Abstract
Impaired nitric oxide (NO) vasodilation (“endothelial dysfunction”) is associated with obesity and thought to be a factor in the development of hypertension. We previously found that NO synthesis inhibition had similar pressor effects in obese hypertensives compared to healthy control during autonomic blockade, suggesting that impaired NO vasodilation is secondary to sympathetic activation. We tested this hypothesis by determining the effect of autonomic blockade (trimethaphan 4 mg/min IV) on NO-mediated vasodilation (increase in forearm blood flow, FBF, to intrabrachial acetylcholine) compared to endothelial independent vasodilation (intrabrachial sodium nitroprusside) in obese hypertensive subjects (30<BMI<40 kg/m2). Acetylcholine and sodium nitroprusside were given at equipotent doses (10, 30 and 50 mcg/min and 1, 2 and 3 mcg/min, respectively) to 14 obese subjects (49±3.6 years, 34±1 kg/m2, 165/94 ± 7/6 mm Hg), on separate occasions one month apart, randomly assigned. Autonomic blockade increased basal FBF (from 3.9±0.7 to 5.2±1.2 ml/100mL/min, p=0.078). As expected, nitric oxide-mediated vasodilation was blunted on the intact day compared to NO-independent vasodilation; FBF increased from 3.6±0.6 to 10.1±1.1 with the highest dose of nitroprusside, but only from 3.7±0.4 to 7.2±0.8 ml/100mL/min with the highest dose of acetylcholine, p<0.05. In contrast, FBF responses to acetylcholine were restored by autonomic blockade, and were no longer different to nitroprusside (from 6.2±1.1 to 11.4±1.6 ml/100mL/min, and from 5.2±0.9 to 12.5±0.9 respectively, p= 0.58). Our results support the concept that sympathetic activation contributes to the impairment in NO-mediated vasodilation seen in obesity hypertension, and provides further rationale to explore it as a therapeutic target.
Keywords: autonomic nervous system, endothelial function, nitric oxide, hypertension, and obesity
There is increasing evidence supporting a role of the sympathetic nervous system in the development and maintenance of hypertension, and this is particularly true in obesity-associated hypertension. Not all obese subjects have increase sympathetic activity1, and in fact there are selected populations (Pima Indians) with high prevalence of obesity and low sympathetic activity as measured by muscle sympathetic nerve activity as compared to Caucasians2. Furthermore, it is also possible that in some cases of monogenic obesity, sympathetic activity might not be increased or even could be decreased as is the case in congenital leptin leptin deficiency 3. Nonetheless, the preponderance of evidence among white populations, in whom most of the studies have been done, supports the concept that sympathetic activation accompanies obesity-associated hypertension and that there is a selective increase in sympathetic activity regulating vasomotor tone. Even though regional sympathetic nerve activity might be heterogeneous in obesity, and whole body norepinephrine spillover is not increased in all states of obesity2, obese hypertensive subjects have been shown to have increased regional spillover to vascular beds that are relevant to hypertension, such as the kidneys, and the heart4, 5. Furthermore, microneurographic recordings of sympathetic nerve traffic innervating the skeletal muscle vasculature (MSNA), which is tightly coupled to baroreflex regulation, have consistently shown a strong positive relationship with body weight, body mass index or percentage body fat 6–8.
Local modulation of vascular tone also plays a role in blood pressure regulation. Among the factors involved, nitric oxide (NO) is arguably the most potent local vasodilator; we previously found that endogenous NO can restrain blood pressure by at least 30 mm Hg in normal subjects. Previous studies have consistently shown that obesity is associated with impaired nitric oxide (NO) vasodilatory function, using either intrarterial infusions of nitric oxide mediated vasodilators (acetylcholine) or responses to shear stress (flow mediated dilation) 9–12. Impaired NO vasodilatory function, therefore, is a prime suspect in the development of obesity-hypertension.
These observations raise the possibility for an interaction between sympathetic activity and NO vasodilatory function. In this regard, Hijmering et al. 13 found that sympathetic activation induced by lower body negative pressure impaired flow mediated dilation in healthy subjects. Similarly, Engelke showed that sympathetic activation induced by lower body negative pressure resulted in an acute impairment in NO-mediated dilation in response to intrabrachial infusion of acetylcholine in the forearm 14, also in healthy subjects. Furthermore, we previously found that NO synthesis inhibition with L-NMMA produced similar increases in blood pressure in obese hypertensives compared to healthy controls if autonomic function was blocked, whereas impaired NO-mediated dilation would have resulted in a lower pressor response, as was the case in smokers 15. The objective of the present study, therefore, was to test the hypothesis that sympathetic activity contributes to impaired NO-mediated vasodilation in obesity hypertension, so that obese hypertensive subjects would have decreased vasodilation in response to intra-arterial acetylcholine (endothelial-dependent vasodilator) infusion as compared to intra-arterial sodium nitroprusside (endothelial independent vasodilator), and that this impairment would disappear with ganglionic autonomic withdrawal with trimethaphan.
METHODS
Subjects
The study was reviewed and approved by the Vanderbilt University Institutional Review Board and written informed consent was obtained from each subject before initiating the study. The study was registered in ClinicalTrilas.gov prior to enrolling (ClinicalTrials.gov identifier: NCT01137253).
We enrolled obese hypertensive volunteers from the Vanderbilt University Clinical Research Center volunteer database and Research Match 16. Subjects 18 to 60 years old of either gender with a BMI between 30–40 kg/m2 were considered eligible for the study. Current smokers, subjects with diabetes, patients with clinically apparent coronary or peripheral vascular disease and pregnant women were excluded. All medications including antihypertensives were withdrawn at least 7 days before each study day with the exception of oral contraceptives. Subjects abstained from caffeine and other substances that are known to have an effect on the autonomic nervous system for ≥72 hours before testing. During the screening visit, all subjects underwent a clinical examination, ECG, urinalysis, and routine laboratory testing (i.e. blood count and chemistry).
Study Design
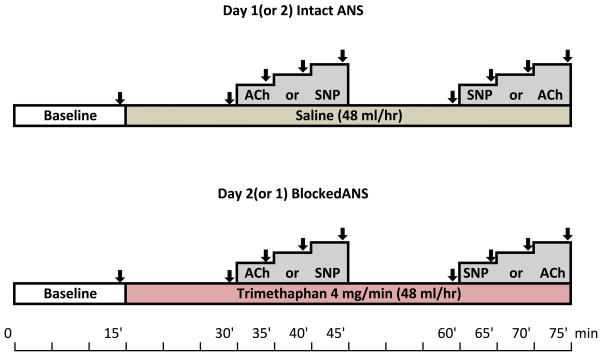
Forearm vasodilation was assessed on two separate days, one month apart using identical instrumentation, as previously described 17; once with intact autonomic function and once during autonomic blockade (Figure 1). Both the order of the study days and the infusions were randomized using a single-blind crossover design with a computer generated randomization code. Subjects were blinded as to which treatment (trimethaphan or saline) will be received; the order of the intra-arterial infusions (SNP or ACh) was randomized in both study days.
Figure 1. Study Design.
Subjects were randomized to either the intact day (saline) or the blocked day (trimethaphan). The order of the vasodilators given (Acetylcholine, ACh; or sodium nitroprusside, SNP) was also randomize. Subjects were blinded as to which treatments were receiving each day.
Instrumentation
For each study day, subjects were fasted and in the supine position. A catheter was inserted into the right antecubital vein for drug administration. An indwelling catheter was placed into the left brachial artery for intrarterial drug administration and connected through 3-way valves to a pressure transducer for continuous blood pressure measurements. Blood pressure was also measured in the finger using the volume clamp method (BMEYE, Nexfin, Edwards Lifesciences), and every five minutes with an automated oscillometric brachial cuff (Vital-Guard 450C, Ivy Biomedical Systems). Heart rate (HR) was determined from continuous ECG monitoring. Cardiovascular signals were digitized using a Windaq system (DA-220; DATAQ Instruments). Forearm blood flow (FBF) was measured using venous occlusion mercury-in silastic strain-gauge plethysmography 18 (Hokanson EC4, DE; Hokanson Inc).
Study Protocol
After instrumentation subjects were allowed to rest in a quiet room for 15 minutes to ensure that all cardiovascular parameters had returned to resting values before taking baseline measurements (Figure 1). The subjects then received an intravenous infusion of either saline (48 ml/hr, “intact study day”) or trimethaphan (4 mg/min, “autonomic blocked day”). Infusions were maintained throughout the study. We have shown previously that this dose induces complete autonomic blockade 19.
After 15 minutes a second set of measurements were taken, and then increasing dosages of the vasodilator acetylcholine, or sodium nitroprusside were administered intrabrachially in random order. Acetylcholine was infused at rates of 10, 30 and 50 μg/min. Nitroprusside was infused at rates of 1, 2, and 3 μg/min. The infusions were given in increasing doses for 5 minutes each, while monitoring the subject’s heart rate, and blood pressure. FBF was measured before infusions and during the last minute of each dose. Sufficient time was allowed between infusions to allow parameters to return toward baseline values.
Measurements
Subjects were warned ahead of time of all procedures done, and that they may produce discomfort, in order to avoid anticipatory reactions. To measure FBF the forearm was elevated to at least 10 cm above the level of the right atrium to ensure that the forearm veins were drained at the beginning of each flow measurement. The wrist cuff was inflated to 200 mm Hg 30 seconds before each series of forearm flow measurements to exclude blood flow through the hand. Four consecutive measurements were performed within one minute, and the last 3 measurements were averaged for the determination of the FBF for each study period. Mean arterial pressure (MAP) was calculated as SBP+2/3DBP and measured immediately after FBF measurements using intrarterial blood pressure recordings. Forearm vascular resistance (FVR) was calculated as MAP/FBF, whereas forearm vascular conductance (FVC) was calculated as FBF/MAP×100 and expressed as arbitrary units.
Statistical Analysis
Unless noted otherwise data are presented as mean±S.E.M. The main outcome selected at priori was endothelial function measured as the difference in the deltas in FBF in response to acetylcholine (endothelial dependent vasodilator) vs. sodium nitroprusside (endothelial independent vasodilator) with intact autonomic nervous system (treatment 1) or with the ANS blocked (treatment 2). Wilcoxon signed rank test was used to prove the null hypothesis that there will be no differences in the deltas in FBF between interventions (intact vs. blocked): H0:[ΔIntact−ΔBlocked]=0.
Where
ΔIntact=[ΔFBFAChIntact−ΔFBFSNPIntact]; ΔFBFAChIntact=FBFPeakAch−FBFSaline; ΔFBFSNPIntact=FBFPeakSNP−FBFSaline.
ΔBlocked=[ΔFBFAChBlocked−ΔFBFSNPBlocked]; ΔFBFACh=FBFPeakAch−FBFTrMT; ΔFBFSNP=FBFPeakSNP−FBFTrMT.
Sample sized was determined, using FBF in response to ACh from our previous studies. With a SD of 3.4 3.4 ml/100ml/min, during intrabrachial infusion of acetylcholine (30 μg/min), using a paired t test with a two-sided type I error probability of 0.05, it was determined that studying 14 subjects will have >95% power to detect a difference in FBF of 4 ml/100ml/min when comparing the before and after response to autonomic blockade. In addition, to adjust for gender and age, a random mixed-effects model was used to take into account correlations among repeated measures, to examine whether and to what extent the change in FBF differed by dose and drug (ACh or SNP) in each of the two study days (intact vs. blocked). All of the tests were 2-tailed, and a P value of <0.05 was considered significant. Analyses were performed with SPSS statistical software (Version 22.0.0, SPSS Inc.).
RESULTS
Demographics
We screened a total of 38 subjects. Twenty of them did not qualify because were either no longer hypertensive after two weeks of medication withdrawal or were taking more than three antihypertensive medications. Eighteen subjects qualified and were enrolled in the study, but 4 were not able to complete both study days study. Fourteen (eight male, 49±2.7 years old, table 1) obese (BMI, 34±0.9 kg/m2) hypertensive subjects completed both study days. As expected, the percentage of body fat (measured by DEXA scan) was high (40±1.4 %). Other parameters are shown in Table 1. Seven subjects were on antihypertensive medications and the others were medication-naive. Seated blood pressure was 131±3.6/83±3.1 mm Hg at screening day while on treatment, and 156±6.2/90±3.9 mm Hg after medication withdrawal of at least one week.
Table 1.
Demographic and baseline characteristics
| Parameters | |
|---|---|
| Gender, F/T (%) | 6/14 (42) |
| Age, years | 48.8 ± 2.5 |
| Weight, kg | 102.0 ± 3.9 |
| BMI, kg/m2 | 34.1 ± 0.9 |
| Body Fat, % | 40.2 ± 1.4 |
| Systolic BP, mm Hg | 156.6 ± 6.2 |
| Diastolic BP, mm Hg | 90.0 ± 3.9 |
| Heart rate, bpm | 69.6 ± 3.3 |
| Glucose, mg/dL | 92.9 ± 4.2 |
| Insulin, mU/dL | 14.3 ± 2.6 |
| Triglycerides, mg/dL | 125.1 ± 25.2 |
| Cholesterol, mg/dL | 174.6 ± 12.3 |
| HDL Cholesterol, mg/dL | 43.4 ± 3.8 |
| LDL Cholesterol, mg/dL | 104.0 ± 10.7 |
| Systolic BP off medications* | 155.5 ± 6.2 |
| Diastolic BP off medications* | 89.6 ± 3.9 |
Values are expressed as mean ± S.E.M and were obtained during the screening visit, except for those marked with an asterisk (*), which were obtained at baseline the first study day.
Study day results
At baseline there were no differences between study days in any of the variables measured (table 2). Supine blood pressure was 153±5/88±3 and 156±5/90±4 mm Hg in the intact and blocked days, respectively, and FBF was 2.8±0.3 and 3.2±0.4 mL/100mL/min, respectively.
Table 2.
Differences in hemodynamic parameters between study days and between baseline and during infusions.
| Parameters | Intact | Autonomic Blockade | p (saline-trimt) | ||
|---|---|---|---|---|---|
|
| |||||
| Baseline | Saline | Baseline | Trimethaphan | ||
| SBP, mm Hg | 153 ± 5.0 | 153 ± 5.2 | 156 ± 5.3 | 111 ± 4.50.003 | 0.003 |
| DBP, mm Hg | 88 ± 3.0 | 89 ± 3.2 | 90 ± 3.8 | 70 ± 2.80.003 | 0.003 |
| HR, bpm | 65 ± 3.6 | 62 ± 2.4 | 65 ± 3.5 | 78 ± 2.60.003 | 0.008 |
| CO, L/min | 6.2 ± 0.6 | 6.5 ± 0.6 | 6.6 ± 0.7 | 5.7 ± 0.4 | 0.779 |
| SV, mL | 94 ± 5.7 | 97 ± 5.6 | 99 ± 7.3 | 77 ± 3.80.016 | 0.05 |
| TPR, dyn×sec×cm−5 | 1571 ± 128 | 1503 ± 121 | 1567 ± 131 | 1255 ± 86.30.021 | 0.036 |
| MAP | 110 ± 3.3 | 111 ± 3.5 | 112 ± 4.0 | 84 ± 3.10.003 | 0.003 |
| FBF | 2.8 ± 0.3 | 3.2 ± 0.4 | 3.2 ± 0.4 | 5.4 ± 0.70.001 | 0.016 |
| FVR | 49.7 ± 7.4 | 42.5 ± 6.7 | 44.8 ± 6.8 | 20.0 ± 3.20.003 | 0.004 |
| FVC | 2.5 ± 0.3 | 2.9 ± 0.4 *0.041 | 2.6 ± 0.3 | 1.97 ± 0.80.003 | 0.004 |
Values are expressed as mean ± S.E.M. The “p (before-after)” column reflects differences between study days (intact vs. blocked) during the infusions (saline or trimethaphan)
p <0.05 for the comparison between baseline and infusion for each study day.
No significant differences were observed between study days at baseline.
Autonomic blockade lowered blood pressure, stroke volume, total peripheral resistance, and forearm vascular resistance and conductance, while increasing heart rate and forearm blood flow, not only at baseline but during intra-arterial infusions of both vasodilators (ACh and SNP), shifting upward the dose response curve (figure 2). Cardiac output was not different, neither between days or before and after autonomic blockade.
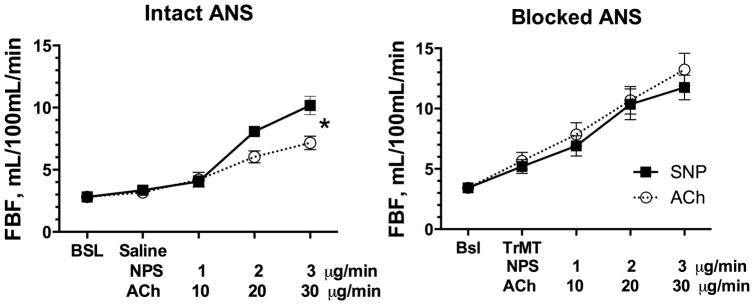
Figure 2.
Effect of increasing doses on sodium nitroprusside (SNP) or acetylcholine (Ach, x-axis) on forearm blood flow (FBF, y-axis) in obese hypertensive subjects studied on two separate occasions, during intravenous infusion of saline (intact autonomic nervous system, ANS, left panel) or the ganglionic blocker trimethaphan (TrMT, blocked ANS, right panel). FBF dilation to ACh was significantly decrease compared to SNP when the ANS was intact (*, p<0.05 by mixed effects model), but not if ANS was blocked by trimethaphan.
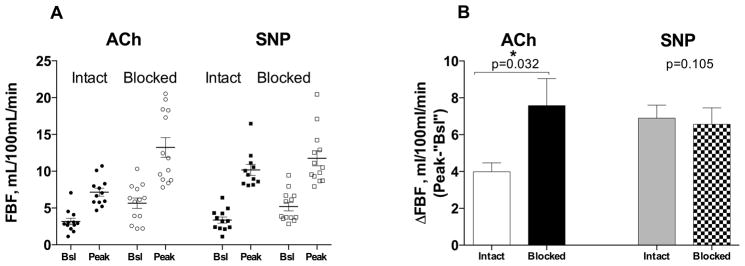
Neither intrabrachial infusions of acetylcholine or sodium nitroprusside had a statistically significant systemic vasodepressor effect (no dose, drug or interaction effect for MAP, table 3). It is worth noticing, however that during autonomic blockade, the highest dose of SNP tended to reduce MAP by 9 mm Hg, while ACh only reduced it by 3 mm Hg. As expected FBF increased with both drugs, but the vasodilatation induced by acetylcholine was blunted compared to sodium nitroprusside on the intact day (Figure 2, p dose=0.001, p drug<0.001, p drug×dose=0.016, by mixed effects model). This difference was no longer observed on the blocked day (p dose<0.001, p drug=0.318, p drug×dose=0.873, by mixed effects model). ACh 50 μg/min increased FBF from 3.17±0.44 to 7.16±0.54 ml/100mL/min during the intact (saline infusion) day, and from 5.66±0.71 to 13.24±1.35 ml/100mL/min during the blocked (trimethaphan infusion) day (Figure 3A). SNP 3 μg/min increased FBF from 3.36±0.42 to 10.17±0.74 ml/100mL/min during the intact day, and from 5.19±0.57 to 11.75±1.02 ml/100mL/min during the blocked (trimethaphan infusion) day (Figure 3A). The peak increase in FBF induced by these drugs was statistically significant between study days only for ACh (3.99±0.49, vs. 7.58±1.47 ml/100mL/min, for the intact vs. block days, p=0.032, Figure 3B). In contrast the differences in vasodilation induced by SNP was minimal between study days (6.90±0.70, vs. 6.56±0.89 ml/100mL/min, for the intact and blocked days, p=0.105). The difference in ΔFBF the intact day (ΔFBFIntact) between ACh (ΔFBFAChIntact) and SNP (ΔFBFSNPIntact) and the blocked day (ΔFBFBlocked) between ACh (ΔFBFAChBlocked) and SNP (ΔFBFSNPBlocked) was statistically significant (3.43±1.13 vs. −0.80±1.63 mL/100mL/min, for the intact and blocked days respectively, p=0.027 by Wilcox sign-rank test).
Table 3.
Differences between study days
| Parameters | Intact | Autonomic Blockade | ||
|---|---|---|---|---|
|
| ||||
| ACh | SNP | ACh | SNP | |
| MAP, mm Hg | ||||
| Dose 1 | 109 ± 4.4 | 108 ± 4.8 | 81 ± 3.1 | 83 ± 3.4 |
| Dose 2 | 107 ± 4.1 | 108 ± 4.5 | 81 ± 3.6 | 78 ± 4.1 |
| Dose 3 | 108 ± 3.8 | 106 ± 4.2 | 78 ± 3.2 | 74 ± 3.4 |
| P dose | 0.922 | 0.253 | ||
| P drug | 0.819 | 0.532 | ||
| P drug×dose | 0.998 | 0.644 | ||
| FBF, ml/100 mL | ||||
| Dose 1 | 4.2 ± 0.6 | 4.1 ± 0.5 | 7.9 ± 1.0 | 6.9 ± 0.8 |
| Dose 2 | 6.0 ± 0.5 | 8.1 ± 0.4 | 10.7 ± 1.2 | 10.4 ± 1.3 |
| Dose 3 | 7.2 ± 0.5 | 10.2 ± 0.7 | 13.2 ± 1.3 | 11.8 ± 1.0 |
| P dose | 0.001 | 0.000086 | ||
| P drug | 0.000 | 0.318 | ||
| P drug×dose | 0.016 | 0.873 | ||
| FVR, (MAP/FBF) | ||||
| Dose 1 | 36.6 ± 7.4 | 33.7 ± 5.4 | 13.2 ± 1.9 | 13.8 ± 1.2 |
| Dose 2 | 19.4 ± 1.5 | 13.5 ± 1.2 | 9.2 ± 1.2 | 8.6 ± 0.6 |
| Dose 3 | 16.5 ± 1.6 | 10.9 ± 0.8 | 6.9 ± 0.8 | 7.0 ± 0.6 |
| P dose | 0.000 | 0.000 | ||
| P drug | 0.117 | 0.985 | ||
| P drug×dose | 0.959 | 0.872 | ||
| FVC, AU | ||||
| Dose 1 | 3.8 ± 0.7 | 3.6 ± 0.5 | 9.3 ± 1.4 | 7.8 ± 0.7 |
| Dose 2 | 5.6 ± 0.5 | 7.8 ± 0.5 | 13.1 ± 1.9 | 12.4 ± 1.0 |
| Dose 3 | 6.6 ± 0.7 | 9.7 ± 0.8 | 16.9 ± 2.3 | 15.6 ± 1.6 |
| P dose | 0.000 | 0.000 | ||
| P drug | 0.001 | 0.346 | ||
| P drug×dose | 0.029 | 0.971 | ||
Values are expressed as mean ± S.E.M. The “p dose” reflects the effects of the different doses for both drugs, the “p drug” reflects the effects of each drug, the “p drug×dose” reflects the combined effect of both drug and the different doses.
Figure 3.
Panel A shows individual forearm blood flow (FBF) at baseline (Bsl), and the peak responses to acetylcholine (ACh, circles) and sodium nitroprusside (SNP, closed squares), during the intact (closed) and blocked (open) days. Panel B shows the change in FBF (peak minus saline or trimethaphan) for both ACh and SNP. The peak increase in FBF induced by these drugs was statistically significant between study days only for ACh (*, p<0.05 by Wilcoxon sign-rank test).
DISCUSSION
Our results suggest that acute removal of sympathetic nervous system activity in obese hypertensive subjects, results in reversal of endothelial dysfunction, as assessed by NO-mediated vasodilation induced by intrarterial infusion of acetylcholine. When obese hypertensive subjects were studied with an intact autonomic nervous system, forearm vasodilation to intrarterial acetylcholine was blunted compared to sodium nitroprusside, but this difference was no longer present when the autonomic nervous system was pharmacologically blocked.
These results are in agreement with previous studies showing that transient increases in sympathetic activity result in acute impairment of NO-mediated vasodilation function in healthy subjects 13, 14. We believe this study shows for the first time that sympathetically mediated vasoconstriction might be at least partly responsible for the apparent endothelial dysfunction seen in obesity associated hypertension. This is also in agreement with our previous observation that blockade of NO synthase with L-NMMA results in similar increases in blood pressure in normotensive and hypertensive individuals studied during autonomic blockade 20, whereas a primary NO impairment would results in a blunted increase in blood pressure in response to L-NMMA, as we observed in smokers 15. Taken together, these findings support the hypothesis that sympathetic activation, known to occur in obesity, tonically contributes to a reduction in NO function.
Ex vivo studies using arterioles from hypertensive and pre-hypertensive subjects have shown a normal response to NO-mediated vasodilators 21, 22. Deng et al, using precontracted small arteries dissected from gluteal subcutaneous fat biopsies, have shown that response to ACh was normal in pre-hypertensive subjects, Angus et al, have shown the same in resistance arteries taken from hypertensive subjects. Furthermore, Angus et al., found an increased reactivity to NE in these subjects. Taken together with our results, these observations suggest that early in the hypertensive disease process there is a functional state of endothelial dysfunction as a consequence of increased sympathetically mediated vasoconstriction, and that it can be reversed by sympathoinhibition. In this regard, it has been shown that transient increased in sympathetic activity achieved by lower body negative pressure, impairs both ACh and SNP vasodilation in healthy subjects, and that local sympatholysis, reverses this effect14. Engelke et al proposed that the sympathetic activity could restrain pharmacological vasodilation. Furthermore, it has been proposed, that physiological increases in sympathetic activity (aging), could also result in a reduction of the vasodilatory effect of intra-arterial ACh or SNP 23. The molecular mechanisms by which sympathetic activity impairs NO-mediated dilation cannot be inferred from our studies. It is possible that removal of sympathetic vasoconstriction with ganglionic blockade improves NO production by increasing sheer stress. The lack of improvement in endothelial-independent vasodilation with sodium nitroprusside (Figure 3B) demonstrates the selectivity of this effect and that it was not simply the result of a nonspecific increase in basal blood flow.
It is equally likely that as the disease progresses a structural endothelial dysfunction state develops as a result of vascular damage. We should note that we excluded patients with clinically apparent coronary or peripheral vascular disease. Our findings, therefore, in no way contradict the impact that atherosclerosis, inflammation, immunity, and oxidative stress have on endothelial dysfunction and hypertension. It should be noted that sympathetic activation could contribute to inflammation with formation of reactive oxygen species and consequent reduction in NO production 24. Sympathetic activity can also impair NO signaling by reducing eNOS activity 25.
Studies in autonomic failure patients have validated the specificity of trimethaphan actions through a autonomic blockade; patients with supine hypertension because of pure autonomic failure have exaggerated responses to most vasodilators 26–30 but not to trimethaphan 31 suggesting that the non-specific effects of high dose trimethaphan (e.g., histamine release) are not seen at the doses used in these studies32. The clinical relevance of our finding is that targeting sympathetic activity in the treatment of hypertension should result in improved NO function. An obvious limitation of our proof of concept study is that only acute effects were studied. It is encouraging, however, that uncontrolled studies suggest that chronic treatment with the central sympatholytic moxonidine appears to improve flow mediated dilation 33. The use of traditional central sympatholytics is limited by their side effect profile, and there has been little interest in development of new drugs that reduce sympathetic activity. There is, however, renewed interest in targeting sympathetic activity in hypertension. After disappointing initial results, a multicenter trial of renal denervation is under way (EnligHTN IV, clinicaltrials.gov identifier: NCT01903187), and novel experimental approaches are being developed such as reducing chemoreceptor input surgically (clinicaltrials.gov identifier: NCT01745172) or pharmacologically 34. Furthermore, we have recently shown that autonomic blockade improves insulin sensitivity in obese hypertensives likely through an increase in glucose uptake by the muscle 35. This effect could also be explained by an improvement in substrate availability resulting from insulin-mediated vasodilation, which is NO-dependent 36. This hypothesis requires validation, but regardless of the mechanism, it is possible that targeting sympathetic activation in obesity hypertension would have beneficial vascular (endothelial dysfunction) and metabolic (insulin resistance) effects.
In conclusion, we report that acute autonomic withdrawal results in apparent reversal of endothelial dysfunction, as evidence by improvement of acetylcholine-mediated dilation in obese hypertensives. These results are consistent with the concept that sympathetic activation contributes to impaired NO-mediated dilation in obesity, and we hope they add to the renewed interest in targeting sympathetic activation in the treatment of obesity hypertension.
Perspectives.
Endothelial dysfunction is one of the earliest markers for the development of arteriosclerosis and cardiovascular disease; it can be predictive of subsequent development of acute coronary syndrome. Increased sympathetic activity could elicit an endothelial dysfunction state, which we believe to be reversible. Identifying and therefore being able to prevent the progression of endothelial dysfunction to endothelial damage is of the utmost importance. The mechanism by which increased sympathetic activity induces endothelial dysfunction in obesity has not been elucidated, not fully studied. Here we propose the first approximation to a better understanding of the phenomena and propose that, an increased sympathetic activity is responsible early in the disease process for developing endothelial dysfunction.
Novelty and Significance.
What Is New?
Increased sympathetic activity, as seen in obesity-associated hypertension can be responsible early in the disease process for developing endothelial dysfunction. We are showing that endothelial dysfunction can be reversed during autonomic blockade
What Is Relevant?
Increased sympathetic activity could elicit an endothelial dysfunction state, which has the potential of being reversible by targeting the sympathetic nervous system. This in turn, will help prevent the progression of endothelial dysfunction to endothelial damage.
Summary
In this randomized crossover study, the effect of autonomic blockade on endothelial function have been studied. We have shown that acute pharmacological autonomic blockade, restores endothelial function in the forearm of obese hypertensive human subjects.
Acknowledgments
Sources of Support: This work was supported in part by National Institutes of Health grants Grant K23 HL-95905, P01 HL056693 and, UL1 RR024975 (Clinical and Translational Science Award).
Footnotes
Conflict(s) of Interest/Disclosure(s): None
References
- 1.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–776. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 2.Weyer C, Pratley RE, Snitker S, Spraul M, Ravussin E, Tataranni PA. Ethnic differences in insulinemia and sympathetic tone as links between obesity and blood pressure. HTN. 2000;36:531–537. doi: 10.1161/01.hyp.36.4.531. [DOI] [PubMed] [Google Scholar]
- 3.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: Multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 4.Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- 5.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 6.Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE, Anderson EA. Reduced sympathetic nervous activity. A potential mechanism predisposing to body weight gain. J Clin Invest. 1993;92:1730–1735. doi: 10.1172/JCI116760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tataranni PA, Cizza G, Snitker S, Gucciardo F, Lotsikas A, Chrousos GP, Ravussin E. Hypothalamic-pituitary-adrenal axis and sympathetic nervous system activities in pima indians and caucasians. Metabolism. 1999;48:395–399. doi: 10.1016/s0026-0495(99)90092-6. [DOI] [PubMed] [Google Scholar]
- 8.Vollenweider P, Randin D, Tappy L, Jequier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest. 1994;93:2365–2371. doi: 10.1172/JCI117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arcaro G, Zamboni M, Rossi L, Turcato E, Covi G, Armellini F, Bosello O, Lechi A. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int J Obes Relat Metab Disord. 1999;23:936–942. doi: 10.1038/sj.ijo.0801022. [DOI] [PubMed] [Google Scholar]
- 10.Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: Protective effect of vitamin c. Diabetes. 2001;50:159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams IL, Wheatcroft SB, Shah AM, Kearney MT. Obesity, atherosclerosis and the vascular endothelium: Mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord. 2002;26:754–764. doi: 10.1038/sj.ijo.0801995. [DOI] [PubMed] [Google Scholar]
- 13.Hijmering ML, Stroes ESG, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. Journal of the American College of Cardiology. 2002;39:683–688. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- 14.Engelke KA, Williams MM, Dietz NM, Joyner MJ. Does sympathetic activation blunt nitric oxide-mediated hyperemia in the human forearm? Clin Auton Res. 1997;7:85–91. doi: 10.1007/BF02267752. [DOI] [PubMed] [Google Scholar]
- 15.Gamboa A, Shibao C, Diedrich A, Choi L, Pohar B, Jordan J, Paranjape SY, Farley G, Biaggioni I. Contribution of endothelial nitric oxide to blood pressure in humans. HTN. 2007;49:170–177. doi: 10.1161/01.HYP.0000252425.06216.26. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Lane L, Biaggioni I. Clinical research subject recruitment: The volunteer for vanderbilt research program http://www.Volunteer.Mc.Vanderbilt.Edu. Journal of the American Medical Informatics Association : JAMIA. 2005;12:608–613. doi: 10.1197/jamia.M1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamboa A, Abraham R, Diedrich A, Shibao C, Paranjape SY, Farley G, Biaggioni I. Role of adenosine and nitric oxide on the mechanisms of action of dipyridamole. Stroke. 2005;36:2170–2175. doi: 10.1161/01.STR.0000179044.37760.9d. [DOI] [PubMed] [Google Scholar]
- 18.Gamboa A, Ertl AC, Costa F, Farley G, Manier ML, Hachey DL, Diedrich A, Biaggioni I. Blockade of nucleoside transport is required for delivery of intraarterial adenosine into the interstitium: Relevance to therapeutic preconditioning in humans. Circulation. 2003;108:2631–2635. doi: 10.1161/01.CIR.0000101927.70100.41. [DOI] [PubMed] [Google Scholar]
- 19.Diedrich A, Jordan J, Tank J, Shannon JR, Robertson R, Luft FC, Robertson D, Biaggioni I. The sympathetic nervous system in hypertension: Assessment by blood pressure variability and ganglionic blockade. J Hypertens. 2003;21:1677–1686. doi: 10.1097/00004872-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Gamboa A, Okamoto LE, Diedrich A, Choi L, Robertson D, Farley G, Paranjape S, Biaggioni I. Sympathetic activation and nitric oxide function in early hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1438–1443. doi: 10.1152/ajpheart.01020.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angus JA, Jennings GL, Sudhir K. Enhanced contraction to noradrenaline, serotonin and nerve stimulation but normal endothelium-derived relaxing factor response in skin small arteries in human primary hypertension. Clinical and Experimental Pharmacology & Physiology. Supplement. 1992;19:39–47. doi: 10.1111/j.1440-1681.1992.tb02809.x. [DOI] [PubMed] [Google Scholar]
- 22.Deng LY, Li JS, Schiffrin EL. Endothelium-dependent relaxation of small arteries from essential hypertensive patients: Mechanisms and comparison with normotensive subjects and with responses of vessels from spontaneously hypertensive rats. Clin Sci (Lond) 1995;88:611–622. doi: 10.1042/cs0880611. [DOI] [PubMed] [Google Scholar]
- 23.Hart EC, Wallin BG, Barnes JN, Joyner MJ, Charkoudian N. Sympathetic nerve activity and peripheral vasodilator capacity in young and older men. Am J Physiol Heart Circ Physiol. 2014;306:H904–909. doi: 10.1152/ajpheart.00181.2013. [DOI] [PubMed] [Google Scholar]
- 24.Andersson DC, Fauconnier J, Yamada T, Lacampagne A, Zhang SJ, Katz A, Westerblad H. Mitochondrial production of reactive oxygen species contributes to the beta-adrenergic stimulation of mouse cardiomycytes. J Physiol. 2011;589:1791–1801. doi: 10.1113/jphysiol.2010.202838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.da Cunha NV, Pinge-Filho P, Panis C, Silva BR, Pernomian L, Grando MD, Cecchini R, Bendhack LM, Martins-Pinge MC. Decreased endothelial nitric oxide, systemic oxidative stress, and increased sympathetic modulation contribute to hypertension in obese rats. American Journal of Physiology-Heart and Circulatory Physiology. 2014;306:H1472–1480. doi: 10.1152/ajpheart.00520.2013. [DOI] [PubMed] [Google Scholar]
- 26.Biaggioni I, Onrot J, Stewart CK, Robertson D. The potent pressor effect of phenylpropanolamine in patients with autonomic impairment. JAMA. 1987;258:236–239. [PubMed] [Google Scholar]
- 27.Gamboa A, Shibao C, Diedrich A, Paranjape SY, Farley G, Christman B, Raj SR, Robertson D, Biaggioni I. Excessive nitric oxide function and blood pressure regulation in patients with autonomic failure. HTN. 2008;51:1531–1536. doi: 10.1161/HYPERTENSIONAHA.107.105171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson D. Contraindication to the use of ocular phenylephrine in idiopathic orthostatic hypotension. American Journal of Ophthalmology. 1979;87:819–822. doi: 10.1016/0002-9394(79)90361-1. [DOI] [PubMed] [Google Scholar]
- 29.Robertson D, Hollister AS, Carey EL, Tung CS, Goldberg MR, Robertson RM. Increased vascular beta2-adrenoceptor responsiveness in autonomic dysfunction. J Am Coll Cardiol. 1984;3:850–856. doi: 10.1016/s0735-1097(84)80264-8. [DOI] [PubMed] [Google Scholar]
- 30.Shannon J, Jordan J, Costa F, Robertson RM, Biaggioni I. The hypertension of autonomic failure and its treatment. HTN. 1997;30:1062–1067. doi: 10.1161/01.hyp.30.5.1062. [DOI] [PubMed] [Google Scholar]
- 31.Shannon JR, Jordan J, Diedrich A, Pohar B, Black BK, Robertson D, Biaggioni I. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101:2710–2715. doi: 10.1161/01.cir.101.23.2710. [DOI] [PubMed] [Google Scholar]
- 32.Fahmy NR, Soter NA. Effects of trimethaphan on arterial blood histamine and systemic hemodynamics in humans. Anesthesiology. 1985;62:562–566. doi: 10.1097/00000542-198505000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Topal E, Cikim AS, Cikim K, Temel I, Ozdemir R. The effect of moxonidine on endothelial dysfunction in metabolic syndrome. Am J Cardiovasc Drugs. 2006;6:343–348. doi: 10.2165/00129784-200606050-00007. [DOI] [PubMed] [Google Scholar]
- 34.Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L, Nightingale A. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. HTN. 2013;61:5–13. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]
- 35.Gamboa A, Okamoto LE, Arnold AC, Figueroa RA, Diedrich A, Raj SR, Paranjape SY, Farley G, Abumrad N, Biaggioni I. Autonomic blockade improves insulin sensitivity in obese subjects. HTN. 2014;64:867–874. doi: 10.1161/HYPERTENSIONAHA.114.03738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]