Abstract
We have previously demonstrated that cryopreservation and thawing lead to altered MSC functionalities. Here, we further analyzed MSC's fitness post freeze-thaw. We have observed that thawed MSC can suppress T-cell proliferation when separated from them by transwell membrane and the effect is lost in a MSC:T-cell coculture system. Unlike actively growing MSCs, thawed MSCs were lysed upon coculture with activated autologous PBMCs and the lysing effect was further enhanced with allogeneic PBMCs. The use of DMSO-free cryoprotectants or substitution of HSA with human platelet lysate in freezing media and use of autophagy or caspase inhibitors did not prevent thaw defects. We tested the hypothesis that IFNγ pre-licensing before cryobanking can enhance MSC fitness post thaw. Post thawing, IFNγ licensed MSCs inhibit T cell proliferation as well as fresh MSCs and this effect can be blocked by 1-methyl Tryptophan, an IDO inhibitor. In addition, IFNγ prelicensed thawed MSCs inhibit the degranulation of cytotoxic T cells while IFNγ unlicensed thawed MSCs failed to do so. However, IFNγ prelicensed thawed MSCs do not deploy lung tropism in vivo following intravenous injection as well as fresh MSCs suggesting that IFNγ prelicensing does not fully rescue thaw-induced lung homing defect. We identified reversible and irreversible cryoinjury mechanisms that result in susceptibility to host T-cell cytolysis and affect MSC's cell survival and tissue distribution. The susceptibility of MSC to negative effects of cryopreservation and the potential to mitigate the effects with IFNγ prelicensing may inform strategies to enhance the therapeutic efficacy of MSC in clinical use.
Keywords: Mesenchymal Stromal cells, Cryopreservation, Thawing, heat shock, actin, autophagy, DMSO, Indoleamine 2, 3-dioxygenase, T cell responses, Immune suppression
Introduction
Mesenchymal stromal cells (MSCs) are adult multipotent stem cells that can be derived from multiple tissue sources such as bone marrow, umbilical cord, adipose tissue, placenta. Of these, bone marrow derived MSCs are the most commonly tested in clinical trials[1] for their anti-inflammatory and regenerative properties[2, 3]. MSCs in bone marrow maintain the hematopoietic niche, regulate marrow immune homeostasis and can differentiate in to bone, cartilage, adipocytes[4]. Despite their low frequency in marrow, 1 in 100,000 marrow nucleated cells, MSCs can be readily and rapidly expanded under standard cell culture conditions for use as a more-than-minimally-manipulated cellular pharmaceutical[5]. Safety of MSC infusion has been well proven in early phase clinical trials but clear demonstration of efficacy is an ongoing concern[6, 7]. Large industry sponsored phase II/III clinical trials have failed to meet primary end points of clinical benefit and hence it is necessary to revisit approaches for the manufacturing and use of MSC in humans[8, 9]. Mechanistic analysis of transfused MSCs and their interaction with immune responders, host inflammatory cues and identifying cellular defects not only will inform surrogate measure of potency but also provide novel translational insights to develop cell therapy platforms[10]. Along with variations in the function of MSC from different donors, culture expansion induced senescence and allogenecity and the effect of cryopreservation and thawing, (a usual practice used in cell therapy clinical trials) impact transfused MSC's fitness and, need to be studied in detail[9].
Of 49 MSC clinical studies published with cell manufacturing methodologies after 2007, 19 used thawed MSCs from cryopreservation[11]. Despite feasibility, this approach yields cell products that may deploy altered potency relative to their counterparts prior to cryopreservation or post culture rescue[9, 12, 13]. Retrospective clinical result analysis have demonstrated that 100% of patients respond to the treatment with MSCs derived from actively growing culture while only 50% of the comparable patient populations show responsiveness to thawed MSCs[14]. Others and we had shown that thawed MSCs from cryopreservation display attenuated immunosuppressive activities compared to actively growing/culture rescued MSCs[14, 15]. In order to explore other mechanisms by which cryopreservation causes injury to MSCs we have investigated the lung homing properties of cryopreserved MSCs and demonstrated that thawing induced a defect in the polymerization of actin cytoskeleton which attenuated their engraftment potential in vivo. However culture rescue for 24-48 hours post thaw reverses these acquired defects[12, 16]. An important unanswered question is: how does freeze thawing alter MSC's interaction with immune responders or in vivo distribution? To address this, we here performed a rigorous mechanistic analysis of the effect of cryopreservation methods and thawing on structural and biochemical MSC fitness and their susceptibility to T cell mediated lysis. In addition, in our efforts to optimize methods to mitigate freeze-thawing induced MSC's dysfunction, we investigated if cytokine prelicensing rescues freeze-thawing induced MSC defects.
Materials and Methods
MSC Isolation and culture
Bone marrow aspirates were collected from iliac crest of consenting subjects enrolled in an IRB-approved study. Mononuclear cells were isolated from the bone marrow aspirates (1:2 diluted with PBMCs) using Ficoll density gradient centrifuged at 400g for 20 minutes. Collected mononuclear cells were plated incomplete human MSC medium (α-MEM, 10% human platelet lysate, 100 U/ml penicillin/streptomycin/Amphotericin B) at 200,000 cell/cm2. Non-adherent hematopoietic cells were removed by changing the medium after 3 days of culture and MSCs were allowed to expand for 7 days. MSCs were passaged weekly and reseeded at 1000 cells/cm2. After the third passage, MSC cultures were assayed by flow cytometric analysis for the absence of CD45+ and CD31+ contaminating cells and expression of CD44, CD73, CD90, and CD105 (BD Bioscience, St Jose, CA). All assays were performed using MSC between passage 2 and 6.
Preparation of human Platelet lysate
Human platelet lysate (hPL) were prepared from outdated platelet pheresis products obtained from Emory University Blood Bank with American Red Cross consent and an Emory IRB waiver. In brief, platelets were lysed using a double freeze thaw procedure to release their contents. Pools of five lysed platelet units were filtered through a 40 μm PALL blood transfusion filter (PALL BIOMEDICAL, INC Fajardo, PR) and then aliquoted and spun for 20 minutes at 4000×g at room temperature, filtered (40 μm) then re-calcified to 20mM CaCl2. Following centrifugation, platelet lysate was filtered to 0.2 μm then stored at -80°C till use[17].
MSC cryopreservation and thawing
MSCs at 70-80% confluence were trypsinized and washed with complete medium. For IFNγ prelicensing, recombinant human IFNγ (Invitrogen, USA) was added at the concentration of 20ng/ml in the MSC culture medium 48 hours prior to cryopreservation. Similarly caspase inhibitor Z-VAD-FMK 50uM (MBL laboratories, USA) or 3-Methyl Adenine 1mM (Sigma, USA) was used for the pretreatment of MSCs before cryopreservation. The cell pellet was resuspended with freezing media slowly at the concentration of 5-10×106 cells/ml. The following freezing media were tested: 5% Human Serum Albumin (HSA) in Plasmalyte/aMEM (Hyclone USA), 5%, 20%, 40%, 90% hPL in aMEM with 10% DMSO (Cellgro,Mediatech Inc.,VA). For DMSO free animal protein free freezing media, we have used CryoSOfree™ DMSO-free Cryopreservation Medium (Sigma,USA). According to manufacturer description, CryoSOfree™ is a animal protein free cryoprotectant contatining polyampholytes with an appropriate ratio of amino and carboxyl groups[18]. The cells were placed in the freezing container Nalgene® Mr. Frosty (Sigma,USA) at the cooling rate of 1°C/min. For step-down freezing we have used the following program in CryoMed™ Controlled-Rate Freezer (Themoscientific, USA). 1. Wait at 4.0°C; 2. Ramp 1.0°C/min. until Sample = -6.0°C; 3. Ramp 25.0°C/min. until Chamber = -50.0°C; 4. Ramp 25.0°C/min. until Chamber = -14.0°C; 5. Ramp 1.0°C/min. until Chamber = -45.0°; 6. Ramp 10.0°C/min. until Chamber = -90.0°C; 7. Hold -90.0°C for 5.0 minutes. The cells were then transferred in to liquid nitrogen. For thawing, the cells were kept at 37C water bath for one minute and immediately transferred in to MSC complete medium for centrifugation two times. Viable cell count is then determined by mixing equal volumes of 0.4% Trypan blue and Cell mixture and analyzed either using a hemacytometer or by automated cell counting (Invitrogen Countess, USA). A secondary measure of viability of MSCs cultures was monitored by FACS analysis using 7-aminoactinomycin D (7-AAD) and analyzed through FACScanto II cytometer (BD, USA). Prestoblue dye reduction assay was performed according to manufacturer instructions. Briefly MSCs and PrestoBlue® Cell Viability Reagent were incubated for three hours and absorbance were read at 570 nm and 600 nm wavelengths. Percent reduction of PrestoBlue™ reagent was calculated according to the manufacturer instructions (Life technologies, USA).
IDO detection
For the IDO mRNA expression analysis, the cells were subjected to total RNA extraction using RNeasy plus mini kit and total cDNA was prepared using Quantitect reverse Transcription kit (Qiagen, USA). Sybr green (Perfecta Sybr green fast mix, Quanta biosciences, USA) real time PCR was performed with IDO primers (5′GCCCTTCAAGTGTTTCACCAA, 5′CCAGCCAGACAAATATATGCGA and GAPDH primers (5′CTCTCTGCTCCTCCTGTTCGAC, 5′TGAGCGATGTGGCTCGGCT) with ABI 7500 fast real-time PCR system thermal cycler. For Western Blot analysis, proteins were detected using primary rabbit anti-human IDO1 (1:1,000; EMD Millipore Corporation, Billerica, MA) or rabbit anti-human β-actin (1:1,000; Cell Signaling Technology, Inc, Danvers, MA), and secondary horseradish peroxide-coupled goat anti-rabbit IgG h + l (1:10,000; Bethyl Laboratories, Inc., Montgomery, TX) and revealed using ECL system (Amersham Pharmacia Biotech, Piscataway, NJ).
Heat shocking and actin depolymerization
Adherent MSCs were treated with Cytochalasin D (Sigma) at a concentration of 2 uM for 2 hours at 37C in a 5% CO2 incubator or heat shocked for 3 hours at 42C in a 5% CO2 incubator. For double treatment MSCs were kept for initial 2hours with cytochalasin D at 42C incubator and continued one more hour after washing Cytochalasin D. Heatshocked and control cells were subjected to quantify HSP70A and 70B mRNA levels with the primers as described previously[15].
MSC and T cell coculture
Live or thawed MSCs and PBMCs were cocultured at the indicated ratio in 96 well plates. PBMCs were prepared from healthy individuals by Ficoll density gradient were resuspended in RPMI-1640 complete medium (10% heat inactivated serum, 100 U/ml penicillin/streptomycin, L-glutamine, 10mM HEPES). PBMCs were activated with 500ng/ml Staphylococcal enterotoxin B (SEB) (Sigma aldrich, USA) or 2ul dynabeads (Life technologies, Norway). For IDO blocking 1-methyl-DL-tryptophan (1mM concentration) (Sigma Aldrich, USA) were added to the coculture. For non-contact MSC and PBMC culture, MSCs and SEB/bead activated PBMCs were cultured in the bottom and transwell respectively using Corning® Costar® 0.4uM Transwell® cell culture inserts. For Ki67 Proliferation assay, cells were incubated for 4 days and were subjected to intracellular Ki67 staining according to manufacturer instructions (BD Biosciences, St Jose, CA). Degranulation assay was performed with CD107 antibody (BD Biosciences, St Jose, CA) staining during stimulation at the indicated time points for 12 hours with monensin and brefeldin A. Intracellular flow cytometry staining was performed with of BD Cytofix and Cytoperm procedures according to the manufacturer instructions and with the antibodies APCCy7-antiCD3, PerCP-antiCD8 and APC-IFNγ (BD Biosciences, St Jose, CA).
MSC survival assays
CFSE labeled live and thawed MSCs were cocultured with/without SEB activated PBMCs in indicated ratios in 96 well or trans well plates. PBMC and MSC numbers were kept constant and variable for dose escalating ratio indications. 4 days post coculture, bound cells were trypsinized and counted (CFSE+ events) in flow cytometry using Accucheck counting Beads (Thermo scientific, USA). MSCs cultured in the absence of PBMCs were used to calculate % survival of MSCs in the presence of PBMCs. Single cell cytotoxicity assay was performed according to manufacturer's instructions (PanToxiLux™ is OncoImmunin, Inc., USA) and MSCs were detected in coculture with PBMCs based on CD45 negative population in flow cytometry.
Ex Vivo Bioluminescent Imaging
Trafficking of luciferase transgenic murine MSCs to the lungs was measured by ex vivo bioluminescent imaging of whole organs 24 hours following cell injection via intravenous route. Briefly, both lungs were excised from each animal and placed into a single well in a 24 well plate. The lungs were then bathed in 350 μL of a 15 mg/mL luciferin solution (Perkin Elmer, Waltham, MA). Immediately following luciferin addition, the plate was imaged using an IVIS Spectrum imager (Perkin Elmer, Waltham, MA). Quantification of MSC accumulation was performed using Living Image software (Perkin Elmer, Waltham, MA) by creating a region of interest over each well. Measurements are recorded as photons/s/cm2. Percentage binding of +/-IFNγ cryo was calculated based on live MSCs.
Statistical analysis
Data were analyzed with the GraphPad Prism 5.0 software. An unpaired two-sided t-test was used to determine significance between the means of two groups, while a one-way ANOVA using Tukey's Multiple Comparison Test was used to compare multiple groups simultaneously. A two-sided P value <0.05 was considered statistically significant.
Results
Defective inhibition of T cell proliferation by frozen-thawed human MSCs is cell contact dependent
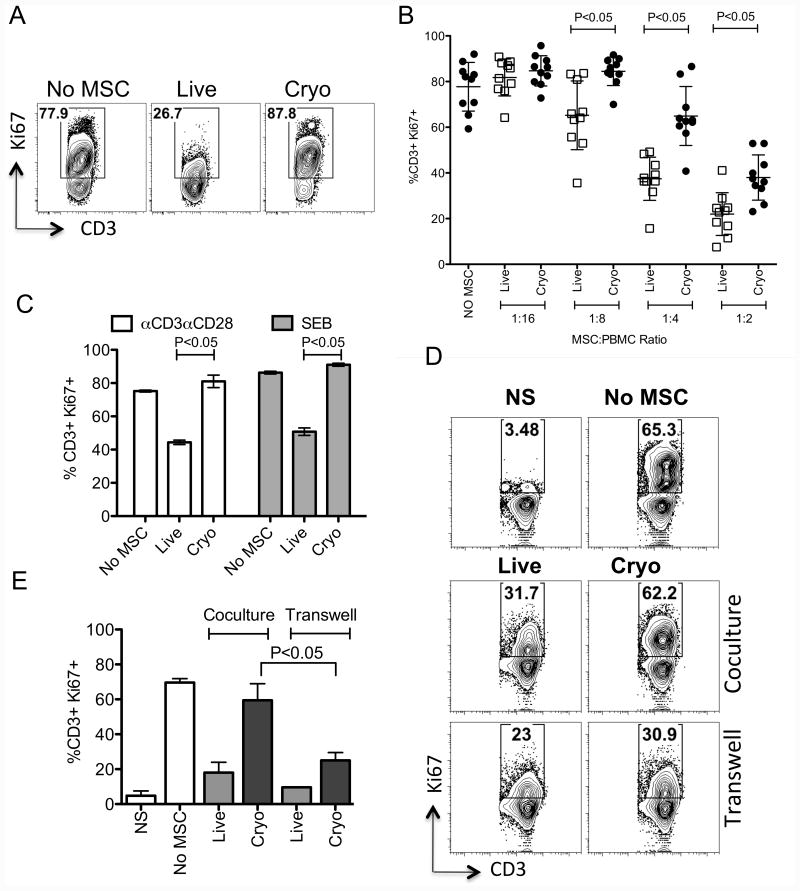
We analyzed the immunosuppressive effect of bone marrow derived human MSCs obtained from both actively growing and frozen-thawed cultures (Hereafter called Live and Cryo MSCs respectively). Results of multiple (n=10) experiments with independent MSC donor pairs (Live and Cryo) demonstrate that Live MSCs inhibit SEB activated Ki67+ T cell proliferation more efficiently than Cryo MSCs (%CD3+Ki67+: No MSC (78±11), Live MSC (38±9), Cryo MSC (65±13)) (Fig. 1A, 1B). Although the inhibitory effect of Live and Cryo MSCs were observed in a dose dependent manner, Live MSCs are superior to Cryo MSCs in inhibiting T cell proliferation at all MSC:T cell ratio tested (Fig. 1A, 1B). T cells in PBMC preparations can be activated either with SEB or aCD3aCD28 coated beads while both of these stimulants induce T cell activation in a distinct manner [19, 20]. In order to define the effect of distinct modes of T cell activation on Live and Cryo MSC's comparative veto function, we cocultured SEB and aCD3aCD28 activated PBMCs in the presence and absence of Live and Cryo MSCs. Our results demonstrated that the functional defect of Cryo MSC's in attenuating T cell proliferation is independent of the mode of T cell activation (Fig. 1C). MSCs inhibit T cells both by contact and non-contact dependent mechanisms[3, 21]. We investigated whether the defect in immunosuppressive properties of MSC is due to either contact or non-contact interaction with immune responders. To test this hypothesis, we cultured activated PBMCs with/without Live or Cryo MSCs using either direct coculture or co-culture in which the T cells and MSC were separated by a two-chamber transwell system. Our results demonstrated that Cryo MSCs inhibit T cell proliferation more efficiently in the transwell culture when compared to coculture system (%CD3+Ki67+: No MSC (70±2), Cryo MSC-Coculture (60±10), Cryo MSC-Transwell (25±5)) (Fig. 1D, E). Our results suggest that activated PBMCs blunted the immunosuppressive properties of Cryo MSCs through a direct contact dependent interaction.
Figure 1. Frozen-thawed human MSCs display cell contact-dependent attenuated immunosuppressive properties on T cells.
PBMCs co-cultured in the presence and absence of MSCs derived from actively growing culture (Live) or thawed from cryopreservation (Cryo) were stimulated with SEB. 4 days post, T cell proliferation was measured by Ki67 intracellular staining. (A) Representative FACS plot and (B) cumulative % of T cell proliferation (CD3+Ki67+) in the presence of variable MSC and PBMC ratio is shown. Cumulative is plotted from multiple independent experiments tested with unique PBMC and MSC donors. (C) Live and Cryo MSCs were cocultured with SEB and aCD3aCD28 activated PBMCs. 4 days post culture, T cell proliferation was evaluated. Live and cryo MSCs were cultured with SEB activated T cells in a contact and non-contact dependent coculture and transwell system respectively. (A) Representative FACS plot and (B) cumulative % of T cell proliferation (CD3+Ki67+) in the coculture and transwell system is shown. Cumulative is plotted from three independent experiments with unique MSC and PBMC donors. P value <0.05 was considered statistically significant based upon two-tail T-tests.
Alternative cryopreservation methods and formulations do not rescue frozen-thawed MSC's immunosuppressive defects
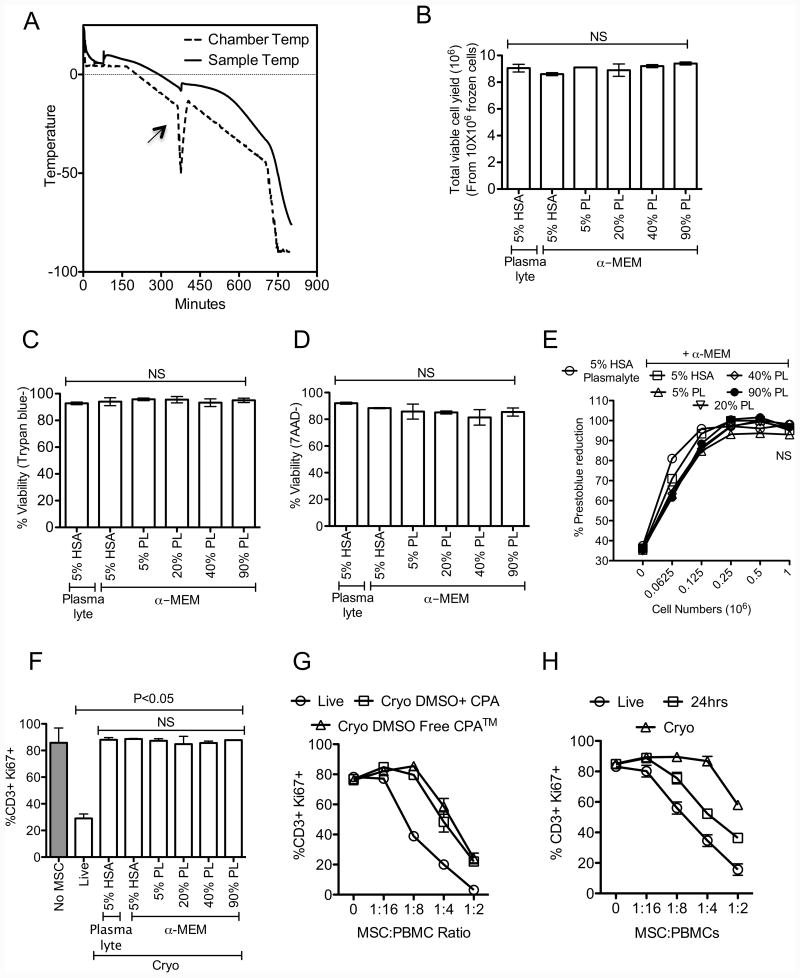
Research laboratories routinely use cell-freezing containers with isopropanol to cryopreserve mammalian cells in mechanical freezer while clinical stem cell storage facilities typically use controlled rate freezers. Although both of these methods of cryopreservation yield comparable clinical outcome with hematopoietic stem cells[22-24], effects on MSC's functionality post freeze-thaw are unknown. We tested the effect of a controlled freezing program on MSC functionality post freeze-thaw (Fig 2A). Standard media formulation for hematopoietic stem cell freezing is 5% HSA and 10% DMSO diluted in Plasmalyte. Considering that human platelet lysate is utilized for culture expansion and production of pharmaceutical grade MSCs[17, 25], we have tested its use in freeze media formulations as a substitute for human serum albumin along with controlled freezing conditions. Our results demonstrate that addition of various concentration (5%-90%) of human platelet lysate in cryopreservation media did not significantly enhance MSC recovery (Fig. 2B), viability (Fig. 2C, D) and metabolic activity (Fig. 2E) post freeze-thaw. In addition, MSCs thawed from cryopreserved cultures containing platelet lysate display attenuated immunosuppressive properties compared to Live MSCs (%CD3+Ki67+: No MSC(86±11), Live MSC(30±3), Cryo MSCs (5%HSA in α-MEM(88±2), 5%HSA in PL(89±1), 5%PL(87±2), 20%PL(85±6), 40%PL(86±2), 90%PL(87±1))) (Fig. 2F). Our results also demonstrate that MSCs frozen-thawed from cryopreserved cultures containing DMSO-free cryoprotectant are defective in attenuating T cell proliferation as well (Fig. 2G), suggesting that use of controlled rate freezing methods and substitution of HSA with hPL and DMSO with alternates do not improve upon standard methodologies. However sub culturing MSCs post freeze-thaw for at least 24 hours rescues their immunosuppressive properties (Fig. 2H) suggesting that freeze-thaw induced cellular defects are reversible.
Figure 2. Freeze-thawing attenuates immunosuppressive properties of human MSCs independent of freezing methods.
(A) Freezing program that was used to cryopreserve MSCs in a step down freezer. Chamber and sample temperature during step-down freezing is shown. An arrow shows alignment of “heat of fusion” between chamber and sample temperature. Live or cryopreserved MSCs with the indicated formulations of cryoprotectant were thawed (B) Total viable cell yield (C) Trypan blue viability (D) 7-AAD negative viability, (E) Prestoblue reduction potential was investigated. Cryo MSC populations were cocultured with SEB activated PBMCs and Live MSCs were used as controls. 4 days post culture (F) T cell proliferation (%CD3+Ki67+) was measured in flow cytometry. (G) Live or Cryo MSCs cryopreserved with 10% DMSO or DMSO free cryoprotectant, (H) Live, thawed and 24-hour culture rescued MSCs were and subjected to test their inhibitory effect on T cell proliferation as indicated above. T cell proliferation was measured 4 days post culture. Representative experiments are shown from two independent experiments performed on two unique MSC donors with independent methods. One-way ANOVA using the Tukey's Multiple Comparison Test was used to compare multiple groups. P value <0.05 was considered statistically significant.
Heat shock response and actin depolymerization effects on MSC's immunosuppressive properties
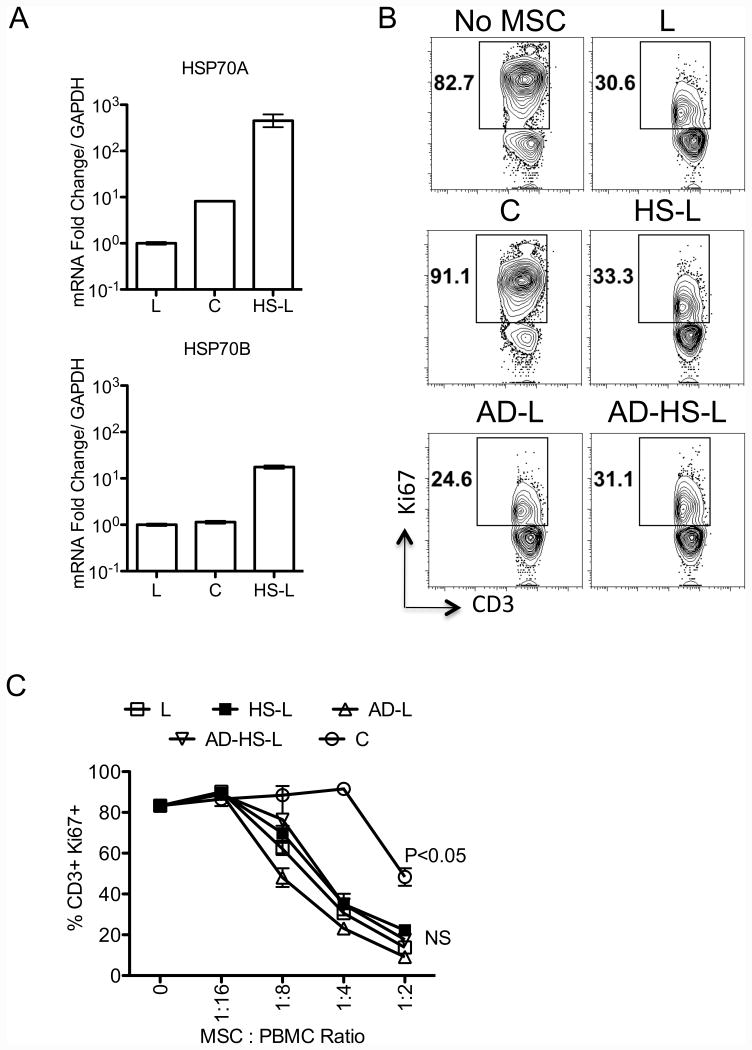
Our published data demonstrated that freeze-thawing induces a molecular genetic heat shock response and depolymerizes cytoskeleton structure in MSCs [16]. Here we further investigated if freeze-thaw induced heat shock response and/or defective actin polymerization affect MSC's immunosuppressive properties independently of freeze/thaw cycle. We show that incubation of MSCs in an incubator set at 42 degree celsius induces HSP70A and HSP70B mRNA in freshly cultured Live MSCs (Fig. 3A). As demonstrated previously, Cytochalasin D-treatment depolymerizes actin cytoskeleton structures, shrinks dendrites and eventually circularizes MSCs (Supporting information 1). Our results demonstrate both heat shocked and cytochalasin D treated MSCs inhibit T cells similar to control cells (Fig. 3B, C). In addition, we also observed that combined treatment of heat-shock and actin depolymerization do not confer attenuated immunosuppressive properties to MSCs (%CD3+Ki67+: No MSC(83±1), Live MSCs(30±1), Cryo MSCs(91±1), actin depleted Live MSCs(23±2), heat shocked Live MSCs(35±2), actin depleted and heat-shocked Lived MSCs(35±5))(Fig. 3B, C) suggesting that freeze-thaw defect in MSC in vitro immune suppression is independent of HS and F-actin cytoskeletal structure and that additional anomalies conferred by cell thawing are in play.
Figure 3. Freeze-thawing induced heat shock response and disruption of actin polymerization do not modulate human MSC's immunosuppressive properties.
Live (L) MSCs were subjected in to three treatments namely, Cytochalasin D (2mM) for 2 hours to depolymerize actin cytoskeleton (AD-L), cultured in a 42C incubator for three hours to induce heat shock response (HS-L) and both (AD-HS-L). (A) Cryo (C), L, and HS-L MSCs were tested for the mRNA expression of heat shock proteins HSP70A, HSP70B. Expression level of HSP70A, HSP70B mRNA relative to GAPDH was evaluated by the quantitative SYBR green real time PCR. Delta-delta CT method was applied to calculate the fold induction of HSP70A, HSP70B over the Live (L) control. MSCs derived from conditions L, C, HS-L, AD-L, AD-HS-L were cocultured with SEB activated PBMCs with the indicated ratios for 4days and T cell proliferation was measured by Ki67 intracellular staining. (B) Representative FACS plot and (C) Dose dependent effect of MSCs on of T cell proliferation (CD3+Ki67+) is shown. A representative experiment is shown from two independent experiments performed on two unique MSC donors. P value <0.05 was considered statistically significant based upon two-tail T-tests.
Frozen-Thawed MSCs are susceptible to lysis by activated PBMCs
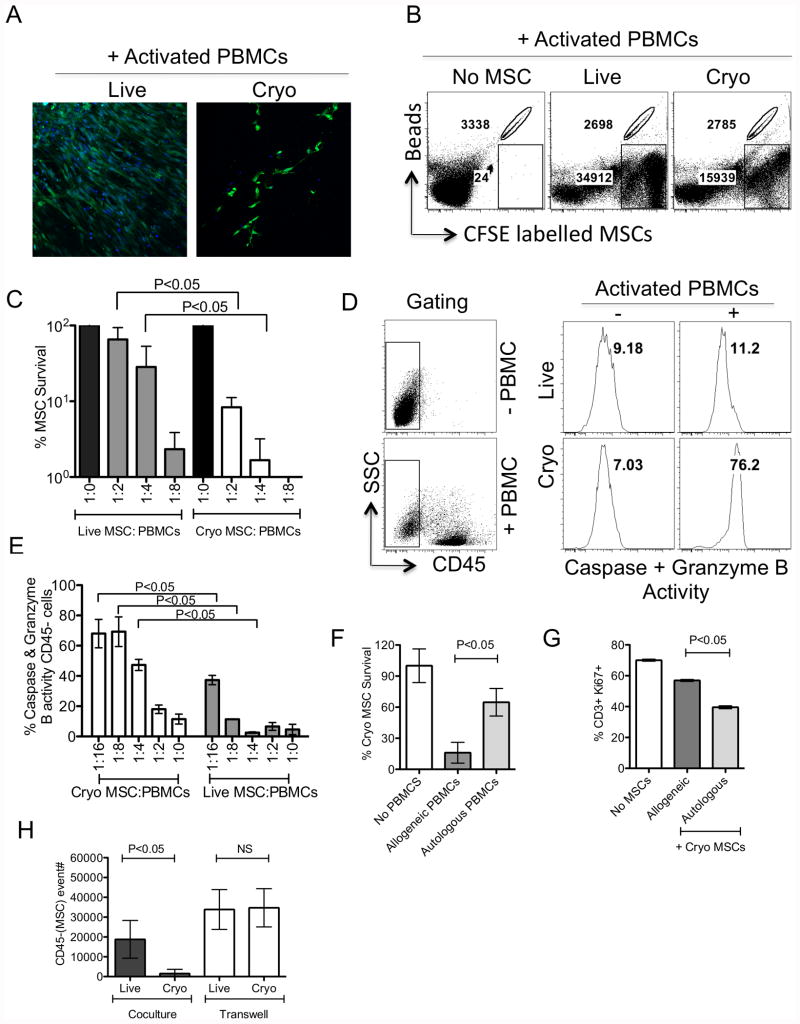
To further decipher the mechanism of attenuated immunosuppressive activity by Cryo MSCs, we performed microscopic analysis of Cryo and Live MSCs (CFSE labeled) in culture with activated PBMCs. Interestingly, we have observed that Cryo MSCs were lysed in coculture with activated allogeneic PBMCs (Fig. 4A, Supporting information 2). In order to quantify the magnitude of lysis by activated PBMCs, we counted CFSE+ Live and Cryo MSCs, retrieved upon coculture with activated PBMCs, using flow cytometer with counting bead normalization to calculate the absolute numbers of live MSC per culture. Our results demonstrate that activated PBMCs lyse Cryo MSCs more so than actively growing live counter parts and this effect is dose dependent on the ratio of MSCs and activated PBMCs (% survival of Live (65±28%) or Cryo (8±2%) MSCs upon coculture with activated PBMCs) (Fig 4B, C). Cytotoxic lymphocytes mediate cell killing by activating granzyme B driven cell death[26]. To measure this effect in MSCs, we performed a single cell cytotoxic assay reflecting serine protease activity[27]. Our results demonstrate that Cryo MSCs cultured with activated PBMCs exhibit higher level of serine protease activities compared to actively growing live MSCs suggesting that activated PBMC mediated killing of Cryo MSCs (% serine protease activities in Live (11±1%) or Cryo (69±10%) MSCs upon coculture with activated PBMCs) (Fig. 4D, 4E). Next we have compared the effect of autologous vs allogeneic activated PBMCs on thawed MSCs. Our results demonstrate although Cryo autologous MSCs are also susceptible to lysis upon culture with activated PBMCs, the lysing effect is substantial less than that seen with co-culture of MHC mismatched MSC:PBMC (% survival of autologous (60±13%) and allogeneic (16±10%) Cryo MSCs) (Fig. 4F). We also tested the inhibitory effect of thawed autologous and allogeneic MSCs on T cell proliferation and our results demonstrate that autologous Cryo MSCs are superior to allogeneic MSCs in inhibiting T cell proliferation (Fig. 4G). Next, we have investigated the lysis of Cryo MSCs by activated allogeneic PBMCs in a transwell system. Our results demonstrate that Cryo MSCs show statistically significant better survival following co-culture with activated PBMCs in a transwell system than Cryo MSCs indirect contact with activated T cells (Fig. 4H).
Figure 4. Frozen-thawed MSCs are susceptible to lysis by activated T cells.
CFSE labeled Live and Cryo MSCs were cocultured with SEB activated PBMCs in indicated ratios. (A) Microscopic images show the survival of CFSE labeled (Green) Live and Cryo MSCs in the coculture. Nuclei were stained with DAPI (blue). Plate bound MSCs from the coculture were trypsinized and event counts were recorded in flow cytometry with the normalization of counting beads. Live and Cryo MSC count in the absence of PBMCs were used for normalization and calculation of % survival of Live and Cryo MSCs in the presence of PBMCs. (B) Representative FACS plot and (C) dose dependent effect of activated PBMCs on Live and Cryo MSC's survival is shown. Serine protease activity was measured on Live and Cryo MSCs cocultured with/without activated PBMCs. (D) Representative FACS plot and (E) dose dependent effect of activated PBMCs on the serine protease activity in Live and Cryo MSCs were shown. (F) % Survival of CFSE labeled thawed MSCs cocultured with autologous and allogeneic PBMCs were shown. (G) Effect of Cryo MSCs in inhibiting autologous and allogeneic T cell proliferation (CD3+Ki67+) is shown. (H) Relative CFSE count of Live and Cryo MSCs in the presence of activated PBMCs cocultured or separated by a transwell system is shown. A representative experiment is shown from at least three independent experiments performed on one to three unique MSC donors. P value <0.05 was considered statistically significant based upon two-tail T-tests.
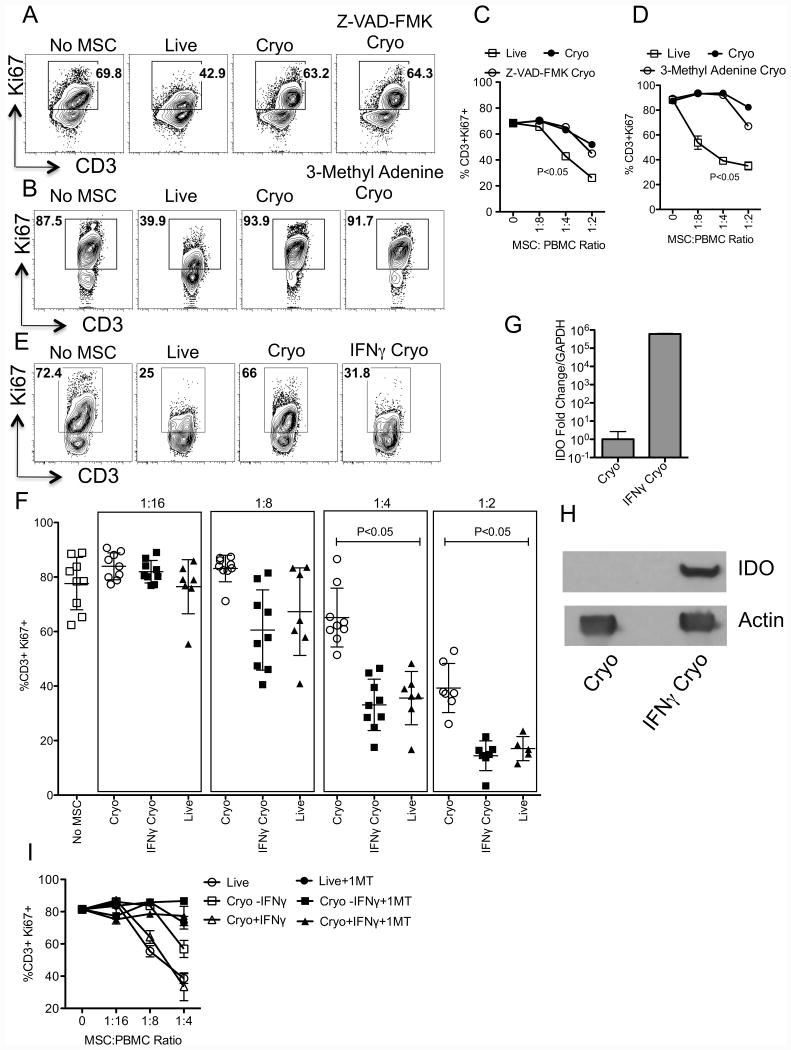
IFNγ but not inhibitors of autophagy and caspase pretreatment prior to cryopreservation enhances thawed MSC's immunosuppressive properties
We tested if pretreatment of MSCs with inhibitors of autophagy and caspase pathways, prior to cryopreservation would rescue frozen-thawed MSC's immunosuppressive properties. Our results demonstrate that frozen-thawed MSCs pretreated with Z-VAD-FMK (Pan caspase inhibitor) or 3-Methyl Adenine (autophagy inhibitor) prior to cryopreservation were defective in inhibiting T cell proliferation (Fig. 5A-D). Published data demonstrates that IFNγ prelicensing enhances MSC's immunosuppressive properties[20, 28-30]. Hence, we have investigated if IFNγ prelicensing prior to cryopreservation rescues thawed MSC's immunosuppressive properties. Our cumulative results demonstrate that IFNγ prelicensed frozen-thawed MSCs (Hereafter called IFNγ Cryo) inhibit T cell proliferation as well as actively growing Live MSCs (%CD3+Ki67+: No MSC (77±9), Live MSC (36±10), Cryo MSC (65±3) IFNγ Cryo MSC(33±10)) (Fig. 5E, F). We demonstrated that IFNγ Cryo MSCs express IDO RNA and protein, which is preserved at thawing (Fig 5G, H). Blocking of IDO catalytic activity with 1-Methyl Tryptophan (1MT) negates the suppressive effect of IFNγ Cryo MSCs on T-cell proliferation (Fig. 5I) suggesting that intracellular IDO protein and its activity are preserved during freeze-thawing and play a significant role on IFNγ Cryo MSC's immunosuppressive properties.
Figure 5. IFNγ prelicensing but not inhibitors of autophagy and caspase rescues frozen-thawed MSC's defective immunosuppressive properties.
+/- IFNγ, Z-VAD-FMK, 3-Methyl adenine(3-MA) pretreated MSCs were cryopreserved and thawed to compare with live MSC's immunosuppressive potential. MSC populations (Live, Cryo and IFNγ/Z-VAD-FMK/3-MA Cryo) were cocultured with SEB activated PBMCs in indicated ratios. PBMC and MSC numbers were kept constant and variable for escalating ratios. 4 days post, T cell proliferation was measured by flow cytometry. Representative and dose dependent effect of (A, C) Z-VAD-FMK and (B, D) 3-MA treated thawed MSCs on T cell proliferation (CD3+Ki67+) is shown. Similar results were obtained in another experiment. (E) Representative FACS plot and (F) cumulative % of T cell proliferation (CD3+Ki67+) with +/-IFNγ Cryo, Live MSC and PBMC ratio is shown. Cumulative is plotted from multiple independent experiments tested with unique PBMC and MSC donors. (G) +/- IFNγ licensed cryopreserved human MSCs were thawed and RNA was extracted to quantitate the expression levels of IDO mRNA by quantitative sybr-green real time PCR. GAPDH mRNA levels were used as an internal control. Delta-delta CT method was applied to calculate the fold change. (H) Western blot analysis to show the IDO expression at protein levels in IFNγ cryo MSCs. Actin was used as an internal control. (I) Live, cryo and IFNγ cryo MSCs were cocutured with activated PBMCs in the presence and absence of IDO blocker, 1-Methyl Tryptophan (1MT). 4 days post, T cell proliferation was measured by flow cytometry. P value <0.05 was considered statistically significant based upon two-tail T-tests.
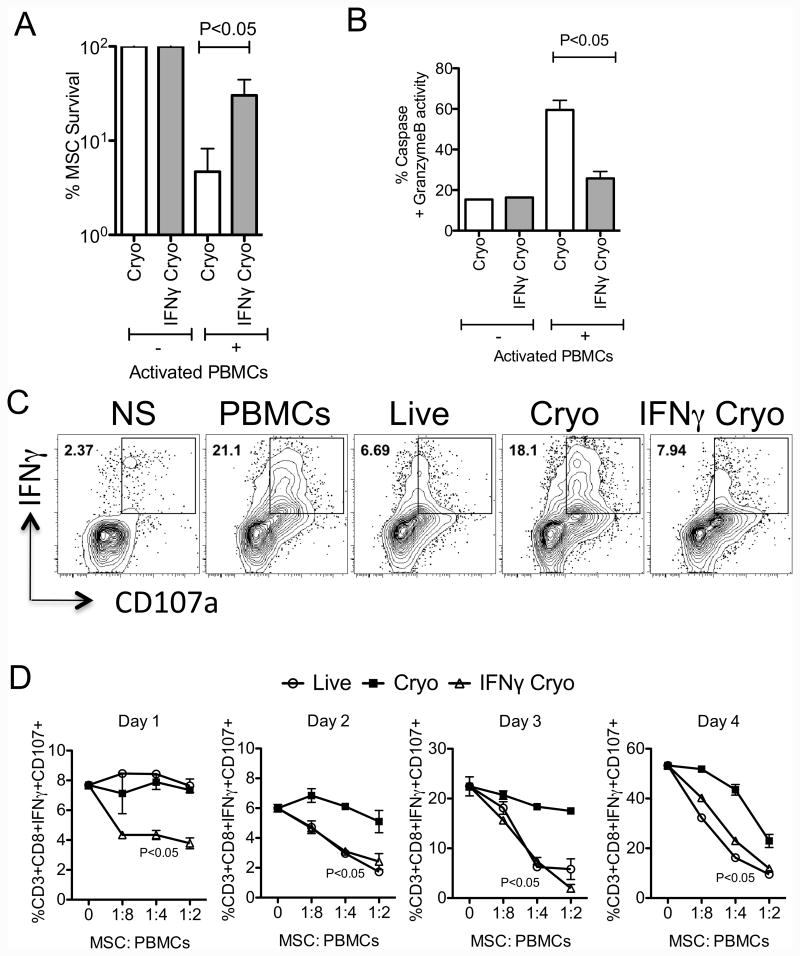
IFNγ prelicensing prior to cryopreservation enhances thawed MSC's survival through inhibition of T cell degranulation
To further investigate the immunosuppressive mechanism of IFNγ Cryo MSCs, we have compared the survival of +/- IFNγ Cryo MSCs cocultured with activated PBMCs. Our results demonstrate that IFNγ prelicensing enhances frozen-thawed MSC's survival following coculture with activated PBMCs(Cryo MSCs 5±2%, IFNγ Cryo MSCs 30±7%) (Fig. 6A). Next we have investigated the effect IFNγ of prelicensing on pro-cytotoxic serine protease activities of thawed MSCs. Our results demonstrate that IFNγ prelicensing attenuates serine protease activity on thawed MSCs upon coculture with activated PBMCs(Cryo MSCs 60±5%, IFNγ Cryo MSCs 26±4%) (Fig. 6B). To further delineate IFNγ dependent thawed MSC's resistance to killing by activated T cells, we analyzed the degranulation potential of co-cultured T cells. We utilized CD107a expression as a surrogate of perforin and granzyme B release by activated T cells[31]. Our results demonstrate that IFNγ Cryo MSCs significantly inhibit the degranulation CD3+CD8+IFNγ+T cells (Fig. 6C). Next we investigated the kinetics of T cell degranulation in the presence and absence of live and +/-IFNγ Cryo MSCs. IFNγ Cryo MSCs inhibit T cell degranulation at all the tested time points (Fig. 6D). However, Live and Cryo MSCs fail to inhibit T cell degranulation immediately at day 1, while live cells gain anti-degranulation properties in subsequent time points and Cryo MSCs display defective in all the tested time points (Fig. 6D). In agreement with previous studies[14, 15, 20], these results suggest that in contrast to thawed counterparts, live MSCs re-activated by IFNγ acquire inhibitory properties to attenuate ongoing T cell degranulation.
Figure 6. IFNγ prelicensing rescues frozen-thawed MSC's survival through inhibition of cytotoxic T cell degranulation.
CFSE labeled +/- IFNγ Cryo MSCs were cocultured with SEB activated PBMCs for 4 days in seeding ratio of 1:4. Trypsinzied CFSE+ cells were counted in flow cytometry using counting bead normalization. +/- IFNγ Cryo MSC count in the absence of PBMCs were used for normalization and calculation of % survival of +/- IFNγ Cryo MSCs in the presence of PBMCs. Live MSC count in the presence of PBMCs were used for normalization and calculation of % survival of +/- IFNγ Cryo MSCs with PBMCs. (A) Relative % survival of live, cryo and IFNγ cryo MSCs cocultured with SEB activated PBMCs is shown. (B) Percentage serine protease activity in cryo and IFNγ cryo MSCs cocultured with activated SEB cells is shown. SEB activated PBMCs were cocultured in the presence and absence of live, cryo and IFNγ cryo MSCs. 12-14 hours prior to the indicated time point BFA, monensin and antibody to CD107 were added to the culture. Cells were subsequently stained with antibodies to CD3, CD8 and IFNγ for flow cytometry. (C) Representative and (D) kinetics of % of CD3+CD8+CD107+IFNγ+T cells is shown. Similar results were obtained in a repeat experiment with another MSC donor. P value <0.05 was considered statistically significant based upon two-tail T-tests.
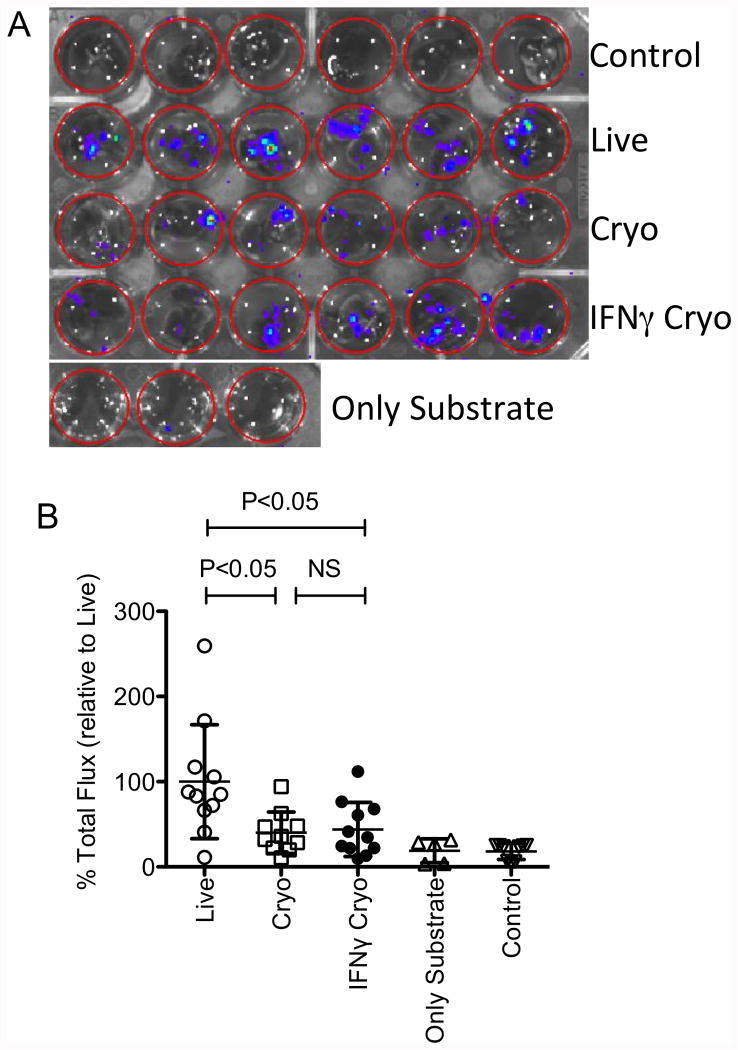
IFNγ prelicensing prior to cryopreservation does not fully rescue thawed MSC's defective lung tropic properties
Our published results demonstrate that human MSCs are readily detectable in mouse lungs for up to 24 hours post transfusion with a rapid decline thereafter[16]. Utilizing this property as a surrogate of MSC's in vivo binding to lung microvasculature, we also demonstrated that the binding potential of Cryo MSCs immediately post-thaw was compromised compared to live MSCs[16]. Here we have investigated the effect of IFNγ prelicensing prior to cryopreservation on thawed MSC's in vivo lung tropic properties following tail vein injection. We have utilized MSCs from luciferase transgenic L2G85.B6 [32] animals to detect their persistence in vivo using bioluminescence imaging. We performed ex vivo lung imaging at 24 hours post IV infusion of 1 million luciferase transgenic MSCs. Live and +/- IFNγ Cryo MSCs were infused and IFNγ effect on MSCs were confirmed with PDL1 expression at post thaw (Supporting information 3). Our cumulative results of two independent experiments demonstrated that syngeneic Live MSCs deploy superior lung tropism than thawed MSCs (Fig. 7A,B). However IFNγ Cryo MSCs are not significantly superior to unlicensed counterparts (% Total flux relative to lung tropism of Live MSCs: Cryo MSCs(40±24), IFNγ Cryo MSCs(44±32)) suggest that IFNγ prelicensing does not fully rescue frozen-thawed MSC biodistributive properties.
Figure 7. IFNγ prelicensing does not rescue frozen-thawed MSC's defective engraftment properties.
1×106 of cryo, IFNγ cryo and Live MSCs derived from luciferase transgenic B6 animals were injected intravenously into C57BL/B6 mice via the tail vein. 24 hours post infusion, the animals were sacrificed and the lungs were excised placed in 24 well plate. D-Luciferin substrate was added at the same time to each well and the plate was subjected to bioluminescence imaging. (A) Representative imaging and (B) cumulative relative % binding of MSCs are shown from two independent experiments (n=5 or 6 animals/group). Cumulative Mean±SD are shown with a P value of <0.05 was considered statistically significant in prism software. One outlier in cryo group was removed based on the extreme studentized deviate method or Grubbs' test in graph pad prism software.
Discussion
There are no published prospective randomized clinical trials using intravenous infused MSCs, which have met their primary endpoint of efficacy, all indications confounded. Yet, there are literally thousands of pre-clinical studies in robust animal models of disease demonstrating the substantial utility of MSC for an array of ailments spanning immune disorders to tissue repair, which unambiguously meet scientific standards of proof. This raises the question of what are the drivers of this discrepancy in outcomes. Most animal studies typically utilize syngeneic MSCs (akin to autologous in humans), but also allogenic as well and this is reflected in human studies. A near universal discriminator though is that virtually all animal studies (murine being the dominant) typically utilize metabolically, fit, log phase of growth MSCs straight from culture, whilst near universally human trials utilize cryobanked MSCs administered to subjects within hours following retrieval from cryostorage and thaw. We have proposed that this substantial methodological difference may explain, in part, the distinct outcomes between pre-clinical studies and pivotal human trials. Our hypothesis is that frozen-thawed MSCs display an altered physiological state in the 24 hours following retrieval from cryostorage, which significantly alters their properties as a pharmaceutical agent when given intravenously. The mechanistic understanding of freeze-thaw induced alterations in MSC biochemistry could inform on remedies to restore desirable pharmaceutical properties including potency, distribution and persistence in vivo following intravenous transfusion in subjects.
A novel observation here made is that Cryo MSCs are susceptible to contact-dependent apoptosis when co-cultured with activated T-cells. It has been shown that MSC mediated induction of tolerance on T cells is partly through contact dependent cell surface factors such as PDL1, PDL2, CD95L, ICAM-1 and VCAM-1 both in vivo and in vitro[20, 33-35] and therefore that Cryo MSCs will be compromised in their suppressive properties due to their poor survival upon contact with inflammatory immune responders. In addition, MSCs distribute and bind to the capillaries of lungs immediately post infusion where it has been proposed that they interact with tissue resident and infiltrating lymphocytes and monocytes [36-39]. The shortened in vivo persistence of Cryo MSCs in lungs may partly/entirely due to their interaction with lytic immune responders.
Our earlier study had shown that freeze-thawing affects polymerization of actin and also induces a heat shock response in MSCs[15, 16]. We also demonstrated that depolymerization of actin cytoskeleton in actively growing MSC attenuates their binding in vivo[16]. Interestingly, actin depleted MSCs still inhibit T proliferation in vitro suggesting that the mechanisms of freeze-thawing induced defect on MSC's binding and immunosuppressive properties are distinguishable to each other. In addition, heat shocked live MSCs with or without actin depletion still inhibit T cell responses comparable to control live MSCs suggesting that the heat shock response is a passive bystander effect of freeze-thawing and does not obviate MSC's immunosuppressive properties on its own. Altogether, identically thawed MSCs retain their immune suppressive effect on T-cell proliferation if they are separated by a physical barrier suggestive that the heat shock response and cytoskeletal disruption in themselves do not abort their suppressor function, but rather that frozen-thawed MSCs and granzyme+ T-cells make poor bedfellows. Thus, we speculate that freeze-thawing induced alterations in the plasma membrane, intracellular pH, and mitochondrial depolarization likely potentiate MSC's susceptibility to T cell mediated killing[40-43] and may also promote sensitivity to complement-mediated lysis [14].
MSCs are responsive to IFNγ derived from host lymphoid effector cells and deploy immunosuppressive properties [44] and in vitro and in vivo studies demonstrated that both infusion of IFNγ receptor KO MSCs and absence of IFNγ abolishes MSC efficacy [45, 46]. Frozen-thawed MSCs are not only susceptible to lysis by lymphoid cytotoxic mediators as we have demonstrated here but also display hyporesponsivess to IFNγ [14, 15], which collectively suggest that freeze-thawing would greatly affect MSC's transient therapeutic effect in the recipient.
Frozen-thawed MSC preparations contain a higher percentage of apoptotic cells (around 10-15%) than live counterparts[16]. Despite normalizing for viability of live and frozen-thawed cells in immunosuppressive and in vivo binding assays, we still observed dysfunctionality of thawed cells suggesting an intrinsic defect. However, we also noted that frozen-thawed MSCs inhibit T cell proliferation at high MSC:T-cell ratios, which likely provide a straightforward explanation for discrepant results from other studies that failed to observe inferior functionality of frozen-thawed cells[47-49]. We can speculate partial clinical efficacy may possibly be achieved by infusing higher doses of thawed MSCs to palliate for their blunted function, however there are practical logistic limits considering that most clinical trials use to 1-10 million MSCs/Kg (typically < 2 million cells/kg) in single or multiple infusions.
There is also now a growing literature, which supports the theory that MSCs undergo reversible changes post freeze-thaw, which can affect their pharmaceutical and biological properties in the hours following their retrieval from cryostorage. Indeed, it has been shown that frozen-thawed human MSCs increase a blood clotting response and induce acute instant blood-mediated inflammatory reaction (IBMIR) in vitro, are susceptible to complement-mediated lysis and have a blunted ability to support chondrocyte growth when delivered in vivo [14, 50]. We have further tested if freeze-thawing leads to shedding of GPI-anchored molecules, which play a role in complement deactivation. We observed that freeze-thawing or IFNγ pre-treatment do not alter surface expression of GPI anchored molecules on MSCs (Supporting information 4) relative to fresh cells suggesting that alternate mechanisms are at play leading to MSC susceptibility to complement as suggested by others[14].
DMSO for cryopreservation of stem cells has been successfully used in the past, notwithstanding its association with transfusion associated clinical side effects[51-55]. An obvious empirical remedy to mitigate the negative effect on freeze-thaw on MSC function was to test whether alternative-freezing solutions using best practice controlled rate freezing methods could rescue the susceptibility of MSCs to T-cell driven apoptosis, akin to other studies which had aimed to enhance the quality of frozen-thawed MSCs by utilizing variable cryopresevation/thawing techniques[56-59] and cryoprotectant formulas with the addition of sugars, anti-oxidants, apoptotic inhibitors, 3D scaffolds[13, 60, 61]. Although majority of these studies investigated intrinsic functions of MSCs and viability at post-thaw, their interactions with immune responders are largely unknown. Despite the substitution of HSA for human platelet lysate as well as the substitution of DMSO for alternate cryoprotectants, we did not improve MSCs susceptibility to T-cell killing.
Considering the potential role of induced autophagy and priming of caspase activation post thaw in MSC lysis, we tested whether adding specific inhibitors to both processes as part of freezing media as well as post thaw would increase MSC resilience. Neither the use of 3-Methyl Adenine or Z-VAD-FMK negated MSCs susceptibility to T-cell lysis. Interestingly, pre-activation of MSCs with IFNγ before freezing led to an IDO-driven resistance to in vitro T-cell lysis post thaw, but failed to improve upon meaningful rescue of shortened persistence in vivo following intravenous transfusion. The latter data suggests that cytoskeletal anomalies on their own may be dominant in shortening MSC lifespan in vivo as we have previously shown. The tissue tropism of intravenously transfused MSCs requires adhesion molecule mediated arrest within the vasculature of respective tissue and eventual extravasation[62]. We have previously shown that human MSCs express cell surface adhesive molecules, including: α4, α5, β1 integrin components and CD63. Although their role in tissue homing is unclear, thawing does not lead to reduced expression on cell surface[16]. In addition, IFNγ prelicensing does not modulate expression of these molecules except for increase of ICAM-1 and VCAM-1 (Supporting information 5)[35] and these may play a role in IFNγ-driven immune suppression in vitro [35, 62], however their role in altering MSC distribution and engraftment remain to be identified. Whether IFNγ activated frozen-thawed MSCs lead to improved pharmaceutical effect in disease models remains to be determined.
As an aggregate, these data demonstrate that thawed MSCs deploy an altered physiology, which as a whole mitigates their potency as a cell pharmaceutical. We demonstrate that frozen-thawed MSCs are susceptible to lysis by activated T cells, allogeneic more so than autologous, which supports the hypothesis that fitness of MSCs at time of infusion in human subjects may affect their life span and potency. This phenomenon is reversible since culture rescue of frozen-thawed MSCs fully regain their fitness and resist bystander lysis by activated T-cells. Moreover, culture rescue for as little as 24 hours fully restores immune modulatory and biodistributive properties of MSCs as well. MSC's pharmacological effects in vivo are likely transient considering the lack of sustained engraftment, as they neither replicate in vivo nor form ectopic tissue[63]. This latter observation suggest that akin to prevalent pre-clinical animal data, that the use of metabolically fit human MSCs may allow for deployment of their full therapeutic potential as part of clinical trials thereby enhancing a positive bias towards validation of efficacy. These observations also provide some mechanistic rationale towards developing remedies, which mitigate the defects transiently acquired post thaw.
Supplementary Material
Acknowledgments
We would like to thank Shala Yuan for technical assistance and the Emory Personalized Immunotherapy Center (EPIC) for providing human MSCs and platelet lysate reagents. The study was supported by a Georgia Cancer Coalition Award to JG. Part of this research was performed as a project for the Immune Tolerance Network (NIH/NIAID Contract # N01 AI15416). This research project was also supported in part by the Emory University Integrated Cellular Imaging Microscopy Core of the Winship Cancer Institute comprehensive cancer center grant, P30CA138292.
Footnotes
Author Contributions: R.C. designed the research plan, performed most experiments, analyzed results and wrote the manuscript. I.B.C Prepared MSCs from multiple donors and provided Platelet lysate. M.G. Prepared cryopreservation formulations and performed cryobanking of MSCs. C.P and E.W. helped with luciferase imaging. C.L. Performed western blot for IDO. A.K. provided scientific guidance and editorial input. J.G. designed the research plan, analyzed results and wrote the manuscript.
References
- 1.Galipeau J, Krampera M. The challenge of defining mesenchymal stromal cell potency assays and their potential use as release criteria. Cytotherapy. 2015;17:125–127. doi: 10.1016/j.jcyt.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013;13:856–867. doi: 10.2174/1566524011313050016. [DOI] [PubMed] [Google Scholar]
- 3.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 6.Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kallekleiv M, Larun L, Bruserud O, et al. Co-transplantation of multipotent mesenchymal stromal cells in allogeneic hematopoietic stem cell transplantation: A systematic review and meta-analysis. Cytotherapy. 2016;18:172–185. doi: 10.1016/j.jcyt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Luk F, de Witte SF, Bramer WM, et al. Efficacy of immunotherapy with mesenchymal stem cells in man: a systematic review. Expert Rev Clin Immunol. 2015;11:617–636. doi: 10.1586/1744666X.2015.1029458. [DOI] [PubMed] [Google Scholar]
- 9.Galipeau J. The mesenchymal stromal cells dilemma-does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Galipeau J, Krampera M, Barrett J, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18:151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikebe C, Suzuki K. Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols. BioMed research international. 2014;2014:951512. doi: 10.1155/2014/951512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollock K, Sumstad D, Kadidlo D, et al. Clinical mesenchymal stromal cell products undergo functional changes in response to freezing. Cytotherapy. 2015;17:38–45. doi: 10.1016/j.jcyt.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, et al. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology. 2015;71:181–197. doi: 10.1016/j.cryobiol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Moll G, Alm JJ, Davies LC, et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32:2430–2442. doi: 10.1002/stem.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francois M, Copland IB, Yuan S, et al. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy. 2012;14:147–152. doi: 10.3109/14653249.2011.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinnadurai R, Garcia MA, Sakurai Y, et al. Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem cell reports. 2014;3:60–72. doi: 10.1016/j.stemcr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Copland IB, Garcia MA, Waller EK, et al. The effect of platelet lysate fibrinogen on the functionality of MSCs in immunotherapy. Biomaterials. 2013;34:7840–7850. doi: 10.1016/j.biomaterials.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 18.Matsumura K, Hyon SH. Polyampholytes as low toxic efficient cryoprotective agents with antifreeze protein properties. Biomaterials. 2009;30:4842–4849. doi: 10.1016/j.biomaterials.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Herman A, Kappler JW, Marrack P, et al. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 20.Chinnadurai R, Copland IB, Patel SR, et al. IDO-independent suppression of T cell effector function by IFN-gamma-licensed human mesenchymal stromal cells. J Immunol. 2014;192:1491–1501. doi: 10.4049/jimmunol.1301828. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 22.Choi CW, Kim BS, Seo JH, et al. Long-term engraftment stability of peripheral blood stem cells cryopreserved using the dump-freezing method in a -80 degrees C mechanical freezer with 10% dimethyl sulfoxide. International journal of hematology. 2001;73:245–250. doi: 10.1007/BF02981945. [DOI] [PubMed] [Google Scholar]
- 23.Galmes A, Gutierrez A, Sampol A, et al. Long-term hematological reconstitution and clinical evaluation of autologous peripheral blood stem cell transplantation after cryopreservation of cells with 5% and 10% dimethylsulfoxide at -80 degrees C in a mechanical freezer. Haematologica. 2007;92:986–989. doi: 10.3324/haematol.11060. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Oteyza J, Bornstein R, Corral M, et al. Controlled-rate versus uncontrolled-rate cryopreservation of peripheral blood progenitor cells: a prospective multicenter study. Group for Cryobiology and Biology of Bone Marrow Transplantation (CBTMO), Spain. Haematologica. 1998;83:1001–1005. [PubMed] [Google Scholar]
- 25.Griffiths S, Baraniak PR, Copland IB, et al. Human platelet lysate stimulates high-passage and senescent human multipotent mesenchymal stromal cell growth and rejuvenation in vitro. Cytotherapy. 2013;15:1469–1483. doi: 10.1016/j.jcyt.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Packard BZ, Telford WG, Komoriya A, et al. Granzyme B activity in target cells detects attack by cytotoxic lymphocytes. J Immunol. 2007;179:3812–3820. doi: 10.4049/jimmunol.179.6.3812. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Chahroudi A, Silvestri G, et al. Visualization and quantification of T cell-mediated cytotoxicity using cell-permeable fluorogenic caspase substrates. Nat Med. 2002;8:185–189. doi: 10.1038/nm0202-185. [DOI] [PubMed] [Google Scholar]
- 28.Duijvestein M, Wildenberg ME, Welling MM, et al. Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549–1558. doi: 10.1002/stem.698. [DOI] [PubMed] [Google Scholar]
- 29.Rafei M, Birman E, Forner K, et al. Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol Ther. 2009;17:1799–1803. doi: 10.1038/mt.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinnadurai R, Copland IB, Ng S, et al. Mesenchymal Stromal Cells Derived From Crohn's Patients Deploy Indoleamine 2,3-dioxygenase-mediated Immune Suppression, Independent of Autophagy. Mol Ther. 2015;23:1248–1261. doi: 10.1038/mt.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods in cell biology. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura R, Baker J, Beilhack A, et al. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood. 2008;112:2563–2574. doi: 10.1182/blood-2007-06-092817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.English K, Ryan JM, Tobin L, et al. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren G, Zhao X, Zhang L, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathias LJ, Khong SM, Spyroglou L, et al. Alveolar macrophages are critical for the inhibition of allergic asthma by mesenchymal stromal cells. J Immunol. 2013;191:5914–5924. doi: 10.4049/jimmunol.1300667. [DOI] [PubMed] [Google Scholar]
- 39.Ko JH, Lee HJ, Jeong HJ, et al. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc Natl Acad Sci U S A. 2016;113:158–163. doi: 10.1073/pnas.1522905113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giugliarelli A, Sassi P, Urbanelli L, et al. Cryopreservation of cells: FT-IR monitoring of lipid membrane at freeze-thaw cycles. Biophysical chemistry. 2016;208:34–39. doi: 10.1016/j.bpc.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Reardon AJ, Elliott JA, McGann LE. Investigating membrane and mitochondrial cryobiological responses of HUVEC using interrupted cooling protocols. Cryobiology. 2015;71:306–317. doi: 10.1016/j.cryobiol.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Raynel S, Padula MP, Marks DC, et al. Cryopreservation alters the membrane and cytoskeletal protein profile of platelet microparticles. Transfusion. 2015;55:2422–2432. doi: 10.1111/trf.13165. [DOI] [PubMed] [Google Scholar]
- 43.Alekseenko LL, Zemelko VI, Domnina AP, et al. Sublethal heat shock induces premature senescence rather than apoptosis in human mesenchymal stem cells. Cell stress & chaperones. 2014;19:355–366. doi: 10.1007/s12192-013-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francois M, Galipeau J. New insights on translational development of mesenchymal stromal cells for suppressor therapy. J Cell Physiol. 2012;227:3535–3538. doi: 10.1002/jcp.24081. [DOI] [PubMed] [Google Scholar]
- 45.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 46.Polchert D, Sobinsky J, Douglas G, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruz FF, Borg ZD, Goodwin M, et al. Freshly thawed and continuously cultured human bone marrow-derived mesenchymal stromal cells comparably ameliorate allergic airways inflammation in immunocompetent mice. Stem Cells Transl Med. 2015;4:615–624. doi: 10.5966/sctm.2014-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luetzkendorf J, Nerger K, Hering J, et al. Cryopreservation does not alter main characteristics of Good Manufacturing Process-grade human multipotent mesenchymal stromal cells including immunomodulating potential and lack of malignant transformation. Cytotherapy. 2015;17:186–198. doi: 10.1016/j.jcyt.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Castelo-Branco MT, Soares ID, Lopes DV, et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7:e33360. doi: 10.1371/journal.pone.0033360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moll G, Hult A, von Bahr L, et al. Do ABO blood group antigens hamper the therapeutic efficacy of mesenchymal stromal cells? PLoS One. 2014;9:e85040. doi: 10.1371/journal.pone.0085040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lasky LC, Van Buren N, Weisdorf DJ, et al. Successful allogeneic cryopreserved marrow transplantation. Transfusion. 1989;29:182–184. doi: 10.1046/j.1537-2995.1989.29289146840.x. [DOI] [PubMed] [Google Scholar]
- 52.Stiff PJ, Koester AR, Weidner MK, et al. Autologous bone marrow transplantation using unfractionated cells cryopreserved in dimethylsulfoxide and hydroxyethyl starch without controlled-rate freezing. Blood. 1987;70:974–978. [PubMed] [Google Scholar]
- 53.Heal JM, Brightman A. Cryopreservation of hematopoietic progenitor cells collected by hemapheresis. Transfusion. 1987;27:19–22. doi: 10.1046/j.1537-2995.1987.27187121465.x. [DOI] [PubMed] [Google Scholar]
- 54.Galvao J, Davis B, Tilley M, et al. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:1317–1330. doi: 10.1096/fj.13-235440. [DOI] [PubMed] [Google Scholar]
- 55.Forbes GM, Sturm MJ, Leong RW, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 56.Li Q, Wang Y, Deng Z. Pre-conditioned mesenchymal stem cells: a better way for cell-based therapy. Stem Cell Res Ther. 2013;4:63. doi: 10.1186/scrt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pravdyuk AI, Petrenko YA, Fuller BJ, et al. Cryopreservation of alginate encapsulated mesenchymal stromal cells. Cryobiology. 2013;66:215–222. doi: 10.1016/j.cryobiol.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Lee SY, Huang GW, Shiung JN, et al. Magnetic cryopreservation for dental pulp stem cells. Cells Tissues Organs. 2012;196:23–33. doi: 10.1159/000331247. [DOI] [PubMed] [Google Scholar]
- 59.Haack-Sorensen M, Kastrup J. Cryopreservation and revival of mesenchymal stromal cells. Methods Mol Biol. 2011;698:161–174. doi: 10.1007/978-1-60761-999-4_13. [DOI] [PubMed] [Google Scholar]
- 60.Heng BC, Clement MV, Cao T. Caspase inhibitor Z-VAD-FMK enhances the freeze-thaw survival rate of human embryonic stem cells. Bioscience reports. 2007;27:257–264. doi: 10.1007/s10540-007-9051-2. [DOI] [PubMed] [Google Scholar]
- 61.Bissoyi A, Pramanik K. Role of the apoptosis pathway in cryopreservation-induced cell death in mesenchymal stem cells derived from umbilical cord blood. Biopreservation and biobanking. 2014;12:246–254. doi: 10.1089/bio.2014.0005. [DOI] [PubMed] [Google Scholar]
- 62.Ren G, Roberts AI, Shi Y. Adhesion molecules: key players in Mesenchymal stem cell-mediated immunosuppression. Cell adhesion & migration. 2011;5:20–22. doi: 10.4161/cam.5.1.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.