Abstract
Background/Aims:
This study aimed to investigate the prevalence of chronic kidney disease (CKD) and associated risk factors in a high-risk population in Korea.
Methods:
A total of 6,045 participants aged ≥ 65 years (mean age, 73.0 ± 5.5) with diabetes or hypertension were enrolled. Participants were screened for CKD, which was defined as the presence of albuminuria (urine albumin-to-creatinine ratio ≥ 30 mg/g) or an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2.
Results:
The prevalence of CKD was 39.6% (women, 40.3%; men, 38.4%). Albuminuria was detected in 22.6% of participants, whereas eGFR < 60 mL/min/1.73 m2 was found in 24.6% of participants. The prevalence of CKD by stage was 4.4% for stage 1, 10.4% for stage 2, 23.4% for stage 3, 0.9% for stage 4, and 0.3% for stage 5. Older age, concomitant diabetes and hypertension, higher body mass index, higher systolic and diastolic blood pressure, and higher hemoglobin A1c levels were independently associated with the presence of CKD in multivariate-adjusted analyses that included with age, sex, body mass index, hypertension, diabetes, and smoking.
Conclusions:
The prevalence of CKD was very high in the present high-risk Korean population. Our results suggest that a screening method for early detection of CKD in high-risk populations is needed in Korea.
Keywords: Kidney diseases, Risk factors, Epidemiology, Aged, Korea
INTRODUCTION
Chronic kidney disease (CKD) is increasingly recognized as a global public health problem. The burden of CKD has been increasing worldwide over the last decade and is expected to increase futher [1]. According to data from the National Health and Nutrition Examination Surveys (NHANES), the prevalence of CKD in the United States increased by 13.1% from 1999 to 2004, compared with 8.8% from 1988 to 1994 [2,3]. European studies suggest that the prevalence of CKD in different European areas is 9.2% to 12.5% [4]. Similar to Western countries, the prevalence of CKD in Asia is also increasing, with recent studies suggesting that the prevalence of CKD in the adult population is 12.9% in Japan and 11.8% in China [5,6].
CKD is associated with a higher risk of hospitalization, cardiovascular events, cardiovascular mortality, non-cardiac mortality, and all-cause mortality [7,8]. The cost of health services for patients with CKD is 1.8 times higher than that for patients without CKD. In 2010, the total medicare expenditure for patients with end stage renal disease (ESRD) in the United States exceeded 28 billion US dollars [9]. Although the health problems and medical care costs related with CKD are significant, the NHANES 1999 to 2000 survey suggested that overall, only 2.0% of the adult population self-reports an awareness of weak or failing kidneys [3]. Therefore, many studies have emphasized the importance of early CKD detection through general population screening and referral to a nephrologist [10,11].
According to data from the Korean NHANES, the prevalence of CKD in the general adult population in Korea has increased from 8.9% to 13.7% from 2001 to 2006 [12,13]. Since 1986, the prevalence and incidence of ESRD have been increasing owing to an increase in CKD patients with risk factors such as older age, diabetes, and hypertension [14]. However, only a limited number of studies have investigated the prevalence of CKD in Korean patients with these risks factors. Therefore, this cross-sectional study aimed to investigate the prevalence of CKD and associated risk factors in a high-risk adult population.
METHODS
Study population
We used data from the Hypertension, Diabetes Registration and Management system, operated by the Korea Center for Disease Control and Prevention. This computerized registration systems collects data on management and health education from citizens aged ≥ 30 years with hypertension or diabetes who visited health centers as well as primary or secondary private institutions from 2007 in five cities (Daegu, Gwangmyeong, Namnyangju, Hanam, and Ansan). We performed a cross-sectional study using data from 2011 of 6,187 participants aged ≥ 65 years. Finally, 6,045 subjects with information on renal function and kidney damage markers such as albuminuria were included in this analysis to assess the prevalence of CKD in a high-risk adult population.
This study was approved by the Institutional Review Board of Chonnam National University Hospital, Gwangju, Republic of Korea (CNUH-2014-100). This Institutional Review Board waived the need for consent given the retrospective design of the project. The study was performed in accordance with the Helsinki Declaration of 1975, as revised in 2000.
Measurements
The subjects visited the local hospital in each city. Health examination data included anthropometric measurements, blood pressure (BP), blood chemistry, and urine test results. Information on age, sex, birth date, and other pertinent medical data was obtained. Anthropometric measurements, including height and weight, were determined, and body mass index (BMI) was calculated by dividing the weight (kg) by the height (m2). BP was measured twice using a standard protocol; the second measurement was performed after a 15-minute rest period. A third measurement was performed if the difference between the first to measurements was > 5 mmHg. Blood and urine samples were collected in the morning after an overnight fast ≥ 8 hours. Serum creatinine was measured using the modified Jaffe kinetic reaction in all participating hospitals. Urine albumin was measured by immunoturbidimetry and urine creatinine by the kinetic Jaffe method.
Definitions
Albuminuria was defined as a urine albumin to creatinine ratio (mg albumin/g creatinine, uACR) ≥ 30 mg/g. Microalbuminuria was defined as a uACR of 30 to 299 mg/g, and macroalbuminuria was defined as a uACR ≥ 300 mg/g. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [15]. Reduced renal function was defined as an eGFR < 60 mL/min/1.73 m2. CKD was defined as reduced renal function, or presence of albuminuria. CKD stages were defined as follows: stage 1, eGFR ≥ 90 mL/min/1.73 m2 and albuminuria; stage 2, eGFR 60 to 89 mL/min/1.73 m2 and albuminuria; stage 3, eGFR 30 to 59 mL/min/1.73 m2; stage 4, eGFR 15 to 29 mL/min/1.73 m2; and stage 5, eGFR < 15 mL/min/1.73 m2 [16].
Diabetes was defined as the use of glucose lowering medicine, fasting plasma glucose ≥ 126 mg/dL, or hemoglobin A1c ≥ 6.5%. Hypertension was defined as the use of antihypertensive medication, systolic BP ≥ 140 mmHg, or diastolic BP ≥ 90 mmHg. BMI was categorized into quartiles (Q): Q1, BMI < 22.6 kg/m2; Q2, BMI 22.6 to 24.4 kg/m2; Q3, BMI 24.4 to 26.5 kg/m2; and Q4, BMI ≥ 26.5 kg/m2.
Statistical analysis
Data are presented as mean ± standard deviation for continuous variables, and as proportions for categorical variables. Patients’ characteristics were compared using the chi-square test for categorical variables. Student t tests were used to compare continuous variables. Multivariate logistic regression models were used to estimate the odds ratio (OR) and 95% confidence interval (CI) to determine the relationship between CKD and risk factors, adjustments for age, sex, BMI, hypertension, diabetes, and smoking. Statistical analysis was performed with PASW SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). All p values < 0.05 were considered statistically significant.
RESULTS
Participant characteristics
The sample included 6,045 participants (women, 61.9%), with mean age of 73.0 ± 5.5 years (73.5 ± 5.5 in women; 72.3 ± 5.2 in men). The proportions of participants in the 65 to 69, 70 to 79, and ≥ 80 years age groups were 26.9%, 57.4%, and 15.7% in women, respectively, and 34.2%, 56.4%, and 9.4%, in men, respectively. Hypertension was more common in women, whereas diabetes and smoking were more common in men. BMI was higher in women. On the other hand, diastolic BP and serum creatinine levels were higher in men. Systolic BP, hemoglobin A1c, uACR, and eGFR were not different between women and men (Table 1).
Table 1.
Clinical characteristics of the participants
| Characteristic | Overall | Women | Men | p value |
|---|---|---|---|---|
| No. of subjects | 6,045 | 3,744 (61.9) | 2,301 (38.1) | |
| Age, yr | 73.0 ± 5.5 | 73.5 ± 5.6 | 72.3 ± 5.2 | < 0.001 |
| Age group, yr | < 0.001 | |||
| 65-69 | 1,792 (29.6) | 1,006 (26.9) | 786 (34.2) | |
| 70-79 | 3,447 (57.0) | 2,149 (57.4) | 1,298 (56.4) | |
| ≥ 80 | 806 (13.3) | 589 (15.7) | 217 (9.4) | |
| Hypertension | 5,485 (90.7) | 3,435 (91.7) | 2,050 (89.1) | 0.001 |
| Diabetes | 2,832 (46.8) | 1,673 (44.7) | 1,159 (50.4) | < 0.001 |
| Current smoker | 420 (6.9) | 43 (1.1) | 377 (16.4) | < 0.001 |
| Body mass index, kg/m2 | 24.6 ± 3.3 | 24.9 ± 3.4 | 24.3 ± 3.1 | < 0.001 |
| Systolic blood pressure, mmHg | 127 ± 13 | 127 ± 13 | 127 ± 13 | 0.134 |
| Diastolic blood pressure, mmHg | 77 ± 9 | 76 ± 8 | 77 ± 9 | 0.001 |
| Hemoglobin A1c | 7.0 ± 1.3 | 7.1 ± 1.3 | 7.0 ± 1.3 | 0.470 |
| Creatinine, mg/dL | 0.9 ± 0.4 | 0.8 ± 0.4 | 1.1 ± 0.4 | < 0.001 |
| Urine microalbumin to creatinine ratio, mg/g | 49.6 ± 360.8 | 49.0 ± 429.4 | 50.7 ± 203.3 | 0.604 |
| eGFR, mL/min/1.73 m2 | 78.0 ± 51.6 | 78.0 ± 37.2 | 77.8 ± 68.9 | 0.988 |
| Albuminuria | 1,355 (22.6) | 842 (22.6) | 513 (22.6) | 0.980 |
| eGFR catergory | 0.067 | |||
| ≥ 90 | 1,440 (23.8) | 926 (24.7) | 514 (22.3) | |
| 60-89 | 3,111 (51.5) | 1,883 (50.3) | 1,228 (53.4) | |
| 30-59 | 1,417 (23.4) | 893 (23.8) | 524 (22.8) | |
| 15-29 | 57 (0.9) | 32 (0.9) | 25 (1.1) | |
| < 15 | 20 (0.3) | 10 (0.3) | 10 (0.4) | |
| Chronic kidney disease | 2,392 (39.6) | 1,508 (40.3) | 884 (38.4) | 0.151 |
Values are presented as number (%) or mean ± SD.
eGFR, estimated glomerular filtration rate.
Prevalence of kidney damage markers and CKD
The overall prevalences of albuminuria and reduced renal function were 22.6% and 24.6%, respectively. The prevalence of albuminuria was not different between women and men. In the sub-analysis, the prevalence of macroalbuminuria was higher in men (microalbuminuria, 20.7% vs. 19.4%, p = 0.237; macroalbuminuria, 1.9% vs. 3.2%, p = 0.002). The prevalence of reduced renal function was not different between women and men (25.0% vs. 24.3%, p = 0.552) (Table 1).
In the present study, 39.6% of participants (n = 2,392) had CKD, of which 4.4% had stage 1, 10.4% had stage 2, 23.4% had stage 3, 0.9% had stage 4, and 0.3% had stage 5 CKD (Table 2). A significant difference was not found between the prevalence of CKD in women and men (40.3% vs. 38.4%, p = 0.151) (Table 1). Table 3 shows the prevalence of CKD in the high-risk population stratified by age, eGFR, diabetes, and hypertension status. Overall, 17.4% (n = 1,054) of participants had an eGFR of 45 to 59 mL/min/1.73 m2, and 6.0% (n = 363) had an eGFR of 30 to 44 mL/min/1.73 m2.
Table 2.
Participants’ distribution based on kidney function, hematuria, albuminuria, and prevalence of chronic kidney disease, by stage
| Kidney function |
Albuminuria within each level of eGFR, % |
Chronic kidney disease |
||||
|---|---|---|---|---|---|---|
| eGFR | No. (%) | Normal | Microalbuminuria | Macroalbuminuria | Stage | No. (%) |
| ≥ 90 | 1,440 (23.8) | 82.2 | 16.4 | 1.5 | 1 | 268 (4.4) |
| 60-89 | 3,111 (51.5) | 80.0 | 18.3 | 1.7 | 2 | 630 (10.4) |
| 30-59 | 1,417 (234) | 67.8 | 28.0 | 4.2 | 3 | 1,417 (23.4) |
| 15-29 | 57 (0.9) | 40.0 | 36.0 | 24.0 | 4 | 57 (0.9) |
| <15 | 20 (0.3) | 58.8 | 29.4 | 11.8 | 5 | 20 (0.3) |
| Total | 6,045 (100.0) | 76.6 | 20.0 | 2.4 | All | 2,392 (39.6) |
eGFR, estimated glomerular filtration rate.
Table 3.
Prevalence of chronic kidney disease in the high-risk population by age, estimated glomerular filtration rate, diabetes, and hypertension
| Age, yr | Estimated glomerular filtration rate, mL/min/1.73 m2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Diabetes and hypertension |
Diabetes, not hypertension |
Hypertension, not diabetes |
|||||||
| 45-59 | 30-44 | < 30 | 45-59 | 30-44 | < 30 | 45-59 | 30-44 | < 30 | |
| 65-69 | 96 (4.2) | 29 (1.3) | 8 (0.4) | 18 (3.2) | 5 (0.9) | 3 (0.5) | 106 (3.3) | 7 (0.2) | 2 (0.1) |
| 70-79 | 274 (12.1) | 99 (4.4) | 26 (1.1) | 47 (8.4) | 15 (2.7) | 3 (0.5) | 242 (7.5) | 98 (3.0) | 10 (0.3) |
| ≥ 80 | 95 (4.2) | 58 (2.6) | 14 (0.6) | 10 (1.8) | 5 (0.9) | 0 | 166 (5.2) | 47 (1.5) | 10 (0.3) |
Values are presented as number (%). Estimated glomerular filtration rate, mL/min/1.73 m2 by Chronic Kidney Disease Epidemiology Collaboration equation.
Prevalence of kidney damage markers and CKD according to age, sex, BMI, and comorbidity
The prevalence of albuminuria, reduced renal function, and CKD increased with age in both women and men (p < 0.001) (Fig. 1). Moreover, the prevalences of albuminuria, reduced renal function, and CKD within each group were significantly higher in subjects with diabetes and hypertension (p < 0.001) (Fig. 2). CKD was more common in non-smokers (p = 0.028). The prevalences of reduced renal function, albuminuria, and CKD by sex and BMI classification were not different. Reduced renal function, albuminuria, and CKD were associated with increased systolic BP (p < 0.001). Reduced renal function (p < 0.001) and CKD (p = 0.013) were more common in subjects with increased diastolic BP. The prevalences of albuminuria (p < 0.001) and CKD (p = 0.002) increased with increasing hemoglobin A1c level (Table 4).
Figure 1.
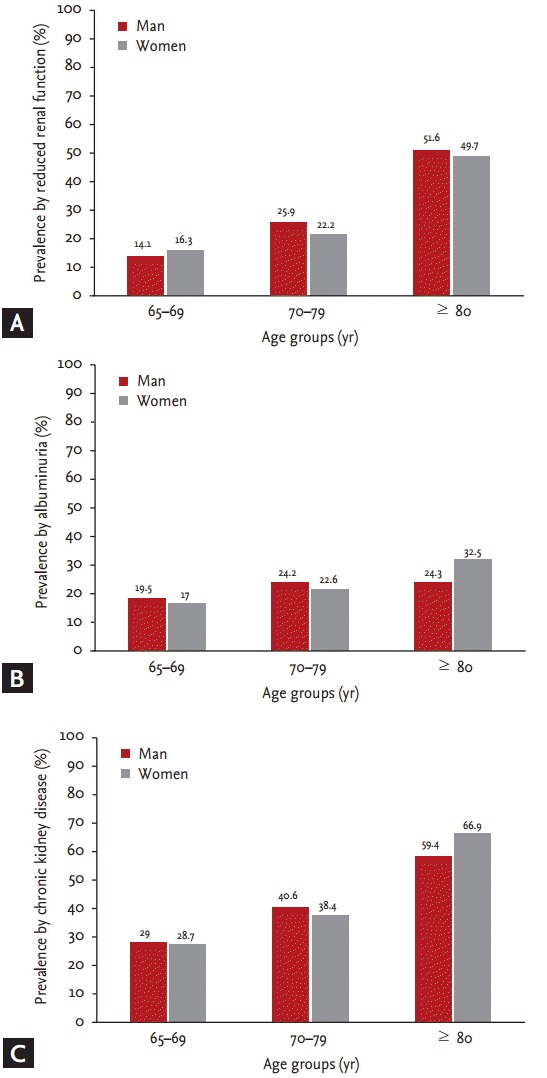
Prevalence of kidney damage markers and chronic kidney disease according to age groups. (A) Prevalence by reduced renal function. (B) Prevalence by albuminuria. (C) Prevalence by chronic kidney disease.
Figure 2.
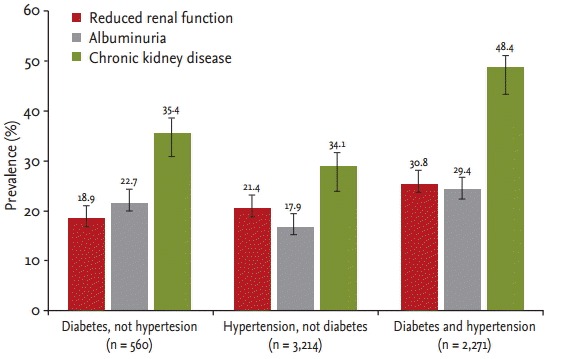
Prevalence of kidney damage markers and chronic kidney disease according to diabetes and hypertension status in the current study.
Table 4.
Prevalence of kidney damage markers and chronic kidney disease according to age, sex, body mass index, and comorbidity
| Variable | Reduced renal function | Albuminuria | Chronic kidney disease |
|---|---|---|---|
| Age group, yr | |||
| 65-69 | 275 (15.3) | 321 (18.1) | 517 (28.9) |
| 70-79 | 814 (23.6) | 794 (23.2) | 1,352 (39.2) |
| ≥ 80 | 405 (50.2) | 240 (30.3) | 523 (64.9) |
| Sex | |||
| Men | 559 (24.3) | 513 (22.6) | 884 (38.4) |
| Women | 935 (25.0) | 842 (22.6) | 1,508 (40.3) |
| Smoking | |||
| No smoker | 1,406 (25.0) | 1,266 (22.7) | 2,247 (39.9) |
| Current smoker | 88 (21.0) | 89 (21.3) | 145 (34.5) |
| Body mass index, kg/m2 | |||
| Q1 | 386 (25.6) | 350 (23.4) | 616 (40.8) |
| Q2 | 351 (23.6) | 316 (21.4) | 550 (36.9) |
| Q3 | 358 (23.5) | 353 (23.4) | 601 (39.5) |
| Q4 | 399 (26.3) | 336 (22.4) | 625 (41.1) |
| Systolic blood pressure, mmHg | |||
| < 120 | 270 (28.1) | 172 (18.0) | 380 (39.5) |
| 120-139 | 930 (22.8) | 900 (22.3) | 1,548 (38.0) |
| 140-159 | 245 (28.9) | 233 (27.8) | 383 (45.1) |
| ≥ 160 | 49 (31.8) | 49 (32.5) | 80 (51.9) |
| Diastolic blood pressure, mmHg | |||
| < 80 | 636 (26.8) | 504 (21.5) | 949 (40.0) |
| 80-89 | 678 (22.1) | 702 (23.1) | 1,174 (38.2) |
| 90-99 | 158 (30.1) | 127 (24.3) | 232 (44.2) |
| ≥ 100 | 22 (31.0) | 21 (30.4) | 36 (50.7) |
| Hemoglobin A1C, % | |||
| < 6 | 116 (29.8) | 79 (20.6) | 164 (42.2) |
| 6-6.9 | 357 (27.1) | 319 (24.5) | 564 (42.9) |
| 7-7-9 | 199 (27.9) | 210 (29.7) | 331 (46.4) |
| 8-8.9 | 72 (25.7) | 102 (37.1) | 139 (49.6) |
| ≥ 9 | 85 (34.3) | 91 (37.9) | 137 (55.2) |
Values are presented as number (%).
Q, quartile.
Factors associated with kidney damage markers and CKD prevalence
In the statistical models adjusted for age, sex, BMI, diabetes, hypertension, and smoking, the 70 to 79 years, and ≥ 80 years age groups had higher ORs for CKD ([OR, 1.571; 95% CI, 1.387 to 1.780; p < 0.001]; [OR, 4.774; 95% CI, 3.980 to 5.727; p < 0.001], respectively), compared with that in the group 65 to 69 years old. Moreover, diabetes (OR, 1.245; 95% CI, 1.025 to 1.513; p = 0.029) and concomitant diabetes and hypertension (OR, 1.859; 95% CI, 1.659 to 2.082; p < 0.001) had higher ORs for CKD, compared with subjects with hypertension. The OR for developing CKD adjusted by BMI quartile was higher in the Q4 quartile (OR, 1.256; 95% CI, 1.078 to 1.464; p = 0.004), compared with subjects in the Q2 quartile. Furthermore, CKD was strongly associated with systolic BP levels ≥ 140 mmHg, diastolic BP levels ≥ 90 mmHg and hemoglobin A1c levels ≥ 7.
Reduced renal function was independently associated with older age, concomitant diabetes and hypertension, male sex, BMI Q4, systolic BP levels of 120 to 139 mmHg, diastolic BP levels of 80 to 99 mmHg, and hemoglobin A1c levels ≥ 9. Predictors of albuminuria were older age, diabetes, concomitant diabetes and hypertension, higher systolic and diastolic BP, and higher hemoglobin A1c levels (Table 5).
Table 5.
Factors associated with kidney damage markers and chronic kidney disease
| Variable | Odds ratio (95% confidence interval) |
||
|---|---|---|---|
| Reduced renal function | Albuminuria | Chronic kidney disease | |
| Age group, yr | |||
| 65-69 | 1 | 1 | 1 |
| 70-79 | 1.686 (1.448-1.963)a | 1.349 (1.166-1.562)a | 1.571 (1.387-1.780)a |
| ≥ 80 | 5.844 (4.815-7.093)a | 2.011 (1.684-2.454)a | 4.774 (3.980-5.727)a |
| HT/DM | |||
| HT, not DM | 1 | 1 | 1 |
| DM, not HT | 1.026 (0.811-1.299) | 1.447 (1.160-1.805)b | 1.245 (1.025-1.513)c |
| DM and HT | 1.670 (1.470-1.897)a | 1.910 (1.679-2.174)a | 1.859 (1.659-2.082)a |
| Sex | |||
| Men | 1 | 1 | 1 |
| Women | 0.874 (0.766-0.997)c | 0.959 (0.839-1.096) | 0.953 (0.848-1.071) |
| Smoking | |||
| No smoker | 1 | 1 | 1 |
| Current smoker | 0.843 (0.650-1.095) | 0.936 (0.725-1.209) | 0.849 (0.678-1.063) |
| Body mass index, kg/m2 | |||
| Q1 | 1.017 (0.855-1.211) | 1.097 (0.921-1.308) | 1.105 (0.948-1.288) |
| Q2 | 1 | 1 | 1 |
| Q3 | 1.023 (0.859-1.220) | 1.147 (0.963-1.366) | 1.152 (0.989-1.343) |
| Q4 | 1.237 (1.040-1.470)c | 1.083 (0.907-1.293) | 1.256 (1.078-1.464)b |
| Systolic blood pressure, mmHg | |||
| < 120 | 1 | 1 | 1 |
| 120-139 | 0.745 (0.632-0.880)a | 1.336 (1.112-1.605)b | 0.942 (0.811-1.094) |
| 140-159 | 0.972 (0.784-1.203) | 1.754 (1.398-2.202)a | 1.219 (1.003-1.482)c |
| ≥ 160 | 1.051 (0.715-1.545) | 2.136 (1.454–3.138)a | 1.541 (1.080-2.200)c |
| Diastolic blood pressure, mmHg | |||
| < 80 | 1 | 1 | 1 |
| 80-89 | 0.815 (0.716-0.929)b | 1.160 (1.016-1.323)c | 0.993 (0.886-1.113) |
| 90-99 | 1.329 (1.069-1.652)c | 1.262 (1.005-1.584)c | 1.344 (1.100-1.640)b |
| ≥ 100 | 1.352 (0.796-2.296) | 1.770 (1.042-3.004)c | 1.745 (1.071-2.844)c |
| Hemoglobin A1C, % | |||
| < 6 | 1 | 1 | 1 |
| 6-6.9 | 0.941 (0.726-1.220) | 1.327 (1.002-1.758)c | 1.105 (0.871-1.402) |
| 7-7.9 | 1.044 (0.785-1.387) | 1.815 (1.344-2.451)a | 1.360 (1.048-1.763)c |
| 8-8.9 | 0.951 (0.663-1.363) | 2.585 (1.814-3.684)a | 1.603 (1.162-2.211)b |
| ≥ 9 | 1.599 (1.119-2.286)c | 2.765 (1.917-3.988)a | 2.192 (1.568-3.064)a |
Adjusted with age, sex, body mass index, hypertension, diabetes, and smoking.
HT, hypertension; DM, diabetes; Q, quartile.
p < 0.001.
p < 0.01.
p < 0.05.
DISCUSSION
To our knowledge, the present study is the first to investigate the prevalence of reduced renal function, albuminuria, and CKD in a high-risk adult population in Korea. The prevalences of reduced renal function, albuminuria, and CKD in the study population were 24.6%, 22.6%, and 39.6%, respectively. Factors such as older age, diabetes, concomitant diabetes and hypertension, higher BMI, higher systolic and diastolic BP, and higher hemoglobin A1c levels were associated with an increased prevalence of CKD.
The prevalence of reduced renal function in the high-risk population in our study was similar to that reported for elderly US and Polish populations, which were 26.2% and 21.2%, respectively [2,17]. The prevalence of reduced renal function in subjects aged ≥ 55 years with diabetes or hypertension was 37.1% in the HUNT (Nord-Trondelag Health Study) II (Norway) and 54.8% in the elderly general population from the AusDiab (Australian Diabetes, Obesity and Lifestyle Study) survey (Australia) [18,19]. In the present study, the prevalence of reduced renal function was 18.9% in subjects with diabetes and 21.4% in subjects with hypertension (Fig. 2). Although different tools were used to calculate the eGFR, the prevalence of reduced renal function in the high-risk population was similar to that in previous studies.
The KDIGO (Kidney Disease: Improving Global Outcomes) guidelines recommend uACR monitoring as the best index of early vascular endothelial injury [16]. In the general population, the prevalences of albuminuria according to uACR were 10.1% in America and 6.3% in China [3,6]. The prevalences of albuminuria in the elderly Polish population were 12.3% overall, 17.6% in subjects with diabetes, and 12.9% in those with hypertension [17]. Our study showed that prevalence of albuminuria was 22.6% overall, 22.7% in subjects with diabetes, 17.9% in subjects with hypertension, and 29.4% in subjects with diabetes and hypertension. The subjects with diabetes had a 1.447-fold increased risk for albuminuria than those with hypertension. Although there were differences between the high-risk populations in our study and previous studies, the prevalence of kidney damage markers in the high-risk population was considerably higher overall than in the general population.
The prevalence of CKD among the high-risk population in our study was 39.6%. In another study, the prevalence of CKD was 4 times higher (45.7% vs. 11.6%) in the elderly patients than in younger participants [20]. Furthermore, a high prevalence of CKD (34% to 42.8%) was reported in outpatients with high-risk factors [21]. Although the differences in area characteristics, screening tools, and sampling methods might have resulted in different prevalence rates [22], these results suggest that the prevalence of CKD in high-risk groups is considerably higher than that in the general population. Thus, some studies suggest that screening for CKD in high-risk populations is more cost-effective than in the general population [23]. Therefore, our results suggest that a screening method for early detection of CKD in high-risk populations is warranted in Korea, where the prevalence of CKD is increasing.
Advanced age is a well-known risk factor for CKD. The prevalence of reduced renal function increase with increasing age [3,17,18]. In Asia, CKD stage 3 is reportedly more common in elderly patients than in younger patients (33.5% vs. 4.1%) [20]. In a 10-year population-based study of 58,000 patients (median age, 75.0 years; range, 67.0 to 81.2) with CKD stage 3, older age was an independent predictor of renal survival and change in eGFR [24]. In the AusDiab survey, older age was a predictor of kidney damage markers, such as proteinuria (OR, 2.5; 95% CI, 1.9 to 3.2) and reduced renal function (OR, 101.5; 95% CI, 61.4 to 162.9) [19]. Similarly, our results suggest that CKD stage 3 (23.4%) is the most common stage in subjects with CKD and that older age in high-risk subjects is, a predictor of kidney damage markers.
A number of studies have suggested that smoking increases the risk of CKD [25,26]. However, in the present study, CKD was more common in nonsmokers, and after adjustment for several potential confounding factors, smoking was not associated with the risk of CKD. According to recent data from the NHANES, the rate of current smoking was lowest in those aged ≥ 60 years [27]. Because our study population included patients aged ≥ 65 years with chronic disease, the rate of current smoking was relatively lower than the NHANES data. Also, our questionnaire did not obtain detailed information on tobacco use, such as the amount used, start and stop dates, and changes in use over time. This could explain the lack of relationship between smoking and risk of CKD in present study.
A high BMI was an independent predictor of developing reduced renal function in the Framingham cohort [28]. A community-based screening program in Okinawa found that the cumulative incidence of ESRD increased 1.48-fold in individuals in the highest BMI quartile compared with those in the lowest BMI quartile [29]. In a 12-year Southeast Asian cohort study, high BMI (> 24.9 kg/m2) was an independent risk factor for the development of reduced renal function [30]. In our study, high BMI (> 26.5 kg/m2) was shown to be an independent risk factor for the development of reduced renal function and CKD. Although not significant, a similar trend for the development of reduced renal function and CKD existed with both low and high BMI (< 22.6 and > 24.4 kg/ m2) compared with other studies [29,30].
It is known that hypertension and diabetes are risk factors for new-onset kidney disease [28,31]. Our study confirms that concomitant or uncontrolled hypertension and diabetes are significantly related to the prevalence of kidney damage markers or CKD.
The present study has several limitations. First, selection bias could have occurred with the inclusion of older patients or a high-risk subgroup [32]. However, we have corrected the selection bias using multivariate logistic regression models. Second, a cross-sectional study design makes it difficult to infer causality between risk factors and the development of CKD. Third, our definition of CKD—defined as albuminuria or an eGFR < 60 mL/min/1.73 m2 —did not include other kidney damage makers. Moreover, repeated measurements of creatinine levels and albuminuria were not performed. Overall, these factors could have led to underestimation or overestimation of the prevalence of CKD.
In conclusion, the prevalence of CKD in a high-risk population was higher than that in the general population. Older age, concomitant diabetes and hypertension, uncontrolled underlying diseases, and higher BMI were identified as significant risk factors. Therefore, Korean individuals with these risk factors are at high-risk of developing CKD, and would benefit from early detection of CKD to prevent disease progression and associated cardiovascular events, cardiovascular mortality, non-cardiac mortality, and all-cause mortality, especially in high-risk groups.
KEY MESSAGE
1. The prevalence of chronic kidney disease (CKD) was very high in the high-risk Korean adults. CKD stage 3 was most common.
2. Older age, concomitant diabetes and hypertension, higher body mass index, and uncontrolled underlying diseases were independently associated with the presence of CKD.
3. These findings suggest that a screening method for early detection of CKD in high-risk populations is warranted in Korea.
Acknowledgments
This research was supported by a grant (CRI 14031-1) Chonnam National University Hospital Biomedical Research Institute, by a fund under the Korea Centers for Disease Control and Prevention (2012-E33024-00), by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (2013R1A2A2A01067611), and by the Pioneer Research Center Program through the NRF funded by the Ministry of Science, ICT & Future Planning (2014M3C1A3053036).
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives: a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 4.de Jong PE, van der Velde M, Gansevoort RT, Zoccali C. Screening for chronic kidney disease: where does Europe go? Clin J Am Soc Nephrol. 2008;3:616–623. doi: 10.2215/CJN.04381007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imai E, Horio M, Watanabe T, et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13:621–630. doi: 10.1007/s10157-009-0199-x. [DOI] [PubMed] [Google Scholar]
- 6.Chen N, Wang W, Huang Y, et al. Community-based study on CKD subjects and the associated risk factors. Nephrol Dial Transplant. 2009;24:2117–2123. doi: 10.1093/ndt/gfn767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 8.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 9.Hunsicker LG. The consequences and costs of chronic kidney disease before ESRD. J Am Soc Nephrol. 2004;15:1363–1364. doi: 10.1097/01.asn.0000126069.68755.99. [DOI] [PubMed] [Google Scholar]
- 10.Locatelli F, Vecchio LD, Pozzoni P. The importance of early detection of chronic kidney disease. Nephrol Dial Transplant. 2002;17 Suppl 11:2–7. doi: 10.1093/ndt/17.suppl_11.2. [DOI] [PubMed] [Google Scholar]
- 11.John R, Webb M, Young A, Stevens PE. Unreferred chronic kidney disease: a longitudinal study. Am J Kidney Dis. 2004;43:825–835. doi: 10.1053/j.ajkd.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 12.Yu M, Ryu DR, Kim SJ, Choi KB, Kang DH. Clinical implication of metabolic syndrome on chronic kidney disease depends on gender and menopausal status: results from the Korean National Health and Nutrition Examination Survey. Nephrol Dial Transplant. 2010;25:469–477. doi: 10.1093/ndt/gfp483. [DOI] [PubMed] [Google Scholar]
- 13.Kim S, Lim CS, Han DC, et al. The prevalence of chronic kidney disease (CKD) and the associated factors to CKD in urban Korea: a population-based cross-sectional epidemiologic study. J Korean Med Sci. 2009;24 Suppl:S11–S21. doi: 10.3346/jkms.2009.24.S1.S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korean Society of Nephrology. ESRD Registry Committee Renal replacement therapy in Korea: Insan Memorial Dialysis Registry 2005. Korean J Nephrol. 2006;25:S425–S457. [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 17.Chudek J, Wieczorowska-Tobis K, Zejda J, et al. The prevalence of chronic kidney disease and its relation to socioeconomic conditions in an elderly Polish population: results from the national population-based study PolSenior. Nephrol Dial Transplant. 2014;29:1073–1082. doi: 10.1093/ndt/gft351. [DOI] [PubMed] [Google Scholar]
- 18.Hallan SI, Dahl K, Oien CM, et al. Screening strategies for chronic kidney disease in the general population: follow-up of cross sectional health survey. BMJ. 2006;333:1047. doi: 10.1136/bmj.39001.657755.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: the AusDiab kidney study. J Am Soc Nephrol. 2003;14(7 Suppl 2):S131–S138. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 20.Lin MY, Chiu YW, Lee CH, et al. Factors associated with CKD in the elderly and nonelderly population. Clin J Am Soc Nephrol. 2013;8:33–40. doi: 10.2215/CJN.05600612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cases Amenos A, Gonzalez-Juanatey JR, Conthe Gutierrez P, et al. Prevalence of chronic kidney disease in patients with or at a high risk of cardiovascular disease. Rev Esp Cardiol. 2010;63:225–228. doi: 10.1016/s1885-5857(10)70041-4. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 23.Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290:3101–3114. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]
- 24.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69:375–382. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 25.Pinto-Sietsma SJ, Mulder J, Janssen WM, Hillege HL, de Zeeuw D, de Jong PE. Smoking is related to albuminuria and abnormal renal function in nondiabetic persons. Ann Intern Med. 2000;133:585–591. doi: 10.7326/0003-4819-133-8-200010170-00008. [DOI] [PubMed] [Google Scholar]
- 26.Ejerblad E, Fored CM, Lindblad P, et al. Association between smoking and chronic renal failure in a nationwide population-based case-control study. J Am Soc Nephrol. 2004;15:2178–2185. doi: 10.1097/01.ASN.0000135048.35659.10. [DOI] [PubMed] [Google Scholar]
- 27.Korean Centers for Disease Control & Prevention . Cheongju: Korean Centers for Disease Control & Prevention; c2014. The National Health and Nutrition Examination Surveys [Internet] [cited 2014 Dec 17]. Available from: http://www.cdc.go.kr/CDC/contents/CdcKrContentView.jsp?cid=60602&menuIds=HOME001-MNU1130-MNU1639-MNU1640-MNU1645. [Google Scholar]
- 28.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 29.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65:1870–1876. doi: 10.1111/j.1523-1755.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 30.Domrongkitchaiporn S, Sritara P, Kitiyakara C, et al. Risk factors for development of decreased kidney function in a Southeast Asian population: a 12-year cohort study. J Am Soc Nephrol. 2005;16:791–799. doi: 10.1681/ASN.2004030208. [DOI] [PubMed] [Google Scholar]
- 31.Kuo HW, Tsai SS, Tiao MM, Yang CY. Epidemiological features of CKD in Taiwan. Am J Kidney Dis. 2007;49:46–55. doi: 10.1053/j.ajkd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Tripepi G, Jager KJ, Dekker FW, Wanner C, Zoccali C. Bias in clinical research. Kidney Int. 2008;73:148–153. doi: 10.1038/sj.ki.5002648. [DOI] [PubMed] [Google Scholar]


