Abstract
Bradykinin (BK) B2 receptor (R) and angiotensin (Ang) (1–7) MasR mediated effects are physiologically interconnected. The molecular basis for such cross-talk is unknown. It is hypothesized that the cross-talk occurs at the R level. We investigated B2R-MasR heteromerization and the functional consequences of such interaction. B2R fused to the cyan fluorescent protein and MasR fused to the yellow fluorescent protein were transiently co-expressed in HEK293T cells. Fluorescence resonance energy transfer (FRET) analysis showed that B2R and MasR formed a constitutive heteromer, which was not modified by their agonists. B2R or MasR antagonists decreased FRET efficiency, suggesting that the antagonist promoted heteromer dissociation. B2R-MasR heteromerization induced an 8-fold increase in the MasR ligand binding affinity. Upon agonist stimulation, the heteromer was internalized into early endosomes with a slower sequestration rate from the plasma membrane, compared to the single Rs. B2R-MasR heteromerization induced a greater increase in arachidonic acid release and ERK phosphorylation after Ang-(1–7) stimulation, and this effect was blocked by the B2R antagonist. Concerning Akt activity, a significant BK promoted activation was detected in B2R-MasR- but not in B2R-expressing cells. Ang-(1–7) and BK elicited anti-proliferative effects only in cells expressing B2R-MasR heteromers, but not in cells expressing each R alone. Proximity ligarion assay confirmed B2R-MasR interaction in human glomerular endothelial cells supporting the interaction between both Rs in vivo. Our findings provide an explanation for the cross-talk between BK B2R and Ang-(1–7) MasR mediated effects. B2R-MasR heteromerization induces functional changes in the R that may lead to long-lasting protective properties.
Keywords: FRET, Mas receptor, B2 receptor, internalization, heteromerization
The renin-angiotensin system (RAS) as well as the kallikrein-kinin system (KKS) contribute to fluid homeostasis and blood pressure regulation. The RAS is composed of two arms with opposing functions. The pressor arm, represented by the angiotensin type 1 receptor (AT1R), angiotensin-converting enzyme (ACE) and angiotensin (Ang) II, is responsible for the vasoconstrictive, proliferative, fibrotic and hypertensive effects of the RAS. In contrast, the second arm exerts depressor and cardiovascular protective effects through Ang-(1–7), the Ang-(1–7) specific receptor (R) Mas that transduces the main physiological actions of Ang-(1–7), and ACE2 that catalyzes the generation of Ang-(1–7) from Ang II.1–3
Bradykinin (BK), generated by the KKS, exerts cardioprotective, vasodilatory and depressor properties through B2R stimulation.4,5 Several studies showed the existence of a cross-talk between the RAS and the KKS.6,7 For instance, kallikrein catalyzes the generation of kinin from kininogen, but it also acts as a prorenin-activating enzyme leading to an increase in Ang II.6 In addition, ACE not only generates the vasopressor Ang II but is also responsible for the proteolytic degradation of BK.6,7
Not only was a cross talk between both systems reported at the enzymatic level, but also between the components of these systems. For example, Ang-(1–7) exerts kinin like effects and potentiates the effects of BK.6,8–11 In fact, BK was devoid of effect on blood vessels when the specific Ang-(1–7) R, the MasR, was blocked or genetically knocked down.12
On the other hand, the Ang-(1–7) effects such as the neuroinhibitory action on noradrenergic neurotransmission,13 the stimulatory effect on nitric oxide generation14 or the facilitation of the baroreflex control of the heart rate15 completely disappeared when the specific BK R subtype B2 was blocked. However, in not all circumstances the effects of these peptides depend on both B2R and MasR.16 Despite that, the function and the molecular basis for such cross-talk are still unknown.
It could be hypothesized that the cross-talk between both pathways occurs at the R level. Indeed, both B2R and MasR belong to the G protein-coupled receptor (GPCR) family. GPCRs exist as homo- or hetero-oligomers. Such structural organization has been found to be essential for R function. GPCR oligomerization affects important R functions like biosynthesis, plasma membrane diffusion or velocity, ligand-binding, pharmacology, signaling, and trafficking properties.17–19 Taking into account that BK actions are blocked by a MasR antagonist or when MasR is knocked-down, or that Ang-(1–7) response disappears when B2R is blocked, we hypothesized that B2R and MasR may interact directly through a heteromer formation. Our aim was to investigate the heteromerization between B2R and MasR and to determine its functional consequences.
Material and Methods
Cell culture and Transfection
HEK293T cells obtained from the American Type Culture Collection were grown in high-glucose Dulbecco’s Modified’s Eagle Medium, 10 mg/mL sodium pyruvate, 2 mM L-glutamine, 10% (vol/vol) FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37° C in a humidified atmosphere at 95% air and 5% CO2. Cells were transiently transfected with cDNA (3 µg per 9.5-cm2 tissue culture well) encoding for MasR fused to EYFP (MasR-YFP) and B2R fused to ECFP (B2R-CFP) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions and were used 48 h post-transfection. MasR-YFP cDNA was obtained as previously described.20 B2R-CFP cDNA was a gift from Dr. Faussner (Ludwig-Maximilians-University Munich, Munich, Germany). This construct was subcloned into BamHI-XhoI sites of pcDNA3.1 plasmid. The cloned genes were confirmed by restriction analysis and sequencing. Physical and biological containment of both recombinant plasmids conform to the National Institutes of Health guidelines.
Human glomerular endothelial cells (HGEC) were kindly gifted by Dr. María Marta Amaral. HGEC were isolated as previously described21 from kidneys removed from different pediatric patients undergoing nephrectomies performed at Hospital Nacional “Alejandro Posadas”, Buenos Aires, Argentina (written informed consent was obtained from the next of kin, caretakers, or guardians on the behalf of the minors/children participants involved). The Ethics Committee of the University of Buenos Aires approved the use of human renal tissues for research purposes.
Detailed methods are provided in the online-only Data Supplement.
Data analysis
The results are presented as mean ± SEM. One-way ANOVA computation combined with the Bonferroni test was used to analyze data with unequal variance between each group. A probability level of 0.05 was considered significant.
Results
B2R Interacts with MasR
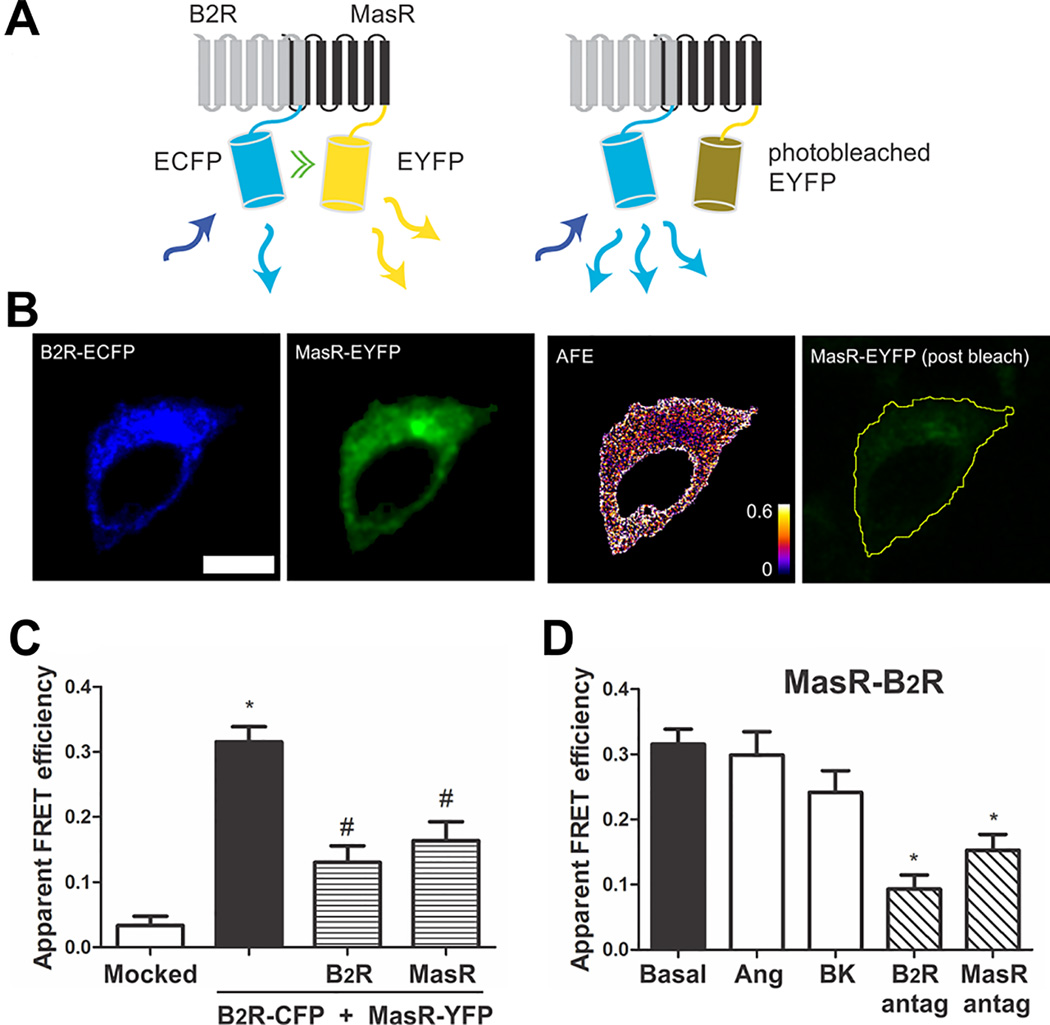
B2R-MasR heteromerization was investigated by fluorescence resonance energy transfer (FRET),22 so we generated fusion proteins of the B2R and MasR sequences tagged at their carboxyl terminus to the enhanced cyan fluorescence protein (ECFP) and enhanced yellow fluorescence protein (EYFP), respectively (see Data Supplement). Human embryonic kidney (HEK) 293T cells were cotransfected with B2R-CFP and MasR-YFP, and interaction between B2R and MasR was evaluated by FRET measured by acceptor photobleaching (APB).22 Figure 1A shows a representative FRET experiment. Fluorescence of YFP and CFP was observed in the cell membrane and cytoplasm of HEK293T cells coexpressing B2R-CFP and MasR-YFP, at pre-photobleaching. Acceptor photobleaching resulted in a decrease in YFP fluorescence and in a positive FRET (Figure 1B). To quantify FRET, we calculated the apparent FRET efficiency, which for tight interactions is proportional to the fraction of the donor in complex with the acceptor. For cells transiently co-expressing B2R-CFP and MasR-YFP with similar donor (ECFP) to acceptor (EYFP) ratio, a mean apparent FRET efficiency of 0.31±0.02 was measured (Figure 1C) demonstrating that both B2R and MasR constitutively interact by a heteromer formation.
Figure 1.
B2R interacts with MasR. (A) Schematic representation of FRET. B2R fused to ECFP (FRET donor) and MasR fused to EYFP (FRET acceptor) are coexpressed in the same cell. Upon excitation of the donor molecule (blue arrow) not only the donor emits the energy (light blue arrow), but also part of the energy is transferred to the acceptor (green double arrow) which also emits (yellow arrows) (left panel). Photodestruction of the acceptor molecule abolishes energy transfer which is evidenced by an increase in the donor emission (light blue arrows) (right panel). (B) Representative images of the FRET measurements (scale bar: 10 µm): B2R-CFP (FRET donor) and MasR-YFP (FRET acceptor) before acceptor photobleaching, apparent FRET efficiency (AFE) and resulting acceptor image after photobleaching. The AFE values can be between 0 and 1 and are proportional to the fraction of interacting B2R molecules. (C) Apparent FRET efficiency of cells transfected with the empty vector (mocked) or the vectors containing the DNA coding for B2R-CFP + MasR-YFP (black bar), B2R-CFP + MasR-YFP + untagged B2R (patterned bar) or B2R-CFP + MasR-YFP + untagged MasR (patterned bar). * P < 0.05 vs. mocked; # P < 0.05 vs. B2R-CFP + MasR-YFP. (D) Influence of ligands on B2R-MasR heteromer formation. Prior to FRET measurements, HEK293T cells transiently co-expressing B2R-CFP + MasR-YFP were incubated without (basal) or with 1 µmol/L BK or Ang-(1–7) or the B2 receptor antagonist Hoe or the Mas receptor antagonist A779 15 min at 37°C. * P < 0.05 vs. basal. Values are expressed as the mean±SEM from four independent experiments. 25 cells were analyzed per experiment.
To further corroborate FRET specificity between B2R and MasR, FRET was evaluated in cells transiently co-expressing B2R-CFP and MasR-YFP with the native B2R (without an ECFP moiety) or with the native MasR (without an EYFP moiety). If an interaction between B2R and MasR exist, a decrease in FRET apparent efficiency would be expected because of the formation of heteromers between B2R-CFP and untagged Mas or MasR-YFP and untagged B2R. As it is shown in Figure 1C, the presence of the untagged Rs resulted in a significant reduction in FRET apparent efficiency, demonstrating that untagged Rs are competing with tagged Rs for heteromer formation, thus reinforcing the interaction between both B2R and MasR. Altogether these results show that FRET arises from the interaction between the R moieties of the fusion proteins, and hence demonstrate the existence of constitutive B2R-MasR heteromers.
The influence of ligands on FRET efficiency was investigated by incubating the cells cotransfected with B2R-CFP plus MasR-YFP with 1 µmol/L BK or Ang-(1–7), for 15 min at 37°C, prior to FRET analysis. The formation of B2R-MasR heteromer was not altered by either of the agonists because FRET was not modified when the cells were stimulated with BK or Ang-(1–7) (Figure 1D), demonstrating that the oligomerization state of B2R-MasR complexes is not modulated by the agonist. On the contrary, the addition of B2R or MasR antagonists decreased FRET efficiency to about 50% of the initial value, indicating that the antagonist promoted the dissociation of the heteromer (Figure 1D).
All the subsequent experiments were carried out with the chimeras B2R-CFP and MasR-YFP, but to make the reading easier, the notation B2R and MasR was used instead.
MasR Displays Higher Affinity to its Ligand when Expressed in Combination with B2R
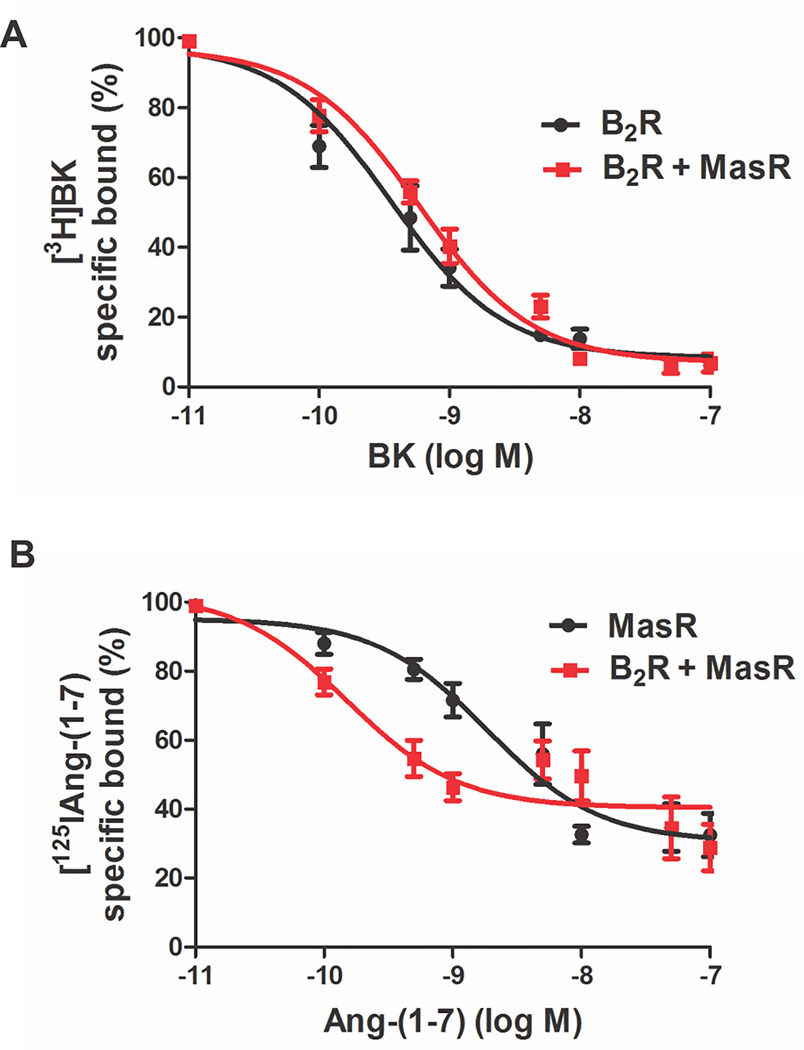
To determine whether B2R-MasR interaction leads to changes in R pharmacology, ligand binding properties of each R were examined. Competition radioligand-binding experiments were performed in B2R-MasR cotransfected cells and cells transfected with either B2R or MasR alone (Figure 2). From these curves, the ligand binding affinity constant was determined. Binding affinity of BK for the specific B2R remained unchanged in B2R- and B2R-MasR expressing cells (KD = 3.56±0.55×10−10 mol/L in B2R transfected cells and 5.8±0.68×10−10 mol/L in B2R-MasR transfected cells) (Figure 2A). Conversely, an 8-fold increase in binding affinity of Ang-(1–7) for its MasR was observed in cells expressing both B2R and MasR (KD = 1.15±0.28 ×10−9 mol/L in MasR-transfected cells and 1.39±0.55×10−10 mol/L in B2R-MasR transfected cells, P<0.05) (Figure 2B). These results suggested that heteromerization modified the pharmacological properties of MasR but not of B2R.
Figure 2.
MasR displays higher affinity to its ligand when expressed in combination with B2R. (A) Competition binding assay with [3H]BK in the presence of increasing concentrations of BK in cells expressing B2R (black line) or B2R-MasR (red line). (B) Competition binding assay with [125I]Ang-(1–7) in the presence of increasing concentrations of Ang-(1–7) in cells expressing MasR (black line) or B2R-MasR (red line). Data represent means+SE from four independent experiments performed in triplicate.
B2R-MasR Heteromer Internalizes into Early Endosomes Upon Agonist Stimulation
To determine whether heteromerization affects the trafficking properties of these Rs, agonist-induced redistribution of Rs from the cell surface (i.e., internalization) was examined. To investigate whether B2R-MasR heteromer was targeted to early endosome, B2R-CFP and MasR-YFP co-transfected cells were stimulated with 1 µmol/L Ang-(1–7) during 15 min and then co-localization with the early endosome marker Rab5 was evaluated. After Ang-(1–7) treatment, B2R and MasR internalization was observed as small bright spots within the cell (Figure S2A and S2B, respectively, of Data Supplement), in a distribution pattern that is characteristic of Rs located in intracellular endocytic vesicles. Quantitative colocalization analysis revealed Pearson correlation coefficient greater than 0.6 indicating that both B2R and MasR colocalized. Thus, B2R and Mas co-internalized upon ligand stimulation. We also evaluated whether the heteromer is endocyted to early endosome upon ligand stimulation. The analysis revealed that a fraction of the oligomer B2R-MasR colocalized with the early endosome marker Rab5 (Figure S2C and S2D of Data Supplement) (Pearsons correlation coefficient: 0.82±0.1, P<0.01 by 1-tail Wilcoxon matched pairs signed ranks test). Similar results were observed after BK stimulation. Altogether, these results showed that the oligomer B2R-MasR was targeted mostly to early endosomes after agonist stimulation.
Ang-(1–7) Induces B2R Internalization when B2R-MasR Heteromer is Formed
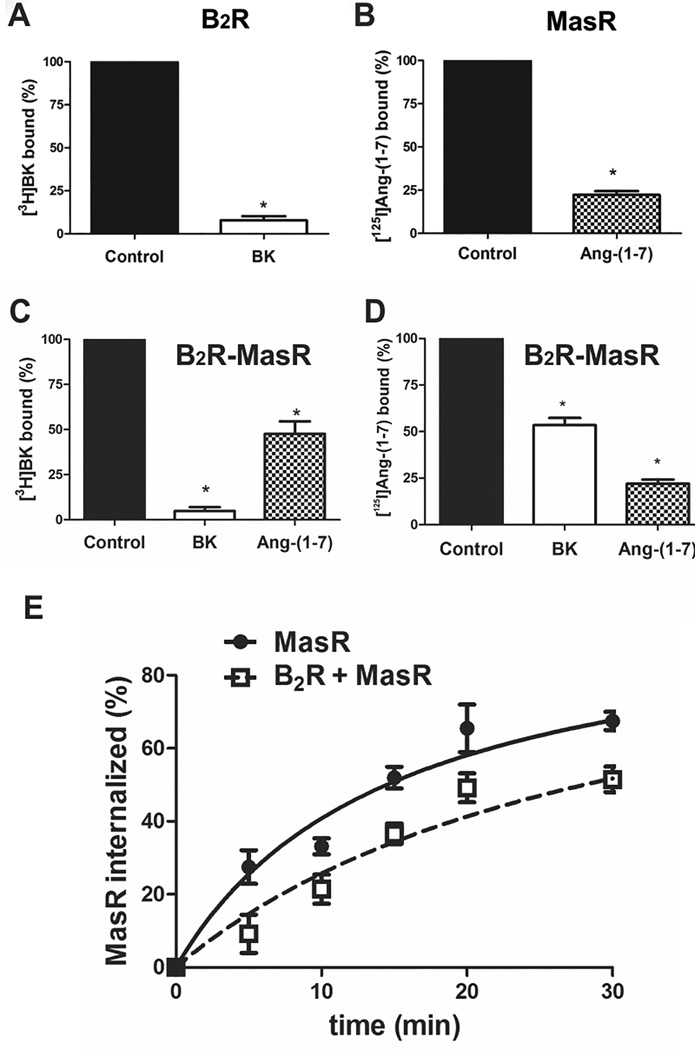
To further confirm the existence of B2R-MasR heteromer and its internalization, we investigated whether selective stimulation of one of the R promotes co-internalization of both Rs. To induce R internalization, cells were stimulated with the selective B2R agonist BK (1 µmol /L) or the selective MasR agonist Ang-(1–7) (1 µmol/L) for 30 min. The amount of B2R or MasR present in the plasma membrane after agonist stimulation was evaluated by radioligand binding assays. B2R transfected cells stimulated with BK or MasR transfected cells stimulated with Ang-(1–7) showed a significant decrease in the amount of Rs present in the plasma membrane (Figure 3A and B, respectively), indicating that the R was internalized upon agonist stimulation. When B2R-MasR transfected cells were stimulated with BK there was a decrease of 91±4% in B2R and 53±3% in MasR in the plasma membrane (Figure 3C and D). Conversely, when B2R-MasR transfected cells were stimulated with Ang-(1–7) there was a decrease of 58±4% in B2R and 82±6% in MasR present in the plasma membrane (Figure 3C and D).
Figure 3.
Agonist-mediated internalization of receptors. Cells expressing (A) B2R, (B) MasR or (C and D) B2R-MasR were incubated in the absence (control, black bar) or presence of (1 µmol/L) BK (white bar) or (1 µmol/L) Ang-(1–7) (patterned bar) for 15 min, and the percentage of B2R (A and C) or MasR (B and D) present in the membrane was analyzed by ligand binding assay as described under Methods. * P < 0.05 vs. control. (E) Effect of B2R coexpression on the time course of MasR sequestration. Cells expressing MasR (solid line) or B2R-MasR (dotted line) were incubated with Ang-(1–7) (1 µmol/L) for the indicated time intervals and receptor sequestration determined as described under Methods. Values are mean+SEM of four different experiments each performed in triplicate.
These results clearly demonstrate that in cells co-expressing both Rs the selective stimulation of one of the GPCRs promotes co-internalization of both Rs.
B2R-MasR Heteromerization Results in a Decreased Rate of Ang-(1–7)-Mediated MasR Sequestration
Since B2R-MasR interaction may lead to changes in the rate of R sequestration from the plasma membrane after agonist stimulation, we evaluated the percentage of internalized MasR after Ang-(1–7) treatment. Cells were stimulated with Ang-(1–7) (1 µmol/L) during different times for the time intervals ranging from 5 min to 40 min and the amount of MasR present in the plasma membrane was determined by radioligand binding assay. R sequestration was then defined as the decrease in specific [125I]Ang-(1–7) binding, compared with the total binding obtained in untreated cells.
Cells transiently co-expressing B2R and MasR revealed a slower sequestration of MasR upon stimulation with Ang-(1–7), compared to cells transiently expressing only MasR (Figure 3E). The rate of Ang-(1–7)-induced MasR internalization was significantly decreased by coexpression of B2R (p<0.05), as the estimated t1/2 was 10.6±1.2 and 16.9±0.8 min in MasR- and in B2R- and MasR-expressing cells, respectively.
B2R-MasR Heteromerization and Downstream Signaling
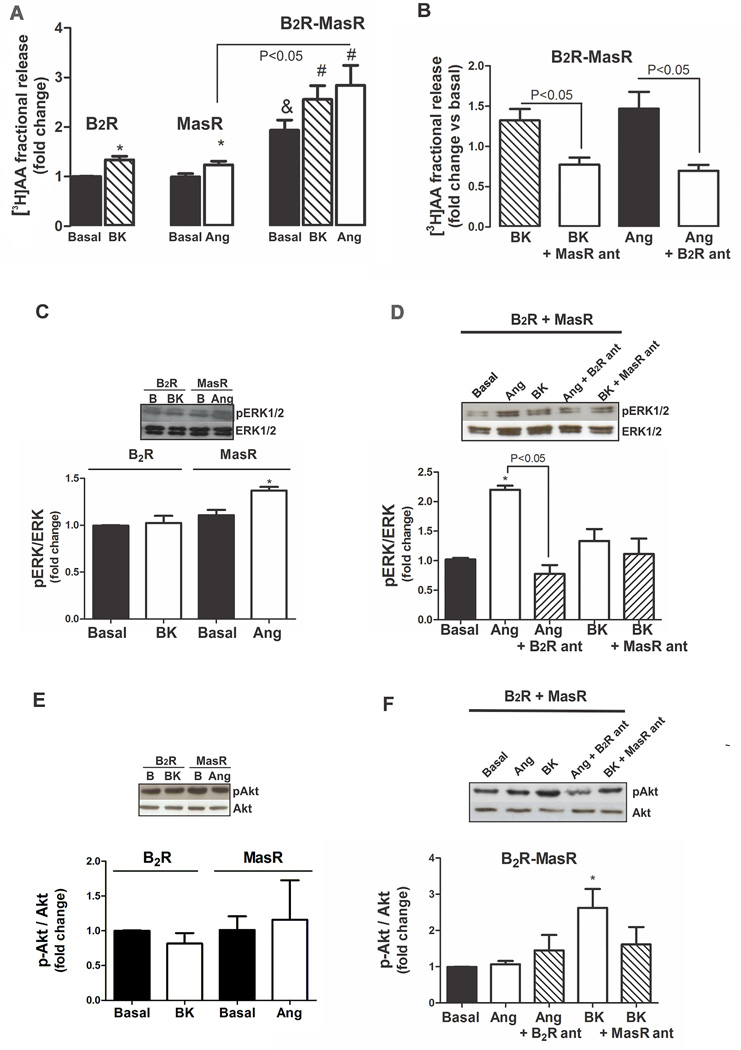
We determined whether heteromerization leads to alterations in functional coupling. AA release in response to BK or Ang-(1–7) was examined in cells expressing individual or both Rs. Cells expressing both B2R and MasR showed an increase in AA release under basal condition which was near 2-fold higher than that observed in cells expressing one of the Rs (Figure 4A). BK or Ang-(1–7) treatment resulted in a significant increase in AA release in cells transiently expressing B2R or MasR alone or together, being the effect elicited by Ang-(1–7) greater in cells expressing both Rs (Figure 4A).
Figure 4.
B2R-MasR heteromerization and downstream signaling. (A) [3H]AA release from cells expressing B2R, MasR or B2R-MasR, incubated in the absence (basal, black bar) or presence of BK (patterned bar) or Ang-(1–7) (white bar). Results are presented as fold change of the response detected in untreated B2R transfected cells. * P < 0.05 vs. untreated B2R- or MasR-transfected cells; & P < 0.05 vs. cells transfected with only one receptor; # P<0.05 vs untreated B2R-MasR transfected cells. (B) [3H]AA release from cells expressing B2R-MasR, incubated with BK (patterned bar) or BK + MasR antagonist (white bar) or Ang-(1–7) (black bar) or Ang-(1–7) + B2R antagonist (white bar). Results are presented as fold change of the response detected in untreated B2R-MasR-transfected cells. Values are mean ± SEM of four independent experiments performed in triplicate. (C–F) Effect of B2R-MasR heteromerization on ERK 1/2 and Akt phosphorylation. ERK 1/2 (C) and Akt (E) phosphorylation in HEK293T cells expressing B2R or MasR incubated in the absence (basal) or presence of BK or Ang-(1–7) (Ang), respectively. ERK 1/2 (D) and Akt (F) phosphorylation in HEK293T cells expressing B2R-MasR incubated in the absence (basal) or presence of Ang-(1–7) (Ang) or Ang-(1–7) + B2R antagonist (ant) or BK or BK + MasR antagonist (ant) for 15 min. * P < 0.05 vs. basal. Results are expressed as fold change of the response detected in basal conditions. Each bar represents the mean ± SEM of 4 independent preparations.
The increase in AA release induced by Ang-(1–7) (1 µmol/L) in cells co-expressing both B2R and MasR was blocked by the B2R antagonist Hoe 140 (10 µmol/L). Conversely, the increase in AA release induced by BK in cells co-expressing both B2R and MasR was blocked by the MasR antagonist A779 (10 µmol/L) (Figure 4B). These results support B2R-MasR heteromerization.
We also investigated whether B2R-MasR heteromerization affects ERK mitogen-activated protein kinase (MAPK) activation. BK (1 µmol/L) had no effect on ERK phosphorylation in cells transiently expressing the B2R alone, while Ang-(1–7) (1 µmol/L) induced a significant increase in ERK phosphorylation in cells transiently expressing the MasR alone (Figure 4C). Cells transiently co-expressing both B2R and MasR showed no change in ERK phosphorylation under basal condition compared to that observed in cells expressing one of the Rs (Figure S3A of Data Supplement), indicating that the B2R-MasR heteromer does not modify constitutive ERK activation. In cells expressing both B2R and MasR, ERK phosphorylation was not modified by BK treatment, but it was increased in response to Ang-(1–7) and this effect was greater compared to cells expressing only MasR. The Ang -(1–7)-stimulated ERK phosphorylation was prevented by pretreatment with the B2R antagonist (Figure 4D).
The effect of B2R-Mas heteromerization on Akt phosphorylation was also evaluated. BK or Ang-(1–7) had no effect on Akt phosphorylation in cells transiently expressing B2R or MasR alone, respectively (Figure 4E). Cells transiently co-expressing both B2R and MasR showed no change in Akt phosphorylation under basal condition compared to that observed in cells expressing one of the Rs (Figure S3B of Data Supplement). Conversely, BK induced an increase in Akt phosphorylation in cells expressing both B2R and MasR, and this effect was partially prevented by pretreatment with the MasR antagonist (Figure 4F).
Altogether, this data supports the presence of B2R-MasR heteromerization and the consequences on downstream signaling.
BK or Ang-(1–7) Induces Anti-Proliferative Effect Only when B2R-MasR Heteromer is Formed
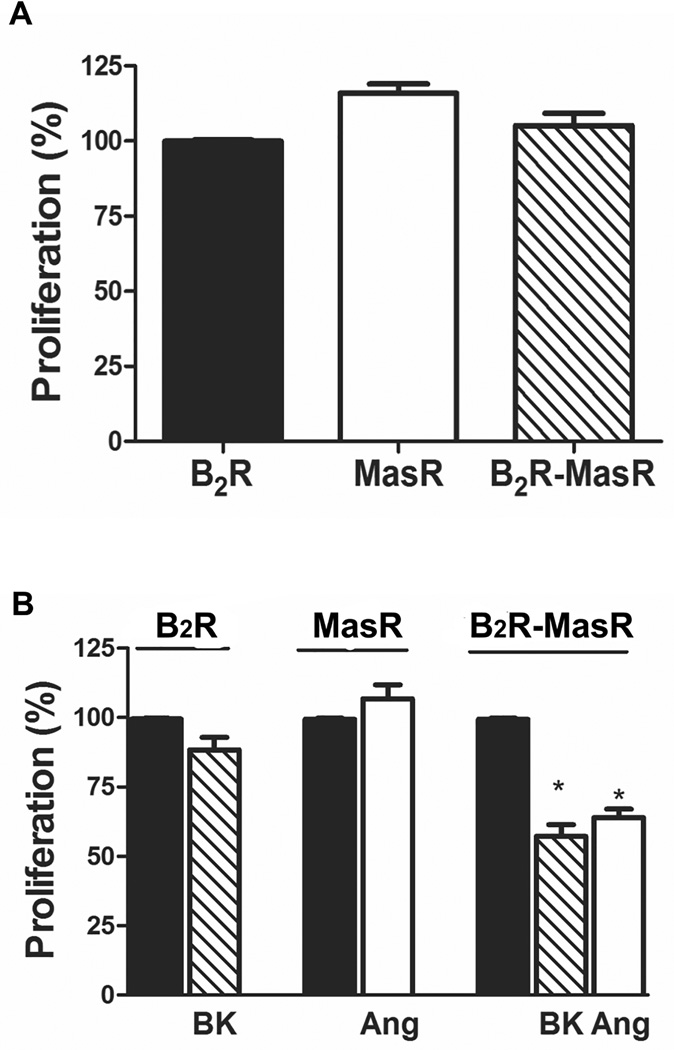
We tested whether B2R-MasR heteromerization may lead to changes in the biological response, e.g. cellular proliferation. Cells expressing individual or both Rs did not show differences in the cellular growth (Figure 5A). BK or Ang-(1–7) (100 nmol/L or 1 µmol/L) were without effect on cellular proliferation in cells expressing only B2R or MasR, respectively (Figure S4 and Figure 5B, respectively). However, in cells co-expressing both Rs, 1 µmol/L BK or Ang-(1–7) induced a decrease in cellular proliferation (43±5% reduction in BK-treated cells and 36±6% reduction in Ang-(1–7)-treated cells) (Figure 5B). There was a tendency to reduce cellular proliferation after incubation with 100 nmol/L BK or Ang-(1–7) (Figure S4 of Supplemental Data) not statistically significant. This reduction was lower than that observed with higher concentrations of the ligands. Thus, BK or Ang-(1–7) induced an anti-proliferative response when B2R heteromerized with MasR.
Figure 5.
B2R-MasR heteromerization and cellular proliferation. Cellular proliferation was evaluated in HEK293T cells expressing B2R, MasR or B2R-MasR incubated in the absence (panel A and black bar in panel B) or presence of BK or Ang-(1–7) (Ang) as described in Methods. Results are expressed as the percentage change of proliferation detected in untreated B2R-transfected cells (A) or in basal conditions (black bars in panel B). * P< 0.05 vs. its corresponding basal. Each bar represents the mean ± SEM of quintuplicate of 4 independent experiments.
B2R-MasR Heteromer Formation in Physiological Conditions
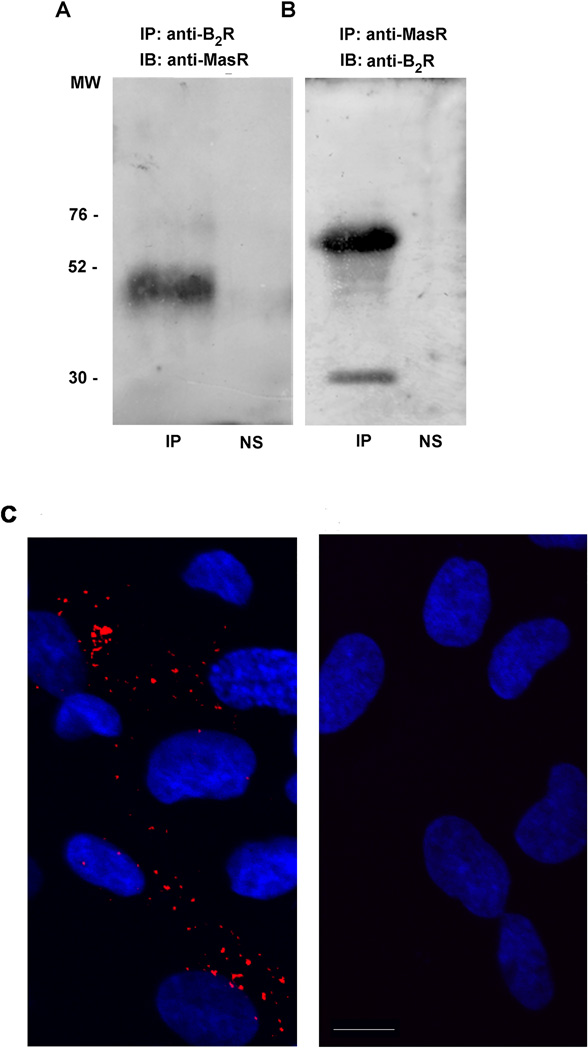
To establish that our results are not specific to transfected HEK293T cells and that B2R-MasR oligomerization also occurs in cells that natively express both Rs, we evaluated B2R-MasR interaction in mesenteric vascular bed from rats. Upon immunoprecipitation of the B2R from mesenteric vascular bed lysate we observed co-precipitation of the MasR (Figure 6A). Conversely, upon immunoprecipitation of the MasR from mesenteric vascular bed lysate we observed co-precipitation of the B2R with two bands detected in this situation (Figure 6B). The lower band may correspond to the immature B2R, as previously suggested.23
Figure 6.
Mas and B2 receptors interact in physiological conditions. Immunoprecipitation of lysates from Wistar rat mesenteric vascular beds were performed using (A) anti-B2R or (B) anti-MasR antibodies. Immunoprecipitates (IP) were analyzed by SDS-PAGE and immunoblotted (IB) using the indicated antibodies. These blots are representative of four different experiments with similar qualitative results. NS: non-specific, which refers to omission of the primary antibody in the coimmunoprecipitation assay (C) B2R and MasR interaction measured by proximity ligation assay (PLA) in human tubulointerstitial and glomerular endothelial cells. Fixed cells were permeabilized with 0.2% Triton X-100 for 1 h, and then blocked in blocking buffer for 1 h. B2R (anti-mouse) and MasR (anti-rabbit) antibodies were diluted in blocking solution, and incubated with the cells overnight at 4°C (A). PLA was conducted using Duolink® In Situ Orange Starter Kit Mouse/Rabbit (Sigma, St. Louis, MO, USA) following manufacturer´s instructions. Cells presented in image B were subjected to the same protocol but without the addition of the primary antibodies. Scale bar=15 µm
To further confirm B2R-MasR interaction, we performed proximity ligation assay (PLA). HGEC cells were incubated in the presence or absence of B2R and MasR antibodies and PLA carried out following manufacturer´s instructions. Figure 6C showed a positive PLA signal observed in cells incubated with the antibodies (left). Conversely, a PLA signal was absent in cells not incubated with the primary antibodies (right). Taken together the data show that B2R and MasR form heteromers in physiological conditions, supporting the interaction between both Rs in vivo.
Discussion
The present study demonstrates that B2R and MasR form constitutive and agonist independent functional heteromers. The results of co-immunoprecipitation experiments in rat mesenteric vascular bed and of positive PLA in human glomerular endothelial cells provide direct evidence that these Rs also form heteromers in vivo (present results).
Previous studies have demonstrated heteromerization of MasR with the AT1R and that this heteromerization inhibited the actions of Ang II.24 In this situation, MasR acts as a physiological antagonist of AT1R. B2R has been described to form constitutive heteromers with AT1R. AT1R-B2R heteromerization was shown to enhance AT1R-stimulated signaling under pathophysiological conditions such as experimental and human pregnancy hypertension.25, 26 However, the universality of the formation of AT1R-B2R heteromers has been questioned.6 B2R also forms heteromers with B1R in co-transfected HEK293 cells and in natively expressing endothelial cells, resulting in significant internalization and desensitization of the B1R response in cells pre-treated with the B2R agonist.27 Concerning B2R and MasR, to our knowledge this is the first evidence showing B2R-MasR heteromerization, which is constitutive and independent of the presence of physiological ligands.
B2R-MasR heteromerization may explain the potentiation in BK or Ang-(1–7) responses when both ligands are incubated together.6, 8–11, 28 Although we could not disregard that BK-Ang-(1–7) interaction may not result only from B2R-MasR heteromerization but also from ACE inactivation by Ang-(1–7). It has been shown that Ang-(1–7) at micromolar levels inhibits ACE thus enhancing BK vasodilatory effects.11, 29,30 In contrast, the addition of B2 or Mas R antagonists decreased FRET efficiency to about 50% of the initial value, suggesting that the antagonist promoted the dissociation of the heteromer and that the formation of B2R-MasR heteromer is essential for agonists to display many of its biological responses. For instance, BK or Ang-(1–7) induced an anti-proliferative response when B2R-MasR heteromer is formed and not when each R (B2R or MasR) is expressed alone (present results) showing the importance of this interaction for some of the effects elicited by BK or Ang-(1–7). B2R-MasR heteromerization may also explain the lack of effect of Ang-(1–7) when B2R is blocked13–15 or the absence of the BK-induced vasodilatory response when MasR is knocked down.12 In agreement, our present study shows that the Ang-(1–7) induced increase in AA release or in ERK phosphorylation was prevented by the B2R antagonist or that the BK-stimulated Akt phosphorylation was prevented by the MasR antagonist.
Heteromerization may result in changes in affinity for a specific ligand, R trafficking, maturation and signaling.17–19 Several reports demonstrated changes in pharmacological properties of the Rs because of heteromerization. Our present study showed that the coexpression of B2R leads to an increase in the affinity of Ang-(1–7) for its R Mas, although the vice versa situation was not observed. This result may explain the potentiation in the vasodilatory or blood pressure lowering responses when BK and Ang-(1–7) are present together.6, 8–11, 28
A receptor which is capable of producing a biological response in the absence of a bound ligand is said to display constitutive activity. Our study showed that constitutive ERK or Akt activarion was not modified by B2R-MasR heteromerization. Conversely, AA release was increased 2-fold in cells expressing both B2R and MasR. We may speculate that in this last situation not only R heteromerization but also a cross-talk between both R may occur, and this may explain the increase in AA release.
B2R-MasR heteromerization also influences downstream signaling. Our present study showed that B2R-MasR heteromerization induced a greater response concerning AA release and ERK phosphorylation upon Ang-(1–7) stimulation. Furthermore, BK stimulated Akt phosphorylation only when both B2R and MasR were co-expressed but not when the B2R was expressed alone, suggesting that this was a “biochemical fingerprint” associated to the heteromer, because it was abolished when heteromerization was disrupted by the cross-antagonist. For instance, β1-adrenergic R (AR) - β2-AR heteromerization induced a reduced ability of the β2-AR to stimulate ERK-phosphorylation31 or the AT1R-D2R heteromerization affected calcium mobilization but not cAMP signaling coupled to AT1R.32 In both examples as it happens in B2R-MasR heteromerization (present results) one receptor modifies downstream signaling of the partner receptor, giving a biochemical fingerprint, which is characteristic of R heteromerization.33
Akt phosphorylation is one of the downstream signaling mediating Ang-(1–7) actions in cultured cells and tissues.1,2 It was surprising to find that Ang-(1–7) stimulation did not elicit Akt activation (present results). Despite that, constitutive Akt activation was present in MasR-transfected cells, suggesting that MasR activation is coupled to Akt activation despite the fact that Ang-(1–7) was absent. We do not have an explanation for the absence of effect of Ang-(1–7) on Akt phosphorylation. Conversely, it does not imply that B2R-MasR interaction does not take place in non-transfected cells because in the present work we provide evidence that this interaction occurs in vascular bed from rats and in renal cells from humans.
R endocytosis serves a variety of purposes, including R downregulation, recycling and relocalization of the cell signaling. B2R follows an internalization pathway involving redistribution to caveolae, through an arrestin-and dynamin-independent pathway.34 However, other evidence showed that B2R was targeted to early endosome following agonist stimulation.35 MasR is internalized upon ligand stimulation into early endosomes via a clathrin-dependent pathway.20 Our study showed that B2R-MasR heteromer was internalized mostly into early endosomes and that stimulation of only one of the Rs was sufficient to promote cointernalization of the entire dimer, reinforcing the concept not only of B2R-MasR heteromer formation but also of cointernalization upon agonist stimulation.
Heteromerization of GPCRs can affect the internalization properties of the individual Rs.17 Our present data demonstrate that B2R-MasR heteromerization results in a delayed sequestration of the MasR when stimulated with its ligand Ang-(1–7). Similar observations were made for other GPCRs.36–38 Rapid desensitization and R internalization and trafficking tightly control the temporal and spatial regulation of GPCR signaling and hence the biological response. Both B2R and MasR have been associated with cardioprotective, vasodilatory and hypotensive responses.1–5 In addition, the present study showed that Ang-(1–7) induced anti-proliferative responses when B2R-MasR heteromer was formed but not when MasR is expressed alone. The delayed sequestration of MasR may cause this R to be present for a longer time in the plasma membrane, thus allowing a long-lasting protective response.
Perspectives
In conclusion, we have demonstrated that B2R and MasR can form heteromers that are internalized upon agonist stimulation. This heteromer revealed a delayed sequestration of the MasR upon stimulation with Ang-(1–7). Furthermore, B2R coexpression induced an increase in the affinity ligand binding properties of MasR. Altogether, these changes in R functional characteristics may lead to long-lasting protective biological properties. B2R-MasR heteromerization leads to altered functional properties in the putative heteromer and suggest that heteromerization may represent a regulatory cross-talk process arising through the creation of a receptor form with distinct functional properties. This is the first time that B2R-MasR heteromerization has been experimentally demonstrated. The existence of B2R-MasR heteromer may help in comprehending the mechanisms underlying BK and Ang-(1–7) cross-talk. Putting such evidence out to the community is an important, necessary, step towards further investigating the functional significance of such interaction in animal models. Furthermore, it would open new strategies for drug development acting through one of these receptors. Compounds targeting B2R-MasR heteromer could provide an alternative pharmacological approach for cardiovascular diseases treatment.
Supplementary Material
Novelty and Significance.
What is New
Both B2R and MasR are associated with cardioprotective, vasodilatory and hypotensive responses. We provide evidence for the first time that B2R form heteromeric complexes with MasR on the cell surface.
What Is Relevant?
B2R-MasR heteromerization increases ligand affinity and induces a delayed sequestration of the complex from the cell membrane, which may allow long-lasting protective biological responses.
Summary
B2R form heteromeric complexes with MasR on the cell surface. B2R-MasR heteromerization increases ligand affinity and induces a delayed sequestration of the complex from the cell membrane. Treatment with MasR or B2R antagonists decreases B2R-MasR interaction while the agonists display antiproliferative effects or Akt activation only when B2R-MasR heteromer is formed. The existence of B2R-MasR heteromer may help comprehending the mechanisms underlying BK and Ang-(1–7) cross-talk and developing drugs targeting these two systems involved in cardiovascular regulation. Compounds targeting B2R-MasR heteromer could provide an alternative pharmacological approach for cardiovascular diseases treatment.
Acknowledgments
We acknowledge the generous gift of HGEC cells from Dr. María Marta Amaral.
Sources of Funding
This work was supported by Universidad de Buenos Aires [20020120100194], Agencia Nacional de Promoción Científica y Tecnológica [2013–2170] and Consejo Nacional de Investigaciones Científicas y Técnicas [11220110101097] and by National Institutes of Health Grant [HL028982].
Footnotes
Conflict of Interest
None.
References
- 1.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1–7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 2013;216:R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 2.Gironacci MM, Cerniello FM, Longo Carbajosa NA, Goldstein J, Cerrato BD. Protective axis of the renin-angiotensin system in the brain. Clin Sci (Lond) 2014;127:295–306. doi: 10.1042/CS20130450. [DOI] [PubMed] [Google Scholar]
- 3.Varagic J, Ahmad S, Nagata S, Ferrario CM. ACE2: angiotensin II/angiotensin-(1–7) balance in cardiac and renal injury. Curr Hypertens Rep. 2014;16:420–436. doi: 10.1007/s11906-014-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su JB. Kinins and cardiovascular diseases. Curr Pharm Des. 2006;12:3423–3435. doi: 10.2174/138161206778194051. [DOI] [PubMed] [Google Scholar]
- 5.Leeb-Lundberg LMF, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- 6.Su JB. Different cross-talk sites between the renin-angiotensin and the kallikrein-kinin systems. J Renin Angiotensin Aldosterone Syst. 2014;15:319–328. doi: 10.1177/1470320312474854. [DOI] [PubMed] [Google Scholar]
- 7.Quitterer U, AbdAlla S. Vasopressor meets vasodepressor: The AT1-B2 receptor heterodimer. Biochem Pharmacol. 2014;88:284–290. doi: 10.1016/j.bcp.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Paula RD, Lima CV, Britto RR, Campagnole-Santos MJ, Khosla MC, Santos RA. Potentiation of the hypotensive effect of bradykinin by angiotensin-(1–7)-related peptides. Peptides. 1999;20:493–500. doi: 10.1016/s0196-9781(99)00031-5. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes L, Fortes ZB, Nigro D, Tostes RC, Santos RA, Catelli De Carvalho MH. Potentiation of bradykinin by angiotensin-(1–7) on arterioles of spontaneously hypertensive rats studied in vivo. Hypertension. 2001;37(2 Pt 2):703–709. doi: 10.1161/01.hyp.37.2.703. [DOI] [PubMed] [Google Scholar]
- 10.Abbas A, Gorelik G, Carbini LA, Scicli AG. Angiotensin-(1–7) induces bradykinin-mediated hypotensive responses in anesthetized rats. Hypertension. 1997;30:217–221. doi: 10.1161/01.hyp.30.2.217. [DOI] [PubMed] [Google Scholar]
- 11.Li P, Chappell MC, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) augments bradykinin-induced vasodilation by competing with ACE and releasing nitric oxide. Hypertension. 1997;29:394–400. doi: 10.1161/01.hyp.29.1.394. [DOI] [PubMed] [Google Scholar]
- 12.Peiró C, Vallejo S, Gembardt F, Palacios E, Novella S, Azcutia V, Rodríguez-Mañas L, Hermenegildo C, Sánchez-Ferrer CF, Walther T. Complete blockade of the vasorelaxant effects of angiotensin-(1–7) and bradykinin in murine microvessels by antagonists of the receptor Mas. J Physiol. 2013;591(Pt 9):2275–2285. doi: 10.1113/jphysiol.2013.251413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gironacci MM, Valera MS, Yujnovsky I, Peña C. Angiotensin-(1–7) inhibitory mechanism of norepinephrine release in hypertensive rats. Hypertension. 2004;44:783–787. doi: 10.1161/01.HYP.0000143850.73831.9d. [DOI] [PubMed] [Google Scholar]
- 14.Costa MA, Lopez Verrilli MA, Gomez KA, Nakagawa P, Peña C, Arranz C, Gironacci MM. Angiotensin-(1–7) upregulates cardiac nitric oxide synthase in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H1205–H1211. doi: 10.1152/ajpheart.00850.2009. [DOI] [PubMed] [Google Scholar]
- 15.Bomtempo CA, Santos GF, Santos RA, Campagnole-Santos MJ. Interaction of bradykinin and angiotensin-(1–7) in the central modulation of the baroreflex control of the heart rate. J Hypertens. 1998;16(12 Pt 1):1797–1804. doi: 10.1097/00004872-199816120-00013. [DOI] [PubMed] [Google Scholar]
- 16.Santos RA, Campagnole-Santos MJ, Baracho NC, Fontes MA, Silva LC, Neves LA, Oliveira DR, Caligiorne SM, Rodrigues AR, Gropen Júnior C. Characterization of a new angiotensin antagonist selective for angiotensin-(1–7): evidence that the actions of angiotensin-(1–7) are mediated by specific angiotensin receptors. Brain Res Bull. 1994;35:293–298. doi: 10.1016/0361-9230(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 17.Ciruela F, Vallano A, Arnau JM, Sánchez S, Borroto-Escuela DO, Agnati LF, Fuxe K, Fernández-Dueñas V. G protein-coupled receptor oligomerization for what? J Recept Signal Transduct Res. 2010;30:322–330. doi: 10.3109/10799893.2010.508166. [DOI] [PubMed] [Google Scholar]
- 18.Ferré S, Casadó V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin JP, Guitart X. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev. 2014;66:413–434. doi: 10.1124/pr.113.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milligan G. The role of dimerisation in the cellular trafficking of G-protein-coupled receptors. Curr Opin Pharmacol. 2010;10:23–29. doi: 10.1016/j.coph.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Gironacci MM, Adamo HP, Corradi G, Santos RA, Ortiz P, Carretero OA. Angiotensin (1–7) induces MAS receptor internalization. Hypertension. 2011;58:176–181. doi: 10.1161/HYPERTENSIONAHA.111.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amaral MM, Sacerdoti F, Jancic C, Repetto HA, Paton AW, Paton JC, Ibarra C. Action of shiga toxin type-2 and subtilase cytotoxin on human microvascular endothelial cells. PLoS One. 2013;8:e70431. doi: 10.1371/journal.pone.0070431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grecco HE, Bastiaens PI. Imaging protein states in cells. In: Goldman RD, Swedlow J, Spector D, editors. Live Cell Imaging: A Laboratory Manual Vol. 6. Cold Spring Harbor Laboratory Press; 2010. pp. 95–118. [Google Scholar]
- 23.Abd Alla J, Reeck K, Langer A, Streichert T, Quitterer U. Calreticulin enhances B2 bradykinin receptor maturation and heterodimerization. Biochem Biophys Res Commun. 2009;387:186–190. doi: 10.1016/j.bbrc.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111:1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- 25.AbdAlla S, Lother H, Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407:94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- 26.AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med. 2001;7:1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Brovkovych V, Zhang Y, Tan F, Skidgel RA. Downregulation of kinin B1 receptor function by B2 receptor heterodimerization and signaling. Cell Signal. 2015;27:90–103. doi: 10.1016/j.cellsig.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda S, Masumori-Maemoto S, Wada A, Ishii M, Brosnihan KB, Umemura S. Angiotensin(1–7) potentiates bradykinin-induced vasodilatation in man. J Hypertens. 2001;19:2001–2009. doi: 10.1097/00004872-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Tom B, de Vries R, Saxena PR, Danser AH. Bradykinin potentiation by angiotensin-(1–7) and ACE inhibitors correlates with ACE C- and N-domain blockade. Hypertension. 2001;38:95–99. doi: 10.1161/01.hyp.38.1.95. [DOI] [PubMed] [Google Scholar]
- 30.Raffai G, Khang G, Vanhoutte PM. Angiotensin-(1–7) augments endothelium-dependent relaxations of porcine coronary arteries to bradykinin by inhibiting angiotensin-converting enzyme 1. J Cardiovasc Pharmacol. 2014;63:453–460. doi: 10.1097/FJC.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 31.Lavoie C, Mercier JF, Salahpour A, Umapathy D, Breit A, Villeneuve LR, Zhu WZ, Xiao RP, Lakatta EG, Bouvier M, Hébert TE. Beta 1/beta 2-adrenergic receptor heterodimerization regulates beta 2-adrenergic receptor internalization and ERK signaling efficacy. J Biol Chem. 2002;277:35402–35410. doi: 10.1074/jbc.M204163200. [DOI] [PubMed] [Google Scholar]
- 32.Martínez-Pinilla E, Rodríguez-Pérez AI, Navarro G, Aguinaga D, Moreno E, Lanciego JL, Labandeira-García JL, Franco R. Dopamine D2 and angiotensin II type 1 receptors form functional heteromers in rat striatum. Biochem Pharmacol. 2015;96:131–142. doi: 10.1016/j.bcp.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Ferré S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, Mackie K, Milligan G, Pfleger KD, Pin JP, Volkow ND, Waldhoer M, Woods AS, Franco R. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takano M, Matsuyama S. Intracellular and nuclear bradykinin B2 receptors. Eur J Pharmacol. 2014;732:169–172. doi: 10.1016/j.ejphar.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Bachvarov DR, Houle S, Bachvarova M, Bouthillier J, Adam A, Marceau F. Bradykinin B2 receptor endocytosis, recycling, and down-regulation assessed using green fluorescent protein conjugates. J Pharmacol Exp Ther. 2001;297:19–26. [PubMed] [Google Scholar]
- 36.Gregan B, Jürgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem. 2004;279:27679–27687. doi: 10.1074/jbc.M403601200. [DOI] [PubMed] [Google Scholar]
- 37.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavoie C, Mercier JF, Salahpour A, Umapathy D, Breit A, Villeneuve LR, Zhu WZ, Xiao RP, Lakatta EG, Bouvier M, Hébert TE. Beta 1/beta 2-adrenergic receptor heterodimerization regulates beta 2-adrenergic receptor internalization and ERK signaling efficacy. J Biol Chem. 2002;277:35402–35410. doi: 10.1074/jbc.M204163200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.