Abstract
Essential tremor (ET) patients have abnormal climbing fiber (CF) synapses in the parallel fiber territory in the cerebellum, and these abnormal CF synapses are inversely correlated with tremor severity. We therefore examined CF synaptic pathology in ET cases with and without thalamic DBS and assessed the association with tremor severity. We found that CF synaptic pathology was inversely correlated with tremor severity in ET cases without DBS, and this correlation disappeared in ET cases with DBS. Our data suggest that DBS might have effects in modulating excitatory synapses in ET cerebellum, in addition to its symptomatic effects on tremor.
Keywords: essential tremor, climbing fiber, deep brain stimulation
Introduction
Climbing fibers (CFs) and parallel fibers (PFs) are two excitatory inputs to Purkinje cells (PCs), and the proper distribution of these two types of excitatory synapses on PC dendrites is critical for the regulation of PC physiology. We recently observed an increase in CF synapses on the thin, distal PC dendritic branchlets in ET vs. control cerebella (i.e., the abnormal presence of CF extension into the PF-PC synaptic territory).1 Interestingly, the percentage of CF-PC synapses on thin PC dendritic branchlets was inversely correlated with tremor severity,1,2 suggesting that these abnormal CF-PC synapses may be linked in some way to the mechanism of tremor.
Thalamic deep brain stimulation (DBS) is an effective surgical procedure for ET and has become an important treatment option. Despite this, its mechanisms of action are not fully understood. One theoretic possibility is that DBS modulates synaptic plasticity in ET. In general, CF-PC synapses are highly plastic and are dynamically regulated in response to their synaptic activity;3,4 therefore, it is conceivable that DBS could disrupt abnormal brain oscillatory networks and affect the organization of CF-PC synapses in ET. In the current study, we evaluated whether the inverse correlation between tremor severity and abnormal CF-PC synapses, recently observed in ET cases without DBS treatment1,2 (see above), would be altered in age-matched ET cases who had undergone DBS treatment.
Methods
We selected ET cases with data on quantitative measurement of tremor severity (see below). We first chose brains of 13 available ET cases who had undergone thalamic DBS placement and, as 1:2 matching is more powerful than 1:1 matching, we chose 26 age-matched ET brains without DBS placement from the New York Brain Bank (1:2 matching). As described in detail, the clinical diagnosis of ET was initially assigned by treating neurologists, and then confirmed by an Essential Tremor Centralized Brain Repository (ETCBR) study neurologist (EDL) using medical records, a detailed, videotaped, neurological examination, and ETCBR diagnostic criteria.5 None of these cases had a history of traumatic brain injury or heavy ethanol use, as previously defined.6 We excluded ET cases with diagnoses of dystonia or Parkinson’s disease. All subjects signed informed consent of the Institutional Review Board of Columbia University and Yale University.
The severity of action tremor in the arms and hands was quantified using a valid clinical rating scale (total tremor score [TTS] (range 0-36)) on the videotaped examination,2 which generally occurred within six months of death. In DBS cases, TTS was determined when the DBS stimulator was turned off. For the cases with unilateral DBS, we utilized tissue from the cerebellar hemisphere contralateral to thalamic DBS placement.
All cases underwent a standard neuropathological assessment as detailed previously,5 including ratings of neurofibrillary tangles using Braak and Braak staging (Braak AD stage),7 and Consortium to Establish a Registry for Alzhemier’s disease rating for neuritic plaques (CERAD),8 because some ET cases have Alzheimer’s disease-related pathology.9 We did not include ET cases with Lewy bodies10 or pathological changes of progressive supranuclear palsy.11
A standard 3 × 20 × 25 mm parasagittal neocerebellar block was obtained, which contained the anterior and posterior quadrangulate lobules of cerebellar cortex and underlying dentate nucleus.1 Paraffin-embedded cerebellar sections (7 μm) were labeled with dual immunofluorescence for vesicular glutamate transporter type 2 (VGlut2) and calbindin to visualize CF-PC synapses. In each case, we randomly chose 5 PCs using computer-generated random digits and then imaged the complete PC dendritic arbor from the base of the molecular layer to the pial surface. We quantified 1) the total VGlut2 puncta on a gjven PC dendritic arbor and 2) VGlut2 puncta on PC dendritic branchlets < 1μm in diameter (i.e., abnormal CF-PC synapses that distributed to the PF-PC synaptic territory), and we divided the number of VGlut2 puncta on PC dendritic branchlets < 1μm in diameter by the total number of VGlut2 puncta to obtain the percentage of VGlut2 puncta on thin PC branchlets (%CFPC1).1, 2 (Figure 1A). PC counts and PC axonal torpedo counts were performed as previously described.10 We used chi-square tests and t-tests to compare clinical and pathological features between DBS and non-DBS cases. We assessed the association between TTS and %CFPC1 using Pearson’s correlation coefficients. Our previous study demonstrated that CF pathology could be affected by gender and voice tremor;2 therefore, in a linear regression model using %CFPC1 as the outcome variable, we adjusted for gender, voice tremor, as well as age, DBS, TTS, and DBS × TTS as independent variables. Since ET cases with tremor onset more than 70 years old might represent a different subgroup,12,13 we performed an additional linear regression analysis excluding these cases (n = 3 in non-DBS cases only).
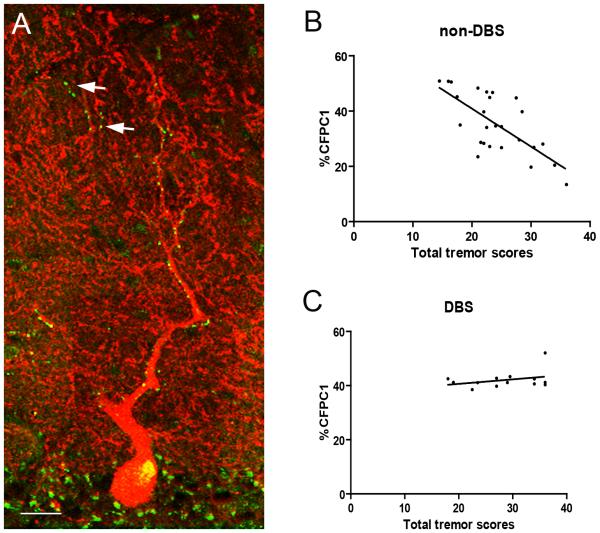
Figure 1.
Deep brain stimulation (DBS) and climbing fiber – Purkinje cell (CF-PC) pathology in essential tremor (ET). (A) Dual immunofluorescence with anti-VGlut2 (Alexa 488, green) and anti-calbindin D28k antibody (Alexa 594, red) of a 7 μm thick cerebellar section in an ET case. Each PC dendritic arbor was imaged, from the PC layer to the pial surface, and reconstructed in Image J (National Institutes of Health, Bethesda). VGlut2 puncta followed the CFs and were distributed over proximal, thick PC dendrites and occasionally VGlut2 puncta localized over the distal, thin PC branchlets (arrows) Scale bar: 25μm. (B) The percentage of VGlut2 puncta on PC branchlets < 1μm (%CFPC1) inversely correlated with the total tremor score in ET cases without DBS. (C) %CFPC1 was not correlated with the total tremor score in ET cases with DBS.
Results
ET cases with DBS and without DBS had a similar age of death, gender, disease duration, brain weight, postmortem interval, Braak AD stage and CERAD plaque score. PC counts and PC axonal torpedoes were also similar between these two groups. ET cases with DBS had a significantly higher %CFPC1 than ET cases without DBS (Table 1).
Table 1.
Clinical and pathological features in 26 ET cases without DBS vs. 13 ET cases with DBS
| non-DBS | DBS | p-value | |
|---|---|---|---|
| N | 26 | 13 | |
| Age at death (yrs) | 86.08 ± 6.63 | 85.08 ± 6.36 | 0.6551 |
| Disease duration (yrs) | 44.88 ± 22.29 | 44.46 ± 25.68 | 0.9591 |
| Gender | 0.7252 | ||
| Male | 8 (30.8%) | 5 (38.5%) | |
| Female | 18 (69.2%) | 8 (61.5%) | |
| TTS | 24.04 ± 5.53 | 28.58 ± 6.43 | 0.0281 |
| Voice tremor | 0.0052 | ||
| No | 19 (73.1%) | 3 (23.1%) | |
| Yes | 7 (26.9%) | 10 (76.9%) | |
| Brain weight (g) | 1189.40 ± 171.76 | 1228.18 ± 122.49 | 0.4731 |
| Postmortem interval (h) | 181.71 ± 197.22 | 190.22 ± 136.14 | 0.8521 |
| Braak AD stage | 0.6572 | ||
| 0 | 2 (7.7%) | 0 (0.0%) | |
| I | 7 (26.9%) | 2 (15.4%) | |
| II | 8 (30.8%) | 3 (11.5%) | |
| III | 5 (19.2%) | 5 (19.2%) | |
| IV | 3 (11.5%) | 2 (7.7%) | |
| V | 0 (0.0 %) | 0 (0.0%) | |
| VI | 1 (3.8 %) | 1 (3.8%) | |
| CERAD plaque score | 0.4262 | ||
| 0 | 12 (46.2%) | 4 (30.8%) | |
| A | 5 (19.2%) | 5 (38.5%) | |
| B | 4 (15.4%) | 3 (23.1%) | |
| C | 5 (19.2%) | 1 (7.7%) | |
| %CFPC1 | 35.35 ± 10.85 | 42.07 ± 3.28 | 0.0071 |
| Purkinje axonal torpedoes | 14.31 ± 10.38 [median = 13.00 ] |
24.92 ± 22.03 [median = 18.00] |
0.1353 |
| Purkinje cell count* | 8.44 ± 1.58 | 8.69 ± 1.38 | 0.6201 |
Values represent mean ± standard deviation [median] or number (percentage).
%CFPC1: the percentage of climbing fiber-Purkinje cell synapses on Purkinje cell (PC) dendrites of < 1μm thickness. % CFPC1 was calculated by dividing the number of VGlut2 puncta on PC dendritic branchlets < 1μm in diameter by the total number of VGlut2 puncta on a given PC dendritic arbor.
Postmortem interval: hours between death and placement of brain in a cold room or upon ice.
AD = Alzheimer’s disease; CERAD = the Consortium to establish a Registry for Alzheimer’s disease; g = grams; h = hours; TTS = total tremor score; yrs: years
Mean number of Purkinje cells (PCs) per 100x microscopic field, among 15 sampled fields.
Independent sample t-test
Chi-square test
Mann-Whitney test
Our primary analysis was to evaluate whether the inverse correlation between tremor severity and abnormal CF-PC synapses, recently observed in ET cases without DBS treatment,1,2 would be altered in ET cases who had undergone DBS treatment. There was a robust, inverse correlation between TTS and %CFPC1 in non-DBS cases (r = −0.70, p < 0.001) (Figure 1B). By contrast, TTS and %CFPC1 were not correlated in DBS cases (r = 0.33, p = 0.28) (Figure 1C), and the %CFPC1 remained at a similar level as seen in mild non-DBS ET cases. In a linear regression model adjusting for age and gender, we found a significant interaction between TTS and DBS (beta = 17.71, p < 0.001) that could account for the variability of %CFPC1. When we additionally added voice tremor to this model, the interaction between TTS and DBS remained similar (beta = 13.41, p = 0.001). The interaction still remained strong even when we excluded ET cases with tremor onset ≥ 70 years (beta = 14.62, p = 0.001). These results suggested that the association between CF pathology and tremor severity differs in ET cases with and without DBS.
Discussion
We found that in ET cases without DBS, the abnormal CF-PC synaptic number was robustly and inversely associated with tremor severity whereas in ET cases who had undergone DBS, this association was not observed. Our findings raise the possibility that DBS might result in underlying brain changes in ET.
The increase in distal CF-PC synapses in ET indicates a disturbed balance in the normal competitive activity-based mechanism between CF and PF excitatory transmission that maintains these distinct synaptic domains on PCs. Distal CF-PC synapses have been observed in the context of PC degeneration in animal models.4 In mild ET (i.e. low TTS), CF-PC distal synaptic contacts are increased; however, as ET progresses and tremor becomes more severe, there is distal pruning of PC spines and dendrites,14 which may lead to a gradual decrease in the distal distribution of CF-PC synapses. By contrast, as we show here, CFs remained in distal synaptic locations in ET cases treated with DBS, even with more severe tremor, and at a similar extent as seen in mild non-DBS ET cases. Based on our disease progression model in non-DBS cases, this raises the interesting possibility that DBS may favorably reduce the PC dendritic pruning process in ET. The interaction of CF synaptic organization and PC dendritic pruning in the presence of DBS is likely to be very complex. Furthermore, the mechanism by which tremor is treated with DBS remains obscure.
The persistence of distal CF synapses in ET DBS cases could also reflect a direct role of DBS to alter synaptic activity in cerebellum. The thalamus has been proposed as a brain region that passively receives the oscillatory rhythm propagated from the cerebellum in ET,15 and thalamic DBS might suppress tremor by disrupting this oscillatory rhythm propagated to the motor cortex. However, mounting evidence has indicated that thalamic DBS, in both animal models and ET patients, can also cause synaptic plasticity locally and network re-organization in brain areas connected to the stimulated sites, including cerebellum.16-18 Additionally, a unique aspect of CF synaptic transmission is a strong and synchronous release of glutamate at CF-PC synapses that can put PCs at risk for excitotoxic damage,19 and the presence of distal CF-PC synapses in ET may then further potentiate dendritic damage. Thus, if thalamic DBS reduces abnormal oscillatory activity in CFs, this may protect PCs from excitotoxic damage and aid to improve PC physiology.
The strength of our study is that we utilized clinically and pathologically well-characterized ET cases from a single brain bank with a uniform tissue processing protocol, and we assessed CF-PC pathology in the same region of the cerebellum in all cases. The weakness in our study is that we do not have detailed information on the DBS setting (i.e. intermittent vs. continuous stimulation) to assess the effects of different stimulation paradigms on CF-PC synaptic pathology. In addition, a rebound increase in tremor severity can be seen in ET cases when DBS is shut off acutely;20 therefore, TTS in DBS patients might not reflect the true baseline tremor severity in ET cases with DBS. Nonetheless, we expect that rebound phenomenon would be similar in each ET-DBS case; therefore, the association between of TTS and CF pathology might not be dramatically altered in our study. Finally, we do not have information on the severity of tremor in ET-DBS cases whose DBS was turned on; this would have allowed us to explore the association between the DBS responsiveness and CF pathology. Further studies on CF pathology of a larger sample size with different DBS stimulation settings and tremor severity will be necessary to advance our understanding of the functional aspect of ET.
DBS has been proposed to alter the underlying synaptic re-organization in other movement disorders such as dyskinesia in Parkinson’s disease and dystonia.16,21 Along these lines, our study raises the possibility that thalamic DBS in ET, in addition to its tremor suppressing effects, might directly affect cerebellar synaptic plasticity. The complex and dynamic interplay between CF-PC synaptic pathology, tremor severity and DBS therapy requires further investigation.
Acknowledgements
We thank Dr Arnulf H. Koeppen in Veterans Affairs Medical Center, Albany, New York, USA who provided the rabbit polyclonal anti-VGluT2 antibody. Dr. Kuo has received funding from NINDS K08 NS08738 (principal investigator), Louis V. Gerstner Jr. Scholar Award, Parkinson’s Disease Foundation, American Brain Foundation Research Fellowship, American Parkinson’s Disease Association, International Essential Tremor Foundation, NIEHS pilot award ES009089, and the Smart Foundation. Dr. Wang has received funding from the Jiangsu Government Scholarship for Overseas Studies, Qing Lan Project supported by the Jiangsu Provincial Department of Education, China and the Key Discipline Development Project of Jiangsu Province, China: JX10617801 (principal investigator). Dr. Louis has received research support from the National Institutes of Health: NINDS R01 NS042859 (principal investigator), NINDS R01 NS39422 (principal investigator), NINDS R01 NS086736 (principal investigator), NINDS R01 NS073872 (principal investigator), NINDS R01 NS085136 (principal investigator) and NINDS R01 NS088257 (principal investigator). Dr. Faust has received funding from NINDS NINDS R01 NS042859 (co-investigator), NINDS R01 NS085136 (co-investigator) and NINDS R01 NS088257 (co-investigator).
Footnotes
Author Contributions
Conception and design of the study: SHK, EDL, PLF
Acquisition and analysis of data: SHK, CYL, JW, JYL, MKP, RJL, WPW, JG,
Drafting the text or preparing the figures: SHK, EDL, PLF
Potential Conflicts of Interest
All authors report no disclosures
References
- 1.Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain. 2014;137:3149–3159. doi: 10.1093/brain/awu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis RJ, Lin CY, Faust PL, Koeppen AH, Kuo SH. Climbing fiber synaptic changes correlate with clinical features in essential tremor. Neurology. 84:10. doi: 10.1212/WNL.0000000000001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cesa R, Scelfo B, Strata P. Activity-dependent presynaptic and postsynaptic structural plasticity in the mature cerebellum. J Neurosci. 2007;27:4603–4611. doi: 10.1523/JNEUROSCI.5617-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cesa R, Strata P. Axonal and synaptic remodeling in the mature cerebellar cortex. Prog Brain Res. 2005;148:45–56. doi: 10.1016/S0079-6123(04)48005-4. [DOI] [PubMed] [Google Scholar]
- 5.Babij R, Lee M, Cortes E, Vonsattel JPG, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–3061. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25:228–235. [PubMed] [Google Scholar]
- 7.Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18:S85–8. doi: 10.1016/s0197-4580(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 8.Mirra SS. The CERAD neuropathology protocol and consensus recommendations1 for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging. 1997;18:S91–4. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 9.Pan JJ, Lee M, Honig LS, Vonsattel JP, Faust PL, Louis ED. Alzheimer's-related changes in non-demented essential tremor patients vs. controls: links between tau and tremor? Parkinsonism Relat Disord. 2014;20:655–8. doi: 10.1016/j.parkreldis.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 11.Louis ED, Babij R, Ma K, Cortés E, Vonsattel JP. Essential tremor followed by progressive supranuclear palsy: postmortem reports of 11 patients. J Neuropathol Exp Neurol. 2013;72:8–17. doi: 10.1097/NEN.0b013e31827ae56e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deuschl G, Petersen I, Lorenz D, Christensen K. Tremor in the elderly: Essential and aging-related tremor. Mov Disord. 2015;30:1327–1334. doi: 10.1002/mds.26265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muthuraman M, Deuschl G, Anwar AR, Mideksa KG, von Helmolt F, Schneider SA. Essential and aging-related tremor: Differences of central control. Mov Disord. 2015;30:1673–80. doi: 10.1002/mds.26410. [DOI] [PubMed] [Google Scholar]
- 14.Louis ED, Lee M, Babij R, et al. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain. 2014;137:3142–3148. doi: 10.1093/brain/awu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helmich RC, Toni I, Deuschl G, Bloem BR. The pathophysiology of essential tremor and Parkinson's tremor. Curr Neurol Neurosci Rep. 2013;13:378. doi: 10.1007/s11910-013-0378-8. doi: 10. [DOI] [PubMed] [Google Scholar]
- 16.Herrington TM, Cheng JJ, Eskandar EN. Mechanisms of deep brain stimulation. J Neurophysiol. 2016;115:19–38. doi: 10.1152/jn.00281.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paek SB, Min HK, Kim I, et al. Frequency-dependent functional neuromodulatory effects on the motor network by ventral lateral thalamic deep brain stimulation in swine. Neuroimage. 2015;105:181–8. doi: 10.1016/j.neuroimage.2014.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein JC, Barbe MT, Seifried C, et al. The tremor network targeted by successful VIM deep brain stimulation in humans. Neurology. 2012;78:787–95. doi: 10.1212/WNL.0b013e318249f702. [DOI] [PubMed] [Google Scholar]
- 19.Slemmer JE, De Zeeuw CI, Weber JT. Don't get too excited: mechanisms of glutamate-mediated Purkinje cell death. Prog Brain Res. 2005;148:367–90. doi: 10.1016/S0079-6123(04)48029-7. [DOI] [PubMed] [Google Scholar]
- 20.Patel N, Ondo W, Jimenez-Shahed J. Habituation and rebound to thalamic deep brain stimulation in long-term management of tremor associated with demyelinating neuropathy. Int J Neurosci. 2014;124:919–25. doi: 10.3109/00207454.2014.895345. [DOI] [PubMed] [Google Scholar]
- 21.Williams NR, Okun MS. Deep brain stimulation (DBS) at the interface of neurology and psychiatry. J Clin Invest. 2013;123:4546–56. doi: 10.1172/JCI68341. [DOI] [PMC free article] [PubMed] [Google Scholar]