Abstract
The development of novel T cell therapies to target leukemia has facilitated the translation of this approach for hematologic malignancies. Different methods of manufacturing leukemia-specific T cells have evolved, along with additional measures to increase the safety of this therapy. This is an overview of expanded T cell therapeutics with a focus on how the manufacturing strategies have been refined, and where the research is heading.
Keywords: Cell therapy, leukemia antigens, ex vivo expanded T cells
INTRODUCTION
T cells are versatile effectors involved in the adaptive cell-mediated immune response. The great diversity generated in the construction of their receptors allows recognition of antigens on both malignant and pathogen-infected cells. These cells directly lyse their targets and secrete immunostimulatory cytokines that recruit and activate other cells [1]. T cells can home to tumor sites, recognize tumor cells with potentially high specificity and confer long term antitumor immunity [2]. The early studies that examined the graft versus tumor effects in allogeneic hematopoietic stem cell transplantation provided one of the first areas of evidence that these cells can be used to elicit a response against cancer.
THE GRAFT VERSUS LEUKEMIA EFFECT: ALLOTRANSPLANTS TO DLI
A study by a group from the Atomic Energy Research Establishment [3] first showed evidence for a graft versus leukemia effect coinciding with a syndrome now known as graft versus host disease (GVHD). This was demonstrated in leukemia mouse models, since mice treated with total body irradiation and allogeneic splenocytes concomitantly developed GVHD [3, 4]. Another group showed that splenocytes derived from donor mice pretreated by injections of leukemic cells conferred protection in recipients [4, 5]. The antileukemic effect was further confirmed in 1981, when a group in Seattle led by E. Donnall Thomas observed in over two hundred bone marrow transplant recipients that lower relapse rates occurred in those who developed GVHD post transplant [4, 6].
Strategies to enhance the graft versus leukemia (GVL) effect confirmed the crucial role of lymphocytes for tumor elimination [4, 7]. The use of donor lymphocyte infusions (DLI) to mediate antileukemia effects is a potent immunotherapeutic approach in some settings [4, 8–10]. For example, while early attempts failed to separate GVL from GVHD [10], the results from the studies using DLI for CML showed promising effects [4, 8]. Hence, both allogeneic stem cell transplantation and donor lymphocyte infusions demonstrate the potency of adoptive cell therapy for leukemia, [11] especially CML [4, 12, 13].
In acute leukemia, however, poorer responses to DLI are thought to arise from deficiencies in antigen presentation by malignant cells, as well as from complications related to GVHD [4, 11]. Recent efforts to limit GVHD, while also limiting immune suppression, have been explored. For example, administration of cyclophosphamide post transplant resulted in a reduced incidence of graft versus host disease and minimized the use of additional post graft immune suppression in an attempt to better preserve the GVL effect [14].
Several methodologies have been developed that seek to separate cells involved in GVL from cells involved in GVHD including: (i) the depletion of alloreactive cells (for example with anti CD25-immunotoxin [15]) (ii) photodynamic purging, [16] or (iii) the introduction of suicide genes [17].
Depletion
Prior incubation of allogeneic donor lymphocytes with recipient cells theoretically results in upregulation of activation markers (like CD25 and CD134) - which could then allow selection of the responding allogeneic cells prior to infusion into the recipient. In the case of clinical trials with the anti CD25 immunotoxin, targeting CD25 resulted in improved T cell reconstitution and lower rates of GVHD [18]. A related strategy targeting CD134-expressing alloreactive cells showed that depletion of alloreactive T cells mediating GVHD did not concurrently deplete tumor antigen-specific T cells [19].
Photodynamic Purging
Photodynamic purging of alloreactive cells makes use of a photosensitizing agent whose entry and exit into cells is altered following activation (in this case, following exposure to alloreactive targets). The photosensitizing agent is effectively trapped in responding allogeneic cells, and following exposure to the appropriate wavelength of light, apoptosis is induced in susceptible cells [20]. A clinical trial using this approach, however, showed delayed immune reconstitution and increased risks for infections and relapse [21].
Modification With Suicide Genes
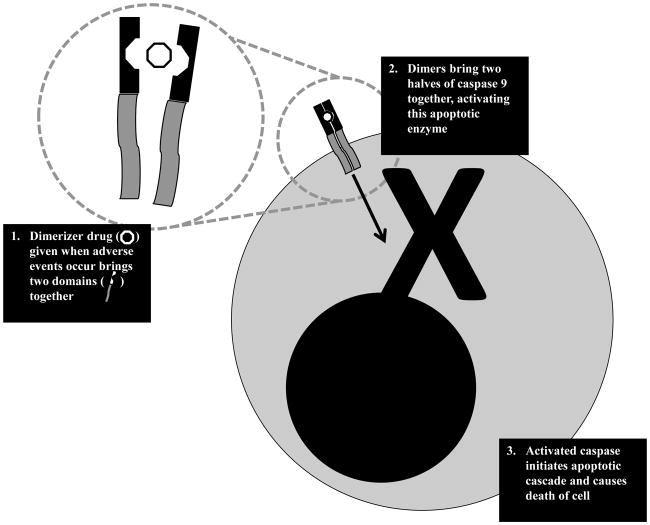
A different approach to separating GVL from GVHD takes advantage of different sensitivities to alloreactive targeting. A model of susceptibility to alloreactive T cells proposes that hematopoietic cells (including leukemic targets) are more prone to alloreactive T cells than gut, liver, and other epithelial cells that feature prominently in GVHD [22]. Administering donor T cells should target cancer cells first, and if they start targeting normal host cells, a signal can be delivered to eliminate them from circulation. Such a signal can be provided by suicide genes - the first of which involves the herpes simplex virus thymidine kinase system. This kinase is activated by ganciclovir administration, and confers susceptibility to cells transduced with this transgene to ganciclovir cytotoxicity. Following infusion, the first signs of GVHD serves as a signal to administer ganciclovir – which results in elimination of the GVHD causing cells. In a study from Italy, the antitumor activity of donor lymphocytes was mediated by HSV-TK-transduced cells in 65% of patients who received the T cells post allogeneic HSCT for hematologic malignancies [23]. One limitation of the HSV-TK approach is that the foreign HSV transgene elicits immune reactions, and the apoptotic signal takes a while to exert its effect. Therefore, efforts to improve suicide gene strategies resulted in a landmark study from Baylor College of Medicine utilizing an inducible caspase system (whose activation results in apoptosis) activated by an inert synthetic drug (dimerizer). In a clinical trial of five patients with acute leukemia receiving donor lymphocytes following haploidentical stem cell transplants, administration of the dimerizer drug eliminated GVHD-causing alloreactive T cells within 30 minutes (see Fig. 1) [24].
Fig. 1.
Schematic of mechanisms used to activate iCaspase suicide gene system. Cells are genetically modified to express caspase 9 that has been separated into two halves, and require dimerization to be functional. Dimerization occurs as a result of coupling with a domain that is brought together by an inert dimerizer drug. Upon iCaspase dimerization, the protein becomes activated and initiates the apoptotic cascade.
Another strategy to extend GVL and limit GVHD involves the generation of antigen specific T cells, where selective expansion of antigen specific populations crowds out potentially allogeneic responses that cause GVHD. To dissociate GVL from GVHD, products containing ex vivo expanded T cells have thus been developed [4, 25].
THE GRAFT VERSUS LEUKEMIA EFFECT: ALLOTRANSPLANTS TO DLI
Ex vivo expanded T cells, in contrast to genetically modified T cells (expressing chimeric antigen receptors, for example, discussed briefly at the end of this review) use their endogenous T cell receptors to recognize antigens in the context of MHC molecules. Not all targetable antigens in leukemia are expressed on the surface, so the ability of ex vivo expanded T cells to target overexpressed intracellular proteins provides a mechanism for increasing GVL in high risk leukemic patients.
The use of ex vivo expanded T cells for therapy grew from studies with antiviral T cells, where pioneering studies by investigators led to the prevention and treatment of viral infections post transplant and the prevention and treatment of EBV-associated post transplant lymphoproliferative disease [26, 27].
EBV-specific T cells, in particular, have had great success in the clinics. At Baylor College of Medicine, these cells been given to more than 120 patients either at high risk for post transplant lymphoproliferative disease (PTLD) or with active disease after allogeneic HSCT [28–31]. The infused cells were shown to expand 1000-fold or more in vivo, [28–31] persist for a decade, [29] prevent lymphoma in the immunocompromised host, [32] and eradicate established disease (even as single agent) [29, 32]. Successful use of EBV-specific T cells led to subsequent attempts to develop “off the shelf” therapies. In a study using allogeneic EBV CTL, infusion of products matching with the donor through at least one HLA allele, showed more than 50% response rates among patients who already failed standard therapies [4, 33]. In another study, more than 60% of patients achieved complete and partial remissions after infusion of EBV-specific T cells [4, 34]. Based on these successes using donor-derived antigen specific T-cells for EBV associated diseases, investigators have attempted to develop a similar approach to target leukemia.
THE MANUFACTURE OF EX VIVO EXPANDED LEUKEMIA-SPECIFIC T CELLS
Several leukemia specific antigens have been identified, reviewed in greater depth elsewhere in literature [35]. The ex vivo expansion of T cells relies on repeated stimulations with these defined antigens to expand clinically significant numbers of T cells from an initial (often small) volume of blood [36]. Studies show that ex vivo expansion of T cells decreases alloreactivity in vitro [37], likely due to cell death or outgrowth by the antigen-specific T cells. Indeed, any residual alloreactivity detected did not translate into clinical morbidity [38]. Several studies have shown that T-cells targeting leukemia- and lymphoma-associated antigens can be generated from donor and patient PBMC and show cytolytic activity against lymphoma cell lines and primary tumor cells in vitro [39–44]. The ex vivo expansion of T cells requires (i) a cell source, (ii) optimized culture conditions, and (iii) specific target antigens.
Cell Sources
In the case of autologous T cells, the population being expanded has theoretically been previously primed against the leukemia antigen, and expansion should be closer to that seen in the virus-specific T cell setting. Indeed, leukemia-specific T cells can be detected and expanded from patients with leukemia [39]. One major drawback to the use of autologous T cells is the immunoediting that may have occurred in vivo: where leukemic cells recognized by the patient’s leukemia-specific T cells have already undergone negative selection, and those that remain do not express immunogenic antigens [22]. Allogeneic T cells, on the other hand, more closely resemble DLI, and may be used to target additional antigens besides leukemia-specific antigens (minor histocompatibility antigens, in particular). The disadvantage with the use of allogeneic ex vivo expanded T cells is the fact that they are derived from a naïve population: requiring optimized priming conditions and subsequent massive expansions.
Optimized Culture Conditions
In general, the culture conditions for expanding leukemia antigen-specific T cells is more complex than the ones used for expanding virus-specific T cells – owing to the nature of their targets. As self proteins, leukemia-specific antigen targets need to prime CTL in vitro. Antigen presenting cells play an important role in priming; consequently more potent antigen presenting cells like dendritic cells have been used in this setting. In the cord blood setting for example, the use of dendritic cells have been shown to prime otherwise naïve populations against an antigen [45]. More recently, the use of artificial antigen presenting cells genetically modified to express costimulatory molecules like CD137 and CD80 have been used to expand tumor antigen specific T cells [46]. The choice of cytokines also influences the product. Because of the need for priming (or reversing anergy, in the case of autologous/patient-derived T cells), generation of tumor-specific CTL has shown increased needs for cytokines, including IL12, IL6, IL7, IL15, and IL2 (generation of virus-specific CTLs, in contrast, requires only IL2 or IL4 and IL7, depending on the specific virus) [40, 42, 47, 48]. An unfortunate consequence of prolonged culture in vitro is exhaustion of the T-cells and shortening of telomeres. From previous T cell therapy studies, “younger cells” with longer telomeres have been shown to correlate with both in vivo persistence and anti-tumor activity [49]. To address this, alternative culture conditions utilizing gas permeable devices have been evaluated [50]. These newer bioreactors are capable of rapidly expanding antigen-specific cells to clinically significant numbers, drastically decreasing the time required to expand T-cells ex vivo and thus increasing the proportion of T-cells that are not exhausted [50]. Recently, several T-cell populations have been identified that appear to have prolonged in vivo persistence and better cytolytic activity. Naïve T-cells and a putative memory stem cell compartment appear to both allow for improved expansion of antigen-specific cells. To generate antigen-specific CTL from naïve populations, Albrecht et al. used CD45RA selection followed by culture in the presence of IL21 [51]. To generate antigen-specific CTL from the memory stem cell compartment, Gattinoni et al. activated Wnt signaling using GSK3b inhibitors to arrest differentiation of cells and allow for expansion of cell populations with better therapeutic properties [52]. Efforts to utilize this population in the clinics using more GMP compliant protocols are currently underway [53].
Target Antigens
In the virus-specific CTL setting, early trials used virus-infected cells (e.g. EBV transformed lymphoblastoid cell lines) to present viral antigens to responding T cells [32, 54, 55]. Subsequent trials, however, took advantage of overlapping peptide libraries spanning entire viral proteins – the 15 amino acid peptides overlapping by 11 amino acids. This allowed for virus-free generation of T cells while conserving most of the CD4 and CD8 epitopes within the immunogenic proteins [56, 57]. In the leukemia-specific CTL setting, recipient cells expressing non-self antigens, peptides, and overlapping peptide pools have been used extensively as antigens. These antigens fall into two broad categories: minor histocompatibility antigens and leukemia specific antigens (see Table 1). Minor histocompatibility antigens are non-self peptides expressed differently among individuals resulting from nucleotide polymorphisms [58]. These antigens can be recognized by allogeneic T cells. Leukemia-specific antigens, on the other hand, are antigens that are either mutated in leukemic cells (e.g. bcr-abl), lineage-restricted (e.g. CD19), or overexpressed in leukemia as a result of the genomic instability or malignant phenotype but absent or minimally expressed in healthy tissue (e.g. survivin). Identification of leukemia-specific antigens is an ongoing effort in several laboratories [59].
Table 1.
Targeting minor histocompatibility antigens vs. leukemia specific antigens.
| T Cells Targeting Minor Histocompatibility Antigens | T Cells Targeting Leukemia-specific Antigens | |
|---|---|---|
| Targets | Minor histocompatibility proteins expressed as a result of genetic polymorphisms | Unique or upregulated proteins expressed specifically by tumors and not expressed or minimally expressed by healthy cells |
| HLA Restriction | Yes | No |
| Cell Source | Allogeneic only | Autologous and allogeneic |
| Other Advantages | Potent allogeneic T cells available in matched donors | Targets can be unique and required by tumors for malignant phenotype |
| Disadvantages | Antigens potentially present in all cells, thus may increase risks for GVHD Difficult to identify antigens for different donor-recipient pairs |
Difficult to grow/not as immunogenic Downregulation by tumors occur Some expression in some healthy cells |
T CELLS TARGETING MINOR HISTOCOMPATIBILITY ANTIGENS
In many ways, minor histocompatibility antigen-specific T cells are a direct extension of donor lymphocyte infusions, whose effector T cells presumably recognize at least some of these same antigens in recipients. Several minor histocompatibility antigens (mHAg) representing genetic polymorphisms that resulted in slightly different MHC presentation among individuals have been tested in vitro and in murine models.
Different groups have shown T cells recognizing mHAgs can kill leukemic blasts derived from patients in vitro. Falkenburg et al. showed that T cell clones recognizing the mHAgs HA-1, HA-2, HA-3, HA-4, HA-5, and H-Y can lyse myeloid leukemic cells and inhibit clonogenic leukemic growth in vitro [60]. Mutis et al. showed that HA-1 and HA-2 synthetic peptide pulsed dendritic cells can prime naïve T cells from HA-1/HA-2-negative healthy donors which can subsequently lyse tumor cells derived from ALL and AML patients [61].
Difficulties with the manufacture of minor histocompatibility antigen-specific T cells, however, have hampered their translation into the clinics. What is more, the limited number of identified mHAgs present a less than ideal pool from which to select a target that can be applicable to multiple donor-recipient combinations in the post transplant leukemia population (i.e. a substantial number of mHAg targets need to be identified to account for many potential donor-recipient HLA types) [58]. To facilitate mHAg discovery, Riddell and Bleakely in Seattle stimulated CD8 T cells from GCSF mobilized PBSC with recipient cells, and selected for T cell clones by testing their ability to lyse recipient (but not donor) lymphoblastoid cell lines. T cell clones identified were subsequently screened for their ability to lyse fibroblasts and a panel of unrelated lymphoblastoid cells sharing at least one HLA allele with the recipient. Subsequent analysis allowed for characterization of leukemia associated minor antigens [62].
A similar approach was used in a recent clinical trial where Warren et al. used ex vivo expanded donor-derived CD8+ T cells directed against unknown minor histocompatibility antigens following leukemia relapse post allogeneic transplant. The transplant donor T cells were stimulated with recipient peripheral blood mononuclear cells, depleted of CD4+ T cells, and further selected for reactive clones capable of lysing EBV-transformed cells from the recipient but not fibroblasts or EBV-transformed cells from the donor. Seven patients who relapsed at a median of seven months post transplant received cytoreductive chemotherapy followed by mHAg-specific CD8+ T cells. Four of the 7 patients had transient complete remissions. However, while MHAg-specific T cells were detected in the bone marrow, persistence was minimal. Further, the GVL effect observed occurred concurrently with GVHD since of the six patients previously diagnosed with GVHD or BOOP, four had a recurrence of their GVHD or BOOP post T cell therapy. Further, pulmonary toxicity was seen in patients who received high T-cell doses likely due to the expression of minor H antigens on respiratory epithelial cells [63]. Nevertheless, this study demonstrated that generating T cells recognizing minor histocompatibility antigens from healthy donor cells is feasible, and these cells are capable of mediating antileukemic activity.
As the clinical trial above emphasizes, very few minor histocompatibility antigens are solely expressed in hematologic cells making the choice of mHAgs as targets difficult as a tumor-specific T-cell therapy approach [63]. Even the most specific mHAgs will target healthy hematopoietic cells as well as malignant cells. Although these studies suggest that this strategy is superior to DLI, mHAg-specific CTL can potentially target healthy recipient cells and cause GVHD. More ideal targets are leukemia associated antigens (LAAs) which are restricted to leukemic cells, either overexpressed relative to healthy cells or uniquely seen in malignant cells.
T CELLS TARGETING LEUKEMIA-ASSOCIATED ANTIGENS
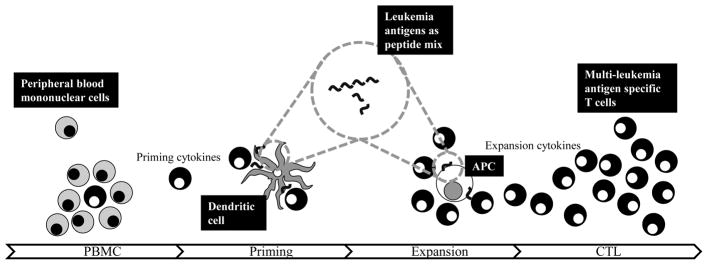
Leukemia-associated antigens (LAAs) comprise a diverse group of antigens seen in acute leukemias, including cancer/testis antigens like MAGE A3, developmental proteins like WT1, and prosurvival/antiapoptotic proteins like survivin. Compared to mHAgs, LAAs are self proteins – requiring even more potent priming conditions in vitro (see Fig. 2).
Fig. 2.
Schematic of manufacture of multileukemia antigen specific T cells. Peripheral blood mononuclear cells (PBMC) from blood serve as source of effector cells and antigen presenting cells (APC), including the most potent - dendritic cells. To expand the rare cell with the appropriate specificity against leukemia antigens, an overlapping peptide library spanning the antigens of choice, in this case, is presented by dendritic cells in the presence of cytokines to prime the naïve response ex vivo. After this initial stimulation, cells are then expanded either by dendritic cells or alternative APC (e.g. PHA blasts with artificial antigen presenting cells) to expand the selected leukemia specific T cell. Subsequent expansion steps in the presence of cytokines will generate multi-leukemia antigen T cells.
Studies have suggested that expansion of LAA-specific T cells in the peripheral blood of patients post allogeneic HSCT contribute to the GVL effect [64, 65]. Based on these results, several groups have explored the potential to ex vivo expand these LAA-specific T cells from healthy donors for adoptive T cell transfer post allogeneic HSCT. WT1 (Wilm’s tumor antigen 1) in particular, appears to be a promising target. WT1 is overexpressed in in 60–100% of ALL [66] and 73–100% of AML [66]. Altered expression of WT1 in patients with ALL was associated with increased relapse [67]. In AML, increased WT1 expression correlated with increased disease burden, relapse, and poor overall survival [68–71]. While WT1 is also seen in healthy hematopoietic stem cells, anti-WT1 cytotoxic therapy did not result in adverse effects in this population [66].
WT-1 appears to be essential to leukemic cells – with increased expression correlating with enhanced proliferation [72]. Several studies showed that WT-1 is an immunogenic protein, with WT-1 specific T cells capable of lysing WT-1 expressing leukemia cells [40, 48, 72–74]. O’Reilly’s group at MSKCC mapped WT-1 epitope responses in healthy donors, and showed majority of WT-1 specific T cells from healthy donors were capable of lysing partially HLA-matched leukemic targets [72]. A group from Osaka University expanded HLA-A2-positive WT-1 specific T cells that lysed HLA-A2-positive WT-1 expressing leukemia cells [73], as well as established a CD4-T cell clone recognizing WT-1-expressing hematopoietic cells and apoptotic WT1 expressing cells presented by autologous dendritic cells [74]. Another group from Universitätsklinikum Benjamin-Franklin was able to demonstrate spontaneous WT1 specific T cells from patients with AML [75]. Another group at Baylor College of Medicine and NIH manufactured multi leukemia specific antigens (recognizing WT-1, proteinase 3, PRAME, neutrophil elastase, and MAGE A3) from healthy donors with the goals of enhancing GVL post stem cell transplant. Immunogenic peptides of WT-1 were mapped in several donors, and in vitro experiments showed that partially HLA-matched leukemic blasts were lysed and killed by these T cells. This approach can thus be used in the transplant setting without limitation to certain HLA types [40]. Additionally, tumor-specific T cells targeting WT1, survivin, MAGE A3, and PRAME were also expanded from 50 patients with ALL receiving maintenance therapy despite low lymphocyte counts. Specificity was observed in more than 90% of patients after three stimulations (as measured by IFNg ELISPOT), and reductions in autologous leukemia blasts in coculture experiments demonstrated tumor-lysing abilities of these generated T cells [48].
Preliminary findings from a phase I trial show that these T cells are well tolerated and effective. Infusions of WT-1 specific CTL into patients with AML, ALL, or MDS following allogeneic HSCT were able to transiently reduce or eliminate cells expressing WT1, without mediating toxicities or GVHD. These cells were generated from healthy donors of allo HSCT recipients, using autologous dendritic cells pulsed with a pool of overlapping peptides spanning the WT1 protein [76]. Additionally, another trial safely administered HLA A2 restricted T cells specific for WT1 into leukemia patients and showed some clinical efficacy (5 with CR post CTL while 3 had relapse and 3 progressed) [77].
Other groups are evaluating other antigens or epitopes. At the NHLBI and the MD Anderson Cancer Center, Molldrem et al. targeted PR-1 by stimulating HLA A2+ T cells with a PR-1 peptide presented by an HLAA2+ T2 cell line. Cells were capable of lysing CML and AML blasts, with only background killing of normal allogeneic marrow cells [78]. A group from Gutenberg University expanded HLA A1+ autologous T cells from a patient with FLT3-ITD AML with an immunogenic peptide within the mutated receptor tyrosine kinase, and expanded T cells were able to target both FLT3-ITD transfected cells and the patient’s own AML blasts but not wild type FLT3-expressing targets [79]. Yasukawa’s group from Ehime University in Japan expanded HLA A2+ T cells targeting a single peptide derived from Aurora-A-kinase (a kinase implicated in tumorigenesis) that were capable of targeting both leukemia cell lines and primary leukemia cells but not healthy hematopoietic stem cells [80]. Finally, another group at Baylor College of Medicine targeted the cancer/testis antigen PRAME (seen in most hematologic malignancies) by using dendritic cells and artificial antigen presenting cells that were an overlapping peptide library spanning the protein to stimulate CTLs. The resulting CTLs had high avidity against PRAME could be generated from healthy donors and patients with PRAME+ malignancies, and could lyse PRAME-expressing leukemic blasts and leukemic progenitor cells [44].
GENETIC MODIFICATION OF EXPANDED T CELLS
One of the main problems using T-cells that recognize tumor associated antigens through their endogenous TCR is that T cells derived from both cancer patients and healthy donors require multiple stimulations to increase the LAA-specific T cell population. This is due to the fact that LAA-specific T-cells with sufficient and therapeutic affinity directed against tumor antigens are rare because of central and peripheral tolerance mechanisms [81].
Alternatively, researchers have used genetic modification to allow expression of such TCRs from a high affinity clone into other T-cells. This involves the cloning and subsequent transfer of the α and β genes of the TCR from patients with detectable high affinity T-cell clones. This abTCR construct can then be cloned into a viral vector to transduce T cells enabling these cells to now have the high affinity and specificity characteristics of the original donor clone [82].
Several leukemia antigens (both mHAgs and leukemia-specific antigens) have been targeted successfully by TCR-modified T cells, including some of the most commonly targeted mHAgs (e.g. HA-1 and HA-2) and leukemia-specific antigens (e.g. WT1) [83–85].
A T cell clone derived from a patient where HA-2 specific T cells emerged following DLI post allo HSCT provided the TCR sequences that were used to transduce T cells with a retroviral vector. TCR-modified T cells were able to recognize and lyse HA-A2 positive CML cells [83]. A novel approach was explored by a group in Japan to target HA-1. Instead of transducing T cells with a TCR recognizing HA-1, the group used the single chain variable fragment of an antibody which recognized HA-1 like a TCR in the context of MHC presentation. The antibody, derived from immunized animals administered with HA-1 antigen and B2-microglobulin peptides, was coupled to CD28 and CD3 zeta domains (like chimeric antigen receptors discussed below). Pseudo-TCR-transduced T cells induced potent inflammatory cytokines in response to leukemia cells expressing HA-1 [84].
The transfer of TCRs recognizing WT1 is not as straightforward since most TCRs against a self protein will still possess suboptimal binding efficiencies. To facilitate identification of a highly avid TCR against WT1, Stauss’ group in London used the T cell receptor derived from alloreactive T-cells obtained from HLA A2-negative donors to target WT1 expressed by leukemias in HLA-A2+ patients. The retrovirus WT1 construct was then used to transduced T cells derived from HLA A2+ donors. WT1-TCR-expressing T cells acquired specificity against the WT1 antigen, and were able to eliminate leukemia cells from patients in a NOD/SCID murine model [86].
More potently, T cells engineered to express a chimeric antigen receptor (CAR) targeting the CD19 molecule on B cell malignancies elicited rapid and sustained responses observed by several groups [87–90] providing some of the most potent demonstrations of the clinical utility of T cell therapies. Chimeric antigen receptor T cells are beyond the scope of this review.
COUNTERACTING/OPTIMIZING THE IN VIVO TUMOR ENVIRONMENT
Ex vivo expanded T cells rely heavily on antigen presentation in leukemic cells to mediate their cytotoxicity. Unfortunately, leukemias can modulate antigen presentation (by downregulation of tumor antigen, MHC, or costimulatory molecule expression) and the immune environment (by secreting immunosuppressive cytokines) to counteract the T cell response.
Several strategies have been identified to subvert these tumor-mediated processes. To prevent loss of tumor antigen presentation, multiple tumor antigens can be targeted. Indeed, such an approach has been shown to be feasible using overlapping peptide libraries spanning multiple tumor proteins [40, 42]. T cells simultaneously recognizing multiple leukemia antigens (proteinase 3 (Pr3), preferentially expressed antigen in melanoma, Wilms tumor gene 1 (WT1), human neutrophil elastase (NE) and melanoma-associated antigen A3) can be generated from a single culture platform [40]. Another approach to mitigating loss of antigen expression is forced re-expression using epigenetic modifying drugs. In AML, tumors and tumor cell lines upregulated antigen expression following co-culture in vitro with epigenetic modifying drugs, and similarly, in patients, the frequency of MAGE-specific T cells increased following therapy with epigenetic-modifying drugs [91]. To counteract TGFb blockade the group at Baylor College of Medicine generated a retrovirus vector expressing the dominant-negative TGFβ type II receptor (DNR) that eliminates signaling from the endogenous receptor. T cells genetically modified to express the TGFb DNR showed resistance to the immunosuppressive properties of the cytokine [92].
Finally, early findings from the T cell immunotherapy field - particularly with the use of tumor infiltrating lymphocytes and chimeric antigen receptor expressing T cells - suggest that a lymphodepleted environment allows for better expansion of infused T cells: less competition, the absence of a cytokine sink, and elimination of regulatory cells have all been cited as potential reasons for this phenomenon [93]. Recently, immunomodulatory drugs have been shown to influence endogenous immune activity. Lenalidomide, anti CTLA4, and anti PD1 have been shown to enhance antitumor immunity, [94, 95] and combinations with ex vivo expanded T cells are potential new strategies.
SAFETY OF T CELL THERAPIES
The use of ex vivo expanded CTL for acute leukemias is a very promising therapeutic approach, but as the experience with chimeric antigen expressing T cells highlights, the occurrence of severe adverse events [96, 97] is a reminder that T cells are extremely potent.
Ex vivo expanded T cells can potentially mediate a variety of unintended effects, as a result of inflammatory mediator release or cytokine secretion [98]. These cells can release significant amounts of cytokines like TNF-α, IL-1β, and IL-6 which may damage the host’s environment [99]. Lethal consequences have occurred in the CAR setting. For example, one patient, who received a CAR T cell targeting ERBB2 experienced respiratory distress immediately following cell infusion, and died five days later despite medical intervention. Patient serum showed markedly elevated levels of IFNg, GMCSF, TNFa, IL6, and IL10 [97]. Additionally, a rapid elimination of bulky malignancy may lead to tumor lysis syndrome since another patient who received CAR T cells targeting CD19 showed increased levels of uric acid, phosphorus, and lactate dehydrogenase approximately three weeks following infusion. Although there were accompanying sings of an acute kidney injury the patient responded well to the treatment they received for this complication [89].
Another risk with the use of ex vivo expanded antigen specific T cells is their potential for causing GVHD although this should be less than with DLI given their specificity. Fortunately, the same approaches to limit DLI (discussed above) can be used to limit the alloreactive T cell effects. These include suicide gene strategies such as the herpes simplex thymidine kinase (HSVtk) system and the iCaspase 9 system. These suicide genes can be introduced into T cells and activated when necessary (using ganciclovir and dimerizer drug, respectively) to prevent GVHD.
CONCLUSION
Cancer immunotherapy has been heralded as 2013 “breakthrough of the year,” [100] and promising studies using ex vivo expanded T cell immunotherapies increase optimism that they will be an important option for patients with cancer. Improved identification of leukemia antigens, optimal culture conditions, and methods to overcome the inhospitable tumor microenvironment should result in better T cell therapies for patients with acute leukemia.
Supplementary Material
Acknowledgments
This work was supported by the Leukemia and Lymphoma Society (SCOR Grant), the Hyundai Hope on Wheels Grant Award, the CPRIT RO1 RP100469 (to CMB), and NCI PO1 CA148600e02 (CMB) awards.
Footnotes
Send Orders for Reprints to reprints@benthamscience.ae
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
References
- 1.Janeway C. Immunobiology: the immune system in health and disease. 6. Garland Science; New York: 2005. p. xxiii.p. 823. [Google Scholar]
- 2.Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med. 2012;4(127):127ps8. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes DW, Corp MJ, Loutit JF, et al. Treatment of murine leukaemia with X rays and homologous bone marrow; preliminary communication. Br Med J. 1956;2(4993):626–7. doi: 10.1136/bmj.2.4993.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz CR, Bollard CM. T Cell Immunotherapy for Cancer. Academic Press; 2015. [Google Scholar]
- 5.Fefer A, Einstein AB, Cheever MA. Adoptive chemoimmunotherapy of cancer in animals: a review of results, principles, and problems. Ann N Y Acad Sci. 1976;277:492–504. doi: 10.1111/j.1749-6632.1976.tb41723.x. [DOI] [PubMed] [Google Scholar]
- 6.Weiden PL, Sullivan KM, Flournoy N, et al. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304(25):1529–33. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 7.Brunschwig A, Southam CM, Levin AG. Host resistance to cancer. Clinical experiments by homotransplants, autotransplants and admixture of autologous leucocytes. Ann Surg. 1965;162(3):416–25. doi: 10.1097/00000658-196509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462–5. [PubMed] [Google Scholar]
- 9.Slavin S, Eckerstein A, Weiss L. Adoptive immunotherapy in conjunction with bone marrow transplantation-amplification of natural host defence mechanisms against cancer by recombinant IL-2. Nat Immun Cell Growth Regul. 1988;7(3):180–4. [PubMed] [Google Scholar]
- 10.Sullivan KM, Storb R, Buckner CD, et al. Graft-versus-host disease as adoptive immunotherapy in patients with advanced hematologic neoplasms. N Engl J Med. 1989;320(13):828–34. doi: 10.1056/NEJM198903303201303. [DOI] [PubMed] [Google Scholar]
- 11.Yee C. Adoptive T-cell therapy of cancer. Hematol Oncol Clin North Am. 2006;20(3):711–33. doi: 10.1016/j.hoc.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Dazzi F, Szydlo RM, Cross NC, et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood. 2000;96(8):2712–6. [PubMed] [Google Scholar]
- 13.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–50. [PubMed] [Google Scholar]
- 14.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amrolia PJ, Muccioli-Casadei G, Huls H, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108(6):1797–808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perruccio K, Topini F, Tosti A, et al. Photodynamic purging of alloreactive T cells for adoptive immunotherapy after haploidentical stem cell transplantation. Blood cells molecules & diseases. 2008;40(1):76–83. doi: 10.1016/j.bcmd.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Bonini C, Ferrari G, Verzeletti S, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276(5319):1719–24. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 18.Amrolia PJ, Mucioli-Casadei G, Huls H, et al. Add-back of allodepleted donor T cells to improve immune reconstitution after haploidentical stem cell transplantation. Cytotherapy. 2005;7(2):116–25. doi: 10.1080/14653240510018181. [DOI] [PubMed] [Google Scholar]
- 19.Ge X, Brown J, Sykes M, et al. CD134-allodepletion allows selective elimination of alloreactive human T cells without loss of virus-specific and leukemia-specific effectors. Biol Blood Marrow Transplant. 2008;14(5):518–30. doi: 10.1016/j.bbmt.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perruccio K, Topini F, Tosti A, et al. Photodynamic purging of alloreactive T cells for adoptive immunotherapy after haploidentical stem cell transplantation. Blood Cells Mol Dis. 2008;40(1):76–83. doi: 10.1016/j.bcmd.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Mielke S, Nunes R, Rezvani K, et al. A clinical-scale selective allodepletion approach for the treatment of HLA-mismatched and matched donor-recipient pairs using expanded T lymphocytes as antigen-presenting cells and a TH9402-based photodepletion technique. Blood. 2008;111(8):4392–402. doi: 10.1182/blood-2007-08-104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent K, Roy DC, Perreault C. Next-generation leukemia immunotherapy. Blood. 2011;118(11):2951–9. doi: 10.1182/blood-2011-04-350868. [DOI] [PubMed] [Google Scholar]
- 23.Ciceri F, Bonini C, Marktel S, et al. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007;109(11):4698–707. doi: 10.1182/blood-2006-05-023416. [DOI] [PubMed] [Google Scholar]
- 24.Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365(18):1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy-Nasser AA, Bollard CM. T cell therapies following hematopoietic stem cell transplantation: surely there must be a better way than DLI? Bone Marrow Transplant. 2007;40(2):93–104. doi: 10.1038/sj.bmt.1705667. [DOI] [PubMed] [Google Scholar]
- 26.Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114(19):4283–92. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121(26):5113–23. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345(8941):9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 29.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–35. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12(10):1160–6. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 31.Heslop HE, Ng CY, Li C, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2(5):551–5. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 32.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549–55. [PubMed] [Google Scholar]
- 33.Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110(4):1123–31. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 34.Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119(11):2644–56. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anguille S, Van Tendeloo VF, Berneman ZN. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia. 2012;26(10):2186–96. doi: 10.1038/leu.2012.145. [DOI] [PubMed] [Google Scholar]
- 36.Bollard CM, Kuehnle I, Leen A, et al. Adoptive immunotherapy for posttransplantation viral infections. Biol Blood Marrow Transplant. 2004;10(3):143–55. doi: 10.1016/j.bbmt.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Bollard CM, Rooney CM, Heslop HE. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat Rev Clin Oncol. 2012;9(9):510–9. doi: 10.1038/nrclinonc.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melenhorst JJ, Leen AM, Bollard CM, et al. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood. 2010;116(22):4700–2. doi: 10.1182/blood-2010-06-289991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber G, Caruana I, Rouce RH, et al. Generation of tumor antigen-specific T cell lines from pediatric patients with acute lymphoblastic leukemia - implications for immunotherapy. Clin Cancer Res. 2013;19(18):5079–91. doi: 10.1158/1078-0432.CCR-13-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber G, Gerdemann U, Caruana I, et al. Generation of multileukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia. 2013;27(7):1538–47. doi: 10.1038/leu.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber G, Karbach J, Kuci S, et al. WT1 peptide-specific T cells generated from peripheral blood of healthy donors: possible implications for adoptive immunotherapy after allogeneic stem cell transplantation. Leukemia. 2009;23(9):1634–42. doi: 10.1038/leu.2009.70. [DOI] [PubMed] [Google Scholar]
- 42.Gerdemann U, Katari U, Christin AS, et al. Cytotoxic T lymphocytes simultaneously targeting multiple tumor-associated antigens to treat EBV negative lymphoma. Mol Ther. 2011;19(12):2258–68. doi: 10.1038/mt.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quintarelli C, Dotti G, De Angelis B, et al. Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia. Blood. 2008;112(5):1876–85. doi: 10.1182/blood-2008-04-150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quintarelli C, Dotti G, Hasan ST, et al. High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells. Blood. 2011;117(12):3353–62. doi: 10.1182/blood-2010-08-300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanley PJ, Cruz CR, Savoldo B, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114(9):1958–67. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ngo MC, Ando J, Leen AM, et al. Complementation of antigen-presenting cells to generate T lymphocytes with broad target specificity. J Immunother. 2014;37(4):193–203. doi: 10.1097/CJI.0000000000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruz CR, Gerdemann U, Leen AM, et al. Improving T-cell therapy for relapsed EBV-negative Hodgkin lymphoma by targeting upregulated MAGE-A4. Clin Cancer Res. 2011;17(22):7058–66. doi: 10.1158/1078-0432.CCR-11-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber G, Caruana I, Rouce RH, et al. Generation of tumor antigen-specific T cell lines from pediatric patients with acute lymphoblastic leukemia-implications for immunotherapy. Clin Cancer Res. 2013;19(18):5079–91. doi: 10.1158/1078-0432.CCR-13-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou J, Shen X, Huang J, et al. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J immunol. 2005;175(10):7046–52. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vera JF, Brenner LJ, Gerdemann U, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010;33(3):305–15. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albrecht J, Frey M, Teschner D, et al. IL-21-treated naive CD45RA+ CD8+ T cells represent a reliable source for producing leukemia-reactive cytotoxic T lymphocytes with high proliferative potential and early differentiation phenotype. Cancer Immunol Immunother. 2011;60(2):235–48. doi: 10.1007/s00262-010-0936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290–7. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cieri N, Camisa B, Cocchiarella F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121(4):573–84. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 54.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362(9393):1375–7. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 55.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038–44. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 56.Trivedi D, Williams RY, O’Reilly RJ, et al. Generation of CMV-specific T lymphocytes using protein-spanning pools of pp65-derived overlapping pentadecapeptides for adoptive immunotherapy. Blood. 2005;105(7):2793–801. doi: 10.1182/blood-2003-05-1433. [DOI] [PubMed] [Google Scholar]
- 57.Gerdemann U, Keirnan JM, Katari UL, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012;20(8):1622–32. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bleakley M, Riddell SR. Exploiting T cells specific for human minor histocompatibility antigens for therapy of leukemia. Immunol Cell Biol. 2011;89(3):396–407. doi: 10.1038/icb.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berlin C, Kowalewski DJ, Schuster H, et al. Mapping the HLA ligandome landscape of acute myeloid leukemia: a targeted approach toward peptide-based immunotherapy. Leukemia. 2014;30(4):1003–4. doi: 10.1038/leu.2016.1. [DOI] [PubMed] [Google Scholar]
- 60.Falkenburg JH, Goselink HM, van der Harst D, et al. Growth inhibition of clonogenic leukemic precursor cells by minor histocompatibility antigen-specific cytotoxic T lymphocytes. J Exp Med. 1991;174(1):27–33. doi: 10.1084/jem.174.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mutis T, Blokland E, Kester M, et al. Generation of minor histocompatibility antigen HA-1-specific cytotoxic T cells restricted by nonself HLA molecules: a potential strategy to treat relapsed leukemia after HLA-mismatched stem cell transplantation. Blood. 2002;100(2):547–52. doi: 10.1182/blood-2002-01-0024. [DOI] [PubMed] [Google Scholar]
- 62.Bleakley M, Otterud BE, Richardt JL, et al. Leukemia-associated minor histocompatibility antigen discovery using T-cell clones isolated by in vitro stimulation of naive CD8+ T cells. Blood. 2010;115(23):4923–33. doi: 10.1182/blood-2009-12-260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Warren EH, Fujii N, Akatsuka Y, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115(19):3869–78. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rezvani K, Yong AS, Mielke S, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111(1):236–42. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrett AJ, Rezvani K, Solomon S, et al. New developments in allotransplant immunology. Hematol Am Soc Hematol Educ Program. 2003:350–71. doi: 10.1182/asheducation-2003.1.350. [DOI] [PubMed] [Google Scholar]
- 66.Rosenfeld C, Cheever MA, Gaiger A. WT1 in acute leukemia, chronic myelogenous leukemia and myelodysplastic syndrome: therapeutic potential of WT1 targeted therapies. Leukemia. 2003;17(7):1301–12. doi: 10.1038/sj.leu.2402988. [DOI] [PubMed] [Google Scholar]
- 67.Boublikova L, Kalinova M, Ryan J, et al. Wilms’ tumor gene 1 (WT1) expression in childhood acute lymphoblastic leukemia: a wide range of WT1 expression levels, its impact on prognosis and minimal residual disease monitoring. Leukemia. 2006;20(2):254–63. doi: 10.1038/sj.leu.2404047. [DOI] [PubMed] [Google Scholar]
- 68.Alonso-Dominguez JM, Tenorio M, Velasco D, et al. Correlation of WT1 expression with the burden of total and residual leukemic blasts in bone marrow samples of acute myeloid leukemia patients. Cancer Genet. 2012;205(4):190–1. doi: 10.1016/j.cancergen.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 69.Bergmann L, Miething C, Maurer U, et al. High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood. 1997;90(3):1217–25. [PubMed] [Google Scholar]
- 70.Nomdedeu JF, Hoyos M, Carricondo M, et al. Bone marrow WT1 levels at diagnosis, post-induction and post-intensification in adult de novo AML. Leukemia. 2013;27(11):2157–64. doi: 10.1038/leu.2013.111. [DOI] [PubMed] [Google Scholar]
- 71.Pozzi S, Geroldi S, Tedone E, et al. Leukaemia relapse after allogeneic transplants for acute myeloid leukaemia: predictive role of WT1 expression. Br J Haematol. 2013;160(4):503–9. doi: 10.1111/bjh.12181. [DOI] [PubMed] [Google Scholar]
- 72.Doubrovina E, Carpenter T, Pankov D, et al. Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1(+) leukemias. Blood. 2012;120(8):1633–46. doi: 10.1182/blood-2011-11-394619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oka Y, Elisseeva OA, Tsuboi A, et al. Human cytotoxic T-lymphocyte responses specific for peptides of the wild-type Wilms’ tumor gene (WT1) product. Immunogenetics. 2000;51(2):99–107. doi: 10.1007/s002510050018. [DOI] [PubMed] [Google Scholar]
- 74.Fujiki F, Oka Y, Tsuboi A, et al. Identification and characterization of a WT1 (Wilms Tumor Gene) protein-derived HLA-DRB1*0405-restricted 16-mer helper peptide that promotes the induction and activation of WT1-specific cytotoxic T lymphocytes. J Immunother. 2007;30(3):282–93. doi: 10.1097/01.cji.0000211337.91513.94. [DOI] [PubMed] [Google Scholar]
- 75.Scheibenbogen C, Letsch A, Thiel E, et al. CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood. 2002;100(6):2132–7. doi: 10.1182/blood-2002-01-0163. [DOI] [PubMed] [Google Scholar]
- 76.O’Reilly RJ, Dao T, Koehne G, et al. Adoptive transfer of unselected or leukemia-reactive T-cells in the treatment of relapse following allogeneic hematopoietic cell transplantation. Semin Immunol. 2010;22(3):162–72. doi: 10.1016/j.smim.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chapuis AG, Ragnarsson GB, Nguyen HN, et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Trans Med. 2013;5(174):174ra27. doi: 10.1126/scitranslmed.3004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Molldrem J, Dermime S, Parker K, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88(7):2450–7. [PubMed] [Google Scholar]
- 79.Graf C, Heidel F, Tenzer S, et al. A neoepitope generated by an FLT3 internal tandem duplication (FLT3-ITD) is recognized by leukemia-reactive autologous CD8+ T cells. Blood. 2007;109(7):2985–8. doi: 10.1182/blood-2006-07-032839. [DOI] [PubMed] [Google Scholar]
- 80.Ochi T, Fujiwara H, Yasukawa M. Aurora-A kinase: a novel target both for cellular immunotherapy and molecular target therapy against human leukemia. Expert Opin Ther Targets. 2009;13(12):1399–410. doi: 10.1517/14728220903307483. [DOI] [PubMed] [Google Scholar]
- 81.Casucci M, Bondanza A, Falcone L, et al. Genetic engineering of T cells for the immunotherapy of haematological malignancies. Tissue Antigens. 2012;79(1):4–14. doi: 10.1111/j.1399-0039.2011.01799.x. [DOI] [PubMed] [Google Scholar]
- 82.Govers C, Sebestyen Z, Coccoris M, et al. T cell receptor gene therapy: strategies for optimizing transgenic TCR pairing. Trends Mol Med. 2010;16(2):77–87. doi: 10.1016/j.molmed.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Heemskerk MH, Hoogeboom M, de Paus RA, et al. Redirection of antileukemic reactivity of peripheral T lymphocytes using gene transfer of minor histocompatibility antigen HA-2-specific T-cell receptor complexes expressing a conserved alpha joining region. Blood. 2003;102(10):3530–40. doi: 10.1182/blood-2003-05-1524. [DOI] [PubMed] [Google Scholar]
- 84.Inaguma Y, Akahori Y, Murayama Y, et al. Construction and molecular characterization of a T-cell receptor-like antibody and CAR-T cells specific for minor histocompatibility antigen HA-1H. Gene Ther. 2014;21(6):575–84. doi: 10.1038/gt.2014.30. [DOI] [PubMed] [Google Scholar]
- 85.Xue SA, Gao L, Hart D, et al. Elimination of human leukemia cells in NOD/SCID mice by WT1-TCR gene-transduced human T cells. Blood. 2005;106(9):3062–7. doi: 10.1182/blood-2005-01-0146. [DOI] [PubMed] [Google Scholar]
- 86.Xue SA, Gao L, Thomas S, et al. Development of a Wilms’ tumor antigen-specific T-cell receptor for clinical trials: engineered patient’s T cells can eliminate autologous leukemia blasts in NOD/SCID mice. Haematologica. 2010;95(1):126–34. doi: 10.3324/haematol.2009.006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122(25):4129–39. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cruz CR, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected-virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase I study. Blood. 2013;122(17):2965–73. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N England J Med. 2011;365(8):725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goodyear O, Agathanggelou A, Novitzky-Basso I, et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010;116(11):1908–18. doi: 10.1182/blood-2009-11-249474. [DOI] [PubMed] [Google Scholar]
- 92.Bollard CM, Rossig C, Calonge MJ, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99(9):3179–87. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 93.Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Br J Cancer. 2014;111(12):2214–9. doi: 10.1038/bjc.2014.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bodera P, Stankiewicz W. Immunomodulatory properties of thalidomide analogs: pomalidomide and lenalidomide, experimental and therapeutic applications. Recent Pat Endocr Metab Immune Drug Discov. 2011;5(3):192–6. doi: 10.2174/187221411797265890. [DOI] [PubMed] [Google Scholar]
- 96.Brentjens R, Yeh R, Bernal Y, et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18(4):666–8. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Food and Drug Administration. [accessed January 21, 2012];Proposed approach to regulation of cellular and tissue-based products. 1997 doi: 10.1089/scd.1.1997.6.195. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM062601.pdf. [DOI] [PubMed]
- 99.Ferrara JL. Cytokine dysregulation as a mechanism of graft versus host disease. Current Opin Immunol. 1993;5(5):794–9. doi: 10.1016/0952-7915(93)90139-j. [DOI] [PubMed] [Google Scholar]
- 100.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–3. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.