Abstract
Spontaneous bacterial peritonitis is a complication of ascitic patients with end-stage liver disease (ESLD); spontaneous fungal peritonitis (SFP) is a complication of ESLD less known and described. ESLD is associated to immunodepression and the resulting increased susceptibility to infections. Recent perspectives of the management of the critically ill patient with ESLD do not specify the rate of isolation of fungi in critically ill patients, not even the antifungals used for the prophylaxis, neither optimal treatment. We reviewed, in order to focus the epidemiology, characteristics, and, considering the high mortality rate of SFP, the use of optimal empirical antifungal therapy the current literature.
Keywords: Cirrhosis, Critically ill patient, Spontaneous fungal peritonitis, Life-threatening infections, Fungal ascitis, Nosocomial spontaneous peritonitis
Core tip: Spontaneous bacterial peritonitis (SBP) occurs in patients with end-stage liver disease (ESLD); spontaneous fungal peritonitis (SFP) is a complication of ESLD less known and described. Patients with SFP had a significantly worse prognosis than those with SBP. The incidence accounts from 0% to 13% of patients with ESLD and spontaneous peritonitis. Data are conflicting regarding fungi distribution between nosocomial and non-nosocomial infections. Candida spp. are the most frequent fungal infectious agent isolated. Previous SBP antibiotic prophylaxis, hepatorenal syndrome, low ascitic fluid protein (< 1 g/dL), elevated acute physiology and chronic health evaluation II and serum lactate also significantly adversely impact hospital mortality.
INTRODUCTION
Spontaneous bacterial peritonitis (SBP) occurs in patients with end-stage liver disease (ESLD); however, spontaneous fungal peritonitis (SFP) is a complication of ESLD less known and described. A diagnosis of SFP is based on large numbers of neutrophil granulocytes (> 250 cells/mL) of ascitic fluid and diagnostic investigation to exclude other causes of intra-abdominal infection[1], whereas we define fungal ascitis as fungal culture positive in ascitic fluid in the presence of ascitic neutrophil counts lower than 250 neutrophils/mL. Hospital-acquired (HA) spontaneous peritonitis (SP), both HA-SBP and HA-SFP, is peritonitis that occurs 48-72 h after hospitalization in the absence of signs of infection at hospital admission.
EPIDEMIOLOGY
Asia
Hwang et al[1] evaluated ESLD patients with SP between 2000 and 2005 in a Korean tertiary care center: 401 patients with SBP and 15 with SFP (3.6%); eleven of the 15 SFP was polymicrobial (Table 1). SFP was more common in nosocomial SP and in patients with higher Child-Turcotte-Pugh (CTP). The most commonly fungus found was Candida spp (8 patients C. albicans; 1 patient C. tropicalis; 1 patient C. glabrata), followed by Cryptococcus neoformans (5 patients). More than two-thirds of patients (11 patients, 73.3%) with fungal infection died within the first month after diagnosis of SP. All 10 patients showed no improvement with empirical antimicrobial therapy and died within a month. Of 5 patients who showed improvement with empirical antimicrobial therapy, only one died in the first month for gastrointestinal bleeding; the remaining four patients survived. HA-SBP and community-acquired SBP (CA-SBP) occurred in 151 and 265 patients, respectively. Distribution of fungi between HA-SFP vs CA-SFP was 12 vs 3, respectively. The mean value of CTP score was 12.5 ± 2.0 in the SFP cohort and 11.1 ± 1.7 in the SBP cohort[1].
Table 1.
Polymicrobial infections
| Ref. | HA-SBP definition | Study design | Data provided by the author | Setting | Patients with polymicrobial infections | Fungal polymicrobial infections |
| Friedrich et al[9], 2015 | PMN > 250 | Retrospective | No | University Hospital | 24/138 | N/A |
| > 48 h of | cohort | |||||
| hospitalization | ||||||
| Li et al[4], 2015 | PMN > 250 | Retrospective | No | University Hospital | 16/306 | N/A |
| > 48 h of | cohort | |||||
| hospitalization | ||||||
| Hwang et al[1], 2014 | PMN > 250 | Retrospective | No | University Hospital | N/A | 11/15 |
| > 72 h of | cohort | |||||
| hospitalization | ||||||
| Ariza et al[12], 2012 | PMN > 250 | Retrospective | No | University Hospital | 15/261 | N/A |
| > 48 h of | cohort | |||||
| hospitalization | ||||||
| Umgelter et al[11], 2009 | PMN > 50 | Prospective | Yes | University Hospital | 4/41 | 2/2 |
| > 48 h of | cohort | |||||
| hospitalization | ||||||
| Bert et al[8], 2003 | PMN > 250 | Retrospective | No | University Hospital | 7/78 | N/A |
| > 48 h of | cohort | |||||
| hospitalization |
HA: Hospital-acquired; SBP: Spontaneous bacterial peritonitis; PMN: Polymorphonuclear; N/A: Not available.
In another retrospective study conducted in Korea, between January 1st 2003 and December 1st 2010, ninety-five ESLD patients with SP were included. Among the forty-seven pathogens isolated, one (2.2%) was a Candida spp. The patient with Candida spp in ascitic fluid had hepatocellular carcinoma and died of liver failure shortly after admission[2].
Cheong et al[3] evaluated the clinical difference between SP acquired in the hospital or in the community in patients with ESLD between 2000 and 2007 in a Korean tertiary care center: HA-SBP occurred in 126 and CA-SBP occurred in 110 patients. Distribution of fungi between HA-SFP vs CA-SFP was 2.4% (3 patients) vs 0%.
Li et al[4] evaluated the drug resistance profile of pathogens isolated by ascitic fluid of 288 Chinese patients with ESLD between 2011 and 2013. Three hundred and six pathogens were isolated: 207 non-nosocomial and 99 nosocomial infections. Fungi were found in the ascitic fluid of nine patients (2.9%); there was significant difference regarding fungi distribution between nosocomial (7.1%, 7 patients) and non-nosocomial (0.9%, 2 patients) cases (P = 0.004).
Jindal et al[5] recently evaluated the outcome of carbapenem- vs cephalosporin-regimen in Indian cirrhotic patients with SP. A total of 175 patients were enrolled, of these two patients (1.1%) had SFP (1 patient with Candida spp and 1 patient with Aspergillus spp) and were treated with success.
Europe
Piano and Angeli reviewed microbiological data between 2007 and 2009 of a tertiary care center of northern Italy. Of sixty-nine culture positive SP, two (3%) were SFP. Fluconazole-susceptible C. albicans was isolated in the two cases[6].
In an observational study conducted in 4 university hospitals in north-eastern France, between January 1st 2010 and December 31st 2011, one hundred and ninety ESLD patients had ascites (median age 61.5 years, 58.5% CTP C): 268 ascitic fluid positive culture were obtained. Of these 140 were bacterascites and 57 SBP. Fungi were found in 2.1% of patients with bacterascites and none of SP patients. Bacterascites seems be considered a serious condition given the mortality rate (close to 20%). The authors concluded that bacterascites is probably a surrogate marker of advanced liver disease[7].
In order to evaluate the different etiology between of HA- and CA-SBP, ninety-five SP episodes were reviewed from a French Liver Unit. Seventy-eight microorganisms were found (39 isolates in each group) including 1 yeast (C. albicans). Distribution of C. albicans between HA-SFP vs CA-SFP was 0% vs 2.5% (1 patient)[8].
Friedrich et al[9] evaluated the drug resistance profile of pathogens isolated from ascitic fluid of 311 ESLD patients (hospitalized in a German tertiary care center) with their first episode of SP between 2007 and 2013. A total of 138 pathogens were isolated (49 non-nosocomial and 89 nosocomial). Fungal infections, Candida spp, were found in 10 patients (7.2%); C. albicans (3.6%) is the most frequent fungal infectious agent isolated. Interestingly, there was no significant difference regarding Candida spp distribution between nosocomial (9.0%, 8 patients) and non-nosocomial (4.1%, 2 patients) cases (P = 0.287).
Reuken et al[10] reviewed retrospectively 244 positive ascitic fluid culture isolated from ESLD patients between 2000 and 2011 in a German tertiary hospital, of these 90 were documented as monomicrobial SP. Fungal infections, Candida spp, were found in 3 patients (3.3%) of the ninety with SP.
Umgelter et al[11] analyzed prospectively 41 positive ascitic fluid culture isolated from ESLD patients between 2000 and 2011 in a German university medical center. C. albicans was found in 2 patients (4.8%) both in association with bacterial infections (Table 1). All C. albicans were susceptible to fluconazole.
In a retrospective observational study on a cohort of cirrhotic patients with SP conducted in a Spanish teaching hospital, between 2001 and 2009, 261 ascitic fluid culture positive SP were evaluated. The authors excluded from the analysis 15 cultures because polymicrobial, so SFP in this cohort could be underestimated. Distribution of C. albicans between HA-SFP vs CA-SFP was 0% vs 0.005% (1 patient)[12].
Africa
In a prospective study carried out in an Egyptian intensive care unit (ICU) from January to August 2013, 46 patients with ESLD were enrolled. Three patients had a polymorphonuclear (PMN) cell count greater than 250 cells/mL in ascitic fluid, of these 3 patients 1 patient had ascitic and blood culture negative, 2 patients (4.3%) had fungal growth in ascitic fluid: 1 patient had ascitic and blood culture positive for A. niger and 1 patient had ascitic culture positive for C. albicans and blood culture positive for C. albicans and C. tropicalis. Three (6.5%) patients had a PMN cell count lower than 250 cells/mL in ascitic fluid, of these 1 had ascitic and blood culture positive for C. albicans, 1 had ascitic culture positive for C. albicans and blood culture negative, 1 had ascitic culture positive for A. niger and blood culture negative. Of these 6 patients only 1 patient who had ascitic and blood culture negative died. Independent risk factors for a fungal infection were found to be previous antibiotic prophylaxis for SBP, hepatorenal syndrome and low protein ascites with total protein concentration of less than 1 g per deciliter. Patients with SFP presented worse prognosis than patients with SBP[13].
North America/miscellaneous
Karvellas et al[14] conducted a retrospective cohort study involving cirrhotic patients with SBP from 28 hospitals of Canada, United States and Saudi Arabia between 1996 and 2011 presenting with septic shock: a positive culture (blood or ascitic fluid) was found in 86 (68%) of 126 patients enrolled (53 HA-SFP vs 73 CA-SFP), the most common pathogens isolated were Escherichia coli (27.3%) followed by Candida spp (11.1%): 9 C. albicans and 2 C. glabrata/tropicalis. No one of these 11 patients survived to hospital discharge. SP-associated septic shock has a poor prognosis (mortality 80%). Appropriate antimicrobial therapy should be given as soon as possible: non-administration corresponds to an increase of 1.86 times hospital mortality per hour. Others hospital mortality risk factors are elevated acute physiology and chronic health evaluation II (APACHE II) and serum lactate.
CURRENT EVIDENCE
Fungi are common saprophytes of the human organism, being ubiquitously on skin and mucous membranes. Antibiotics (used for the prevention of SBP in patients with ascites) acting on the intestinal bacterial flora produce an excessive growth of fungi especially of the intestinal tract[15] with subsequent “translocation” from the gut lumen across the mucosa into the peritoneal cavity. Immunosuppression and malnutrition, common in ESLD patients, promote this process.
Differences between SBP and SFP: compared with SBP patients, the CTP score seems to be higher in SFP patients[1]. Patients with SFP had significantly higher mortality than the patients with SBP[1,13]. Patients who do not respond to empiric antimicrobial therapy (if this does not cover the fungus) have a very poor prognosis (mortality 100%)[1]. Data are conflicting regarding fungi distribution between nosocomial and non-nosocomial infections, cases of fungal peritonitis is not clearly more common in HA-[1,4] than CA-SP[9]. The number of isolates is so low that any analysis is underpowered so a meta-analysis of observational studies could clarify the fungi distribution between nosocomial and non-nosocomial infections.
C. albicans is the most frequent fungal infectious agent isolated[1,2,9-11,14] following by C. neoformans[1] and Aspergillus spp[5,13]. Fungal infection is often polymicrobial (73.3%-100% of cases), on the contrary polymicrobial bacterial infections affecting 5.2%-17.4% of cases (Table 1).
Risk factors for hospital mortality in SFP are SBP antibacterial prophylaxis, hepatorenal syndrome, low protein ascites with total protein concentration of less than 1 g per deciliter[13], elevated APACHE II and serum lactate[14].
Bremmer et al[16] in an historic cohort study including 25 patients (21 liver transplanted and 4 not liver transplanted), with isolation of Candida spp in ascitic fluid, found that C. albicans (48%; 12 out of 25) is the commonest pathogen, less frequently C. glabrata (20%), C. parapsilosis (16%), C. tropicalis (12%) and C. zeylanoides (4%). In the study, 28-d mortality was significantly higher in patients with elevated Charlson Comorbidity Index, Model for End-Stage Liver Disease (MELD) and APACHE II scores. There was no significant difference regarding 28-d mortality between fungal ascitis and SFP; conversely there was significant difference regarding 28-d mortality between patients who do not underwent liver transplantation (14 out of 21) and patients who underwent liver transplantation (0 out of 4). This study suggests that antifungal therapy used to treat SFP, could be a “bridge” to liver transplantation.
Saludes et al[17] recently reported 7 episodes of Candida spp isolation in ascites of cirrhotic patients detected in a Spanish hospital during the past 15 years. C. albicans was isolated in 5 patients (71.4%) and C. glabrata in 2 patients (28.6%). All patients were CTP C, per year mortality was 100% and 3 patients died in the first 10 d of diagnosis.
Choi et al[18] reviewed the clinical and laboratory features of all cirrhotic patients whose ascites samples were positive for Candida spp. A total of 21 cirrhotic patients was identified. Patients were regarded as having peritonitis if they had 1 or more clinical symptom(s) or sign(s) in the absence of any other possible explanation. Ten patients (47.6%) were classified into the spontaneous Candida-related peritonitis group, and the remaining 11 patients (52.4%) were classified into asymptomatic fungal ascitis. Mortalities were higher in the spontaneous Candida peritonitis group at discharge (50.0% vs 27.3%), 6-mo (90% vs 45.5%) and 1-year (100% vs 54.5%) (P = 0.007). Receiver-operating characteristic curve analysis revealed that the cut-off value of ascitic fluid PMN cell count of 315 cells/mL had the highest diagnostic accuracy with both sensitivity and specificity.
FUTURE PROSPETIVES
The available data suggest that the SFP could affect negatively the prognosis of patients with SP, therefore new diagnostic and therapeutic strategies are required. Candida spp. Is associated with a severe outcome when manifested with peritonitis[18]. In a recent clinical trial, fluconazole was added in patients with HA-SBP with no response to meropenem and daptomycin. In this study, never previously proposed, the authors added empiric antifungal therapy in a therapeutic HA-SBP protocol[19], although in the latest guidelines no mention is made about the use of antifungals in ESLD patients[20,21]. Actually start antifungal therapy as soon as possible improves prognosis in patients with invasive candidiasis[22,23].
Mortality from SFP is increased in case of severe underlying diseases and/or if initial antimicrobial therapy is inappropriate[14,24-26]. Karvellas et al[14] state that non-administration of an appropriate antimicrobial therapy corresponds to an increase of 1.86 times hospital mortality per hour. Unfortunately, it is not possible to extrapolate from this study the subgroup of SFP, but we can assume that septic shock has a worse outcome.
Area of uncertainty that remains for clinicians is the management of fungal ascitis: studies report no differences in mortality rates among patients with ascitic cell count upper or lower 250 cells/mL[16], or higher mortality in the SFP group but with a fungal ascitis mortality ranging from 27.3% at discharge to 54.5% after 1 year of discharge[18], conversely bacterascites show lower mortality rates than SBP[7].
As we recently proposed, given the low incidence of the SFP, a prophylaxis would be unuseful[27]. Treatment should be considered in absence of a positive culture in patients with a higher Charlson Comorbidity Index, MELD and APACHE II scores. Patients with a positive fungal culture of the ascitic fluid independently of PMN count should be treated.
Echinocandins are recommended for patients with HA-SFP or patients with CA-SFP and severe underlying illness given the poor prognosis of inappropriate antimicrobial therapy[28]. De-escalation to fluconazole is recommended when sensitivity tests are available[29].
Echinocandins should be considered as empirical or preemptive systemic antifungal therapy for patients with suspected SFP. The de-escalation to fluconazole reduces pharmaceutical costs and emerging of resistant microorganisms[30].
Micafungin in a different setting of patients with ESLD (liver transplant patients with a MELD score ≥ 20) showed non inferiority to standard antifungal prophylaxis, although renal function showed a better performance in micafungin group[31]. In conclusion an algorithm should be proposed for the treatment of patients with suspected SFP (Figure 1).
Figure 1.
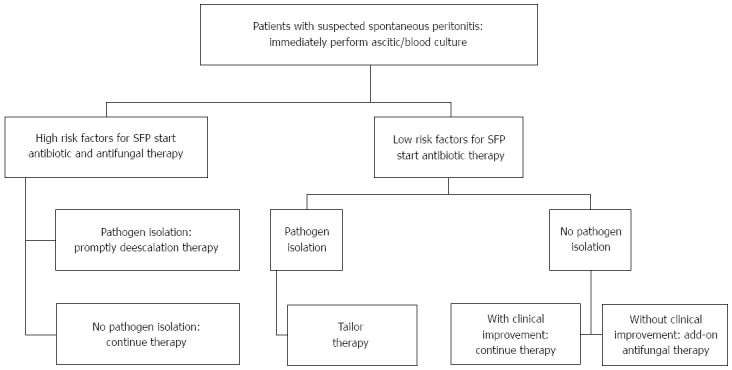
Spontaneous fungal peritonitis management algorithm. Risk factors for fungal diseases[32]: Surgery, total parenteral nutrition, fungal colonisation, renal replacement therapy, infection and/or sepsis, mechanical ventilation, diabetes, and APACHE II or III score; Add-on: consider adding empiric antifungal therapy. APACHE: Acute physiology and chronic health evaluation.
ACKNOWLEDGMENTS
The authors deeply thank Professor Andreas Umgelter for his personal data.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Conflict-of-interest statement: None of the authors have any conflict of interest in connection with this study.
Peer-review started: March 25, 2016
First decision: May 12, 2016
Article in press: August 1, 2016
P- Reviewer: Gong ZJ, Jarcuska P, Zapater P S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Hwang SY, Yu SJ, Lee JH, Kim JS, Yoon JW, Kim YJ, Yoon JH, Kim EC, Lee HS. Spontaneous fungal peritonitis: a severe complication in patients with advanced liver cirrhosis. Eur J Clin Microbiol Infect Dis. 2014;33:259–264. doi: 10.1007/s10096-013-1953-2. [DOI] [PubMed] [Google Scholar]
- 2.Tsung PC, Ryu SH, Cha IH, Cho HW, Kim JN, Kim YS, Moon JS. Predictive factors that influence the survival rates in liver cirrhosis patients with spontaneous bacterial peritonitis. Clin Mol Hepatol. 2013;19:131–139. doi: 10.3350/cmh.2013.19.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheong HS, Kang CI, Lee JA, Moon SY, Joung MK, Chung DR, Koh KC, Lee NY, Song JH, Peck KR. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin Infect Dis. 2009;48:1230–1236. doi: 10.1086/597585. [DOI] [PubMed] [Google Scholar]
- 4.Li YT, Yu CB, Huang JR, Qin ZJ, Li LJ. Pathogen profile and drug resistance analysis of spontaneous peritonitis in cirrhotic patients. World J Gastroenterol. 2015;21:10409–10417. doi: 10.3748/wjg.v21.i36.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jindal A, Kumar M, Bhadoria AS, Maiwall R, Sarin SK. A randomized open label study of ‘imipenem vs. cefepime’ in spontaneous bacterial peritonitis. Liver Int. 2016;36:677–687. doi: 10.1111/liv.12985. [DOI] [PubMed] [Google Scholar]
- 6.Piano S, Angeli P. Echinocandins vs fluconazole in the treatment of spontaneous fungal peritonitis. Hepatology. 2016 Epub ahead of print. [Google Scholar]
- 7.Piroth L, Pechinot A, Di Martino V, Hansmann Y, Putot A, Patry I, Hadou T, Jaulhac B, Chirouze C, Rabaud C, et al. Evolving epidemiology and antimicrobial resistance in spontaneous bacterial peritonitis: a two-year observational study. BMC Infect Dis. 2014;14:287. doi: 10.1186/1471-2334-14-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bert F, Andreu M, Durand F, Degos F, Galdbart JO, Moreau R, Branger C, Lambert-Zechovsky N, Valla D. Nosocomial and community-acquired spontaneous bacterial peritonitis: comparative microbiology and therapeutic implications. Eur J Clin Microbiol Infect Dis. 2003;22:10–15. doi: 10.1007/s10096-002-0840-z. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich K, Nüssle S, Rehlen T, Stremmel W, Mischnik A, Eisenbach C. Microbiology and resistance in first episodes of spontaneous bacterial peritonitis: implications for management and prognosis. J Gastroenterol Hepatol. 2016;31:1191–1195. doi: 10.1111/jgh.13266. [DOI] [PubMed] [Google Scholar]
- 10.Reuken PA, Pletz MW, Baier M, Pfister W, Stallmach A, Bruns T. Emergence of spontaneous bacterial peritonitis due to enterococci - risk factors and outcome in a 12-year retrospective study. Aliment Pharmacol Ther. 2012;35:1199–1208. doi: 10.1111/j.1365-2036.2012.05076.x. [DOI] [PubMed] [Google Scholar]
- 11.Umgelter A, Reindl W, Miedaner M, Schmid RM, Huber W. Failure of current antibiotic first-line regimens and mortality in hospitalized patients with spontaneous bacterial peritonitis. Infection. 2009;37:2–8. doi: 10.1007/s15010-008-8060-9. [DOI] [PubMed] [Google Scholar]
- 12.Ariza X, Castellote J, Lora-Tamayo J, Girbau A, Salord S, Rota R, Ariza J, Xiol X. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J Hepatol. 2012;56:825–832. doi: 10.1016/j.jhep.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Hassan EA, Abd El-Rehim AS, Hassany SM, Ahmed AO, Elsherbiny NM, Mohammed MH. Fungal infection in patients with end-stage liver disease: low frequency or low index of suspicion. Int J Infect Dis. 2014;23:69–74. doi: 10.1016/j.ijid.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Karvellas CJ, Abraldes JG, Arabi YM, Kumar A; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Appropriate and timely antimicrobial therapy in cirrhotic patients with spontaneous bacterial peritonitis-associated septic shock: a retrospective cohort study. Aliment Pharmacol Ther. 2015;41:747–757. doi: 10.1111/apt.13135. [DOI] [PubMed] [Google Scholar]
- 15.Bernhardt H, Zimmermann K, Knoke M. The continuous flow culture as an in vitro model in experimental mycology. Mycoses. 1999;42 Suppl 2:29–32. [PubMed] [Google Scholar]
- 16.Bremmer DN, Garavaglia JM, Shields RK. Spontaneous fungal peritonitis: a devastating complication of cirrhosis. Mycoses. 2015;58:387–393. doi: 10.1111/myc.12321. [DOI] [PubMed] [Google Scholar]
- 17.Saludes P, Araguás C, Sánchez-Delgado J, Dalmau B, Font B. Isolation of Candida spp. from ascites in cirrhotic patients. Enferm Infecc Microbiol Clin. 2015 doi: 10.1016/j.eimc.2015.05.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Choi SH, Soo Kim Y, Chung JW, Choo EJ, Kwak YG, Lee YS, Kim MN, Woo JH, Ryu J, Kim NJ. Clinical significance of untreated Candida species isolated from ascites in cirrhotic patients. Scand J Infect Dis. 2004;36:649–655. doi: 10.1080/00365540410020866. [DOI] [PubMed] [Google Scholar]
- 19.Piano S, Fasolato S, Salinas F, Romano A, Tonon M, Morando F, Cavallin M, Gola E, Sticca A, Loregian A, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: Results of a randomized, controlled clinical trial. Hepatology. 2016;63:1299–1309. doi: 10.1002/hep.27941. [DOI] [PubMed] [Google Scholar]
- 20.Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 21.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 24.Esposito S, Leone S, Carosi G. Analysis of current guidelines for intra-abdominal infections. J Chemother. 2009;21 Suppl 1:30–35. doi: 10.1179/joc.2009.21.Supplement-1.30. [DOI] [PubMed] [Google Scholar]
- 25.Leone S, Stefani S, Venditti M, Grossi P, Colizza S, De Gasperi A, Scaglione F, Sganga G, Esposito S. Intra-abdominal infections: model of antibiotic stewardship in an era with limited antimicrobial options. Int J Antimicrob Agents. 2011;38:271–272. doi: 10.1016/j.ijantimicag.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Leone S, Bisi L, Rossi M, Gori A. Comment on “Management of infections in cirrhotic patients: report of a consensus conference” S Fagiuoli et al. [Dig liver dis 2014; 46: 204-212] Dig Liver Dis. 2014;46:573–574. doi: 10.1016/j.dld.2014.01.155. [DOI] [PubMed] [Google Scholar]
- 27.Fiore M, Leone S. Use of antifungals in critically ill cirrhotic patients with spontaneous peritonitis. J Hepatol. 2016;64:986–987. doi: 10.1016/j.jhep.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Sartelli M, Viale P, Catena F, Ansaloni L, Moore E, Malangoni M, Moore FA, Velmahos G, Coimbra R, Ivatury R, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3. doi: 10.1186/1749-7922-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah DN, Yau R, Weston J, Lasco TM, Salazar M, Palmer HR, Garey KW. Evaluation of antifungal therapy in patients with candidaemia based on susceptibility testing results: implications for antimicrobial stewardship programmes. J Antimicrob Chemother. 2011;66:2146–2151. doi: 10.1093/jac/dkr244. [DOI] [PubMed] [Google Scholar]
- 30.Fiore M, Andreana L, Leone S. Pre-emptive therapy of spontaneous fungal peritonitis. Hepatology. 2016 doi: 10.1002/hep.28448. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Saliba F, Pascher A, Cointault O, Laterre PF, Cervera C, De Waele JJ, Cillo U, Langer RM, Lugano M, Göran-Ericzon B, Phillips S, Tweddle L, Karas A, Brown M, Fischer L; TENPIN (Liver Transplant European Study Into the Prevention of Fungal Infection) Investigators; TENPIN Liver Transplant European Study Into the Prevention of Fungal Infection Investigators. Randomized trial of micafungin for the prevention of invasive fungal infection in high-risk liver transplant recipients. Clin Infect Dis. 2015;60:997–1006. doi: 10.1093/cid/ciu1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muskett H, Shahin J, Eyres G, Harvey S, Rowan K, Harrison D. Risk factors for invasive fungal disease in critically ill adult patients: a systematic review. Crit Care. 2011;15:R287. doi: 10.1186/cc10574. [DOI] [PMC free article] [PubMed] [Google Scholar]


