Abstract
Direct oral anticoagulants are at least as effective as vitamin K antagonists for the prevention and treatment of thromboembolism. Unfortunately, differently from vitamin K antagonists, they have the great drawback of lacking specific antidotes in the case of bleeding or emergency situations such as trauma, stroke requiring thrombolysis, and urgent surgery. The progressive development of antidotes for these new drugs, which, it is hoped, will become available in the near future, will allow better and safer management of the rapid reversal of their anticoagulant effect.
Keywords: direct oral anticoagulants, bleeding, anticoagulation reversal, fresh-frozen plasma, prothrombin complex concentrates
Introduction
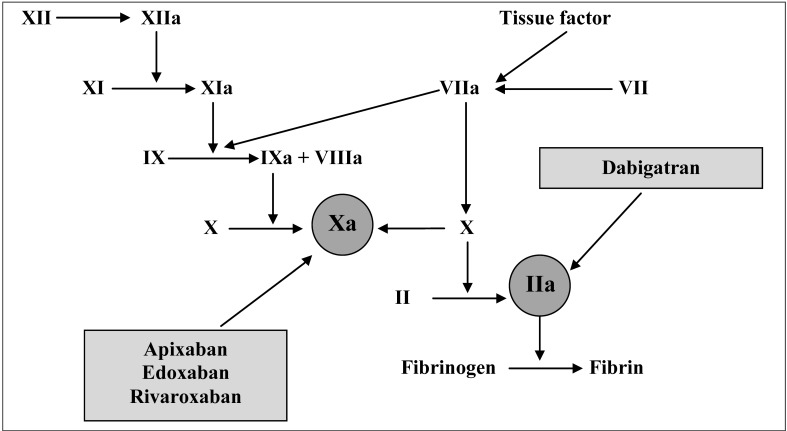
Anticoagulant therapy is a fundamental approach for the prevention and treatment of thromboembolic diseases and the currently available therapeutic armamentarium includes unfractionated heparin, low-molecular-weight heparins and vitamin K antagonists (VKA)1–3. Moreover, a new class of direct oral anticoagulants (DOAC) has been developed in the last decade to overcome the main limitations of traditional anticoagulants, i.e. parenteral administration and onset of thrombocytopenia for heparins, and the need for routine International Normalised Ratio (INR) monitoring and susceptibility to a number of food and drug interactions for VKA4–6. Two types of DOAC are currently available: the factor Xa inhibitors apixaban, edoxaban, and rivaroxaban and the thrombin inhibitor dabigatran (see Figure 1 and Table I for the description of their mechanisms of action and main characteristics, respectively)7. Unlike VKA, which block the formation of multiple, active vitamin K-dependent coagulation factors, DOAC antagonise the activity of a single step in coagulation7. In addition to their above mentioned practical advantages, at the moment there is robust evidence from several phase 3 trials that DOAC are at least as effective as VKA for the prevention and treatment of thromboembolism8–23. However, as the most feared complication of VKA is the onset of severe haemorrhage, especially intracerebral24, the comparison between these two classes of drugs is based mainly on the ability of DOAC to reduce the incidence of life-threatening bleeding complications25–28.
Figure 1.
Mechanisms of action of direct oral anticoagulants.
Table I.
Characteristics of the new oral anticoagulants.
| Characteristics | Direct thrombin inhibitor | Factor Xa inhibitors | ||
|---|---|---|---|---|
|
| ||||
| Dabigatran | Apixaban | Edoxaban | Rivaroxaban | |
|
|
||||
| Prodrug | Yes | No | No | No |
| Bioavailability (%) | 3–7 | 50 | 62 | 80 |
| Time to peak concentration (hours) | 1–3 | 1–3 | 1–3 | 2–4 |
| Half-life (hours) | 12–17 | 8–15 | 8–10 | 7–13 |
| Renal clearance (%) | 80 | 25 | 35 | 36 |
| Elimination | Urine and faeces | Urine and faeces | Urine and faeces | Urine and faeces |
| Dosing regimen | 110–150 mg twice daily | 2.5–5 mg twice daily | 15–30 mg once daily | 10–30 mg once daily |
| Metabolism | P-glycoprotein | P-glycoprotein, CYP3A4 | P-glycoprotein, CYP3A4 | P-glycoprotein, CYP3A4 |
| Antidotes under development | Idarucizumab, modified thrombin molecules | Andexanet alfa, Aripazine | Andexanet alfa, Aripazine | Andexanet alfa, Aripazine |
In this review, after a brief discussion of the safety of DOAC, we summarise the current knowledge on the management of bleeding complications associated with the use of DOAC. A section is also dedicated to the current status of development of specific antidotes. Finally we propose a treatment algorithm based on the literature and our personal experience.
Safety of direct oral anticoagulants
The results of large, randomised licensing trials have shown that bleeding rates with DOAC are generally the same or even lower than the bleeding rates with VKA and a number of meta-analyses pooling together the data from such trials have confirmed these findings29–36. Thanks to their efficacy and safety profile37, DOAC are being increasingly used in clinical practice worldwide38–40. In parallel, to validate the data from phase 3 trials, a number of real-world studies have recently been completed and their results have been published. A nationwide Danish prospective cohort study, assessing the safety and efficacy of dabigatran vs warfarin for the treatment of patients with atrial fibrillation in everyday clinical practice, found that the rates of major bleeding and gastrointestinal bleeding were similar between the dabigatran and warfarin groups: for major bleeding the adjusted hazard ratio (aHR) with 95% confidence interval (95% CI) was 0.82 (0.59–1.12) in the dabigatran 110 mg group, and 0.77 (95% CI: 0.51–1.13) in the dabigatran 150 mg group, while for gastrointestinal bleeding the aHR was 0.60 (95% CI: 0.37–0.93) in the dabigatran 110 mg group, and 1.12 (95% CI: 0.67–1.83) in the dabigatran 150 mg group41.
However, a study carried out in the USA in a large population of elderly Medicare patients, comparing the safety of dabigatran (75 or 150 mg twice daily) vs warfarin, showed that dabigatran was associated with a significantly reduced risk of ischaemic stroke (aHR: 0.80; 95% CI: 0.67–0.96), intracranial haemorrhage (aHR: 0.34; 95% CI: 0.26–0.46) and death (aHR: 0.86; 95% CI: 0.77–0.96), but with an increased risk of major gastrointestinal bleeding (aHR: 1.28; 95% CI: 1.14–1.44)42. A similar gastrointestinal bleeding risk between the DOAC dabigatran and rivaroxaban and warfarin was observed in two studies conducted in the USA on large populations of commercially insured adults43,44, although particular caution was recommended when prescribing such novel oral anticoagulants to older people (>75 years) due to an increased gastrointestinal bleeding risk44. In the Dresden prospective registry, the observed 6.1% of rivaroxaban-related major bleeding was lower and the outcome (6.3% of bleeding-related case fatality rates at day 90) better than that reported for VKA45. An update from the same registry showed that only a small proportion (5.3%) of reported bleeding events observed with DOAC were major46. Overall, these post-marketing, real-life efficacy data document that a number of DOAC-associated bleeding events do occur. The management of such events can be a major concern among physicians because of the lack of specific antidotes (see below).
Recently, various reviews and the opinions of panels of experts on the treatment of DOAC-related bleeding have been published with the aim of filling the gap consequent to the lack of evidence based on clinical trials26,27,47–49.
Management of bleeding associated with direct oral anticoagulants
Since their introduction, one of the potential downsides of DOAC administration has been the absence of specific antidotes to reverse their anticoagulant effects. Until an antidote becomes available for clinical use, supportive care remains the pillar of the treatment of haemorrhagic complications; however, based on experience with VKA-related bleeding24,50–53, the use of fresh-frozen plasma, prothrombin complex concentrates (PCC), or recombinant activated factor VIIa (rFVIIa) has been proposed54,55. In addition, although it is not usually necessary to monitor the anticoagulant effects of DOAC, an assessment of coagulation status is necessary in the case of major bleeding, trauma, urgent surgery or overdose (for the most appropriate tests for the quantitative measurement of the anticoagulant activity of DOAC, see reference 28).
In most cases of DOAC-associated mild bleeding, considering their short half-life, drug discontinuation, investigation of the source of bleeding, and general supportive measures can be adopted. The general management of major bleeding includes prompt control of the haemorrhage by mechanical compression, surgical or endoscopic haemostasis, radiological interventional procedures, transfusion of blood components and haemodynamic support with fluid replacement as well as the use of adjunctive haemostatic agents (i.e., antifibrinolytics or desmopressin)28.
Other possible therapies exploited include haemodialysis and activated charcoal. Haemodialysis may reverse the anticoagulant effects of dabigatran overdose because of the low protein binding (35%) of this drug56 and, in a single-centre study in patients with end-stage renal disease, it proved to be effective in removing approximately 70% of a single 50-mg dose of dabigatran at 4 hours57. However, it is not effective for rivaroxaban or apixaban because these drugs are highly protein bound (95% and 87%, respectively)58,59. Oral activated charcoal can be used if a recent (<2–3 hours) overdose of dabigatran is suspected, as shown by in vitro data60, but no data are available on factor Xa inhibitors.
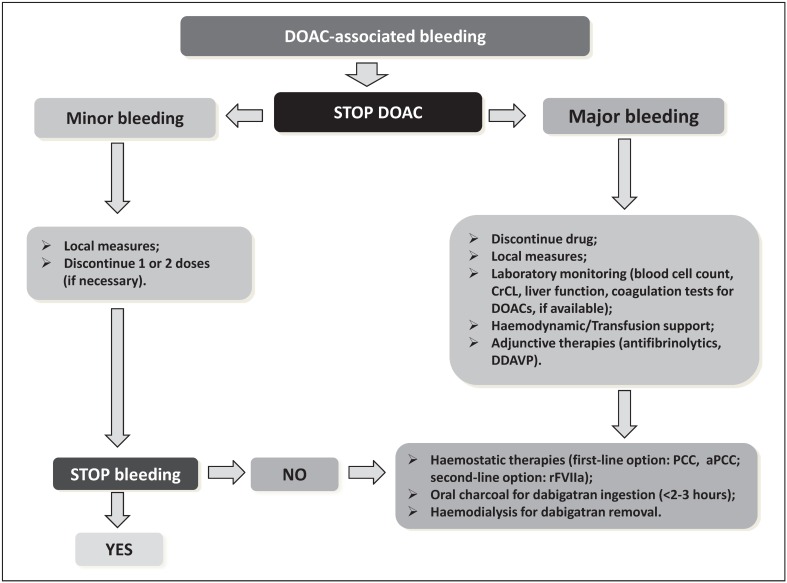
This review focuses on the use of non-specific procoagulant agents and specific antidotes (though currently still at various stages of clinical development) that can be used for the urgent reversal of anticoagulation with DOAC in severe acute haemorrhage61. Figure 2 presents a proposed treatment algorithm for patients with DOAC-associated bleeding patients or at high risk of bleeding.
Figure 2.
Treatment algorithm for patients with direct oral anticoagulant-associated bleeding or at high risk of bleeding.
DOAC: direct oral anticoagulant; CrCl: creatinine clearance; DDAVP: desmopressin; PCC: prothrombin complex concentrate; aPCC: activated prothrombin complex concentrate; rFVIIa: recombinant activated factor VII; hs: hours.
Non-specific procoagulant agents
In the case of serious bleeding, in the absence of specific antidotes, non-specific procoagulant agents (PCC, activated prothrombin complex concentrates [aPCC)], and rFVIIa) have been used for their capacity to reverse the anticoagulant effects of DOAC.
PCC contain various amounts of vitamin K-dependent coagulation factors (factors II, IX and X and proteins C and S) and are classified as three-factor PCC or as four-factor PCC, if they contain factor VII62.
Pre-clinical studies on the efficacy of PCC in the reversal of the anticoagulant effect of DOAC have yielded conflicting results63. On the one hand, a mice model of intracerebral haemorrhage64 and a rabbit model of kidney injury65 showed good effects of PCC in limiting haematoma growth and blood loss associated with dabigatran while another study demonstrated that four-factor PCC was able to reduce rat (tail vein) bleeding time significantly66.
On the other hand, successive studies pointed out that PCC had no effect on dabigatran-related bleeding in mice67 or on the reversal of the effects of rivaroxaban in a rabbit model of hepatosplenic bleeding, despite partial correction of laboratory parameters68. Moreover, in separate studies, the addition of PCC to rivaroxaban-spiked plasma in vitro did not change abnormal coagulation tests, thromboelastometry or thrombin generation tests69,70. An ex vivo randomised, double-blind, placebo-controlled study, aimed at reversing the effects of dabigatran and rivaroxaban in healthy male volunteers, showed that four-factor PCC (50 IU/kg) failed to reverse the prolongation of the activated partial thromboplastin time, ecarin clotting time, or thrombin time in participants treated with dabigatran but corrected the prothrombin time prolongation and abnormal endogenous thrombin potential in those treated with rivaroxaban71. Four-factor PCC also improved the thrombin peak and velocity indices in thrombin generation of blood from healthy male volunteers pre-treated with apixaban72.
The result of an open-label, single-centre, parallel-group study showed that both four-factor PCC and three-factor PCC can at least partially reverse the anticoagulant effects of rivaroxaban. Administration of either four-factor or three-factor PCC to 35 healthy volunteers in the presence of rivaroxaban appeared to be safe and well tolerated, with no signs of thromboembolic events73.
The three-factor PCC was also able to reverse edoxaban-induced thrombin generation inhibition in a phase 1, single-dose, placebo-controlled trial74. However, we must underline that all human studies carried out so far in this clinical setting have exploited healthy volunteers without active bleeding. Therefore, we still do not know whether and how the corrections of the laboratory tests modified by DOAC translate into a clinical benefit. Furthermore, at the moment, we do not have robust data to recommend four-factor PCC over three-factor PCC or vice versa in this setting, although data supporting the use of four-factor PCC are probably more consistent71,72.
Finally, from the analysis of the available literature data, PCC (50 IU/kg) seems to be more effective for bleeding associated with oral anti-FXa inhibitors than for that associated with oral direct thrombin inhibitors. PCC should be preferred over fresh-frozen plasma for DOAC reversal as they provide a more rapid and sustained replacement of multiple factors in a small volume and also theoretically carry a lower risk of transmitting infections because of the viral inactivation process62.
aPCC (factor VIII inhibitor bypassing activity, FEIBA) contain factors II, VII, IX and X, which are activated during the industrial production process. Several animal and in vitro studies on the reversal of the anticoagulant effects of dabigatran, rivaroxaban and apixaban demonstrated that aPCC are able to correct some abnormal clot-based coagulation tests, thromboelastometry parameters and thrombin generation indices75–78. In fact, while PCC, aPCC and rFVIIa corrected the prolongation of the prothrombin time and reduced bleeding time in rivaroxaban-treated rats, only aPCC reduced bleeding time in primates treated with rivaroxaban77. In an ex vivo study of healthy male volunteers, carried out by Marlu and colleagues79, both four-factor PCC and FEIBA were able to reverse the anticoagulant effect of dabigatran and rivaroxaban but aPCC were more efficacious. Another ex vivo experimental study showed that FEIBA, at a dose of 50 IU/kg, corrected thrombin generation in the plasma of patients treated with dabigatran80. Another study showed that PCC, aPCC and rFVIIa all corrected the prolongation of prothrombin time when added to plasma from patients receiving rivaroxaban, but only PCC and aPCC modified all abnormal thrombin generation indices81. A recent study by Martin and collaborators showed that aPCC was more effective than PCC or rFVIIa in reversing, in vitro, the effects of apixaban. aPCC rapidly triggered the development of an apparently normal fibrin network and corrected latency and quantitative parameters, whereas PCC or rFVIIa had only a partial effect82.
Therefore, aPCC (50 IU/kg, maximum 200 IU/kg/day) seems promising in reversing the effects of DOAC, especially for dabigatran. However, as data from well-designed clinical studies are lacking, the aPCC-associated increased thromboembolic risk should be evaluated carefully.
rFVIIa has also been used to reverse the effect of DOAC. Although it was not able to stop bleeding following treatment with dabigatran or rivaroxaban in animal models, in vitro studies suggest a variable effect on rivaroxaban- and apixaban-induced haemostatic abnormalities64,68,72,83. Similarly to PCC, rFVIIa also failed to reverse bleeding induced by rivaroxaban overdose68, while both aPCC and rFVIIa significantly shortened the bleeding time in edoxaban-treated rats84. In an in vitro study, rFVIIa corrected prothrombin time prolongation at a low concentration of rivaroxaban (80 ng/μL) and thromboelastometry results at a high concentration of rivaroxaban (200 ng/μL)70. In another in vitro study analysing the efficacy of PCC, aPCC and rFVIIa in reversing the anti-haemostatic activity of apixaban, rFVIIa was the most effective in correcting clotting time prolongations72. In ex vivo-treated plasma samples from healthy volunteers rFVIIa was unable to reverse the anticoagulant effect of dabigatran79. Although clinical data are lacking, results from pre-clinical studies seem to indicate that rFVIIa is less effective than other haemostatic agents (i.e., activated or non-activated PCC) for the treatment of DOAC-associated bleeding, especially when dealing with oral direct thrombin inhibitors85. Furthermore, like aPCC, rFVIIa enhances the risk of thrombosis. Therefore, rFVIIa (90 μg/kg) should be considered as a second-line therapeutic option for the treatment of DOAC-associated bleeding, to be used after the failure of PCC or aPCC (Figure 2).
Specific antidotes
Several specific antidotes for DOAC are currently under development and seem to be promising in early clinical trials evaluating their efficacy and safety55.
Idarucizumab, a humanised monoclonal antibody fragment (aDabi-Fab), has been produced by Boehringer Ingelheim GmbH (Ingelheim am Rhein, Germany) to reverse the anticoagulant effect of dabigatran and is currently in phase 3 clinical development86,87. It has a binding affinity that is about 350 times higher than the binding affinity of dabigatran for thrombin but no procoagulant or anticoagulant effects and a short half-life. A randomised, placebo-controlled, double-blind, proof-of-concept phase 1 study involving 47 healthy male volunteers documented the rapid, complete and sustained reversal of the effects of dabigatran following administration of idarucizumab, with no adverse events88. A phase 3 study (RE-VERSE AD; NCT02104947) is currently recruiting patients in more than 35 countries worldwide and aims at assessing the effect of idarucizumab, at a dose of 5 g, on the anticoagulant action of dabigatran in patients treated with dabigatran etexilate who have either uncontrolled bleeding needing urgent medical intervention (group A) or need emergency surgery or procedures necessitating rapid reversal of the anticoagulant effect of dabigatran (group B). An interim analysis including 90 patients who received idarucizumab (51 patients in group A and 39 in group B) showed that the antibody fragment rapidly and completely reversed the anticoagulant activity of dabigatran in 88 to 98% of patients89. Similarly to previous studies involving more than 200 healthy volunteers who were treated with aDabi-Fab88,90,91, no safety concerns were reported. A possible theoretical challenge is linked to the presence of natural antibodies binding to the cleavage site of Fab fragments in about 15% of normal subjects92. They would not be able to block the drug effect but the complexes of dabigatran, idarucizumab, and the anti-Fab antibody would no longer be filtered by the kidney, because of their dimension, thus prolonging the resistance to new doses of dabigatran. This problem could be solved simply by changing the anticoagulant, in the case that further anticoagulation is necessary. Another possible very low risk is the formation of anti-idiotype antibodies against this antidote, which may sterically mimic dabigatran and could become an endogenous thrombin inhibitor.
Modified thrombin molecules such as an active site-mutated S195A thrombin (S195A-IIa) and its trypsinised derivative (γT-S195A-IIa) can also play a role in the reversal of dabigatran’s effects but clinical data are still lacking93.
As far as antidote development for the direct FXa inhibitors is concerned, Portola Pharmaceuticals Inc., (San Francisco, CA, USA) has recently produced a modified, recombinant activated FX (andexanet alpha, PRT064445, Annexa™-A), which is catalytically inactive but retains high-affinity binding to factor Xa inhibitors94. In a phase 1 study on 32 healthy volunteers randomised to receive single escalating intravenous bolus doses of andexanet alfa or placebo, the anti-FXa activity of rivaroxaban, which was added ex vivo, was reversed in a dose-dependent manner with no adverse events95. Preliminary results of a phase 2, double-blind, placebo-controlled trial showed that a bolus dose of andexanet alfa antagonised the anti-FXa activity of apixaban and rivaroxaban in healthy volunteers96,97. In the randomised, double-blind, placebo-controlled phase 3 ANNEXA-A (Andexanet Alfa a Novel Antidote to the Anticoagulant Effects of FXa Inhibitors - Apixaban) study, evaluating the safety and efficacy of this antidote in older healthy volunteers (50–75 years), andexanet alfa produced rapid, sustained and nearly complete reversal of the anticoagulant effect of apixaban with no serious adverse effects98,99. Finally, in January 2015, a phase 3 prospective open-label study to evaluate the effect of andexanet in patients receiving FXa-inhibitors who have acute major bleeding started100. However, as according to the currently available data different doses of andexanet alpha seem to be required for the two direct FXa inhibitors apixaban and rivaroxaban96, it may be challenging to use the appropriate dosage in an emergency clinical condition. An additional possible concern, as with all structurally modified proteins, is immunogenicity of this antidote92.
Another reversal agent is aripazine, or PER977, a small, synthetic, water-soluble, cationic molecule developed by Perosphere Inc. (Danbury, CT, USA), which binds non-covalently to anticoagulants, inhibiting the anticoagulant effects of low molecular weight heparins, fondaparinux, the direct oral FXa inhibitors and dabigatran through hydrogen bonding and charge-charge interactions101. Unfortunately, at the moment, only limited information about this potential antidote is available. In a human plasma ex vivo model, PER977 reversed the anticoagulant effect of rivaroxaban, apixaban, and enoxaparin, measured according to anti-FXa activity, in a dose-dependent manner across a range of concentrations101. A phase 1 study evaluating the safety, tolerability, pharmacokinetics and pharmacodynamic characteristics of PER977 administered alone or following a single dose of edoxaban has recently been completed102, while a phase 2 trial evaluating the safety, tolerability and effect on coagulation tests of escalating doses of PER977 in subjects receiving edoxaban is currently underway103. According to the first in vivo human data, “baseline hemostasis was restored from the anticoagulated state within 10 to 30 minutes after administration of 100 to 300 mg of PER977 and was sustained for 24 hours”104. Additional phase 2 clinical studies are ongoing. Currently, according to Greinacher et al.92, the main challenges for this antidote are its unclear mode of action and monitoring of reversal therapy, because it may reverse bleeding without reversing the altered clotting assays.
Conclusions
DOAC are at least as effective as VKA for the prevention and treatment of thromboembolism. In addition, post-marketing data show that the safety of DOAC in the real world is similar to that observed in the published, large clinical trials, although particular caution is needed when dealing with elderly patients, who have a higher risk of bleeding, particularly from the gastrointestinal tract. Thanks to their proven efficacy and predictable anticoagulant effect, without need for routine monitoring, the DOAC are gradually replacing VKA for several indications. However, unlike VKA, which can be reversed by PCC, they have the important disadvantage of lacking specific antidotes in the case of emergency situations such as trauma, stroke requiring thrombolysis, and urgent surgery. The progressive development of antidotes for these new drugs which, it is hoped, will become available in the near future will allow better, quicker, and safer management of the reversal of the drugs’ effects. Meanwhile, shared treatment policies and algorithms can play a key role in the case emergency reversal is needed.
Footnotes
The Authors declare no conflict of interest.
References
- 1.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835–46. doi: 10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- 2.Mannucci PM, Franchini M. Old and new anticoagulant drugs: a minireview. Ann Med. 2011;43:116–23. doi: 10.3109/07853890.2010.539250. [DOI] [PubMed] [Google Scholar]
- 3.Franchini M, Liumbruno GM, Bonfanti C, Lippi G. The evolution of anticoagulant therapy. Blood Transfus. 2016;14:175–84. doi: 10.2450/2015.0096-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franchini M, Mannucci PM. A new era for anticoagulants. Eur J Intern Med. 2009;20:562–8. doi: 10.1016/j.ejim.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Franchini M, Mannucci PM. New anticoagulants for treatment of venous thromboembolism. Eur J Intern Med. 2012;23:692–5. doi: 10.1016/j.ejim.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Ageno W, Gallus AS, Wittkowsky A, et al. American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141( 2 Suppl):e44S–88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weitz JI, Eikelboom JW, Samama MM American College of Chest Physicians. New antithrombotic drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141( 2 Suppl):e120S–51S. doi: 10.1378/chest.11-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007;5:2178–85. doi: 10.1111/j.1538-7836.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–56. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 10.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 11.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 12.Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- 13.Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–98. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 14.Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 15.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 16.Fuji T, Wang CJ, Fujita S, et al. Safety and efficacy of edoxaban, an oral factor Xa inhibitor, versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial. Thromb Res. 2014;134:1198–204. doi: 10.1016/j.thromres.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Fuji T, Wang CJ, Fujita S, et al. Safety and efficacy of edoxaban, an oral factor Xa inhibitor, for thromboprophylaxis after total hip arthroplasty in Japan and Taiwan. J Arthroplasty. 2014;29:2439–46. doi: 10.1016/j.arth.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Hokusai-VTE Investigators. Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–15. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 19.Giugliano RP, Ruff CT, Braunwald E, et al. ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–75. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 21.Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–86. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 22.The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 23.Patel MR, Mahaffey KW, Garg J, et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 24.Masotti L, Di Napoli M, Godoy DA, et al. The practical management of intracerebral hemorrhage associated with oral anticoagulant therapy. Int J Stroke. 2011;6:228–40. doi: 10.1111/j.1747-4949.2011.00595.x. [DOI] [PubMed] [Google Scholar]
- 25.Dzik WS. Reversal of drug-induce anticoagulation: old solutions and new problems. Transfusion. 2012;52:45S–55S. doi: 10.1111/j.1537-2995.2012.03690.x. [DOI] [PubMed] [Google Scholar]
- 26.Miesbach W, Seifried E. New direct oral anticoagulants - current therapeutic options and treatment recommendations for bleeding complications. Thromb Haemost. 2012;108:625–32. doi: 10.1160/TH12-05-0319. [DOI] [PubMed] [Google Scholar]
- 27.Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood. 2014;123:1152–8. doi: 10.1182/blood-2013-09-529784. [DOI] [PubMed] [Google Scholar]
- 28.Franchini M, Bonfanti C, Mannucci PM. Management of bleeding associated with new oral anticoagulants. Semin Thromb Hemost. 2015;41:788–801. doi: 10.1055/s-0035-1556046. [DOI] [PubMed] [Google Scholar]
- 29.Bloom BJ, Filion KB, Atallah R, Eisenberg MJ. Meta-analysis of randomized controlled trials on the risk of bleeding with dabigatran. Am J Cardiol. 2014;113:1066–74. doi: 10.1016/j.amjcard.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 30.Miller CS, Grandi SM, Shimony A, et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110:453–60. doi: 10.1016/j.amjcard.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 31.Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381–91. doi: 10.1161/CIRCULATIONAHA.112.115410. [DOI] [PubMed] [Google Scholar]
- 32.Kwong JS, Lam YY, Yan BP, Yu CM. Bleeding of new oral anticoagulants for stroke prevention in atrial fibrillation: a meta-analysis of randomized controlled trials. Cardiovasc Drugs Ther. 2013;27:23–35. doi: 10.1007/s10557-012-6426-9. [DOI] [PubMed] [Google Scholar]
- 33.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 34.Fox BD, Kahn SR, Langleben D, et al. Efficacy and safety of novel oral anticoagulants for treatment of acute venous thromboembolism: direct and adjusted indirect meta-analysis of randomised controlled trials. BMJ. 2012;345:e7498. doi: 10.1136/bmj.e7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Hulle T, Kooiman J, den Exter PL, et al. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12:320–8. doi: 10.1111/jth.12485. [DOI] [PubMed] [Google Scholar]
- 36.Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood. 2014;124:2450–8. doi: 10.1182/blood-2014-07-590323. [DOI] [PubMed] [Google Scholar]
- 37.Camm AJ, Lip GY, De Caterina R, et al. ESC Committee for Practice Guidelines (CPG) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 38.Franchini M, Velati C. The use of novel oral anticoagulants: the debate continues! Blood Transfus. 2015;13:170–1. doi: 10.2450/2015.0059-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riva N, Ageno W. Which patients with venous thromboembolism should receive non-vitamin K antagonist oral anticoagulants? The majority. Blood Transfus. 2015;13:181–3. doi: 10.2450/2015.0057-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prandoni P. The treatment of venous thromboembolism with novel oral anticoagulants: warnings and limitations. Blood Transfus. 2015;13:178–80. doi: 10.2450/2015.0002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsen TB, Rasmussen LH, Skjøth F, et al. Efficacy and safety of dabigatran etexilate and warfarin in “real-world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61:2264–73. doi: 10.1016/j.jacc.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for non-valvular atrial fibrillation. Circulation. 2015;131:157–64. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 43.Chang HY, Zhou M, Tang W, et al. Risk of gastrointestinal bleeding associated with oral anticoagulants: population based retrospective cohort study. BMJ. 2015;350:h1585. doi: 10.1136/bmj.h1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857. doi: 10.1136/bmj.h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beyer-Westendorf J, Förster K, Pannach S, et al. Rates, management, and outcome of rivaroxaban bleeding in daily care: results from the Dresden NOAC registry. Blood. 2014;124:955–62. doi: 10.1182/blood-2014-03-563577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beyer-Westendorf J. Pattern and management of bleeding complications with new oral anticoagulants. Results of the prospective Dresden NOAC Registry ( NCT01588119) [Abstract] J Thromb Haemost. 2013;11:774. [Google Scholar]
- 47.Fenger-Eriksen C, Münster AM, Grove EL. New oral anticoagulants: clinical indications, monitoring and treatment of acute bleeding complications. Acta Anaesthesiol Scand. 2014;58:651–9. doi: 10.1111/aas.12319. [DOI] [PubMed] [Google Scholar]
- 48.Liew A, Eikelboom JW, O’Donnell M, Hart RG. Assessment of anticoagulation intensity and management of bleeding with old and new oral anticoagulants. Can J Cardiol. 2013;29:S34–44. doi: 10.1016/j.cjca.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Siegal DM, Cuker A. Reversal of novel oral anticoagulants in patients with major bleeding. J Thromb Thrombolysis. 2013;35:391–8. doi: 10.1007/s11239-013-0885-0. [DOI] [PubMed] [Google Scholar]
- 50.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of plasma and platelets. Blood Transfus. 2009;7:132–50. doi: 10.2450/2009.0005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the use of antithrombin concentrates and prothrombin complex concentrates. Blood Transfus. 2009;7:325–34. doi: 10.2450/2009.0116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liumbruno GM, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion management of patients in the peri-operative period. I. The pre-operative period. Blood Transfus. 2011;9:19–40. doi: 10.2450/2010.0074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franchini M, Liumbruno GM, Lanzoni M, et al. Clinical use and the Italian demand for prothrombin complex concentrates. Blood Transfus. 2013;11( 4 Suppl):s94–100. doi: 10.2450/2013.015s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Enriquez A, Lip GY, Baranchuk A. Anticoagulation reversal in the era of the non-vitamin K oral anticoagulants. EP Europace. 2015:euv030. doi: 10.1093/europace/euv030. [DOI] [PubMed] [Google Scholar]
- 55.Mo Y, Yam FK. Recent advances in the development of specific antidotes for target-specific oral anticoagulants. Pharmacotherapy. 2015;35:198–207. doi: 10.1002/phar.1532. [DOI] [PubMed] [Google Scholar]
- 56.PRADAXA (dabigatran etexilate mesylate) capsules for oral use [package insert] Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.; 2013. [Accessed on 08/07/2015]. Available at: http://bidocs.boehringeringelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. [Google Scholar]
- 57.Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49:259–68. doi: 10.2165/11318170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 58.Xarelto (rivaroxaban) prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc.; [Accessed on 08/07/2015]. Available at: www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100. [Google Scholar]
- 59.Eliquis (apixaban) prescribing information. Princeton, NJ: Bristol-Myers Squibb Company; [Accessed on 08/07/2015]. Available at: http://packageinserts.bms.com/pi/pi_eliquis.pdf. [Google Scholar]
- 60.van Ryn J, Sieger P, Kink-Eiband M, et al. Adsorption of dabigatran etexilate in water or dabigatran in pooled human plasma by activated charcoal in vitro [Abstract]. 51st ASH Annual Meeting and Exposition; 2009; n.1065. [Google Scholar]
- 61.Gehrie E, Tormey C. Novel oral anticoagulants: efficacy, laboratory measurement, and approaches to emergent reversal. Arch Pathol Lab Med. 2015;139:687–92. doi: 10.5858/arpa.2013-0677-RS. [DOI] [PubMed] [Google Scholar]
- 62.Franchini M, Lippi G. Prothrombin complex concentrates: and update. Blood Transfus. 2010;40:812–24. doi: 10.2450/2010.0149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin complex concentrates (PCCs): what is the evidence. Thromb Haemost. 2014;11:189–98. doi: 10.1160/TH13-05-0431. [DOI] [PubMed] [Google Scholar]
- 64.Zhou W, Schwarting S, Illanes S, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke. 2011;42:3594–9. doi: 10.1161/STROKEAHA.111.624650. [DOI] [PubMed] [Google Scholar]
- 65.Pragst I, Zeitler SH, Doerr B, et al. Reversal of dabigatran anticoagulation by prothrombin complex concentrate (Beriplex P/N) in a rabbit model. J Thromb Haemost. 2012;10:1841–8. doi: 10.1111/j.1538-7836.2012.04859.x. [DOI] [PubMed] [Google Scholar]
- 66.van Ryn J, Kink-Eiband M, Clemens A. The successful reversal of dabigatran-induced bleeding by coagulation factor concentrates in a rat tail bleeding model do not correlate with ex vivo markers of anticoagulation [Abstract] Blood. 2011;118:2316. [Google Scholar]
- 67.Herzog E, Kaspereit F, Krege W, et al. Non-clinical safety and efficacy of prothrombin complex concentrates (PCC) for the reversal of dabigatran mediated anticoagulation [Abstract] J Thromb Haemost. 2013;11( Suppl 2):693. [Google Scholar]
- 68.Godier A, Miclot A, Le Bonniec B, et al. Evaluation of prothrombin complex concentrate and recombinant activated factor VII to reverse rivaroxaban in a rabbit model. Anesthesiology. 2012;116:94–102. doi: 10.1097/ALN.0b013e318238c036. [DOI] [PubMed] [Google Scholar]
- 69.Dinkelaar J, Molenaar PJ, Ninivaggi M, et al. In vitro assessment, using thrombin generation, of the applicability of prothrombin complex concentrate as an antidote for Rivaroxaban. J Thromb Haemost. 2013;11:1111–8. doi: 10.1111/jth.12236. [DOI] [PubMed] [Google Scholar]
- 70.Körber MK, Langer E, Ziemer S, et al. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost. 2014;20:735–40. doi: 10.1177/1076029613494468. [DOI] [PubMed] [Google Scholar]
- 71.Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–9. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 72.Escolar G, Fernandez-Gallego V, Arellano-Rodrigo E, et al. Reversal of apixaban induced alterations in hemostasis by different coagulation factor concentrates: significance of studies in vitro with circulating human blood. PLoS One. 2013;8:e78696. doi: 10.1371/journal.pone.0078696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levi M, Moore KT, Castillejos CF, et al. Comparison of three-factor and four-factor prothrombin complex concentrates regarding reversal of the anticoagulant effects of rivaroxaban in healthy volunteers. J Thromb Haemost. 2014;12:1428–36. doi: 10.1111/jth.12599. [DOI] [PubMed] [Google Scholar]
- 74.Brown KS, Wickremasingha P, Parasrampuria DA, et al. The impact of prothrombin complex concentrate on the coagulopathy effects of edoxaban. JACC. 2014;63:A2095. [Google Scholar]
- 75.Galan AM, Arellano-Rodrigo E, Sanz V, et al. Effects of rivaroxaban and dabigatran on hemostasis and reversion of their antithrombotic effects by different coagulation factors: evidence raised from a clinical study in healthy volunteers [Abstract] J Thromb Haemost. 2013;11( Suppl 2):418. [Google Scholar]
- 76.Arellano-Rodrigo E, Galan AM, Sanz V, et al. Alterations induced by rivaroxaban on hemostasis can be reversed by different coagulation factor concentrates: in vitro experimental studies with steady and circulating human blood [Abstract] J Thromb Haemost. 2013;11( Suppl 2):953. [Google Scholar]
- 77.Perzborn E, Gruber A, Tinel H, et al. Reversal of rivaroxaban anticoagulation by haemostatic agents in rats and primates. Thromb Haemost. 2013;110:162–72. doi: 10.1160/TH12-12-0907. [DOI] [PubMed] [Google Scholar]
- 78.Chan HHW, Atkinson HM, Goncharenko M, et al. Reversal of dabigatran using recombinant activated factor VII and activated prothrombin complex concentrates in thromboelastography assay. J Thromb Haemost. 2011;9:576–7. [Google Scholar]
- 79.Marlu R, Hodaj E, Paris A, et al. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217–24. doi: 10.1160/TH12-03-0179. [DOI] [PubMed] [Google Scholar]
- 80.Khoo TL WC, Kershaw G, Reddel CJ, et al. The use of FEIBA in the correction of coagulation abnormalities induce by dabigatran. Int J Lab Hem. 2013;35:222–4. doi: 10.1111/ijlh.12005. [DOI] [PubMed] [Google Scholar]
- 81.Herrmann R, Thom J, Wood A, et al. Thrombin generation using the calibrated automated thrombinoscope to assess reversibility of dabigatran and rivaroxaban. Thromb Haemost. 2014;111:989–95. doi: 10.1160/TH13-07-0607. [DOI] [PubMed] [Google Scholar]
- 82.Martin AC, Gouin-Thibault I, Siguret V, et al. Multimodal assessment of non-specific hemostatic agents for apixaban reversal. J Thromb Haemost. 2015;13:426–36. doi: 10.1111/jth.12830. [DOI] [PubMed] [Google Scholar]
- 83.Lambourne MD, Eltringham-Smith LJ, Gataiance S, et al. Prothrombin complex concentrates reduce blood loss in murine coagulopathy induced by warfarin, but not in that induced by dabigatran etexilate. J Thromb Haemost. 2012;10:1830–40. doi: 10.1111/j.1538-7836.2012.04863.x. [DOI] [PubMed] [Google Scholar]
- 84.Fukuda T, Honda Y, Kamisato C, et al. Reversal of anticoagulant effects of edoxaban, an oral, direct factor Xa inhibitor, with haemostatic agents. Thromb Haemost. 2012;107:253–9. doi: 10.1160/TH11-09-0668. [DOI] [PubMed] [Google Scholar]
- 85.Lee FMH, Chan AKC, Lau KK, Chan HH. Reversal of new, factor-specific oral anticoagulants by rFVIIa, prothrombin complex concentrate and activated protrhombin complex concentrate: a review of animal and human studies. Thromb Res. 2014;133:705–13. doi: 10.1016/j.thromres.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 86.Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554–62. doi: 10.1182/blood-2012-11-468207. [DOI] [PubMed] [Google Scholar]
- 87.Glund S, Stangier J, Schmohl M, et al. A specific antidote for dabigatran: immediate, complete and sustained reversal of dabigatran induced anticoagulation in healthy male volunteers [Abstract] Circulation. 2013;128:A17765. [Google Scholar]
- 88.Glund S, Stangier J, Schmohl M, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet. 2015;386:680–90. doi: 10.1016/S0140-6736(15)60732-2. [DOI] [PubMed] [Google Scholar]
- 89.Pollack CV, Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511–20. doi: 10.1056/NEJMoa1502000. [DOI] [PubMed] [Google Scholar]
- 90.Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects [abstract] Blood. 2014;124:344. [Google Scholar]
- 91.Glund S, Moschetti V, Norris S, et al. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb Haemost. 2015;113:943–51. doi: 10.1160/TH14-12-1080. [DOI] [PubMed] [Google Scholar]
- 92.Greinacher A, Thiele T, Selleng K. Reversal of anticoagulants: an overview of current developments. Thromb Haemost. 2015;113:931–42. doi: 10.1160/TH14-11-0982. [DOI] [PubMed] [Google Scholar]
- 93.Sheffield WP, Lambourne MD, Eltringham-Smith LJ, et al. qγT -S195A thrombin reduces the anticoagulant effects of dabigatran in vitro and in vivo. J Thromb Haemost. 2014;12:1110–5. doi: 10.1111/jth.12601. [DOI] [PubMed] [Google Scholar]
- 94.Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19:446–51. doi: 10.1038/nm.3102. [DOI] [PubMed] [Google Scholar]
- 95.Crowther MA, Kitt M, McClure M, et al. Randomized, double-blind, placebo-controlled single ascending dose pharmacokinetic and pharmacodynamic study of PRT064445, a universal antidote for factor Xa inhibitors [Abstract] Arterioscler Thromb Vasc Biol. 2013;33:A10. [Google Scholar]
- 96.Crowther MA, Kitt M, Lorenz T, et al. A phase 2 randomized, double-blind, placebo-controlled trial of PRT064445, a novel, universal antidote for direct and indirect factor Xa inhibitors [Abstract] J Thromb Haemost. 2013;11(Suppl):AS20.1. [Google Scholar]
- 97.Crowther MA, Mathur V, Kitt M, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of rivaroxaban induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), an antidote for FXa inhibitors [Abstract] Blood. 2013;122:A3636. [Google Scholar]
- 98.Crowther M, Levy GG, Lu G, et al. ANNEXA™-A: A phase 3 randomized, double-blind, placebo-controlled trial, demonstrating reversal of apixaban-induced anticoagulation in older subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (FXa) inhibitors [Abstract] Circulation. 2014;130:2116. [Google Scholar]
- 99.Crowther M, Gold A, Lu G, et al. ANNEXA™-A PART 2: A phase 3 randomized, double-blind, placebo-controlled trial, demonstrating sustained reversal of apixaban-induced anticoagulation in older subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (FXa) inhibitors [Abstract] J Thromb Haemost. 2015;13( Suppl 2):84. [Google Scholar]
- 100.Recombinant Factor Xa Inhibitor Antidote. ClinicalTrials.gov. [Accessed on 08/07/2015]. NCT02329327. Available at: https://clinicaltrials.gov/ct2/show/NCT02329327.
- 101.Laulicht B, Bakhru S, Lee C, et al. Small molecule antidote for anticoagulants [Abstract] Circulation. 2012;126:10021. [Google Scholar]
- 102.Effects of a Double-Blind, Single Dose of PER977 Administered Alone, and Following a Single Dose of Edoxaban (PER977-P1) ClinicalTrials.gov. [Last accessed on 08/07/2015]. NCT01826266. Available at: https://clinicaltrials.gov/ct2/show/NCT01826266.
- 103.Study of PER977 Administered to Subjects With Steady State Edoxaban Dosing and Re-anticoagulation With Edoxaban. ClinicalTrials.gov. [Last accessed on 08/07/2015]. NCT02207257. Available at: https://clinicaltrials.gov/ct2/show/NCT02207257.
- 104.Ansell JE, Bakhru SH, Laulicht BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban [Letter] N Engl J Med. 2014;371:2141–2. doi: 10.1056/NEJMc1411800. [DOI] [PubMed] [Google Scholar]