAO (60min) |
- | 3, 12, 24, 48 and 72h after reperfusion, SphK1 inhibitor 5C (CAY10621) 2mg/kg, i.v. |
SphK1 but not SphK2 was induced in ischemic brains over 96h after stroke, inhibition or knockdown of Sphk1 improved the outcomes following stroke and suppressed post-ischemic neuroinflammation, SphK1 can also reduce microglia induced inflammation in vitro |
Zheng et al. |
2015 |
| AIS | S1P2 | Male C57BL/6 mice and S1P2−/− mice, tMCAO (90min or 180min) |
Slice of human pial vessels and microvessels |
10min or 1.7h or 4.5h or 7.5h after reperfusion, JTE013 10mg/kg or 30mg/kg, oral gavage |
S1P2 plays a critical role in the induction of cerebrovascular permeability, development of hemorrhage and neurovascular injury in experimental stroke. S1P2 also correlate with MMP-9 expression and regulate endothelium response after injury. |
Kim et al. | 2015 |
| AIS | SphK2 | Male S-D rats, tMCAO (60min) |
- | 3, 6 or 9h after MCAO, Allicin 50mg/kg, i.p. |
Allicin can reduce infarct volume, attenuate brain edema and improve neurological outcome as well as decrease neuronal death. SphK2 mediated mechanism was involved in allicin-induced protection |
Lin et al. | 2015 |
| AIS | S1P1, S1P3, S1P4, S1P5 |
Male C57BL/6 mice, MCA thrombolytic occlusion model (Ref) |
- | (1) permanent occlusion: 45min, 24 and 48h after occlusion, FTY720 0.5mg/kg, i.p.; (2) tPA 30min after thrombin injection: 30min (together with tPA), 24 and 48h after occlusion, FTY720 0.5mg/kg, i.p.; (3) tPA 3h after thrombin injection: 3h (together with tPA), 24 and 48h after occlusion, FTY720 0.5mg/kg, i.p.; |
FTY720 attenuate neurological deficit and decrease infarct volume in thrombolytic occlusion model. Combined with tPA, it can improve neurological outcome and decline the risk of hemorrhagic transformation after delayed tPA administration. |
Campos et al. |
2013 |
| AIS | S1P1, S1P3, S1P4, S1P5 |
- | 47 AIS patients with anterior or middle cerebral artery occlusion, <3h after onset |
Less than 3h after onset, tPA 0.9mg/kg, i.v., FTY720 0.5mg p.o., q.d., for 3 consecutive days and first dose co-administered with tPA. |
Combination of FTY720 and tPA can attenuate reperfusion injury, infarct volume and improve clinical outcome in AIS patients. |
Zhu et al. | 2015 |
| ICH | S1P1, S1P3, S1P4, S1P5 |
CD-1 mice, cICH model |
- | 1h after ICH induction, FTY720 1mg/kg, i.p. |
FTY720 can reduce brain edema and improve neurological function. |
Rolland et al. |
2011 |
| ICH | S1P1, S1P3, S1P4, S1P5 |
Male CD-1 mice, bICH / cICH model; Male S-D rats, cICH model |
- | 1h after ICH induction, FTY720 1mg/kg, i.p., single dose or Q.d. |
FTY720 can improve neurological outcome and brain edema at short-/long-term, and induce fewer lymphocyte infiltration in blood and brain. |
Rolland et al. |
2013 |
| ICH | S1P1, S1P3, S1P4, S1P5 |
Male CD-1 mice, cICH model |
- | 30min after surgery, FTY720 0.5mg/kg, i.p., Q.d., for 2 consecutive days. |
FTY720 can enhance neurological outcome and decrease brain edema, while no difference in inflammatory (CD68+) cells |
Lu et al. | 2014 |
| ICH | S1P1, S1P3, S1P4, S1P5 |
- | 23 patients with primary parenchymal ICH of 5–30ml in basal ganglia, less than 72h prior to admission |
Within 1h after the baseline CT scan, FTY720 0.5mg, p.o., q.d., for 3 consecutive days |
FTY720 can attenuate neurological deficit and decrease circulating lymphocyte counts, as well as decreased perihematoma edema volume and rPHE and showed better long-term recovery |
Fu et al. | 2014 |
| SAH | S1P | Dog SAH model | - | S1P injected into cisterna magna |
S1P induces vasoconstriction in canine basilar artery in vivo and in vitro, may act through activation of Rho/Rho-kinase pathway |
Tosaka et al. |
2001 |
| SAH | SPC | Dog SAH model and single-hemorrhage model |
- | After administering SPC into cisterna magna, Y27632 or EPA was injected intracisternally; During single-hemorrhage model, Y27632 or EPA was injected on day 7 after SAH |
SPC is a novel mediator of Rho-kinase-induced cerebral vasospasm (CV) in vivo. The inhibition of CV induced by SPC or after SAH by eicosapentaenoic acid (EPA) suggests beneficial roles of EPA in the treatment of CV, SPC-ROK pathway may be involved in CV. |
Shirao et al. |
2008 |
| SAH | SPC | Dogs SAH model | CSF of 11 patients with SAH who had undergone surgery within 72h of the onset of SAH and classified as Fisher grade 3 by CT. |
SPC injected into cisterna magna |
SPC concentrations elevated after SAH in CSF of patients, suggested that SPC is involved in development of cerebral vasospasm. SPC rapid diluted in canine CSF indicated a higher concentration of SPC released in CSF than previous study. |
Kurokawa et al. |
2009 |
| SAH | SPC and S1P | - | - | Lysophilized S1P and SPC incubated with rat cerebral arteries. |
SPC but not S1P acts as a proinflammatory mediator in cerebral arteries. |
Wirrig et al. |
2011 |
| SAH | Cer | - | CSF of Fisher 3 grade SAH patients within 48h of the bleed. |
- | Ceramide changes occur in SAH. Elevation of levels of ceramide, particularly C18:0, correlated with the occurrence of symptomatic vasospasm and poor neurological outcome after SAH. |
Testi et al. | 2012 |
| SAH | Ceramide, DHC, S1P, ASMase, NSMase, SMS, S1P-lyase, GCS |
Male S-D rats, SAH model |
CSF of Fisher 3 grade SAH patients within 48h of the bleed. |
- | Activation of ASMase, S1P-lyase, and GCS resulting in a shift in the production of protective S1P in favor of deleterious Cer after SAH |
Testi et al. | 2015 |
| SAH | S1P and TNF-α signaling pathway |
C57BL/6 mice, TNF-α−/− and SphK1−/− mice, SAH model |
- | Immediately after SAH, JTE013, 3 mg/kg, i.p., q .d., for 3 consecutive days |
Vascular smooth muscle cell TNF-α and S1P signaling significantly enhance cerebral artery tone in SAH; anti-TNF-α and anti-S1P treatment may significantly improve outcome |
Yaqi et al. | 2015 |
| SAH | S1P | Rabbit, double hemorrhage SAH model |
- | - | S1P expression in cerebral arteries may be a new indicator during the development of cerebral vasospasm after SAH which can act as a new therapeutic target |
Tang et al. | 2015 |
AIS = Acute ischemic stroke; Cer = Ceramide; GSLs = Glucosylceramide; CNS = Central nervous system; SphK = Sphingosine kinase; tPA = Tissue plasminogen activator; ICH = Intracerebral hemorrhage; cICH = Collagenase infusion ICH model; bICH = autologous blood infusion ICH model; rPHE = relative perihematoma edema; SAH = subarachnoid hemorrhage; SPC = Sphingosylphosphorylcholine; CSF = Cerebrospinal fluid; DHC = Dihydroceramide; S1P = Sphingosine-1-phosphate; ASMase = acid sphingomyelinases; NSMase = neutral sphingomyelinases; SMS = sphingomyelinase synthase; GCS = glucosylceramide synthase
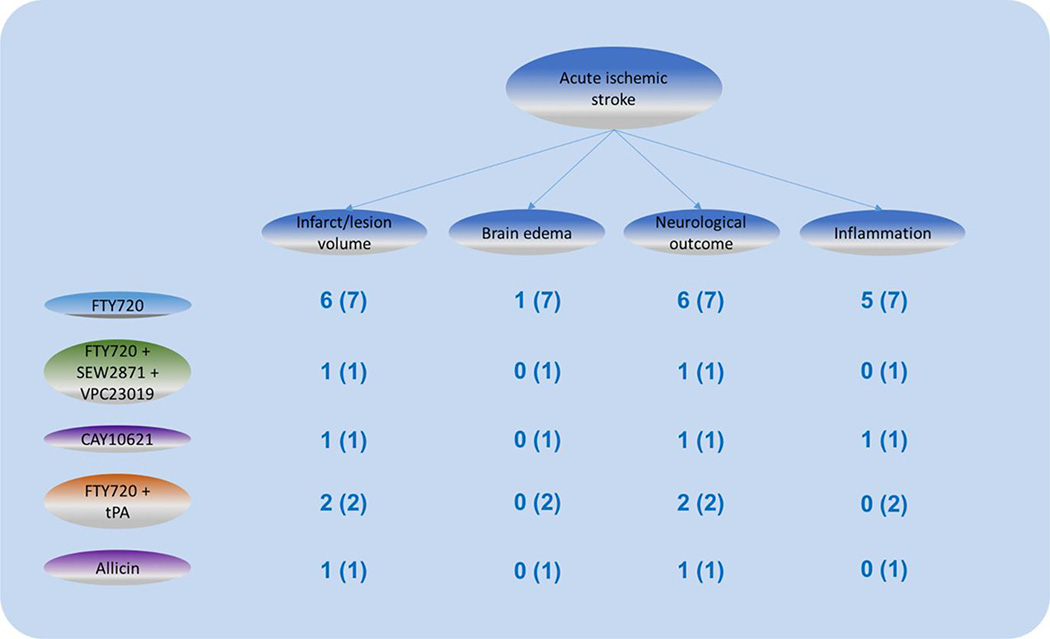
Figure 3. Interventions and outcomes of sphingolipid pathway in ischemic stroke.
Different interventions aiming at sphingolipid pathway in ischemic stroke with outcomes summarized. Numbers represents the amount of studies aiming at this outcome and the total amount of studies of this intervention.
Cerebral Ischemia
Sudden thromboembolic occlusion of a brain vessel can cause hypoperfusion of the corresponding vascular territory leading to AIS and neurological deficits. To date, the only available therapies are tissue plasminogen activator (tPA) and thrombectomy to induce revascularization. However, due to a narrow therapeutic time window (4.5h) for tPA and unstable outcome of thrombectomy, only a small fraction of patients benefit from those therapies.(80–82) There is, therefore, an urgent need for other medical therapies for AIS. After a stroke, the central area of tissue damage gradually expands into surrounding tissue, the penumbra, and it that tissue that may be rescued.(12, 83)
The overall pathology after AIS involves many components including oxidative stress, immune activation, blood-brain barrier (BBB) damage and neuronal death.(84, 85) Mounting evidence indicates that the immune response after AIS plays a critical role in stroke outcome. The release of cell death products, such as Damage associated molecular pattern molecules (DAMPs), activates pattern recognition receptors (PRR), for instance, Toll-like receptors (TLR), resulting in both an innate and an adaptive immune response. There is activation of resident microglia as well as a serial infiltration of inflammatory components into the brain through a damaged BBB, which further aggravates brain tissue damage.(86)
Amplification of initial brain injury by inflammatory mediators continues well beyond 4.5h of stroke onset, the current tPA time window, making it a promising therapeutic target.(84, 87) Early, microglia/macrophages and neutrophils are crucial players in the inflammation.(84, 86) Cytokines, complement and, in delayed brain inflammation, T and B lymphocytes as well as Natural killer (NK) cells, have also been reported to contribute to post-ischemic inflammation.(86–93) Considering the regulatory functions of the sphingolipid signaling pathway in immune system and endothelial biology, interventions targeting that pathway are a promising approach in stroke therapy.
To date, a mounting number of in vivo and in vitro preclinical studies and pilot human studies have shown therapeutic effects of S1P receptor modulation and elucidated possible mechanisms by which the SphK-S1P axis affects stroke.(67, 94–107) For example, preclinically, fingolimod (FTY720), a S1P receptor modulator, has shown short and long-term protection in rodent models of AIS.(94–96) Reduction in lesion size and improvement in neurological outcomes as well as decreased cell death can be observed after fingolimod treatment up to 15 days after onset.(94–96) A reduction of nuclear translocation of apoptosis inducing factor (AIF), caspase-3 cleavage and TUNEL positive neurons, as well as up-regulation of anti-apoptotic pathways such as extracellular signal regulated kinase (ERK1/2), protein kinase B/Akt kinase (PKB/Akt) and Bcl-2 were observed after administration of fingolimod. This supports the hypothesis that fingolimod exerts direct neuronal protection.(95, 96) Moreover, decreased immune cell activation and infiltration (such as activated neutrophils, microglia/macrophages) and adhesion molecule (ICAM-1) expression in the ipsilateral hemisphere has been detected.(94–96) Inspired by such preclinical studies, a pilot study examined the effects of fingolimod in 22 patients at 4.5h after stroke onset. It found evidence of neuroprotection with reduced lymphocyte counts, improved neurological dysfunction, milder lesion enlargement and decreased microvascular permeability,(102) which coincides well with the previous rodent studies.
The effects of fingolimod are cell-type specific. It can regulate the polarization on microglia activation.(108) Decreased ICAM-1 expression in brain microvascular endothelial cells, an indicator of the inflammatory response, was found after fingolimod administration but protection was not found in neuronal cell cultures subjected to conditions to simulate ischemia (glutamate, H2O2 or hypoxia).(96, 109) Considering potential differences between in vitro and in vivo conditions, further exploration of fingolimod cell-specific effects are needed. Interestingly, a reduction of microvascular thrombosis in peri-infarct area was observed after fingolimod administration which identified a correlation between inflammation and thrombus formation, i.e. thromboinflammation.(100) Using Rag−/− mice and cell culture, Kraft et al. found lymphopenia and reduced microvascular thrombosis but not direct neuroprotection after fingolimod intervention.(100) Thus, the mode of action of fingolimod on secondary occlusion and post-stroke coagulation deserves further investigation.
Considering the non-selective function of fingolimod on S1P1, S1P3, S1P4 and S1P5, and corresponding complications, the crucial sphingolipid receptor target in stroke intervention remains blurred and deserves further investigation. Hasegawa et al. used the selective S1P1 agonist SEW2871 and S1P1, S1P3, S1P4 antagonist VPC20319 to test the role of S1P1 in stroke intervention and found that S1P1 activation can reduce neuronal death and plays a critical role in the overall outcome of ischemic stroke.(95) As S1P3 has mutual functions with thrombin and PAR-1 in regulating coagulation and vascular permeability,(3) the thrombus formation after stroke may not only correlate to S1P1 in lymphocyte but also S1P3 in endothelial cells.
S1P2 has also recently been found to be crucial in endothelial function. Experiments using gene knockout and a selective S1PR2 antagonist (JTE013) both in vitro and in vivo in rodent and human specimens indicate it protects against vascular dysfunction after I/R injury.(67) Effects on BBB permeability, matrix metallopeptidase 9 (MMP-9) expression and hemorrhagic transformation were observed, but not on neuronal survive, indicating a critical role of S1P2 in endothelial function. One area that deserves investigation is whether S1P2 may impact glucose-induced hemorrhagic transformation. Hyperglycemia has positive relationship with I/R injury and post-stroke hemorrhagic transformation in animal models and clinically.(110, 111)
The function of SphK should also not be neglected. SphK1 and SphK2, by regulating S1P formation, are key components of the sphingolipid pathway.(2) SphK2 is essential for phosphorylation of fingolimod with its protective effects in ischemic stroke.(97) Gene deletion studies on SphK1 and SphK2 demonstrated that SphK2 provides support in stroke protection with decreased lesion size and better neurological function.(97) The specific mechanisms need further elucidation and further attention on SphK2-S1P axis in stroke intervention should be paid a high priority.
Additionally, combined use of tPA and fingolimod has raised attention and gained partially positive results,(67, 105, 112). The need for a combination approach is based on potential side-effects of tPA therapy, ischemia/reperfusion (I/R) injury and high risk of fatal hemorrhagic transformation, as well as its narrow therapeutic window.(113–115) Therefore, an ideal combination or intervention is of great significance for better use of recanalization and reperfusion by tPA. The function of S1P signal pathway in endothelial cells has been tested by administration of non-selective S1PR modulator fingolimod in rodent and human, as well as mechanism study of S1P2 in endothelial protection.(67, 105, 112) Using a model of thromboembolic stroke, Campos et al. found that fingolimod reduced neurological deficit, infarct volume and especially reduced the risk of hemorrhagic transformation and BBB damage secondary to delayed use of tPA.(105) In contrast, in a stroke model of large hemispheric infarction, no benefit was shown, especially on functional outcome and BBB integrity, when fingolimod was administered alone or in combination with tPA.(116) A disadvantage of this study is the lack of measurement of hemorrhagic transformation and infarct volume. Considering the severe outcome of this 3h MCAO model, different measurement of neurological function may also be applied. Moreover, a multicenter pilot trial examined using fingolimod combined with tPA within 4.5h after stroke onset in patients with hemispheric ischemic stroke stemming from anterior or middle cerebral arterial occlusion. Patients had milder reperfusion injury and improved clinical outcomes.(112) The specific role of S1PR2 in hemorrhagic transformation has been discussed and further manifested the correlation between sphingolipids and hemorrhagic transformation. (67)
Taken as a whole, the function of the sphingolipid pathway in I/R injury has gained much attention and partially positive results. It deserves further investigation and detailed dissection of mechanism. The effects of manipulating the sphingolipid pathway along with tPA administration to reduce I/R injury needs further models to mimic the exact pathology of endothelial function and neurological deterioration after I/R injury with tPA. In addition, the use of a relatively non-selective S1P receptor modulator, fingolimod, makes identification of the exact S1P signaling events involved lack of specify. The possible function of sphingolipid pathway in widening the therapeutic window and its function on large infarction patients, especially on hemorrhagic transformation, still need to be taken into consideration in future studies.
Intracerebral hemorrhage
ICH is a devastating disease with high mortality and morbidity with no current therapy.(117–122) Considering the aging population, the incidence of ICH is expected to increase.(123) ICH is classified as either primary or secondary, based on bleeding cause. Primary ICH, which occurs in ~80% of patients, results from spontaneous blood vessel rupture (primarily due to hypertension), whereas in secondary ICH the underlying cause is another condition such as brain tumors or traumatic brain injury.(124) Irrespective of type, ICH results in a hematoma, edema, inflammation, apoptosis and necrosis.(118, 125)
ICH induces both primary and secondary injury.(126) The primary injury is caused by hematoma formation and its expansion which leads to mechanical damage, compression of surrounding brain tissue, increased intracranial pressure, followed potentially by oligemia, neurotransmitter release, mitochondrial disturbances, and membrane depolarization.(127–129) Secondary injury is, however, and important component. It involves cytotoxic, excitotoxic, oxidative and inflammation pathways, and brain edema.(130–132) Peri-hematoma edema exacerbates the hematoma mass effect, aggravates secondary brain injury and cell death process through subsequent oligemia and inflammatory insults.(117, 123, 124) Edema has the potential to negatively impact ICH outcome.(133–135) Thrombin, inflammatory mediators and the release of clot components, such as hemoglobin and iron, are major causes of cerebral edema and brain injury after ICH.(117–119, 136, 137)
Thrombin is a critical factor in ICH both as a component of the coagulation cascade that limits bleeding but also as a factor that can induce injury mainly through PAR-1.(138) Thus, it can trigger inflammatory cell infiltration, BBB damage and edema, and neuron and astrocyte death.(117–119, 138) The functions of thrombin may be receptor or non-receptor (e.g. fibrinogen cleavage) mediated. There are three receptors, PAR-1, PAR-3 and PAR-4, with PAR-1 being the main subtype mediating several pathological effects.(139) And thrombin inhibition can be protective in hemorrhagic stroke models.(140, 141)
S1P1 plays a role in activated protein C (aPC)-mediated endothelial barrier protection, either by SphK1-mediated S1P production or by EPCR-mediated direct trans-activation of S1P1, while a differential coupling of thrombin-activated PAR-1 with S1P3 leads to endothelial barrier disruption.(3) Thus, whether thrombin or aPC/EPCR is the activating protease in combination with the presence of S1P1 or S1P3 determines PAR-1 signaling specificity in endothelial cells during vascular permeability regulation.(3, 142–146) Therefore, the SphK-S1P signaling pathway plays a critical role in thrombin-induced ICH injury and may be a potential therapeutic target.
Inflammation has emerged as a crucial component during the onset and progression of ICH, with cross-talk between multiple pathways.(87, 124, 133, 147–149) Brain-intrinsic microglia are the first inflammatory cell type to react to brain injury. By releasing inflammatory mediators, they contribute to BBB breakdown and leukocyte influx from blood. These include neutrophils, monocytes and macrophages, followed by lymphocyte populations.(150–153) Activated resident and migrant cells also produce cytokines and chemokines and that, together with the cell death products, further exacerbates the inflammatory process causing peri-hematoma edema and subsequent brain injury.(124, 154, 155) Because of the effects of the sphingolipid pathway on inflammatory and endothelial cells, it may be a target to affect these processes.
In rodents, administration of the S1PR modulator fingolimod ICH reduced the inflammatory response, decreasing CD3+ T lymphocyte infiltration and endothelial ICAM-1 expression, as well as interferon-γ(IFN-γ)and interleukin (IL)-17 levels in brain.(156) A number of studies have also found improvements in neurological function, as well as reductions in brain edema and neuronal death,(156–158) indicating the possible efficacy of targeting to S1PR in ICH. Investigations comparing single and consecutive doses found a better outcome with continuous dosing which suggest a prolonged therapeutic window for ICH. Further studies are needed to define the therapeutic time window and care is needed in relation to long-term dosing and immune suppression.
There has been a randomized, placebo-controlled, pilot study with 23 ICH patients with 5–30 ml hematoma volumes. Patients who were given fingolimod orally for 3 consecutive days had gained improved neurological function, decreased inflammation and peri-hematoma edema.(159) Moreover, short- and long-term benefits have been found with fingolimod administration in neurological deficits, cell death and brain atrophy.(156–158) This supports the concept of targeting the sphingolipid pathway in ICH treatment. However, no significant change in microglia/macrophages was found using CD68 as marker has been observed in a rodent experiment,(157) which leaves a question about the critical target of the S1P signaling pathway in ICH intervention.
Intraventricular hemorrhage (IVH) is bleeding into the brain ventricles. It occurs secondary to ICH and SAH (160)and it is associated with poor prognosis for hemorrhagic stroke.(161) Severe IVH leads to hydrocephalus and has a great risk of hemorrhage-associated morbidity. Unfortunately, no effective therapies exist to date.(162) As with ICH, there is a role for inflammation and the coagulation cascade in IVH-induced brain injury and there is also a role for those processes in IVH-induced hydrocephalus.(163) As the sphingolipid pathway has effects on inflammation and coagulation, it is may be a therapeutic target for IVH- induced hydrocephalus and sphingolipid pathway, but this has not been examined.
Subarachnoid hemorrhage
Although SAH only accounts for ~5% of all strokes, it has a particularly high mortality and morbidity.(164) Early brain injury after SAH, which happens before the onset of delayed vasospasm, can cause death.(165) However, SAH has a biphasic time course, with that initial hemorrhagic insult being followed by delayed cerebral ischemia (DCI) within 4 to 12 days. (166–170) DCI may cause half of the mortality in SAH and it has been a major focus of SAH research.(166–168) Cerebral vasospasm, a potential cause of DCI, is a frequent complication of SAH and it has been regarded as a major determinant of outcome. Angiographic vasospasm is mainly driven by marked alterations in cerebrovascular response which compromise cerebral auto-regulation.(168, 171, 172) However, despite a clear correlation between hypoperfusion and vasospasm, cerebral blood flow deficits do exist in regions without vasospasm.(173) A general amplification of myogenic tone in the cerebral microcirculation may be a more meaningful regulator for SAH outcome by comprehensively altering perfusion deficits.(174) Therapeutic interventions should particularly correct the increased myogenic tone in SAH.
During the past decades, the role of the sphingolipid pathway in SAH has received considerable attention.(32, 37, 38, 175–179) S1P was first found to induce vasoconstriction in canine basilar artery and SPC was determined to be a novel mediator of Rho-kinase induced cerebral vasospasm and act as a proinflammatory mediator in cerebral arteries. Further, SPC cerebrospinal fluid (CSF) levels are elevated in SAH patients.(32, 37, 38, 176) Moreover, sphingolipids and CSF ceramide alterations after SAH have also been detected and correlate with clinical outcome. In the CSF of patients with SAH, ceramide is increased and an activation of sphingomyelinae (ASMase), S1P-lyase, and glucosylceramide synthase (GCS) lead to an alteration in the production of protective (S1P) in favor of deleterious (Ceramide) sphingolipids.(175, 177) Using SphK1−/− mice and S1PR antagonism (JTE013), SAH significantly presented an increase in myogenic tone and JTE013 can fully reverse the myogenic tone augmentation both in vivo and in vitro, which reveals a critical role of S1P signaling pathway as a mediator of increased myogenic tone in SAH.(178) Interestingly, S1P3 receptors are also reported to mediate the potent constriction of cerebral arteries by S1P,(40) thus providing a novel target of S1P1/S1P3 system in regulating the vascular response after SAH. Tang et al. also observed the expression of S1P on the cerebral vasospasm after SAH using a “double hemorrhage” model in rabbit,(179) and they found S1P expression a new marker for the development of cerebral vasospasm. Also, targeting either TNF-α or the S1P signaling pathway after SAH attenuated neuronal apoptosis and improved neurological outcome.(178) Furthermore, the specific role of SphK1 in vasoconstriction has also been demonstrated in isolated basilar arteries.(160, 180) Thus, the sphingolipid pathway plays an important role in SAH and especially vasospasm. However, due to the complicated pathology of SAH, more details on the pathological target and the specific functions of S1P receptors are still needed.
Preconditioning
Preconditioning is a process by which a sublethal noxious stimulation induces a robust protection or tolerance against subsequent lethal injuries.(181–184) It has been observed in multiple organs, including brain. Moreover, a wide range of stimuli can induce preconditioning, e.g. hypoxia, transient ischemia, hyperbaric oxygen, hypothermia, hyperthermia, inhalational anesthetics (isoflurane) or inflammatory agents such as bacterial lipopolysaccharide.(181) Interestingly, cross-talk exists between different stimuli which means one type of preconditioning can protect against a wide range of other stresses.(181) Brain preconditioning involves multiple downstream pathways including: metabolic inhibition, activation of extra- and intra- cellular defense mechanisms, disturbance of neuronal excitatory/ inhibitory balance, and suppression of inflammatory sequelae.(181)
Hypoxia inducible factor (HIF) is a crucial factor involved in preconditioning. It is stabilized by hypoxia and ischemia, as well as non-hypoxia stimuli involved in preconditioning such as isoflurane or lipopolysaccharide (LPS). It exists in three isoforms which bind to hypoxia-responsive elements (HRE) of hypoxia regulated genes to promote their transcription.(1) A relationship between hypoxia preconditioning and the sphingolipid pathway has been shown by several in vivo and in vitro studies.(27–29, 185–193) Ceramide is critical in hypoxia-induced preconditioning both in cultured neurons(28) and in rat brain.(27) It plays a role as a mediator during the protective process and is capable of up-regulating B cell lymphoma 2 (Bcl-2) while decreasing TUNEL positive cells.(27) Additionally, ceramide is critical in the induction of ischemic tolerance in ischemia- and LPS-induced preconditioning.(29, 189) Elements of the sphingolipid pathway downstream of ceramide, especially SphK2, are also involved in preconditioning.(190–192) SphK2 is proposed to act as a proximal trigger to drive S1P mediating alterations in gene expression and, thus, to promote the ischemia-tolerant phenotype.(191) Moreover, SphK2 is also involved in BBB protection during preconditioning.(190) Recent evidence also suggests that cross-talk between HIF and the SphK2 produced S1P signaling can co-upregulate chemokine (C-C motif ) ligand 2 (CCL2) expression, which can contribute to the stroke-tolerant phenotype established by hypoxic and S1P agonist preconditioning.(186)
Isoflurane can also induce preconditioning with protective effects in several type of stroke.(194) Those effects correlate with changes in the sphingolipid pathway,(188) especially activation of the S1P/ PI3K/Akt pathway.(187) Moreover, isoflurane can protect the BBB, an effect mainly mediated by SphK1 expression and S1P1/S1P3 activation.(193) A recent study also revealed that preconditioning-stimulated autophagy is mediated by activation of SphK2, and proposed to function through Beclin 1/ Bcl-2 interaction.(185)
Considering the complicated mechanisms underlying preconditioning and the possible pathways enrolled, the sphingolipid pathway has shown a close relationship to preconditioning-induced protection of preconditioning, and particularly hypoxia, ischemia and isoflurane preconditioning.(1) The possible mechanisms involved may be multi-fold and both upstream and downstream investigations are still necessary.
Post-stroke repair
Post-stroke neurogenesis plays a significant role in overall stroke outcome. It involves three major steps: proliferation, migration and differentiation into neural phenotypes, with proliferation having a major role.(195) Studies on the role of the sphingolipid pathway in recovery have mainly focused on astrogliosis (178) and neural progenitor cells.(196, 197) Brain injury induces astrogliosis which involves transcriptional, biochemical and morphological changes in astrocytes.(198) The glial scar can have both negative and positive impacts on recovery. Modulating the S1P receptor signaling with fingolimod has a protective effect on stroke recovery at later time points using due to reduced astrogliosis, modulation of synaptic morphology and increased neurotrophic factor expression.(191) Significantly, S1P can also induce neural progenitor cells (NPC) migration and regulation of S1P2 can promote migration towards injured brain.(196) S1P also reduces glial cell line derived neurotrophic factor levels.(199) Moreover, S1P has a versatile role in neurogenesis, possibly through a pathway involving MAP kinase (MAPK) and Rho kinase.(197) Additionally, fingolimod can increase brain-derived neurotrophic factor in Rett syndrome(200) and amyloid β-induced memory impairment.(201, 202)
4. Conclusions
Mounting evidence during the past two decades from both animals and humans, both in vivo and in vitro, has indicated a major role of the sphingolipid pathway in stroke pathology. In addition, many studies have shown that it is a potential therapeutic target for stroke, those who are inconsistent with the STAIR and RIGOR criteria are not included.(203, 204) There is much still to learn about the underlying effects of the sphingolipid pathway in stroke and more investigations are still greatly needed to elucidate novel targets and provide a solid foundation for further clinical practice in stroke management.
Search criteria
This review focuses on research in sphingolipid pathway in stroke. We searched Pubmed with the terms “sphingolipid” “sphingosine” “sphingosine 1 phosphate” “stroke” “isch(a)emic stroke” “intracerebral h(a)emorrhage” “subarachnoid h(a)emorrhage” “h(a)emorrhage” “preconditioning” “repair” and retrieved all articles published in English. We selected articles for their conceptual importance and primacy and complied with STAIR and RIGOR criteria. For controversial issues, evidence on both sides was sought.
Acknowledgments
This study was supported by grants NS-073595, NS-079157, NS-084049, NS-090925 and NS-091545 from the National Institutes of Health (NIH) and 973 Program-2014CB541600.
Footnotes
Conflicts of Interest: Na Sun, Richard F. Keep, Ya Hua, and Guohua Xi declare that they have no conflict of interest.
Compliance with Ethics Requirements: All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Brunkhorst R, Vutukuri R, Pfeilschifter W. Fingolimod for the treatment of neurological diseases-state of play and future perspectives. Front Cell Neurosci. 2014;8:283. doi: 10.3389/fncel.2014.00283. PubMed PMID: 25309325. Pubmed Central PMCID: PMC4162362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014 Jun 5;510(7503):58–67. doi: 10.1038/nature13475. PubMed PMID: 24899305. Pubmed Central PMCID: PMC4320971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obinata H, Hla T. Sphingosine 1-phosphate in coagulation and inflammation. Seminars in immunopathology. 2012 Jan;34(1):73–91. doi: 10.1007/s00281-011-0287-3. PubMed PMID: 21805322. Pubmed Central PMCID: 3237867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nature reviews Immunology. 2008 Oct;8(10):753–763. doi: 10.1038/nri2400. PubMed PMID: 18787560. Pubmed Central PMCID: 2600775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aktas O, Kury P, Kieseier B, Hartung HP. Fingolimod is a potential novel therapy for multiple sclerosis. Nature reviews Neurology. 2010 Jul;6(7):373–382. doi: 10.1038/nrneurol.2010.76. PubMed PMID: 20551946. [DOI] [PubMed] [Google Scholar]
- 6.Levkau B. Sphingosine-1-phosphate in the regulation of vascular tone: a finely tuned integration system of S1P sources, receptors, and vascular responsiveness. Circulation research. 2008 Aug 1;103(3):231–233. doi: 10.1161/CIRCRESAHA.108.181610. PubMed PMID: 18669929. [DOI] [PubMed] [Google Scholar]
- 7.Coussin F, Scott RH, Wise A, Nixon GF. Comparison of sphingosine 1-phosphate-induced intracellular signaling pathways in vascular smooth muscles: differential role in vasoconstriction. Circulation research. 2002 Jul 26;91(2):151–157. doi: 10.1161/01.res.0000028150.51130.36. PubMed PMID: 12142348. [DOI] [PubMed] [Google Scholar]
- 8.Prager B, Spampinato SF, Ransohoff RM. Sphingosine 1-phosphate signaling at the blood-brain barrier. Trends in molecular medicine. 2015 Jun;21(6):354–363. doi: 10.1016/j.molmed.2015.03.006. PubMed PMID: 25939882. [DOI] [PubMed] [Google Scholar]
- 9.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends in immunology. 2007 Mar;28(3):102–107. doi: 10.1016/j.it.2007.01.007. PubMed PMID: 17276731. [DOI] [PubMed] [Google Scholar]
- 10.Cipriani R, Chara JC, Rodriguez-Antiguedad A, Matute C. FTY720 attenuates excitotoxicity and neuroinflammation. Journal of neuroinflammation. 2015;12:86. doi: 10.1186/s12974-015-0308-6. PubMed PMID: 25953296. Pubmed Central PMCID: 4429813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pyszko JA, Strosznajder JB. The key role of sphingosine kinases in the molecular mechanism of neuronal cell survival and death in an experimental model of Parkinson's disease. Folia neuropathologica / Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences. 2014;52(3):260–269. doi: 10.5114/fn.2014.45567. PubMed PMID: 25310737. [DOI] [PubMed] [Google Scholar]
- 12.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008 May 10;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. PubMed PMID: 18468545. [DOI] [PubMed] [Google Scholar]
- 13.Hanada K. Co-evolution of sphingomyelin and the ceramide transport protein CERT. Biochimica et biophysica acta. 2014 May;1841(5):704–719. doi: 10.1016/j.bbalip.2013.06.006. PubMed PMID: 23845852. [DOI] [PubMed] [Google Scholar]
- 14.Nixon GF, Mathieson FA, Hunter I. The multi-functional role of sphingosylphosphorylcholine. Progress in lipid research. 2008 Jan;47(1):62–75. doi: 10.1016/j.plipres.2007.11.001. PubMed PMID: 18042469. [DOI] [PubMed] [Google Scholar]
- 15.Kleger A, Liebau S, Lin Q, von Wichert G, Seufferlein T. The impact of bioactive lipids on cardiovascular development. Stem cells international. 2011;2011:916180. doi: 10.4061/2011/916180. PubMed PMID: 21876704. Pubmed Central PMCID: 3159013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature reviews Molecular cell biology. 2008 Feb;9(2):139–150. doi: 10.1038/nrm2329. PubMed PMID: 18216770. [DOI] [PubMed] [Google Scholar]
- 17.Dobrosotskaya IY, Seegmiller AC, Brown MS, Goldstein JL, Rawson RB. Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science. 2002 May 3;296(5569):879–883. doi: 10.1126/science.1071124. PubMed PMID: 11988566. [DOI] [PubMed] [Google Scholar]
- 18.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009 Jan 23;323(5913):524–527. doi: 10.1126/science.1167449. PubMed PMID: 19074308. [DOI] [PubMed] [Google Scholar]
- 19.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochimica et biophysica acta. 2002 May 23;1582(1–3):72–80. doi: 10.1016/s1388-1981(02)00139-7. PubMed PMID: 12069812. [DOI] [PubMed] [Google Scholar]
- 20.Lynch KR. Lysophospholipid receptor nomenclature. Biochimica et biophysica acta. 2002 May 23;1582(1–3):70–71. doi: 10.1016/s1388-1981(02)00138-5. PubMed PMID: 12069811. [DOI] [PubMed] [Google Scholar]
- 21.Kihara A. Synthesis and degradation pathways, functions, and pathology of ceramides and epidermal acylceramides. Prog Lipid Res. 2016 Apr 21;63:50–69. doi: 10.1016/j.plipres.2016.04.001. PubMed PMID: 27107674. [DOI] [PubMed] [Google Scholar]
- 22.Borodzicz S, Czarzasta K, Kuch M, Cudnoch-Jedrzejewska A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015;14:55. doi: 10.1186/s12944-015-0053-y. PubMed PMID: 26076974. Pubmed Central PMCID: PMC4470334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canals D, Roddy P, Hannun YA. Protein phosphatase 1alpha mediates ceramide-induced ERM protein dephosphorylation: a novel mechanism independent of phosphatidylinositol 4, 5-biphosphate (PIP2) and myosin/ERM phosphatase. The Journal of biological chemistry. 2012 Mar 23;287(13):10145–10155. doi: 10.1074/jbc.M111.306456. PubMed PMID: 22311981. Pubmed Central PMCID: 3323024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J, et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO molecular medicine. 2013 Jan;5(1):105–121. doi: 10.1002/emmm.201201283. PubMed PMID: 23180565. Pubmed Central PMCID: 3569657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox TE, Houck KL, O'Neill SM, Nagarajan M, Stover TC, Pomianowski PT, et al. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. The Journal of biological chemistry. 2007 Apr 27;282(17):12450–12457. doi: 10.1074/jbc.M700082200. PubMed PMID: 17308302. [DOI] [PubMed] [Google Scholar]
- 26.Henry B, Ziobro R, Becker KA, Kolesnick R, Gulbins E. Acid sphingomyelinase. Handbook of experimental pharmacology. 2013;(215):77–88. doi: 10.1007/978-3-7091-1368-4_4. PubMed PMID: 23579450. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Ginis I, Hallenbeck JM. The protective effect of ceramide in immature rat brain hypoxia-ischemia involves up-regulation of bcl-2 and reduction of TUNEL-positive cells. J Cereb Blood Flow Metab. 2001 Jan;21(1):34–40. doi: 10.1097/00004647-200101000-00005. PubMed PMID: 11149666. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Ginis I, Spatz M, Hallenbeck JM. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-alpha and ceramide. Am J Physiol Cell Physiol. 2000 Jan;278(1):C144–C153. doi: 10.1152/ajpcell.2000.278.1.C144. PubMed PMID: 10644522. [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann C, Ginis I, Furuya K, Klimanis D, Ruetzler C, Spatz M, et al. Lipopolysaccharide-induced ischemic tolerance is associated with increased levels of ceramide in brain and in plasma. Brain Res. 2001 Mar 23;895(1–2):59–65. doi: 10.1016/s0006-8993(01)02028-5. PubMed PMID: 11259760. [DOI] [PubMed] [Google Scholar]
- 30.Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, et al. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. The Journal of biological chemistry. 2004 Mar 19;279(12):11320–11326. doi: 10.1074/jbc.M309262200. PubMed PMID: 14676210. [DOI] [PubMed] [Google Scholar]
- 31.Lamour NF, Wijesinghe DS, Mietla JA, Ward KE, Stahelin RV, Chalfant CE. Ceramide kinase regulates the production of tumor necrosis factor alpha (TNFalpha) via inhibition of TNFalpha-converting enzyme. The Journal of biological chemistry. 2011 Dec 16;286(50):42808–42817. doi: 10.1074/jbc.M111.310169. PubMed PMID: 22009748. Pubmed Central PMCID: 3234830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurokawa T, Yumiya Y, Fujisawa H, Shirao S, Kashiwagi S, Sato M, et al. Elevated concentrations of sphingosylphosphorylcholine in cerebrospinal fluid after subarachnoid hemorrhage: a possible role as a spasmogen. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2009 Aug;16(8):1064–1068. doi: 10.1016/j.jocn.2009.01.010. PubMed PMID: 19596114. [DOI] [PubMed] [Google Scholar]
- 33.Kim HJ, Kim H, Han ES, Park SM, Koh JY, Kim KM, et al. Characterizations of sphingosylphosphorylcholine-induced scratching responses in ICR mice using naltrexon, capsaicin, ketotifen and Y-27632. European journal of pharmacology. 2008 Mar 31;583(1):92–96. doi: 10.1016/j.ejphar.2008.01.005. PubMed PMID: 18289521. [DOI] [PubMed] [Google Scholar]
- 34.Imokawa G, Takagi Y, Higuchi K, Kondo H, Yada Y. Sphingosylphosphorylcholine is a potent inducer of intercellular adhesion molecule-1 expression in human keratinocytes. The Journal of investigative dermatology. 1999 Jan;112(1):91–96. doi: 10.1046/j.1523-1747.1999.00462.x. PubMed PMID: 9886270. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto R, Arikawa J, Ishibashi M, Kawashima M, Takagi Y, Imokawa G. Sphingosylphosphorylcholine is upregulated in the stratum corneum of patients with atopic dermatitis. Journal of lipid research. 2003 Jan;44(1):93–102. doi: 10.1194/jlr.m200225-jlr200. PubMed PMID: 12518027. [DOI] [PubMed] [Google Scholar]
- 36.Byun HJ, Kang KJ, Park MK, Lee HJ, Kang JH, Lee EJ, et al. Ethacrynic Acid Inhibits Sphingosylphosphorylcholine-Induced Keratin 8 Phosphorylation and Reorganization via Transglutaminase-2 Inhibition. Biomolecules & therapeutics. 2013 Sep 30;21(5):338–342. doi: 10.4062/biomolther.2013.066. PubMed PMID: 24244820. Pubmed Central PMCID: 3825196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shirao S, Fujisawa H, Kudo A, Kurokawa T, Yoneda H, Kunitsugu I, et al. Inhibitory effects of eicosapentaenoic acid on chronic cerebral vasospasm after subarachnoid hemorrhage: possible involvement of a sphingosylphosphorylcholine-rho-kinase pathway. Cerebrovascular diseases. 2008;26(1):30–37. doi: 10.1159/000135650. PubMed PMID: 18511869. [DOI] [PubMed] [Google Scholar]
- 38.Wirrig C, Hunter I, Mathieson FA, Nixon GF. Sphingosylphosphorylcholine is a proinflammatory mediator in cerebral arteries. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011 Jan;31(1):212–221. doi: 10.1038/jcbfm.2010.79. PubMed PMID: 20551970. Pubmed Central PMCID: 3049485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nature reviews Immunology. 2011 Jun;11(6):403–415. doi: 10.1038/nri2974. PubMed PMID: 21546914. Pubmed Central PMCID: 3368251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salomone S, Yoshimura S, Reuter U, Foley M, Thomas SS, Moskowitz MA, et al. S1P3 receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. European journal of pharmacology. 2003 May 23;469(1–3):125–134. doi: 10.1016/s0014-2999(03)01731-x. PubMed PMID: 12782194. [DOI] [PubMed] [Google Scholar]
- 41.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circulation research. 2008 Mar 28;102(6):669–676. doi: 10.1161/CIRCRESAHA.107.165845. PubMed PMID: 18258856. Pubmed Central PMCID: 2659392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. The Journal of experimental medicine. 2010 Jan 18;207(1):17–27. doi: 10.1084/jem.20091619. PubMed PMID: 20026661. Pubmed Central PMCID: 2812554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002 Dec;16(14):1874–1878. doi: 10.1096/fj.02-0548com. PubMed PMID: 12468451. [DOI] [PubMed] [Google Scholar]
- 44.Lai WQ, Wong WS, Leung BP. Sphingosine kinase and sphingosine 1-phosphate in asthma. Bioscience reports. 2011 Apr;31(2):145–150. doi: 10.1042/BSR20100087. PubMed PMID: 21091442. [DOI] [PubMed] [Google Scholar]
- 45.Jolly PS, Rosenfeldt HM, Milstien S, Spiegel S. The roles of sphingosine-1-phosphate in asthma. Molecular immunology. 2002 Sep;38(16–18):1239–1245. doi: 10.1016/s0161-5890(02)00070-6. PubMed PMID: 12217390. [DOI] [PubMed] [Google Scholar]
- 46.Terai K, Soga T, Takahashi M, Kamohara M, Ohno K, Yatsugi S, et al. Edg-8 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience. 2003;116(4):1053–1062. doi: 10.1016/s0306-4522(02)00791-1. PubMed PMID: 12617946. [DOI] [PubMed] [Google Scholar]
- 47.Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, et al. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005 Feb 9;25(6):1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. PubMed PMID: 15703400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, et al. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Molecular cell. 2001 Sep;8(3):693–704. doi: 10.1016/s1097-2765(01)00324-0. PubMed PMID: 11583630. [DOI] [PubMed] [Google Scholar]
- 49.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, et al. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circulation research. 2002 Feb 22;90(3):325–332. doi: 10.1161/hh0302.104455. PubMed PMID: 11861422. [DOI] [PubMed] [Google Scholar]
- 50.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Molecular and cellular biology. 2003 Mar;23(5):1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. PubMed PMID: 12588974. Pubmed Central PMCID: 151702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009 Sep 4;325(5945):1254–1257. doi: 10.1126/science.1176709. PubMed PMID: 19729656. Pubmed Central PMCID: 2850596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010 Jun 24;465(7301):1084–1088. doi: 10.1038/nature09128. PubMed PMID: 20577214. Pubmed Central PMCID: 2946785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. The Journal of clinical investigation. 2000 Oct;106(8):951–961. doi: 10.1172/JCI10905. PubMed PMID: 11032855. Pubmed Central PMCID: 314347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003 Nov 15;102(10):3665–3667. doi: 10.1182/blood-2003-02-0460. PubMed PMID: 12869509. [DOI] [PubMed] [Google Scholar]
- 55.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Molecular and cellular biology. 2005 Dec;25(24):11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. PubMed PMID: 16314531. Pubmed Central PMCID: 1316977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. The Journal of clinical investigation. 2009 Jul;119(7):1871–1879. doi: 10.1172/JCI38575. PubMed PMID: 19603543. Pubmed Central PMCID: 2701879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, et al. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. The Journal of biological chemistry. 2004 Jul 9;279(28):29367–29373. doi: 10.1074/jbc.M403937200. PubMed PMID: 15138255. [DOI] [PubMed] [Google Scholar]
- 58.Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, et al. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. The Journal of biological chemistry. 2002 Jul 12;277(28):25152–25159. doi: 10.1074/jbc.M200137200. PubMed PMID: 12006579. [DOI] [PubMed] [Google Scholar]
- 59.Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, et al. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. The Journal of biological chemistry. 2001 Sep 7;276(36):33697–33704. doi: 10.1074/jbc.M104441200. PubMed PMID: 11443127. [DOI] [PubMed] [Google Scholar]
- 60.MacLennan AJ, Carney PR, Zhu WJ, Chaves AH, Garcia J, Grimes JR, et al. An essential role for the H218/AGR16/Edg-5/LP(B2) sphingosine 1-phosphate receptor in neuronal excitability. The European journal of neuroscience. 2001 Jul;14(2):203–209. doi: 10.1046/j.0953-816x.2001.01634.x. PubMed PMID: 11553273. [DOI] [PubMed] [Google Scholar]
- 61.Herr DR, Grillet N, Schwander M, Rivera R, Muller U, Chun J. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007 Feb 7;27(6):1474–1478. doi: 10.1523/JNEUROSCI.4245-06.2007. PubMed PMID: 17287522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. The Journal of biological chemistry. 2007 Apr 6;282(14):10690–10696. doi: 10.1074/jbc.M700370200. PubMed PMID: 17284444. [DOI] [PubMed] [Google Scholar]
- 63.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. The Journal of clinical investigation. 2007 Sep;117(9):2506–2516. doi: 10.1172/JCI31123. PubMed PMID: 17710232. Pubmed Central PMCID: 1940238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du W, Takuwa N, Yoshioka K, Okamoto Y, Gonda K, Sugihara K, et al. S1P(2), the G protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer research. 2010 Jan 15;70(2):772–781. doi: 10.1158/0008-5472.CAN-09-2722. PubMed PMID: 20068174. [DOI] [PubMed] [Google Scholar]
- 65.Wang F, Okamoto Y, Inoki I, Yoshioka K, Du W, Qi X, et al. Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. The Journal of clinical investigation. 2010 Nov;120(11):3979–3995. doi: 10.1172/JCI42315. PubMed PMID: 20978351. Pubmed Central PMCID: 2964972. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Skoura A, Michaud J, Im DS, Thangada S, Xiong Y, Smith JD, et al. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2011 Jan;31(1):81–85. doi: 10.1161/ATVBAHA.110.213496. PubMed PMID: 20947824. Pubmed Central PMCID: 3013369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim GS, Yang L, Zhang G, Zhao H, Selim M, McCullough LD, et al. Critical role of sphingosine-1-phosphate receptor-2 in the disruption of cerebrovascular integrity in experimental stroke. Nat Commun. 2015;6:7893. doi: 10.1038/ncomms8893. PubMed PMID: 26243335. Pubmed Central PMCID: PMC4587559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michaud J, Im DS, Hla T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. Journal of immunology. 2010 Feb 1;184(3):1475–1483. doi: 10.4049/jimmunol.0901586. PubMed PMID: 20042570. Pubmed Central PMCID: 3068864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levkau B, Hermann S, Theilmeier G, van der Giet M, Chun J, Schober O, et al. High-density lipoprotein stimulates myocardial perfusion in vivo. Circulation. 2004 Nov 23;110(21):3355–3359. doi: 10.1161/01.CIR.0000147827.43912.AE. PubMed PMID: 15545521. [DOI] [PubMed] [Google Scholar]
- 70.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006 Sep 26;114(13):1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. PubMed PMID: 16982942. [DOI] [PubMed] [Google Scholar]
- 71.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. American journal of physiology Heart and circulatory physiology. 2007 Jun;292(6):H2944–H2951. doi: 10.1152/ajpheart.01331.2006. PubMed PMID: 17293497. [DOI] [PubMed] [Google Scholar]
- 72.Takuwa N, Ohkura S, Takashima S, Ohtani K, Okamoto Y, Tanaka T, et al. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovascular research. 2010 Feb 1;85(3):484–493. doi: 10.1093/cvr/cvp312. PubMed PMID: 19755413. Pubmed Central PMCID: 2802201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walter DH, Rochwalsky U, Reinhold J, Seeger F, Aicher A, Urbich C, et al. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arteriosclerosis, thrombosis, and vascular biology. 2007 Feb;27(2):275–282. doi: 10.1161/01.ATV.0000254669.12675.70. PubMed PMID: 17158356. [DOI] [PubMed] [Google Scholar]
- 74.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nature immunology. 2007 Dec;8(12):1337–1344. doi: 10.1038/ni1523. PubMed PMID: 17965716. [DOI] [PubMed] [Google Scholar]
- 75.Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. The Journal of experimental medicine. 2009 Oct 26;206(11):2469–2481. doi: 10.1084/jem.20090525. PubMed PMID: 19808259. Pubmed Central PMCID: 2768857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. The Journal of biological chemistry. 2003 Nov 21;278(47):46832–46839. doi: 10.1074/jbc.M306577200. PubMed PMID: 12954646. [DOI] [PubMed] [Google Scholar]
- 77.Le Stunff H, Peterson C, Liu H, Milstien S, Spiegel S. Sphingosine-1-phosphate and lipid phosphohydrolases. Biochimica et biophysica acta. 2002 May 23;1582(1–3):8–17. doi: 10.1016/s1388-1981(02)00132-4. PubMed PMID: 12069805. [DOI] [PubMed] [Google Scholar]
- 78.Brindley DN, Pilquil C. Lipid phosphate phosphatases and signaling. Journal of lipid research. 2009 Apr;50(Suppl):S225–S230. doi: 10.1194/jlr.R800055-JLR200. PubMed PMID: 19066402. Pubmed Central PMCID: 2674702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.WHO. Neurological Disorders: Public Health Chanllenges. http://www.who.int/mental_health/neurology/neurodiso/en/
- 80.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England journal of medicine. 2008 Sep 25;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. PubMed PMID: 18815396. [DOI] [PubMed] [Google Scholar]
- 81.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010 May 15;375(9727):1695–1703. doi: 10.1016/S0140-6736(10)60491-6. PubMed PMID: 20472172. [DOI] [PubMed] [Google Scholar]
- 82.Donnan GA, Davis SM, Parsons MW, Ma H, Dewey HM, Howells DW. How to make better use of thrombolytic therapy in acute ischemic stroke. Nature reviews Neurology. 2011 Jul;7(7):400–409. doi: 10.1038/nrneurol.2011.89. PubMed PMID: 21670766. [DOI] [PubMed] [Google Scholar]
- 83.Seifert HA, Pennypacker KR. Molecular and cellular immune responses to ischemic brain injury. Transl Stroke Res. 2014 Oct;5(5):543–553. doi: 10.1007/s12975-014-0349-7. PubMed PMID: 24895236. Pubmed Central PMCID: 4125453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shichita T, Sakaguchi R, Suzuki M, Yoshimura A. Post-ischemic inflammation in the brain. Frontiers in immunology. 2012;3:132. doi: 10.3389/fimmu.2012.00132. PubMed PMID: 22833743. Pubmed Central PMCID: 3400935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl Stroke Res. 2014 Aug;5(4):442–453. doi: 10.1007/s12975-014-0336-z. PubMed PMID: 24619488. Pubmed Central PMCID: 4112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nature medicine. 2011 Jul;17(7):796–808. doi: 10.1038/nm.2399. PubMed PMID: 21738161. Pubmed Central PMCID: 3137275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu Y, Liu Q, Anrather J, Shi FD. Immune interventions in stroke. Nature reviews Neurology. 2015 Sep;11(9):524–535. doi: 10.1038/nrneurol.2015.144. PubMed PMID: 26303850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gan Y, Liu Q, Wu W, Yin JX, Bai XF, Shen R, et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proceedings of the National Academy of Sciences of the United States of America. 2014 Feb 18;111(7):2704–2709. doi: 10.1073/pnas.1315943111. PubMed PMID: 24550298. Pubmed Central PMCID: 3932858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, et al. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Annals of neurology. 2013 Sep;74(3):458–471. doi: 10.1002/ana.23815. PubMed PMID: 23674483. Pubmed Central PMCID: 3748165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nature medicine. 2009 Feb;15(2):192–199. doi: 10.1038/nm.1927. PubMed PMID: 19169263. [DOI] [PubMed] [Google Scholar]
- 91.Monson NL, Ortega SB, Ireland SJ, Meeuwissen AJ, Chen D, Plautz EJ, et al. Repetitive hypoxic preconditioning induces an immunosuppressed B cell phenotype during endogenous protection from stroke. Journal of neuroinflammation. 2014;11:22. doi: 10.1186/1742-2094-11-22. PubMed PMID: 24485041. Pubmed Central PMCID: 3926678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. Treatment of experimental stroke with IL-10-producing B-cells reduces infarct size and peripheral and CNS inflammation in wild-type B-cell-sufficient mice. Metabolic brain disease. 2014 Mar;29(1):59–73. doi: 10.1007/s11011-013-9474-3. PubMed PMID: 24374817. Pubmed Central PMCID: 3944055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke. Metabolic brain disease. 2013 Sep;28(3):375–386. doi: 10.1007/s11011-013-9413-3. PubMed PMID: 23640015. Pubmed Central PMCID: 3737266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Czech B, Pfeilschifter W, Mazaheri-Omrani N, Strobel MA, Kahles T, Neumann-Haefelin T, et al. The immunomodulatory sphingosine 1-phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochemical and biophysical research communications. 2009 Nov 13;389(2):251–256. doi: 10.1016/j.bbrc.2009.08.142. PubMed PMID: 19720050. [DOI] [PubMed] [Google Scholar]
- 95.Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke; a journal of cerebral circulation. 2010 Feb;41(2):368–374. doi: 10.1161/STROKEAHA.109.568899. PubMed PMID: 19940275. Pubmed Central PMCID: 2811754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Annals of neurology. 2011 Jan;69(1):119–129. doi: 10.1002/ana.22186. PubMed PMID: 21280082. Pubmed Central PMCID: 3200194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pfeilschifter W, Czech-Zechmeister B, Sujak M, Mirceska A, Koch A, Rami A, et al. Activation of sphingosine kinase 2 is an endogenous protective mechanism in cerebral ischemia. Biochemical and biophysical research communications. 2011 Sep 23;413(2):212–217. doi: 10.1016/j.bbrc.2011.08.070. PubMed PMID: 21872577. [DOI] [PubMed] [Google Scholar]
- 98.Choi JW, Chun J. Lysophospholipids and their receptors in the central nervous system. Biochimica et biophysica acta. 2013 Jan;1831(1):20–32. doi: 10.1016/j.bbalip.2012.07.015. PubMed PMID: 22884303. Pubmed Central PMCID: 3693945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J, Zhang C, Tao W, Liu M. Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (S1P) receptor agonist FTY720 (fingolimod) in animal models of stroke. The International journal of neuroscience. 2013 Mar;123(3):163–169. doi: 10.3109/00207454.2012.749255. PubMed PMID: 23167788. [DOI] [PubMed] [Google Scholar]
- 100.Kraft P, Gob E, Schuhmann MK, Gobel K, Deppermann C, Thielmann I, et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke; a journal of cerebral circulation. 2013 Nov;44(11):3202–3210. doi: 10.1161/STROKEAHA.113.002880. PubMed PMID: 24029635. [DOI] [PubMed] [Google Scholar]
- 101.Hasegawa Y, Suzuki H, Altay O, Rolland W, Zhang JH. Role of the sphingosine metabolism pathway on neurons against experimental cerebral ischemia in rats. Translational stroke research. 2013 Oct;4(5):524–532. doi: 10.1007/s12975-013-0260-7. PubMed PMID: 24187597. Pubmed Central PMCID: 3811943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fu Y, Zhang N, Ren L, Yan Y, Sun N, Li YJ, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proceedings of the National Academy of Sciences of the United States of America. 2014 Dec 23;111(51):18315–18320. doi: 10.1073/pnas.1416166111. PubMed PMID: 25489101. Pubmed Central PMCID: 4280578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin JJ, Chang T, Cai WK, Zhang Z, Yang YX, Sun C, et al. Post-injury administration of allicin attenuates ischemic brain injury through sphingosine kinase 2: In vivo and in vitro studies. Neurochemistry international. 2015 Oct;89:92–100. doi: 10.1016/j.neuint.2015.07.022. PubMed PMID: 26275594. [DOI] [PubMed] [Google Scholar]
- 104.Zheng S, Wei S, Wang X, Xu Y, Xiao Y, Liu H, et al. Sphingosine kinase 1 mediates neuroinflammation following cerebral ischemia. Experimental neurology. 2015 Oct;272:160–169. doi: 10.1016/j.expneurol.2015.03.012. PubMed PMID: 25797575. [DOI] [PubMed] [Google Scholar]
- 105.Campos F, Qin T, Castillo J, Seo JH, Arai K, Lo EH, et al. Fingolimod reduces hemorrhagic transformation associated with delayed tissue plasminogen activator treatment in a mouse thromboembolic model. Stroke. 2013 Feb;44(2):505–511. doi: 10.1161/STROKEAHA.112.679043. PubMed PMID: 23287783. Pubmed Central PMCID: PMC3586809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Herr I, Martin-Villalba A, Kurz E, Roncaioli P, Schenkel J, Cifone MG, et al. FK506 prevents stroke-induced generation of ceramide and apoptosis signaling. Brain research. 1999 May 1;826(2):210–219. doi: 10.1016/s0006-8993(99)01288-3. PubMed PMID: 10224298. [DOI] [PubMed] [Google Scholar]
- 107.Hisaki H, Okazaki T, Kubota M, Nakane M, Fujimaki T, Nakayama H, et al. L-PDMP improves glucosylceramide synthesis and behavior in rats with focal ischemia. Neurological research. 2008 Nov;30(9):979–984. doi: 10.1179/016164108X339396. PubMed PMID: 18691449. [DOI] [PubMed] [Google Scholar]
- 108.Noda H, Takeuchi H, Mizuno T, Suzumura A. Fingolimod phosphate promotes the neuroprotective effects of microglia. Journal of neuroimmunology. 2013 Mar 15;256(1–2):13–18. doi: 10.1016/j.jneuroim.2012.12.005. PubMed PMID: 23290828. [DOI] [PubMed] [Google Scholar]
- 109.Liesz A, Sun L, Zhou W, Schwarting S, Mracsko E, Zorn M, et al. FTY720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PloS one. 2011;6(6):e21312. doi: 10.1371/journal.pone.0021312. PubMed PMID: 21701599. Pubmed Central PMCID: 3119049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang J, Yang Y, Sun H, Xing Y. Hemorrhagic transformation after cerebral infarction: current concepts and challenges. Annals of translational medicine. 2014 Aug;2(8):81. doi: 10.3978/j.issn.2305-5839.2014.08.08. PubMed PMID: 25333056. Pubmed Central PMCID: 4200641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet neurology. 2012 Mar;11(3):261–271. doi: 10.1016/S1474-4422(12)70005-4. PubMed PMID: 22341034. [DOI] [PubMed] [Google Scholar]
- 112.Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, et al. Combination of the Immune Modulator Fingolimod With Alteplase in Acute Ischemic Stroke: A Pilot Trial. Circulation. 2015 Sep 22;132(12):1104–1112. doi: 10.1161/CIRCULATIONAHA.115.016371. PubMed PMID: 26202811. Pubmed Central PMCID: PMC4580515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Annals of neurology. 2004 Oct;56(4):468–477. doi: 10.1002/ana.20199. PubMed PMID: 15389899. [DOI] [PubMed] [Google Scholar]
- 114.Caplan LR. Tissue plasminogen activator for acute ischemic stroke. The New England journal of medicine. 1999 Oct 14;341(16):1240–1241. doi: 10.1056/NEJM199910143411618. PubMed PMID: 10523162. [DOI] [PubMed] [Google Scholar]
- 115.Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA Stroke Study Group. Stroke; a journal of cerebral circulation. 1997 Nov;28(11):2109–2118. doi: 10.1161/01.str.28.11.2109. PubMed PMID: 9368550. [DOI] [PubMed] [Google Scholar]
- 116.Cai A, Schlunk F, Bohmann F, Kashefiolasl S, Brunkhorst R, Foerch C, et al. Coadministration of FTY720 and rt-PA in an experimental model of large hemispheric stroke-no influence on functional outcome and blood-brain barrier disruption. Experimental & translational stroke medicine. 2013;5(1):11. doi: 10.1186/2040-7378-5-11. PubMed PMID: 24499647. Pubmed Central PMCID: 4029477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012 Aug;11(8):720–731. doi: 10.1016/S1474-4422(12)70104-7. PubMed PMID: 22698888. Pubmed Central PMCID: Pmc3884550. Epub 2012/06/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009 May 9;373(9675):1632–1644. doi: 10.1016/S0140-6736(09)60371-8. PubMed PMID: 19427958. Epub 2009/05/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet neurology. 2006 Jan;5(1):53–63. doi: 10.1016/S1474-4422(05)70283-0. PubMed PMID: 16361023. [DOI] [PubMed] [Google Scholar]
- 120.Pandey AS, Xi G. Intracerebral hemorrhage: a multimodality approach to improving outcome. Transl Stroke Res. 2014 Jun;5(3):313–315. doi: 10.1007/s12975-014-0344-z. PubMed PMID: 24764218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xiong XY, Yang QW. Rethinking the roles of inflammation in the intracerebral hemorrhage. Transl Stroke Res. 2015 Oct;6(5):339–341. doi: 10.1007/s12975-015-0402-1. PubMed PMID: 25940771. [DOI] [PubMed] [Google Scholar]
- 122.Schlunk F, Greenberg SM. The Pathophysiology of Intracerebral Hemorrhage Formation and Expansion. Transl Stroke Res. 2015 Aug;6(4):257–263. doi: 10.1007/s12975-015-0410-1. PubMed PMID: 26073700. [DOI] [PubMed] [Google Scholar]
- 123.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010 Feb;9(2):167–176. doi: 10.1016/S1474-4422(09)70340-0. PubMed PMID: 20056489. [DOI] [PubMed] [Google Scholar]
- 124.Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007 May;27(5):894–908. doi: 10.1038/sj.jcbfm.9600403. PubMed PMID: 17033693. Epub 2006/10/13. eng. [DOI] [PubMed] [Google Scholar]
- 125.Selim M, Sheth KN. Perihematoma edema: a potential translational target in intracerebral hemorrhage? Transl Stroke Res. 2015 Apr;6(2):104–106. doi: 10.1007/s12975-015-0389-7. PubMed PMID: 25693976. Pubmed Central PMCID: 4359064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014 Apr;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. PubMed PMID: 24291544. [DOI] [PubMed] [Google Scholar]
- 127.Qureshi AI, Ali Z, Suri MF, Shuaib A, Baker G, Todd K, et al. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Critical care medicine. 2003 May;31(5):1482–1489. doi: 10.1097/01.CCM.0000063047.63862.99. PubMed PMID: 12771622. [DOI] [PubMed] [Google Scholar]
- 128.Lusardi TA, Wolf JA, Putt ME, Smith DH, Meaney DF. Effect of acute calcium influx after mechanical stretch injury in vitro on the viability of hippocampal neurons. Journal of neurotrauma. 2004 Jan;21(1):61–72. doi: 10.1089/089771504772695959. PubMed PMID: 14987466. [DOI] [PubMed] [Google Scholar]
- 129.Graham DI, McIntosh TK, Maxwell WL, Nicoll JA. Recent advances in neurotrauma. Journal of neuropathology and experimental neurology. 2000 Aug;59(8):641–651. doi: 10.1093/jnen/59.8.641. PubMed PMID: 10952055. [DOI] [PubMed] [Google Scholar]
- 130.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011 Jun;42(6):1781–1786. doi: 10.1161/STROKEAHA.110.596718. PubMed PMID: 21527759. Pubmed Central PMCID: PMC3123894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, et al. Cell death in experimental intracerebral hemorrhage: the "black hole" model of hemorrhagic damage. Annals of neurology. 2002 Apr;51(4):517–524. doi: 10.1002/ana.10160. PubMed PMID: 11921058. [DOI] [PubMed] [Google Scholar]
- 132.Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. Journal of neurosurgery. 2002 Feb;96(2):287–293. doi: 10.3171/jns.2002.96.2.0287. PubMed PMID: 11838803. [DOI] [PubMed] [Google Scholar]
- 133.Ziai WC. Hematology and inflammatory signaling of intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2013 Jun;44(6 Suppl 1):S74–S78. doi: 10.1161/STROKEAHA.111.000662. PubMed PMID: 23709738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gebel JM, Jr, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, et al. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2002 Nov;33(11):2631–2635. doi: 10.1161/01.str.0000035284.12699.84. PubMed PMID: 12411653. [DOI] [PubMed] [Google Scholar]
- 135.Butcher KS, Baird T, MacGregor L, Desmond P, Tress B, Davis S. Perihematomal edema in primary intracerebral hemorrhage is plasma derived. Stroke; a journal of cerebral circulation. 2004 Aug;35(8):1879–1885. doi: 10.1161/01.STR.0000131807.54742.1a. PubMed PMID: 15178826. [DOI] [PubMed] [Google Scholar]
- 136.Hatakeyama T, Okauchi M, Hua Y, Keep RF, Xi G. Deferoxamine reduces neuronal death and hematoma lysis after intracerebral hemorrhage in aged rats. Transl Stroke Res. 2013 Oct;4(5):546–553. doi: 10.1007/s12975-013-0270-5. PubMed PMID: 24187595. Pubmed Central PMCID: 3810989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zheng M, Du H, Ni W, Koch LG, Britton SL, Keep RF, et al. Iron-induced necrotic brain cell death in rats with different aerobic capacity. Transl Stroke Res. 2015 Jun;6(3):215–223. doi: 10.1007/s12975-015-0388-8. PubMed PMID: 25649272. Pubmed Central PMCID: 4425582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheng Y, Xi G, Jin H, Keep RF, Feng J, Hua Y. Thrombin-induced cerebral hemorrhage: role of protease-activated receptor-1. Transl Stroke Res. 2014 Aug;5(4):472–475. doi: 10.1007/s12975-013-0288-8. PubMed PMID: 24323711. Pubmed Central PMCID: 3962522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000 Sep 14;407(6801):258–264. doi: 10.1038/35025229. PubMed PMID: 11001069. [DOI] [PubMed] [Google Scholar]
- 140.Kitaoka T, Hua Y, Xi G, Hoff JT, Keep RF. Delayed argatroban treatment reduces edema in a rat model of intracerebral hemorrhage. Stroke. 2002;33(12):3012–3018. doi: 10.1161/01.str.0000037673.17260.1b. [DOI] [PubMed] [Google Scholar]
- 141.Sugawara T, Jadhav V, Ayer R, Chen W, Suzuki H, Zhang JH. Thrombin inhibition by argatroban ameliorates early brain injury and improves neurological outcomes after experimental subarachnoid hemorrhage in rats. Stroke. 2009 Apr;40(4):1530–1532. doi: 10.1161/STROKEAHA.108.531699. PubMed PMID: 19228846. Pubmed Central PMCID: 2743552. Epub 2009/02/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, et al. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. The Journal of biological chemistry. 2005 Apr 29;280(17):17286–17293. doi: 10.1074/jbc.M412427200. PubMed PMID: 15710622. [DOI] [PubMed] [Google Scholar]
- 143.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005 Apr 15;105(8):3178–3184. doi: 10.1182/blood-2004-10-3985. PubMed PMID: 15626732. [DOI] [PubMed] [Google Scholar]
- 144.Bae JS, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007 Dec 1;110(12):3909–3916. doi: 10.1182/blood-2007-06-096651. PubMed PMID: 17823308. Pubmed Central PMCID: 2190610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bae JS, Yang L, Rezaie AR. Lipid raft localization regulates the cleavage specificity of protease activated receptor 1 in endothelial cells. Journal of thrombosis and haemostasis : JTH. 2008 Jun;6(6):954–961. doi: 10.1111/j.1538-7836.2008.02924.x. PubMed PMID: 18284602. [DOI] [PubMed] [Google Scholar]
- 146.Feistritzer C, Schuepbach RA, Mosnier LO, Bush LA, Di Cera E, Griffin JH, et al. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. The Journal of biological chemistry. 2006 Jul 21;281(29):20077–20084. doi: 10.1074/jbc.M600506200. PubMed PMID: 16709569. [DOI] [PubMed] [Google Scholar]
- 147.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Progress in neurobiology. 2010 Dec;92(4):463–477. doi: 10.1016/j.pneurobio.2010.08.001. PubMed PMID: 20713126. Pubmed Central PMCID: 2991407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao H, Garton T, Keep RF, Hua Y, Xi G. Microglia/Macrophage Polarization After Experimental Intracerebral Hemorrhage. Transl Stroke Res. 2015 Dec;6(6):407–409. doi: 10.1007/s12975-015-0428-4. PubMed PMID: 26446073. Pubmed Central PMCID: 4628553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen S, Yang Q, Chen G, Zhang JH. An update on inflammation in the acute phase of intracerebral hemorrhage. Transl Stroke Res. 2015 Feb;6(1):4–8. doi: 10.1007/s12975-014-0384-4. PubMed PMID: 25533878. [DOI] [PubMed] [Google Scholar]
- 150.Hammond MD, Ambler WG, Ai Y, Sansing LH. alpha4 integrin is a regulator of leukocyte recruitment after experimental intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2014 Aug;45(8):2485–2487. doi: 10.1161/STROKEAHA.114.005551. PubMed PMID: 25013026. Pubmed Central PMCID: 4129460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hammond MD, Taylor RA, Mullen MT, Ai Y, Aguila HL, Mack M, et al. CCR2+ Ly6C(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014 Mar 12;34(11):3901–3909. doi: 10.1523/JNEUROSCI.4070-13.2014. PubMed PMID: 24623768. Pubmed Central PMCID: 3951693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Graeber MB. Changing face of microglia. Science. 2010 Nov 5;330(6005):783–788. doi: 10.1126/science.1190929. PubMed PMID: 21051630. [DOI] [PubMed] [Google Scholar]
- 153.Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clinical & developmental immunology. 2013;2013:746068. doi: 10.1155/2013/746068. PubMed PMID: 24223607. Pubmed Central PMCID: 3810327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nature reviews Immunology. 2011 Oct;11(10):658–671. doi: 10.1038/nri3065. PubMed PMID: 21941294. Pubmed Central PMCID: 3620656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hua Y, Wu J, Keep RF, Nakamura T, Hoff JT, Xi G. Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006 Mar;58(3):542–550. doi: 10.1227/01.NEU.0000197333.55473.AD. discussion -50. PubMed PMID: 16528196. [DOI] [PubMed] [Google Scholar]
- 156.Rolland WB, Lekic T, Krafft PR, Hasegawa Y, Altay O, Hartman R, et al. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Experimental neurology. 2013 Mar;241:45–55. doi: 10.1016/j.expneurol.2012.12.009. PubMed PMID: 23261767. Pubmed Central PMCID: 3570752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lu L, Barfejani AH, Qin T, Dong Q, Ayata C, Waeber C. Fingolimod exerts neuroprotective effects in a mouse model of intracerebral hemorrhage. Brain research. 2014 Mar 25;1555:89–96. doi: 10.1016/j.brainres.2014.01.048. PubMed PMID: 24502984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rolland WB, 2nd, Manaenko A, Lekic T, Hasegawa Y, Ostrowski R, Tang J, et al. FTY720 is neuroprotective and improves functional outcomes after intracerebral hemorrhage in mice. Acta neurochirurgica Supplement. 2011;111:213–217. doi: 10.1007/978-3-7091-0693-8_36. PubMed PMID: 21725758. Pubmed Central PMCID: 3569072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fu Y, Hao J, Zhang N, Ren L, Sun N, Li YJ, et al. Fingolimod for the Treatment of Intracerebral Hemorrhage: A 2-Arm Proof-of-Concept Study. JAMA neurology. 2014 Jul 7; doi: 10.1001/jamaneurol.2014.1065. PubMed PMID: 25003359. [DOI] [PubMed] [Google Scholar]
- 160.Gaberel T, Magheru C, Emery E. Management of non-traumatic intraventricular hemorrhage. Neurosurgical review. 2012 Oct;35(4):485–494. doi: 10.1007/s10143-012-0399-9. discussion 94-5. PubMed PMID: 22732889. [DOI] [PubMed] [Google Scholar]
- 161.Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD, Investigators S. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta neurochirurgica Supplement. 2006;96:65–68. doi: 10.1007/3-211-30714-1_16. PubMed PMID: 16671427. [DOI] [PubMed] [Google Scholar]
- 162.Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. Journal of neurology. 2000 Feb;247(2):117–121. doi: 10.1007/pl00007792. PubMed PMID: 10751114. [DOI] [PubMed] [Google Scholar]
- 163.Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res. 2012 Jul;3(Suppl 1):25–38. doi: 10.1007/s12975-012-0182-9. PubMed PMID: 23976902. Pubmed Central PMCID: 3750748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007 Jan 27;369(9558):306–318. doi: 10.1016/S0140-6736(07)60153-6. PubMed PMID: 17258671. Epub 2007/01/30. eng. [DOI] [PubMed] [Google Scholar]
- 165.Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013 Aug;4(4):432–446. doi: 10.1007/s12975-013-0257-2. PubMed PMID: 23894255. Pubmed Central PMCID: 3719879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brilstra EH, Rinkel GJ, Algra A, van Gijn J. Rebleeding, secondary ischemia, and timing of operation in patients with subarachnoid hemorrhage. Neurology. 2000 Dec 12;55(11):1656–1660. doi: 10.1212/wnl.55.11.1656. PubMed PMID: 11113219. [DOI] [PubMed] [Google Scholar]
- 167.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nature reviews Neurology. 2014 Jan;10(1):44–58. doi: 10.1038/nrneurol.2013.246. PubMed PMID: 24323051. [DOI] [PubMed] [Google Scholar]
- 168.Solenski NJ, Haley EC, Jr, Kassell NF, Kongable G, Germanson T, Truskowski L, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Critical care medicine. 1995 Jun;23(6):1007–1017. doi: 10.1097/00003246-199506000-00004. PubMed PMID: 7774210. [DOI] [PubMed] [Google Scholar]
- 169.Zhang JH. Vascular neural network in subarachnoid hemorrhage. Transl Stroke Res. 2014 Aug;5(4):423–428. doi: 10.1007/s12975-014-0355-9. PubMed PMID: 24986148. Pubmed Central PMCID: 4127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Song J, Li P, Chaudhary N, Gemmete JJ, Thompson BG, Xi G, et al. Correlating Cerebral (18)FDG PET-CT Patterns with Histological Analysis During Early Brain Injury in a Rat Subarachnoid Hemorrhage Model. Transl Stroke Res. 2015 Aug;6(4):290–295. doi: 10.1007/s12975-015-0396-8. PubMed PMID: 25833084. [DOI] [PubMed] [Google Scholar]
- 171.Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke; a journal of cerebral circulation. 2010 Oct;41(10):2391–2395. doi: 10.1161/STROKEAHA.110.589275. PubMed PMID: 20798370. [DOI] [PubMed] [Google Scholar]
- 172.Budohoski KP, Czosnyka M, Kirkpatrick PJ, Smielewski P, Steiner LA, Pickard JD. Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nature reviews Neurology. 2013 Mar;9(3):152–163. doi: 10.1038/nrneurol.2013.11. PubMed PMID: 23419369. [DOI] [PubMed] [Google Scholar]
- 173.Dhar R, Scalfani MT, Blackburn S, Zazulia AR, Videen T, Diringer M. Relationship between angiographic vasospasm and regional hypoperfusion in aneurysmal subarachnoid hemorrhage. Stroke; a journal of cerebral circulation. 2012 Jul;43(7):1788–1794. doi: 10.1161/STROKEAHA.111.646836. PubMed PMID: 22492520. Pubmed Central PMCID: 3383942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jaeger M, Schuhmann MU, Soehle M, Nagel C, Meixensberger J. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke; a journal of cerebral circulation. 2007 Mar;38(3):981–986. doi: 10.1161/01.STR.0000257964.65743.99. PubMed PMID: 17272764. [DOI] [PubMed] [Google Scholar]
- 175.Testai FD, Hillmann M, Amin-Hanjani S, Gorshkova I, Berdyshev E, Gorelick PB, et al. Changes in the cerebrospinal fluid ceramide profile after subarachnoid hemorrhage. Stroke; a journal of cerebral circulation. 2012 Aug;43(8):2066–2070. doi: 10.1161/STROKEAHA.112.650390. PubMed PMID: 22713492. [DOI] [PubMed] [Google Scholar]
- 176.Tosaka M, Okajima F, Hashiba Y, Saito N, Nagano T, Watanabe T, et al. Sphingosine 1-phosphate contracts canine basilar arteries in vitro and in vivo: possible role in pathogenesis of cerebral vasospasm. Stroke; a journal of cerebral circulation. 2001 Dec 1;32(12):2913–2919. doi: 10.1161/hs1201.099525. PubMed PMID: 11739995. [DOI] [PubMed] [Google Scholar]
- 177.Testai FD, Xu HL, Kilkus J, Suryadevara V, Gorshkova I, Berdyshev E, et al. Changes in the metabolism of sphingolipids after subarachnoid hemorrhage. Journal of neuroscience research. 2015 May;93(5):796–805. doi: 10.1002/jnr.23542. PubMed PMID: 25597763. Pubmed Central PMCID: 4359096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yagi K, Lidington D, Wan H, Fares JC, Meissner A, Sumiyoshi M, et al. Therapeutically Targeting Tumor Necrosis Factor-alpha/Sphingosine-1-Phosphate Signaling Corrects Myogenic Reactivity in Subarachnoid Hemorrhage. Stroke; a journal of cerebral circulation. 2015 Aug;46(8):2260–2270. doi: 10.1161/STROKEAHA.114.006365. PubMed PMID: 26138121. [DOI] [PubMed] [Google Scholar]
- 179.Tang H, Zhao D, Chen S, Fang M, Wang F, Cui Y, et al. Expression of Sphingosine-1-phosphate (S1P) on the cerebral vasospasm after subarachnoid hemorrhage in rabbits. Acta cirurgica brasileira / Sociedade Brasileira para Desenvolvimento Pesquisa em Cirurgia. 2015 Oct;30(10):654–659. doi: 10.1590/S0102-865020150100000001. PubMed PMID: 26560422. [DOI] [PubMed] [Google Scholar]
- 180.Salomone S, Soydan G, Ip PC, Hopson KM, Waeber C. Vessel-specific role of sphingosine kinase 1 in the vasoconstriction of isolated basilar arteries. Pharmacological research. 2010 Dec;62(6):465–474. doi: 10.1016/j.phrs.2010.09.002. PubMed PMID: 20850539. Pubmed Central PMCID: 2974794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, et al. Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Progress in neurobiology. 2014 Mar;114:58–83. doi: 10.1016/j.pneurobio.2013.11.005. PubMed PMID: 24389580. Pubmed Central PMCID: 3937258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Keep RF, Wang MM, Xiang J, Hua Y, Xi G. Full Steam Ahead with Remote Ischemic Conditioning for Stroke. Transl Stroke Res. 2014 Jul Jul; doi: 10.1007/s12975-014-0363-9. PubMed PMID: 25053258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xi G. Clinical translation of cerebral preconditioning. Transl Stroke Res. 2010 Mar;1(1):2–3. doi: 10.1007/s12975-010-0012-x. PubMed PMID: 24323449. [DOI] [PubMed] [Google Scholar]
- 184.Keep RF, Wang MM, Xiang J, Hua Y, Xi G. Full steam ahead with remote ischemic conditioning for stroke. Transl Stroke Res. 2014 Oct;5(5):535–537. doi: 10.1007/s12975-014-0363-9. PubMed PMID: 25053258. Pubmed Central PMCID: 4187220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sheng R, Zhang TT, Felice VD, Qin T, Qin ZH, Smith CD, et al. Preconditioning stimuli induce autophagy via sphingosine kinase 2 in mouse cortical neurons. The Journal of biological chemistry. 2014 Jul 25;289(30):20845–20857. doi: 10.1074/jbc.M114.578120. PubMed PMID: 24928515. Pubmed Central PMCID: 4110292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wacker BK, Perfater JL, Gidday JM. Hypoxic preconditioning induces stroke tolerance in mice via a cascading HIF, sphingosine kinase, and CCL2 signaling pathway. Journal of neurochemistry. 2012 Dec;123(6):954–962. doi: 10.1111/jnc.12047. PubMed PMID: 23043544. Pubmed Central PMCID: 3514614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhou Y, Lekic T, Fathali N, Ostrowski RP, Martin RD, Tang J, et al. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke; a journal of cerebral circulation. 2010 Jul;41(7):1521–1527. doi: 10.1161/STROKEAHA.110.583757. PubMed PMID: 20508187. Pubmed Central PMCID: 2917259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Altay O, Hasegawa Y, Sherchan P, Suzuki H, Khatibi NH, Tang J, et al. Isoflurane delays the development of early brain injury after subarachnoid hemorrhage through sphingosine-related pathway activation in mice. Critical care medicine. 2012 Jun;40(6):1908–1913. doi: 10.1097/CCM.0b013e3182474bc1. PubMed PMID: 22488000. Pubmed Central PMCID: 3358576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Furuya K, Ginis I, Takeda H, Chen Y, Hallenbeck JM. Cell permeable exogenous ceramide reduces infarct size in spontaneously hypertensive rats supporting in vitro studies that have implicated ceramide in induction of tolerance to ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001 Mar;21(3):226–232. doi: 10.1097/00004647-200103000-00006. PubMed PMID: 11295877. [DOI] [PubMed] [Google Scholar]
- 190.Wacker BK, Freie AB, Perfater JL, Gidday JM. Junctional protein regulation by sphingosine kinase 2 contributes to blood-brain barrier protection in hypoxic preconditioning-induced cerebral ischemic tolerance. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012 Jun;32(6):1014–1023. doi: 10.1038/jcbfm.2012.3. PubMed PMID: 22314269. Pubmed Central PMCID: 3367228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wacker BK, Park TS, Gidday JM. Hypoxic preconditioning-induced cerebral ischemic tolerance: role of microvascular sphingosine kinase 2. Stroke. 2009 Oct;40(10):3342–3348. doi: 10.1161/STROKEAHA.109.560714. PubMed PMID: 19644058. Pubmed Central PMCID: PMC2753710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yung LM, Wei Y, Qin T, Wang Y, Smith CD, Waeber C. Sphingosine kinase 2 mediates cerebral preconditioning and protects the mouse brain against ischemic injury. Stroke; a journal of cerebral circulation. 2012 Jan;43(1):199–204. doi: 10.1161/STROKEAHA.111.626911. PubMed PMID: 21980199. Pubmed Central PMCID: 3246529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Altay O, Suzuki H, Hasegawa Y, Caner B, Krafft PR, Fujii M, et al. Isoflurane attenuates blood-brain barrier disruption in ipsilateral hemisphere after subarachnoid hemorrhage in mice. Stroke; a journal of cerebral circulation. 2012 Sep;43(9):2513–2516. doi: 10.1161/STROKEAHA.112.661728. PubMed PMID: 22773559. Pubmed Central PMCID: 3429639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Deng J, Lei C, Chen Y, Fang Z, Yang Q, Zhang H, et al. Neuroprotective gases--fantasy or reality for clinical use? Progress in neurobiology. 2014 Apr;115:210–245. doi: 10.1016/j.pneurobio.2014.01.001. PubMed PMID: 24440817. [DOI] [PubMed] [Google Scholar]
- 195.Iwai M, Sato K, Omori N, Nagano I, Manabe Y, Shoji M, et al. Three steps of neural stem cells development in gerbil dentate gyrus after transient ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002 Apr;22(4):411–419. doi: 10.1097/00004647-200204000-00005. PubMed PMID: 11919512. [DOI] [PubMed] [Google Scholar]
- 196.Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, Mimuro J, et al. Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke; a journal of cerebral circulation. 2008 Dec;39(12):3411–3417. doi: 10.1161/STROKEAHA.108.514612. PubMed PMID: 18757288. [DOI] [PubMed] [Google Scholar]
- 197.Harada J, Foley M, Moskowitz MA, Waeber C. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. Journal of neurochemistry. 2004 Feb;88(4):1026–1039. doi: 10.1046/j.1471-4159.2003.02219.x. PubMed PMID: 14756825. [DOI] [PubMed] [Google Scholar]
- 198.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends in neurosciences. 2009 Dec;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. PubMed PMID: 19782411. Pubmed Central PMCID: 2787735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yamagata K, Tagami M, Torii Y, Takenaga F, Tsumagari S, Itoh S, et al. Sphingosine 1-phosphate induces the production of glial cell line-derived neurotrophic factor and cellular proliferation in astrocytes. Glia. 2003 Jan 15;41(2):199–206. doi: 10.1002/glia.10180. PubMed PMID: 12509810. [DOI] [PubMed] [Google Scholar]
- 200.Deogracias R, Yazdani M, Dekkers MP, Guy J, Ionescu MC, Vogt KE, et al. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2012 Aug 28;109(35):14230–14235. doi: 10.1073/pnas.1206093109. PubMed PMID: 22891354. Pubmed Central PMCID: 3435172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fukumoto K, Mizoguchi H, Takeuchi H, Horiuchi H, Kawanokuchi J, Jin S, et al. Fingolimod increases brain-derived neurotrophic factor levels and ameliorates amyloid beta-induced memory impairment. Behavioural brain research. 2014 Jul 15;268:88–93. doi: 10.1016/j.bbr.2014.03.046. PubMed PMID: 24713151. [DOI] [PubMed] [Google Scholar]
- 202.Doi Y, Takeuchi H, Horiuchi H, Hanyu T, Kawanokuchi J, Jin S, et al. Fingolimod phosphate attenuates oligomeric amyloid beta-induced neurotoxicity via increased brain-derived neurotrophic factor expression in neurons. PloS one. 2013;8(4):e61988. doi: 10.1371/journal.pone.0061988. PubMed PMID: 23593505. Pubmed Central PMCID: 3625222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res. 2013 Jun;4(3):279–285. doi: 10.1007/s12975-012-0209-2. PubMed PMID: 23658596. Pubmed Central PMCID: PMC3644408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang MM, Xi G, Keep RF. Should the STAIR criteria be modified for preconditioning studies? Transl Stroke Res. 2013 Feb;4(1):3–14. doi: 10.1007/s12975-012-0219-0. PubMed PMID: 23458885. Pubmed Central PMCID: PMC3580874. [DOI] [PMC free article] [PubMed] [Google Scholar]