Abstract
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of cancer-related death worldwide. Besides the lymphatic and haematogenous routes of dissemination, CRC frequently gives rise to transcoelomic spread of tumor cells in the peritoneal cavity, which ultimately leads to peritoneal carcinomatosis (PC). PC is associated with a poor prognosis and bad quality of life for these patients in their terminal stages of disease. A loco-regional treatment modality for PC combining cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy has resulted in promising clinical results. However, this novel approach is associated with significant morbidity and mortality. A comprehensive understanding of the molecular events involved in peritoneal disease spread is paramount in avoiding unnecessary toxicity. The emergence of PC is the result of a molecular crosstalk between cancer cells and host elements, involving several well-defined steps, together known as the peritoneal metastatic cascade. Individual or clumps of tumor cells detach from the primary tumor, gain access to the peritoneal cavity and become susceptible to the regular peritoneal transport. They attach to the distant peritoneum, subsequently invade the subperitoneal space, where angiogenesis sustains proliferation and enables further metastatic growth. These molecular events are not isolated events but rather a continuous and interdependent process. In this manuscript, we review current data regarding the molecular mechanisms underlying the development of colorectal PC, with a special focus on the peritoneum and the role of the surgeon in peritoneal disease spread.
Keywords: Peritoneal carcinomatosis, Pathophysiology, Peritoneal metastatic cascade, Cytoreductive surgery, Peritoneum, Hyperthermic intraperitoneal peroperative chemotherapy
Core tip: Colorectal peritoneal carcinomatosis (PC) is associated with poor prognosis and bad quality of life for patients in their terminal stages of disease. A loco-regional treatment, combining cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy, is associated with significant morbidity and mortality. Therefore, a comprehensive understanding of the molecular events involved in peritoneal disease spread and subsequent careful patient selection is paramount to avoid unnecessary toxicity. This manuscript highlights current data regarding the molecular mechanisms underlying the development of colorectal PC with a special focus on the peritoneum and the role of the surgeon in peritoneal disease spread.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of cancer-related death worldwide[1,2]. Besides the lymphatic and haematogenous routes of dissemination, CRC frequently gives rise to the transcoelomic spread of tumor cells in the peritoneal cavity, which ultimately leads to peritoneal carcinomatosis (PC)[3]. Colorectal PC is associated with a poor prognosis and bad quality of life for these patients in their terminal stages of disease[4-6]. Recent genomic profiling studies have demonstrated distinct gene expression patterns, determining CRC spreading to either the liver, the peritoneum or both[7,8].
The precise incidence of PC is unknown due to the low sensitivity of preoperative imaging techniques (CT, MRI, PET, PET/CT) and heterogeneity of published methods and findings[9,10]. Using a database of 3019 CRC patients, Jayne et al[11] reported that 8% of these patients presented with synchronous PC and 5% presented with metachronous disease. In a recent population-based cohort study from Stockholm County in Sweden, 4.3% of 11124 CRC patients were diagnosed with synchronous PC and 4% with metachronous PC[12]. In the synchronous group, more than 50% of patients presented with peritoneal metastases as the only site of tumor dissemination.
In the past, oncologists and surgeons assumed PC was identical to distant metastases and as such, regarded it as an incurable component of intra-abdominal malignancy only open to palliative treatment options. Systemic chemotherapy in patients with PC resulted in no long-term survival with a median overall survival of 15.2 mo and poor quality of life of these patients in their terminal stages of disease[13,14]. A new loco-regional treatment modality combining cytoreductive surgery (CRS) and hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) has demonstrated promising clinical results in patients with colorectal PC. In this setting, CRS aims to remove all macroscopic tumor through standardized peritonectomy procedures and multivisceral resections, whereas the subsequent intraoperative chemotherapy seeks to eliminate all residual microscopic tumor[15]. This novel approach has demonstrated encouraging clinical results in several phase II and III trials[16-18]. However, CRS and HIPEC are associated with a significant morbidity (grade III-IV complications) of approximately 34% and a 30-d mortality of 4%[19,20]. Therefore, careful patient selection is paramount to avoid unnecessary toxicity[21-23]. The aim of this manuscript is to review current data regarding the molecular mechanisms underlying the development of colorectal PC, with a special focus on the peritoneum.
THE PERITONEUM AS THE FIRST LINE OF DEFENCE IN PC
The peritoneum is the largest and most complex serous membrane of the human body[24]. The visceral peritoneum, covering the intra-abdominal organs and mesenteries, forms a continuous layer with the parietal peritoneum, which lines the abdominal wall and pelvic cavities[24]. It is composed of a monolayer of mesothelial cells supported by a basement membrane that rests on a layer of connective tissue, also referred to as the submesothelium (Figure 1)[25].
Figure 1.
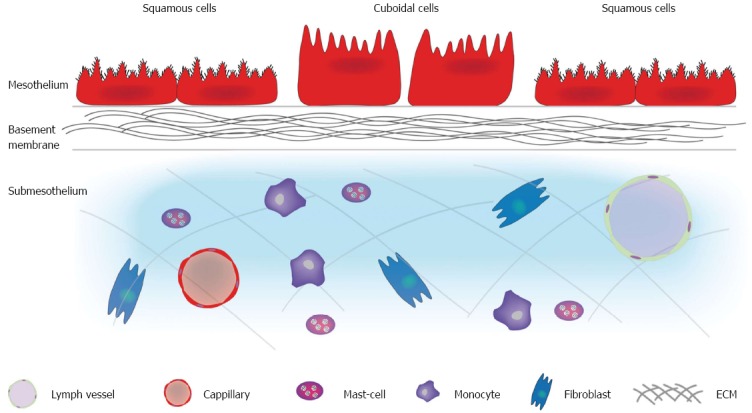
Structure of the peritoneum. The peritoneum is composed of a mesothelium supported by a basement membrane that rests on a layer of submesothelium. The mesothelium consists of a monolayer of either flattened, stretch, squamous-like or cuboidal mesothelial cells. The luminal surface of mesothelial cells has numerous microvilli varying in shape, size and density. Cilia have also been identified on the surface of resting mesothelial cells. The basement membrane consists of a thin laminar network containing type I and IV collagen, proteoglycans and glycoproteins. The submesothelium consists of a complex network of extracellular matrix made up of different types of collagen, glycoproteins, glycosaminoglycans and proteoglycans. Blood vessels, lymphatics, and various cells types (fibroblasts, resident tissue macrophages, and mast cells) are also found is this layer. ECM: Extracellular matrix.
The mesothelium consists of a monolayer of either flattened, stretched, squamous-like or cuboidal mesothelial cells. The latter can be found in various areas including the liver, the spleen, the “milky spots” of the omentum and the peritoneal side of the diaphragm overlying the lymphatic lacunae (cfr. Attachment to distant peritoneum)[26-28]. Cuboidal mesothelial cells are also observed within an injured mesothelium. Both squamous and cuboidal mesothelial cells can be distinguished by their ultrastructural differences. Squamous-like mesothelial cells contain few mitochondria, a poorly developed Golgi apparatus and little rough endoplasmatic reticulum (RER), which are located centrally near the round or oval nucleus[29]. Cuboidal mesothelial cells contain a central prominent nucleolus, abundant mitochondria and RER, a well developed Golgi apparatus, microtubules and microfilaments[30]. The luminal surface of mesothelial cells has numerous microvilli varying in shape, size and density; increasing the functional mesothelial surface area[31]. Cilia have also been identified on the surface of resting mesothelial cells[32]. The mesothelium functions as a dynamic layer that contributes substantially to the structural, functional, and homeostatic properties of the peritoneum[29]. The underlying basement membrane, a thin laminar network containing type I and IV collagen, proteoglycans and glycoproteins, acts as a selective barrier to macromolecules entering the submesothelial layer[29]. The submesothelium consists of a complex network of extracellular matrix (ECM) made up of different types of collagen, glycoproteins, glycosaminoglycans and proteoglycans. Blood vessels, lymphatics, and various cell types (fibroblasts, resident tissue macrophages, and mast cells) are also found in this layer[24,29].
The first major function of the peritoneum is facilitating transport of fluid and cells across the serosal cavities[33]. The microvilli on the luminal surface of the mesothelial cells play an important role in this process as they increase the surface area and bind fluids in their glycosaminoglycan-rich glycocalyx thereby aiding absorption[34]. Secondly, the peritoneum provides a slippery and non-adhesive surface to allow intracoelomic movement[35]. This slippery and non-adhesive surface is established by the secretion of a small amount of sterile fluid containing phosphatidylcholine produced by each mesothelial cell. Thirdly, the peritoneum acts as a first line of defence in host resistance[36,37]. The fourth function of the peritoneum involves tissue repair by releasing growth factors[38,39]. In conclusion, the peritoneum should be considered an organ with a structural and protective function for the contents of the abdominal cavity[24,25,40].
PATHOPHYSIOLOGY OF COLORECTAL PC
The emergence of PC is the result of a molecular crosstalk between tumor cells and host elements, comprising several well-defined steps. First, individual or clumps of tumor cells detach from the primary tumor and gain access to the peritoneal cavity. In the second step, these free tumor cells become susceptible to the regular peritoneal transport along predictable routes. The third step involves the attachment to the distant peritoneum where the tumor cells, during the fourth step, invade the subperitoneal space. The underlying connective tissue provides the necessary scaffold for tumor proliferation. The final step involves angiogenesis, which sustains tumor proliferation and enables further metastatic growth[41]. It is important to realise that these steps, known as the “peritoneal metastatic cascade”, do not necessarily occur in isolation, but rather describe a continuous and interdependent process (Figure 2, Table 1)[41].
Figure 2.
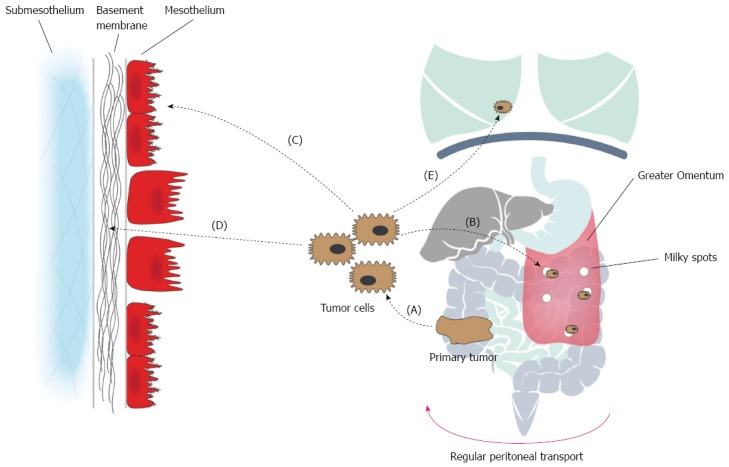
Pathophysiology of colorectal peritoneal carcinomatosis: the peritoneal metastatic cascade. The emergence of PC is the result of a molecular crosstalk between tumor cells and host elements, comprising several well-defined steps. A: Individual or clumps of tumor cells detach from the primary tumor and gain access to the peritoneal cavity. Spontaneous exfoliation of tumor cells from the primary tumor can be promoted by the down-regulation of E-cadherin, increased interstitial fluid pressure, and iatrogenically during surgery; B: The free tumor cells become susceptible to the regular peritoneal transport. Peritoneal transport is due to changes in the intra-abdominal pressure during respiration, gravity and peristalsis of the bowel; which results in a clockwise flow from the pelvis, along the right paracolic gutter and to the subdiaphragmatic space and finally towards the pelvis again; C: Attachment of tumor cells to distant peritoneum occurs via two processes, denominated transmesothelial and translymphatic metastasis. During transmesothelial metastasis, loose tumor cells directly adhere to distant mesothelium through adhesion molecules. During translymphatic metastasis, free tumor cells gain access to the submesothelial lymphatics through lymphatic stomata. Preferential tumor growth in the milky spots of the greater omentum has been observed; D: Tumor cells invade the submesothelium. In areas of absent or rounded (cuboidal) mesothelial cells, tumor cells interact with the laminar network of the basement membrane through integrin-mediated adhesion. Subsequent invasion of the submesothelial tissue occurs via degradation by proteases (MMPs); E: Systemic metastasis. PC: Peritoneal carcinomatosis.
Table 1.
General overview molecules/molecular pathways involved in the peritoneal metastatic cascade
| Steps peritoneal metastatic cascade | Molecules/molecular pathways |
| Detachment from the primary tumor | Spontaneous tumor cell shedding: |
| E-cadherin ↓ | |
| N-cadherin ↑ | |
| EMT | |
| PC1 and PC2 ↑ | |
| Interstitial fluid pressure ↑ | |
| Peroperative seeding tumor cells during surgery | |
| Peritoneal transport | Muscinous ascites |
| Actin microfilament system | |
| Lammelipodia, filipodia | |
| Attachment to distant peritoneum | Transmesothelial dissemination: |
| ICAM-1 ↑, PECAM-1, VCAM-1 ↑ | |
| TNF-α, IL-1β, IL-6, IFN-γ | |
| β1 integrin subunit | |
| CD43, CD44 | |
| Hyaluronan | |
| Translymphatic dissemination: | |
| Lymphatic stomata | |
| Milky spots | |
| Invasion into the subperitoneal space | Rounding of mesothelial cells: |
| HGF/SF ↑ | |
| c-Met ↑ | |
| Destruction of the mesothelial monolayer: | |
| Tumor-induced apoptosis | |
| Fas ligand/Fas | |
| Adherence to the basement membrane: | |
| Integrines | |
| Invasion of the peritoneal-blood barrier: | |
| MMP-1, MMP-2, MMP-7, MMP-9, MMP-13, MMP-14 ↑ | |
| TIMP-1, TIMP-2, TIMP-3, TIMP-4 | |
| uPA/uPAR | |
| plasminogen activator inhibitor -1 and -2 | |
| Proliferation and angiogenesis | Proliferation: |
| EGFR, EGF, TGFα | |
| IGF-1, IGF-Binding Protein-3 | |
| Angiogenesis: | |
| HIF-1α, HIF-1β | |
| VEGF/VEGFR |
E-cadherin: Epithelial-cadherin; N-cadherin: Neural-cadherin; EMT: Epithelial to mesenchyme transition; PC: Polycystin; ICAM: Intercellular adhesion molecule-1; PECAM: Platelet-endothelial cell adhesion molecule-1; VCAM-1: Vascular adhesion molecule-1; TNF-α: Tumor necrosis factor-α; IL-1β: Interleukin-1β; IL-6: Interleukin-6; IFN-γ: Interferon-γ; CD43: Sialophorin; HGF: Hepatocyte growth factor; SF: Scatter factor; MMP: Matrix metalloproteinases; TIMP: Tissue inhibitor metalloproteinases; uPA: Urokinase plasminogen activator; uPAR: Urokinase plasminogen activator receptor; EGFR: Epidermal growth factor receptor; EGF: Epidermal growth factor; TGFα: Tumor growth factor α; IGF-1: Insulin like growth factor-1; HIF: Hypoxia inducible factor; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor.
Detachment of tumor cells from the primary tumor
The metastatic pathway begins with the detachment of individual, or clumps of tumor cells from the primary tumor. This can be the result of spontaneous exfoliation of tumor cells from cancers that have invaded though the full thickness of the bowel wall and its investing serosa[41,42]. Serosal involvement of colon adenocarcinoma (pT4 stage) is an unfavourable independent prognostic marker for the development of PC[43-45]. Spontaneous exfoliation of malignant cells can be promoted by the down-regulation of intracellular adhesion molecules on the tumor cell surfaces, more specifically E (epithelial)-cadherin[46]. E-cadherin belongs to the type I subfamily of cadherins[47]. The general structure comprises an extracellular part, a membrane-spanning domain and a cytoplasmic tail. E-cadherin binds homotypically to E-cadherin on neighbouring cells through its Ca2+-dependent extracellular domains. The cytoplasmic tail associates with p120, α-, β-, and γ-catenin, which is responsible for the connection with the actin cytoskeleton and allows in- and outward signal transduction[46-49]. It has been confirmed that the down-regulation of E-cadherin expression levels is associated with dedifferentiation, progression, and metastasis of CRC[50,51]. This down-regulated tumor suppressor or metastasis suppressor function has also been reported in gastric[52,53] and ovarian cancer[54,55] with PC. Furthermore, reduction of cell-cell adherence, by the loss of E-cadherin, and the upregulation of mesenchymal N (neural)-cadherin are established hallmarks of the epithelial to mesenchyme transition (EMT)[56]. This reversible reprogramming process allows cells to separate, lose their apico-basal polarity typical of epithelial cells, demonstrate heightened resistance to apoptosis, and revert to a more motile mesenchymal phenotype[56]. This is believed to play a major role in the invasion and metastasis of tumor cells[49,57]. Gargalionis et al[58] report that overexpression of the epithelial polycystins PC1 and PC2 in a human colon carcinoma cell line, HCT116, is able to induce EMT-related alteration in E-cadherin, N-cadherin, Snail and Twist (EMT trigger) mRNA expression. PC2 exogenous expression was also found to increase cell migration[58]. PC1 and PC2 are both membrane-spanning proteins, which were first identified as mechanosensors involved in the pathophysiology of polycystic kidney disease[59]. PC1 is a mechanosensor with G-protein coupled receptor properties that perceives extracellular mechanical signals and translates them into biochemical responses[60-63]. PC2 constitutes a mechanosensitive Ca2+ channel[64-67]. Both receptors physically interact through their C-terminal, forming heterodimeric complexes at the cell membrane and at primary cilia[68,69].
Secondly, spontaneous tumor cell shedding from the primary tumor can also occur due to increased interstitial fluid pressure, a well-known phenomenon in solid tumors such as in CRC. Hayashi et al[70] reported that the pressure in the tumor is important, not only for the number of tumor cells shed but, also for the size of emboli shedding into lymphatics around the primary tumor. Interstitial hypertension can be the result of high osmotic pressure, increased vessel permeability and hyperperfusion, rapid cell proliferation, lack of effective lymphatic drainage, hyperplasia around blood vessels and increased production of ECM components[71].
Thirdly, peroperative seeding of viable tumor cells can also be induced iatrogenically during surgery, when the tumor is inadvertently ruptured, opened or cut into. Alternatively, the presence of tumor cells in the peritoneal cavity can be the result of transected lymphatics and blood vessels during the course of surgical resection[72].
Using peritoneal lavage cytology, several studies have aimed to assess the presence of free peritoneal tumor cells in gastric and CRC patients. They relate the presence of these cells to various clinical and pathological parameters, including locoregional recurrence rate and survival[73-77]. A wide range of 2.1% to 52% positive peritoneal cytology is reported across several studies, which reflects the heterogeneity of the techniques and the timing to detect malignant cells in peritoneal washes (Table 2)[45,76,78]. Lloyd et al[75] and Altomare et al[79] performed both pre and post resection peritoneal lavage cytology analysis using polymerase chain reaction. They both report a similar pre-resection rate of positive cytology, 12% to 14%, but differ in the post resection detection rate, 3%[79] and 20%[75]. Recently, Bae et al[76] performed peritoneal lavage cytology in patients with CRC without distant metastasis who underwent curative resection, immediately after making a midline abdominal incision and just before manipulation of the tumor. They reported a rate of positive cytology of 4.1%.
Table 2.
Intra-operative peritoneal lavage: detection method, timing and outcome data
| Ref. | Patients, n | Method of detection | Peritoneal lavage fluid | Timing of sampling | Intraperitoneal free cancer cells |
| Kirstensen et al[73] | 237 | PCR | 200-600 mL 0.9% NaCl | After | 8.01% |
| Nishikawa et al[74] | 410 | Cytology | 200 mL 0.9% NaCl | Before | 7.60% |
| Lloyd et al[75] | 125 | Immunobead RT-PCR | 100 mL 0.9% NaCl | Before | 12.80% |
| After | 29.60% | ||||
| Bae et al[76] | 145 | Cytology | 100 mL 0.9% NaCl | Before | 4.10% |
| Noura et al[77] | 697 | Cytology | 100 mL 0.9% NaCl | Before | 2.20% |
| Altomare et al[79] | 29 | Thin-Prep | 150 mL 0.9% NaCl | Before | 13.80% |
| After | 2.60% | ||||
| RT-PCR | 150 mL 0.9% NaCl | Before | 37.90% | ||
| After | 41.40% | ||||
| Rossi Del Monte et al[78] | 48 | Cytology | 250 mL 0.9% NaCl | Before | 0.00% |
| Immunofluorescence | 250 mL 0.9% NaCl | Before | 17.00% | ||
| qRT-PCR | 250 mL 0.9% NaCl | Before | 42.00% |
PCR: Polymerase chain reaction; RT-PCR: Real-time polymerase chain reaction; qRT-PCR: Quantitative real-time polymerase chain reaction.
Peritoneal transport of tumor cells
Once the tumor cells have been detached from the primary tumor and seeded in the peritoneal cavity, they become susceptible to the regular peritoneal transport. Previously, it was assumed that intraperitoneal cancer dissemination was a random process independent of the physical and biological properties of the tumor and the host[80]. However, several studies report that the direction taken by the loose tumor cells and their ultimate destination are dependent on the anatomic site of the primary tumor and the continued cephalic circulation responsible for the clearance of fluid from the peritoneal cavity[81-83]. The latter is due to changes in intra-abdominal pressure during respiration, gravity and peristalsis of the bowel; which results in a clockwise flow from the pelvis, along the right paracolic gutter and to the subdiaphragmatic space and finally towards the pelvis again[40,81]. As a result, certain areas of the peritoneal cavity; the subphrenic region, lesser sac, mesentery, diaphragm and the paracolic gutters, will have an increased risk of occurrence of metastases[80]. Carmignani et al[81] and Hugen et al[82] report the presence of mucinous ascites to be a prominent facilitator of widespread intraperitoneal cancer distribution with colonic mucinous adenocarcinoma and mucinous colonic adenocarcinoma having different peritoneal surface distribution patterns. The presence of peritoneal adhesions and fibrin entrapment resulting from a surgical trauma are also influencing factors in peritoneal transport. Moreover, during the EMT, malignant tumor cells gain migratory and invasive properties that involve a dramatic reorganisation and activity of the actin microfilament system resulting in the formation of actin-rich membrane protrusions: lammelipodia and filipodia. This process is stimulated by pathological expression of growth factors, their receptors and signalling intermediates, which are the products of proto-oncogenes[56,84].
Attachment to the distant peritoneum
The ultimate destination of intraperitoneal dissemination depends not only on the physical and biological properties of the free tumor cells but also on the tissue that will harbour the mestastatic implantation. The attachment of free CRC cells to the distant peritoneum can occur via two processes, denominated transmesothelial and translymphatic mestastasis.
During transmesothelial dissemination, loose tumor cells directly adhere to the distant mesothelium, the innermost layer of the peritoneum. The possible role of adhesion molecules in tumor-mesothelial interactions has been investigated, based on parallels drawn between the mesothelial cell and the endothelial cell[47]. The mesothelium expresses a distinct pattern of adhesion molecules, which are known to play an important role in leukocyte traffic during peritoneal inflammation and are believed to be exploited by invading tumor cells during the peritoneal metastatic cascade[29].
Mesothelial cells express adhesion molecules belonging to the Immunoglobulin Superfamily: intercellular adhesion molecule-1 (ICAM-1), platelet-endothelial cell adhesion molecule-1 (PECAM-1) and vascular adhesion molecule-1 (VCAM-1)[85-87]. Several pro-inflammatory cytokines released following surgery or secreted by circulating tumor cells [tumor necrosis factor-α, interleukin (IL)-1β, IL-6 and interferon-γ] are known to cause a beneficial environment for the tumor-mesothelial interactions[88-93]. These cytokines enhance the expression of the adhesion molecules, ICAM-1 and VCAM-1, on mesothelial cells and induce the contraction of mesothelial cells, thereby exposing the basement membrane[94]. In these areas of absent or rounded mesothelial cells, the interaction between the tumor cells and the laminar network of the basement membrane is mediated through the β1 integrin subunit[95-97]. In an in vitro study, Ziprin et al[98] demonstrated tumor-mesothelial adhesion by an interaction between mesothelial ICAM-1 and tumor expressed CD43 (sialophorin).
The glycosaminoglycan, hyaluronan, is secreted by the mesothelial cells and subsequently assembled into a pericellular coat[37]. First, the hyaluronan coat protects the mesothelium from vital infections and the cytotoxic effect of lymphocytes. Secondly, hyaluronan is involved in tumor-mesothelial adhesion through the interaction with tumor expressed CD44[29,99]. This interaction is an important step in the peritoneal metastatic cascade of ovarian, colon and CRC[100,101]. CD44 is a cell surface glycoprotein, which is widely expressed in several non-neoplastic cells as well as neoplastic cells and is involved in migration of cells, homotypic and heterotypic cell-cell adhesion. The cd44 gene is composed of 20 exons, 10 of which are variably expressed[102,103]. The smallest and most abundant isoform is the standard form, CD44s. Alternative splicing of 10 variant exons, which account for sequences located in the extracellular part of the CD44, results in the expression of CD44v1 up to CD44v10[104]. The variant isoforms CD44v3 and CD44v6 are believed to play a role in the metastatic cascade of CRC. Expression of CD44v6 is largely restricted to the advanced stages (T3/T4) of CRC and was higher in metastatic cancer than in nonmetastatic cancer[102,105]. Saito et al[106] investigated the clinical importance of CD44s and CD44v6 and their relevance to EMT in 113 patients with stage II/III CRC treated with curative surgery. They report that high expression of CD44v6 is an independent poor prognostic factor for disease-free survival and overall survival.
During translymphatic dissemination, loose tumor cells gain access to the submesothelial lymphatics through openings at the junction of two or more mesothelial cells, the lymphatic stomata. Lymphatic stomata are small openings of lymphatic capillaries, which are involved in immunoregulation but most importantly serve as drainage channels for active absorption of fluids and cells from the serous cavities. They can be found in the greater omentum, appendices epiploicae of the colon, the peritoneal side of the diaphragm, falciform ligament, Douglas pouch and the small bowel mesentery[107]. Specialized structures, called “milky spots”, are also found in these anatomical regions, distributed around the lymphatic stomata. Milky spots are immunocompetent cell aggregates, which resorb peritoneal fluid through their lymphatic stomata and mainly serve as gateways for and providers of macrophages for the abdominal cavity[108-110]. They play a role in the formation of PC as they provide a highly vascular microenvironment, which permits early survival of circulating tumor cells. The production of VEGF by the mesothelium in the milky spots also promotes angiogenesis, contributing to preferential tumor growth in the milky spots[111]. However, the precise mechanisms are not well understood. Lopes Cardozo et al[112] investigated the spread of the syngeneic CC531 colon cancer cells in Wag/Rij rats, after inoculation in the abdominal cavity. They observed tumor cells in the milky spots of the greater omentum within 4 h after intraperitoneal inoculation, demonstrating preferential tumor growth in these immune aggregates.
Invasion into the subperitoneal space
For adhered tumor cells to invade the subperitoneal space, they must first penetrate the mesothelial monolayer. This can occur either at areas of peritoneal discontinuity, by invading the intercellular spaces between adjacent rounded mesothelial cells or by destroying the monolayer.
Rounding of mesothelial cells occurs in response to several pro-inflammatory cytokines, thereby exposing the basement membrane[97]. Hepatocyte growth factor/scatter factor (HGF/SF) produced by mesothelial cells induces detachment, motility and proliferation of these cells in the process of mesothelial wound repair[39]. Binding of HGF to its tyrosine kinase receptor, encoded by the c-MET proto-oncogene, initiates an invasive growth program[113]. This program is required during embryonic development for tissue and organ morphogenesis, but is exploited by tumor cells to promote invasive and metastatic ability[114,115]. Sawada et al[116] already reported that overexpression of c-Met is a prognostic factor in ovarian cancer and targeting this receptor in cultured ovarian carcinoma cells inhibited peritoneal dissemination through an α5β1 integrin-dependent mechanism. In CRC, Osada et al[117] investigated the effect of HGF on progression of liver metastasis. They reported that malignancy with high c-Met expression and under high level of HGF, led to unfavourable patient prognosis and poor survival. The presence of malignant ascites was also reported to contain factors, which induce changes in the morphology of the mesothelial cells, resulting in the separation of cell-cell contacts and the subsequent establishment of the characteristic round morphology[94,118].
Destruction of the mesothelial monolayer can occur through tumor-induced apoptosis. Using a three-dimensional in vitro model of the human peritoneum, Jayne et al[94] demonstrated that CRC cell lines rapidly adhered to the outer mesothelial monolayer. The majority of the adhered tumor cells displayed proliferative growth on the mesothelial surface without invasion. A proportion of the tumor cells invaded the mesothelium, which was characterized by apoptosis of the mesothelial cells involving membrane blebbing, cell shrinkage and nuclear fragmentation. Heath et al[119] explored the role of the death ligand/receptor system, Fas Ligand/Fas, in the process of apoptosis using human mesothelial cells co-cultured in vitro with the SW480 colonic cancer cell line. They demonstrated that invasion of the peritoneal mesothelium occurs via tumor-induced mesothelial apoptosis, at least in part mediated by a Fas-dependent mechanism.
After penetrating the mesothelium, the tumor cells adhere to the basement membrane through integrin-mediated adhesion. Integrins are calcium/magnesium-dependent heterodimer molecules, consisting of an α and a β subunit, located on the cell membrane. They are involved in both homotypic cell-cell and heterotypic cell-ECM adhesion and mediate in- and outward signal transduction to the actin cytoskeleton via cytoplasmic proteins[47].
Subsequent invasion of the peritoneal-blood barrier, the submesothelial tissue between the peritoneal mesothelium and the submesothelial arterial blood capillaries, occurs via degradation by proteases[120]. Tumor cells, mesothelial cells, surrounding fibroblasts, inflammatory cells and macrophages secrete matrix metalloproteinases (MMPs), which are responsible for the degradation of several ECM components[121]. Destruction of the peritoneal-blood barrier by these enzymes results from a disturbed equilibrium between the activation of pro- MMPs and their inhibition by tissue inhibitor metalloproteinases (TIMPs)[47]. In CRC, increased levels of MMP-1, MMP-2, MMP-7, MMP-9, MMP-13 and MMP-14 have been reported to play a role in the formation of PC. The MMPs are a family of zinc- and calcium dependent multifunctional enzymes currently comprising 23 members in humans, either membrane-anchored or secreted[122,123]. Many MMPs have overlapping substrate specificity and are involved in a network of mutual activation by MMPs and plasmin activation (Table 3)[124]. Four types of TIMPs (TIMP-1 - TIMP-4) have been reported, which control the activity of the MMPs[125-129].
Table 3.
Overview matrix metalloproteinases[124]
| MMP | Name | Producing Cells | Substrates |
| MMP-1 | Collagenase | Fibroblasts, synovial cells, chondrocyte | Collagen I, II, III, X |
| MMP-2 | Gelatinase | Fibroblasts, chrondrocyte, mesangium | Gelatin, collagen IV, V, VII, XI |
| endothelial cells, cancer cells | Laminin, fibronectin, elastin | ||
| MMP-3 | Stromelysin-1 | Synovial cells, chondrocyte, fibroblasts | Proteoglycan, collagen III, IV, VII, IX, elastin |
| MMP-7 | Matrilysin | Cancer cells, macrophage | Proteoglycan, gelatin, fibronectin |
| elastin, collagen IV, laminin | |||
| MMP-8 | Neutrophil, Collagenase | Neutrophil | Collagen I, II, III |
| MMP-9 | Gelatinase B | Neutrophil, macrophage, thromboblast | Gelatin, collagen III, IV, V |
| osteoclast, cancer cells, T-lymphocyte | α2 chain, Elastin | ||
| MMP-10 | Stromelysin-2 | Cancer cells, T-lymphocyte | Collagen III, IV, V, fibronectin, gelatin |
| MMP-11 | Stromelysin-3 | Cancer cells, macrophage, mesangium | Fibronectin, laminin, proteoglycan, gelatin |
| MMP-12 | Metalloestelase | Macrophage | Elastin |
| MMP-13 | Collagenase-3 | Chondrocyte, cancer cells | Collagen I, II, III |
| MMP-14 | MT1-MMP | Cancer cells, fibroblasts | Collagen I, II, III, gelatin, Laminin |
| Fibronectin, vitronectin, proteoglycan | |||
| MMP-15 | MT2-MMP | Cancer cells, fibroblasts | Fibronectin, aggrecan, tenascin |
| MMP-16 | MT3-MMP | Neuronal cell | Collagen III, gelatin, fibronectin |
| MMP-17 | MT4-MMP | Unknown | Unknown |
| MMP-20 | Enamelysin | Odontoblast | Amelogenin, gelatin |
| MMP-24 | MT5-MMP | Unknown | Unknown |
| MMP-25 | MT6-MMP | Unknown | Unknown |
| TIMP-1 | Tissue, extracellular fluid | Complex formation with pro-MMP-9 and MMPs | |
| TIMP-2 | Tissue, extracellular fluid | Complex formation with pro-MMP-9, inhibition of MMP-2 degradation |
MMP: Matrix metalloproteinases; TIMP: Tissue inhibitor metalloproteinases.
Elevated expression of MMP1 has been reported in several studies to be correlated with metastasis, reduced overall and/or disease-free survival[129-132]. However, some controversy exists regarding the role of MMP-1. Hettiaratchi et al[133] published a study, including 503 CRC patients and 471 healthy individuals, demonstrating that a single nucleotide polymorphism in the mmp-1 gene promoter resulted in a significantly improved 5-year survival rate.
Groblewska et al[134] suggested that MMP-2 and TIMP-2 play a role in the process of CRC invasion and metastasis. They conducted a study with 72 CRC patients and 68 healthy individuals to assess the serum levels and tissue expression of MMP-2 and TIMP2. MMP-9 has been implicated in the progression, invasion and metastasis of CRC[135,136]. In a study conducted by Alkhamesi et al[137], bidirectional signalling was reported between mesothelial cells and tumor cells in generating cancer invasion. They demonstrated that the interaction of ICAM with its ligand, CD43, plays a vital role in both peritoneal adhesion of tumor cells and the preparation of the right environment for subsequent invasion by increasing the production of MMPs (MMP-2 and MMP-9).
MMP-7 is the smallest member of the MMP family and has been proposed to fulfil a dual role in the progression of peritoneal metastases. On the one hand, MMP-7 can have a potential role in tumor invasion and metastasis by degrading basement membrane and submesothelial components. On the other hand, MMP-7 can promote the development and progression of tumor cells by inhibiting tumor cell apoptosis, decreasing cell adhesion and inducing angiogenesis[138].
Yamada et al[139] reported MMP-13 as a useful predictor of liver metastasis in patients diagnosed with CRC.
Another mediator in the degradation of peritoneal-blood barrier is the urokinase plasminogen activating system, consisting of the urokinase plasminogen activator receptor (uPAR) and the urokinase plasminogen activator (uPA). uPA is a serine protease, which upon activation of the pro-enzyme (pro-uPA) catalyses the reaction in which plasminogen is converted to plasmin. Plasmin is in turn responsible for the degradation of several ECM components and the activation of pro-MMPs[140,141]. The catalytic activity of uPA is controlled by its inhibitors, plasminogen activator inhibitor-1 and plasminogen activator inhibitor-2, through the formation of an enzymatically inactive, trimeric receptor-protease-inhibitor complex[142]. Seetoo et al[143] investigated the expression levels of PAS in a series of human CRC tissues and correlated these results with patient outcome. They report uPA and uPAR to be possible independent predictors of liver metastasis, patient overall survival and cancer-specific survival after resection of colorectal tumors.
Proliferation and angiogenesis
A known hallmark of malignant tumor cells is their ability to trigger proliferation. Sustained proliferation is achieved through the production of growth factors and their receptors by tumor cells and their associated stromal cells, inducing autocrine and paracrine loops[144]. Both the epidermal growth factor receptor (EGFR) and the insulin like growth factor-1 (IGF-1) have been reported to be involved in this process[7,145].
EGFR belongs to the ErbB cell surface receptor family and can be activated by several ligands including EGF and TGFα[146]. Binding of its ligand results in homo- or heterodimerization of various ErbB family members, followed by internalisation of the EFGR receptor complex. Upon autophosphorylation of the EGRF tyrosine kinase domains in the cytoplasmic tails, a transduction signalling cascade is initiated, which in turn regulates tumor cell proliferation, differentiation and survival[147,148]. Yonemura et al[149] reported that gastric tumors with synchronous expression of EGF and EGFR had the highest malignant potential, causing autocrine secretions for self-replication. Ziober et al[150] conducted an in vitro assay with CRC cell lines and demonstrated that TGFα activated autocrine circuits with its receptor, EGFR, and determined growth factor independence of CRC cells[150,151]. Tampellini et al[152] reported that co-expression of immunoreactive EGFR and TGFα was significantly higher in CRC with distant metastases at diagnosis than in CRC presenting at a lower tumor stage.
IGF-1 and its transmembrane receptor are part of a family of cellular modulators that are important in the regulation of growth and development[153]. Varghese et al[7] performed microarray analyses on tumors from patients with CRC metastasized to either the liver or the peritoneum. IGF-1 was exclusively upregulated in tumor samples of patients with peritoneal metastases. Further evidence of the involvement of IGF-1 was provided by Fuchs et al[154]. They reported that increased plasma levels of IGF-Binding Protein-3, an endogenous antagonist of IGF-1, were associated with improved treatment response to first-line chemotherapy and a prolonged time to cancer progression.
Angiogenesis, the growth of new blood vessels from pre-existing vessels, is paramount for tumor growth and the formation of metastases. For their survival, tumor cells rely on the delivery of oxygen from pre-existing blood vessels and nutrients by the recruitment of stromal cells. However, when these tumor cells are located more than 150 μm from the submesothelial capillaries, oxygen and nutrients will not be able to pass the peritoneal-blood barrier resulting in hypoxia-induced apoptosis[155]. Therefore, angiogenesis is induced through the production of angiogenic factors by tumor cells[156,157]. Key players in this process are hypoxia inducible factor-1 (HIF-1) and VEGF.
HIF-1 is a heterodimeric protein composed of HIF-1α and HIF-1β, which activates the transcription of genes involved in the induction of angiogenesis, including VEGF[158,159]. HIF-1β is constitutively expressed and does therefore not depend on the hypoxic status of the cells. The expression of HIF-1α increases exponentially as oxygen levels decline in the cell[160]. In the same study discussed above, Varghese et al[7] reported that HIF-1α was upregulated only in tumor samples from peritoneal metastases. Greijer et al[161] investigated the expression of HIF-1 in normal colorectal mucosa, adenomas and carcinomas. They observed upregulation of HIF-1α in colorectal adenomas and carcinomas. Using immunohistochemistry, Wu et al[162] investigated the clinicopathologic significance of HIF-1 expression in primary human colon cancer. High expression levels correlated positively with an advanced TNM stage and were associated with increased metastatic potential[162].
The VEGF family constitutes five structurally related proteins, VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor. VEGF-C and VEGF-D are important in the process of lymphangiogenesis, while VEGF-A, VEGF-B and placental growth factor are important in neovascularization[163-166]. The most potent pro-angiogenic growth factor, VEGF-A binds to its receptors VEGFR-1 and VEGFR-2 and thereby increases endothelial cell survival, proliferation, migration and differentiation[167,168]. VEGF-A displays sensitivity to hypoxia and its expression in growing tissue is regulated by HIF[169]. In a study conducted by Shaheen et al[170], nude mice were injected with KM12L4 human colon cancer cells to generate PC. The simultaneous blockage of the VEGF and the EGF receptors with anti-VEGFR (DC101) and anti-EGFR (C225) resulted in decreased tumor vascularity, growth, proliferation, formation of ascites and increased apoptosis of both tumor cells and endothelial cells. Using immunohistochemistry, Logan-Collins et al[171] investigated tumor samples from patients undergoing CRS and intraperitoneal hyperthermic perfusion for mucinous adenocarcinoma of appendiceal or colonic origin. They reported that overall survival was better in patients with low VEGF expression than in patients with high VEGF expression.
ROLE OF THE SURGEON IN PERITONEAL DISEASE SPREAD
The above-mentioned pathophysiology describes the formation of PC as a continuous and interdependent process, known as the peritoneal mestastatic cascade. The surgeon plays a dual role in this process, as promotor of PC by breaching the mesothelium during surgery but at the same time as opponent of PC by performing CRS and HIPEC.
The role of the surgeon as promotor of PC was first described by Sugarbaker[22] in what they called the “tumor cell entrapment” hypothesis. This hypothesis explains the rapid peritoneal disease progression in patients who have undergone surgery as sole treatment. In this setting, the surgeon is responsible for: (1) peroperative seeding of malignant tumor cells originating from transected lymphatics and blood vessels; and/or (2) the dissemination of malignant cells when the tumor is inadvertently ruptured, opened or cut into[25,72]; (3) The peritonectomized surfaces and areas where the peritoneal barrier is disrupted during the course of surgical dissection, provide a favourable niche for the re-implantation of these free tumor cells[96]. The tumor cells become entrapped in the local fibrin deposition present on the traumatised peritoneal surfaces, where they can progress in the presence of growth factors involved in wound healing[172,173]. The fibrin is infiltrated by platelets, neutrophils and monocytes as part of this wound healing process. Using a rat model, van den Tol et al[174] reported that growth of intraperitoneally administered rat colon carcinoma cells was enhanced when injected together with lavage fluid from intra-abdominally traumatized animals. Lee et al[175] investigated whether the wound healing response after an abdominal incision leads to locally increased MMP-9 activity, thereby contributing to peritoneal metastasis. Using a murine model, they concluded that wound-associated inflammation enhances pro-MMP-9 expression. This in turn plays a key role in the growth and progression of tumor cells associated with peritoneal metastases[176].
Port-site recurrences or recurrences situated at extraction site skin incisions have been established concerns since the introduction of diagnostic or cancer laparoscopy[177,178]. First, port-site recurrences are abdominal wall recurrences that occur in the subcutaneous tissue as result of slipping of trocars as well as poor extraction techniques[179,180]. Second, the increase in intraperitoneal pressure during laparoscopy promotes tumor invasiveness and tumor growth via a protease-determined pathway. Using an in vitro experiment, Paraskeva et al[181] reported that the exposure of a human colon carcinoma cell line to a laparoscopic environment enhances the production of MMP-2, MMP-9 and uPA.
The surgeon’s role as preventer of PC involves the administration of intraperitoneal chemotherapy following CRS during the peroperative period. Today’s treatment of colorectal PC involves the combined treatment modality of CRS and HIPEC. The rationale behind the use of HIPEC after CRS is to treat viable circulating tumor cells, residual microscopic lesions, and to eliminate viable platelets, leukocytes and monocytes from the peritoneal cavity. The latter reduces the promotion of tumor growth at traumatized peritoneal surfaces during the wound healing process. Combining CRS and HIPEC for the treatment of colorectal PC has demonstrated encouraging clinical results in several phase II en III trials[16-18]. In a multicentre French trial, Elias et al[182] report an overall 1-year, 3-year and 5-year survival rate of 81%, 41% and 27%.
CONCLUSION
PC is the result of a complex molecular crosstalk between cancer cells and host elements, comprising several well-defined steps, known as the peritoneal metastatic cascade. Individual or clumps of tumor cells detach from the primary tumor, gain access to the peritoneal cavity and become susceptible to the regular peritoneal transport. They attach to distant peritoneum, invade the subperitoneal space, where angiogenesis sustains proliferation and enables further metastatic growth. It is important to realise that these molecular events do not necessarily occur in isolation, but rather describe a continuous and interdependent process. Current treatment combines CRS and HIPEC. This approach is associated with significant morbidity and mortality. A comprehensive understanding of the molecular events involved in peritoneal disease spread, subsequent careful patient selection and knowledgeable management of peritoneal surface metastases is therefore paramount to avoid unnecessary toxicity.
ACKNOWLEDGMENTS
Professor Yutaka Yonemura for granting his permission to use his overview of matrix metalloproteinnases, their producing cells and substrates[124]. Professor Lars Grieten for the illustrations.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Belgium
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Authors declare no conflict of interest for this article.
Peer-review started: April 28, 2016
First decision: June 20, 2016
Article in press: August 1, 2016
P- Reviewer: Nowacki M, Sommariva A S- Editor: Yu J L- Editor: A E- Editor: Ma S
References
- 1.Arjona-Sánchez A, Medina-Fernández FJ, Muñoz-Casares FC, Casado-Adam A, Sánchez-Hidalgo JM, Rufián-Peña S. Peritoneal metastases of colorectal origin treated by cytoreduction and HIPEC: An overview. World J Gastrointest Oncol. 2014;6:407–412. doi: 10.4251/wjgo.v6.i10.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 3.Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243:212–222. doi: 10.1097/01.sla.0000197702.46394.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoyagi T, Terracina KP, Raza A, Takabe K. Current treatment options for colon cancer peritoneal carcinomatosis. World J Gastroenterol. 2014;20:12493–12500. doi: 10.3748/wjg.v20.i35.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Easson AM, Bezjak A, Ross S, Wright JG. The ability of existing questionnaires to measure symptom change after paracentesis for symptomatic ascites. Ann Surg Oncol. 2007;14:2348–2357. doi: 10.1245/s10434-007-9370-3. [DOI] [PubMed] [Google Scholar]
- 6.Passot G, Bakrin N, Roux AS, Vaudoyer D, Gilly FN, Glehen O, Cotte E. Quality of life after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy: a prospective study of 216 patients. Eur J Surg Oncol. 2014;40:529–535. doi: 10.1016/j.ejso.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Varghese S, Burness M, Xu H, Beresnev T, Pingpank J, Alexander HR. Site-specific gene expression profiles and novel molecular prognostic factors in patients with lower gastrointestinal adenocarcinoma diffusely metastatic to liver or peritoneum. Ann Surg Oncol. 2007;14:3460–3471. doi: 10.1245/s10434-007-9557-7. [DOI] [PubMed] [Google Scholar]
- 8.Xie T, Cho YB, Wang K, Huang D, Hong HK, Choi YL, Ko YH, Nam DH, Jin J, Yang H, et al. Patterns of somatic alterations between matched primary and metastatic colorectal tumors characterized by whole-genome sequencing. Genomics. 2014;104:234–241. doi: 10.1016/j.ygeno.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Klaver YL, Lemmens VE, Nienhuijs SW, Luyer MD, de Hingh IH. Peritoneal carcinomatosis of colorectal origin: Incidence, prognosis and treatment options. World J Gastroenterol. 2012;18:5489–5494. doi: 10.3748/wjg.v18.i39.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasqual EM, Bertozzi S, Bacchetti S, Londero AP, Basso SM, Santeufemia DA, Lo Re G, Lumachi F. Preoperative assessment of peritoneal carcinomatosis in patients undergoing hyperthermic intraperitoneal chemotherapy following cytoreductive surgery. Anticancer Res. 2014;34:2363–2368. [PubMed] [Google Scholar]
- 11.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 12.Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99:699–705. doi: 10.1002/bjs.8679. [DOI] [PubMed] [Google Scholar]
- 13.Tol J, Koopman M, Rodenburg CJ, Cats A, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, Mol L, Antonini NF, et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol. 2008;19:734–738. doi: 10.1093/annonc/mdm607. [DOI] [PubMed] [Google Scholar]
- 14.Franko JSQ, Meyers JP, Helnemann V, Falcone A, Tebbutt NC, Maughan T, Seymour M, Saltz L, Tournigand C, Diaz-Rubio E, et al. Prognostic value of isolated peritoneal versus other metastatic sites in colorectal cancer patients treated by systemic chemotherapy: Findings from 9,265 patients in the ARCAD database. Am Soc Clin Oncol Educ Book. 2016;34 Suppl 4:abstr 656. [Google Scholar]
- 15.Mirnezami R, Mehta AM, Chandrakumaran K, Cecil T, Moran BJ, Carr N, Verwaal VJ, Mohamed F, Mirnezami AH. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br J Cancer. 2014;111:1500–1508. doi: 10.1038/bjc.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 17.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 18.Glehen O, Mithieux F, Osinsky D, Beaujard AC, Freyer G, Guertsch P, Francois Y, Peyrat P, Panteix G, Vignal J, et al. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol. 2003;21:799–806. doi: 10.1200/JCO.2003.06.139. [DOI] [PubMed] [Google Scholar]
- 19.Kuijpers AM, Mirck B, Aalbers AG, Nienhuijs SW, de Hingh IH, Wiezer MJ, van Ramshorst B, van Ginkel RJ, Havenga K, Bremers AJ, et al. Cytoreduction and HIPEC in the Netherlands: nationwide long-term outcome following the Dutch protocol. Ann Surg Oncol. 2013;20:4224–4230. doi: 10.1245/s10434-013-3145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S, Elias D. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608–5618. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]
- 21.Kwakman R, de Cuba EM, de Winter JP, de Hingh IH, Delis-van Diemen PM, Tijssen M, Rooimans MA, Krijgsman O, Carvalho B, Peters GJ, et al. Tailoring heated intraperitoneal mitomycin C for peritoneal metastases originating from colorectal carcinoma: a translational approach to improve survival. Br J Cancer. 2015;112:851–856. doi: 10.1038/bjc.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugarbaker PH. Management of peritoneal-surface malignancy: the surgeon’s role. Langenbecks Arch Surg. 1999;384:576–587. doi: 10.1007/s004230050246. [DOI] [PubMed] [Google Scholar]
- 23.LaRocca CJ, Tuttle TM. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for colorectal cancer: choosing the right candidates. Expert Rev Anticancer Ther. 2015;15:859–861. doi: 10.1586/14737140.2015.1069187. [DOI] [PubMed] [Google Scholar]
- 24.van der Wal JB, Jeekel J. Biology of the peritoneum in normal homeostasis and after surgical trauma. Colorectal Dis. 2007;9 Suppl 2:9–13. doi: 10.1111/j.1463-1318.2007.01345.x. [DOI] [PubMed] [Google Scholar]
- 25.Sugarbaker PH. Peritoneum as the first-line of defense in carcinomatosis. J Surg Oncol. 2007;95:93–96. doi: 10.1002/jso.20676. [DOI] [PubMed] [Google Scholar]
- 26.Tsilibary EC, Wissig SL. Absorption from the peritoneal cavity: SEM study of the mesothelium covering the peritoneal surface of the muscular portion of the diaphragm. Am J Anat. 1977;149:127–133. doi: 10.1002/aja.1001490111. [DOI] [PubMed] [Google Scholar]
- 27.Mironov VA, Gusev SA, Baradi AF. Mesothelial stomata overlying omental milky spots: scanning electron microscopic study. Cell Tissue Res. 1979;201:327–330. doi: 10.1007/BF00235068. [DOI] [PubMed] [Google Scholar]
- 28.Michailova K, Wassilev W, Wedel T. Scanning and transmission electron microscopic study of visceral and parietal peritoneal regions in the rat. Ann Anat. 1999;181:253–260. doi: 10.1016/S0940-9602(99)80040-5. [DOI] [PubMed] [Google Scholar]
- 29.Mutsaers SE, Wilkosz S. Structure and function of mesothelial cells. Cancer Treat Res. 2007;134:1–19. doi: 10.1007/978-0-387-48993-3_1. [DOI] [PubMed] [Google Scholar]
- 30.Dobbie JW. Morphology of the peritoneum in CAPD. Blood Purif. 1989;7:74–85. doi: 10.1159/000169580. [DOI] [PubMed] [Google Scholar]
- 31.Mutsaers SE, Whitaker D, Papadimitriou JM. Changes in the concentration of microvilli on the free surface of healing mesothelium are associated with alterations in surface membrane charge. J Pathol. 1996;180:333–339. doi: 10.1002/(SICI)1096-9896(199611)180:3<333::AID-PATH659>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 32.Bird SD. Mesothelial primary cilia of peritoneal and other serosal surfaces. Cell Biol Int. 2004;28:151–159. doi: 10.1016/j.cellbi.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Fedorko ME, Hirsch JG, Fried B. Studies on transport of macromolecules and small particles across mesothelial cells of the mouse omentum. II. Kinetic features and metabolic requirements. Exp Cell Res. 1971;69:313–323. doi: 10.1016/0014-4827(71)90230-8. [DOI] [PubMed] [Google Scholar]
- 34.Wang NS. The regional difference of pleural mesothelial cells in rabbits. Am Rev Respir Dis. 1974;110:623–633. doi: 10.1164/arrd.1974.110.5.623. [DOI] [PubMed] [Google Scholar]
- 35.Dobbie JW, Pavlina T, Lloyd J, Johnson RC. Phosphatidylcholine synthesis by peritoneal mesothelium: its implications for peritoneal dialysis. Am J Kidney Dis. 1988;12:31–36. doi: 10.1016/s0272-6386(88)80068-4. [DOI] [PubMed] [Google Scholar]
- 36.Topley N. The host’s initial response to peritoneal infection: the pivotal role of the mesothelial cell. Perit Dial Int. 1995;15:116–117. [PubMed] [Google Scholar]
- 37.Heldin P, Pertoft H. Synthesis and assembly of the hyaluronan-containing coats around normal human mesothelial cells. Exp Cell Res. 1993;208:422–429. doi: 10.1006/excr.1993.1264. [DOI] [PubMed] [Google Scholar]
- 38.Mutsaers SE, Bishop JE, McGrouther G, Laurent GJ. Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol. 1997;29:5–17. doi: 10.1016/s1357-2725(96)00115-x. [DOI] [PubMed] [Google Scholar]
- 39.Warn R, Harvey P, Warn A, Foley-Comer A, Heldin P, Versnel M, Arakaki N, Daikuhara Y, Laurent GJ, Herrick SE, et al. HGF/SF induces mesothelial cell migration and proliferation by autocrine and paracrine pathways. Exp Cell Res. 2001;267:258–266. doi: 10.1006/excr.2001.5240. [DOI] [PubMed] [Google Scholar]
- 40.Wasnik AP, Maturen KE, Kaza RK, Al-Hawary MM, Francis IR. Primary and secondary disease of the peritoneum and mesentery: review of anatomy and imaging features. Abdom Imaging. 2015;40:626–642. doi: 10.1007/s00261-014-0232-8. [DOI] [PubMed] [Google Scholar]
- 41.Jayne D. Molecular biology of peritoneal carcinomatosis. Cancer Treat Res. 2007;134:21–33. doi: 10.1007/978-0-387-48993-3_2. [DOI] [PubMed] [Google Scholar]
- 42.Koppe MJ, Nagtegaal ID, de Wilt JH, Ceelen WP. Recent insights into the pathophysiology of omental metastases. J Surg Oncol. 2014;110:670–675. doi: 10.1002/jso.23681. [DOI] [PubMed] [Google Scholar]
- 43.Keshava A, Chapuis PH, Chan C, Lin BP, Bokey EL, Dent OF. The significance of involvement of a free serosal surface for recurrence and survival following resection of clinicopathological stage B and C rectal cancer. Colorectal Dis. 2007;9:609–618. doi: 10.1111/j.1463-1318.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- 44.Stewart CJ, Morris M, de Boer B, Iacopetta B. Identification of serosal invasion and extramural venous invasion on review of Dukes’ stage B colonic carcinomas and correlation with survival. Histopathology. 2007;51:372–378. doi: 10.1111/j.1365-2559.2007.02787.x. [DOI] [PubMed] [Google Scholar]
- 45.Honoré C, Goéré D, Souadka A, Dumont F, Elias D. Definition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann Surg Oncol. 2013;20:183–192. doi: 10.1245/s10434-012-2473-5. [DOI] [PubMed] [Google Scholar]
- 46.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bracke ME. Role of adhesion molecules in locoregional cancer spread. Cancer Treat Res. 2007;134:35–49. doi: 10.1007/978-0-387-48993-3_3. [DOI] [PubMed] [Google Scholar]
- 48.Cavallaro U, Liebner S, Dejana E. Endothelial cadherins and tumor angiogenesis. Exp Cell Res. 2006;312:659–667. doi: 10.1016/j.yexcr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 49.Bodenstine TM, Welch DR. Metastasis suppressors and the tumor microenvironment. Cancer Microenviron. 2008;1:1–11. doi: 10.1007/s12307-008-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dorudi S, Sheffield JP, Poulsom R, Northover JM, Hart IR. E-cadherin expression in colorectal cancer. An immunocytochemical and in situ hybridization study. Am J Pathol. 1993;142:981–986. [PMC free article] [PubMed] [Google Scholar]
- 51.Pocard M, Debruyne P, Bras-Gonçalves R, Mareel M, Dutrillaux B, Poupon MF. Single alteration of p53 or E-cadherin genes can alter the surgical resection benefit in an experimental model of colon cancer. Dis Colon Rectum. 2001;44:1106–1112. doi: 10.1007/BF02234630. [DOI] [PubMed] [Google Scholar]
- 52.Hippo Y, Yashiro M, Ishii M, Taniguchi H, Tsutsumi S, Hirakawa K, Kodama T, Aburatani H. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 2001;61:889–895. [PubMed] [Google Scholar]
- 53.Yonemura Y, Nojima N, Kaji M, Fujimura T, Itoh H, Ninomiya I, Miyazaki I, Endo Y, Sasaki T. E-cadherin and urokinase-type plasminogen activator tissue status in gastric carcinoma. Cancer. 1995;76:941–953. doi: 10.1002/1097-0142(19950915)76:6<941::aid-cncr2820760606>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 54.Veatch AL, Carson LF, Ramakrishnan S. Differential expression of the cell-cell adhesion molecule E-cadherin in ascites and solid human ovarian tumor cells. Int J Cancer. 1994;58:393–399. doi: 10.1002/ijc.2910580315. [DOI] [PubMed] [Google Scholar]
- 55.Elloul S, Elstrand MB, Nesland JM, Tropé CG, Kvalheim G, Goldberg I, Reich R, Davidson B. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103:1631–1643. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- 56.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 57.Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179:56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- 58.Gargalionis AN, Korkolopoulou P, Farmaki E, Piperi C, Dalagiorgou G, Adamopoulos C, Levidou G, Saetta A, Fragkou P, Tsioli P, et al. Polycystin-1 and polycystin-2 are involved in the acquisition of aggressive phenotypes in colorectal cancer. Int J Cancer. 2015;136:1515–1527. doi: 10.1002/ijc.29140. [DOI] [PubMed] [Google Scholar]
- 59.Ibraghimov-Beskrovnaya O, Natoli TA. mTOR signaling in polycystic kidney disease. Trends Mol Med. 2011;17:625–633. doi: 10.1016/j.molmed.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 60.The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. The European Polycystic Kidney Disease Consortium. Cell. 1994;78:725. [PubMed] [Google Scholar]
- 61.Nims N, Vassmer D, Maser RL. Transmembrane domain analysis of polycystin-1, the product of the polycystic kidney disease-1 (PKD1) gene: evidence for 11 membrane-spanning domains. Biochemistry. 2003;42:13035–13048. doi: 10.1021/bi035074c. [DOI] [PubMed] [Google Scholar]
- 62.Dalagiorgou G, Basdra EK, Papavassiliou AG. Polycystin-1: function as a mechanosensor. Int J Biochem Cell Biol. 2010;42:1610–1613. doi: 10.1016/j.biocel.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Parnell SC, Magenheimer BS, Maser RL, Rankin CA, Smine A, Okamoto T, Calvet JP. The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem Biophys Res Commun. 1998;251:625–631. doi: 10.1006/bbrc.1998.9514. [DOI] [PubMed] [Google Scholar]
- 64.Hoffmeister H, Gallagher AR, Rascle A, Witzgall R. The human polycystin-2 protein represents an integral membrane protein with six membrane-spanning domains and intracellular N- and C-termini. Biochem J. 2011;433:285–294. doi: 10.1042/BJ20101141. [DOI] [PubMed] [Google Scholar]
- 65.Köttgen M, Walz G. Subcellular localization and trafficking of polycystins. Pflugers Arch. 2005;451:286–293. doi: 10.1007/s00424-005-1417-3. [DOI] [PubMed] [Google Scholar]
- 66.Scheffers MS, Le H, van der Bent P, Leonhard W, Prins F, Spruit L, Breuning MH, de Heer E, Peters DJ. Distinct subcellular expression of endogenous polycystin-2 in the plasma membrane and Golgi apparatus of MDCK cells. Hum Mol Genet. 2002;11:59–67. doi: 10.1093/hmg/11.1.59. [DOI] [PubMed] [Google Scholar]
- 67.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 68.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 69.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 70.Hayashi K, Jiang P, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res. 2007;67:8223–8228. doi: 10.1158/0008-5472.CAN-07-1237. [DOI] [PubMed] [Google Scholar]
- 71.Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 72.Hansen E, Wolff N, Knuechel R, Ruschoff J, Hofstaedter F, Taeger K. Tumor cells in blood shed from the surgical field. Arch Surg. 1995;130:387–393. doi: 10.1001/archsurg.1995.01430040049007. [DOI] [PubMed] [Google Scholar]
- 73.Kristensen AT, Wiig JN, Larsen SG, Giercksky KE, Ekstrøm PO. Molecular detection (k-ras) of exfoliated tumour cells in the pelvis is a prognostic factor after resection of rectal cancer? BMC Cancer. 2008;8:213. doi: 10.1186/1471-2407-8-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishikawa T, Watanabe T, Sunami E, Tsuno NH, Kitayama J, Nagawa H. Prognostic value of peritoneal cytology and the combination of peritoneal cytology and peritoneal dissemination in colorectal cancer. Dis Colon Rectum. 2009;52:2016–2021. doi: 10.1007/DCR.0b013e3181b4c46e. [DOI] [PubMed] [Google Scholar]
- 75.Lloyd JM, McIver CM, Stephenson SA, Hewett PJ, Rieger N, Hardingham JE. Identification of early-stage colorectal cancer patients at risk of relapse post-resection by immunobead reverse transcription-PCR analysis of peritoneal lavage fluid for malignant cells. Clin Cancer Res. 2006;12:417–423. doi: 10.1158/1078-0432.CCR-05-1473. [DOI] [PubMed] [Google Scholar]
- 76.Bae SJ, Shin US, Ki YJ, Cho SS, Moon SM, Park SH. Role of peritoneal lavage cytology and prediction of prognosis and peritoneal recurrence after curative surgery for colorectal cancer. Ann Coloproctol. 2014;30:266–273. doi: 10.3393/ac.2014.30.6.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Noura S, Ohue M, Seki Y, Yano M, Ishikawa O, Kameyama M. Long-term prognostic value of conventional peritoneal lavage cytology in patients undergoing curative colorectal cancer resection. Dis Colon Rectum. 2009;52:1312–1320. doi: 10.1007/DCR.0b013e3181a745a4. [DOI] [PubMed] [Google Scholar]
- 78.Rossi Del Monte S, Ranieri D, Mazzetta F, Kazemi Nava A, Raffa S, Torrisi MR, Ziparo V. Free peritoneal tumor cells detection in gastric and colorectal cancer patients. J Surg Oncol. 2012;106:17–23. doi: 10.1002/jso.23052. [DOI] [PubMed] [Google Scholar]
- 79.Altomare DF, Tedeschi M, Rotelli MT, Bocale D, Piscitelli D, Rinaldi M. Lack of prognostic role of pre- and postoperative peritoneal cytology and cytokeratin PCR-expression on local recurrence after curative anterior resection for mid-low rectal cancer. Updates Surg. 2011;63:109–113. doi: 10.1007/s13304-011-0071-x. [DOI] [PubMed] [Google Scholar]
- 80.Sugarbaker PH. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Treat Res. 1996;82:79–100. doi: 10.1007/978-1-4613-1247-5_6. [DOI] [PubMed] [Google Scholar]
- 81.Carmignani CP, Sugarbaker TA, Bromley CM, Sugarbaker PH. Intraperitoneal cancer dissemination: mechanisms of the patterns of spread. Cancer Metastasis Rev. 2003;22:465–472. doi: 10.1023/a:1023791229361. [DOI] [PubMed] [Google Scholar]
- 82.Hugen N, van de Velde CJ, de Wilt JH, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25:651–657. doi: 10.1093/annonc/mdt591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Catalano V, Loupakis F, Graziano F, Torresi U, Bisonni R, Mari D, Fornaro L, Baldelli AM, Giordani P, Rossi D, et al. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer. 2009;100:881–887. doi: 10.1038/sj.bjc.6604955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lindberg U, Karlsson R, Lassing I, Schutt CE, Höglund AS. The microfilament system and malignancy. Semin Cancer Biol. 2008;18:2–11. doi: 10.1016/j.semcancer.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 85.Jonjić N, Peri G, Bernasconi S, Sciacca FL, Colotta F, Pelicci P, Lanfrancone L, Mantovani A. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J Exp Med. 1992;176:1165–1174. doi: 10.1084/jem.176.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klein CL, Bittinger F, Skarke CC, Wagner M, Köhler H, Walgenbach S, Kirkpatrick CJ. Effects of cytokines on the expression of cell adhesion molecules by cultured human omental mesothelial cells. Pathobiology. 1995;63:204–212. doi: 10.1159/000163953. [DOI] [PubMed] [Google Scholar]
- 87.Müller J, Yoshida T. Interaction of murine peritoneal leukocytes and mesothelial cells: in vitro model system to survey cellular events on serosal membranes during inflammation. Clin Immunol Immunopathol. 1995;75:231–238. doi: 10.1006/clin.1995.1076. [DOI] [PubMed] [Google Scholar]
- 88.Alkhamesi NA, Ziprin P, Pfistermuller K, Peck DH, Darzi AW. ICAM-1 mediated peritoneal carcinomatosis, a target for therapeutic intervention. Clin Exp Metastasis. 2005;22:449–459. doi: 10.1007/s10585-005-2893-8. [DOI] [PubMed] [Google Scholar]
- 89.Ksiazek K, Mikuła-Pietrasik J, Catar R, Dworacki G, Winckiewicz M, Frydrychowicz M, Dragun D, Staniszewski R, Jörres A, Witowski J. Oxidative stress-dependent increase in ICAM-1 expression promotes adhesion of colorectal and pancreatic cancers to the senescent peritoneal mesothelium. Int J Cancer. 2010;127:293–303. doi: 10.1002/ijc.25036. [DOI] [PubMed] [Google Scholar]
- 90.Slack-Davis JK, Atkins KA, Harrer C, Hershey ED, Conaway M. Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer Res. 2009;69:1469–1476. doi: 10.1158/0008-5472.CAN-08-2678. [DOI] [PubMed] [Google Scholar]
- 91.van Grevenstein WM, Hofland LJ, Jeekel J, van Eijck CH. The expression of adhesion molecules and the influence of inflammatory cytokines on the adhesion of human pancreatic carcinoma cells to mesothelial monolayers. Pancreas. 2006;32:396–402. doi: 10.1097/01.mpa.0000220865.80034.2a. [DOI] [PubMed] [Google Scholar]
- 92.van Rossen ME, Hofland LJ, van den Tol MP, van Koetsveld PM, Jeekel J, Marquet RL, van Eijck CH. Effect of inflammatory cytokines and growth factors on tumour cell adhesion to the peritoneum. J Pathol. 2001;193:530–537. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH805>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 93.Ziprin P, Ridgway PF, Pfistermüller KL, Peck DH, Darzi AW. ICAM-1 mediated tumor-mesothelial cell adhesion is modulated by IL-6 and TNF-alpha: a potential mechanism by which surgical trauma increases peritoneal metastases. Cell Commun Adhes. 2003;10:141–154. [PubMed] [Google Scholar]
- 94.Jayne DG, O’Leary R, Gill A, Hick A, Guillou PJ. A three-dimensional in-vitro model for the study of peritoneal tumour metastasis. Clin Exp Metastasis. 1999;17:515–523. doi: 10.1023/a:1006606006878. [DOI] [PubMed] [Google Scholar]
- 95.Takatsuki H, Komatsu S, Sano R, Takada Y, Tsuji T. Adhesion of gastric carcinoma cells to peritoneum mediated by alpha3beta1 integrin (VLA-3) Cancer Res. 2004;64:6065–6070. doi: 10.1158/0008-5472.CAN-04-0321. [DOI] [PubMed] [Google Scholar]
- 96.van Grevenstein WM, Hofland LJ, van Rossen ME, van Koetsveld PM, Jeekel J, van Eijck CH. Inflammatory cytokines stimulate the adhesion of colon carcinoma cells to mesothelial monolayers. Dig Dis Sci. 2007;52:2775–2783. doi: 10.1007/s10620-007-9778-4. [DOI] [PubMed] [Google Scholar]
- 97.Yonemura Y, Endou Y, Nojima M, Kawamura T, Fujita H, Kaji M, Ajisaka H, Bandou E, Sasaki T, Yamaguchi T, et al. A possible role of cytokines in the formation of peritoneal dissemination. Int J Oncol. 1997;11:349–358. doi: 10.3892/ijo.11.2.349. [DOI] [PubMed] [Google Scholar]
- 98.Ziprin P, Alkhamesi NA, Ridgway PF, Peck DH, Darzi AW. Tumour-expressed CD43 (sialophorin) mediates tumourmesothelial cell adhesion. Biol Chem. 2004;385:755–761. doi: 10.1515/BC.2004.092. [DOI] [PubMed] [Google Scholar]
- 99.Harada N, Mizoi T, Kinouchi M, Hoshi K, Ishii S, Shiiba K, Sasaki I, Matsuno S. Introduction of antisense CD44S CDNA down-regulates expression of overall CD44 isoforms and inhibits tumor growth and metastasis in highly metastatic colon carcinoma cells. Int J Cancer. 2001;91:67–75. doi: 10.1002/1097-0215(20010101)91:1<67::aid-ijc1011>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 100.Li CZ, Liu B, Wen ZQ, Li HY. Inhibition of CD44 expression by small interfering RNA to suppress the growth and metastasis of ovarian cancer cells in vitro and in vivo. Folia Biol (Praha) 2008;54:180–186. [PubMed] [Google Scholar]
- 101.Ween MP, Oehler MK, Ricciardelli C. Role of versican, hyaluronan and CD44 in ovarian cancer metastasis. Int J Mol Sci. 2011;12:1009–1029. doi: 10.3390/ijms12021009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fujisaki T, Tanaka Y, Fujii K, Mine S, Saito K, Yamada S, Yamashita U, Irimura T, Eto S. CD44 stimulation induces integrin-mediated adhesion of colon cancer cell lines to endothelial cells by up-regulation of integrins and c-Met and activation of integrins. Cancer Res. 1999;59:4427–4434. [PubMed] [Google Scholar]
- 103.Zhao LH, Lin QL, Wei J, Huai YL, Wang KJ, Yan HY. CD44v6 expression in patients with stage II or stage III sporadic colorectal cancer is superior to CD44 expression for predicting progression. Int J Clin Exp Pathol. 2015;8:692–701. [PMC free article] [PubMed] [Google Scholar]
- 104.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA. 1992;89:12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wielenga VJ, Heider KH, Offerhaus GJ, Adolf GR, van den Berg FM, Ponta H, Herrlich P, Pals ST. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993;53:4754–4756. [PubMed] [Google Scholar]
- 106.Saito S, Okabe H, Watanabe M, Ishimoto T, Iwatsuki M, Baba Y, Tanaka Y, Kurashige J, Miyamoto Y, Baba H. CD44v6 expression is related to mesenchymal phenotype and poor prognosis in patients with colorectal cancer. Oncol Rep. 2013;29:1570–1578. doi: 10.3892/or.2013.2273. [DOI] [PubMed] [Google Scholar]
- 107.Wang ZB, Li M, Li JC. Recent advances in the research of lymphatic stomata. Anat Rec (Hoboken) 2010;293:754–761. doi: 10.1002/ar.21101. [DOI] [PubMed] [Google Scholar]
- 108.Krist LF, Eestermans IL, Steenbergen JJ, Hoefsmit EC, Cuesta MA, Meyer S, Beelen RH. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995;241:163–174. doi: 10.1002/ar.1092410204. [DOI] [PubMed] [Google Scholar]
- 109.Shimotsuma M, Shields JW, Simpson-Morgan MW, Sakuyama A, Shirasu M, Hagiwara A, Takahashi T. Morpho-physiological function and role of omental milky spots as omentum-associated lymphoid tissue (OALT) in the peritoneal cavity. Lymphology. 1993;26:90–101. [PubMed] [Google Scholar]
- 110.Cao L, Hu X, Zhang Y, Sun XT. Omental milky spots in screening gastric cancer stem cells. Neoplasma. 2011;58:20–26. doi: 10.4149/neo_2011_01_20. [DOI] [PubMed] [Google Scholar]
- 111.Gerber SA, Rybalko VY, Bigelow CE, Lugade AA, Foster TH, Frelinger JG, Lord EM. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathol. 2006;169:1739–1752. doi: 10.2353/ajpath.2006.051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lopes Cardozo AM, Gupta A, Koppe MJ, Meijer S, van Leeuwen PA, Beelen RJ, Bleichrodt RP. Metastatic pattern of CC531 colon carcinoma cells in the abdominal cavity: an experimental model of peritoneal carcinomatosis in rats. Eur J Surg Oncol. 2001;27:359–363. doi: 10.1053/ejso.2001.1117. [DOI] [PubMed] [Google Scholar]
- 113.Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003;22:309–325. doi: 10.1023/a:1023768811842. [DOI] [PubMed] [Google Scholar]
- 114.Gentile A, D’Alessandro L, Lazzari L, Martinoglio B, Bertotti A, Mira A, Lanzetti L, Comoglio PM, Medico E. Met-driven invasive growth involves transcriptional regulation of Arhgap12. Oncogene. 2008;27:5590–5598. doi: 10.1038/onc.2008.173. [DOI] [PubMed] [Google Scholar]
- 115.Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637–645. doi: 10.1038/nrc1912. [DOI] [PubMed] [Google Scholar]
- 116.Sawada K, Radjabi AR, Shinomiya N, Kistner E, Kenny H, Becker AR, Turkyilmaz MA, Salgia R, Yamada SD, Vande Woude GF, et al. c-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res. 2007;67:1670–1679. doi: 10.1158/0008-5472.CAN-06-1147. [DOI] [PubMed] [Google Scholar]
- 117.Osada S, Matsui S, Komori S, Yamada J, Sanada Y, Ihawa A, Tanaka Y, Tokuyama Y, Okumura N, Nonaka K, et al. Effect of hepatocyte growth factor on progression of liver metastasis in colorectal cancer. Hepatogastroenterology. 2010;57:76–80. [PubMed] [Google Scholar]
- 118.Akedo H, Shinkai K, Mukai M, Mori Y, Tateishi R, Tanaka K, Yamamoto R, Morishita T. Interaction of rat ascites hepatoma cells with cultured mesothelial cell layers: a model for tumor invasion. Cancer Res. 1986;46:2416–2422. [PubMed] [Google Scholar]
- 119.Heath RM, Jayne DG, O’Leary R, Morrison EE, Guillou PJ. Tumour-induced apoptosis in human mesothelial cells: a mechanism of peritoneal invasion by Fas Ligand/Fas interaction. Br J Cancer. 2004;90:1437–1442. doi: 10.1038/sj.bjc.6601635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yonemura Y, Endou Y, Yamaguchi T, Nojima N, Kawamura T, Fujimura T, Obata T, Kim B, Miyazaki I, Sasaki T. Roles of VLA-2 and VLA-3 on the formation of peritoneal dissemination in gastric cancer. Int J Oncol. 1996;8:925–931. doi: 10.3892/ijo.8.5.925. [DOI] [PubMed] [Google Scholar]
- 121.Kataoka H, Tanaka H, Nagaike K, Uchiyama S, Itoh H. Role of cancer cell-stroma interaction in invasive growth of cancer cells. Hum Cell. 2003;16:1–14. doi: 10.1111/j.1749-0774.2003.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 122.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 123.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 124.Yonemura Y, Endo Y, Yamaguchi T, Fujimura T, Obata T, Kawamura T, Nojima N, Miyazaki I, Sasaki T. Mechanisms of the formation of the peritoneal dissemination in gastric cancer. Int J Oncol. 1996;8:795–802. doi: 10.3892/ijo.8.4.795. [DOI] [PubMed] [Google Scholar]
- 125.Garcia-Albeniz X, Pericay C, Alonso-Espinaco V, Alonso V, Escudero P, Fernández-Martos C, Gallego R, Gascón P, Castellví-Bel S, Maurel J. Serum matrilysin correlates with poor survival independently of KRAS and BRAF status in refractory advanced colorectal cancer patients treated with irinotecan plus cetuximab. Tumour Biol. 2011;32:417–424. doi: 10.1007/s13277-010-0136-3. [DOI] [PubMed] [Google Scholar]
- 126.Hilska M, Roberts PJ, Collan YU, Laine VJ, Kössi J, Hirsimäki P, Rahkonen O, Laato M. Prognostic significance of matrix metalloproteinases-1, -2, -7 and -13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in colorectal cancer. Int J Cancer. 2007;121:714–723. doi: 10.1002/ijc.22747. [DOI] [PubMed] [Google Scholar]
- 127.Koskensalo S, Louhimo J, Nordling S, Hagström J, Haglund C. MMP-7 as a prognostic marker in colorectal cancer. Tumour Biol. 2011;32:259–264. doi: 10.1007/s13277-010-0080-2. [DOI] [PubMed] [Google Scholar]
- 128.Ring P, Johansson K, Höyhtyä M, Rubin K, Lindmark G. Expression of tissue inhibitor of metalloproteinases TIMP-2 in human colorectal cancer--a predictor of tumour stage. Br J Cancer. 1997;76:805–811. doi: 10.1038/bjc.1997.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tahara K, Mimori K, Iinuma H, Iwatsuki M, Yokobori T, Ishii H, Anai H, Kitano S, Mori M. Serum matrix-metalloproteinase-1 is a bona fide prognostic marker for colorectal cancer. Ann Surg Oncol. 2010;17:3362–3369. doi: 10.1245/s10434-010-1149-2. [DOI] [PubMed] [Google Scholar]
- 130.Murray GI, Duncan ME, O’Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2:461–462. doi: 10.1038/nm0496–461. [DOI] [PubMed] [Google Scholar]
- 131.Sunami E, Tsuno N, Osada T, Saito S, Kitayama J, Tomozawa S, Tsuruo T, Shibata Y, Muto T, Nagawa H. MMP-1 is a prognostic marker for hematogenous metastasis of colorectal cancer. Oncologist. 2000;5:108–114. doi: 10.1634/theoncologist.5-2-108. [DOI] [PubMed] [Google Scholar]
- 132.Sun Y, Wenger L, Brinckerhoff CE, Misra RR, Cheung HS. Basic calcium phosphate crystals induce matrix metalloproteinase-1 through the Ras/mitogen-activated protein kinase/c-Fos/AP-1/metalloproteinase 1 pathway. Involvement of transcription factor binding sites AP-1 and PEA-3. J Biol Chem. 2002;277:1544–1552. doi: 10.1074/jbc.M100567200. [DOI] [PubMed] [Google Scholar]
- 133.Hettiaratchi A, Hawkins NJ, McKenzie G, Ward RL, Hunt JE, Wakefield D, Di Girolamo N. The collagenase-1 (MMP-1) gene promoter polymorphism - 1607/2G is associated with favourable prognosis in patients with colorectal cancer. Br J Cancer. 2007;96:783–792. doi: 10.1038/sj.bjc.6603630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Groblewska M, Mroczko B, Gryko M, Pryczynicz A, Guzińska-Ustymowicz K, Kędra B, Kemona A, Szmitkowski M. Serum levels and tissue expression of matrix metalloproteinase 2 (MMP-2) and tissue inhibitor of metalloproteinases 2 (TIMP-2) in colorectal cancer patients. Tumour Biol. 2014;35:3793–3802. doi: 10.1007/s13277-013-1502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li CY, Yuan P, Lin SS, Song CF, Guan WY, Yuan L, Lai RB, Gao Y, Wang Y. Matrix metalloproteinase 9 expression and prognosis in colorectal cancer: a meta-analysis. Tumour Biol. 2013;34:735–741. doi: 10.1007/s13277-012-0601-2. [DOI] [PubMed] [Google Scholar]
- 136.Illemann M, Bird N, Majeed A, Sehested M, Laerum OD, Lund LR, Danø K, Nielsen BS. MMP-9 is differentially expressed in primary human colorectal adenocarcinomas and their metastases. Mol Cancer Res. 2006;4:293–302. doi: 10.1158/1541-7786.MCR-06-0003. [DOI] [PubMed] [Google Scholar]
- 137.Alkhamesi NA, Roberts G, Ziprin P, Peck DH. Induction of Proteases in Peritoneal Carcinomatosis, the Role of ICAM-1/CD43 Interaction. Biomark Insights. 2007;2:377–384. [PMC free article] [PubMed] [Google Scholar]
- 138.Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 2006;231:20–27. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- 139.Yamada T, Oshima T, Yoshihara K, Tamura S, Kanazawa A, Inagaki D, Yamamoto N, Sato T, Fujii S, Numata K, et al. Overexpression of MMP-13 gene in colorectal cancer with liver metastasis. Anticancer Res. 2010;30:2693–2699. [PubMed] [Google Scholar]
- 140.Kim TD, Song KS, Li G, Choi H, Park HD, Lim K, Hwang BD, Yoon WH. Activity and expression of urokinase-type plasminogen activator and matrix metalloproteinases in human colorectal cancer. BMC Cancer. 2006;6:211. doi: 10.1186/1471-2407-6-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Klinge U, Rosch R, Junge K, Krones CJ, Stumpf M, Lynen-Jansen P, Mertens PR, Schumpelick V. Different matrix micro-environments in colon cancer and diverticular disease. Int J Colorectal Dis. 2007;22:515–520. doi: 10.1007/s00384-006-0199-1. [DOI] [PubMed] [Google Scholar]
- 142.Fujii T, Obara T, Tanno S, Ura H, Kohgo Y. Urokinase-type plasminogen activator and plasminogen activator inhibitor-1 as a prognostic factor in human colorectal carcinomas. Hepatogastroenterology. 1999;46:2299–2308. [PubMed] [Google Scholar]
- 143.Seetoo DQ, Crowe PJ, Russell PJ, Yang JL. Quantitative expression of protein markers of plasminogen activation system in prognosis of colorectal cancer. J Surg Oncol. 2003;82:184–193. doi: 10.1002/jso.10210. [DOI] [PubMed] [Google Scholar]
- 144.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Davies DE, Farmer S, White J, Senior PV, Warnes SL, Alexander P. Contribution of host-derived growth factors to in vivo growth of a transplantable murine mammary carcinoma. Br J Cancer. 1994;70:263–269. doi: 10.1038/bjc.1994.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 147.Franklin WA, Veve R, Hirsch FR, Helfrich BA, Bunn PA. Epidermal growth factor receptor family in lung cancer and premalignancy. Semin Oncol. 2002;29:3–14. doi: 10.1053/sonc.2002.31520. [DOI] [PubMed] [Google Scholar]
- 148.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 149.Yonemura Y, Sugiyama K, Fushida S, Kamata T, Ohoyama S, Kimura H, Yamaguchi A, Miyazaki I. Tissue status of epidermal growth factor and its receptor as an indicator of poor prognosis in patients with gastric cancer. Anal Cell Pathol. 1991;3:343–350. [PubMed] [Google Scholar]
- 150.Ziober BL, Willson JK, Hymphrey LE, Childress-Fields K, Brattain MG. Autocrine transforming growth factor-alpha is associated with progression of transformed properties in human colon cancer cells. J Biol Chem. 1993;268:691–698. [PubMed] [Google Scholar]
- 151.Awwad RA, Sergina N, Yang H, Ziober B, Willson JK, Zborowska E, Humphrey LE, Fan R, Ko TC, Brattain MG, et al. The role of transforming growth factor alpha in determining growth factor independence. Cancer Res. 2003;63:4731–4738. [PubMed] [Google Scholar]
- 152.Tampellini M, Longo M, Cappia S, Bacillo E, Alabiso I, Volante M, Dogliotti L, Papotti M. Co-expression of EGF receptor, TGFalpha and S6 kinase is significantly associated with colorectal carcinomas with distant metastases at diagnosis. Virchows Arch. 2007;450:321–328. doi: 10.1007/s00428-007-0370-2. [DOI] [PubMed] [Google Scholar]
- 153.LeRoith D, Werner H, Beitner-Johnson D, Roberts CT. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 154.Fuchs CS, Goldberg RM, Sargent DJ, Meyerhardt JA, Wolpin BM, Green EM, Pitot HC, Pollak M. Plasma insulin-like growth factors, insulin-like binding protein-3, and outcome in metastatic colorectal cancer: results from intergroup trial N9741. Clin Cancer Res. 2008;14:8263–8269. doi: 10.1158/1078-0432.CCR-08-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Olcina MM, Leszczynska KB, Senra JM, Isa NF, Harada H, Hammond EM. H3K9me3 facilitates hypoxia-induced p53-dependent apoptosis through repression of APAK. Oncogene. 2016;35:793–799. doi: 10.1038/onc.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier. Cancer Treat Res. 1996;82:53–63. doi: 10.1007/978-1-4613-1247-5_4. [DOI] [PubMed] [Google Scholar]
- 157.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 158.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 159.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 160.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 161.Greijer AE, Delis-van Diemen PM, Fijneman RJ, Giles RH, Voest EE, van Hinsbergh VW, Meijer GA. Presence of HIF-1 and related genes in normal mucosa, adenomas and carcinomas of the colorectum. Virchows Arch. 2008;452:535–544. doi: 10.1007/s00428-008-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu Y, Jin M, Xu H, Shimin Z, He S, Wang L, Zhang Y. Clinicopathologic significance of HIF-1α, CXCR4, and VEGF expression in colon cancer. Clin Dev Immunol. 2010;2010 doi: 10.1155/2010/537531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rosa M, Abdelbaqi M, Bui KM, Nasir A, Bui MM, Shibata D, Coppola D. Overexpression of Vascular Endothelial Growth Factor A in Invasive Micropapillary Colorectal Carcinoma. Cancer Control. 2015;22:206–210. doi: 10.1177/107327481502200212. [DOI] [PubMed] [Google Scholar]
- 164.Yang X, Zhang Y, Hosaka K, Andersson P, Wang J, Tholander F, Cao Z, Morikawa H, Tegnér J, Yang Y, et al. VEGF-B promotes cancer metastasis through a VEGF-A-independent mechanism and serves as a marker of poor prognosis for cancer patients. Proc Natl Acad Sci USA. 2015;112:E2900–E2909. doi: 10.1073/pnas.1503500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen H, Guan R, Lei Y, Chen J, Ge Q, Zhang X, Dou R, Chen H, Liu H, Qi X, et al. Lymphangiogenesis in gastric cancer regulated through Akt/mTOR-VEGF-C/VEGF-D axis. BMC Cancer. 2015;15:103. doi: 10.1186/s12885-015-1109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Funaki H, Nishimura G, Harada S, Ninomiya I, Terada I, Fushida S, Tani T, Fujimura T, Kayahara M, Shimizu K, et al. Expression of vascular endothelial growth factor D is associated with lymph node metastasis in human colorectal carcinoma. Oncology. 2003;64:416–422. doi: 10.1159/000070301. [DOI] [PubMed] [Google Scholar]
- 167.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.De Falco S, Gigante B, Persico MG. Structure and function of placental growth factor. Trends Cardiovasc Med. 2002;12:241–246. doi: 10.1016/s1050-1738(02)00168-8. [DOI] [PubMed] [Google Scholar]
- 169.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 170.Shaheen RM, Ahmad SA, Liu W, Reinmuth N, Jung YD, Tseng WW, Drazan KE, Bucana CD, Hicklin DJ, Ellis LM. Inhibited growth of colon cancer carcinomatosis by antibodies to vascular endothelial and epidermal growth factor receptors. Br J Cancer. 2001;85:584–589. doi: 10.1054/bjoc.2001.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Logan-Collins JM, Lowy AM, Robinson-Smith TM, Kumar S, Sussman JJ, James LE, Ahmad SA. VEGF expression predicts survival in patients with peritoneal surface metastases from mucinous adenocarcinoma of the appendix and colon. Ann Surg Oncol. 2008;15:738–744. doi: 10.1245/s10434-007-9699-7. [DOI] [PubMed] [Google Scholar]
- 172.Zeamari S, Roos E, Stewart FA. Tumour seeding in peritoneal wound sites in relation to growth-factor expression in early granulation tissue. Eur J Cancer. 2004;40:1431–1440. doi: 10.1016/j.ejca.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 173.Sugarbaker PH. A perspective on clinical research strategies in carcinoma of the large bowel. World J Surg. 1991;15:609–616. doi: 10.1007/BF01789207. [DOI] [PubMed] [Google Scholar]
- 174.van den Tol PM, van Rossen EE, van Eijck CH, Bonthuis F, Marquet RL, Jeekel H. Reduction of peritoneal trauma by using nonsurgical gauze leads to less implantation metastasis of spilled tumor cells. Ann Surg. 1998;227:242–248. doi: 10.1097/00000658-199802000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee IK, Vansaun MN, Shim JH, Matrisian LM, Gorden DL. Increased metastases are associated with inflammation and matrix metalloproteinase-9 activity at incision sites in a murine model of peritoneal dissemination of colorectal cancer. J Surg Res. 2013;180:252–259. doi: 10.1016/j.jss.2012.04.074. [DOI] [PubMed] [Google Scholar]
- 176.Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta. 2004;1705:69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 177.Savalgi RS. Port-site metastasis in the abdominal wall: fact or fiction? Semin Surg Oncol. 1998;15:189–193. doi: 10.1002/(sici)1098-2388(199810/11)15:3<189::aid-ssu8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 178.Ouellette JR, Ko AS, Lefor AT. The physiologic effects of laparoscopy: applications in oncology. Cancer J. 2005;11:2–9. doi: 10.1097/00130404-200501000-00002. [DOI] [PubMed] [Google Scholar]
- 179.Steinert R, Lippert H, Reymond MA. Tumor cell dissemination during laparoscopy: prevention and therapeutic opportunities. Dig Surg. 2002;19:464–472. doi: 10.1159/000067598. [DOI] [PubMed] [Google Scholar]
- 180.Jingli C, Rong C, Rubai X. Influence of colorectal laparoscopic surgery on dissemination and seeding of tumor cells. Surg Endosc. 2006;20:1759–1761. doi: 10.1007/s00464-005-0694-4. [DOI] [PubMed] [Google Scholar]
- 181.Paraskeva PA, Ridgway PF, Jones T, Smith A, Peck DH, Darzi AW. Laparoscopic environmental changes during surgery enhance the invasive potential of tumours. Tumour Biol. 2005;26:94–102. doi: 10.1159/000085816. [DOI] [PubMed] [Google Scholar]
- 182.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, Lorimier G, Dubè P, Glehen O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]


