Abstract
Prospective studies indicate that hyperaldosteronism is found in 20% of patients with resistant hypertension (RHTN). A small number of observational studies in normotensive and hypertensive patients suggest a correlation between aldosterone levels and obesity while others could not confirm these findings. The correlation between aldosterone levels and body mass index (BMI) in patients with RHTN has not been previously investigated. Our objective was to determine if BMI is positively correlated with plasma aldosterone concentration (PAC), plasma renin activity (PRA), aldosterone-renin ratio (ARR), and 24-hour urinary aldosterone (24-hr UAldo) African-American (AA) and Caucasian patients. We performed a cross-sectional analysis of a large diverse cohort (n=2170) with RHTN. The relation between PAC, PRA, ARR, 24-hr UAldo and BMI was investigated for the entire cohort, by gender, and race (65.3% Caucasian, 40.3% men). We demonstrate that PAC and ARR were significantly correlated to BMI (p<0.0001) across the first three quartiles, but not from the 3rd to 4th quartile of BMI. PRA was not correlated with BMI. 24-hr UAldo was positively correlated across all quartiles of BMI for the cohort (p<0.0001) and when analyzed by gender (men p<0.0001; women p=0.0013) and race (p<0.05), and stronger for men compared to women (r=0.19, p<0.001 versus r=0.05, p=0.431, p=0.028) regardless of race. In both AA and Caucasian patients, aldosterone levels were positively correlated to increasing BMI, with the correlation being more pronounced in AA and Caucasian men. These findings suggest that obesity, particularly the abdominal obesity typical of men, contributes to excess aldosterone in patients with RHTN.
Keywords: primary aldosteronism, black, visceral obesity, body weight, association
Introduction
Advancements in technology, urbanization, an increasingly sedentary lifestyle, and dietary changes are factors contributing to the rising global prevalence of overweight and obesity in the United States (US) and worldwide, currently affecting two thirds of the adult population in the US1. Obesity is strongly associated with an increased risk of cardiovascular (CV) morbidity and mortality.1, 2 A meta-analysis of 57 prospective studies found that in adults older than 35 years a 5 kg/m2 higher BMI was associated with a 30% higher mortality.3 In this analysis, increasing BMI and increased risk of coronary artery disease, ischemic, hemorrhagic, and total stroke, and chronic kidney disease (CKD)3, were largely attributable to the effects of adiposity on blood pressure (BP), risk of diabetes, and circulating lipid levels. Similarly, cross-sectional and longitudinal studies demonstrate an independent association between increasing body weight and higher BP levels.
Obesity is one of the most common risk factors for resistant hypertension (RHTN).4, 5 RHTN is defined as BP >140/90 mmHg with use of >3 medications, including a diuretic, if tolerated.4 Prospective studies indicate that hyperaldosteronism is found in approximately 20% of African American (AA) and Caucasian patients with RHTN.4, 6 Aldosterone excess has been shown to have deleterious effects on the CV system, especially in the setting of high dietary sodium intake.7 The prevalence of RHTN is increasing in the US and strongly associated with being overweight or obese.8
Increased aldosterone levels in obese patients have been suggested as a possible mechanism of obesity-related hypertension. While a few small studies have shown a significant correlation between plasma aldosterone levels and BMI,9,10, 11 others reported a positive relationship for plasma aldosterone concentration (PAC) and BMI in women but not men12, 13, or for AAs but not Caucasians14, or none at all.15 These prior analyses were all based on PAC as opposed to 24-hr urine excretion of aldosterone as an index of aldosterone status.
We hypothesized that the aldosterone excess commonly observed in patients with RHTN is attributable to increasing adiposity and is reflected in a positive correlation between 24-hr urinary excretion of aldosterone and BMI.
Methods
The study is a cross-sectional analysis of 2170 AA and Caucasian patients with RHTN who were referred to the University of Alabama at Birmingham (UAB) Hypertension Clinic during the 12-year period 2000-2012. RHTN was defined as clinic BP >140/90 mmHg with a concurrent use of 3 or more antihypertensive agents of different classes, including a diuretic, if tolerated.4 All patients underwent a routine physical examination, evaluation of demographic characteristics (age, gender, race), medical history (duration of hypertension, co-morbidities, number and classes of antihypertensive medications), vital signs (clinic BP, heart rate), and weight assessment. Race and height were self-reported. Body weight was measured with a digital scale according to standardized methods. BMI was calculated as weight per height squared (kg/m2). Obesity was defined as BMI ≥30 kg/m2.16
BP was measured by trained personal after at least 5 minutes of rest in a relaxed sitting position, using the auscultatory method with a manual sphygmomanometer (Welch Allyn, Inc., Skaneateles Falls, NY 13153, USA) according to guidelines.17 The arm was supported at heart level and a correctly sized cuff size with the air bladder encircling at least 80% of the arm was used. The BP was measured twice at intervals of at least 1 minute in each arm and the average of two readings in the arm with the higher BP reading was used for final BP value.
Biochemical assessment included serum potassium (s-K+), serum creatinine (s-Crea), PAC, plasma renin activity (PRA), 24-hr urinary aldosterone (24-hr UAldo), potassium (24-hr U-K+), sodium (24-hr U-Na+), and creatinine (24-hr U-Crea). Aldosterone-renin ratio (ARR) was calculated by the formula ARR=PAC/PRA. PAC, PRA, and 24-hr UAldo levels were analyzed by mass spectrometry at Mayo Clinic (Mayo Medical Laboratories, Rochester, MN, USA).18-21 Estimated glomerular filtration rate was calculated with use of the Modification of Diet in Renal Disease formula.22 Patients were instructed to begin the urine collection after the first morning void. All urine was collected in a prepared plastic jug to which 25 mL of 50% acetic acid had been added as a preservative.23 The last urine sample was saved into the container exactly 24 hours after beginning the collection. Patients were instructed to bring the samples to the laboratory early in the morning on the same day they completed the collection. At the time of this laboratory visit, a morning blood sample was collected for measurement of PAC and PRA. Adequacy of the 24-hr urine collection was assessed by measuring 24-hr U-Crea by comparing total creatinine in the sample to predicted creatinine.24 The 24-hr urine collection was obtained while patients were consuming their usual diet and without change in their level of physical activity. Exclusion criteria for data analysis were use of mineralocorticoid receptor antagonist (MRA) at the time biochemical assessment, incomplete urinary collection evaluated by low 24-hr U-Crea, and CKD stage IV –V.24,25
Statistical Analysis
PAC, PRA, ARR, and 24-hr UAldo levels were related to categorized quartiles of BMI for the entire cohort and for gender and race. The quartile cutoffs were Quartile (Q) 1: <28.1kg/m2; Q2 BMI ≥ 28.1 and BMI< 32.1 kg/m2; Q3: BMI ≥32.1 and BMI<36.6 kg/m2; Q4: BMI≥36.6 kg/m2.
The ANCOVA method was used to determine whether population means of PAC, PRA, ARR and 24-hr UAldo levels were equal across BMI quartiles, while controlling for age. Because of their skewed distribution, the levels of PAC, PRA, ARR and 24-hr UAldo were log-transformed and the transformed outcomes met the normal assumption well. The age-adjusted means were compared using t-test with Bonferroni correction for multiplicity. The original scale of the outcomes was obtained by lognormal distribution approximation. Statistical analysis was performed using SAS 9.2 software (SAS Institute Inc., Cary, NC).
This study was approved by the UAB Institutional Review Board and was conducted according to institutional guidelines.
Results
Patient characteristics are shown in Table 1. In this single center cohort of 2170 patients, 2086 patients met inclusion criteria and were included in the analysis (Supplemental Figure S1). Patients were on average 57.0±14.0 years (yrs) of age, 59.5% female, and 35.2% AA. AA patients were significantly younger than Caucasian patients (54±13 versus (vs.) 59±14 yrs, p<0.001) (Table 2). For the entire cohort the mean BMI was 31.1±7.2 kg/m2 (Table 1). Obesity, defined as BMI ≥30 kg/m2, was present in 62.8% of patients. The BMI range within the cohort was 15.5-73.8 kg/m2. Overall, men and women had a similar BMI (31.2±5.6 vs. 31.0±8.1 kg/m2, p=0.42). However, when analyzed by race, Caucasian men had a significantly higher BMI than Caucasian women (30.8±5.3 vs. 28.5±6.7 kg/m2, p<0.001), while in AA patients, AA women were more obese than AA men (34.6±8.5 vs. 32.5±6.1 kg/m2, p<0.001) (Table 2). AA men and women had a significantly higher BMI than Caucasian men and women (33.9±7.9 vs. 29.5±6.2 kg/m2, p<0.001) (Table 2). Patients were treated with an average of 4.2±1.0 antihypertensive agents. The medication regimen included in almost all patients, an angiotensin-converting-enzyme inhibitor or angiotensin II receptor blocker, beta blocker, calcium channel blocker, and thiazide-like diuretic.
Table 1.
Characteristics of patients with resistant hypertension (n=2086) included in the analysis. Values are mean ± SD.
| Parameter | Patients (n=2086) |
|---|---|
| Age - yrs | 57.0±14.0 |
| Women, % | 59.5 |
| African American, % | 35.2 |
| Other*, % | 0.7 |
| BMI - kg/m2 | 31.1±7.2 |
| BMI range - kg/m2 | 15.5-73.8 |
| HTN - yrs | 16.0±11.0 |
| # of antihypertensive agents | 4.2±1.0 |
| SBP - mmHg | 158.0±28.0 |
| DBP - mmHg | 86.0±16.0 |
| Obesity, % | 62.8 |
| HLD, % | 61.7 |
| OSA, % | 27.9 |
| HF, % | 15.2 |
| CKD, % | 18.8 |
| CAD, % | 16.1 |
| Diabetes, % | 27.9 |
| Biochemical analysis | |
| S- Crea - mg/dL | 1.1±0.4 |
| S- K+ - mEq /L | 4.0±0.5 |
| PAC - ng/dl | 10.9±9.0 |
| PRA - ng/ml/h | 3.6±8.0 |
| ARR | 12.8±16.8 |
| 24-hr UAldo - μg | 12.1±9.2 |
| 24-hr UNa+ - mEq | 179.0±84.0 |
| 24-hr UK + - mEq | 63.0±33.0 |
| 24-hr UVol - ml | 2056.0±913.0 |
| 24-hr UCrea - mg | 1609.0±626.0 |
Hispanics, Indian, Native American, Asian;
BMI - body mass index; HTN - hypertension; SBP- systolic blood pressure; DBP - diastolic blood pressure; HLD - Hyperlipidemia; OSA - obstructive sleep apnea; HF- heart failure; CKD- Chronic kidney disease; CAD- coronary artery disease; PAC - plasma aldosterone concentration; PRA-plasma renin activity; ARR - aldosterone-renin-ratio; S-Crea- serum creatinine; S-K+- serum potassium; UAldo - urinary aldosterone; UNa+- urinary sodium; UK+- urinary potassium; UVol- urinary volume; UCrea - urinary creatinine
Table 2.
Patient characteristics are shown by gender and race. Values are mean ± SD.
| Parameter | All n=2086 |
Men n=845 |
Women n=1241 |
AA n=735 |
Caucasians n=1321 |
AA men n=242 |
AA women n=493 |
Caucasian men n=584 |
Caucasian women n=737 |
|---|---|---|---|---|---|---|---|---|---|
| Age –yrs | 57.0±14.0 | 55±13 | 58±15□ | 54±13 | 59±14□ | 52±13 | 54±13* | 57±13 | 61±15□ |
| BMI -kg/m2 | 31.1±7.2 | 31.2±5.6 | 31.0±8.1* | 33.9±7.9 | 29.5±6.2□ | 32.5±6.1 | 34.6±8.5† | 30.8±5.3 | 28.5±6.7□ |
| HTN –yrs | 16.0±11.0 | 16.0±11.0 | 17.0±12.0* | 17.0±11.0 | 16.0±12.0* | 16.0±11.0 | 17.0±11.0* | 16±11.4 | 16.0±12.0* |
|
SBP-
mmHg |
158.0±28.0 | 156.0±28.0 | 160.0±27.6† | 155.0±26.0 | 160.0±29.0† | 154.0±26.0 | 155.0±26.0* | 157.0±29.0 | 163.0±28.0† |
|
DBP-
mmHg |
86.0±16.0 | 88.0±16.0 | 84.0±15.2† | 88.0±15.0 | 84.0±16.0† | 90.0±14.0 | 87.0±15.0* | 87.0±17.0 | 82±15□ |
|
Crea-
mg/dL |
1.1±0.4 | 1.2±0.4 | 1.0±0.3□ | 1.1±0.4 | 1.0±0.4† | 1.3±0.4 | 1.0±0.3□ | 1.2±0.32 | 0.9±0.4□ |
| K+-mEq /L | 4.0±0.5 | 4.0±0.5 | 4.0±0.5* | 3.9±0.5 | 4.1±0.5□ | 3.9±0.5 | 3.9±0.5* | 4.1±0.5 | 4.1±0.5† |
| PAC -ng/dL | 10.9±9.0 | 10.7±7.9 | 11.0±9.6* | 11.4±9.4 | 10.6±8.8† | 11.2±8.7 | 11.5±9.7* | 10.5±7.6 | 10.7±9.6* |
|
PRA-
ng/mL/h |
3.0±5.5 | 3.1±5.5 | 3.0±5.5* | 2.2±4.5 | 3.5±6.0□ | 2.5±4.8 | 2.1±4.3* | 3.4±5.8 | 3.5±6.2* |
| ARR | 13.8±17.7 | 14.6±18.8 | 13.2±16.9* | 16.4±18.5 | 12.3±17.2□ | 16.0±15.5 | 16.5±19.8* | 14.0±20.1 | 11.1±14.5□ |
|
24-hr
Urinary Analysis |
|||||||||
| UAldo-μg | 12.3±11.7 | 14.6±9.9 | 10.4±8.1□ | 12.6±9.3 | 11.7±9.0† | 14.7±10.3 | 11.2±8.4□ | 13.8±9.8 | 9.7±7.8□ |
| UNa+-mEq | 179.0±84.0 | 206.0±88.0 | 157.0±74.0□ | 181.0±86.0 | 178.0±83.0* | 206.0±93.0 | 165.0±78.0□ | 206.0±85.5 | 150.0±70.0□ |
| UK+-mEq | 63.0±3.0 | 74.0±35.0 | 53.0±27.0□ | 55.0±28.0 | 68.0±34.0□ | 64.0±31.0 | 50.0±25.0□ | 79.0±35.7 | 56.0±28.0□ |
| UVol-mL | 2057.0± 910.0 |
2226.0± 961.0 |
1916.0± 839.0□ |
1879.0± 816.0 |
2177.0± 953.0□ |
2027.0± 827.0 |
1792.0± 798.0† |
2327.0± 1012.0 |
2018.0± 860.0† |
| UCrea- mg | 1608.0± 623.0 |
2033.0± 556.0 |
1252.0± 421.0 □ |
1715.0± 642.0 |
1536.0± 601.0□ |
2175.0± 656.0 |
1426.0± 430.0□ |
1961.0± 480.0 |
1107.0± 356.0□ |
BMI - body mass index; HTN - hypertension; SBP - systolic blood pressure; DBP - diastolic blood pressure; PAC - plasma aldosterone concentration; PRA - plasma renin activity; ARR - aldosterone-renin-ratio; Crea - serum creatinine; K+-serum potassium; UAldo - urinary aldosterone; UNa+- urinary sodium; UK+- urinary potassium; UVol - urinary volume; UCrea - urinary creatinine
Non-significant
p<0.05
p<0.001
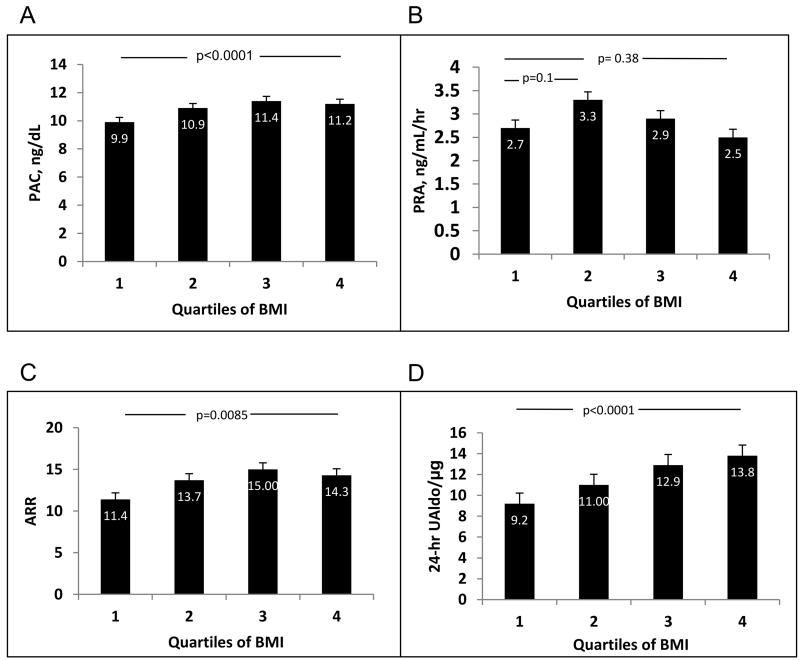
Overall, PAC, ARR, and 24-hr UAldo levels increased significantly with BMI strata (Figure 1). The increases of PAC and ARR were significant between the first and fourth quartiles of BMI (PAC p<0.0001, ARR p=0.0085, respectively) and between quartile 1 vs. 2 and quartile 2 vs. 3, respectively. However, for both parameters, there was no significant difference between the 3rd and 4th quartile of BMI. In contrast, 24-hr UAldo levels manifested a significant positive correlation across all quartiles of BMI. This relation remained highly significant after adjusting for age.
Figure 1.
Increase of mean plasma aldosterone concentration (PAC) (Panel A), plasma renin activity (PRA) (Panel B), aldosterone-renin ratio (ARR) (Panel C), and 24-h urinary aldosterone (24-hr UAldo) levels (Panel D) with increasing quartiles of body mass index (BMI) in patients with resistant hypertension. All panels show Bonferroni corrected p values.
When PRA was related to quartiles of BMI, there was a non-significant trend towards higher PRA between the 1st and 2nd and 1st and 3rd quartiles, respectively (Figure 1) suggesting that factors other than the renin-angiotensin system play an important role in aldosterone release.
Gender and Race
Analysis by Gender
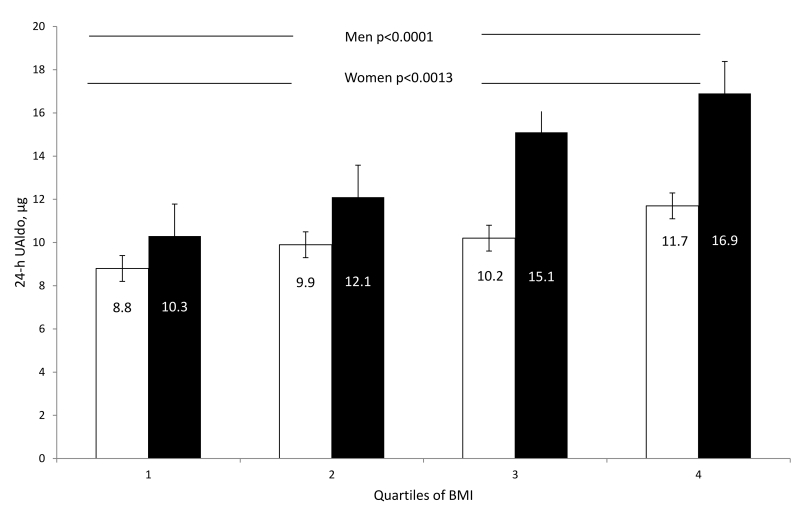
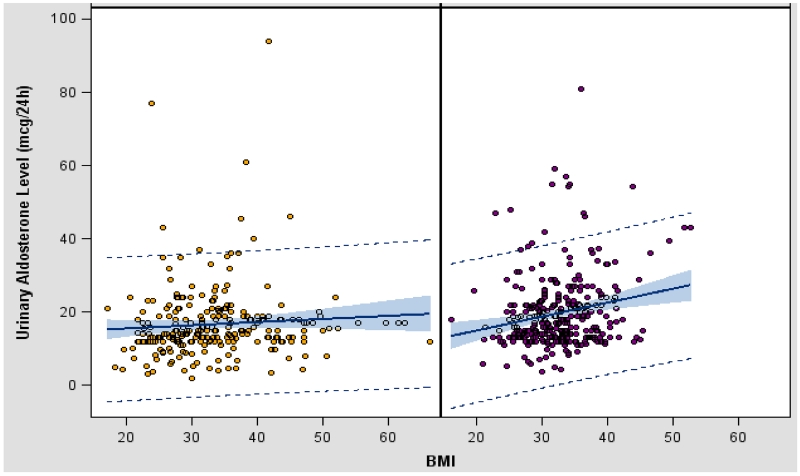
Stratification by gender revealed that men and women had similar PAC, PRA, and ARR levels (Table 2). In contrast, 24-hr UAldo levels were significantly higher in men compared to women (24-hr UAldo 14.6±9.9 vs. 10.4±8.1 μg, p<0.001). This differential was present across all quartiles of BMI (Figure 2), but was exaggerated in the 3rd and 4th quartiles because of greater increases in 24-hr UAldo levels in men with higher BMIs (Figure 2). The correlation between 24-hr UAldo levels and BMI after adjustment for age and race was significantly stronger in men (r=0.19, p<0.001, confidence interval (CI) 95%) compared to women (r=0.05, p=0.431, p=0.028, CI 95%) (Figure 3). This observed gender difference was true for both AA and Caucasian men versus women (Table 2). Regression analysis indicated that after adjusting for age and race, BMI remained a significant predictor of 24-hr UAldo levels. For every unit increase of BMI, there was a 0.352 unit increase of 24-hr UAldo in men compared to a smaller 0.0714 unit increase of 24-hr UAldo in women.
Figure 2.
Mean 24-hr urinary aldosterone (UAldo) levels to quartiles of body mass index (BMI) in men versus women. White columns represent women and black columns represent men. Figure shows Bonferroni corrected p values.
Figure 3.
Scatter plot of the association of 24-hr UAldo levels and BMI in Caucasian and African American women (left panel) versus men (right panel) with a stronger correlation of 24-hr UAldo levels and BMI in men than women after adjustment for age and race (women (r=0.05, p=0.431) versus men (r=0.19, p<0.001), p=0.028). Regression line was drawn with 95% confidence limits of the mean.
Analysis by Race
Univariate analysis showed that AA patients had significantly higher PAC (11.4±9.4 vs. 10.6± 8.8 ng/dl, p<0.05), lower PRA (2.2±4.5 vs. 3.5±6.0 ng/mL/h, p<0.001), higher ARR (16.4±18.5 vs. 12.3±17.2, p<0.001), and higher 24-hr UAldo levels (12.6±9.3 vs. 11.7±9.0 μg, p<0.05) compared to Caucasian patients (Table 2). AAs were also significantly younger (53.5±13 vs. 59±14 yrs, p<0.001), more obese (BMI 33.9±7.9 vs 29.5±6.2 kg/m2, p<0.001), and had lower s-K+ (3.9±0.5 vs. 4.1±0.5 mEq/L, p<0.001) and higher s-Crea levels (1.1±0.4 vs. 1.04±0.4, p<0.05) (Table 2). After multivariate analysis, the difference between 24-hr UAldo levels in AA and Caucasian patients with resistant hypertension was no longer present, indicating that there was no racial difference in obesity-associated aldosterone levels.
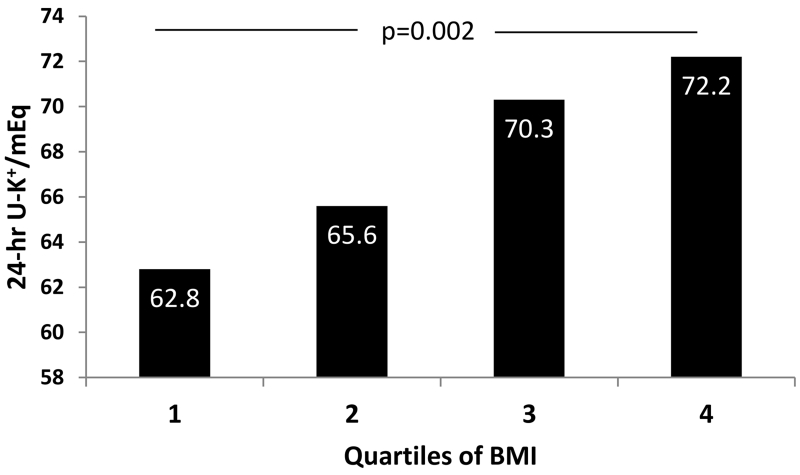
Consistent with increasing aldosterone levels across quartiles of BMI, we found that 24-hr UK+ levels increased significantly with BMI strata (p=0.002) (Figure 4). Stratification by gender and race revealed that 24-hr UK+ levels were significantly higher in men compared to women overall (24-hr UK+ 74.0±35.0 vs. 53.0±27.0 mEq, p<0.001), in AA men vs. AA women (24-hr UK+ 64.0±31.0 vs. 50.0±25.0 mEq, p<0.001), and in Caucasian men vs. Caucasian women (24-hr UK+ 79.0±36.0 vs. 56.0±28.0 mEq, p<0.001) (Table 2). This functional relevance of higher aldosterone levels in obese patients indexed by increased 24-hr UK+ excretion was consistent with aldosterone’s kaliuretic action and affected obese more than non-obese individuals (Figure 4). This effect was independent of BP medication, including diuretics (Supplemental Table S1).
Figure 4.
Increasing 24-hr urinary potassium (24-hr U-K+) excretion across quartiles of body mass index (BMI) in AA and Caucasian patients with resistant hypertension.
Discussion
The current analysis of a large, diverse cohort of patients with RTHN has several novel findings: 1) BMI positively correlates with 24-hr UAldo levels in men and women, with the relation being stronger in the former; 2) men have significantly higher aldosterone levels than women 3) AA and Caucasian individuals manifest a similar positive correlation between 24-hr UAldo excretion and increasing BMI that is also most evident in men compared to women in both races.
This study is unique in having such a large and diverse cohort of patients with RHTN in relation to BMI and aldosterone status; is the first to base such a large analysis on 24-hr UAldo levels, a more integrated assessment of aldosterone release than PAC; and, is limited to patients with RHTN, a subgroup of patients known to commonly have aldosterone excess.4, 6
In this study we also demonstrate the functional relevance of higher aldosterone levels in obese patients in showing that increased 24-hr UK+ excretion is consistent with aldosterone’s kaliuretic action and affects obese individuals significantly more than non-obese individuals independent of blood pressure medication, including diuretics.
Prior data on the clinical relevance of aldosterone excess reported by others and us has indicated that AA and Caucasian patients with RHTN have a high prevalence of hyperaldosteronism that contributes importantly to antihypertensive treatment resistance4,6, CV and cardio-metabolic complications.26-28 Cross-sectional studies have shown that comorbidities such as obstructive sleep apnea (OSA) are associated with hyperaldosteronism and obesity and commonly found in individuals with RHTN.26, 29
We have previously shown that hyperaldosteronism is found in 20% of AA and Caucasian patients with RHTN.6 Separately, in a prospective study of 108 patients with RHTN we have demonstrated that high aldosterone is associated with left and right ventricular end-diastolic volumes measured by cardiac magnetic resonance imaging (MRI), that is, being greater in high versus normal aldosterone patients (p<0.05).26 We have also demonstrated that the treatment with a MRA reduces BP,30 intra-cardiac volumes and left ventricular mass as measured by cardiac MRI in patients with hyperaldosteronism.26
We and others have also reported that the prevalence of OSA is increased in obese patients with RHTN and high aldosterone levels compared to patients without hyperaldosteronism.31-33 These findings were confirmed in a small interventional study, where we further demonstrated that treatment with MRA reduces the severity of OSA in patients with RHTN.29, 34
In previous studies investigating aldosterone levels and BMI in normotensive and hypertensive individuals, but not in patients with RHTN, a gender-dependent relation between plasma aldosterone and BMI was found, while others did not observe such a relation. Specifically, several studies could only confirm a positive relation in women but not men.35 Similarly, when analyzed by race, the relationship of PAC and BMI has been reported in AAs but not Caucasians14, or not at all.15 A few small studies reported a positive relation of PAC and BMI.9, 10,36 However, these reports did not include sub-analysis by gender.
The current study findings are novel in finding that aldosterone levels are positively correlated with BMI; that this correlation, independent of race, is more pronounced in men compared to women; and that the relation is seemingly independent of renin. This observation is in contrast to the majority of small studies that found no relationship of aldosterone with BMI overall or in women overall or in AA women. The positive finding for both genders in our study might be explained through the analysis of such a large cohort and by use of PAC, PRA, ARR and 24-hr UAldo levels as parameters, the latter being a more integrated assessment and therefore better index of aldosterone release than PAC.
The finding of similar aldosterone levels in both, AAs and Caucasians patients with resistant hypertension is in line with other comparative studies investigating aldosterone excretion in Caucasian and AA normotensive and hypertensive individuals.37,38 However, other groups have found lower aldosterone levels in AA children and adolescents.39,40
In our study, an important gender difference is reported for the first time in that men had significantly higher 24-hr UAldo levels than women and the relation between 24-hr UAldo and BMI was more pronounced in men compared to women. One interpretation of this gender difference is that visceral adipose tissue, more characteristic of men, may be a stronger mediator of aldosterone release than peripheral fat.41 Egan et al. reported that waist-hip ratio correlated with aldosterone levels in adult men and women, also suggesting that visceral fat may be an important mediator of aldosterone release.42,39
Additionally, conditions such as hyperlipidemia, insulin resistance, and diabetes are more prevalent in persons with central obesity. Likewise, metabolic syndrome has been shown to be more prevalent in obese patients with primary aldosteronism.28 These factors and others are associated with pathologic fat distribution and function and may play a role in the observed aldosterone dysregulation.
Higher aldosterone levels especially in a high sodium setting are associated with CV complications and impaired surrogate markers such as endothelial dysfunction and arterial stiffness the latter being increased to a greater extent in women with obesity and diabetes than in men. 7,30,43-48
We have previously shown that low-dose spironolactone provides significant additive BP reduction in AA and Caucasian individuals with RHTN.30 We have also demonstrated that patients with RHTN and PA have impaired endothelium-dependent vascular function and that after 3 months of treatment with spironolactone endothelium-dependent vascular function was significantly improved independently of blood pressure change.46 Recently, it has been shown that a high in fat and sodium diet contributes to cardiac diastolic dysfunction and aortic stiffening in young female mice, abnormalities that were prevented by MR antagonism.47, 48 Subsequent experimental studies by the same laboratory have shown that the endothelial MR mediates diet-induced aortic stiffness in females, but not males, and deletion of the endothelial MR prevented diet-induced aortic fibrosis and stiffness in females.49 These studies suggest that environmental factors such as high dietary sodium can disturb normal MR physiology, especially in females compared to males. However, further studies are needed to elucidate the mechanisms of environmental effects and to what extent they relate to humans.
Potential mechanisms by which adipocytes may contribute to excess aldosterone synthesis and secretion include both generalized stimulation of the renin-angiotensin-aldosterone system and, separately, production and release of products stimulating local and/or adrenal aldosterone synthesis. Adipocytes have all components of the renin-angiotensin system and thus produce locally generated angiotensin II. 50-52 A growing body of evidence suggests that adipocytes produce and release factors, that may stimulate aldosterone release independent of renin-angiotensin. 53, 54, 55-58,59 Ehrhart-Bornstein et al. have provided evidence of adipocyte secretory products that directly stimulate adrenocortical aldosterone secretion. In their studies, placing human adrenocortical cells into medium exposed to isolated adipocytes resulted in a 7-fold increase in aldosterone secretion.56 This stimulatory effect was not inhibited by valsartan, an angiotensin II receptor blocker, indicating an effect independent of angiotensin II. Subsequent studies by the same investigator group has demonstrated that human adipocytes induce an ERK1/2 MAP kinases-mediated upregulation of steroidogenic acute regulatory protein (StAR) and an associated angiotensin II sensitization of human adrenocortical cells.57
Other factors such as high fat diet, fatty acid oxidation products, adipokines and others also function as potential aldosterone stimulating factors. In an experimental study with obese, diabetic rats, Jeon et al. demonstrated that complement-C1q TNF-related protein 1 (CTRP1) functioned as a potent aldosterone-stimulating factor55 and was expressed at high levels in adipose tissue and in the zona glomerulosa inducing a dose-dependent increase in angiotensin II-dependent aldosterone release.
Fatty acid oxidation products or endogenous ones from adipocytes could also stimulate aldosterone synthesis.59 Goodfriend et al. have shown that oxidized derivatives of linoleic acid stimulate release of aldosterone from isolated rat adrenal cells, with one specific derivative, 12,13-epoxy-9-keto-10(trans)-octadecenoic acid, being particularly potent,59 suggesting that free fatty acids, serve as potent stimuli of synthesis and release of aldosterone independent of angiotensin II. Similarly, obesity and high fat diets are both associated with increased aldosterone levels and MR expression which suggests that in there is a pathophysiologic important dysregulation of ligand and receptor. MR activation has been suggested to potentiate white adipose tissue inflammation, brown fat dysfunction, oxidative stress, fibrosis, and insulin resistance. 28, 60-62
Recently, Huby et al. demonstrated that leptin, a fat-cell derived hormone (adipokine), is a direct regulator of aldosterone secretion, regulating aldosterone synthase (CYP11B2) expression and production and promotes endothelial dysfunction and cardiac fibrosis.63 A generalized stimulation of the renin-angiotensin-aldosterone system is supported by weight loss studies that demonstrate reductions in components of the pathway, including aldosterone.64
Similarly, treatment with MRAs has been shown to be effective in patients with the metabolic syndrome in reducing insulin resistance, BP, collagen formation, and other adverse metabolic consequences of adipocyte dysfunction.65 These studies linking adiposity to aldosterone secretion provide support for the hypothesis that obesity contributes directly to inappropriate release of aldosterone, resulting in a state of relative aldosterone excess.
Our study is strengthened by having analyzed a very large cohort of patients with RHTN, a subgroup of hypertensive individuals known to have high rates of aldosterone excess.4 Limitations of our study include its cross-sectional design, having evaluated patients while taking their normal antihypertensive medications, and the lack of a non-resistant hypertensive control group.
The current findings support the hypothesis that adiposity stimulates aldosterone release independent of renin in showing that 24-hr UAldo excretion, but not PRA is positively related to BMI. In fact, the current data demonstrated a negative relation between BMI and PRA. Such an inverse relation would be consistent with an adipose-related secretagogue stimulating aldosterone release, promoting sodium and fluid retention and thereby leading to suppression of the renin-angiotensin system. The finding that the relation between BMI and aldosterone is stronger in men compared to women suggests that abdominal adiposity, more typical of men, may be the predominant source of the hypothesized aldosterone secretagogue(s). Such a possibility is consistent with a large body of literature demonstrating that visceral adipose tissue is much more hormonally active than subcutaneous adipose tissue.66
The finding of increased aldosterone levels with increasing BMI is consistent with weight loss studies which demonstrated significant decreases in aldosterone levels after successful weight reduction.9,13,35 Both PAC and PRA decreased in some of these studies, consistent with a generalized stimulation of the renin-angiotensin-aldosterone system. However, in other studies, a decrease of renin levels with weight loss was not observed. The findings of the latter studies suggest weight-related changes in aldosterone levels, independent of renin, suggesting that adiposity may stimulate aldosterone secretion, at least in part, through other mechanisms than renin-angiotensin stimulation.9
Perspectives
Hyperaldosteronism is common in patients with resistant hypertension. We found that in a large cohort of AA and Caucasian patients with RHTN, there was a consistent and significant correlation between 24-hr UAldo levels and BMI. The correlation was stronger in men than women, independent of race and renin, suggesting that factors other than the renin-angiotensin system and, perhaps, more related to visceral obesity, typical of men, might be causative. Confirmation of identification of hypothesized factors that stimulate aldosterone release in obese patients will provide important insight into the growing problem of obesity-related hypertension, and especially, resistant hypertension.
Supplementary Material
Novelty and Significance.
1) What is new?
BMI positively correlates with 24-hr UAldo levels in men and women, with the relation being stronger in the former
Men have significantly higher aldosterone levels than women
AA and Caucasian individuals manifest a similar positive correlation between 24-hr UAldo excretion and increasing BMI that is also most evident in men compared to women in both races and is independent of renin
We demonstrate the functional relevance of higher aldosterone levels in obese patients in showing that increased 24-hr UK+ excretion is consistent with aldosterone’s kaliuretic action and affects obese individuals significantly more than non-obese individuals independent of blood pressure medication, including diuretics.
2) What is relevant
As with aldosterone’s impact on cardiovascular prognosis, these data suggest a renin and race-independent role and effects of aldosterone secretion in individuals with obesity and resistant hypertension.
Summary.
In a large cohort of AA and Caucasian patients with RHTN, there was a consistent and significant correlation between 24-hr UAldo levels and BMI in obese compared to non-obese individuals. The correlation of aldosterone and BMI was stronger in men than women, independent of race and renin, suggesting that factors other than the renin-angiotensin system and more related to visceral obesity, typical of men, might be causative. Confirmation of identification of these hypothesized factors that stimulate aldosterone release in obese patients will provide important insight into the growing problem of obesity-related hypertension, and especially, resistant hypertension.
Acknowledgements
Funding Sources
This work was supported by NIH grants NIH RO1 HL113004 (DAC, TD), NIH grant UL1TR001417 (PL), 3T32DK062710-10S1 (ML)
Footnotes
Author contributions: All authors contributed to each of the following aspects of the study: 1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work, 2) Drafting the work or revising it critically for important intellectual content, 3) Final approval of the version to be published, 4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Drs. Dudenbostel and Li take responsibility for the accuracy and integrity of the data analysis.
Disclosures
None.
References
- 1.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L, NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 2.Dagenais GR, Yi Q, Mann JF, Bosch J, Pogue J, Yusuf S. Prognostic impact of body weight and abdominal obesity in women and men with cardiovascular disease. Am Heart J. 2005;149:54–60. doi: 10.1016/j.ahj.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Prospective Studies C. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM, American Heart Association Professional Education C Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Evans JC, Larson MG, O’Donnell CJ, Roccella EJ, Levy D. Differential control of systolic and diastolic blood pressure : factors associated with lack of blood pressure control in the community. Hypertension. 2000;36:594–599. doi: 10.1161/01.hyp.36.4.594. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–896. doi: 10.1161/01.hyp.0000040261.30455.b6. [DOI] [PubMed] [Google Scholar]
- 7.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 8.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocchini AP, Katch VL, Grekin R, Moorehead C, Anderson J. Role for aldosterone in blood pressure regulation of obese adolescents. Am J Cardiol. 1986;57:613–618. doi: 10.1016/0002-9149(86)90845-3. [DOI] [PubMed] [Google Scholar]
- 10.Andronico G, Cottone S, Mangano MT, Ferraro-Mortellaro R, Baiardi G, Grassi N, Ferrara L, Mule G, Cerasola G. Insulin, renin-aldosterone system and blood pressure in obese people. Int J Obes Relat Metab Disord. 2001;25:239–42. doi: 10.1038/sj.ijo.0801483. [DOI] [PubMed] [Google Scholar]
- 11.Rossi GP, Belfiore A, Bernini G, Fabris B, Caridi G, Ferri C, Giacchetti G, Letizia C, Maccario M, Mannelli M, Palumbo G, Patalano A, Rizzoni D, Rossi E, Pessina AC, Mantero F, Primary Aldosteronism Prevalence in hYpertension Study I Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93:2566–2571. doi: 10.1210/jc.2008-0251. [DOI] [PubMed] [Google Scholar]
- 12.Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7:355–362. doi: 10.1002/j.1550-8528.1999.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 13.Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304:930–933. doi: 10.1056/NEJM198104163041602. [DOI] [PubMed] [Google Scholar]
- 14.Kidambi S, Kotchen JM, Krishnaswami S, Grim CE, Kotchen TA. Aldosterone contributes to blood pressure variance and to likelihood of hypertension in normal-weight and overweight African Americans. Am J Hypertens. 2009;22:1303–1308. doi: 10.1038/ajh.2009.167. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Madrazo S, Padmanabhan S, Mayosi BM, Watkins H, Avery P, Wallace AM, Fraser R, Davies E, Keavney B, Connell JM. Familial and phenotypic associations of the aldosterone Renin ratio. J Clin Endocrinol Metab. 2009;94:4324–4333. doi: 10.1210/jc.2009-1406. [DOI] [PubMed] [Google Scholar]
- 16.Obes Res. Suppl 2. Vol. 6. National Institutes of Health; 1998. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report; pp. 51S–209S. [PubMed] [Google Scholar]
- 17.Jones DW, Hall JE. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and evidence from new hypertension trials. Hypertension. 2004;43:1–3. doi: 10.1161/01.HYP.0000110061.06674.ca. [DOI] [PubMed] [Google Scholar]
- 18.Fredline VF, Kovacs EM, Taylor PJ, Johnson AG. Measurement of plasma renin activity with use of HPLC-electrospray-tandem mass spectrometry. Clin Chem. 1999;45:659–664. [PubMed] [Google Scholar]
- 19.Taylor PJ, van Rosendal SP, Coombes JS, Gordon RD, Stowasser M. Simultaneous measurement of aldosterone and cortisol by high-performance liquid chromatography-tandem mass spectrometry: application to dehydration-rehydration studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1195–1198. doi: 10.1016/j.jchromb.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Fredline VF, Taylor PJ, Dodds HM, Johnson AG. A reference method for the analysis of aldosterone in blood by high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. Anal Biochem. 1997;252:308–313. doi: 10.1006/abio.1997.2340. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RL, Machacek D, Singh RJ. Validation of a high-throughput liquid chromatography-tandem mass spectrometry method for urinary cortisol and cortisone. Clin Chem. 2002;48:1511–1519. [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayo Clinic MML; Rochester, MN, USA: [Accessed 10/23/2015]. http://www.mayomedicallaboratories.com/test-catalog/Specimen/8556. [Google Scholar]
- 24.Ix JH, Wassel CL, Stevens LA, Beck GJ, Froissart M, Navis G, Rodby R, Torres VE, Zhang YL, Greene T, Levey AS. Equations to estimate creatinine excretion rate: the CKD epidemiology collaboration. Clin J Am Soc Nephrol. 2011;6:184–191. doi: 10.2215/CJN.05030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young WF., Jr. Pheochromocytoma and primary aldosteronism: diagnostic approaches. Endocrinol Metab Clin North Am. 1997;26:801–827. doi: 10.1016/s0889-8529(05)70283-8. [DOI] [PubMed] [Google Scholar]
- 26.Gaddam K, Corros C, Pimenta E, Ahmed M, Denney T, Aban I, Inusah S, Gupta H, Lloyd SG, Oparil S, Husain A, Dell’Italia LJ, Calhoun DA. Rapid reversal of left ventricular hypertrophy and intracardiac volume overload in patients with resistant hypertension and hyperaldosteronism: a prospective clinical study. Hypertension. 2010;55:1137–1142. doi: 10.1161/HYPERTENSIONAHA.109.141531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiera F, Sharma AM. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol. 2003;35:807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 28.Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, Rabbia F, Federspil G, Mulatero P. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91:454–459. doi: 10.1210/jc.2005-1733. [DOI] [PubMed] [Google Scholar]
- 29.Gonzaga CC, Gaddam KK, Ahmed MI, Pimenta E, Thomas SJ, Harding SM, Oparil S, Cofield SS, Calhoun DA. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6:363–368. [PMC free article] [PubMed] [Google Scholar]
- 30.Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–930. doi: 10.1016/s0895-7061(03)01032-x. [DOI] [PubMed] [Google Scholar]
- 31.Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131:453–459. doi: 10.1378/chest.06-1442. [DOI] [PubMed] [Google Scholar]
- 32.Florczak E, Prejbisz A, Szwench-Pietrasz E, Sliwinski P, Bielen P, Klisiewicz A, Michalowska I, Warchol E, Januszewicz M, Kala M, Witkowski A, Wiecek A, Narkiewicz K, Somers VK, Januszewicz A. Clinical characteristics of patients with resistant hypertension: the RESIST-POL study. J Hum Hypertens. 2013;27:678–685. doi: 10.1038/jhh.2013.32. [DOI] [PubMed] [Google Scholar]
- 33.Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, Leung RS, Bradley TD. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 34.Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM, Calhoun DA. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2010;24:532–537. doi: 10.1038/jhh.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodfriend TL, Egan BM, Kelley DE. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins Leukot Essent Fatty Acids. 1999;60:401–405. doi: 10.1016/s0952-3278(99)80020-9. [DOI] [PubMed] [Google Scholar]
- 36.Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, Garg R. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92:4472–4475. doi: 10.1210/jc.2007-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez-Sanchez CE, Holland OB. Urinary tetrahydroaldosterone and aldosterone-18-glucuronide excretion in white and black normal subjects and hypertensive patients. J Clin Endocrinol Metab. 1981;52:214–219. doi: 10.1210/jcem-52-2-214. [DOI] [PubMed] [Google Scholar]
- 38.Fisher ND, Gleason RE, Moore TJ, Williams GH, Hollenberg NK. Regulation of aldosterone secretion in hypertensive blacks. Hypertension. 1994;23:179–184. doi: 10.1161/01.hyp.23.2.179. [DOI] [PubMed] [Google Scholar]
- 39.Pratt JH, Jones JJ, Miller JZ, Wagner MA, Fineberg NS. Racial differences in aldosterone excretion and plasma aldosterone concentrations in children. N Engl J Med. 1989;321:1152–1157. doi: 10.1056/NEJM198910263211703. [DOI] [PubMed] [Google Scholar]
- 40.Murro DG, Beavers M, Harshfield GA, Kapuku GK. Aldosterone contributes to elevated left ventricular mass in black boys. Pediatr Nephrol. 2013;28:655–660. doi: 10.1007/s00467-012-2367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 42.Egan BM, Stepniakowski K, Goodfriend TL. Renin and aldosterone are higher and the hyperinsulinemic effect of salt restriction greater in subjects with risk factors clustering. Am J Hypertens. 1994;7:886–893. doi: 10.1016/0895-7061(94)P1710-H. [DOI] [PubMed] [Google Scholar]
- 43.Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120:893–901. [PubMed] [Google Scholar]
- 44.Schmidt BM, Schmieder RE. Aldosterone-induced cardiac damage: focus on blood pressure independent effects. Am J Hypertens. 2003;16:80–86. doi: 10.1016/s0895-7061(02)03199-0. [DOI] [PubMed] [Google Scholar]
- 45.Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6:261–273. doi: 10.1038/nrneph.2010.30. [DOI] [PubMed] [Google Scholar]
- 46.Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109:2857–2861. doi: 10.1161/01.CIR.0000129307.26791.8E. [DOI] [PubMed] [Google Scholar]
- 47.Bostick B, Habibi J, DeMarco VG, Jia G, Domeier TL, Lambert MD, Aroor AR, Nistala R, Bender SB, Garro M, Hayden MR, Ma L, Manrique C, Sowers JR. Mineralocorticoid receptor blockade prevents Western diet-induced diastolic dysfunction in female mice. Am J Physiol Heart Circ Physiol. 2015;308:H1126–1135. doi: 10.1152/ajpheart.00898.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-Dose Mineralocorticoid Receptor Blockade Prevents Western Diet-Induced Arterial Stiffening in Female Mice. Hypertension. 2015;66:99–107. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial Mineralocorticoid Receptor Mediates Diet-Induced Aortic Stiffness in Females. Circ Res. 2016;118:935–943. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiland F, Verspohl EJ. Local formation of angiotensin peptides with paracrine activity by adipocytes. J Pept Sci. 2009;15:767–776. doi: 10.1002/psc.1174. [DOI] [PubMed] [Google Scholar]
- 51.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 52.Gorzelniak K, Engeli S, Janke J, Luft FC, Sharma AM. Hormonal regulation of the human adipose-tissue renin-angiotensin system: relationship to obesity and hypertension. J Hypertens. 2002;20:965–973. doi: 10.1097/00004872-200205000-00032. [DOI] [PubMed] [Google Scholar]
- 53.Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Correa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–1078. doi: 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]
- 54.Schinner S, Willenberg HS, Krause D, Schott M, Lamounier-Zepter V, Krug AW, Ehrhart-Bornstein M, Bornstein SR, Scherbaum WA. Adipocyte-derived products induce the transcription of the StAR promoter and stimulate aldosterone and cortisol secretion from adrenocortical cells through the Wnt-signaling pathway. Int J Obes (Lond) 2007;31:864–870. doi: 10.1038/sj.ijo.0803508. [DOI] [PubMed] [Google Scholar]
- 55.Jeon JH, Kim KY, Kim JH, Baek A, Cho H, Lee YH, Kim JW, Kim D, Han SH, Lim JS, Kim KI, Yoon do Y, Kim SH, Oh GT, Kim E, Yang Y. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J. 2008;22:1502–11. doi: 10.1096/fj.07-9412com. [DOI] [PubMed] [Google Scholar]
- 56.Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, Hauner H, McCann SM, Scherbaum WA, Bornstein SR. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci U S A. 2003;100:14211–14216. doi: 10.1073/pnas.2336140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krug AW, Vleugels K, Schinner S, Lamounier-Zepter V, Ziegler CG, Bornstein SR, Ehrhart-Bornstein M. Human adipocytes induce an ERK1/2 MAP kinases-mediated upregulation of steroidogenic acute regulatory protein (StAR) and an angiotensin II-sensitization in human adrenocortical cells. Int J Obes (Lond) 2007;31:1605–1616. doi: 10.1038/sj.ijo.0803642. [DOI] [PubMed] [Google Scholar]
- 58.Vleugels K, Schinner S, Krause D, Morawietz H, Bornstein SR, Ehrhart-Bornstein M, Krug AW. ERK1/2 MAPKs and Wnt signaling pathways are independently involved in adipocytokine-mediated aldosterone secretion. Exp Clin Endocrinol Diabetes. 2011;119:644–648. doi: 10.1055/s-0031-1284367. [DOI] [PubMed] [Google Scholar]
- 59.Goodfriend TL, Ball DL, Egan BM, Campbell WB, Nithipatikom K. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension. 2004;43:358–363. doi: 10.1161/01.HYP.0000113294.06704.64. [DOI] [PubMed] [Google Scholar]
- 60.Whaley-Connell A, Johnson MS, Sowers JR. Aldosterone: role in the cardiometabolic syndrome and resistant hypertension. Prog Cardiovasc Dis. 2010;52:401–409. doi: 10.1016/j.pcad.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, Fujita T. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol. 2006;17:3438–3446. doi: 10.1681/ASN.2006080944. [DOI] [PubMed] [Google Scholar]
- 62.Marzolla V, Armani A, Zennaro MC, Cinti F, Mammi C, Fabbri A, Rosano GM, Caprio M. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Mol Cell Endocrinol. 2012;350:281–288. doi: 10.1016/j.mce.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemele EJ. The Adipocyte-Derived Hormone Leptin is a Direct Regulator of Aldosterone Secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis. Circulation. 2015;132:2134–2145. doi: 10.1161/CIRCULATIONAHA.115.018226. [DOI] [PubMed] [Google Scholar]
- 64.Dall’Asta C, Vedani P, Manunta P, Pizzocri P, Marchi M, Paganelli M, Folli F, Pontiroli AE. Effect of weight loss through laparoscopic gastric banding on blood pressure, plasma renin activity and aldosterone levels in morbid obesity. Nutr Metab Cardiovasc Dis. 2009;19:110–114. doi: 10.1016/j.numecd.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150:776–783. doi: 10.7326/0003-4819-150-11-200906020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.