Abstract
The antigens of the ABO system are expressed on red blood cell membranes as well as on the surface of several other normal and pathological cells and tissues. Following the first clinical observations more than 60 years ago, the role of ABO blood group in cancer biology has been intensely studied by several investigators, and it is now widely recognised that ABO antigens are associated with the risk of developing several types of tumours, namely pancreatic and gastric cancers. However, whether this association also affects the clinical outcome of cancer patients is less certain. In this narrative review, based on literature data, we discuss the role of ABO blood types as prognostic biomarkers in different types of cancers. The current knowledge of the underlying pathogenic mechanisms of the association is also analysed.
Keywords: ABO blood group, cancer, prognosis, outcome, survival
Introduction
The ABO blood group is by far the most important among human blood group systems1. The ABO gene is located on chromosome 9q34 and encodes two alleles (i.e., A and B) for specific glycosyltransferases which catalyse the covalent linkage of N-acetylgalactosamine or D-galactose to a common precursor side chain (i.e., the H determinant), which is finally converted into A or B antigen2–4. Unlike A and B alleles, the O variant encodes a non-functional glycosyltransferase, thus leaving the H antigen virtually unmodified in this instance4. The ABO blood group antigens are defined by carbohydrate moieties on the extracellular surface of red blood cell membranes5. However, along with their expression on erythrocytes, these antigens are also highly expressed on the surface of a large number of human cells and tissues, including epithelia, sensory neurons, platelets and the vascular endothelia6. The term histo-blood group ABO is often used to reflect the ubiquitous distribution of ABO antigens. It is biologically plausible that the clinical significance of the ABO system could expand beyond immunohaematology, transfusion and transplantation medicine. Indeed, there is growing evidence from the recent scientific literature of an important involvement of the ABO blood group system in the development of cardiovascular, infectious and neoplastic diseases, as well as in several other human disorders7–12.
More specifically, although a number of studies have found evidence of an association between ABO blood group antigens and various types of cancers13,14, so far there is limited understanding of the prognostic value in cancer patients. Moreover, the underlying molecular mechanisms are largely unknown. The relationship between ABO blood groups and survival from cancer is addressed in this review through analysis of the pathogenic mechanisms and the published clinical data.
Search methods
We systematically reviewed the scientific literature for published studies evaluating the association between ABO blood group antigens and outcome in patients with various types of cancers. The MEDLINE® electronic database was searched without temporal limits using English language restriction. The Medical Subject Heading and keywords used were the following: “ABO blood group”, “cancer”, “survival”, “prognosis”, “outcome”, “disease progression” and “life-expectancy”. We also hand-searched the reference lists of the most relevant items to identify further eligible studies not captured in the initial literature search. Search terms were also applied to abstracts from the latest international congresses on cancer.
Pathogenic mechanisms
The underlying mechanisms by which the ABO blood group or the closely linked genetic variants of the ABO locus may interplay with cancer development and progression are still poorly understood and remain the matter of research9. One plausible hypothesis encompasses a dysregulation of the enzymatic activity of the ABO glycosyltransferases, which are specifically involved in the processes of intercellular adhesion and cellular membrane signalling as well as in the immune response to the host15–17. The alteration of these surface molecules may promote the process of malignancy18, through a mechanism analogous to the well-known role played by the ABO glycosyltransferases in modulating the circulating plasma levels of von Willebrand factor and the consequent increased risk of venous thromboembolism19,20. This association is particularly intriguing, also considering that von Willebrand factor was recently found to be an important modulator of angiogenesis and apoptosis, which, in turn, are processes involved in tumorigenesis21.
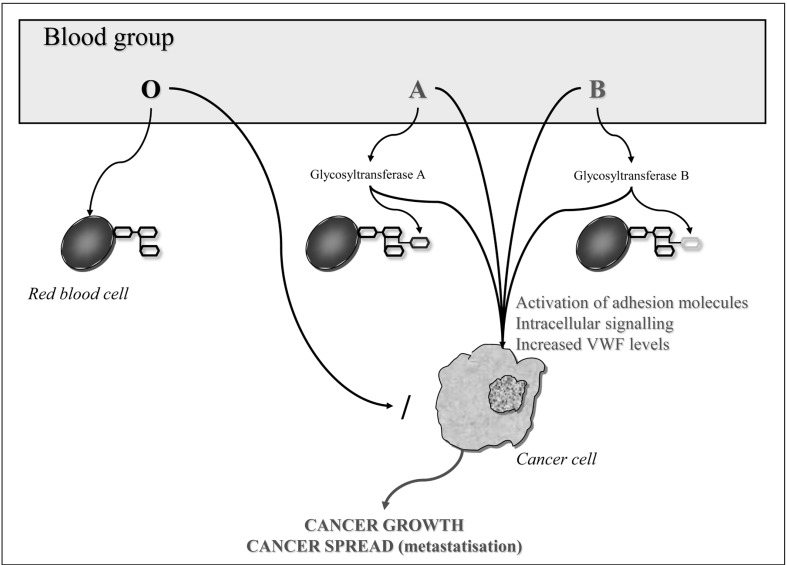
Alterations in the host inflammatory state due to ABO blood group antigens provide a further potential mechanism by which blood type may influence the progression and spread of malignancy22. Recent studies reported an association between polymorphisms at the ABO gene locus and circulating levels of tumour necrosis factor-alpha23, soluble intercellular adhesion molecule (ICAM)-124,25, E-selectin26,27 and P-selectin25. All these adhesion molecules are important mediators of chronic inflammation and immune cell recruitment. They may, therefore, provide a biological basis for the postulated influence of ABO on cancer survival, by directly linking ABO blood group and tumour initiation and spread9. The expression of soluble ICAM-1, which inhibits lymphocyte attachment to endothelial cells by binding to the ICAM ligands on circulating cells, is significantly reduced in patients with non-O blood group (particularly blood group A) compared to the expression in those with blood group O28,29. Since some cancer cells use similar mechanisms for adhesion to endothelial cells and subsequent metastatisation30, the decreased soluble ICAM levels in patients with non-O blood groups may promote metastatic spread of tumours. In support of this hypothesis, a number of studies showed decreased survival in non-O blood type cancer patients and a favourable prognosis in those carrying O blood type31,32. Figure 1 summarises the mechanisms involved in the association between ABO antigens and cancer risk.
Figure 1.
Mechanisms of the association between ABO antigens and cancer.
VWF: von Willebrand factor.
Epidemiological and clinical data
A number of epidemiological studies have assessed the relationship between ABO blood group antigens and survival in patients with various types of cancers (Table I)14.
Table I.
Characteristics of the main studies investigating the effect of ABO blood group on survival in patients with cancers.
| First author, yearref. | Study design | Population | Results |
|---|---|---|---|
| Dandona, 201038 | Retrospective | 417 patients undergoing resection for pancreatic cancer | No statistically significant difference in OS among different blood groups (p=0.196) was found |
| Ben, 201139 | Prospective | 1,431 patients with pancreatic cancer | The median OS in patients with blood type O was significantly longer than that of patients with non-O blood types (16.0 months vs 11.0 months, p=0.001) |
| Rahbari, 201231 | Prospective | 627 patients undergoing resection for pancreatic cancer | Blood group O was independently associated with OS (HR: 0.78; 95% CI: 0.62–0.99; p=0.037) |
| Wang, 201240 | Prospective | 488 patients with pancreatic cancer | ABO blood type was not associated with OS (non-O vs O HR: 1.150; 95% CI: 0.931–1.420; p=0.194) |
| Kaffenberger, 201232 | Retrospective | 900 patients undergoing surgery for renal cell carcinoma | In a multivariate analysis, non-O blood type was significantly associated with decreased OS (HR: 1.68; 95% CI: 1.18–2.39, p=0.004) |
| de Martino, 201442 | Retrospective | 556 patients undergoing surgery for renal cell carcinoma | ABO blood type was not associated with OS (non-O vs O HR: 0.72; 95% CI: 0.48–1.10; p=0.13) |
| Lee, 201543 | Retrospective | 3,172 patients undergoing surgery for renal cell carcinoma | ABO blood group was not associated with OS (p=0.990) |
| Orihuela, 198744 | Retrospective | 494 patients with superficial bladder cancer | Progression to advanced disease was more frequent among patients with O blood type than in those with other groups (37% vs 12–16%; p<0.05) |
| Klatte, 201445 | Retrospective | 931 patients with non-muscle invasive bladder cancer | Patients with O blood type had worse recurrence and progression rates than those with type A (p=0.015 and 0.031) or B (p=0.004 and 0.075), respectively |
| Raitanen, 199346 | Retrospective | 261 patients with bladder cancer | No differences in the mortality rate were observed among patients with different ABO blood types |
| Yamada, 199347 | Retrospective | 538 patients with bladder cancer | No significant differences among blood groups for stage, histological grade or survival rate were observed |
| Klatte, 201449 | Retrospective | 7,906 patients with bladder cancer undergoing radical cystectomy | No relevant association of ABO blood type with prognosis was detected by multivariable analysis |
| Ouyang, 201351 | Retrospective | 2,117 patients with nasopharyngeal carcinoma | Blood type A patients had significantly lower OS compared with non-O blood types (HR: 1.49, 95% CI: 1.03–2.17; p=0.036) |
| Zhang, 201452 | Retrospective | 1,601 patients with non-metastatic nasopharyngeal carcinoma | ABO blood group was not associated with OS (HR non-A vs A: 1.136, 95% CI: 0.840–1.537; p=0.408) |
| Lee, 199156 | Retrospective | 164 patients with non-small cell lung cancer | Median OS was significantly shorter in patients with primary tumour negative for blood group antigen A compared with those with antigen A-positive tumour (p<0.001) |
| Graziano, 199757 | Retrospective | 260 patients with resected early-stage non-small cell lung cancer | Median OS was significantly shorter in patients with primary tumours negative for blood group antigen A compared with those with antigen A-positive tumours (38 months vs 98 months, p<0.01) |
| Leòn-Atance, 201259 | Retrospective | 402 patients with stage I non-small cell lung cancer | The 5-year cumulative survival was 73% for patients expressing blood group antigen A vs 53% for patients with loss of expression (p=0.03) |
| Nozoe, 200461 | Retrospective | 284 patients with esophageal squamous cell carcinoma | No association between ABO blood group and prognosis was observed |
| Sun, 201462 | Retrospective | 511 patients with with esophageal squamous cell carcinoma | Among patients who had ever smoked, B/O blood group independently correlated with unfavourable survival (B/O vs A/AB: HR: 1.565, 95% CI: 1.110–2.205; p=0.011) |
| Qiu, 201166 | Retrospective | 474 patients with gastric adenocarcinoma/404 controls | No significant difference between ABO blood group and OS was observed (5-year OS B/O vs A/AB: 52.8% vs 43.0%; p=0.157) |
| Cao, 201468 | Retrospective | 1,555 patients with surgically resected colon cancer | Patients with AB blood type had a better mean OS than those with non-AB blood type (113.9 months vs 106.1 months; p<0.001). |
| Holdsworth, 198570 | Retrospective | 1,001 patients with invasive breast cancer | Patients with B and AB blood types had a poorer OS than those with O and A blood groups (p=0.015) |
| Gates, 201173 | Prospective | 3,107 patients with breast cancer | No association between blood type and breast-cancer specific mortality was found |
| Klimant, 201174 | Retrospective | 426 patients undergoing surgery for breast cancer | After adjusting for age, disease stage and treatment, no significant differences were observed in 5-year OS and disease-free survival |
| Yu, 201275 | Retrospective | 468 patients with triple-negative breast cancer | Compared to women with blood type O, there was no significant difference in breast-cancer specific mortality for blood types A, B, or AB |
OS: overall survival; HR: hazard ratio; CI: confidence interval.
Pancreatic cancer
As far as regards pancreatic cancer, Amundadottir and colleagues identified the contribution of genetic variations in the ABO locus of 9q34 to pancreatic carcinogenesis in a two-stage genome-wide association study33, and their results were then replicated by Rizzato and collaborators34. Furthermore, the lower incidence of pancreatic cancer among patients with blood group O observed in some studies35–37 has raised the question as to whether ABO blood group status may correlate with the outcome of patients who develop this type of neoplasia. In a study of 417 patients, Dandona and colleagues confirmed the existence of an increased risk of developing pancreatic cancer in patients with blood groups others than O, but did not observe a significant effect of ABO blood group on overall survival38. In another prospective study conducted in 1,431 Chinese patients, Ben and colleagues found that the median overall survival of patients with blood type O was significantly longer than that of individuals with non-O blood types among patients undergoing potentially curative resection of pancreatic cancer39. A multivariate analysis revealed that blood group O was an independent predictor of long-term survival in a study based on 627 patients undergoing resection for pancreatic ductal adenocarcinoma31. By contrast, another study failed to find evidence of an impact of ABO blood type on the prognosis of pancreatic cancer patients40.
Urinary tract cancers
The ABO antigens are expressed on the surfaces of several normal and tumour tissues, including the kidneys and renal cell carcinoma lines41. It is not, therefore, surprising that the relationship between ABO blood group and renal cell carcinoma has been assessed in a number of studies. In a recent study conducted in 900 patients undergoing surgery for loco-regional renal cell carcinoma, ABO blood group was found to be independently associated with overall survival32. In particular, non-O blood type was identified as an independent predictor of mortality32. In another study, O blood group was associated with the absence of lymph node metastasis, although this finding did not generate a favourable impact on prognosis42. A similar conclusion was reached in a more recent study conducted on 3,172 renal cell cancer patients43. Other investigators focused their research on the prognostic value of ABO blood group in patients with bladder cancer, but results were inconsistent (Table I)44–49. Indeed, although some studies found that ABO blood type was a prognostic biomarker44,45, others failed to demonstrate a significant association46–49. Curiously and at variance from studies on renal cell carcinoma, patients with O blood type and bladder cancer seemed to have a worse clinical outcome than those with blood types other than O44,45.
Respiratory tract cancers
As far as regards nasopharyngeal carcinomas, some investigators demonstrated that blood type A is not only associated with an increased risk of developing this cancer50, but also with a poorer prognosis. More specifically, patients with blood type A had a significantly lower overall survival rate than those with non-A types51, although this relationship was not confirmed by others52. No significant association between ABO blood group and the incidence or mortality of laryngeal carcinoma53 or malignant mesethelioma54 was observed by other investigators. With regards to lung cancer, a study conducted in 81 patients with non-metastatic local-advanced non-small cell lung cancer found no significant effect of ABO blood group on prognosis55. More interesting data emerged from studies evaluating blood group antigen expression in tumour cells. Lee and colleagues assessed the prognostic value of immuno-histochemically altered expression of ABO blood-group antigens in tumour samples from 164 patients who underwent curative surgery for non-small cell lung cancer. They found that the survival of 28 patients with blood group A or AB with primary tumours negative for blood group A was shorter than that of 43 patients with antigen A-positive tumours and that of 93 patients with blood group B or O56. Similar findings were made in three subsequent studies, suggesting that the loss of A antigen is a powerful predictor of short survival in lung cancer patients57–59. Finally, a study conducted by Moldvay and colleagues in 227 patients with resected non-small cell lung carcinoma found that positive A+B+H antigen staining of cancer cells was associated with better survival, whereas an O blood type was an independent marker of shorter survival60.
Gastrointestinal tract cancers
As regards oesophageal squamous cell carcinoma, in a study conducted by Nozoe and Colleagues in 284 patients, non-O blood groups correlated with poorly differentiated grades of the tumour and AB blood group was associated with advanced stage and large tumour size61. However, no correlation was found between different ABO blood groups and overall survival. By contrast, in another study the B/O group was found to independently correlate with unfavourable survival among patients who had ever smoked62. As regards gastric cancer, after the initial report by Aird and colleagues on the correlation with blood type A63, large, prospective, population-based studies have consistently documented an increased risk of gastric cancer in individuals with blood type A64,65, although this association apparently did not affect patients’ survival66. It should, however, be noted that the progression of gastric cancer and survival related to ABO blood group might also be associated with other factors, such as infection with Helicobacter pylori67. Finally, patients with surgically resected colon cancer with AB blood type were more likely to have a better survival than patients with non-AB blood types68.
Breast cancer
Several investigators have assessed the relationship between ABO blood group and breast cancer. A recent meta-analysis of 14 studies including 9,665 breast cancer patients and 244,768 controls suggested that Caucasian people with blood type A may have a higher risk of this cancer than Caucasians with other blood groups69. The earlier observation, published by Holdsworth and colleagues70, that patients with blood groups B and AB had a significantly increased incidence of breast cancer and a poorer overall survival compared with those with types O and A, was partially replicated by a further study conducted by Costantini and colleagues71, wherein patients with O blood type had a significantly lower risk of death than patients with non-O blood types. The a study by Stamatakos and colleagues, the worst prognosis was found to be associated with A blood type, particularly Rh negative72. More recent studies failed to confirm this association. In a prospective analysis of 67,697 women in the Nurses’ Health Study, Gates and colleagues identified 3,107 incident cases of invasive breast cancer, but no significant association was found between ABO blood type and breast cancer risk or survival73. In a retrospective population-based study by Klimant et al., including 426 patients undergoing surgical therapy for breast cancer, no significant differences in overall and disease-free survival could be demonstrated among the subjects with different blood type groups74. Finally, another study revealed no association between ABO blood group and cancer survival in 468 patients with triple-negative (i.e., oestrogen receptor-negative, progesterone-receptor negative and HER2 non-amplified) breast cancer75.
Conclusions
A large body of literature seems to describe a link between ABO blood type and cancer risk. A number of plausible mechanisms have been proposed in support of this link, including inflammation, immunosurveillance for malignant cells, intercellular adhesion and membrane signalling. Whatever the mechanism implicated, it now seems consolidated that altered ABO glycosyltransferase activity plays a key role in carginogenesis, mainly by affecting cell proliferation, tumour invasion and metastatic spread76,77. Besides the experimental and clinical evidence of this association, the results from studies evaluating the prognostic value of ABO antigens in various cancers are quite conflicting. Possible reasons to explain the heterogeneity of findings across the studies include limitations in study design (the great majority of them are retrospective) and differences in population characteristics, as well as small study populations. Probably, the most consistent evidence on the prognostic role of ABO blood group concerns pancreatic cancer, in which O blood type seems to be protective against cancer development and progression. For lung cancer patients, expression of blood group antigen A in tumour cells was reported to be a favourable prognostic factor. Although additional experimental studies are needed to unravel the pathogenic mechanisms linking ABO blood types with cancer development, further prospective studies with larger number of patients are advisable to gain a better understanding of the role played by ABO blood group antigens as prognostic factors in various types of cancers.
Footnotes
The Authors declare no conflict of interest.
References
- 1.Storry JR, Olsson ML. The ABO blood group system revisited: a review and update. Immunohematology. 2009;25:48–59. [PubMed] [Google Scholar]
- 2.Yamamoto F, Clausen H, White T, et al. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990;345:229–33. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto F, Cid E, Yamamoto M, Blancher A. ABO research in the modern era of genomics. Transfus Med Rev. 2012;26:103–18. doi: 10.1016/j.tmrv.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Lowe J. The blood group-specific human glycosyltransferases. Baillieres Clin Haematol. 1993;6:465–90. doi: 10.1016/s0950-3536(05)80155-6. [DOI] [PubMed] [Google Scholar]
- 5.Franchini M, Liumbruno GM. ABO blood group: old dogma, new perspectives. Clin Chem Lab Med. 2013;51:1545–53. doi: 10.1515/cclm-2013-0168. [DOI] [PubMed] [Google Scholar]
- 6.Eastlund T. The histo-blood group ABO system and tissue transplantation. Transfusion. 1998;38:975–88. doi: 10.1046/j.1537-2995.1998.381098440863.x. [DOI] [PubMed] [Google Scholar]
- 7.Anstee DJ. The relationship between blood groups and disease. Blood. 2010;115:4635–43. doi: 10.1182/blood-2010-01-261859. [DOI] [PubMed] [Google Scholar]
- 8.Liumbruno GM, Franchini M. Beyond immunohaematology: the role of the ABO blood group in human diseases. Blood Transfus. 2013;11:491–9. doi: 10.2450/2013.0152-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchini M, Favaloro EJ, Targher G, Lippi G. ABO blood group, hypercoagulability, and cardiovascular and cancer risk. Crit Rev Clin Lab Sci. 2012;49:137–49. doi: 10.3109/10408363.2012.708647. [DOI] [PubMed] [Google Scholar]
- 10.Franchini M, Mannucci PM. ABO blood group and thrombotic vascular disease. Thromb Haemost. 2014;112:1103–9. doi: 10.1160/TH14-05-0457. [DOI] [PubMed] [Google Scholar]
- 11.Dentali F, Sironi AP, Ageno W, et al. Non-O blood type is the commonest genetic risk factor for VTE: results from a meta-analysis of the literature. Semin Thromb Hemost. 2012;38:535–48. doi: 10.1055/s-0032-1315758. [DOI] [PubMed] [Google Scholar]
- 12.Dentali F, Sironi AP, Ageno W, et al. ABO blood group and vascular disease: an update. Semin Thromb Hemost. 2014;40:49–59. doi: 10.1055/s-0033-1363460. [DOI] [PubMed] [Google Scholar]
- 13.Liumbruno GM, Franchini M. Hemostasis, cancer, and ABO blood group: the most recent evidence of association. J Thromb Thrombolysis. 2014;38:160–6. doi: 10.1007/s11239-013-1027-4. [DOI] [PubMed] [Google Scholar]
- 14.Franchini M, Lippi G. The intriguing relationship between the ABO blood group, cardiovascular disease, and cancer. BMC Med. 2015;13:7. doi: 10.1186/s12916-014-0250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Zhang HS, Cordon-Cardo C, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: II. Blood group-related antigens. Int J Cancer. 1997;73:50–6. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim Biophys Acta. 1999;1473:247–66. doi: 10.1016/s0304-4165(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 17.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 18.Roseman S. Reflections on glycobiology. J Biol Chem. 2001;276:41527–42. doi: 10.1074/jbc.R100053200. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins PV, O’Donnell JS. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion. 2006;46:1836–44. doi: 10.1111/j.1537-2995.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- 20.Franchini M, Crestani S, Frattini F, et al. ABO blood group and von Willebrand factor: biological implications. Clin Chem Lab Med. 2014;52:1273–6. doi: 10.1515/cclm-2014-0564. [DOI] [PubMed] [Google Scholar]
- 21.Franchini M, Frattini F, Crestani S, et al. Von Willebrand factor and cancer: a renewed interest. Thromb Res. 2013;131:290–2. doi: 10.1016/j.thromres.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melzer D, Perry JR, Hernandez D, et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs) PLoS Genet. 2008;4:e1000072. doi: 10.1371/journal.pgen.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pare G, Chasman DI, Kellogg M, et al. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet. 2008;4:e1000118. doi: 10.1371/journal.pgen.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbalic M, Dupuis J, Dehghan A, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19:1863–72. doi: 10.1093/hmg/ddq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paterson AD, Lopes-Virella MF, Waggott D, et al. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler Thromb Vasc Biol. 2009;29:1958–67. doi: 10.1161/ATVBAHA.109.192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi L, Cornelis MC, Kraft P, et al. Genetic variants in ABO blood group region, plasma soluble E-selectin levels, and risk of type 2 diabetes. Hum Mol Genet. 2010;19:1856–62. doi: 10.1093/hmg/ddq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witkowska AM, Borawska MH. Soluble intercellular adhesion molecule-1 (sICAM-1): an overview. Eur Cytokine Netw. 2004;15:91–8. [PubMed] [Google Scholar]
- 29.Rieckmann P, Michel U, Albrecht M, et al. Soluble forms of intercellular adhesion molecule-1 (ICAM-1) block lymphocyte attachment to cerebral endothelial cells. J Neuroimmunol. 1995;60:9–15. doi: 10.1016/0165-5728(95)00047-6. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi H, Boelte KC, Lin PC. Endothelial cell adhesion molecules and cancer progression. Curr Med Chem. 2007;14:377–86. doi: 10.2174/092986707779941032. [DOI] [PubMed] [Google Scholar]
- 31.Rahbari NN, Bork U, Hinz U, et al. AB0 blood group and prognosis in patients with pancreatic cancer. BMC Cancer. 2012;12:319. doi: 10.1186/1471-2407-12-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaffenberger SD, Morgan TM, Stratton KL, et al. ABO blood group is a predictor of survival in patients undergoing surgery for renal cell carcinoma. BJU Int. 2012;110:E641–6. doi: 10.1111/j.1464-410X.2012.11366.x. [DOI] [PubMed] [Google Scholar]
- 33.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41:986–90. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizzato C, Campa D, Giese N, et al. Pancreatic cancer susceptibility loci and their role in survival. PLoS ONE. 2011;6:e27921. doi: 10.1371/journal.pone.0027921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101:424–31. doi: 10.1093/jnci/djp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolpin BM, Kraft P, Gross M, et al. Pancreatic cancer risk and ABO blood group alleles: results from the pancreatic cancer cohort consortium. Cancer Res. 2010;70:1015–23. doi: 10.1158/0008-5472.CAN-09-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakao M, Matsuo K, Hosono S, et al. ABO blood group alleles and the risk of pancreatic cancer in a Japanese population. Cancer Sci. 2011;102:1076–80. doi: 10.1111/j.1349-7006.2011.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dandona M, Gao F, Linehan DC, Wang-Gillam A. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2010;102:135–7. doi: 10.1093/jnci/djp447. [DOI] [PubMed] [Google Scholar]
- 39.Ben Q, Wang K, Yuan Y, Li Z. Pancreatic cancer incidence and outcome in relation to ABO blood groups among Han Chinese patients: a case-control study. Int J Cancer. 2011;128:1179–86. doi: 10.1002/ijc.25426. [DOI] [PubMed] [Google Scholar]
- 40.Wang DS, Wang ZQ, Zhang L, et al. Are risk factors associated with outcomes in pancreatic cancer? PLoS One. 2012;7:e41984. doi: 10.1371/journal.pone.0041984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breimer ME, Mölne J, Nordén G, et al. Blood group A and B antigen expression in human kidneys correlated to A1/A2/B, Lewis, and secretor status. Transplantation. 2006;82:479–85. doi: 10.1097/01.tp.0000231697.15817.51. [DOI] [PubMed] [Google Scholar]
- 42.de Martino M, Waldert M, Haitel A, et al. Evaluation of ABO blood group as a prognostic marker in renal cell carcinoma (RCC) BJU Int. 2014;113:E62–6. doi: 10.1111/bju.12436. [DOI] [PubMed] [Google Scholar]
- 43.Lee C, You D, Sohn M, et al. Prognostic value of ABO blood group in patients with renal cell carcinoma: single-institution results from a large cohort. J Cancer Res Clin Oncol. 2015;141:1441–7. doi: 10.1007/s00432-015-1908-3. [DOI] [PubMed] [Google Scholar]
- 44.Orihuela E, Shahon RS. Influence of blood group type on the natural history of superficial bladder cancer. J Urol. 1987;138:758–9. doi: 10.1016/s0022-5347(17)43363-5. [DOI] [PubMed] [Google Scholar]
- 45.Klatte T, Xylinas E, Rieken M, et al. Impact of ABO blood type on outcomes in patients with primary nonmuscle invasive bladder cancer. J Urol. 2014;191:1238–43. doi: 10.1016/j.juro.2013.11.106. [DOI] [PubMed] [Google Scholar]
- 46.Raitanen MP, Tammela TL. Relationship between blood groups and tumour grade, number, size, stage, recurrence and survival in patients with transitional cell carcinoma of the bladder. Scand J Urol Nephrol. 1993;27:343–7. doi: 10.3109/00365599309180445. [DOI] [PubMed] [Google Scholar]
- 47.Yamada T, Fukui I, Yokokawa M, Oshima H. A study of prognosis and clinicopathology of bladder cancer to blood group type of host patients in Japan. Scand J Urol Nephrol. 1993;27:199–203. doi: 10.3109/00365599309181249. [DOI] [PubMed] [Google Scholar]
- 48.Llopis B, Ruiz JL, Server G, et al. ABO blood groups and bladder carcinoma. Eur Urol. 1990;17:289–92. doi: 10.1159/000464061. [DOI] [PubMed] [Google Scholar]
- 49.Klatte T, Xylinas E, Rieken M, et al. Effect of ABO blood type on mortality in patients with urothelial carcinoma of the bladder treated with radical cystectomy. Urol Oncol. 2014;32:625–30. doi: 10.1016/j.urolonc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Sheng L, Sun X, Zhang L, Su D. ABO blood group and nasopharyngeal carcinoma risk in a population of Southeast China. Int J Cancer. 2013;133:893–7. doi: 10.1002/ijc.28087. [DOI] [PubMed] [Google Scholar]
- 51.Ouyang PY, Su Z, Mao YP, et al. Prognostic value of ABO blood group in southern Chinese patients with established nasopharyngeal carcinoma. Br J Cancer. 2013;109:2462–6. doi: 10.1038/bjc.2013.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang YX, Kang SY, Chen G, et al. ABO blood group, Epstein-Barr virus infection and prognosis of patients with non-metastatic nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2014;15:7459–65. doi: 10.7314/apjcp.2014.15.17.7459. [DOI] [PubMed] [Google Scholar]
- 53.Adam SI, Wilson KM, Overholser SM, et al. Are laryngeal squamous cell carcinoma incidence and patient mortality a function of ABO blood grouping? A retrospective study. J Laryngol Otol. 2012;126:180–4. doi: 10.1017/S0022215111002507. [DOI] [PubMed] [Google Scholar]
- 54.Utkan G, Ürün Y, Cangir AK, et al. Clinicopathological features of patients with malignant mesothelioma in a multicenter, case-control study: no role for ABO-Rh blood groups. Asian Pac J Cancer Prev. 2013;14:249–53. [PubMed] [Google Scholar]
- 55.Unal D, Eroglu C, Kurtul N, et al. ABO blood groups are not associated with treatment response and prognosis in patients with local advanced non- small cell lung cancer. Asian Pac J Cancer Prev. 2013;14:3945–8. doi: 10.7314/apjcp.2013.14.6.3945. [DOI] [PubMed] [Google Scholar]
- 56.Lee JS, Ro JY, Sahin AA, et al. Expression of blood-group antigen A--a favourable prognostic factor in non-small-cell lung cancer. N Engl J Med. 1991;324:1084–90. doi: 10.1056/NEJM199104183241603. [DOI] [PubMed] [Google Scholar]
- 57.Graziano SL, Tatum AH, Gonchoroff NJ, et al. Blood group antigen A and flow cytometric analysis in resected early-stage non-small cell lung cancer. Clin Cancer Res. 1997;3:87–93. [PubMed] [Google Scholar]
- 58.Ulger AF, Keklik T, Kumbasar OO, et al. Prognostic significance of blood group antigen expression of tumor tissue in lung cancer patients. Tumori. 2002;88:395–9. doi: 10.1177/030089160208800509. [DOI] [PubMed] [Google Scholar]
- 59.León-Atance P, Moreno-Mata N, González-Aragoneses F, et al. Prognostic influence of loss of blood group A antigen expression in pathologic stage I non-small-cell lung cancer. Arch Bronconeumol. 2012;48:49–54. doi: 10.1016/j.arbres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Moldvay J, Scheid P, Wild P, et al. Predictive survival markers in patients with surgically resected non-small cell lung carcinoma. Clin Cancer Res. 2000;6:1125–34. [PubMed] [Google Scholar]
- 61.Nozoe T, Ezaki T, Baba H, et al. Correlation of ABO blood group with clinicopathologic characteristics of patients with esophageal squamous cell carcinoma. Dis Esophagus. 2004;17:146–9. doi: 10.1111/j.1442-2050.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- 62.Sun P, Chen C, Zhang F, et al. The ABO blood group predicts survival in esophageal squamous cell carcinoma in patients who ever smoked: a retrospective study from China. Tumour Biol. 2014;35:7201–8. doi: 10.1007/s13277-014-1960-7. [DOI] [PubMed] [Google Scholar]
- 63.Aird I, Bentall HH, Fraser RJA. A relationship between cancer of the stomach and the ABO blood groups. BMJ. 1953;1:799–801. doi: 10.1136/bmj.1.4814.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edgren G, Hjalgrim H, Rostgaard K, et al. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: a cohort study. Am J Epidemiol. 2010;172:1280–5. doi: 10.1093/aje/kwq299. [DOI] [PubMed] [Google Scholar]
- 65.Etemadi A, Kamangar F, Islami F, et al. Mortality and cancer in relation to ABO blood group phenotypes in the Golestan Cohort Study. BMC Med. 2015;13:8. doi: 10.1186/s12916-014-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu MZ, Zhang DS, Ruan DY, et al. A relationship between ABO blood groups and clinicopathologic characteristics of patients with gastric adenocarcinoma in China. Med Oncol. 2011;28( Suppl 1):S268–73. doi: 10.1007/s12032-010-9735-5. [DOI] [PubMed] [Google Scholar]
- 67.Rizzato C, Kato I, Plummer M, et al. Risk of advanced precancerous lesions in Helicobacter pylori infected subjects is influenced by ABO blood group and cagA status. Int J Cancer. 2013;133:315–22. doi: 10.1002/ijc.28019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cao X, Wen ZS, Sun YJ, et al. Prognostic value of ABO blood group in patients with surgically resected colon cancer. Br J Cancer. 2014;111:174–80. doi: 10.1038/bjc.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miao S-Y, Zhou W, Chen L, et al. Influence of ABO blood group and rhesus factor on breast cancer risk: a meta-analysis of 9665 breast cancer patients and 244768 controls. Asia-Pac J Clin Oncol. 2014;10:101–8. doi: 10.1111/ajco.12083. [DOI] [PubMed] [Google Scholar]
- 70.Holdsworth PJ, Thorogood J, Benson EA, Clayden AD. Blood group as a prognostic indicator in breast cancer. Br Med J. 1985;290:671–3. doi: 10.1136/bmj.290.6469.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costantini M, Fassio T, Canobbio L, et al. Role of blood groups as prognostic factors in primary breast cancer. Oncology. 1990;47:308–12. doi: 10.1159/000226839. [DOI] [PubMed] [Google Scholar]
- 72.Stamatakos M, Kontzoglou K, Safioleas P, et al. Breast cancer incidence in Greek women in relation to ABO blood groups and Rh factor. Int Semin Surg Oncol. 2009;6:14. doi: 10.1186/1477-7800-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gates MA, Xu M, Chen WY, et al. ABO blood group and breast cancer incidence and survival. Int J Cancer. 2011;130:2129–37. doi: 10.1002/ijc.26220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klimant E, Glurich I, Mukesh B, Onitilo AA. Blood type, hormone receptor status, HER2/neu status, and survival in breast cancer: a retrospective study exploring relationship in a phenotypically well-defined cohort. Clin Med Res. 2011;3/4:111–8. doi: 10.3121/cmr.2011.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu J, Gao F, Klimberg VS, Margenthaler JA. ABO blood type/Rh factor and the incidence and outcomes for patients with triple-negative breast cancer. Ann Surg Oncol. 2012;19:3159–64. doi: 10.1245/s10434-012-2533-x. [DOI] [PubMed] [Google Scholar]
- 76.Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim Biophys Acta. 1999;1473:247–66. doi: 10.1016/s0304-4165(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 77.Dabelsteen E. Cell surface carbohydrates as prognostic markers in human carcinomas. J Pathol. 1996;179:358–69. doi: 10.1002/(SICI)1096-9896(199608)179:4<358::AID-PATH564>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]