Abstract
Background
Safety of double-erythrocyte (2RBC) collection and reasons for ceasing 2RBC donation were retrospectively analysed in the blood donor population of Basel, Switzerland.
Methods
Donors with at least 1 2RBC apheresis were included in the study. Minimal requirements were Hb ≥140 g/L and body weight ≥70 kg; serum ferritin (SF) values were measured routinely, but were not part of the selection criteria. 2RBC collections were performed with ALYX devices at 6-month intervals. Adverse events (AEs) were systematically recorded and classified according to the ISBT EHN 2008 criteria. Data of procedures were retrieved from the ALYX software. Demographics, apheresis data and AEs were analysed with descriptive statistics.
Results
Data of 4,377 2RBC aphereses performed in 793 donors (779 males) between 1st January 2003 and 31st May 2015 were evaluated. Mean donor age at first 2RBC donation was 44 years (standard deviation [SD] 21), median number of donations was 4 (interquartile range [IQR] 8); 32% of the donors underwent a single procedure. There were 161 AEs, mostly local haematomas (55%) and vasovagal reactions (20%); fatigue was reported in 6% of the cases and was more frequent than citrate toxicity. Two severe AEs were observed. The most frequent reasons for abandoning 2RBC donation were low SF levels and donor choice (both 11%), but most donors simply did not reply to invitations (16%). Overall, procedure-related causes (AEs, low SF levels, no time for apheresis, inadequate venous access) were observed in 14% of the cases. At the end of the observation period, 40% of the donors were still active blood donors, but only 20% were donating 2RBC.
Discussion
2RBC donation is overall safe. Donor retention was low over a period of 11 years. An important reason for abandoning 2RBC was the detection of low SF levels. The impact of fatigue on donor retention and the course of iron stores after repeated 6-monthly 2RBC apheresis require further investigation.
Keywords: double red blood cell apheresis, adverse events, donor retention
Introduction
Improving the safety of blood donation and the care of donors who experience an adverse event (AE) is of paramount importance for blood collecting institutions, both because of the ethical concern of protecting healthy volunteers, and because of the consequences of complications concerning blood donation on donor behaviour. There is a large body of evidence to show the negative impact of AEs on donor return, and therefore on the preservation of the pool of active blood donors1–4. From a practical point of view, retention of long-term active, experienced donors is particularly important because they are safer and more cost-effective than newly recruited donors5. A significant negative impact of even non-serious complications of blood donation on the likelihood of donor return within 12 months has been well described in both first and repeat whole blood (WB) donors6.
Double red blood cell unit (2RBC) collection by apheresis is an established method for allogeneic and autologous blood donation. Because of concerns of a possible higher incidence of AEs, in particular vasovagal reactions, and of a higher risk of iron deficiency anaemia in 2RBC donors, stricter eligibility criteria are adopted for this type of collection. Although criteria vary between the different national directives, they include larger total blood volume, higher haemoglobin (Hb) cut-offs and longer interdonation intervals compared to WB donation. According to the Swiss directives, 2RBC donors must have an estimated total blood volume of at least 5.0 L (body weight ≥70 kg and height >165 cm) and a pre-donation Hb level of at least 140 g/L, and the minimal interval between two aphereses is 6 months for both males and females. The Council of Europe criteria are less restrictive (estimated total blood volume of at least 4.5 L, minimal interval between two aphereses 4 months for males and 6 months for females)7, and are similar to the American Red Cross criteria (estimated total blood volume of approximately 4.6 L for males and 5.6 L for females, a minimal required haematocrit of 40%, with minimal donation interval of 16 weeks for both sexes)8.
Large studies on the incidence and severity of AEs associated with 2RBC apheresis show an overall more favourable safety profile of this procedure compared to conventional WB donation, with a very low incidence of severe complications even in young blood donors, who represent a subgroup particularly prone to side effects9–12.However, if minor reactions are also considered, the incidence of AEs in 2RBC apheresis is actually higher than in WB donation6,11, as we also observed in carriers of HFE-mutations with hyperferritinaemia undergoing repeated 2RBC apheresis at shorter intervals13. Similarly, in the study of Rader et al.6, male 2RBC donors were more likely to experience AEs than male WB donors. Interestingly, this analysis showed that, although the occurrence of AEs after 2RBC apheresis had a smaller impact on donor retention compared to repeat WB donors, the return rate of 2RBC donors was overall inferior.
In January 2003, we implemented the automated collection of RBC by apheresis in our centre along with the systematic recording of AEs and the routine measurement of serum ferritin (SF) levels to monitor the course of iron stores in our donors. We present the results of a retrospective evaluation of safety of 2RBC collection by apheresis, describing the incidence and type of AEs, and analysing the reasons that induced donors to discontinue 2RBC donation.
Materials and methods
Subjects
The analysis included donors aged 18–65 years who underwent at least one 2RBC collection. According to the Swiss directives, aphereses can be performed in subjects up to 65 years of age, while the donor physician can allow collection also in older donors in individual cases. All subjects, except those donating for autologous transfusion, were repeat donors with at least one WB donation before their first apheresis. We previously described our experience with frequent 2RBC apheresis in subjects with proven or suspected hereditary haemochromatosis at our centre13. Because the donation policy in this particular group was adapted to the individual donor’s characteristics and SF levels, subjects and procedures of this previous study were excluded from the description of demographics, donation number per donor and reasons for ceasing 2RBC, but were included in the evaluation of AEs. Eligibility for 2RBC was assessed according to the criteria of the Swiss regulations and in compliance with the European Directives. For both males and females, the minimal required body weight was 70 kg and the minimal Hb level 140 g/L as assessed on a capillary blood sample. The interval between two 2RBC aphereses was 6 months for both genders. SF levels were not part of the selection criteria, and deferral of 2RBC donors or changing to another type of donation due to low SF was at the discretion of the donor physician or due to donor choice.
Collection procedures and blood products
Aphereses were performed with an ALYX device (Fenwal-Baxter, Zürich, Switzerland) programmed for the collection of 360 mL RBC. No plasma or platelet components were collected additionally. Oral calcium supplementation was not provided routinely, but only if requested in case of symptoms of citrate toxicity. The anticoagulant used was ACD-A, the extracorporeal volume during apheresis was approximately 400 mL and the ACD-A to whole blood ratio 1:11. Volume loss was replaced with saline. Inline leukocyte depletion of the collected RBCs and addition of storage solution (SAG-M) was performed automatically at the end of the collection. Data of the last 100 collections could be retrieved from each ALYX device. Data of 291 procedures, including processed blood volume, duration of procedures, volume of anticoagulant and saline infused to the donor, RBC volume collected and RBC amount not returned to the donor, were obtained for analysis after exclusion of collections that were interrupted for any reason.
Definition and classification of adverse events
Data on AEs were systematically collected as of January 2007. AEs recorded before January 2007 were retrospectively classified. Complications of blood donation were defined according to indications of the European Directorate for the Quality of Medicines and HealthCare (EDQM)7. AEs were classified as immediate if occurring at the collection site, and delayed if occurring after the donor had left the centre, and were recorded either by the collection staff or reported later by the donor. Severe AEs (SAEs) were defined as events that were fatal, life-threatening, disabling, incapacitating, had a prolonged course or required any medical intervention other than that of the donor physician. AEs were recorded on a standardised form by the collection staff, reviewed by the donor physician who assigned the appropriate category and severity grade, and reported the event(s) in the donor’s files. Type of complication, severity grade and imputability of AEs were classified according to the criteria of the International Society of Blood Donation, European Haemovigilance Network 2008 (ISBT EHN 2008)14, which were modified for easier internal application. Modifications of the original version were a more detailed description of the severity grades in order to facilitate classification, and the addition of a separate category for cases where the procedures were interrupted because of poor flow. Multiple AEs were recorded in the appropriate categories as separate AEs. As from 2012, all complications were cumulatively reported once yearly to the national haemovigilance competent authority (Swissmedic), with SAEs being notified separately within 15 days of occurrence.
Analysis of adherence to 2RBC donation
Possible or clear reasons for abandoning 2RBC donation (such as permanent or temporary deferral, AEs, donor choice, donor relocation and any further information that could possibly influence donor return) were retrieved from the donors’ records. Where no explanation for ceasing 2RBC donation was found, donors, even if no longer active, were contacted by telephone by members of the collection staff and asked specifically about the reason(s) for this.
Statistical analysis
Demographics of the donor population, incidence, type and severity of AEs, and procedure-related data were analysed with descriptive statistics. The rate of AEs was calculated as the number of respective complications divided by the total number of donations performed during the observation period, also including the procedure performed in carriers of hereditary haemochromatosis (as described above).
Results
From 1st January 2003 to 31st May 2015 a total of 4,858 2RBC aphereses were performed in 861 donors. Excluding the procedures of carriers of HFE-mutations with elevated SF levels who underwent 2RBC at an individually-defined frequency, there were 4,377 2RBC collections in 793 donors (779 males, 14 females) performed under standard conditions. Donors’ and donation characteristics are presented in Table I. Mean age was 44 years (SD: 21) at first 2RBC donation and 48 years (SD: 22) at last 2RBC donation. There was only one male under the age of 20 years and 4 females under the age of 50 years.
Table I.
Demographics of donors and characteristics of double-erythrocyte (2RBC) donations by apheresis in the period from 1st January 2003 to 31st May 2015.
| Donor characteristics | N. |
|---|---|
| All donors with >1 2RBCa | 861 |
| Included in the analysisb | 793 |
| Gender (m/f) | 779/14 |
| Age at first 2RBC, years (mean [SD]) | 44 (21) |
| Age at last 2RBC, years (mean [SD]) | 48 (22) |
|
| |
| 2RBC collections | N. |
|
| |
| All 2RBC collectionsa | 4,858 |
| Included in the analysisb | 4,377 |
| Purpose of donation: | |
| - allogeneic transfusion | 4,350 (99%) |
| - autologous transfusion | 22 |
| - otherc | 5 |
| Collections per donor, n. median (interquartile range) | 4 (8) |
Including carriers of hereditary haemochromatosis donating according to individually adapted frequency and eligibility criteria.
Data on demographics and collections refer to subjects donating according to standard procedures (excluding carriers of hereditary haemochromatosis).
Laboratory use and validation of the apheresis device in donors temporarily not eligible for blood donation.
SD: standard deviation.
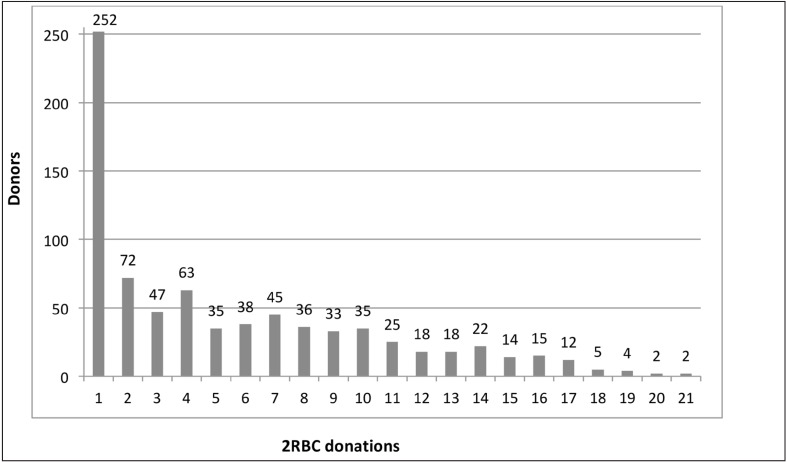
Blood components of 4,350 procedures were used for allogeneic transfusion, 22 donations were performed for autologous collection, and 5 for other purposes in subjects temporarily not eligible for blood donation (laboratory purposes, validation of the device). Figure 1 shows the number of donors grouped per number of 2RBC donations. Median number of collections per donor was 4 (IQR 8); 252 subjects (32%) underwent a single 2RBC collection. All autologous donations but one (n=21) were performed as single procedures.
Figure 1.
Number of 2RBC donations by donor in the period 01/01/2003–31/05/2015.
2RBC: double-erythrocyte.
Procedure-related data
Table II summarises the data of 291 procedures obtained from the ALYX software. Mean collection duration was 25 minutes (SD 3), and mean volume of processed blood was 1,035 mL (SD 52). The mean overall volume loss (including total RBC loss and plasma in the collection bag and in the collection set) was 500 mL (SD 16), comparable or slightly greater than the net volume loss after WB donation. The total RBC loss, including the RBCs collected (mean 361 mL, SD 6) and the mean RBC volume remaining in the set after completion of the apheresis was 387 mL (SD 5). Volume replacement included the infusion of saline and ACD-A to the donor during the procedure, and amounted to a mean 542 mL (SD 36).
Table II.
Procedure-related data of 291 2RBC collections.
| Parameter | Mean (SD) | Median (range) |
|---|---|---|
| Duration of procedure (min) | 25 (3) | 24 (20–35) |
| Blood volume processed (mL) | 1,035 (52) | 1,057 (890–1244) |
| Saline infused to donor (mL) | 432 (29) | 439 (332–497) |
| Anticoagulant (ACD-A) infused to donor (mL) | 126 (9) | 126 (112–244) |
| RBC collected (mL) | 361 (6) | 361 (258–365) |
| Plasma loss (mL) | 113 (10) | 113 (98–241) |
| Total RBC loss (mL)a | 387 (5) | 386 (382–457) |
| Total volume loss (mL)b | 500 (16) | 499 (401–698) |
| Total volume infused to the donor (mL)c | 542 (36) | 582 (258–714) |
Including RBC collected and RBC lost in collection set.
Including RBC loss and plasma loss.
Including saline and ACD-A. Data were obtained from the ALYX devices. Procedures that had to be interrupted for any reason were not included in the analysis.
2RBC: double-erythrocyte; SD: standard deviation; RBC: red blood cells.
Adverse events
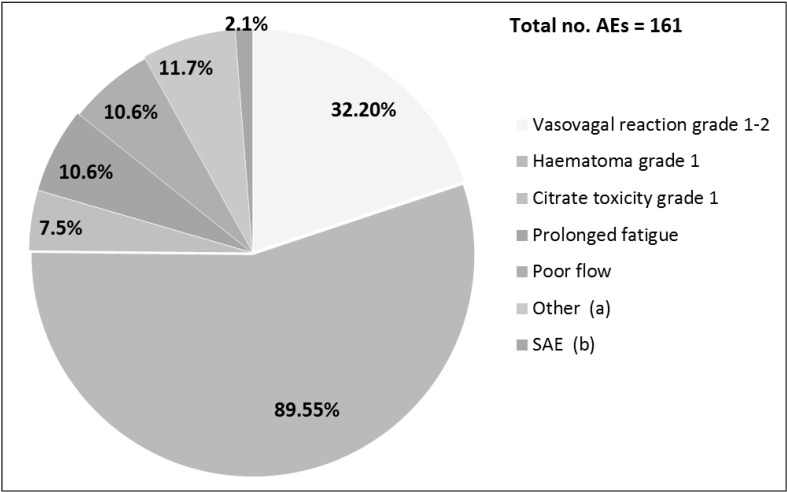
Complications were recorded in 161 of 4,858 apheresis (3.3%) and 157 donors (18%). Frequency and type of AEs are presented in Figure 2.
Figure 2.
Adverse events (AEs) of 2RBC donation.
The incidence of AEs was calculated in relation to the total number of 2RBC collection performed (n=4,858) and on the total number of donors with at least one 2RBC donation (n=861). The numbers indicate the frequency of AEs grouped by type of complication. SAE were defined were defined as events that were fatal, life -threatening, disabling, incapacitating, had a prolonged course or required any medical intervention other than that of the donor physician. a Other: local pain (n=5), headache (n=2), nerve injury (n=1); bSAE: myocardial infarction and complicated delayed vasovagal reaction. 2RBC: double-erythrocyte.
The most frequent side effects were local haematomas grade 1 (n=89, 55% of the cases), followed by vasovagal reactions of immediate type grade 1–2 (n=32, 20%). Further AEs were poor flow and fatigue (10 cases each), while other complications, including citrate toxicity, were less frequent. There were two donors with an SAE, both of severity grade 3: a 64-year old female had a delayed vasovagal reaction and required hospitalisation; a 49-year old male with no known risk factors for arterial coronary disease experienced a myocardial infarction 16–18 hours after his last 2RBC donation. He had previously donated blood regularly, including 10 2RBC aphereses, without complications. His Hb level at the time of his last 2RBC donation was 178 g/L. The procedure was completed without technical problems, thus the total blood loss was not likely to have exceeded 500 mL. Both donors with an SAE were permanently deferred. While the association between the delayed vasovagal reaction and the apheresis performed a few hours before was considered to be definitely confirmed, the imputability of the 2RBC donation in the case of the acute coronary event was evaluated as being “possible”.
Beside these two SAEs, complications led to cessation of 2RBC donation in 32 additional donors. In all cases, AEs were grade 1–2 and included prolonged fatigue after donation (n=9), local haematoma (n=7), immediate vasovagal reaction (n=7), citrate toxicity grade 1–2 (n=5), headache after apheresis (n=2), delayed vasovagal reaction and paraesthesia after local nerve injury (n=1 each). Of the 34 with an AE who did not undergo further 2RBC apheresis, 14 were still active blood donors at the end of the observation period.
Reasons for ceasing 2RBC donations
Overall, within the observation period, 2RBC donations were discontinued by 641 of 793 donors (80%). Reasons for abandoning 2RBC are listed in Table III. In the most frequent cases (n=101, 16%) donors did not respond to our invitations with no further explanation given; 88 subjects (14%) became ineligible for blood donation, mostly due to their medical condition; 84 donors (14%) recruited at the time of implementation of the procedure underwent only one apheresis and continued donating WB or platelets/plasma; in 21 cases (3%), a single collection was performed for autologous use; 19 subjects (2%) with blood group AB or B were switched to donate platelets or plasma.
Table III.
Reasons for discontinuing 2RBC donation and status of 2RBC donors at the end of the study period.
| N. | % | |
|---|---|---|
| Donors with >1 RBC donation | 793 | 100 |
| Donors discontinuing 2RBC donations | 641 | 80 |
| Reasons | ||
| No reply to invitation | 101 | 16 |
| Not eligible for blood donationa | 88 | 14 |
| 2RBC donation at implementation of procedure only | 84 | 14 |
| SF levels ≤30 ng/mL | 73 | 11 |
| Donor choice/preference for other type of donation | 72 | 11 |
| Reason not documented | 52 | 8 |
| Age >65 years | 46 | 7 |
| Donor relocation/administrative | 41 | 6 |
| Procedure-related AEb | 34 | 5 |
| Autologous donation | 22 | 3 |
| Donor asked for platelet/plasma apheresis (blood group B or AB) | 19 | 3 |
| Other, related to 2RBCc | 5 | <1 |
| Low Hb levelsd | 4 | <1 |
| Total reasons related to 2RBC apheresise | 112 | 14 |
| Active donors as at 31/05/2015: | 314 | 40 |
| - donating 2RBC (and WB or platelets/plasma) | 152 (8) | 20 |
| - donating other than 2RBC | 154 | 20 |
Results refer to subjects donating according to standard procedures (carriers of hereditary haemochromatosis are excluded).
Medical condition (n=73); positive infectious disease markers (n=6); medication (n=3); transfusions after 1980 (n=2); piercing, elevated ALAT, vCJD risk, past HBV infection with inadequate antibody titre (n=1 each).
Fatigue (n=9); vasovagal reaction of immediate type (n=7); haematoma (n=7); citrate toxicity (n=5); headache (n=2); delayed vasovagal reaction, prolonged paresthesia of arm, acute coronary syndrome, and complicated delayed vasovagal reaction (n=1 each).
No time for apheresis procedure (n=4); inadequate venous access (n=1).
Below value required for 2RBC (n=3); below value required for blood donation (n=1).
Including SF levels ≤30 ng/mL.
2RBC: double-erythrocyte; RBC: red blood cell; SF: serum ferritin; AE: adverse events; hb: haemoglobin; ALAT: alanine aminotransferase; vCJD: variant Creutzfeldt-Jakob disease.
Importantly, for 112 individuals, a reason directly related to the procedure was documented. Seventy-three donors (12%) were advised against further 2RBC apheresis due to low SF levels. Of these, 46 subjects already had SF levels ≤30 ng/mL at their first apheresis, while in 25 cases, iron deficiency was detected during examinations outside the blood collection centre. Thirty-four subjects (5%) ceased 2RBC donation because of AEs (as described above), for 4 donors the time requested for the apheresis procedure was considered to be too long, and in 1 case venous access was inadequate.
Further reasons for discontinuing 2RBC collections were age above the limit for apheresis (n=46, 7%), relocation, and preference for another type of donation. Four subjects were excluded from 2RBC apheresis because of low Hb levels: in 3 cases there was no anaemia but Hb was below the minimal requirement of 140 g/L; in one subject with anaemia, further investigations revealed impaired renal function, and the donor was permanently deferred. Finally, in 52 cases (8%), no specific reason was documented. Over a period of almost 12 years, 40% of the donors with at least 1 2RBC donation remained active, and 20% continued 2RBC apheresis, with 8 of these also regularly donating WB and/or platelets or plasma.
Discussion
In this study, we provide a retrospective analysis of the incidence and type of AEs and of the adherence of donors to 2RBC apheresis, a type of donation that, according to recent data15,16, is increasingly performed.
A striking observation of our study is that, in the vast majority of cases (80%), 2RBC donation was abandoned at some time, most frequently after a first apheresis (32%). In 2003, at the time of the implementation of the procedure, recruitment was directed at repeat WB donors fulfilling the eligibility criteria for 2RBC. Although in general experienced donors were willing to agree to a new type of donation, a significant proportion (14%) of the subjects who tried 2RBC apheresis preferred to continue donating WB or platelets/plasma. It is possible that, in some cases where no other clear reason was found (i.e. medical condition, reaching the upper age limit for apheresis, or switching to platelet or plasma donation because of blood group B or AB), experienced donors were reluctant to change their donation habit. Similar experiences were also described in early works on RBC collection, where the longer duration of the procedure and the less flexible appointment system were also indicated as possible explanations of the relatively high drop-out rate of 2RBC donors17.
Considering that the permanence at the site of donation for an uncomplicated WB collection at our centre is usually longer than 30 minutes (including the time for blood withdrawal, for resting before leaving the donation room, and for refreshments), the overall time required for a 2RBC apheresis with a mean duration of 25 minutes is obviously longer. On the other hand, subjects regularly undergoing 2RBC apheresis every 6 months give the same number of RBC components in one year as those donating WB at the maximal frequency allowed in Switzerland (every 3 months for males and every 4 months for females). We observed that time was not a major issue among the subjects who discontinued 2RBC donation; only 4 donors declared they did not have enough time for the apheresis procedure. However, we believe that this point should be explicitly addressed when recruiting new donors for 2RBC apheresis.
Our data confirm an overall low incidence of complications in 2RBC collection. In a previous analysis at our centre, the cumulative rate of AEs and SAEs for 2RBC apheresis was 1.9% and 0.1%, respectively, a value that lies between that of WB donation (0.7% and 0.07%) and of platelet apheresis (2.8% and 0.2%) (data not shown). Similarly, larger studies described a higher complication rate in 2RBC collection compared to WB donation, when also minor side effects were reported6,11, and an overall very low incidence of SAEs10.
After local haematomas, vasovagal reactions were the most frequent AEs observed, with no immediate complications of severity greater than grade 2. Limiting the volume of blood collection is one of the most important measures for protecting the donor from vasovagal reactions, and according to the AABB and to the Council of Europe, no more than 15% of the estimated donor’s blood volume must be removed. In a donation with the ALYX, the maximal extra-corporeal volume during the procedure is approximately 400 mL, but no net fluid loss occurs if the apheresis is completed, since fluid replacement is always performed. Thus, selecting donors with a minimal total blood volume of up to 5.0 L, as required by the Swiss regulations, appears to offer the donor adequate protection. Nevertheless, vasovagal reactions were the second most frequent AE. Among the preventive measures that can be applied, in the case of an isovolaemic 2RBC apheresis, those addressing psychological factors, such as paying attention to the donors and keeping their mind occupied during the procedure2, are likely to be more efficacious than other interventions directed to circulatory aspects.
The small amount of citrate infused (mean 126 mL) to subjects who were almost exclusively males with a body weight >70 kg, and the short duration of the procedure, explain the very low incidence of citrate toxicity in our cohort, with no events greater than grade 1, even without routine calcium supplementation. However, short- and long-term effects of repeated citrate exposure on bone metabolism, as described in frequent platelet donors18,19, represent a theoretical safety issue also for 2RBC donation, especially for those subjects who in addition undergo platelet and/or plasma apheresis. Due to the factors mentioned above, and to the much lower donation frequency, it can be postulated that 2RBC apheresis have a limited effect on bone metabolism compared to frequent platelet or plasma donation, but no data for 2RBC apheresis on this aspect of donation safety are available so far.
In 2RBC collection with the ALYX device, a mean RBC loss of 387 mL occurs, corresponding to less than the amount of RBC withdrawn with 2 WB donations. Accordingly, fast-developing iron loss is inevitably presented; but the recovery of iron stores after 2RBC apheresis, and the corresponding impact on Hb levels, greatly depends on donation frequency, and is incomplete when 2RBC apheresis is performed at a 4-month interval20,21. In our study, an important proportion of subjects (12%) abandoned 2RBC donation on medical advice because of low SF levels (generally below 30 ng/mL), even in the absence of anaemia and of symptoms of iron deficiency. This practice largely reflects the uncertainty about the course of Hb and SF levels in repeated 2RBC donors, with a perceived greater risk for iron deficiency anaemia associated with this procedure compared to conventional WB donation. Although only very few donors of our cohort were excluded from 2RBC apheresis because of inadequate Hb levels, it is possible that in a larger number of subjects the decrease in Hb values below critical levels was prevented by an earlier deferral due to low SF. Interestingly, 25 cases already had low SF levels at their first apheresis. To our knowledge, there are no published data on the effects of 2RBC collection in subjects with low base-line iron stores. Such evaluations, as well as a comparison of different 2RBC donation frequencies on the course of Hb and SF levels, would be extremely valuable in order to optimise donation strategies targeted at preventing iron deficiency anaemia and thus avoiding donor loss.
An interesting observation was the occurrence of fatigue, which was reported as an AE only by a small but important proportion of donors (6%), and was more frequent than an expected side effect such as citrate toxicity. In our experience, only 10 cases of fatigue were recorded as AEs, and only 9 donors explicitly indicated fatigue as the reason for abandoning 2RBC apheresis. However, fatigue is a frequent symptom after WB donation22, and represents a possibly relevant AE also in 2RBC donors, causing per se self-exclusion1,2. One may speculate that fatigue was the reason for discontinuing 2RBC apheresis at least in some of the donors who were lost for reasons that were not clear. Prolonged fatigue may reflect a transitory situation of tissue iron deficiency or “relative” anaemia23,24, even in the presence of normal Hb and SF values. The perception of this symptom can be very different in non-anaemic subjects, and does not show a reliable correlation with the extent of iron depletion as expressed by low SF levels, as we also observed in female blood donors with iron deficiency25. In our opinion, the real impact of fatigue on abandoning blood donation, and specifically 2RBC donation, has been under-estimated and needs further analysis in order to provide appropriate donor counselling.
Overall, having experienced an AE was a not a frequent reason for abandoning 2RBC donation (5% of the cases), although, as discussed above, symptoms were most probably under-reported by the donor, particularly if occurring some time after the procedure. In this respect, carrying out a post-donation interview would allow complications to be recognised, as suggested by previous experiences with WB donors1,26.
Taken together, one reason for the loss of 2RBC donors directly related to the procedure (AE, low ferritin levels, and, rarely, time issues and inadequate venous access) was recognised in a minority of the cases. In the greater proportion of cases where no reason could be found (no reply to invitation, reason not clear, or not documented), the adherence to 2RBC donation is likely to have been influenced also by socio-economic factors, reflecting the general behaviour of the blood donor population living in the urban area of our region, as recently described27, and by psychological aspects, such as social inertia. A lack of motivation despite a positive attitude towards blood donation represents also a specific donor characteristic associated with return behaviour28.
The small size of our donor cohort and the retrospective nature of the data allow only a descriptive analysis of donor safety and retention to be made. Our data were collected in a population of almost exclusively experienced, middle-aged male donors. There were only 16 first-time 2RBC donors, all donating for autologous transfusion, and these included 6 of the 14 females included in the analysis. Thus, we are not able to draw any conclusion on the incidence of AEs and retention of donors under 20 years of age and on females, two categories of subjects at higher risk for AEs12. Despite these limitations, our work provides an accurate picture of the possible complications and of donor behaviour in a homogeneous cohort of subjects undergoing 2RBC donation according to standards that are particularly protective towards the donor. In Basel, the systematic recording of AEs in blood donors according to the ISBT EHN 2008 criteria was introduced several years ago, and recently became mandatory in Switzerland for the survey of donor safety nationwide. Such evaluations not only provide a basis for a critical revision of the donation processes within single institutions, but also allow multicentric data collection and comparison, and the elaboration of possible common strategies for preventing AEs, which could enhance donor retention. However, while haemovigilance reporting systems for the safety of blood transfusion have been implemented in many countries, systematic surveys of donation safety are much less widespread among blood collection establishments.
Conclusions
In conclusion, in our experience, 2RBC donation is safe and overall well tolerated, but with a high drop-out rate for reasons that are multifactorial and still not completely understood. Some aspects of 2RBC apheresis, such as the real impact of fatigue and iron depletion on donor retention, the feasibility of 2RBC collections in younger donors and females, and the impact of different donation policies (i.e. different donation frequency) on donor return require further investigation. In order to continue to explore these unresolved issues, further systematic survey of donor safety and attitude is essential.
Acknowledgements
We thank Fenwal/Fresenius for providing and analysing data of the ALYX procedures. We thank the Basel Swiss Red Cross Blood Transfusion Centre research pool for their financial support.
Footnotes
Authorship contributions
KK, ZJ and VP collected data, LI, KK and MS performed the statistics, LI and KK wrote the manuscript, AH, JS, AOM and AB revised the manuscript.
The Authors declare no conflicts of interest.
References
- 1.Newman BH, Pichette S, Pichette D, et al. Adverse effects in blood donors after whole-blood donation: a study of 1000 blood donors interviewed 3 weeks after whole-blood donation. Transfusion. 2003;43:598–603. doi: 10.1046/j.1537-2995.2003.00368.x. [DOI] [PubMed] [Google Scholar]
- 2.Newman BH, Newman DT, Ahmad R, et al. The effect of whole-blood donor adverse events on blood donor return rates. Transfusion. 2006;46:1374–9. doi: 10.1111/j.1537-2995.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Dongen A, Abraham C, Ruiter RA, et al. The influence of adverse reactions, subjective distress, and anxiety on retention of first-time blood donors. Transfusion. 2013;53:337–43. doi: 10.1111/j.1537-2995.2012.03810.x. [DOI] [PubMed] [Google Scholar]
- 4.Veldhuizen I, Atsma F, van Dongen A, et al. Adverse reactions, psychological factors, and their effect on donor retention in men and women. Transfusion. 2012;52:1871–79. doi: 10.1111/j.1537-2995.2011.03551.x. [DOI] [PubMed] [Google Scholar]
- 5.Popovsky MA. Anemia, iron depletion, and the blood donor: it’s time to work on the donor’s behalf. Transfusion. 2012;52:688–2. doi: 10.1111/j.1537-2995.2012.03562.x. [DOI] [PubMed] [Google Scholar]
- 6.Rader AW, France CR, Carlson B. Donor retention as a function of donor reactions to whole-blood and automated double red cell collections. Transfusion. 2007;47:995–1001. doi: 10.1111/j.1537-2995.2007.01223.x. [DOI] [PubMed] [Google Scholar]
- 7.Council of Europe. Guide to the Preparation, Use and Quality Insurance of Blood Components. 17th ed. Strasbourg: Council of Europe Publishing; 2013. European Directorate for the Quality of Medicine and Health Care. [Google Scholar]
- 8.American Red Cross. Eligibility Requirements. [Accessed on 25/08/2015]. Available at: http://www.redcrossblood.org/donating-blood/eligibility-requirements.
- 9.Popovsky MA. Safety of RBC apheresis and whole blood donation in allogeneic and autologous blood donors. Transfus Apher Sci. 2006;34:205–11. doi: 10.1016/j.transci.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Wiltbank TB, Giordano GF. The safety profile of automated collections: an analysis of more than 1 million collections. Transfusion. 2007;47:1002–5. doi: 10.1111/j.1537-2995.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 11.Eder AF, Dy BA, Kennedy JM, et al. The American Red Cross donor hemovigilance program: complications of blood donation reported in 2006. Transfusion. 2008;48:1809–19. doi: 10.1111/j.1537-2995.2008.01811.x. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin RJ, Dy BA, Kennedy JM, et al. The relative safety of automated two-unit red blood cell procedures and manual whole-blood collection in young donors. Transfusion. 2009;49:1874–83. doi: 10.1111/j.1537-2995.2009.02237.x. [DOI] [PubMed] [Google Scholar]
- 13.Stefashyna O, Stern M, Infanti L, et al. Pattern of care of blood donors with early-uncomplicated hereditary haemochromatosis in a Swiss blood donation centre. Vox Sang. 2014;106:111–7. doi: 10.1111/vox.12078. [DOI] [PubMed] [Google Scholar]
- 14.Working Group on Complications Related to Blood Donation, International Society of Blood Transfusion Working Party on Haemovigilance, European Haemovigilance Network. Standard for Surveillance of Complications Related to Blood Donation. 2008. [Google Scholar]
- 15.van der Poel C, Janssen M, Behr-Gross M-E. Directorate for the Quality of Medicines and Healthcare of the Council of Europe (EDQM) The Collection, Testing and Use of Blood Components in Europe, 2007 Report. Strasbourg: Council of Europe Publishing; 2011. [Google Scholar]
- 16.Whitaker BI, Hinkins S. The 2011 National Blood Collection and Utilization Survey Report. Rockville, MD: US Department of Health and Human Services; 2013. [Google Scholar]
- 17.Harrison JF. Automated red cell collection--quality and value. Transfus Med. 2006;16:155–64. doi: 10.1111/j.1365-3148.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 18.Bolan CD, Greer SE, Cecco SA, et al. Comprehensive analysis of citrate effects during plateletpheresis in normal donors. Transfusion. 2001;41:1165–71. doi: 10.1046/j.1537-2995.2001.41091165.x. [DOI] [PubMed] [Google Scholar]
- 19.Amrein K, Katschnig C, Sipurzynski S, et al. Apheresis affects bone and mineral metabolism. Bone. 2010;46:789–95. doi: 10.1016/j.bone.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Radtke H, Mayer B, Rocker L, et al. Iron supplementation and 2-unit red blood cell apheresis: a randomized, double-blind, placebo-controlled study. Transfusion. 2004;44:1463–7. doi: 10.1111/j.1537-2995.2004.04045.x. [DOI] [PubMed] [Google Scholar]
- 21.Mendrone A, Jr, Arrais CA, Almeida Neto C, et al. Impact of allogeneic 2-RBC apheresis on iron stores of Brazilian blood donors. Transfus Apher Sci. 2009;41:13–7. doi: 10.1016/j.transci.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson-Sojka B, Sojka P. The blood-donation experience: perceived physical, psychological and social impact of blood donation on the donor. Vox Sanguinis. 2003;84:120–8. doi: 10.1046/j.1423-0410.2003.00271.x. [DOI] [PubMed] [Google Scholar]
- 23.Brownlie T, Utermohlen V, Hinton PS, et al. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am J Clin Nutr. 2002;75:734–42. doi: 10.1093/ajcn/75.4.734. [DOI] [PubMed] [Google Scholar]
- 24.Brownlie T, Utermohlen V, Hinton PS, et al. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79:437–43. doi: 10.1093/ajcn/79.3.437. [DOI] [PubMed] [Google Scholar]
- 25.Pittori C, Buser A, Gasser UE, et al. A pilot iron substitution programme in female blood donors with iron deficiency without anaemia. Vox Sanguinis. 2011;100:303–11. doi: 10.1111/j.1423-0410.2010.01427.x. [DOI] [PubMed] [Google Scholar]
- 26.Sinclair KS, Campbell TS, Carey PM, et al. An adapted postdonation motivational interview enhances blood donor retention. Transfusion. 2010;50:1778–86. doi: 10.1111/j.1537-2995.2010.02618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volken T, Buser A, Holbro A, et al. Blood donor to inactive donor transition in the Basel region between 1996 and 2011: a retrospective cohort study. Vox Sanguinis. 2015;109:155–62. doi: 10.1111/vox.12269. [DOI] [PubMed] [Google Scholar]
- 28.Wevers A, Wigboldus DHJ, de Kort W, et al. Characteristics of donors who do or do not return to give blood and barriers to their return. Blood Transfus. 2014;12( Suppl 1):s37–43. doi: 10.2450/2013.0210-12. [DOI] [PMC free article] [PubMed] [Google Scholar]