Abstract
Background
An accurate assessment of hemoglobin (Hb) values before donation is unavoidable for safeguarding donors’ safety and fulfilling the current specifications of Hb content in blood bags. This study was hence aimed to compare a finger-prick method for Hb measurement in capillary blood with Hb assessment in venous blood using a hematological analyser.
Materials and methods
The study populations consisted in 1,014 consecutive blood donors, who had paired measurement of Hb values with HemoCue on capillary blood and UniCel DxH800 in venous blood.
Results
A significant overestimation was found with HemoCue compared to UniCel DxH800, but the correlation between methods was significant (comprised between 0.600 and 0.759; all p<0.01) and the bias always lower than the quality specifications. The prevalence of Hb values below the gender-specific thresholds for blood donation was also not significantly different (p=0.186).
Discussion
It can hence be concluded that the finger-prick method evaluated is a safe and reliable means for screening blood donors.
Keywords: haemoglobin, anaemia, blood donation, screening
Introduction
The assessment of haemoglobin (Hb) concentration in blood donors just before donation is virtually mandatory in most countries. The current Hb cut-off values set by the European Union (EU) for blood donation are 125 g/L and 135 g/L in women and men, respectively1. These thresholds have been fixed for the purpose of protecting blood donors from becoming anaemic, but also to guarantee that the collected blood units fulfil the required standards of Hb content2,3.
It is, therefore, noteworthy that the use of inaccurate methods for measuring Hb may generate at least two important negative outcomes. First, falsely decreased Hb values may increase deferral rates and hence worsen the blood shortage, whereas falsely increased Hb values may have an impact on donor’s health or even aggravate a potential anaemic state4,5.
Due to low cost, ease of use and rapidity, capillary blood collected with finger-prick methods is widely used to determine Hb level across a number of healthcare settings. Nevertheless, some drawbacks of this practice have been highlighted, such as the lack of standardisation among the methods and a trend to overestimating Hb compared to values obtained in paired blood specimens6. As such, the aim of this study was the comparison of a finger-prick method to measure Hb level in capillary blood before donation with the reference approach based on Hb measurement in venous blood using a haematology analyser.
Materials and methods
The study population consisted of 1,014 consecutive blood donors (mean age: 41 years, range: 18–65 years) referred to the Blood Collection Unit in the Laboratory of Clinical Pathology of the Hospital of Merano, Italy. Of these 1,014 donors, 224 were women (mean age: 39.2 years, range: 18–64 years) and 790 were men (mean age: 41.8 years, range: 18–65 years) The capillary Hb screening was performed by appropriately trained nurses, using a photometric technique method (HemoCue Hb 301 System®, HemoCue AB, Ängelholm, Sweden)7. In brief, after skin disinfection of the third finger, the digital pulp was pricked using a lancet. The first two drops of blood were eliminated, and the third was used for measurement of the Hb level with HemoCue. The accurate functioning of the test system was systematically monitored with daily quality control testing, as recommended by the manufacturer. The HemoCue photometer is automatically calibrated following the International Council for Standardization in Haematology (ICSH) reference method for Hb and does not need additional calibration. The Hb concentration is determined by measuring the absorbance of whole blood at an Hb/Hb2 isobestic point. The device uses a double wavelength measuring method, 506 nm and 880 nm, for compensation of turbidity7. For the purpose of this study, the haematology analyser UniCel DxH800 (Beckman Coulter, Fullerton, CA, USA) was used as the standard reference technique for measuring the concentration of Hb in blood. Specifically, venous blood was drawn into a polyethylene terephthalate plastic tube (Venosafe, Terumo Europe N.V, Leuven, Belgium) containing K2-EDTA by appropriately trained nurses and conveyed to the core laboratory where the Hb measurement was performed within 3 hours of collection. The UniCel DxH800 is a fully automated analyser which uses a cyanide-free reagent and oxyhaemoglobin technique for Hb measurement, as described elsewhere8. Surfactants are used to lyse the erythrocytes. The Hb released by the red blood cells is then incubated with an antioxidant and a stabilising reagent, and converted into a stable haemochromogen, which is finally measured with absorbance technique.
The normal distribution of values was verified with the D’Agostino-Pearson test and the results were then reported as mean, standard deviation and 95% confidence interval (CI) of the mean. The differences between values obtained with HemoCue and the reference haematology analyser were assessed with a paired Student’s t-test, Spearman’s correlation and Passing and Bablock regression analysis. Statistical significance was set at p<0.05. Percentage variations from the standard reference were also analysed with Bland-Altman plots and compared with the current quality specifications for desirable bias for Hb, as derived from the intra-individual and inter-individual variations9. The reference interval of Hb in the blood donor population was also calculated using a non-parametric percentile method, as currently suggested by the Clinical and Laboratory Standards Institute (CLSI) document C28-A310.
The investigation was based on pre-existing samples, so that ethical permission and informed consent were unnecessary. All the samples were made anonymous before evaluation. The study was performed in accordance with the Declaration of Helsinki and under the terms of all relevant local legislations.
Results
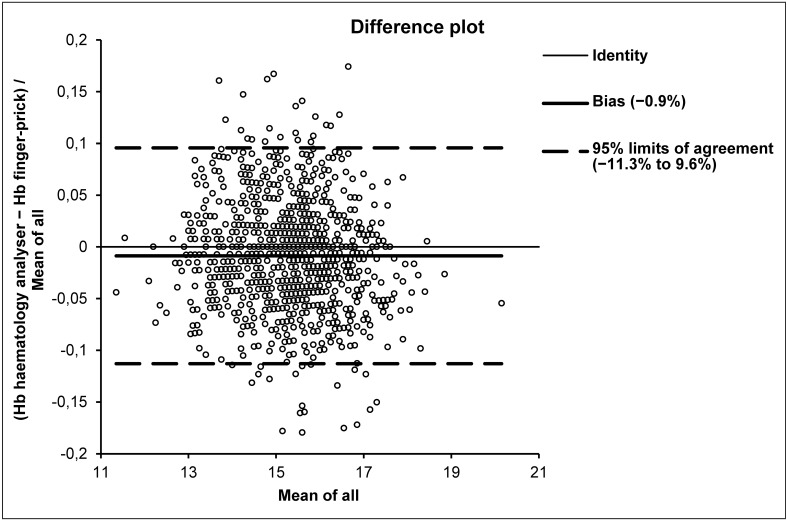
The results of this study are shown in Table I. The Hb data obtained with finger-prick and the haematology analyser followed a normal distribution. A statistically significant difference was found between Hb values obtained with the two methods in the entire cohort, as well as in the subpopulations of women and men. The correlation and regression analysis in the entire cohort as well as in women and men yielded excellent results. Specifically, the Spearman’s coefficient of rank correlation (rho) was 0.759 (95% CI: 0.732 to 0.784; p<0.01), the intercept was −0.921 (95% CI: −1.725 to 0.200) and the slope was 1.071 (95% CI: 1.000 to 1.125) in the entire cohort. In men the rho was 0.645 (95% CI 0.602 to 0.684; p<0.01), the intercept −1.572 (95% CI −2.744 to −0.407) and the slope 1.111 (95% CI 1.036 to 1.187), whereas in women the rho was 0.600 (95% CI: 0.509 to 0.678; p<0.01), the intercept 0.100 (95% CI: −2.336 to 1.382) and the slope 1.000 (95% CI: 0.909 to 1.182) (Table I). Although the Hb values were found to be significantly higher with the HemocCue than with the reference haematology analyser, the mean bias calculated with Bland-Altman plots analysis (Figure 1) was always lower than the current quality specifications10 for Hb (i.e. ±1.84%). It is also worth noting that the prevalence of blood donors with Hb values below the gender-specific thresholds for blood donations did not significantly differ when measured with HemoCue (1/1,014) or with the haematology analyser (4/1,014; p=0.186). The reference ranges for Hb calculated using HemoCue were: lower limit 139 g/L (90% CI: 138 to 14) and upper limit 181 g/L (90% CI: 179 to 184) in men; lower limit 126 g/L (90% CI: 122 to 128) and upper limit 157 mg/dL (90% CI: 155 to 159) in women.
Table I.
Haemoglobin (Hb) values measured in 1,014 blood donors in capillary blood using the finger-prick method (HemoCue) and in venous blood using a reference haematology analyser.
| All donors (n=1,014) | Women (n=244) | Men (n=790) | |
|---|---|---|---|
|
Hb values (g/d) with finger-prick method Mean±SD – (95% CI for mean) |
153.6±12.7 (152.8 to 154.4) | 140.2±8.7 (139.0 to 141.3) | 157.4±10.9 (156.7 to 158.2) |
|
Hb values (g/L) with haematology analyser Mean±SD – (95% CI for mean) |
152.2±11.8 (151.5 to 152.9) | 139.2±8.5 (138.1 to 140.3) | 156.0±9.8 (155.3 to 156.6) |
|
p value - Paired t-test Haematology analyser vs finger-prick method |
p<0.0001 | p=0.0466 | p<0.0001 |
|
Mean % bias and (95% confidence limit of agreement) Haematology analyser vs finger-prick method |
−0.9 (9.6; −11.3) | −0.9 (9.6; −11.4) | −0.7 (9.7; −11.1) |
|
Passing and Bablok Coefficient of rank correlation (rho) y= finger prick; x= haematology analyser |
y = −0.921+1.071 x rho=0.759 |
y = −0.100+1.000 x rho=0.600 |
y = −1.572+1.111 x rho=0.645 |
Figure 1.
Bland-Altman plots of haemoglobin (Hb) values measured in 1,014 blood donors in capillary blood using the finger-prick method (HemoCue) and in venous blood using a reference haematology analyser.
Discussion
Good quality Hb assessment before a blood donation is essential both to safeguard donors’ safety and to fulfil the current criteria or specifications for Hb content in blood bags2. Several lines of evidence now confirm that the largest number of laboratory errors emerge from the manually intensive activities of the pre-analytical phase rather than from the analytical and post-analytical phases11. This is particularly true for apparently straightforward tests such as Hb measurement with finger-prick methods, since these techniques are not always fool-proof.
Several studies have shown that the Hb concentration measured in capillary blood is higher than that assessed in venous blood4–6,12. In accordance with these findings, the results of our study also showed that capillary Hb measured with HemoCue exhibited a significant trend toward overestimation compared to values measured in venous blood. This is obviously due to the fact that the composition of capillary blood obtained by the finger-stick technique differs from that of blood obtained with a standard venipuncture, and the former technique is also influenced by a number of specific factors such as skin thickness, temperature and potential effects of squeezing the digital pulp12. Nevertheless, the bias observed between capillary and venous blood values was clinically meaningless compared to the current quality specifications, and the deferral rate that would have resulted from paired measurement in venous and capillary blood did not reach statistical significance. It can, therefore, be concluded that finger-prick methods represent a safe and reliable means for screening blood donors, and venous blood testing may be limited to subjects with values very close to the blood donation thresholds (i.e., ±0.9%) (Table I). The safety of this strategy is also supported by data published by Ziemann et al.13, who showed that currently available techniques for capillary Hb screening would allow reliable assessment of pre-donation Hb under routine conditions.
Conclusions
It is worth noting that this approach also has several advantages and strengths. First, it has been reported that the HemoCue test system may provide a more accurate assessment of Hb than automated haematology analysers, which is attributable to the fact that Hb is measured directly by the former instrument, whereas marked sample dilution is systematically performed when analysing blood with conventional haemocytometers14. Moreover, HemoCue is a compact instrument that can be transported easily and located in different healthcare setting, thus abolishing the need for more expensive and complicated core laboratory instruments. The collection of capillary blood is also more practical that routine venipuncture, since it does not require specialised phlebotomists, reduces the discomfort and potential complications of a venipuncture and, even more importantly, overcomes the problems caused by blood drawing in patients with difficult veins which are also a cause of important diagnostic errors15.
Acknowledgements
We thank Monika Alber, Monica Nardelli, Stefania Vielmetti, Anita Waldboth, Maria Kienzl, Sabine Trafoier and Anna Maria Haller for the great work they do every day in our institution.
Footnotes
Authorship contributions
MD and GL conceived and designed the study, analysed the data, performed the statistical analysis and drafted the manuscript; RC, EMZ, AJ, SP and RH reviewed the literature, acquired data, interpreted the results and critically revised the manuscript. All Authors read and approved the final version of the manuscript.
The Authors declare no conflicts of interest.
References
- 1.Council of Europe. Guide to the Preparation, Use and Quality Assurance of Blood Components. 17th ed. Strasbourg: Council of Europe Publishing; 2013. [Google Scholar]
- 2.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of red blood cells. Blood Transfus. 2009;7:49–64. doi: 10.2450/2008.0020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee. Guidelines for the Blood Transfusion Services in the United Kingdom. 8th ed. Norwich: The Stationery Office; 2013. [Google Scholar]
- 4.Sawant RB, Bharucha ZS, Rajadhyaksha SB. Evaluation of hemoglobin of blood donors deferred by the copper sulphate method for hemoglobin estimation. Transfus Apher Sci. 2007;36:143–8. doi: 10.1016/j.transci.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Gómez-Simón A, Navarro-Núnez L, Pérez-Ceballos EL, et al. Evaluation of four rapid methods for hemoglobin screening of whole blood donors in mobile collection settings. Transfus Apher Sci. 2007;36:235–42. doi: 10.1016/j.transci.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Pagliaro P, Belardinelli A, Boko V, et al. A non-invasive strategy for haemoglobin screening of blood donors. Blood Transfus. 2014;12:458–63. doi: 10.2450/2014.0284-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchis-Gomar F, Cortell-Ballester J, Pareja-Galeano H, et al. Hemoglobin point-of-care testing: the HemoCue system. J Lab Autom. 2013;18:198–205. doi: 10.1177/2211068212457560. [DOI] [PubMed] [Google Scholar]
- 8.Hedley BD, Keeney M, Chin-Yee I, Brown W. Initial performance evaluation of the UniCel® DxH 800 Coulter® cellular analysis system. Int J Lab Hematol. 2011;33:45–56. doi: 10.1111/j.1751-553X.2010.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricos C, Alvarez V, Cava F, et al. Current databases on biologic variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59:491–500. doi: 10.1080/00365519950185229. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline. 3rd ed. Clinical and Laboratory Standards Institute; Wayne: 2008. CLSI document C28-A3. [Google Scholar]
- 11.Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med. 2006;44:358–65. doi: 10.1515/CCLM.2006.073. [DOI] [PubMed] [Google Scholar]
- 12.Patel AJ, Wesley R, Leitman SF, Bryant BJ. Capillary versus venous haemoglobin determination in the assessment of healthy blood donors. Vox Sang. 2013;104:317–23. doi: 10.1111/vox.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziemann M, Lizardo B, Geusendam G, Schlenke P. Reliability of capillary hemoglobin screening under routine conditions. Transfusion. 2011;51:2714–9. doi: 10.1111/j.1537-2995.2011.03183.x. [DOI] [PubMed] [Google Scholar]
- 14.Von Schenk H, Falkensson M, Lundberg B. Evaluation of Hemocue, a device for determining haemoglobin. Clin Chem. 1987;33:2307–8. [PubMed] [Google Scholar]
- 15.Lippi G, Avanzini P, Aloe R, Cervellin G. Blood collection from intravenous lines: is one drawing site better than others? Lab Med. 2014;45:172–5. doi: 10.1309/lm2xcv5sqml1ontm. [DOI] [PubMed] [Google Scholar]