Abstract
Objective
To evaluate the feasibility and preliminary effect of metacognitive strategy training (MCST) on cognitive performance and on neural connectivity in the frontal-parietal network in women with chemotherapy-induced cognitive impairment (CICI) following treatment for breast cancer.
Methods
Single group, pre/post study was conducted. After completing the baseline assessment battery and neuroimaging, participants completed a 12-session MCST intervention. Following the completion of the intervention, subjects completed the same assessment battery and neuroimaging that was completed at baseline within four weeks post-intervention. The key inclusion/exclusion criteria for this study included: completed chemotherapy for treatment for breast cancer, no other neurological or psychiatric diagnoses, self-reported CICI, and no contraindications for the use of MRI.
Results
MCST had a small to large positive effect on all primary (cognitive) and secondary (quality of life and psychosocial) behavioral outcome measures (r = −.12 to −.88). There was also a positive change in functional connectivity in a frontal-parietal cognitive control network connection in 6 of the 10 subjects, which was correlated to changes on the behavioral measures.
Conclusions
This study found that MCST was associated with a positive effect on cognitive performance and neural connectivity in women with CICI following treatment for breast cancer.
Keywords: Breast cancer, Metacognitive-Strategy Training, Neuroimaging, Occupational Therapy, Cancer Survivorship
Introduction
Breast cancer is the most common malignancy in women with an estimated incidence of 234,840 in 2015, accounting for 29% of female cancer incidence in the United States [1]. The use of chemotherapy has led to significant improvements in survival in breast cancer patients[2,3] and is administered to the majority of patients with early stage disease to reduce the risk of recurrence [4]. In spite of the survival benefits, chemotherapy has been associated with decreased productivity, impaired community involvement, and poor role-functioning resulting from cognitive dysfunctions following treatment [5–9]. Cancer survivors have cognitive deficits in several domains after chemotherapy but most often in executive function (planning, problem solving, multitasking). Women with such executive function (EF) deficits following breast cancer treatment report changes in everyday life activities, such as work/productivity, community involvement, driving, and financial management [5–9]. These cognitive changes are referred to as chemotherapy-induced cognitive impairments (CICI) or “chemobrain.” The rate of CICI in the published literature ranges 16–75% [10,11].
Clinically, executive function impairment related to CICI is most often identified using self-report measures and neuropsychological assessments; however, self-report measures do not provide an objective method to compare cognitive abilities between participants, and neuropsychological assessments have limited ability to capture real-world cognitive performance [12–15]. Non-invasive neuroimaging is a promising complementary assessment to identify deficits in EF associated with CICI and a relatively new neuroimaging method being used for this purpose is resting-state functional connectivity MRI (rs-fcMRI). During rs-fcMRI, intrinsic brain activity is imaged when subjects are at wakeful rest and not performing a task, to understand the functional organization of the brain [16,17]. Using rs-fcMRI, alterations in global and regional functional connectivity have been reported in breast cancer survivors [18,19]. Using rs-fcMRI, our research team recently found that breast cancer survivors who report CICI, compared to those who do not, show weaker functional connectivity between two regions of the frontal-parietal executive control network [20]. In addition, weaker functional connectivity correlated with greater levels of reported cognitive impairment [20].
There are two common intervention approaches to address cognitive impairment: the compensation model and the restoration model [21]. The premise of restoration-based methods is that through repetitive practice of cognitive retraining activities, overall cognitive performance will improve [21]. Recent clinical studies evaluating the use of restoration-based methods with cancer survivors, including breast cancer survivors, has found that these methods can improve cognitive function [22,23]. Unfortunately, these studies and several others suggest that this restoration approach has little impact on everyday life performance [22–26]. In contrast to the restoration model, the compensation model includes interventions like metacognitive-strategy training (MCST). MCST interventions tend to be targeted at the performance and participation level to help participants improve/learn new skills to complete everyday life activities, and are usually delivered by occupational therapists. There is some existing evidence that suggests that MCST has more of a positive effect on EF impairments and activity performance than remediation/retraining-based approaches [27]. One of the concepts and theories driving MCST is experience-dependent neuroplasticity, which postulates that learning new skills leads to functional changes in the brain [28]. Current evidence suggests that the MCST approach directly targets the action of the frontal-parietal network, a cognitive control network involved in flexible moment-to-moment task control that also reflects compositional coding to enable transfer of knowledge to novel tasks [29–31]. MCST approaches help individuals learn general strategies that they can employ in multiple contexts to improve their performance which requires flexible cognitive control and the ability to transfer knowledge to novel tasks [32]. Given the results of our preliminary study that showed the negative impact of CICI on this frontal-parietal network and what is known about metacognitive-strategy training [20], the purpose of this study was to evaluate the feasibility and preliminary effect of MCST on cognitive performance and on neural connectivity in the frontal-parietal network in women with CICI following treatment for breast cancer.
Methods
A single group, pre/post study was conducted. Participants were recruited from breast cancer patients in the clinical database at Washington University Faculty Practice Plan Division of Breast Oncology at Washington University in St. Louis. This study was reviewed and approved by the Washington University Human Research Protection Office (HRPO) and the Protocol Review Monitoring Committee (PRMC) at Siteman Cancer Center.
Participants
Participants were recruited from the Siteman Cancer Center at Washington University School of Medicine. Inclusion criteria: (1) females 35–70 years old; (2) self-reported CICI (Global Rating of Cognition dysfunction as “Moderately” “Strongly “or “Extremely” and a Cognitive Failures Questionnaire (CFQ) score >30); (3) completed adjuvant (or neoadjuvant) chemotherapy at least 6 months prior to participation; (4) able to read, write, and speak English fluently; (5) able to provide valid informed consent; (6) have a life expectancy of greater than 6 months at time of enrollment; (7) females diagnosed with breast cancer (invasive ductal or lobular BrCA Stages I, II, or III) and completed chemotherapy within the preceding two years; (8) on stable doses (i.e., no changes in past 90 days) of medications that are known to impact cognitive function (i.e., anti-depressants). Exclusion criteria: (1) prior cancer diagnoses of other sites with evidence of active disease within the past year; (2) active diagnoses of any acute or chronic brain-related neurological conditions that can alter normal brain anatomy or function (e.g., Parkinson’s disease, dementia, cerebral infarcts); (3) severe depressive symptoms (Personal Health Questionnaire (PHQ-9) score of ≥21); (4) history of traumatic brain injury; (5) weigh over 350 pounds (weight limit of MRI machine); (6) received skull-based radiation treatment within the past year for any reason; (7) implanted metal objects not compatible with MRI, electrodes, pacemakers, intracardiac lines, or medication pumps; (8) history of claustrophobia or inability to lie flat that will preclude undergoing MRI; (9) any medical condition which would render the study unsafe or not in the best interest of the participant; and (10) male gender. The clinical coordinators in the breast oncology division identified female breast cancer patients based on criteria related to age, diagnosis, co-morbidities, and language. The remaining inclusion/exclusion criteria were evaluated by the research coordinator.
No study of which we are aware has examined the effect of metacognitive strategy training for CICI at a brain network level of investigation. Therefore, effect sizes of alterations in cortical network correlations, which are required for sample size determination, are unknown. The purpose of this pilot study was to evaluate if MCST could have a measureable effect on subjective and objective cognitive performance and also on neural connectivity, as measured by resting state functional connectivity MRI. A sample of 14 participants was determined based on the experience of the research team as the minimum sample necessary in a repeated measures design to detect a change in signal in the neuroimaging data.
Assessment and Intervention Procedures
Breast cancer patients who received chemotherapy at Siteman Cancer Center at Washington University School of Medicine and met the inclusion/exclusion criteria for the study were identified and contact information was forwarded to our research team. Patients who were interested were asked to complete a screening battery over the phone or via a web-based survey to evaluate eligibility and identify if they have CICI (Global Rating of Cognition dysfunction as “Moderately” “Strongly “or “Extremely” and a Cognitive Failures Questionnaire (CFQ) score >30). Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Washington University in St. Louis [33]. REDCap is a mature, secure web-based application designed to support data capture for research studies.
Those patients with self-reported CICI were then scheduled for a face-to-face assessment to determine final eligibility. After providing informed consent, eligible patients were asked to complete the baseline assessment battery and were scheduled for the neuroimaging assessment. After completing the baseline assessment battery and neuroimaging, participants completed a 12-session, or until their goals were met, metacognitive strategy training intervention with a trained occupational therapist. Each session lasted 45 minutes and the intervention was completed over the course of 12 to 14 weeks. Participants may have completed less sessions if they met their baseline goals before the completion of 12 sessions. Following the completion of the intervention, subjects completed the same assessment battery and neuroimaging that was completed at baseline within four weeks post-intervention.
Intervention Description: Cognitive Orientation to daily Occupational Performance (CO-OP) CO-OP: A Metacognitive-Strategy Training Treatment Approach
Cognitive Orientation to daily Occupational Performance (CO-OP) is a metacognitive-strategy training treatment approach that incorporates both global and domain-specific cognitive strategies [34]. Complete details about the CO-OP approach have been previously published [35]. CO-OP has seven key features: cognitive strategy use, patient-chosen goals, dynamic performance analysis, guided discovery, enabling principles, parent/significant other involvement, and intervention format [34]. In the first meeting, the patient selects 3 activities to be the focus of treatment and baseline level of performance for each activity is established. In the second meeting, when CO-OP actually begins, the approach is introduced to the patient and the global cognitive strategy (GOAL-PLAN-DO-CHECK) is learned. In all subsequent sessions this strategy is used as the main problem-solving framework to facilitate skill acquisition. The patient identifies a GOAL, and then is guided by the therapist to develop his/her own PLAN to potentially achieve the goal. The client is then asked to DO the plan (if feasible during the therapy session otherwise asked to complete at home prior to the next treatment session), and subsequently to CHECK to see if the plan worked, i.e. the goal was achieved. If the goal was not achieved, as is often the case initially, the patient is guided to analyze what went wrong and modify the plan. Thereby, the lack of success is associated with the wrong plan, rather than a problem with his or her personal capacity. Throughout, the therapist actively seeks opportunities to promote generalization of skills and strategies to the natural environment and transfer to novel skills.
Behavioral Outcome Measures
All participants completed the baseline assessment (approximately 90 minutes) with a blind rater. This same assessment battery was used post-intervention. Table 1 below is an overview of all the behavioral outcome assessments. The primary outcome measures are subjective and objective measures of cognitive performance.
Table 1.
Baseline Assessment Battery
| Measures | Description |
|---|---|
| Primary Outcome Measures | |
| Cognitive Failures Questionnaire (CFQ)[58] | CFQ measures subjective lapses in motor function, memory, and perception. This questionnaire contains 25 items and scores range from 0 to 100. |
| Dysexecutive Questionnaire (DEX)[59] | The DEX measures behavioral changes associated with having executive dysfunction. The questionnaire contains 20-items |
| Delis-Kaplan Executive Function System (DKEFS)[60]-Trailmaking Subtest | The DKEFS is the only objective scaled EF battery available. The DKEFS has 9 stand-alone tests; The Trailmaking Condition 4 subtest score was used. The Trailmaking Condition 4 subtest measures cognitive flexibility. |
| Secondary Outcome Measures | |
| PROMIS-57 Profile v1.0[61] | The PROMIS measures subjective changes in physical function, fatigue, and satisfaction with social roles. |
| Canadian Occupational Performance Measure (COPM)[62] | The COPM measures changes in performance of tasks by measuring the clients’ perceived performance and satisfaction with their level of participation. |
| Personal Health Questionnaire (PHQ-9)Depression[63] | The PHQ-9 measures depressive symptoms |
| Montreal Cognitive Assessment (MOCA)[64] | The MOCA measures general cognitive status. It is a publically-available screening tool used to screen for dementia. |
| Self-Efficacy Gauge (SEG)[65] | SEG measures an individual’s confidence in everyday life activities. |
Neuroimaging Outcome Measurement
Resting-state functional-connectivity MRI (rs-fcMRI) and anatomical images were collected using a Siemens 3T Tim Trio MRI scanner. The anatomical T1-weighted magnetization prepared rapid gradient echo (MP-RAGE) image was acquired across 176 sagittal slices (TR=2400ms; TE=3.09ms; flip angle=8°; inversion time [TI] =1000ms; 1 × 1 × 1 mm voxels). An asymmetric spin-echo echo-planar pulse sequence (EPI) (TR=2200ms, TE=27ms, flip angle=90°, 4×4×4 mm voxels) captured images of blood oxygenation level-dependent (BOLD) contrast responses across 36 odd-even, contiguously interleaved, bicommissurally aligned axial slices.[36,37] Three 164-frame (6 minute) EPI runs recorded spontaneous brain activity while participants are awake, not performing a task, with their eyes open in a darkened room.
Image Preprocessing
EPI image preprocessing started with compensation for systematic slice-dependent differences from interleaved odd-even slice acquisition and alignment of the time for each slice to the beginning of each volume acquisition using sinc interpolation. Next, corrections for intensity differences within runs utilized a whole brain mean signal intensity normalized to mode 1000. These time and intensity-adjusted slices were realigned within and across runs using rigid body correction for inter-frame head motion [38–41]. The across-run-realigned slices were resampled to 3mm3 voxels and registered to an atlas template by computing 12 parameter affine transforms between an average from the first frames of each EPI run and the atlas template using the individual’s MP-RAGE image as an intermediary [42]. This atlas template was created using MP-RAGE structural images from 12 normal middle-age individuals (mean 48 yrs, SD ±10.7) and registered to Talairach atlas space [43,44] based on spatial normalization methods [45]. Each subjects’ second scan was cross-day realigned to the MP-RAGE of the first scan.
For rs-fcMRI analyses, additional preprocessing steps were applied in MATLAB (2012a, The Mathworks, Natick, MA) to reduce noise from sources unlikely to reflect neural activity [46]. These steps included demeaning and detrending each BOLD run, temporal filtering with a bandpass filter to remove frequencies <0.009Hz and >0.08Hz, and spatial smoothing with a 6 mm full width at half-maximum Gaussian kernel. Using linear regression, BOLD signal per voxel was adjusted for 24 motion-related and 6 tissue-related sources of nuisance variance. The motion regressors are the six previously computed linear corrections for head movement, their squares, and the same for the immediately preceding timepoint, as derived by Volterra expansion.32 The tissue-related regressors were a global whole-brain signal averaged over all voxels, signals in the ventricles and white matter, and their associated temporal derivatives [47]. The subject’s own anatomy, as segmented using Freesurfer version 5 [48], was used for whole-brain, ventricle, and white matter masks [49,50,39,40]. We applied a volume censoring method [46], which removed frames of data with >.3mm of frame by frame displacement (FD), as well as episodes with fewer than 5 contiguous frames with <.3mm FD [51]. In addition, BOLD runs with fewer than 30 frames meeting these requirements were eliminated. Only the 10 subjects with 119 or more frames of good data in both scans were retained for analysis. Spatial smoothing and temporal filtering as well as nuisance variable regression were repeated on the original preprocessed data, leaving out the censored frames and interpolating across the gap [52]. Since the motion-correction parameters used are more lenient than recommended, we plotted the difference between scan days in each region pair correlation versus the distance between regions for 264 regions sampling the entire brain [16] to check for the distance-dependent artifact often caused by even sub-millimeter head motion [46,53].
Analysis
Feasibility data were analyzed using descriptive statistics. Behavioral data analysis was conducted using SPSS version 20.[54] The data were cleaned and checked for accuracy. The data were found to not be normally distributed so non-parametric analysis methods were used. Distribution of the scores for each of the tests pre-intervention and post-intervention were described using median and range. The difference between pre-post test scores was calculated for each subject for each test. Median of the difference and 95% CI interval was calculated using Student version of MINITAB® Release 14.11.1. Wilcoxon signed rank test was used to test for significant differences in pre-post test scores. Non-parametric effect size (r) calculations were also performed on the behavioral data [55].
For neuroimaging data analysis, timecourses were calculated for each subject and each scan for the two frontoparietal control regions which showed a difference between impaired and non-impaired breast cancer survivors [20], and also for 264 regions covering the brain [16]. Fisher z-transformed Pearson correlation coefficients calculated between two region’s timecourses in a single scan serve as a measure of functional connectivity between them. The Fisher z-transform normalizes the distribution of values, to satisfy the assumptions of the Student’s t-test and comparisons using the Gibbs distribution. Functional connectivity across the brain was compared between days using Object Oriented Data Analysis (OODA) [56], which uses an iterative approach and comparison to the Gibbs distribution to assess the significance of differences found in a multiple dimensional approach. Spearman rho correlations were also calculated to evaluate the relationship between the changes in functional connectivity in the previously reported connection [20] and changes in the behavioral outcome measures.
Results
Feasibility
Based on the initial inclusion/exclusion criteria related to age, gender, and diagnosis, 127 women were referred to our study. From this sample, 72 women either did not report having CICI symptoms or did not show up for their scheduled testing. Another 38 women were unable to be reached via the contact information they had listed in their medical record. The remaining 17 women started treatment and 14 completed treatment; on average, those who completed the intervention completed an average of 9.79 (SD = 2.04) sessions. Therefore, our recruitment rate was 13.4% of our potential sample of breast cancer survivors; however, it was already anticipated that only a certain percentage of this sample would meet the criterion of having CICI. Our attrition rate was 17.6%. Due to the small sample no comparisons were made to determine if the three women who did not complete the study differed from the remaining sample. One women dropped out after active disease was found during a follow-up appointment and the remaining two dropout secondary to conflicts with work schedules.
Participants Results
Our sample is described in the table below (see Table 2). Overall, our sample was fairly young, well-educated, mostly Caucasian, and mostly working full-time during their participation in this study. The median time duration since completion of chemotherapy was slightly less than 12 months.
Table 2.
Study sample characteristics (n = 14)
| Variable | Median (Min-Max) or Percentage |
|---|---|
| Age (Years) | 50.50 (36 to 65) |
| Time since completion of chemotherapy (months) | 9.5 (7 to 34) |
| n (%) | |
| Race | |
| Caucasian | 12 (86%) |
| African American | 1 (7%) |
| Asian | 1 (7%) |
| Highest level of education | |
| High School or Associate Degree | 2 (14%) |
| Bachelor’s Degree | 3 (21%) |
| Master’s or Doctoral Degree | 9 (65%) |
| Work Status | |
| Full-time | 12 (86%) |
| Part-time | 1 (7%) |
| Retired | 1 (7%) |
Behavioral Results
Table 3 displays distribution of the data for the behavioral outcome measures as well as distribution of the difference of the scores pre-intervention as compared to post intervention. The results show that CO-OP had a medium to very large effect on all the primary and secondary behavioral outcome measures in our sample (n = 14) with the exception of sleep function.
Table 3.
Behavioral Outcomes
| Assessment | Pre-Score Median (Min-Max) | Post-Score Median (Min-Max) | Median of difference (pre-post) (95%CI) | Effect size (r)b | Interpretation |
|---|---|---|---|---|---|
| Primary Outcome Measures | |||||
| Cognitive Failures Questionnaire (CFQ) | 50 (39–68) | 36 (15–49) | 15 (8.9 to 25.2) | −.85 | Decrease in subjective cognitive symptoms |
| Delis-Kaplan Executive Function System (DKEFS)-Trailmaking Condition 4 | 12 (1–13) | 12 (7–14) | −1 (−2.1 to 0) | −.50 | Improvement in objective EF (cognitive flexibility) |
| Dysexecutive Questionnaire (DEX) | 23 (3–39) | 11 (0–33) | 9 (4 to 16) | −.75 | Improvement in subjective executive performance |
| Secondary Outcome Measures | |||||
| Montreal Cognitive Assessment (MOCA) | 28 (21–30) | 28 (21–30) | 0(−1.05 to 0.05) | −.28 | Stable general cognitive function |
| The Canadian Occupational Performance Measure (COPM) | 4.8 (2.6–7.3) | 7.7 (5.8–9.7) | −3 (−3.3 to −1.6) | −.88 | Improvement in self-rated performance of activities |
| 2.8 (1.4–5.5) | 8.0 (3.5–10.0) | −4.5 (−5.3 to −3.3) | −.88 | Improvement in self-rated satisfaction with performance of activities | |
| Personal Health Questionnaire (PHQ-9)-Depression | 6.5 (1–13) | 4.5 (0–11.0) | 1.5 (0.9 to 4.1) | −.53 | Decrease in depressive symptoms approaching significance |
| NIH-PROMIS 57-Physical Function | 25.8 (20.2–37.5) | 24.7 (20.2–32.7) | 3.6 (2.9 to 4.8) | −.88 | Improvement in self-reported physical function |
| NIH-PROMIS 57-Anxiety | 53.8 (37.1–61.4) | 45.9 (37.1–61.4) | 0 (−0.1 to 9.0) | −.65 | Decrease in reported anxiety symptoms |
| NIH-PROMIS 57-Depression | 44.7 (38.2–59.4) | 44.7 (38.2–56.8) | 2.3 (0 to 9.3) | −.53 | Decrease in reported depression symptoms |
| NIH-PROMIS 57-Fatigue | 51.5 (41.1–65.3) | 52.0 (33.1–58.5) | 7.9 (1.6 to 10.7) | −.70 | Decrease in fatigue symptoms |
| NIH-PROMIS 57-Sleep Function | 50.2 (30.5–63.0) | 49.0 (30.5–63.0) | 0 (−2.9 to 5.3) | −.12 | No change in sleep function |
| NIH-PROMIS 57-Satisfaction with Participation in Social Roles | 45.3 (37.7–65.6) | 53.4 (41.0–65.6) | −3.8 (−10.0 to 0) | −.67 | Improvement in satisfaction with participation |
| NIH-PROMIS 57-Pain Interference | 48.9 (40.7–62.8) | 56.6 (40.7–67.7) | −1.25 (−15.9 to 3.5) | −.30 | Increase in pain interference |
Effect Size r: .1, small effect; .3 medium effect; .5 large effect [55]
Given the limited sample size, the individual effect of the intervention on the CFQ was also qualitatively evaluated and plotted in a bar graph in Figure 1. As depicted in Figure 1, there was evident variation in terms of response to the CO-OP intervention on this subjective outcome measure; however the majority of the participants had improvements on their subjective score on the CFQ (positive change on the CFQ indicated improved cognitive function).
Fig. 1.
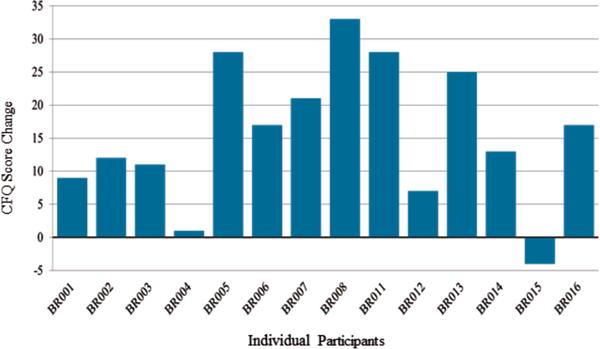
Change in CFQ Score Pre/Post
Neuroimaging Results
Ten of the 14 subjects had a sufficient number of good frames of MRI data in both before and after treatment scans to be analyzed further. The amount of data kept did not differ between the two scans (paired t-test p=.59). The plot of change in functional connectivity between scans vs the distance between regions for the 264 regions showed no distance dependent artifact, justifying the use of slightly relaxed motion parameters to include more subjects.
Using object oriented data analysis (OODA) [56] for comparing subjects before and after treatment across all 264 regions was not sensitive enough to detect a difference in functional connectivity (p=.79). However, a one-tailed, paired t-test on the connection between the two frontal parietal control regions previously described [20] showed trend level significance (p=.054), demonstrating the expected increase in functional connectivity strength after treatment in 6 of the 10 subjects, and minimal decreases in 3 others (see Figure 2).
Fig. 2.
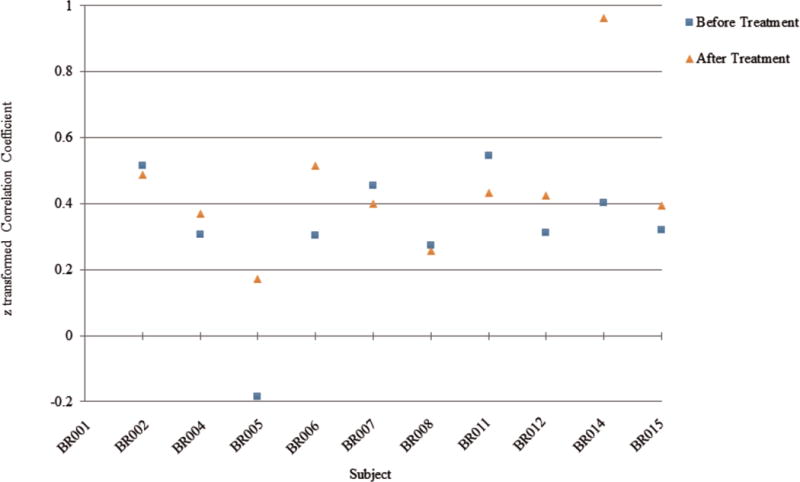
Connection strength before and after treatment
The change score, post-intervention minus pre-intervention, in the PHQ-9 correlated strongly, rs = 0.663, with the change in connection strength between the frontal-parietal (FP) regions evaluated. Additionally, both the DKEFS Trailmaking Condition 4 (rs = −.0369) and the DEX (rs = .596) change scores correlated moderately with the change in connection strength between the frontal-parietal (FP) regions evaluated. Finally, there was a measureable but weak correlation between the CFQ change score and the change in connection strength between the frontal-parietal (FP) regions evaluated, rs = 0.201.
Discussion
The results from this study suggest that the use of CO-OP is feasible with this population. Overall, our recruitment and retention rates were acceptable and along with self-report outcome measures demonstrate that the women in this study found value in the intervention. CO-OP was alsoassociated with a positive effect on improving subjective and objective cognitive performance, subjective activity performance, and quality of life. Further, we were able to measure a positive change in functional connectivity in the one frontal-parietal cognitive control brain network connection previously reported [20] and this change was correlated with changes on a few of the behavioral measures. The results from the study are consistent with previous work using CO-OP with individuals with cognitive deficits following stroke that found positive changes in activity performance and satisfaction [57] and also changes in objective cognitive performance [27]. This study however is the first to evaluate the effect on a particular brain system, i.e., the frontal-parietal cognitive control network, of using CO-OP to address CICI. Given the limitations in how EF is often measured clinically, these data provide support for continued investigation into the use of functional neuroimaging as an objective way to assess mechanistic changes in higher-level cognitive function in women with CICI.
An important strength of the study is its repeated measures, within patient design. However, the study had several limitations. First, while a single group pre/post study was appropriate for this early-stage investigation, the lack of an active control group for comparison limits the ability to conclude the changes we observed were due to the intervention and not due to other non-specific effects of CO-OP or other factors, e.g, passage of time and/or learning effect. The next phase of this investigation should include an active control group to confirm the effects identified in this study were due to the CO-OP intervention. Second, the sample had substantial heterogeneity in terms of age, time since completion of chemotherapy, and differences in response to the intervention on both the behavioral and neuroimaging measures. As shown in Figure 2, some individuals had over a 30-point reduction in reported cognitive problems after the intervention while one person actually had a 4-point increase in reported cognitive problems. The present study provides information to inform the sample size for future studies, which must enroll sufficient number of subjects to control for resonse differences and better identify the responders vs. the non-responders to the intervention. Also, the self-report outcomes measures of cognitive function used in this study, the CFQ and DEX, have limited psychometric development which needs to be addressed in future studies. Finally, while functional neuroimaging was able to detect a positive change in functional connectivity in 6 of the 10 subjects, the use of resting-state functional connectivity as an outcome measure was exploratory and needs considerable further investigation to evaluate its utility as a measure of the mechanistic action of CO-OP.
While there are several methodological and design limitations of this pilot study, these results do support the continued investigation of CO-OP for women with cognitive impairment after chemotherapy for breast cancer. Future studies need to investigate the findings of this study with an active control group and an attempt to control for the confounding factors, e.g., hormonal treatment, different chemotherapy treatment regimens, etc., that may have influenced the results of this study. Overall, this study found that the use of a metacognitive strategy training intervention (CO-OP) is associated with a positive effect on patient outcomes and functional connectivity in a cognitive control network previously demonstrated to be negatively impacted after chemotherapy for breast cancer [20].
Acknowledgments
This study was funded by the McDonnell Center for Systems Neuroscience, Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and the Mallinckrodt Institute of Radiology at the Washington University School of Medicine. Research reported in this publication was also supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number U54HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University. TW received salary support from the National Center for Medical Rehabilitation Research (NCMRR) in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health under award number K23HD073190. The electronic databased system used in this publication, RedCap, is also supported by the National Institutes of Health Clinical and Translational Science Award (CTSA) Grant UL1 TR000448 and Siteman Comprehensive Cancer Center and the National Cancer Institute (NCI) Cancer Center Support Grant P30CA091842. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would also like to thank the faculty, staff, and students in the Performance, Participation, and Neurorehabilitation Laboratory.
Footnotes
Conflicts of Interests
The authors have no conflicts of interest to report.
Contributor Information
Timothy J. Wolf, University of Missouri.
Meghan Doherty, Washington University in St. Louis.
Dorina Kallogjeri, Washington University in St. Louis.
Rebecca S. Coalson, Washington University in St. Louis.
Joyce Nicklaus, AbbVie Clinical Pharmacology Research Unit (ACPRU).
Cynthia X. Ma, Washington University in St. Louis.
Bradley L. Schlaggar, Washington University in St. Louis.
Jay Piccirillo, Washington University in St. Louis.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2015–2016. American Cancer Society; 2015. http://www.cancer.org/research/cancerfactsfigures/breastcancerfactsfigures/breast-cancer-facts-and-figures-2011-2012. Accessed November 15, 2015. [Google Scholar]
- 2.De Laurentiis M, Cancello G, D’Agostino D, Giuliano M, Giordano A, Montagna E, Lauria R, Forestieri V, Esposito A, Silvestro L, Pennacchio R, Criscitiello C, Montanino A, Limite G, Bianco AR, De PS. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26(1):44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- 3.Mieog JS, van der Hage JA, van d V. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007;(2):CD005002. doi: 10.1002/14651858.CD005002.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Goldstein LJ, Gradishar WJ, Hayes DF, Hudis CA, Jahanzeb M, Kiel K, Ljung BM, Marcom PK, Mayer IA, McCormick B, Nabell LM, Pierce LJ, Reed EC, Smith ML, Somlo G, Theriault RL, Topham NS, Ward JH, Winer EP, Wolff AC. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7(2):122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 5.Reid-Arndt SA, Hsieh C, Perry MC. Neuropsychological functioning and quality of life during the first year after completing chemotherapy for breast cancer. Psychooncology. 2010;19(5):535–544. doi: 10.1002/pon.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid-Arndt SA, Yee A, Perry MC, Hsieh C. Cognitive and psychological factors associated with early posttreatment functional outcomes in breast cancer survivors. Journal of psychosocial oncology. 2009;27(4):415–434. doi: 10.1080/07347330903183117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronis DL, Duffy SA, Fowler KE, Khan MJ, Terrell JE. Changes in quality of life over 1 year in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134(3):241–248. doi: 10.1001/archoto.2007.43. [DOI] [PubMed] [Google Scholar]
- 8.Schou I, Ekeberg O, Sandvik L, Hjermstad MJ, Ruland CM. Multiple predictors of health-related quality of life in early stage breast cancer. Data from a year follow-up study compared with the general population. Qual Life Res. 2005;14(8):1813–1823. doi: 10.1007/s11136-005-4344-z. [DOI] [PubMed] [Google Scholar]
- 9.Tobias JS, Monson K, Gupta N, MacDougall H, Glaholm J, Hutchison I, Kadalayil L, Hackshaw A. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66–74. doi: 10.1016/S1470-2045(09)70306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tannock IF, Ahles TA, Ganz PA, van D. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. Journal of Clinical Oncology. 2004;22(11):2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 11.Wieneke MH, Dienst ER. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psycho-Oncology. 1995;4(1):61–66. doi: 10.1002/pon.2960040108. [DOI] [Google Scholar]
- 12.Lezak MD. Neuropsychological Assessment. In: Lewinsohn LTP, editor. Geropsychological assessment and treatment. Springer; New York: 1986. [Google Scholar]
- 13.Lezak MD, Howieson D, Loring D. Neuropsychological Assessment. 4. Oxford; New York: 2004. [Google Scholar]
- 14.Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114(Pt 2):727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- 15.Wolf T, Morrison M, Matheson L. Initial Development of a Work-Related Assessment of Dysexecutive Syndrome: the Complex Task Performance Assessment. WORK. 2008;31(2):221–228. [PubMed] [Google Scholar]
- 16.Power JD, Cohen AL, Nelson S, Wig G, Barnes K, Church J, Vogel A, Laumann T, Miezin FM, Schlaggar BL. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo B, Krienen F, Sepulcre J, Sabuncu M, Lashkari D, Hollinshead M, Roffman J, Smoller J, Zöllei L, Polimeni J. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruno J, Hosseini S, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiology of disease. 2012;48(3):329–338. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesler S, Wefel J, Hosseini S, Cheung M, Watson C, Hoeft F. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proceedings of the National Academy of Sciences. 2013;110(28):11600–11605. doi: 10.1073/pnas.1214551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccirillo JF, Hardin FM, Nicklaus J, Kallogjeri D, Wilson M, Ma CX, Coalson RS, Shimony J, Schlaggar BL. Cognitive Impairment after Chemotherapy Related to Atypical Network Architecture for Executive Control. Oncology. 2015;88(6):360–368. doi: 10.1159/000370117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schagen SB, Wefel JS. Chemotherapy-related changes in cognitive functioning. EJC Supplements. 2013;11(2):225–232. doi: 10.1016/j.ejcsup.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherrier M, Anderson K, David D, Higano C, Gray H, Church A, Willis S. A randomized trial of cognitive rehabilitation in cancer survivors. Life sciences. 2013;93(17):617–622. doi: 10.1016/j.lfs.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ercoli L, Petersen L, Hunter A, Castellon S, Kwan L, Kahn-Mills B, Embree L, Cernin P, Leuchter A, Ganz P. Cognitive rehabilitation group intervention for breast cancer survivors: results of a randomized clinical trial. Psycho-Oncology. 2015;24(11):1360–1367. doi: 10.1002/pon.3769. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Mott LA. Cognitive-behavioral management of chemotherapy-related cognitive change. Psycho-Oncology. 2007;16(8):772–777. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poppelreuter M, Weis J, Bartsch HH. Effects of specific neuropsychological training programs for breast cancer patients after adjuvant chemotherapy. Journal of psychosocial oncology. 2009;27(2):274–296. doi: 10.1080/07347330902776044. [DOI] [PubMed] [Google Scholar]
- 26.Von Ah D, Carpenter J, Saykin A, Monahan P, Wu J, Yu M, Rebok G, Ball K, Schneider B, Weaver M, Tallman E, Unverzagt F. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Research and Treatment. 2012;135(3):799–809. doi: 10.1007/s10549-012-2210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf T, Polatajko H, Baum C, Rios J, Cirone D, Doherty M, McEwen S. Combined cognitive-strategy and task-specific training impacts cognition and upper extremity function in sub-acute stroke: An exploratory randomized controlled trial. American Journal of Occupational Therapy. 2016;70:7002290010. doi: 10.5014/ajot.2016.017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May A. Experience-dependent structural plasticity in the adult human brain. Trends in Cognitive Sciences. 2011;15(10):475–482. doi: 10.1016/j.tics.2011.08.002. http://dx.doi.org/10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16(9):1348–1355. doi: 10.1038/nn.3470. http://www.nature.com/neuro/journal/v16/n9/abs/nn.3470.html#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanto TP, Gazzaley A. Fronto-parietal network: flexible hub of cognitive control. Trends in Cognitive Sciences. 2013;17(12):602–603. doi: 10.1016/j.tics.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haskins E. Cognitive Rehabilitation Manual: Translating Evidence-Based Recommendations into Practice. Vol. 1. American Congress of Rehabilitation Medicine; Reston, VA: 2012. [Google Scholar]
- 33.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polatajko HJ, McEwen SE, Ryan JD, Baum CM. Comparing skill acquisition using a cognitive-based treatment approach to contemporary occupational therapy in stroke: A pilot randomized controlled trial. Archives of Physical Medicine and Rehabilitation. 2010;90(10):e31. [Google Scholar]
- 35.Polatajko H, Mandich A. Enabling occupation in children: The Cognitive Orientation to daily Occupational Performance (CO-OP) approach. CAOT Publications ACE; Ottawa, ON: 2004. [Google Scholar]
- 36.Kwong K, Belliveau J, Chesler D, Goldberg I, Weisskoff R, Poncelet B, Kennedy D, Hoppel B, Cohen M, Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa S, Tank D, Menon R, Ellermann J, Kim S, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Sciences. 1992;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox MD, Snyder A, Zacks J, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nature neuroscience. 2005;9(1):23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- 39.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van E, D C, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vincent JL, Kahn I, Snyder A, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of neurophysiology. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 42.Ojemann J, Akbudak E, Snyder A, McKinstry RC, Raichle ME, Conturo T. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6(3):156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- 43.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain 3-Dimensional proportional system: an approach to cerebral imaging. Thieme Medical; New York, NY: 1988. [Google Scholar]
- 44.Talairach J, Tournoux P, Missir O. Referentially oriented cerebral MRI anatomy: an atlas of stereotaxic anatomical correlations for gray and white matter. G. Thieme Verlag; Stuttgart, New York, NY: 1993. [Google Scholar]
- 45.Lancaster J, Glass T, Lankipalli B, Downs H, Mayberg H, Fox P. A modality-independent approach to spatial normalization of tomographic images of the human brain. Human brain mapping. 1995;3(3):209–223. [Google Scholar]
- 46.Power JD, Barnes K, Snyder A, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friston K, Williams S, Howard R, Frackowiak R, Turner R. Movement-related effects in fMRI time-series. Magnetic resonance in medicine. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 48.Reuter M, Schmansky N, Rosas H, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birn R, Diamond J, Smith M, Bandettini P. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31(4):1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 50.Cordes D, Haughton V, Arfanakis K, Carew J, Turski P, Moritz C, Quigley M, Meyerand M. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. American Journal of Neuroradiology. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 51.Smyser C, Inder T, Shimony JS, Hill J, Degnan A, Snyder A, Neil J. Longitudinal analysis of neural network development in preterm infants. Cerebral cortex. 2010:bhq035. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Power JD, Mitra A, Laumann T, Snyder A, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satterthwaite T, Wolf D, Ruparel K, Erus G, Elliott M, Eickhoff S, Gennatas E, Jackson C, Prabhakaran K, Smith A. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 2013;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.IBM. SPSS for Windows, Rel 20.0. IBM Corporation; 2011. [Google Scholar]
- 55.Fritz C, Morris P, Richler J. Effect size estimates: current use, calculations, and interpretation. Journal of experimental psychology: General. 2012;141(1):2. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 56.La Rosa P, Brooks T, Deych E, Shands B, Prior F, Larson-Prior L. Shannon W. Gibb’s Distribution for Statistical Analysis of Graphical Data with a Sample Application to fcMRI Brain Images. Statistics in Medicine. doi: 10.1002/sim.6757. (in press) [DOI] [PubMed] [Google Scholar]
- 57.McEwen S, Polatajko H, Baum C, Rios J, Cirone D, Doherty M, Wolf T. Combined Cognitive-Strategy and Task-Specific Training Improve Transfer to Untrained Activities in Subacute Stroke An Exploratory Randomized Controlled Trial. Neurorehabilitation and neural repair. 2015;29(6):526–536. doi: 10.1177/1545968314558602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Broadbent DE, Cooper PF, Fitzgerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(Pt 1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 59.Wilson B, Alderman N, Burgess P, Ernslie H, Evans J. Behavioral Assessment of the Dysexecutive Syndrome. Journal of Occupational Psychology, Employment and Disability. 2003;5(2):33–37. [Google Scholar]
- 60.Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. Journal of the International Neuropsychological Society. 2004;10(2):301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- 61.Bandura A. Self-Efficacy. In: Ramachaudran VS, editor. Encyclopedia of human behavior. Vol. 4. Academic Press; New York: 1994. pp. 71–81. [Google Scholar]
- 62.Law M, Baptiste S, Carswell A, McColl A, Polatajko H, Pollock N. Canadian Occupational Therapy Performance Measure (COPM) Canadian Association of Occupational Therapists; Ottawa, ON, Canada: 2005. [Google Scholar]
- 63.Kroenke K, Spitzer R, Williams J. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 65.Gage M, Noh S, Polatajko HJ, Kaspar V. Measuring perceived self-efficacy in occupational therapy. The American Journal of Occupational Therapy. 1994;48(9):783–790. doi: 10.5014/ajot.48.9.783. [DOI] [PubMed] [Google Scholar]


