Abstract
Given the expression of ABO blood group antigens on the surface of a wide range of human cells and tissues, the putative interplay of the ABO system in human biology outside the area of transfusion and transplantation medicine constitutes an intriguing byway of research. Thanks to evidence accumulated over more than 50 years, the involvement of the ABO system in the pathogenesis of several human diseases, including cardiovascular, infectious and neoplastic disorders, is now acknowledged. However, there is controversial information on the potential association between ABO blood type and adverse pregnancy outcomes, including pre-eclampsia and related disorders (eclampsia, HELLP syndrome and intrauterine growth restriction), venous thromboembolism, post-partum haemorrhage and gestational diabetes. To elucidate the role of ABO antigens in pregnancy-related complications, we performed a systematic review of the literature published in the past 50 years. A meta-analytical approach was also applied to the existing literature on the association between ABO status and pre-eclampsia. The results of this systematic review are presented and critically discussed, along with the possible pathogenic implications.
Keywords: ABO blood group, pre-eclampsia, eclampsia, HELLP syndrome, venous thromboembolism
Introduction
Along with their expression on red blood cells, ABO blood group antigens (namely, A, B, AB and O) are highly expressed by a large number of human cells and tissues including epithelia, platelets, vascular endothelia and neurons1,2. For this reason, a number of investigators have addressed whether this biological characteristic of the ABO system has clinical significance beyond that in transfusion and transplantation medicine. In fact, there is now a large body of evidence supporting the notion that ABO antigens are actively involved in the pathogenesis of various systemic diseases, including neoplastic, infectious, neurological and cardiovascular disorders3–8. While the non-O blood type-related increased circulating levels of von Willebrand factor (VWF), factor VIII (FVIII), cholesterol and several inflammatory cytokines (e.g., tumour necrosis factor-alpha, soluble intercellular adhesion molecule 1, E-selectin, P-selectin and interleukin-6) have been suggested as the most likely mechanisms for explaining the association between ABO blood group and arterial or venous thrombosis9,10, the pathogenic mechanisms underlying the other ABO blood type-associated disorders are still largely unexplored. In addition, a number of studies have investigated the association between maternal ABO blood type and pregnancy complications, including pre-eclampsia and related disorders (e.g., eclampsia, the haemolysis, elevated liver enzyme, low platelets [HELLP] syndrome, intrauterine foetal growth restriction [IUGR]), venous thromboembolism (VTE), post-partum haemorrhage and gestational diabetes, although contradictory results have emerged. In order to elucidate the role of ABO blood types in these pregnancy-related disorders, we performed a systematic review of the existing literature.
Search methods
We systematically reviewed the scientific literature for published studies evaluating the interplay between maternal ABO blood types and pregnancy outcomes. We searched the MEDLINE and EMBASE electronic databases for the last 50 years (January 1965 – August 2015) with English language restriction. Only full-text articles were considered for this systematic review. The Medical Subject Heading and key words used were the following: “ABO blood group”, “pregnancy”, “preeclampsia”, “eclampsia”, “venous thromboembolism”, “deep-vein thrombosis”, “pulmonary embolism”, “gestational diabetes”, “post-partum hemorrhage”, “pregnancy-induced hypertension” and “hemolysis, elevated liver enzymes, low platelets syndrome”, “HELLP syndrome” “intrauterine fetal growth restriction” and “IUGR”. We also hand-searched the reference lists of the most relevant items to identify any further eligible studies not captured in the initial literature search. All prospective and retrospective studies were included in the final analysis. Due to the paucity of published data, a meta-analysis of retrieved data (including more than three studies evaluable) was only possible for the association between ABO blood groups and pre-eclampsia.
ABO blood group and pre-eclampsia
Pre-eclampsia, defined as hypertension (≥90 mmHg) accompanied by proteinuria (≥300 mg/24 hours) after 20 weeks of gestational age11, is one of the leading causes of maternal and foetal morbidity and mortality, since it can progress to eclampsia (characterised by the occurrence of convulsions), HELLP syndrome, and may be associated with fibrin deposition in the placental microcirculation and consequent IUGR (defined as neonatal birth weight below the 10th percentile)12,13. Although various studies have investigated a possible relationship between maternal ABO antigens and pre-eclampsia14–24, there is no consensus on whether a true association does exist. In a study conducted by May, British women with A blood type had a 2.7-fold higher risk of pre-eclampsia compared with O type individuals16. Similarly, Spinillo and Colleagues19 and Hiltunen and Colleagues22 found that the risk of pre-eclampsia was increased by 2.1- to 3.1-fold in Italian and Finnish gravidas with AB blood type compared to that in women with O blood type. In a study conducted in pregnant Thai women, the risk of pre-eclampsia was 1.7-fold higher in women with A and AB blood types compared to the risk in O type individuals24. However, an impact of ABO blood types on pregnancy complications was not observed by Scott and Colleagues in a study conducted in the USA17 and, more recently, by Witsenburg and Colleagues20 and Clark and Colleagues21, in two studies of Dutch and Scottish women, respectively.
Systematic review and meta-analysis
A systematic review of the literature initially identified 117 potentially relevant citations in the last 50 years. Following the exclusion process (87 references were excluded as not relevant, 8 after language restriction and 13 because they did include relevant information, such as definitions of diseases or ABO blood group distribution in both patients and controls), a total of nine studies (7 case-control studies, 2 cohort studies) were identified and considered eligible for this systematic review16–24. A meta-analysis was then performed including these nine eligible studies. Table I summarises the main characteristics of the studies.
Table I.
Association between ABO blood type and pre-eclampsia: a systematic literature review.
| First author, yearref. | Study design | Cases N (%) |
Controls N (%) |
Main results |
|---|---|---|---|---|
| May, 197316 | Case-control | 47 10 O (21.3), 37 non-O (78.7), A 31 |
400 182 O (45.5), 218 non-O (54.5) A 172 |
A blood group was associated with a 2.7-fold increased OR of pre-eclampsia compared with O blood type |
| Scott, 197617 | Case-control | 46 22 O (47.8), 24 non-O (52.2) 15 A, 7 B, 2 AB |
4,494 2,139 O (47.6), 2,355 non-O (52.4) 1,705 A, 511 A, 139 AB |
No association between ABO blood group and the risk of pre-eclampsia |
| Amin, 198918 | Case-control | 368 106 O (28.8), 262 non-O (71.2) 112 A, 112 B, 38 AB |
342 123 O (36.0), 219 non-O (64.0) 98 A, 93 B, 28 AB |
Significant reduction (p<0.05) in group O in cases compared with healthy controls |
| Spinillo, 199519 | Case-control | 204 74 O (36.3), 130 non-O (63.7) 84 A, 30 B, 16 AB |
744 288 O (38.7), 456 non-O (61.3) 356 A, 80 B, 20 AB |
Maternal AB blood group (8% in cases vs 3% in controls) was associated with an increased risk (adjusted OR 3.07, 95% CI: 1.48–6.36) of severe pre-eclampsia |
| Witsenburg, 200520 | Case-control | 36* 11 O (30.6), 25 non-O (69.4) |
272 111 O (40.8), 161 non-O (59.2) |
No effect of ABO blood group was observed on the risk of pregnancy complications (pre-eclampsia, HELLP syndrome and PIH) |
| Clark, 200821 | Prospective cohort | 66 32 O (48.5), 32 non-O (51.5) |
3,919 2,055 O (52.4), 1,864 non-O (47.6) |
No effect of ABO blood type on the risk of pre-eclampsia |
| Hiltunen, 200822 | Case-control | 248 72 O (29.0), 176 non-O (71.0) 104 A, 40 B, 32 AB |
679 217 O (32.0), 462 non-O (68.0) 294 A, 124 B, 44 AB |
AB blood group was associated with an increased risk of pre-eclampsia (OR 2.1, 95% CI: 1.3–3.5) compared with non-AB blood group |
| Lee, 201223 | Cohort | 37,814 13,881 O (36.7), 23,933 non-O (63.3) 17,408 A, 4,430 B, 2,095 AB |
641,926 243,041 O (37.9), 398,885 non-O (62.1) 291,453 A, 74,147 B, 33,285 AB |
Women with AB blood group had the highest risk of developing pre-eclampsia (OR 1.10, 95% CI: 1.04–1.16) and severe pre-eclampsia (OR 1.18, 95% CI: 1.07–1.30) |
| Phaloprakarn, 201324 | Case-control | 350 105 O (30.0), 245 non-O (70.0) 100 A, 113 B, 32 AB |
5,320 1,956 O (36.8), 3,364 non-O (63.2) 1,153 A, 1,832 B, 379 AB |
Blood types A (28.6% in cases and 21.7% in controls) and AB (9.1% in cases and 7.1% in controls) were associated with an increased risk of pre-eclampsia compared with blood type O (RR for A blood type: 1.7, 95% CI: 1.3–2.3, p=0.001; RR for AB blood type: 1.6, 95% CI: 1.1–2.6, p=0.01) |
Combined group with pre-eclampsia, HELLP syndrome and PIH.
RR: relative risk; OR: odds ratio; CI: confidence interval; HELLP: haemolysis, elevated liver enzymes, low platelets; PIH: pregnancy-induced hypertension.
Methods
The main outcome was the incidence of pre-eclampsia or eclampsia. The case-control studies were evaluated by study odds ratio (OR) meta-analytical pooling, whereas the cohort studies were evaluated by pooling the study relative risk. The Mantel-Haenszel method of weighting was used. The heterogeneity was quantified by the I2 statistic25. When heterogeneity was large (I2>50%), the DerSimonian-Laird random effects procedure was followed26. The influence of each individual study on the overall meta-analysis summary estimate was assessed by sensitivity analysis. This was based on single study omission, extended to all studies, in turn; this procedure was limited to case-control studies. The difference of prevalence of the group O in cases and in controls was also evaluated by meta-analytical pooling.
Results and discussion
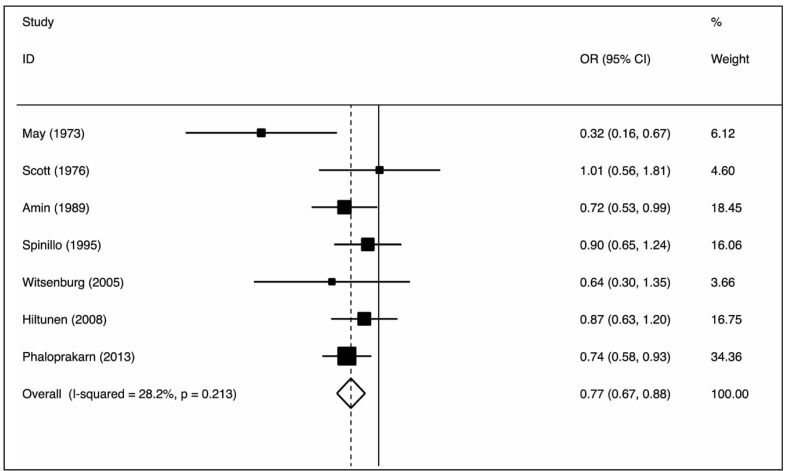
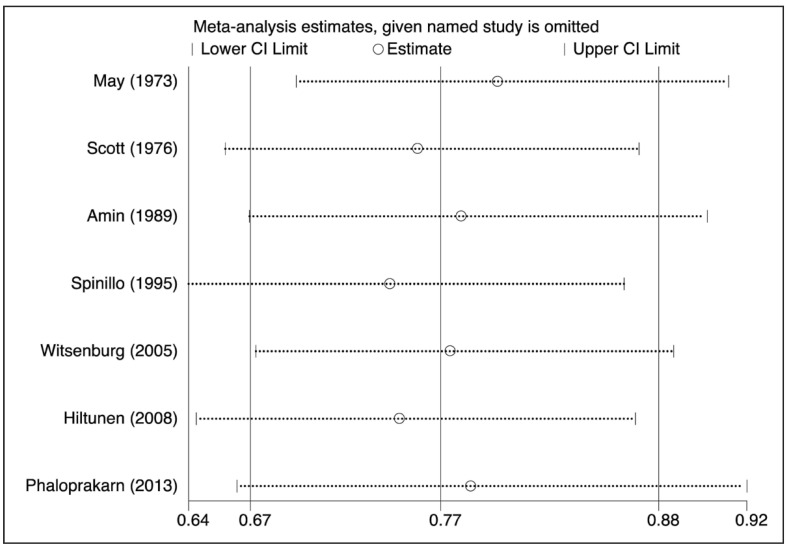
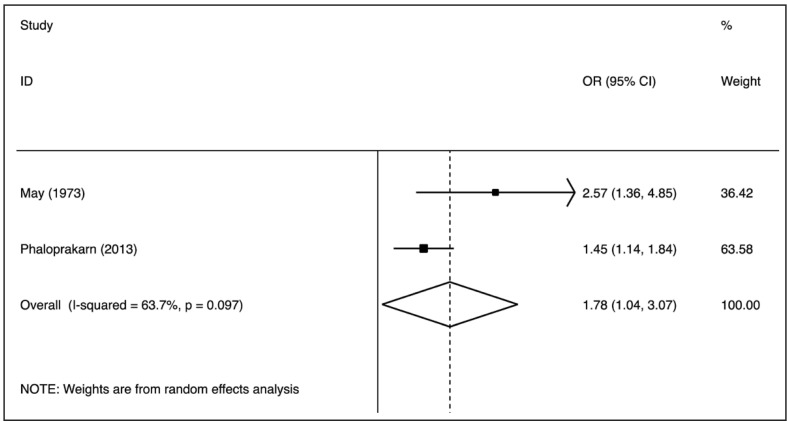
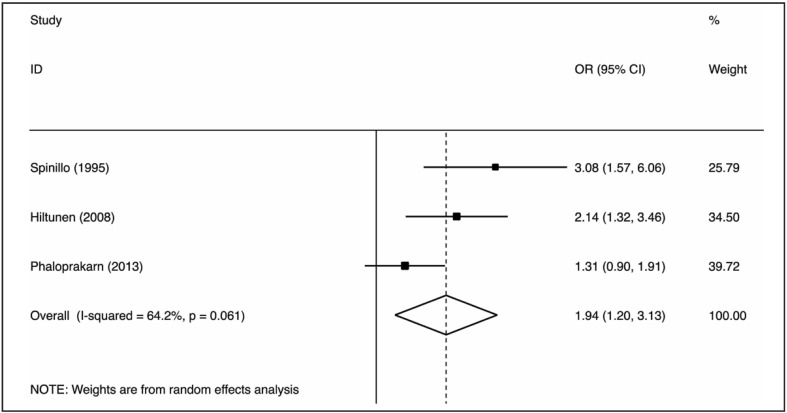
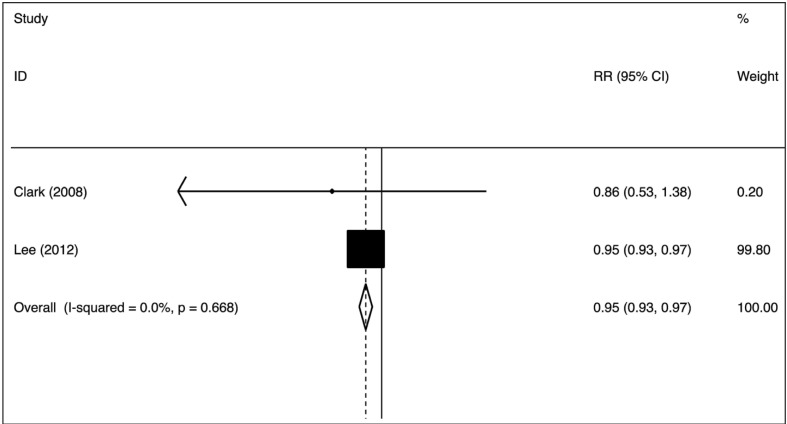
The nine case-control studies were analysed to evaluate the association between group O exposure and outcome by OR pooling (Figure 1). The effect size was 0.77 (95% confidence interval [CI]: 0.67–0.88), which is significantly lower than one, implying a lower prevalence of this blood group in patients with pre-eclampsia (p=0.000) in comparison to non-O group women. The heterogeneity was low (I2=28.2%). These findings suggest that group O was associated with lower odds of the outcome. The pooled difference of prevalence of group O in cases and in controls was −6.6% (95% CI: −10.9 to −2.3%). No single study was essential for the overall significance after sensitivity analysis (Figure 2). Of these case-control studies, two were evaluable for a differential prevalence of group A, and three for a differential prevalence of group AB (Figures 3 and 4). For group A the effect size was 1.78 (95% CI: 1.04–3.07), significantly higher than one, thus implying a higher prevalence of this blood group among the patients with pre-eclampsia (p=0.037). The heterogeneity was substantial (I2=63.7%). For group AB the effect size was 1.94 (95% CI: 1.20–3.13), also higher than one (p=0.007). The heterogeneity was also substantial (I2=64.2%). These data indicate that having blood group A or AB was associated with higher odds of the adverse outcome. The two cohort studies were submitted to evaluation of probability of pre-eclampsia due to group O by RR pooling (Figure 5). The effect size was 0.95 (95% CI: 0.93–0.97). Again, the resulting estimation was significantly lower than one, implying a lower probability of pre-eclampsia in this group than in non-O group women (p=0.000). The heterogeneity was low (I2=0%).
Figure 1.
Association of the group O with pre-eclampsia (OR meta-analytical pooling, fixed effect).
OR: odds ratio; CI: confidence interval.
Figure 2.
Sensitivity analysis of the association of group O with pre-eclampsia (OR meta-analytical pooling, fixed effect).
No study, if omitted, changed the overall effect to non-significance. Indeed, the 95% CI never overlaps the null value of one.
OR: odds ratio; CI: confidence interval.
Figure 3.
Association of group A with pre-eclampsia (OR meta-analytical pooling, random effects).
OR: odds ratio; CI: confidence interval.
Figure 4.
Association of group AB with pre-eclampsia (OR meta-analytical pooling, random effects).
OR: odds ratio; CI: confidence interval.
Figure 5.
Putative causal effect of group O vs non-O on pre-eclampsia (relative risk meta-analytical pooling, fixed effect).
RR: risk ratio; CI: confidence interval.
Taken together, the results of the pooled analysis of the data indicate that O blood type may exert some protective effects against the development of pre-eclampsia compared with non-O blood type. However, a note of caution is necessary. The prevalence of group O was highly variable, ranging from 21 to 48% in the cases and from 32 to 48% in the controls, which may suggest the presence of heterogeneity in the ethnic background of the study participants. The very low number of studies supporting the investigations on group A and AB is another important aspect. This holds true also for the causal effect of group O in the cohort studies, which were dominated by the overwhelming weight (99.8%) of the study by Lee and Colleagues23. Taken together, the results of our systematic review and meta-analysis are seemingly divergent from those published by Clark and Wu in 200814, in which a consistent positive influence of ABO blood group on pre-eclampsia could not be found in the 17 eligible studies (O vs non-O blood group: pooled OR of 1.01, 95% CI: 0.91–1.12). A possible explanation for this difference probably lies in the inclusion criteria used in our meta-analysis (inclusion of studies published in the last 50 years), thus including more recent studies with a better design and a more accurate definition of the patient populations.
Although the definitive pathogenic mechanism is still unknown, the existence of a pro-thrombotic state may be regarded as the most plausible factor linking pre-eclampsia with ABO blood types. Indeed, while non-O blood groups are well recognised risk factors for thrombosis due to the higher levels of VWF and FVIII compared to those in subjects with O blood type, a number of studies identified a causative role of hypercoagulability in the risk and severity of pre-eclampsia27. Indeed, several investigators have consistently reported that VWF and/or FVIII levels are increased in pre-eclamptic women compared to those in women with healthy pregnancies28–31. This finding is in keeping with the epidemiological observation of an association between the risk of developing pre-eclampsia and AB blood type19,22–24, which is characterised by the highest VWF and FVIII levels among the different antigens of the ABO system32. Notably, reduced levels of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13), an enzyme responsible for cleavage and clearance of ultralarge VWF (ULVWF, the most haemostatically active form of VWF) from the circulation, have also been reported in patients with pre-eclampsia or HELLP syndrome in some studies33–35, but not in others36,37. The resulting increased levels of ULVWF may trigger platelet aggregation, thus causing diffuse occlusion of placental arterioles, a key event in the pathogenesis of pre-eclampsia and related disorders38. The endothelial injury and dysfunction then activates endothelial cells, which in turn release active VWF that can perpetuate and even worsen placental insufficiency through a vicious circle34,39. However, besides the imbalance between ADAMTS13 activity and VWF levels, other mechanisms may come into play. The metabolism of placental protein 13 (PP13), a placenta-specific galectin exerting important immune biological functions at the maternal-foetal interface and now considered an early biomarker of severe pre-eclampsia, is regulated by ABO blood groups. PP13 binds to the beta-galactosides (N-acetyl-galactosamine, galactose and fucose) located at terminal positions on ABO antigens and this sequestration process may reduce PP13 levels in the first trimester, thus predisposing to pregnancy complications, including pre-eclampsia. The effect seems to be more pronounced in pregnant women with the AB blood group, because the concomitant presence of A and B antigenic determinants results in more efficient binding to PP13, thus lowering PP13 serum levels40. Finally, another putative mechanism could involve up-regulation, by polymorphisms in the ABO locus, of circulating levels of the aforementioned inflammatory cytokines, which are also strongly associated with pre-eclampsia41–43. All these mechanisms are biologically plausible and, as one does not exclude the other, it is possible that they be concomitantly present44.
ABO blood group and disorders related to pre-eclampsia
Fewer studies have specifically addressed the relationship between ABO blood types, HELLP syndrome and IUGR, two conditions sharing the same pathogenesis as pre-eclampsia (i.e., uteroplacental insufficiency). As regards HELLP syndrome, a retrospective study conducted on 547 Turkish women with pre-eclampsia found that the incidence of HELLP syndrome was two times higher in O Rh-negative patients than in the overall study population (48% vs 24%, OR 3.1, 95% CI: 1.28–7.43)45. By contrast, no association between any ABO blood type and HELLP syndrome was observed in the study by Witsenburg and Colleagues20, although a separate analysis with pre-eclampsia and pregnancy-induced hypertension was not performed. As regards IUGR, a prospective case-control study conducted by Clark and Greer showed no association between this condition and ABO genotype or phenotype, although a significant relationship was observed between maternal secretor/Lewis status and occurrence of foetal growth restriction (secretor vs non-secretor: OR 1.70, 95% CI: 1.08–2.69; Lewis(b) vs Lewis(a): OR 1.80, 95% CI: 1.15–2.83)46. Non-significant effects on foetal growth were observed in the Glasgow Outcome APC resistance and Lipid (GOAL) study when non-O group subjects were compared with O group subjects21. Similar conclusions were drawn in the study by Witsenburg and Colleagues20, despite the fact that FVIII levels were higher in the IUGR group and thus associated with a nearly 3-fold higher risk of IUGR. Interestingly, a recent study found a negative combined influence of smoking and A blood type on birth weight47, although no significant relationship between ABO blood type and low birth weight was observed in another investigation24.
ABO blood group, venous thromboembolism and haemorrhagic complications
It is now well acknowledged that non-O blood type individuals have an approximately 2-fold higher risk of developing VTE than have O type subjects48–51. In contrast, individuals with O blood type have VWF plasma levels 25–35% lower than those in non-O individuals, thus exhibiting a modestly increased risk of haemorrhage52. Only a few studies have analysed this issue in the context of pregnancy and the puerperium. The first observation dates back to 1969 and was made by Jick and Colleagues53, who conducted a case-control study in women from the UK, Sweden and USA, and reported a 2.1 relative risk of VTE (95% CI: 1.5–3.1) among pregnant women with non-O blood group compared with those with blood group O. A similar study performed in 1970 by Talbot and Colleagues54 found a relative risk of 1.7 (95% CI: 1.1–2.6) in a subgroup of pregnant British women. In a subsequent study conducted in the same geographical area, compared with the incidence in subjects with O blood type, blood group A was found to increase the incidence of both antenatal VTE (adjusted OR: 1.9, 95% CI: 1.2–3.0) and post-natal VTE (adjusted OR: 1.6, 95% CI: 1.2–2.2)55. Increased risk estimates of VTE for women with blood groups A and AB were found in both pregnancy (adjusted OR: 3.9, 95% CI: 1.5–9.7 and 2.2, 95% CI: 0.4–12.5, respectively) and in the puerperium (adjusted OR: 2.4, 95% CI: 1.0–4.9 and 2.7, 95% CI: 0.8–9.3, respectively) in a nested case-control study conducted in Denmark by Larsen and Colleagues56. As far as regards the ABO-related risk of bleeding, a case-control study carried out by Chauleur and Colleagues in France found that O blood group was independently associated with a significant risk of severe post-partum haemorrhage (adjusted OR: 1.84, 95% CI: 1.32–2.57)57. Despite these results, no influence of ABO blood groups on the risk of VTE or post-partum haemorrhage was observed in the aforementioned prospective GOAL study21.
ABO blood group and gestational diabetes
Due to the influence of ABO antigens on circulating levels of E-selectin, P-selectin, tumour necrosis factor-α, soluble intercellular adhesion molecule-1 and interleukin-641,42 combined with the observed association between these biomarkers and insulin resistance and the development of type 2 diabetes mellitus58, several epidemiological studies have explored the relationship between ABO blood group and diabetes, but their findings were mostly inconsistent59–66. Nevertheless, only a few investigators have analysed this association in a subset of pregnant women. A recent study on 792 healthy Iranian women reported that those with AB blood type had higher fasting glucose levels in the second trimester than those with A blood type67. Another study identified a higher prevalence of O blood type in pregnant Turkish women with gestational diabetes mellitus than in non-diabetic pregnant women (52.2% vs 33.3%)68. By contrast, no significant association was found between ABO blood group and risk of developing gestational diabetes mellitus in a retrospective cohort study on 5320 pregnant women who attended a tertiary care centre in Thailand24. Finally, a more recent prospective study conducted in China reported that AB blood group was a protective factor against gestational diabetes mellitus (AB vs non-AB blood types: OR: 1.44, 95% CI: 1.13–1.83)69.
Conclusions
Several studies have convincingly proven that the ABO blood type not only plays a role in transfusion and transplantation medicine, but is implicated in the pathogenesis of a kaleidoscope of human disorders. The results of this systematic review support for the first time the existence of a consistent influence of ABO status on the risk of developing pre-eclampsia. Specifically, women with a non-O blood type were found to have a moderately increased risk of this condition compared with the risk in those with O blood type. The systematic analysis of the literature data also suggests that non-O pregnant women have an increased incidence of VTE compared with that in pregnant women with O blood type. Less evidence is available for the association with other adverse pregnancy outcomes, reflecting the paucity of published clinical data. Thus, further prospective studies including large populations of patients are warranted to assess the role of ABO blood group in identifying women at risk of developing pre-eclampsia or other pregnancy-related complications. Experimental investigations are also needed to unravel the underlying pathogenic mechanisms of these interactions.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Storry JR, Olsson ML. The ABO blood group system revisited: a review and update. Immunohematology. 2009;25:48–9. [PubMed] [Google Scholar]
- 2.Liumbruno GM, Franchini M. Beyond immunohaematology: the role of the ABO blood group in human diseases. Blood Transfus. 2013;11:491–9. doi: 10.2450/2013.0152-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franchini M, Mannucci PM. ABO blood group and thrombotic vascular disease. Thromb Haemost. 2014;112:1103–9. doi: 10.1160/TH14-05-0457. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto F, Cid E, Yamamoto M, Blancher A. ABO research in the modern era of genomics. Transfus Med Rev. 2012;26:103–18. doi: 10.1016/j.tmrv.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Anstee DJ. The relationship between blood groups and disease. Blood. 2010;115:4635–43. doi: 10.1182/blood-2010-01-261859. [DOI] [PubMed] [Google Scholar]
- 6.Franchini M, Favaloro EJ, Targher G, Lippi G. ABO blood group, hypercoagulability, and cardiovascular and cancer risk. Crit Rev Clin Lab Sci. 2012;49:137–49. doi: 10.3109/10408363.2012.708647. [DOI] [PubMed] [Google Scholar]
- 7.Franchini M, Lippi G. The intriguing relationship between the ABO blood group, cardiovascular disease, and cancer. BMC Med. 2015;13:7. doi: 10.1186/s12916-014-0250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dentali F, Sironi AP, Ageno W, et al. ABO blood group and vascular disease: an update. Semin Thromb Hemost. 2014;40:49–59. doi: 10.1055/s-0033-1363460. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins PV, O’Donnell JS. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion. 2006;46:1836–44. doi: 10.1111/j.1537-2995.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Chen C, Ke X, et al. Analysis of circulating cholesterol levels as a mediator of an association between ABO blood group and coronary heart disease. Circ Cardiovasc Genet. 2014;7:43–8. doi: 10.1161/CIRCGENETICS.113.000299. [DOI] [PubMed] [Google Scholar]
- 11.Pregnancy WGoHBPi. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–22. [PubMed] [Google Scholar]
- 12.Davey DA, MacGillivray I. The classification and definitions of the hypertensive disorders of pregnancy. Am J Obstet Gynaecol. 1988;158:892–8. doi: 10.1016/0002-9378(88)90090-7. [DOI] [PubMed] [Google Scholar]
- 13.Bakketeig LS. Current growth standards, definitions, diagnosis and classification of fetal growth retardation. Eur J Clin Nutr. 1998;52( Suppl 1):S1–4. [PubMed] [Google Scholar]
- 14.Clark P, Wu O. ABO(H) blood groups and pre-eclampsia. A systematic review and meta-analysis. Thromb Haemost. 2008;100:469–74. [PubMed] [Google Scholar]
- 15.Alpoim PN, de Barros Pinheiro M, Junqueira DRG, et al. Preeclampsia and ABO blood groups: a systematic review and meta-analysis. Mol Biol Rep. 2013;40:2253–61. doi: 10.1007/s11033-012-2288-2. [DOI] [PubMed] [Google Scholar]
- 16.May D. Maternal blood group A and pre-eclampsia. Br Med J. 1973;4:738. doi: 10.1136/bmj.4.5894.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott JR, Beer AE, Stastny P. Immunogenetic factors in preeclampsia and eclampsia. Erythrocyte, histocompatibility, and Y-dependent antigens. JAMA. 1976;235:402–4. [PubMed] [Google Scholar]
- 18.Amin NS, Tahir SA, Abadi NA, Kubba K. Association of blood groups with preeclamptic toxemia. Med Sci Res. 1989;17:861–2. [Google Scholar]
- 19.Spinillo A, Capuzzo E, Baltaro F, et al. Case-control study of maternal blood group and severe preeclampsia. J Hum Hypertens. 1995;9:623–5. [PubMed] [Google Scholar]
- 20.Witsenburg C, Rosendaal F, Middledorp J, et al. Factor VIII levels and the risk of pre-eclampsia, HELLP syndrome, pregnancy related hypertension and severe intrauterine growth retardation. Thromb Res. 2005;115:387–92. doi: 10.1016/j.thromres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Clark P, Walker I, Govan L, et al. The GOAL study: a prospective examination of the impact of factor V Leiden and ABO(H) blood groups on haemorrhagic and thrombotic pregnancy outcomes. Br J Haematol. 2008;140:236–40. doi: 10.1111/j.1365-2141.2007.06902.x. [DOI] [PubMed] [Google Scholar]
- 22.Hiltunen LM, Laivuori H, Rautanen A, et al. Blood group AB and factor V Leiden as risk factors for pre-eclampsia: a population-based nested case-control study. Thromb Res. 2009;124:167–73. doi: 10.1016/j.thromres.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Lee BK, Zhang Z, Wikman A, et al. ABO and RhD blood groups and gestational hypertensive disorders: a population-based cohort study. BJOG. 2012;119:1232–7. doi: 10.1111/j.1471-0528.2012.03421.x. [DOI] [PubMed] [Google Scholar]
- 24.Phaloprakarn C, Tangjitgamol S. Maternal ABO blood group and adverse pregnancy outcomes. J Perinatol. 2013;33:107–11. doi: 10.1038/jp.2012.73. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J, Thompson S, Deeks J, Altman DTI. Measuring inconsistency in meta-analyses. BMJ. 2003;32:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Robertson L, Wu O, Langhorne P, et al. Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study. Thrombophilia in pregnancy: a systematic review. Br J Haematol. 2006;132:171–96. doi: 10.1111/j.1365-2141.2005.05847.x. [DOI] [PubMed] [Google Scholar]
- 28.Thornton CA, Bonnar J. Factor VIII-related antigen and factor VIII coagulant activity in normal and pre-eclamptic pregnancy. Br J Obstet Gynaecol. 1977;84:919–23. doi: 10.1111/j.1471-0528.1977.tb12521.x. [DOI] [PubMed] [Google Scholar]
- 29.Pekonen F, Rasi V, Ammala M, et al. Platelet function and coagulation in normal and pre-eclamptic pregnancy. Thromb Res. 1986;43:553–60. doi: 10.1016/0049-3848(86)90075-7. [DOI] [PubMed] [Google Scholar]
- 30.Dunlop W, Hill L, Landon M, et al. Clinical relevance of coagulation and renal changes in pre-eclampsia. Lancet. 1978;2:346–9. doi: 10.1016/s0140-6736(78)92944-6. [DOI] [PubMed] [Google Scholar]
- 31.Fournie A, Monrizies M, Pontonnier G, et al. Factor VIII complex in normal pregnancy, pre-eclampsia and fetal growth retardation. BJOG. 1981;88:250–4. doi: 10.1111/j.1471-0528.1981.tb00977.x. [DOI] [PubMed] [Google Scholar]
- 32.Sousa NC, Anicchino-Bizzacchi JM, Locatelli MF, et al. The relationship between ABO groups and subgroups, factor VIII and von Willebrand factor. Haematologica. 2007;92:236–9. doi: 10.3324/haematol.10457. [DOI] [PubMed] [Google Scholar]
- 33.Lattuada A, Rossi E, Calzarossa C, et al. Mild to moderate reduction of a von Willebrand factor cleaving protease (ADAMTS-13) in pregnant women with HELLP microangiopathic syndrome. Haematologica. 2003;88:1029–34. [PubMed] [Google Scholar]
- 34.Hulstein JJ, van Runnard Heimel PJ, Franx A, et al. Acute activation of the endothelium results in increased levels of active von Willebrand factor in hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. J Thromb Haemost. 2006;4:2569–75. doi: 10.1111/j.1538-7836.2006.02205.x. [DOI] [PubMed] [Google Scholar]
- 35.Alpoim PN, Gomes KB, Godoi LC, et al. ADAMTS13, FVIII, von Willebrand factor, ABO blood group assessment in preeclampsia. Clin Chim Acta. 2011;412:2162–6. doi: 10.1016/j.cca.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Molvarec A, Rigo J, Jr, Boze T, et al. Increased plasma von Willebrand factor antigen levels but normal von Willebrand factor cleaving protease (ADAMTS13) activity in preeclampsia. Thromb Haemost. 2009;101:305–11. [PubMed] [Google Scholar]
- 37.Zhang D, Xiao J, Huang H, et al. Von Willebrand factor antigen and ADAMTS13 activity assay in pregnant women and severe preeclamptic patients. J Huazhong Univ Sci Technolog Med Sci. 2010;30:777–80. doi: 10.1007/s11596-010-0657-4. [DOI] [PubMed] [Google Scholar]
- 38.Naljayan MV, Karumanchi SA. New developments in the pathogenesis of preeclampsia. Adv Chronic Kidney Dis. 2013;20:265–70. doi: 10.1053/j.ackd.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dusse LM, Alpoim PN, Lwaleed BA, et al. Is there a link between endothelial dysfunction, coagulation activation and nitric oxide synthesis in preeclampsia? Clin Chim Acta. 2013;415:226–9. doi: 10.1016/j.cca.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Than NG, Romero R, Meiri H, et al. PP13, maternal ABO blood groups and the risk assessment of pregnancy complications. PLoS One. 2011;6:e21564. doi: 10.1371/journal.pone.0021564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbalic M, Dupuis J, Dehghan A, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19:1863–72. doi: 10.1093/hmg/ddq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiechl S, Paré G, Barbalic M, et al. Association of variation at the ABO locus with circulating levels of soluble intercellular adhesion molecule-1, soluble P-selectin, and soluble E-selectin: a meta-analysis. Circ Cardiovasc Genet. 2011;4:681–6. doi: 10.1161/CIRCGENETICS.111.960682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szarka A, Rigó J, Jr, Lázár L, et al. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinheiro MB, Carvalho MG, Martins-Filho OA, et al. Severe preeclampsia: are hemostatic and inflammatory parameters associated? Clin Chim Acta. 2014;427:65–70. doi: 10.1016/j.cca.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 45.Sezik M, Toyran H, Yapar EG. Distribution of ABO and Rh blood groups in patients with HELLP syndrome. Arch Gynecol Obstet. 2002;267:33–6. doi: 10.1007/s00404-001-0263-6. [DOI] [PubMed] [Google Scholar]
- 46.Clark P, Greer IA. The influence of maternal Lewis, Secretor and ABO(H) blood groups on fetal growth restriction. J Thromb Haemost. 2011;9:2411–5. doi: 10.1111/j.1538-7836.2011.04515.x. [DOI] [PubMed] [Google Scholar]
- 47.Gloria-Bottini F, Cozzoli E, Neri A, et al. Effect of smoking and ABO blood groups on maternal age at child bearing and on birth weight. Eur J Obstet Gynecol Reprod Biol. 2011;159:83–6. doi: 10.1016/j.ejogrb.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 48.Wu O, Bayoumi N, Vickers MA, Clark P. ABO(H) blood groups and vascular disease: a systematic review and meta-analysis. J Thromb Haemost. 2008;6:62–9. doi: 10.1111/j.1538-7836.2007.02818.x. [DOI] [PubMed] [Google Scholar]
- 49.Dentali F, Sironi AP, Ageno W, et al. Non-O blood type is the commonest genetic risk factor for VTE: results from a meta-analysis of the literature. Semin Thromb Hemost. 2012;38:535–48. doi: 10.1055/s-0032-1315758. [DOI] [PubMed] [Google Scholar]
- 50.Rosendaal FR, Reitsma PH. Genetics of venous thrombosis. J Thromb Haemost. 2009;7(Suppl 1):301–4. doi: 10.1111/j.1538-7836.2009.03394.x. [DOI] [PubMed] [Google Scholar]
- 51.Franchini M, Makris M. Non-O blood group: an important genetic risk factor for venous thromboembolism. Blood Transfus. 2013;11:164–5. doi: 10.2450/2012.0087-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dentali F, Sironi AP, Ageno W, et al. Relationship between ABO blood group and hemorrhage: a systematic literature review and meta-analysis. Semin Thromb Hemost. 2013;39:72–82. doi: 10.1055/s-0032-1329550. [DOI] [PubMed] [Google Scholar]
- 53.Jick H, Slone D, Westerholm B, et al. Venous thromboembolic disease and ABO blood type. A cooperative study. Lancet. 1969;1:539–42. doi: 10.1016/s0140-6736(69)91955-2. [DOI] [PubMed] [Google Scholar]
- 54.Talbot S, Wakley EJ, Ryrie D, Langman MJ. ABO blood-groups and venous thromboembolic disease. Lancet. 1970;1:1257–9. doi: 10.1016/s0140-6736(70)91741-1. [DOI] [PubMed] [Google Scholar]
- 55.Simpson EL, Lawrenson RA, Nightingale AL, Farmer RD. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. BJOG. 2001;108:56–60. doi: 10.1111/j.1471-0528.2001.00004.x. [DOI] [PubMed] [Google Scholar]
- 56.Larsen TB, Johnsen SP, Gislum M, et al. ABO blood groups and risk of venous thromboembolism during pregnancy and the puerperium. A population-based, nested case–control study. J Thromb Haemost. 2005;3:300–4. doi: 10.1111/j.1538-7836.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 57.Chauleur C, Cochery-Nouvellon E, Mercier E, et al. Some hemostasis variables at the end of the population distributions are risk factors for severe postpartum hemorrhages. J Thromb Haemost. 2008;6:2067–74. doi: 10.1111/j.1538-7836.2008.03168.x. [DOI] [PubMed] [Google Scholar]
- 58.Hu FB, Meigs JB, Li TY, et al. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 59.Rahman M. Non-association of ABO blood groups with diabetes mellitus in Bangladesh. Bangladesh Med Res Counc Bull. 1976;2:144–6. [PubMed] [Google Scholar]
- 60.Koley S. The distribution of the ABO blood types in patients with diabetes mellitus. Anthropologist. 2008;10:129–32. [Google Scholar]
- 61.McConnell R, Pyke D, Roberts JA. Blood groups in diabetes mellitus. Br Med J. 1956;1:772–6. doi: 10.1136/bmj.1.4970.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andersen J, Lauritzen E. Blood groups and diabetes mellitus. Diabetes. 1960;9:20–4. doi: 10.2337/diab.9.1.20. [DOI] [PubMed] [Google Scholar]
- 63.Kamil M, Al-Jamal HA, Yusoff NM. Association of ABO blood groups with diabetes mellitus. Libyan J Med. 2010;5 doi: 10.3402/ljm.v5i0.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okon UA, Antai AB, Osim EE, Ita SO. The relative incidence of diabetes mellitus in ABO/Rhesus blood groups in South-Eastern Nigeria. Niger J Physiol Sci. 2008;23:1–3. doi: 10.4314/njps.v23i1-2.54897. [DOI] [PubMed] [Google Scholar]
- 65.Sidhu LS, Malhotra P, Singh SP. ABO and Rh blood groups in diabetes mellitus. Anthropol Anz. 1988;46:269–75. [PubMed] [Google Scholar]
- 66.Fagherazzi G, Gusto G, Clavel-Chapelon F, et al. ABO and Rhesus blood groups and risk of type 2 diabetes: evidence from the large E3N cohort study. Diabetologia. 2015;58:519–22. doi: 10.1007/s00125-014-3472-9. [DOI] [PubMed] [Google Scholar]
- 67.Seyfizadeh N, Yousefi B, Borzoueisileh S, et al. Is there association between ABO blood group and the risk factors of unfavorable outcomes of pregnancy? J Matern Fetal Neonatal Med. 2015;28:578–82. doi: 10.3109/14767058.2014.927424. [DOI] [PubMed] [Google Scholar]
- 68.Donma MM. Macrosomia, top of the iceberg: the charm of underlying factors. Pediatr Int. 2011;53:78–84. doi: 10.1111/j.1442-200X.2010.03198.x. [DOI] [PubMed] [Google Scholar]
- 69.Zhang C, Li Y, Wang L, et al. Blood group AB is protective factor for gestational diabetes mellitus: a prospective population-based study in Tianjin, China. Diabetes Metab Res Rev. 2015;31:627–37. doi: 10.1002/dmrr.2650. [DOI] [PubMed] [Google Scholar]