Abstract
Interleukin 10 (IL10) is a poor prognostic marker in several cancers. Its role in breast cancer is not well elucidated. The present study is designed to see the expression of IL10 in breast cancer tissue and to evaluate its correlation with the established markers of prognosis. Sixty female patients who underwent surgery for breast cancer were enrolled for the study. Immediately after surgery, 2–5 g of tumour tissue and similar volume of peritumoural normal breast tissue were collected for IL10 assay. IL10 expression was assayed by immunohistochemistry. IL10 expressing tumours and IL10 non expressing tumours were compared. Chi square/Fisher exact test and student’s t test were used to compare the data. p- valueless than 0.05 was considered as statistically significant. Thirty six patients (60 %) of carcinoma breast showed IL 10 expression in tumour tissue as compared to no IL 10 expression in any peritumouralnormal breast tissue (p < 0.01). IL10 expression had statistically significant correlation with locally advanced disease, tumour grade, HER2 + ve tumours and ER-ve, PR-ve, HER2 + ve breast cancer subtypes (p = 0.001, 0.001, 0.001 and 0.01 respectively). No correlation could be found with patient’s age, tumour size, tumour histology and ER and PR status. Correlation of IL10 expressing tumours with several established poor prognostic markers of breast cancer may indicate the possible association of IL10 expression with poor prognosis. Large studies with long term follow up are needed to substantiate the association of IL10 with poor prognosis.
Keywords: Breast cancer, Interleukin 10, Breast cancer prognosis
Introduction
Prognosis and treatment of breast cancer are commonly determined by patient’s age, tumour size, lymph node status, tumour grade, molecular markers like estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) status. In the last few decades, there has been substantial progress in breast cancer management due to the advent of potent chemotherapy and ER, PR and HER2 status guided therapeutic regimes. However, the prognosis of breast cancer is still considered as poor [1]. To improve prognostications and therapeutic guidance, there has been continuous search for newer molecular markers [2]. Cytokines and its implications in breast cancer is an evolving area of active research.
Cytokines are low molecular weight glycoproteins and they play an important role in cancer pathogenesis [3, 4]. Interleukin 10 (IL10) is a cytokine secreted by almost all leukocytes including T cells, monocytes, macrophages, keratinocytes and by tumour cells [4, 5]. It plays a dual role on cancer pathogenesis. On the one hand, they are highly immunosuppressive, producing a conducive environment for cancer progression while on the other hand, they exhibit cancer inhibiting anti-angiogenic property [6, 7]. Several tumours express IL10. Raised serum concentrations of IL10 in lung cancer, bone sarcoma, gastric cancer, colon cancer, pancreatic cancer, hepatocellular carcinoma, malignant melanoma and diffuse B cell lymphoma are associated with adverse disease stage or with poor prognosis. Raised serum concentration of IL10 and the resulting immunosuppression seem to be a common feature in progressive cancer [4].
The IL10 expression in the breast cancer and its role in breast cancer prognosis are not well elucidated. A few studies have documented IL10 expression in breast cancer [8–11]. Serum IL10 concentration is raised in breast cancer patients compared to normal healthy females [12], while other failed to show any association between breast cancer and serum IL10 concentration [13]. IL10 expressing tumours are often ER-ve and associated with apoptotic marker Bax, suggesting a possible aggressive behaviour of IL 10 expressing tumours [9, 10].
This study has been designed to evaluate expression of IL10 in breast cancer and its correlation with known prognostic markers of breast cancers i.e. patient’s age, tumour stage, tumour grade and ER, PR and HER2 status.
Materials and Methods
This was a prospective observational study conducted between November 2011 and March 2013. Prior approval from the Institute’s Ethics Committee and informed consent from the patients were obtained. The female patients with breast cancer and planned for surgery were included in the study. Patients with in-situ cancers and patients with tumour size less than 1 cm were excluded. Sixty patients were enrolled. The clinical and demographic profiles of the patients were recorded in detail. Radiologically, tumours were graded according to BI-RADS scores as BI-RADS 0-6 [14]. Patients were classified according to American Joint Committee on Cancer Staging of Breast Cancer (AJCC 7th Edition). Patients with locally advanced carcinoma breast received neo adjuvant chemotherapy. The response to neo-adjuvant chemotherapy was assessed using the Response Evaluation Criteria in Solid Tumour (RECIST) criteria [15]. Patients underwent either a breast conserving surgery or mastectomy with axillary lymph node dissection. Depending upon the final histopathology of the tumour, patients were offered adjuvant chemo therapy, radiotherapy and endocrine therapy as per the treatment protocol of the department. Patients were followed up for six months.
IL 10 expression in the tumour as well as in the peritumour normal breast tissue was studied by immunohistochemistry. The details of histopathological and immunohistochemical analysis are described below.
Sample Collection and Immunohistochemistry
Two to five grams of tumour tissue and another sample from the normal peri-tumoural breast tissue were taken from the resected breast immediately after surgery. Specimen were fixed in 10 % buffered formalin, processed and embedded in paraffin. Paraffin section was stained with hematoxylin and eosin staining for histopathology and grading of the tumour (Bloom-Richardson Grading). Immunohistochemistry was done for detection of cytokine IL 10, ER, PR and HER2 following a standardized protocol described earlier [16]. A set of 5 μm-thick paraffin sections were cut. Formalin fixed tissues were deparaffinized in xylene and rehydrated gradually by dipping in 100 %, 95 % and 70 % alcohol and distilled water. Antigen retrieval was done by microwave treatment in citrate buffer (10 mM, PH-6.0). It was followed by serum blocking, application of diluted (1:100) primary antibody IL-10 (Abcam, Cat no. ab34843) and incubation overnight at 4 °C. The secondary antibody application and staining was done following a standard protocol using Vectastain universal kit. (Cat. No. pk 8800) and Diaminobenzidine (DAB) substrate kit(cat. No. SK-4100). The sections were then counterstained with hematoxylin, followed by dehydration and finally mounted in DPX and evaluated under microscope. Immunoreactivity in the tissue was judged independently by two experts who were blinded to the clinical data. Negative controls were included in each slide run with omission of primary and secondary antibodies. Interleukin10 expression in tumour tissue was regarded as positive when 10 % or more tumor cells were positive.
Statistical Analysis
The categorical data was analyzed using Chi square/Fisher exact test wherever applicable. The ordinal data like tumour grading/BIRADS was analyzed by applying Chi square linear trend test. The student’s t test was applied to compare continuous data in two groups. Computer software (SPSS/PC 20 version) was used to analyze the data. All the p- values less than 0.05 was considered as statistically significant.
Results
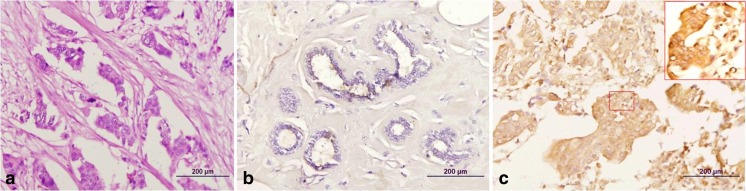
Thirty six patients (60 %) showed IL 10 expression in tumour tissue as compared to no IL 10 expression in any peritumour normal breast tissue which was statistically significant (p < 0.01) (Table 1) (Fig. 1).
Table 1.
Comparison of IL10 expression between tumour tissue and peri-tumour breast tissue of breast cancer patients
| Study group | IL 10 positive (n) | IL 10 negative (n) | p-value |
|---|---|---|---|
| Tumour tissue of breast cancer patients | 36(60 %) | 24(40 %) | <0.01* |
| Peri-tumour breast tissue of breast cancer patients | 0 | 60(100 %) |
*Fisher’s Exact test
Fig. 1.
Photomicrograph showing (a) infiltrating duct carcinoma (H&E), (b) peritumoral tissue with ductal epithelium negative for IL10 immunohistochemistry and (c) strongly positive expression of IL10 in the tumor cells in the same case
Comparison of IL10 Positive (+Ve) and IL 10 Negative (-Ve) Breast Cancer Patients
The patients with IL10 + ve cancers and patients with IL10-ve cancers had similar demographic and clinical profile (Table 2). Radiological characteristics (BIRADS Score) were also comparable (Table 3). However, patients with locally advanced breast cancer had statistically significant higher IL 10 expression as compared to patients with early breast cancer (72 % vs 29 %; p = 0.001) (Table 3).
Table 2.
Comparison of demographic profile and clinical parameter of IL10 positive and IL10 negative breast cancer patients
| Demographic and clinical profile | IL10 positive Mean ± SD (Range) | IL10 negative Mean ± SD (Range) | p-value |
|---|---|---|---|
| n = 36 | n = 24 | ||
| Age (years) | 47.7 ± 9.8 (28–67) | 46.7 ± 9.1(32–50) | 0.69* |
| Post menopausal(n) | 25 | 14 | 0.27*** |
| Menopausal age (years) | 45.8 ± 2.02 (42–51) | 43.7 ± 5.16 (32–49) | 0.65* |
| Duration of lump (months) | 6.47 ± 4.8 (1–24) | 6.9 ± 5.57 (1–18) | 0.13* |
| Size of the lump(cm) Mean ± SD (Range) | 5.2 ± 2.3 (2–10) | 5.9 ± 3.4 (2–8) | 0.23* |
| No regional lymph nodes (n) | 13 | 6 | 0.23** |
| Palpable N1 lymph nodes(n) | 19 | 13 | |
| Palpable N2 Lymph nodes(n) | 4 | 5 |
*Student’s t test, **Fisher exact test. ***Chi square test
Table 3.
Radiological and Clinico-pathological correlation between IL10 positive and IL10 negative breast cancer patients
| Grading system | IL 10 positive | IL10 negative | p value |
|---|---|---|---|
| (n = 36) | (n = 24) | ||
| BIRADS Scoring | |||
| BIRADS-3 | 2(5.6 %) | 1(4.2 %) | 0.18*** |
| BIRADS-4 | 2(5.6 %) | 5(20.8 %) | |
| BIRADS-5 | 27(75 %) | 17(70 %) | |
| BIRADS-6 | 5(20.8 %) | 1(4.2 %) | |
| Clinical Staging | |||
| Early breast cancer(EBC) | 7(28 %) | 25(71 %) | 0.001* |
| Locally advanced breast cancer(LABC) | 18(72 %) | 10(29 %) | |
| Response to NACT (Clinical) | |||
| Complete Response (CR) | 7(35 %) | 2(25 %) | 0.83** |
| Partial Response (PR) | 12(60 %) | 5(62 %) | |
| No Change (NC) | 1(5 %) | 1(13 %) | |
| Histology | |||
| Invasive ductal carcinoma | 32(89 %) | 22(92 %) | 0.64** |
| Invasive lobular carcinoma | 4(11 %) | 2(8 %) | |
| Histological grading | |||
| Grade I | 2(7 %) | 6(19 %) | 0.001*** |
| Grade II | 6(21 %) | 17(55 %) | |
| Grade III | 21(72 %) | 8(23 %) | |
| Hormone Receptor status | |||
| ER + | 15(42 %) | 11(46 %) | 0.75* |
| ER- | 21(58 %) | 13(54 %) | |
| PR+ | 14(39 %) | 13(54 %) | 0.24* |
| PR - | 22(61 %) | 11(46 %) | |
| HER2+ | 26(72 %) | 9(38 %) | 0.001* |
| HER2- | 10(28 %) | 15(62 %) | |
*Chi-square test, **Fisher’s Exact test, *** Chi-square linear trend test
Twenty eight patients received neo adjuvant chemotherapy. No association of IL 10 positivity with response to neoadjuvant chemotherapy could be seen (Fisher exact test, p = 0.83) (Table 3).
Fifty four patients had invasive ductal carcinoma and six patients had lobular carcinoma. The histological type had no correlation with IL 10 expression (Table 3). There was significant linear by linear association of IL 10 positivity and grade of tumour. There was increasing trend of IL 10 expression towards Grade III tumour; odds ratio being 1,1.6 and 7.88 in Grade I ,Grade II and Grade III respectively. (linear by linear association, p = 0.001)(Table 3).
Thirty five patients were HER2 + ve breast cancer and 26 of these patients had IL10 expression in the tumour tissue, which was statistically significant (p = 0.001, Chi square test). There was no statistically significant correlation of IL 10 expression with ER + ve and PR + ve breast cancer (p = 0.75 and 0.24 respectively) (Table 3).
A subgroup analysis was done to see the patterns of IL 10 expression in ER, PR and HER2 subtypes. The tumours were classified as ER/PR+,HER2+ (Luminal B); ER/PR+,HER2-(Luminal A); ER/PR-,HER2+; ER/PR-,HER2-(Triple negative) [17]. We found statistically significant correlation between IL 10 expression and ER/PR-, HER2+ subtype. (Fisher exact test, p = 0.01) (Table 4).
Table 4.
Comparison of ER, PR, HER2 subtypes between IL10 positive and IL10 negative breast cancer patients
| Subtypes | Included groups | IL 10 positive | IL 10 negative | p value |
|---|---|---|---|---|
| n = 36 | n = 24 | |||
| ER/PR+,HER2+ (Luminal B) | ER+/PR+,HER2+; ER−/PR+,HER2+; ER+/PR-,HER2+ | 10(28 %) | 7(29 %) | 0.91* |
| ER/PR+,HER2- (Luminal A) | ER+/PR+,HER2-; ER−/PR+,HER2-; ER+/PR-,HER2- | 5(14 %) | 8(33 %) | 0.09* |
| ER/PR-, HER2+ | ER−/PR-,HER2+ | 17(47 %) | 2(8 %) | 0.01** |
| ER/PR-,HER2- (Triple negative) | ER−/PR-,HER2- | 4(11 %) | 7(29 %) | 0.10** |
*Chi-square test, **Fisher’s Exact test for categorical variable
Follow Up
Fifty eight patients completed 6 months follow up. One patient had loco-regional recurrence with satellite nodules at three months followup. She had IL 10 expression in her tumour. The difference was not significant statistically (p = 1.00). The patient was given palliative radiotherapy and chemotherapy.
At six months follow up, three patients developed metastatic disease. One patient had IL 10 expression whereas the other two had no IL 10 expression in tumour tissue, this difference was not significant statistically (p = 0.056).
The patient who had IL10 positive cancer presented with ipsilateral satellite nodule and contralateral right breast lump. The biopsy of right breast lump was invasive ductal carcinoma, ER+,PR+ and HER2 + ve. This tumour also had IL 10 expression. She received chemotherapy and local radiotherapy. Subsequently, she developed progressive disease and underwent modified radical mastectomy on the right side too.
The second patient with the metastasis did not show IL 10 expression in her tumour. She had stage IIIA tumour and received neoadjuvant chemotherapy and underwent modified radical mastectomy. She developed metastasis in the lung after 6 months.
The third patient with metastasis also did not show IL 10 expression in her tumour. She received neoadjuvant chemotherapy followed by modified radical mastectomy and adjuvant radiotherapy. She developed vertebral metastasis.
Discussion
IL10 expression in breast cancer and its role in breast cancer prognosis are not well elucidated. Our study has shown that there is significantly higher expression of IL10 in tumour cells compared to the peritumoral breast parenchyma in patients with breast cancer. A few other studies have also shown similar results. In a study of 26 breast cancer patients, IL 10 mRNA was detected in 16 patients. IL10 mRNA was also detected in two normal peri-tumoural breast tissue samples [8]. Lianes -Fernandez et al. showed strong expression of IL 10 in 23 out of 27 breast cancer patients by immunohistochemistry [9]. In another study of 105 breast cancer and 13 healthy breast tissue samples, IL10 expression was seen only in breast cancer tissue and none of the normal healthy tissue had IL10 expression [10]. 60 % of our breast cancer patients expressed IL10. Strategies to neutralise IL10 in the tumour microenvironment might have the potential for targeted therapy [9, 20]. However, the complex role of IL10 in cancer initiation and progression makes its therapeutic potential debatable [6, 18]. On the one hand, IL10 is a strong immunosuppressing cytokine [4]. It inhibits the presentation of tumour associated antigen by tumour cells and antigen presentation by macrophages and Langerhan’s cells [19]. The T cell proliferation and functions are also inhibited by IL10 [20, 21]. Thus, the IL10 produced by the tumour cells is helping them to suppress the host immune response and prevent their detection and elimination by the host immune system [11]. On the other hand, IL10 also exhibits tumour inhibiting anti-angiogenic action [6, 7]. This dual nature of IL 10 complicates any attempt to manage breast cancer with IL10 as therapeutic target [5], and it’s an area for further research.
Another interesting aspect of our study is that expression of IL10 might serve as a biomarker for poor prognosis in breast cancer. In several cancers serum IL10 concentration is associated with advanced disease stage and negative prognosis [4]. In breast cancer, although, the role of IL10 as a biomarker for prognosis is not elucidated, a possible aggressive behaviour of IL10 expressing tumours has been suggested by a few researchers. Lianes -Fernandez et al. noted statistically significant correlation of IL 10 expression in breast tissue with apoptotic marker Bax. The author suggested that IL10 might be an anti-apoptotic factor; its expression along with high expression of Bcl2 family protein in the tumour micro-environment, might reflect the aggressiveness of the tumour [9]. Chavey et al. found no correlation of IL10 expression with patient’s age, tumour size, histological type, lymph node and HER2 status of the tumour. They showed a significant correlation of IL10 expression with tumour grade, ER-ve and PR-ve tumours. These authors also suggest the possible association of IL10 expression with aggressive behaviour of the tumours [10].
Our findings of significant correlation of IL 10 expression with locally advanced disease, tumour grade, HER2 + ve tumour and with ER-ve, PR-ve, HER2 + ve sub-types of breast cancer have prognostic significance. HER2 + ve breast cancers are associated with increase cell proliferation and motility, increased angiogenesis and tumour invasiveness and decreased apoptosis. HER2 + ve tumours are also more likely to be of high grade [22]. Fortunately, HER2 expressing tumours are amenable to highly effective targeted therapy. These targeted therapies have improved the prognosis in these group of patients, however, in absence of targeted therapy, HER2 over expressing tumours are associated with worse prognosis [23, 24]. Our findings of positive association of IL10 expression with higher grade and HER2 + ve tumours also supports the theory that IL10 expressing tumours are aggressive tumours [9, 10].
IL10 expressing tumours correlate significantly with ER-ve, PR-ve and HER2 + ve sub types of breast cancer. Triple negative tumours and ER-ve, PR-ve and HER2 + ve tumours are of worse prognosis among the different sub types [17, 25, 26]. A few studies even associated ER-ve, PR-ve and HER2 + ve sub types with worst prognosis among the different subtypes [27, 28]. ER-ve, PR-ve and HER2 + ve tumours are also associated with increased risk of loco regional recurrence following breast conservation surgery [29]. In absence of long term outcome data of IL10 expressing tumours, these clinical evidences indirectly suggest that IL10 expression may be a bio marker of poor prognosis. We could not find any co relation of IL10 expression with local or distant failure, however, the study was small with limited follow up of 6 months.
Conclusion
There is significantly higher expression of IL10 in tumour cells in comparison to normal breast tissue in patients with breast cancer. We did not find any correlation of IL10 expression with patient’s age, tumour size, lymph node status, tumour histology and ER and PR status. IL10 expression correlates statistically significantly with locally advanced disease, tumour grade, HER2 + ve status and ER-ve, PR-ve and HER2 + ve subtypes of breast cancer. Due to the short follow up of the study, correlation of IL10 expression with clinical outcome in terms of disease free or overall survival could not be ascertained. Nevertheless, this study has put forward an additional insight that IL10 may be a crucial bio marker of poor prognosis in breast cancer. Large studies with long term follow up are needed to substantiate the association of IL10 with poor prognosis.
Compliance with Ethical Standards
Work has been approved by Institute’s Ethics Committee.
Conflict of Interest Statement
There is no conflict of interest.
Funding Source
None
Contributor Information
Hemanga Kumar Bhattacharjee, Email: dr_hkb75@yahoo.com.
Virinder Kumar Bansal, Email: drvkbansal@gmail.com.
Bikash Nepal, Email: bikash.nepal3@gmail.com.
Sandeep Srivastava, Email: bio.sandeep@gmail.com.
Amit K. Dinda, Email: dindaaiims@gmail.com
Mahesh C. Misra, Email: mcmisra@gmail.com
References
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Hicks D, Turner B. Pathologic diagnosis, immunohistochemistry, multigene assays and breast cancer treatment: progress toward"precision" cancer therapy. Biotech Histochem. 2015;90:81–92. doi: 10.3109/10520295.2014.978893. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Ding Q, Shi Y, et al. The interleukin-10-1082 promoter polymorphism and cancer risk: a meta-analysis. Mutagenesis. 2012;27:305–312. doi: 10.1093/mutage/ger078. [DOI] [PubMed] [Google Scholar]
- 4.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 5.Hamidullah, Changkija B, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Res Treat. 2012;133:11–21. doi: 10.1007/s10549-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 6.Mocellin S, Marincola F, Rossi CR, Nitti D, Lise M. The multifaceted relationship between IL10 and adaptive immunity: putting together the pieces of a puzzle. Cytokine Growth Factor Rev. 2004;15:61–76. doi: 10.1016/j.cytogfr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Huang S, Ullrich SE, Bar-Eli M. Regulation of tumor growth and metastasis by interleukin-10: the melanoma experience. J Interf Cytokine Res. 1999;19:697–703. doi: 10.1089/107999099313532. [DOI] [PubMed] [Google Scholar]
- 8.Venetsanakos E, Beckman I, Bradley J, Skinner JM. High incidence of interleukin 10 mRNA but not interleukin 2 mRNA detected in human breast tumours. Br J Cancer. 1997;75:1826–1830. doi: 10.1038/bjc.1997.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llanes-Fernández L, Alvarez-Goyanes RI, Arango-Prado Mdel C, et al. Relationship between IL-10 and tumor markers in breast cancer patients. Breast. 2006;15:482–489. doi: 10.1016/j.breast.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Chavey C, Bibeau F, Gourgou-Bourgade S, et al. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9:R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heckel MC, Wolfson A, Slachta CA, et al. Human breast tumor cells express IL-10 and IL-12p40 transcripts and proteins, but do not produce IL-12p70. Cell Immunol. 2011;266:143–153. doi: 10.1016/j.cellimm.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Kozłowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–84. [PubMed] [Google Scholar]
- 13.Lyon DE, McCain NL, Walter J, Schubert C. Cytokine comparisons between women with breast cancer and women with a negative breast biopsy. Nurs Res. 2008;57:51–58. doi: 10.1097/01.NNR.0000280655.58266.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberl MM, Fox M, Edge CH, Carter SB, C, Mahoney, M. C BI-RADS classification for management of abnormal mammograms. J Am Board Fam Med. 2006;19:161–164. doi: 10.3122/jabfm.19.2.161. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh R, Sharma A, Mitra DK, Agarwal SK, Dinda AK, Saxena A. Study of CC chemokine receptor 5 in renal allograft rejection. Indian J Nephrol. 2013;23:196–200. doi: 10.4103/0971-4065.111848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features andsurvival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2008.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son KS, Kang HS, Kim SJ, et al. Hypomethylation of the interleukin-10 gene in breast cancer tissues. Breast. 2010;19:484–488. doi: 10.1016/j.breast.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Toomey D, Harmey J, Condron C, Kay E, Bouchier-Hayes D. Phenotyping of immune cell infiltrates in breast and colorectal tumours. Immunol Investig. 1999;28:29–41. doi: 10.3109/08820139909022721. [DOI] [PubMed] [Google Scholar]
- 20.Halak BK, Maguire HC, Jr, Lattime EC. Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res. 1999;59:911–917. [PubMed] [Google Scholar]
- 21.Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010;21(5):315–324. doi: 10.1016/j.cytogfr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 23.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neuoncogene. Sci. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 24.Figueroa-Magalhães MC, Jelovac D, Connolly RM, Wolff AC. Treatment of HER2-positive breast cancer. Breast. 2014;23:128–136. doi: 10.1016/j.breast.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su Y, Zheng Y, Zheng W, et al. Distinct distribution and prognostic significance of molecular subtypes of breast cancer in Chinese women: apopulation-based cohort study. BMC Cancer. 2011;11:292. doi: 10.1186/1471-2407-11-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puig-Vives M, Sánchez MJ, Sánchez-Cantalejo J, et al. Distribution and prognosis of molecular breast cancer subtypes defined by immunohistochemical biomarkers in a Spanish population-based study. Gynecol Oncol. 2013;130:609–614. doi: 10.1016/j.ygyno.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 27.Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptorand Her2/neu-overexpressing phenotypes. Hum Pathol. 2006;37:1217–1226. doi: 10.1016/j.humpath.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer study. Clin Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WJl J, HX J, HY F, YP Y, Chen K, FX S. HER2-enriched tumors have the highest risk of local recurrence in Chinese patients treated with breast conservation therapy. Asian Pac J Cancer Prev. 2014;15:315–320. doi: 10.7314/APJCP.2014.15.1.315. [DOI] [PubMed] [Google Scholar]