Abstract
Exposure to diisocyanates (dNCOs), such as methylene diphenyl diisocyanate (MDI) can cause occupational asthma (OA). Currently, lab tests for dNCO specific IgE are specific, but not sensitive, which limits their utility in diagnosing dNCO asthma. This may be due to variable preparation and poor characterization of the standard antigens utilized in these assays. The aim of this study was to produce and characterize a panel of antigens prepared using three different commonly employed methods and one novel method. The conjugates were examined for recognition by anti-MDI monoclonal antibodies (mAbs) in varying enzyme linked immunosorbant assay (ELISA) formats, extent of crosslinking, total amount of MDI, the sites of MDI conjugation, relative shape/charge, and reactivity with human serum with antibodies from sensitized, exposed workers. Results indicate that while there are minimal differences in the total amount of MDI conjugated, the extent of crosslinking, and the conjugation sites, there are significant differences in the recognition of differently prepared conjugates by mAbs. Native and denaturing polyacrylamide gel electrophoresis demonstrate differences in the mobility of different conjugates, indicative of structural changes that are likely important for antigenicity. While mAbs exhibited differential binding to different conjugates, polyclonal serum antibodies from MDI exposed workers exhibited equivalent binding to different conjugates by ELISA. While differences in the recognition of the different conjugates exist by mAb detection, differences in antigenicity could not be detected using human serum from MDI-sensitized individuals. Thus, although dNCO conjugate preparation can, depending on the immunoassay platform, influence binding of specific antibody clones, serologic detection of the dNCO-exposure-induced polyclonal antibody response may be less sensitive to these differences.
Keywords: diisocyanate, methylene diphenyl diisocyanate, MDI, ELISA, enzyme linked immunosorbant assay
INTRODUCTION
Methylene diphenyl diisocyante (MDI) is used in a variety of industrial and household products including polyurethanes, adhesives, elastomers, sealants, and foams. Exposure to MDI can lead to sensitization and subsequently allergic contact dermatitis or allergic asthma, depending on the site of exposure. Exposure to diisocyanates is one of the leading reported causes of occupational asthma (OA) in the US. Sensitization to MDI poses a significant problem in the manufacturing and construction sectors, as exposures to minute levels of MDI in sensitized individuals can trigger severe asthmatic responses, which can be fatal. It is critical to worker health to develop and standardize bio-monitoring methods to identify exposure and sensitization of individuals to MDI.
Currently, diagnosis of MDI-associated occupational asthma (MDI-OA) is based on a clinical diagnosis of asthma and a patient history of exposure to MDI. Specific inhalation challenge (SIC) can confirm a MDI-OA diagnosis and distinguish it from environmental asthma, although this test is rarely performed in the US. Additional monitoring methods include measuring the levels of the MDI-specific antibodies in the blood. The presence of MDI specific IgG is more common in affected individuals, with a prevalence of 43% in confirmed cases of MDI-OA vs. 8% in non-MDI-OA cases.(1) In addition, approximately 20% of patients with confirmed MDI-OA are MDI-IgE seropositive with a 96–98% diagnostic specificity.(1–7) Measuring the levels of the MDI byproduct, methylene dianiline (MDA), in hydrolyzed urine or blood is another method employed to monitor workers for exposure to MDI, however, this method does not reveal sensitization status, and is rather a reflection of exposure(8, 9). Furthermore, sandwich ELISAs have been developed and proposed as an additional biomonitoring method(10). These could be used to either measure MDI-conjugated proteins within blood or urine as an indicator of exposure, or to measure MDI-specific antibodies as an indicator of sensitization. For measuring MDI-specific antibodies, a mAb would be used to capture a standard antigen and workers’ serum would be screened for MDI-specific antibodies that bind the standard antigen. Our labs have developed mAbs that are specific to MDI for use in the development of sandwich ELISAs(11, 12).
When developing a sandwich ELISA, several features must be optimized, including the standard antigen and its reactivity with the mAbs. Several aspects must be considered when developing a standard antigen.(1) The choice of carrier protein is important; it has been demonstrated by multiple groups that human serum albumin (HSA) is the optimal carrier protein and that reduced immunoreactivity can be observed when using other carriers.(3, 13) The method to produce the conjugate, such as in a liquid phase or vapor phase, is also important,(13, 14) as is the diisocyanate (dNCO) and molar ratio used to produce standard antigens.(1, 3, 15) Thorough characterization of the standard antigens used in dNCO immunoassays is rarely performed, with generally only one or two measures examined. Characterization methods have varied and included both chemical and immunological examinations of dNCO adducts such as sodium dodecyl sulfate polyacrylamide electrophoresis (SDS-PAGE), proteomics, immunoassays, measurement of free amines, and measurements of total MDI bound. The inconsistencies in the methods used to produce standard antigen and the lack of thorough characterization of the standard antigens may contribute to the variable results observed for MDI-specific IgE in cohorts of MDI exposed individuals. Here we sought to investigate different methods for dNCO preparation and characterize the resulting antigens using multiple chemical and immunological techniques.
METHODS
Reagents
All chemicals, unless otherwise noted, were purchased from Sigma Aldrich. Fatty acid free, globulin free ≥99% pure HSA was used.
Conjugate preparation
MDI-HSA conjugates were prepared using the drip, fast dispense, and infusion methods (described below) at a 20:1 ratio by adding 50 µL of a 3.78 mg/mL solution of MDI prepared in either dry ethylene glycol dimethyl ether (EGDE) or dry acetone to 5 mL of 0.5 mg/mL HSA in PBS. After preparation, all conjugates were dialyzed in water overnight using a 12–14 kDa molecular weight cut off dialysis membrane (SpectraPor) and stored at 4°C.
Drip conjugates were prepared by slowly dripping MDI into the HSA while vortexing. The conjugates were vortexed for an additional hour at room temperature. To prepare the fast dispense conjugates, MDI was expelled quickly and directly into the HSA followed by a 3–5 second vortex. The drip and fast dispense conjugations were prepared by 3 laboratorians (denoted A, J and L) on 3 different days from the same stock reagents prepared fresh immediately prior to use to evaluate intra-personal conjugation preparation variability. Infusion conjugates (n=3) were prepared using a syringe pump (Harvard Apparatus Pump 11) set to a flow rate of 50 µL/min. MDI in solvent was loaded into the syringe, which was connected to polyether ether ketone (PEEK) tubing with an inner diameter of 0.030 inches. The tip of the PEEK tubing was placed half way down the 15 mL conical tube containing HSA and the MDI was pumped into the HSA at 50 µL/min for 1 minute while vortexing. 2-phase conjugates (n=3) were prepared by adding 5 mL of the MDI dissolved in hexane to 5 mL of HSA which was stirring at medium speed in a glass cylinder on a stir plate. Solutions were stirred overnight and the aqueous phase was collected and subjected to dialysis as described above.
Enzyme Linked Immunosorbent Assay (ELISA)
MDI-MDI Sandwich ELISA
Plates were coated with anti-murine IgM µ chain antibody (Jackson) diluted to 4 µg/mL in carbonate coating buffer (CCB; 100 mM sodium carbonate pH 9.6). The plate was incubated with 2 µg/mL of MDI-specific mouse IgM mAb 15D4-1C4-1C3 in 3% skim milk PBST (SMPBST)(12). The plate was blocked with SMPBST and then incubated with 25 µg/mL of each conjugate. The wells were incubated with 1 µg/mL of the MDI specific mouse IgG1 mAb DA5 followed by incubation with anti-murine IgG1 biotinylated antibody (Jackson) at a 1:10,000 dilution. Finally, the plate was incubated with a 1:5000 dilution of alkaline phosphatase (AP)-conjugated streptavidin before being incubated with 4-nitrophenyl phosphate disodium salt hexahydrate substrate (PNPP). Absorbance was measured at 405 nm and recorded when control samples reached an absorbance reading between 2.3 and 2.5 absorbance units.
MDI-HSA Sandwich ELISA
MDI-HSA sandwich ELISAs were carried out as described previously and above with the following modifications. The plate was coated with an anti-murine IgG1 antibody at 4 µg/mL. 2 µg/mL of the anti-MDI mAb DA5(11) was added to the wells for capture of the conjugates. A 1:5000 dilution of biotinylated anti-HSA antibody (Rockland) was used followed by AP-conjugated streptavidin and substrate as described above.
MDI-IgG mAb Direct ELISA
Plates were coated with the conjugates diluted to 10 µg/mL in CCB. 2 µg/mL mAb DA5 was added followed by a 1:10000 dilution of the anti-murine IgG1 biotinylated antibody (Jackson). The plate was developed as described above.
MDI-IgG or MDI-IgE from Human Serum
Human serum samples from previously identified sensitized workers were obtained from Dr. A.V. Wisnewski from published(16) and unpublished studies. Plates were coated with 10 µg/mL of the prepared conjugates or mock treated HSA in CCB. Human serum diluted 1:10 for IgE or 1:500 for IgG was used followed by biotinylated anti-human IgE (1:1000) (SouthernBiotech) or HRP conjugated anti-human IgG (1:2000) (BD Pharminigen) antibodies. For IgE, the plate was incubated with a 1:5000 dilution of AP-conjugated streptavidin antibody before being incubated with PNPP. For IgG, the plate was developed using tetramethylbenzidine (TMB) substrate. Controls using unconjugated HSA had absorbance readings ranging from 0.05–0.1 for IgG and 0.06 for IgE.
Inhibition ELISAs
Human serum diluted 1:500 or mAb DA5 diluted to 1 µg/mL were incubated with 2-fold decreasing amounts of conjugate, starting at 10 or 25 µg, respectively. Plates were coated with 10 µg/mL of a standard drip antigen in CCB and then with the 2-fold dilutions of conjugates and mAb or human serum. Either anti-murine IgG1 biotinylated antibody (1:10,000) (Jackson) for the mAb or HRP-conjugated anti-human IgG (1:2000) (BD Pharminigen) for the serum were used. Serum plates were then developed using TMB as described above. MAb plates were incubated with a 1:5000 dilution of AP-conjugated streptavidin antibody and developed as described above.
SDS-PAGE
Ten micrograms of MDI-HSA conjugates were separated on 10% acrylamide native or denaturing gels at 100 V for 1.5–2 hrs using Tris-Glycine based gel and buffer chemistry. Gels were stained using Imperial Stain (Life Technologies) and destained in water.
TNBS Assay
Samples or standards were diluted 1:1 with saturated borate buffer (pH 9.3). Twenty-five microliters of a 1:5.68 dilution of picrylsulfonic acid (2,4,6 trinitrobenxenesulfonic acid; TNBS) was added to each sample in a 96 deep-well polystyrene flat-bottom plate. Samples were mixed by pipetting and incubated at room temperature for 30 minutes. Absorbance was measured at 340 and 420 nm.
LC-MS/MS Analysis
LC-MS/MS analysis was performed as previously described(17, 18). Briefly, samples were brought to 45 mM ammonium bicarbonate, reduced with tributylphosphine (TBP), and alkylated with iodoacetamide (IAA) according to manufacturer’s instructions (Pierce). The samples were digested overnight at 30°C after the addition of 1.5 µL of porcine trypsin (1 µg/µL) (Sigma) dissolved in acetic acid. Peptides were separated on a Waters (Milford, MA) nanoACQUITY ultra-performance liquid chromatography (UPLC) system and directed to the nanoelectrospray source of a Waters SYNAPT MS quadrupole time-of-flight (qTOF) mass spectrometer. Collision-induced dissociation (CID) was performed and spectra were acquired in an “MSe” fashion(19). Data were analyzed with BioPharmaLynx v. 1.2 (Waters). Custom modifiers were created for two bound forms of MDI as described previously(17, 20).
Acid Hydrolysis and GC-MS Analysis of MDA
Acid hydrolysis was carried out as previously described with the following modifications(9). 1 mL of the MDI-HSA conjugate was incubated with 1 mL of 6 M hydrochloric acid (HCl) and 3200 ng of dideuterated MDA (MDDA) and incubated at 100°C overnight. 2.5 mL of 17.6 M sodium hydroxide was added to the hydrolysis product and vortexed. The sample was cooled on ice for 10 minutes before the addition of 1 mL of toluene. Samples were vortexed and placed at room temperature for 1 hr. 0.5 mL of the top phase was derivatized through the addition of 20 µL of pentafluoropropionic anhydride (PFPA) according to manufactures instructions. 200 µL of the organic top layer was analyzed using an Agilent 6890 gas chromatograph coupled to an Agilent 5975C mass spectrometer (MSD) using a 30-m Rxi-5MS column (Restek, Bellefonte, PA). Samples were injected (1 µL) in splitless mode into a 250° C inlet with a 6.0 minute solvent delay. Analytes were eluted from the column using 0.8 mL/min ultra-high purity helium as the carrier gas and an oven temperature program as follows: 70° C for 3.0 min and then ramped at 25° C/min to a final temperature of 300° C. The MSD source temperature was maintained at 200° C, and the quadrupole temperature was maintained at 150° C. The MSD was operated in selected ion monitoring mode (SIM), the monitored m/z were 132.0, 165.0, 166.1, 168.1, 489.1, 490.2, and 492.2. Quantitation was done using the sum of the areas under the curve for the selected ions. Standards and samples were blank corrected using acid hydrolyzed HSA without MDA and samples were normalized based on the MDDA internal standard.
Statistical Analyses
All analyses were performed using SAS/STAT software, version 9.3 of the SAS system for Windows. Conjugation methods were analyzed with mixed model analyses of variance (ANOVA) using the Proc Mixed procedure. Method and solvent were included as fixed effect variables and arranged as two-factor ANOVA’s and included an interaction term. For the group effort experiments, both batch and person were included in the model as random effects. For the experiments performed by a single individual, only batch was included as a random variable. Correlations were performed using Proc Corr. All differences were considered significant at p 2-phase ≈ fast. The results of both sets of sandwich ELISAs are in agreement with each other and the data are correlative (Figure 1 C; p 1000ng MDA/mg HSA) in the amount of MDA detected were observed for samples Drip-J. However, large deviations were also observed in samples Fast-J, Infusion 2, Infusion 3, and 2-phase-1, indicating that while conjugates prepared by the same method had similar immunoreactivity in the sandwich ELISAs, there was greater variability in the amount of MDA quantified. Furthermore, the total MDA quantified did not correlate with the mAb ELISA results (data not shown). On average, across all methods, 4.2±1.5 with a range of 2.7–5.8 moles of MDI per mole HSA was observed. This is consistent with previous observations at similar molar substitution ratios.(3, 21)
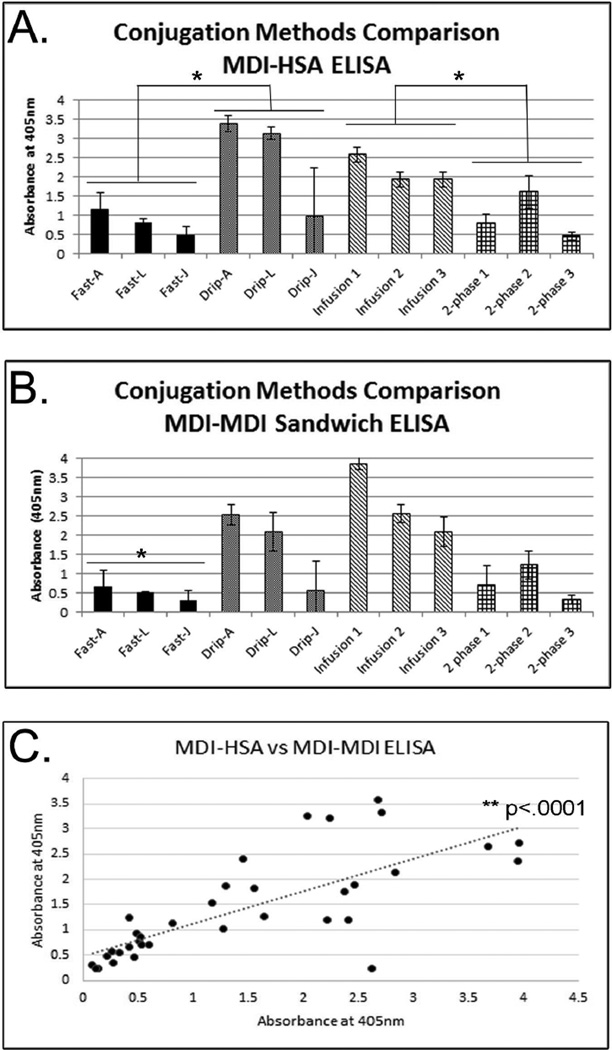
FIGURE 1. Immunoreactivity of MDI-HSA Conjugates.
(A) MDI-HSA ELISA and (B) MDI-MDI ELISA analyses of the immunoreactivity of conjugates prepared using different methods. For (A) and (B) IgM anti-MDI mAb 15D4 was used as a capture antibody. Conjugates prepared using either the fast, drip, infusion, or 2-phase methods were used as antigen and either an anti-HSA antibody (A) or anti-MDI IgG mAb DA5 (B) were used as the detection antibody. Results show the average of three different conjugates prepared the same way. ELISAs are corrected using unconjugated HSA as a control. For (B) fast method ELISA absorbances were significantly less than that from drip, infusion or 2-phase methods. (C) Correlation between results from (A) on the y-axis and (B) on the x-axis. * denotes p<.05; ** denotes p<.0001.
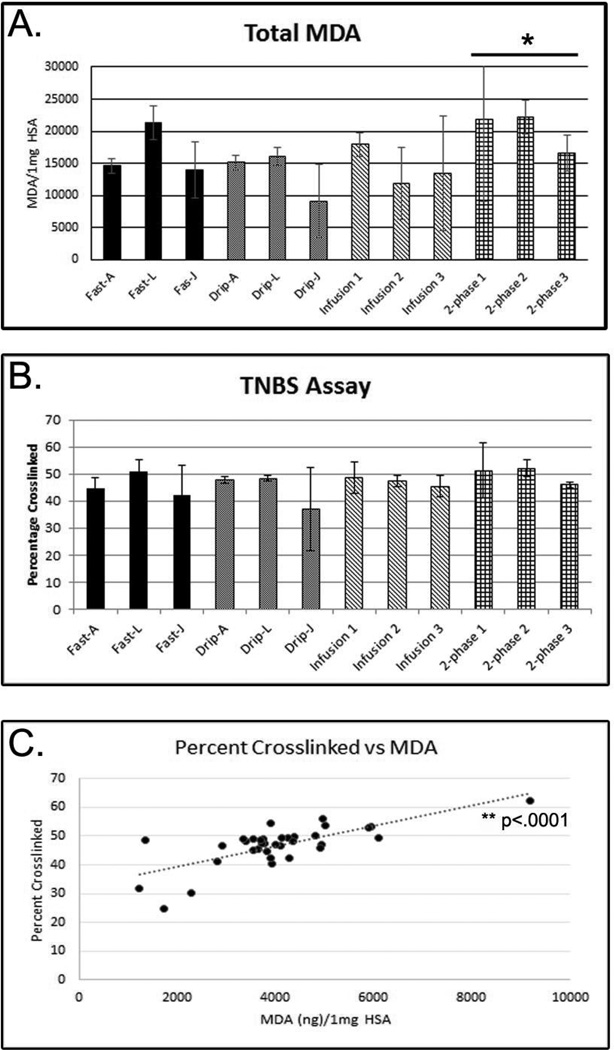
Picrylsulfonic acid (TNBS) reacts with free amines to yield a colorimetric change that can be measured by spectrometry. Here, reactivity of MDI conjugates with TNBS was used to determine the relative abundance of free amines in each sample and an estimate of the percentage of crosslinked amines was determined by comparison with unconjugated HSA. The results demonstrate that all samples had approximately the same amount of crosslinking with no statistically significant differences observed between any of the conjugation methods (Figure 2 B). Just as with the ELISA and the acid hydrolysis assays, large deviations (>15%) in crosslinking were observed for the Drip-J samples. The results from the TNBS assay correlate well with the results from the acid hydrolysis assay (Figure 2 C; p<0.0001) and suggest that similar amounts of MDI and crosslinking are present in each conjugate, regardless of the preparation method.
FIGURE 2. Chemical Measures of Conjugation.
(A) Quantification of the amount of MDA present in acid hydrolyzed samples. Results are shown as the number of micrograms of MDA per 1 mL of conjugate. (B) The percentage of crosslinking was assessed by measuring the abundance of free amines in each sample compared to unconjugated HSA. Results from (A) and (B) show the average of three different conjugates prepared the same way. (C) Correlation between results from (A) on the x-axis and (B) on the y-axis. * denotes p<.05 difference between 2-phase and infusion conjugates; ** denotes p<.0001.
Structural evaluation of dNCO adducts
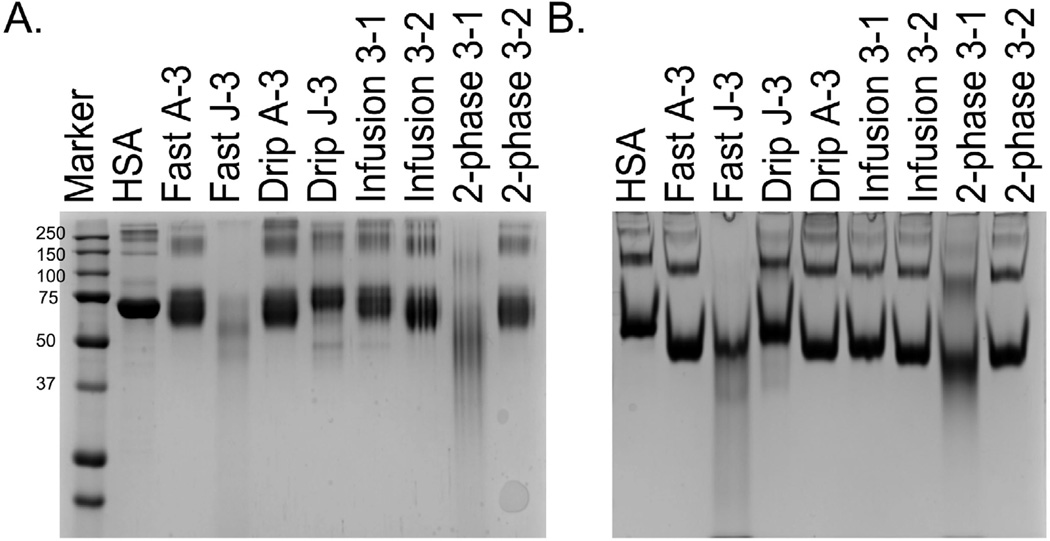
MDI conjugates were next examined by denaturing and native polyacrylamide gel electrophoresis to qualitatively assess inter- and intra- molecular crosslinking. Figure 3 A shows the results from 8 representative conjugates, 2 from each method, on a denaturing gel while Figure 3 B shows the results from the same conjugates on a native gel. Table I displays the results of the ELISAs, acid hydrolysis, and TNBS assay for each of the conjugates examined by gel electrophoresis. In general, there is variability in the migration of the conjugates through both the native and denaturing gels. The migration pattern does not necessarily reflect the results of the ELISAs, nor the amount of MDA or crosslinking observed. For example, Fast A-3, Drip A-3, and Infusion 3–1 have similar mobility on both the denaturing and native gels. These samples have an absorbance of 0.41, 2.67, and 1.64 on the MDI-MDI ELISA, 15.7, 14.8, and 11.2 µg MDA, and 40, 47.5, and 41% crosslinking, respectively (Table I). One interesting exception is sample 2-phase 3–1, which showed smearing and increased mobility on both the native and denaturing gels, indicative of high levels of inter- and intra-molecular crosslinking, and had 62% crosslinking and 36.7 µg of MDA, which were, by far, the highest values observed for any sample. However, this sample had only an absorbance of 1.28 and 1.01 on the MDI-MDI and MDI-HSA ELISAs, respectively. Thus, while large differences in the extent of modification can easily be observed by gel electrophoresis, many of the nuances reflecting the amount of MDI, crosslinking, and immunoreactivity cannot be observed by electrophoresis. Conjugates prepared using the infusion method had the most consistent appearance on denaturing gels (data not shown), implying that using an automated pump will remove inter- and intra-personal variability allowing for a more consistent preparation of the conjugates.
FIGURE 3. Electrophoretic Mobility of Conjugates.
10 µg of each conjugate was loaded onto a denaturing (A) or native (B) 8% Tris-Glycine gel. The Fast and Drip samples are designated by (user A or J)-(sample number:3). The infusion and 2-phase samples are designated by (MDI stock number:3)-(sample number: 1 or 2).
TABLE I.
ELISA, acid hydrolysis, and TNBS assay results for the 8 conjugates in Figure 3.
| Method | MDI-MDI ELISA (OD) |
MDI-HSA ELISA (OD) |
MDA/HSA (µg/mg) |
TNBS (%Crosslinked) |
|---|---|---|---|---|
| Fast A3 | .041 | .066 | 15.72 | 40.27 |
| Fast J3 | 0.61 | 0.71 | 17.55 | 49.69 |
| Drip A3 | 2.67 | 3.59 | 14.87 | 47.50 |
| Drip J3 | 0.12 | 0.24 | 4.90 | 31.74 |
| Infusion 3_1 | 1.64 | 1.26 | 11.29 | 41.00 |
| Infusion 3_2 | 2.41 | 1.20 | 17.39 | 48.38 |
| 2-phase 3_1 | 1.28 | 1.01 | 36.73 | 62.25 |
| 2-phase 3_2 | 0.82 | 1.13 | 23.59 | 52.85 |
Identification of MDI binding sites
Significant differences between the fast, drip, infusion, and 2–phase methods were observed using sandwich ELISAs, while differences between these methods were not observed when using chemical measures (i.e. TNBS or acid hydrolysis). This discrepancy may reflect changes in immunoreactivity due to variances in MDI-HSA binding sites. Using UPLC-MS/MS, the MDI binding sites on HSA were determined for three conjugates prepared using each method and are reported in Table II.(17, 18, 20) The most abundantly modified site amongst all samples was K73* (* represents hydrolyzed MDI; no * indicates MDI that has been cross-linked to two amines). The top three most abundant sites are signified with a XX. Nine sites of modification were identified for the fast method, 11 for the drip, 8 for the infusion, and 13 for the 2-phase. Interestingly, one of the most abundant sites modified using the 2 phase method was K233*, which was not observed for any other conjugate and has not been reported in the literature.(17, 21) This modification could occur as a result of a conformational change that occurs at the hydrophobic/hydrophilic interface. Additionally, while the 2-phase conjugates were only moderately antigenic in the ELISAs, they had the highest number of modified sites observed, and also had a higher percentage of modified versus unmodified peptides (data not shown), indicating that while they are not very immunoreactive, they are extensively modified. When examining sites of modification, there did not seem to be a trend between the conjugates that were more immunoreactive (drip and infusion) compared to those that were less immunoreactive (fast and 2-phase). One residue, K557, was modified in both the fast and 2-phase methods and was absent from the drip and infusion conjugates, although this residue was not one of the most abundantly modified. While correlations between immunoreactivity and the sites of modification were not defined, it remains possible that modification of K557 could result in reduced reactivity with the mAbs, and account for the differences observed in the ELISAs.
Table II.
Sites of MDI Modification on Human Serum Albumin.
| Fast | Drip | Infusion | 2-Phase | |
|---|---|---|---|---|
| K73 | X | XX | X | X |
| K73* | XX | XX | XX | XX |
| K162* | X | |||
| K190* | X | X | X | |
| K199* | X | X | ||
| K212* | XX | X | XX | XX |
| K233* | XX | |||
| K262* | X | XX | X | |
| K276 | X | X | X | |
| K378* | X | |||
| K402* | X | X | X | X |
| K413/414 | X | |||
| K414* | XX | XX | X | |
| K432 | X | |||
| K466* | X | |||
| K523/524 | X | |||
| K541* | X | X | ||
| K577 | X | X | ||
|
Total # of identified Modifications |
9 | 11 | 8 | 13 |
The X denotes the site was identified while XX denotes it was identified as one of the top three most abundantly modified sites.
Characterization of antigenicity
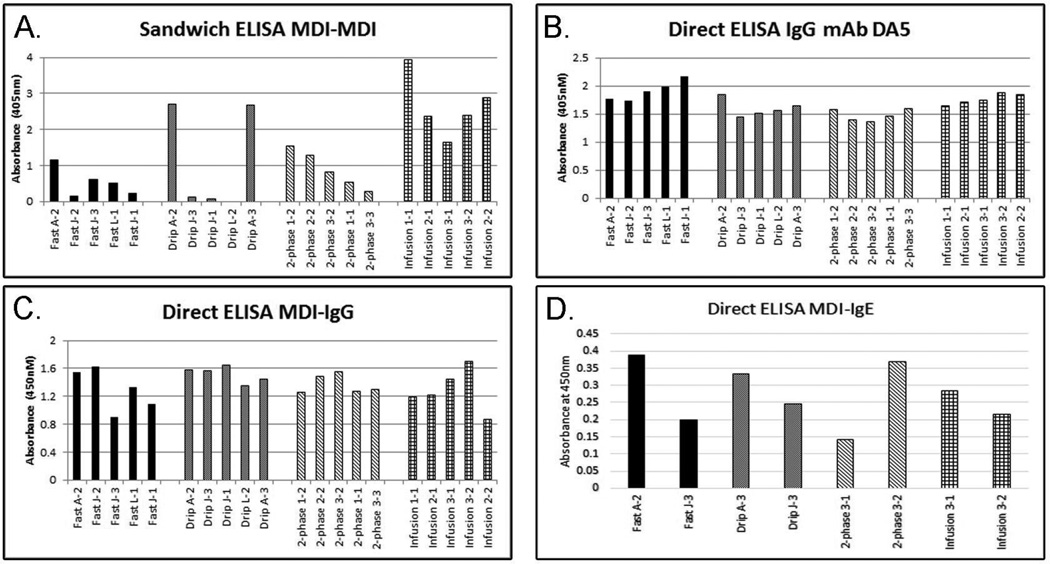
The data presented thus far suggest that there are differences in immunoreactivity between different conjugates and the mAbs despite similarities in the amount of MDI present and the level of crosslinking. To determine whether there were also differences in immunoreactivity to the IgG mAb DA5, a direct ELISA was performed using the conjugates directly bound to the plate and DA5 as the detection antibody. Unlike the MDI-MDI sandwich ELISA (Figures 1 A & 4 A), very little difference was observed in immunoreactivity using DA5 (Figure 4 B). Statistically, the fast method produced more immunoreactive conjugates than the drip or 2-phase methods (p<0.05), however the improved reactivity appeared to be minor (0.2–0.4 absorbance units; range = 1.5–1.9 between methods). The results from the IgG mAb DA5 direct ELISA did not correlate with the results from the sandwich MDI-MDI ELISA, suggesting that the results of the sandwich ELISA were largely dependent on antigenic differences detectable by mAb 15D4.
Figure 4. Immunoreactivity of Conjugates.
Five individual conjugates from each group were chosen based on results from Figure 1 B. (A) Immunoreactivity of conjugates in a sandwich ELISA with IgM mAb 15D4 as a capture and IgG1 mAb DA5 as a detection antibody. (B) Immunoreactivity of conjugates coated directly on the plate and detected using mAb DA5. (C & D) Antigenicity of the conjugates with human serum IgG (C) or IgE (D) from a representative sensitized individual. All assays are corrected using unconjugated HSA as a control.
To examine the clinical implications of the differently prepared conjugates, MDI-IgG and IgE positive human serum was used to screen select conjugates for antigenicity (Figure 4 C & D). Figure 4 C shows results from a representative serum sample screened for MDI specific IgG with the differently prepared conjugates. The results show no correlation with the MDI-MDI sandwich ELISA (Figure 4 A & C). In general, minor differences in human IgG antigenicity were observed across all samples, suggesting that the method for preparing antigen minimally effects serology results. Similar results were observed for all 4 human serum samples tested (data not shown). Differences in antigenicity in specific conjugates were observed and were variable across all serum samples tested (data not shown), suggesting that there may be a preferential antigen, but it likely varies on an individual basis, perhaps supporting a role for using a mixed antigen for specific antibody detection.
Figure 4 D shows the results from screening 8 conjugates for antigenicity to human MDI-specific IgE. Variances in antigenicity to MDI-IgE from an exposed worker were observed using the different conjugates, suggesting that differences in immunogenicity exist. Again, there is no correlation between the observed results and those from the MDI-MDI or MDI-HSA sandwich ELISAs. Interestingly, the 2-phase 3–1 sample and the Fast J3 sample showed reduced reactivity with human IgE and increased mobility and streaking on native and denaturing polyacrylamide gels (Figure 3 A & B), implying the immunogenic form of MDI-HSA may not have extensive intra-molecular crosslinking.
To examine whether the absence of differences in the antigenicity of the conjugates observed for DA5 or for MDI-specific serum IgG were the result of antibody excess, inhibition ELISAs were performed. Either DA5 or MDI-IgG/IgE positive human serum were incubated with increasing concentrations of the different conjugates before being used for detection of a drip antigen. IC50 values were estimated and are reported in Table III. Results for the DA5 inhibition ELISA demonstrate increased inhibition by conjugates 2-phase 3–1 and Fast J-3, moderate inhibition by conjugates Drip A3, 2-phase 3–2, and Infusion 3–2, and weak inhibition by conjugates Fast A3, Infusion 3–1, and Drip J3. Interestingly, the conjugates with the increased inhibition (2-phase 3–1 and Fast J-3) showed increased mobility and streaking on polyacrylamide gels (Figure 3 A & B) and decreased antigenicity to human MDI-IgE (Figure 4 D). This suggests that DA5 has increased affinity for intra-molecular crosslinked molecules, but that this may not be reflective of human IgE antibodies. When the same conjugates were examined for inhibition using human serum IgG, no differences in inhibition were observed (Table III; IC50 values are reported as ng of antigen.). The inhibition ELISA was repeated on three different human serum samples, which all demonstrated similar results (data not shown).
Table III.
Inhibition ELISAs.
| Fast A-3 |
Fast J-3 |
Drip A-3 |
Drip J-3 |
Infusion 3–1 |
Infusion 3–2 |
2-Phase 3–1 |
2-Phase 3–2 |
|
|---|---|---|---|---|---|---|---|---|
| Mab DA5 | >25 | 5.17 | 21.93 | >25 | >25 | 16.28 | 6.79 | 17.20 |
| Human Serum IgG | 0.22 | 0.28 | 0.24 | 0.29 | 0.24 | 0.22 | 0.35 | 0.25 |
IC50 values are given in g.
DISCUSSION
This study characterized MDI–albumin conjugates prepared using different methods for potential use as antigens in clinical serology. The findings within suggest significant differences in mAb specificity that could be important when developing clinical immunoassays using mAbs.(12) The data herein suggest that DA5 preferentially recognizes conjugates with extensive intra-molecular crosslinking of HSA (Figures 3 A, 4 B and 5 A). Extensive intra-molecular crosslinking was observed for two samples prepared by the fast and 2-phase methods (Figure 3 A), but was not consistently observed using any of the preparation methods, although the 2-phase method produced more conjugates with extensive intra-molecular crosslinking than any of the other methods (data not shown). Furthermore, this specificity was only observed under conditions of limiting antibody. Conversely, ELISAs data demonstrated that the IgM 15D4 exhibits preferential binding to epitopes on the drip and infusion conjugates that is either not present or less abundant in the fast and 2-phase conjugates (Figure 1). Evaluation by SDS-PAGE, TNBS, acid hydrolysis, and LC-MS/MS did not reveal any observable differences between these conjugates. One parameter that has not been tested is the extent of polymerization of the MDI on the HSA. It is possible that some methods result in fewer modified HSA molecules that have larger polymers bound which would not be distinguished by any of the chemical methods used in this study. These findings suggest that for clinical assay development, particularly for sandwich ELISA development, it is imperative to characterize the immunoreactivity of the standard antigens with the mAbs to ensure the highest level of sensitivity is achieved.(10–12, 22)
While large differences in immunoreactivity were observed between differently prepared conjugates by mAb 15D4, significant differences in antigenicity between the differently prepared conjugates and serum antibodies were not readily observed. Four human serum samples were screened in a direct ELISA against 20 different conjugates coated directly on the plate. Some differences in antigenicity between serum IgG and different conjugates were observed, although these differences did not correlate with the method used to prepare the conjugates (Figure 4 B & C). Also, the variances observed in antigenicity were not consistent between subject samples. Inhibition ELISAs demonstrated that the lack of difference in antigenicity between the conjugates was not the result of having excess antibody in the assay (Table III). IgE ELISA results indicate that the immunogenic form(s) of MDI-protein producing sensitization may not have appreciable levels of intra-molecular crosslinking (Figure 3 and 4 D). Further testing of additional serum samples will be needed to determine if this result is observed in other MDI-exposed workers. Taken together, this data suggests that, for the purposes of screening human serum samples using a direct ELISA, the method for antigen preparation may have less influence than the conjugation ratio or carrier used. This may be the result of the polyclonal nature of serum antibody responses. It is therefore, not surprising that differences in immunoreactivity exist between differently prepared antigens and mAbs but not between the differently prepared antigens and serum antibodies.
It is important to note that several limitations to this study exist. The human samples used in these studies were previously identified as positive for MDI antibodies using a method similar to the drip method for antigen preparation. It would be interesting to test the differently prepared conjugates with serum from patients that have been identified as having MDI-OA by SIC but tested negative for MDI-IgE. Furthermore, human serum samples were limited and allowed for IgE analysis of only one individual. Future studies examining a worker population for MDI-IgE using conjugates that differ in the method of preparation, and perhaps a mixture of conjugates, should be performed to truly assess whether particular conjugates produce increased sensitivity in ELISAs for detection of MDI-sensitized individuals.
CONCLUSIONS
The results from these studies suggest that while different methods of conjugate preparation result in variances in the conjugates that can be observed by ELISAs with mAbs, there is little to no discrimination between the differently prepared conjugates by the polyclonal antibody population in serum from exposed workers.
REFERENCES
- 1.Ott MG, Jolly AT, Burkert AL, Brown WE. Issues in diisocyanate antibody testing. Crit Rev Toxicol. 2007;37(7):567–585. doi: 10.1080/10408440701419553. [DOI] [PubMed] [Google Scholar]
- 2.Karol MH, Alarie YC. Serologic test for toluene diisocyanate (TDI) antibodies. J Occup Med. 1978;20(6):383. [PubMed] [Google Scholar]
- 3.Wass U, Belin L. Immunologic specificity of isocyanate-induced IgE antibodies in serum from 10 sensitized workers. J Allergy Clin Immunol. 1989;83(1):126–135. doi: 10.1016/0091-6749(89)90487-9. [DOI] [PubMed] [Google Scholar]
- 4.Tee RD, Cullinan P, Welch J, Burge PS, Newman-Taylor AJ. Specific IgE to isocyanates: a useful diagnostic role in occupational asthma. J Allergy Clin Immunol. 1998;101(5):709–715. doi: 10.1016/S0091-6749(98)70181-2. [DOI] [PubMed] [Google Scholar]
- 5.Cartier A, Grammer L, Malo JL, Lagier F, Ghezzo H, Harris K, et al. Specific serum antibodies against isocyanates: association with occupational asthma. J Allergy Clin Immunol. 1989;84(4 Pt 1):507–514. doi: 10.1016/0091-6749(89)90364-3. [DOI] [PubMed] [Google Scholar]
- 6.Keskinen H, Tupasela O, Tiikkainen U, Nordman H. Experiences of specific IgE in asthma due to diisocyanates. Clin Allergy. 1988;18(6):597–604. doi: 10.1111/j.1365-2222.1988.tb02911.x. [DOI] [PubMed] [Google Scholar]
- 7.Budnik LT, Preisser AM, Permentier H, Baur X. Is specific IgE antibody analysis feasible for the diagnosis of methylenediphenyl diisocyanate-induced occupational asthma? Int Arch Occup Environ Health. 2013;86(4):417–430. doi: 10.1007/s00420-012-0772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocker J. Biological monitoring for isocyanates. Ann Occup Hyg. 2011;55(2):127–131. doi: 10.1093/annhyg/meq083. [DOI] [PubMed] [Google Scholar]
- 9.Dalene M, Skarping G, Brunmark P. Assessment of occupational exposure to 4,4’-methylenedianiline by the analysis of urine and blood samples. Int Arch Occup Environ Health. 1995;67(2):67–72. doi: 10.1007/BF00572228. [DOI] [PubMed] [Google Scholar]
- 10.Lemons AR, Bledsoe TA, Siegel PD, Beezhold DH, Green BJ. Development of sandwich ELISAs for the detection of aromatic diisocyanate adducts. J Immunol Methods. 2013;397(1–2):66–70. doi: 10.1016/j.jim.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wisnewski AV, Liu J. Molecular determinants of humoral immune specificity for the occupational allergen, methylene diphenyl diisocyanate. Mol Immunol. 2013;54(2):233–237. doi: 10.1016/j.molimm.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemons AR, Siegel PD, Mhike M, Law BF, Hettick JM, Bledsoe TA, et al. A murine monoclonal antibody with broad specificity for occupationally relevant diisocyanates. J Occup Environ Hyg. 2014;11(2):101–110. doi: 10.1080/15459624.2013.843783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wisnewski AV, Stowe MH, Cartier A, Liu Q, Liu J, Chen L, et al. Isocyanate vapor-induced antigenicity of human albumin. J Allergy Clin Immunol. 2004;113(6):1178–1184. doi: 10.1016/j.jaci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Ye YM, Kim CW, Kim HR, Kim HM, Suh CH, Nahm DH, et al. Biophysical determinants of toluene diisocyanate antigenicity associated with exposure and asthma. J Allergy Clin Immunol. 2006;118(4):885–891. doi: 10.1016/j.jaci.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Campo P, Wisnewski AV, Lummus Z, Cartier A, Malo JL, Boulet LP, et al. Diisocyanate conjugate and immunoassay characteristics influence detection of specific antibodies in HDI-exposed workers. Clin Exp Allergy. 2007;37(7):1095–1102. doi: 10.1111/j.1365-2222.2007.02745.x. [DOI] [PubMed] [Google Scholar]
- 16.Wisnewski AV, Liu J, Redlich CA. Connecting glutathione with immune responses to occupational methylene diphenyl diisocyanate exposure. Chem Biol Interact. 2013;205(1):38–45. doi: 10.1016/j.cbi.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hettick JM, Siegel PD. Determination of the toluene diisocyanate binding sites on human serum albumin by tandem mass spectrometry. Anal Biochem. 2011;414(2):232–238. doi: 10.1016/j.ab.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Hettick JM, Ruwona TB, Siegel PD. Structural elucidation of isocyanate-peptide adducts using tandem mass spectrometry. J Am Soc Mass Spectrom. 2009;20(8):1567–1575. doi: 10.1016/j.jasms.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty AB, Berger SJ, Gebler JC. Use of an integrated MS--multiplexed MS/MS data acquisition strategy for high-coverage peptide mapping studies. Rapid Commun Mass Spectrom. 2007;21(5):730–744. doi: 10.1002/rcm.2888. [DOI] [PubMed] [Google Scholar]
- 20.Hettick JMaSPD. Comparative analysis of aromatic diisocyanate conjugation to human albumin utilizing multiplexed tandem mass spectrometry. Int. J. Mass Spectrometry. 2012;309:168–175. [Google Scholar]
- 21.Mhike M, Chipinda I, Hettick JM, Simoyi RH, Lemons A, Green BJ, et al. Characterization of methylene diphenyl diisocyanate-haptenated human serum albumin and hemoglobin. Anal Biochem. 2013;440(2):197–204. doi: 10.1016/j.ab.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruwona TB, Johnson VJ, Hettick JM, Schmechel D, Beezhold D, Wang W, et al. Production, characterization and utility of a panel of monoclonal antibodies for the detection of toluene diisocyanate haptenated proteins. J Immunol Methods. 2011;373(1–2):127–135. doi: 10.1016/j.jim.2011.08.011. [DOI] [PubMed] [Google Scholar]