Abstract
AIM
To investigate the cost effectiveness of routine small bowel biopsies (SBBs) in patients with iron deficiency anemia (IDA) independent of their celiac disease (CD) serology test results.
METHODS
We used a state transition Markov model. Two strategies were compared: routine SBBs during esophagogastroduodenoscopy (EGD) in all patients with IDA regardless their celiac serology status (strategy A) vs SBBs only in IDA patients with positive serology (strategy B). The main outcomes were quality adjusted life years (QALY), average cost and the incremental cost effectiveness ratio (ICER). One way sensitivity analysis was performed on all variables and two way sensitivity analysis on selected variables were done. In order to validate the results, a Monte Carlo simulation of 100 sample trials with 10, and an acceptability curve were performed.
RESULTS
Strategy A of routine SBBs yielded 19.888 QALYs with a cost of $218.10 compared to 19.887 QALYs and $234.17 in strategy B. In terms of cost-effectiveness, strategy A was the dominant strategy, as long as the cost of SBBs stayed less than $67. In addition, the ICER of strategy A was preferable, providing the cost of biopsy stays under $77. Monte Carlo simulation demonstrated that strategy A yielded the same QALY but with lower costs than strategy B.
CONCLUSION
Our model suggests that EGD with routine SBBs is a cost-effective approach with improved QALYs in patients with IDA when the prevalence of CD is 5% or greater. SBBs should be a routine screening tool for CD among patients with IDA, regardless of their celiac antibody status.
Keywords: Celiac disease, Iron deficiency anemia, Cost-effectiveness, Esophagogastroduodenoscopy, Markov model
Core tip: Common practice, and currents recommendations are to perform celiac serology tests and endoscopies to iron defeciency patients. Obtaining duodenal biopsies is usually reserved to patients with positive celiac serology. We aimed to investigate the cost effectiveness of routine duodenal biopsies in each and every patient with iron deficiency anemia, regardless serology status. We found this strategy to have a higher quality adjusted life years, a lower cost and a higher incremental cost effectiveness ratio over the common strategy of selected duodenal biopsies strategy.
INTRODUCTION
Iron deficiency anemia (IDA) is a common presentation of celiac disease (CD) found in as many as 50% of the patients at the time of diagnosis[1-4]. However, the need for routine duodenal biopsies in IDA patients, independent of their celiac serology results, is still debated[5-8].
In 2000, The British Society of Gastroenterology guidelines recommended that in the absence of overt blood loss or any other obvious cause for IDA, all patients with IDA should undergo esophagogastroduodenoscopy (EGD), including obtaining small bowel biopsies (SBBs)[9]. A decade later, the revised guidelines published by the same group, recommended that in IDA patients SBBs should be obtained only if celiac serology was positive or not performed[10]. This recommendation is based on the estimation that for every 330 biopsies taken from patients with negative celiac serology, one patient with CD would be diagnosed, meaning an additional cost of £35000 (in prices of 2011)[10]. No guidelines exist in the literature regarding screening for CD among those with IDA in the United States.
On the other hand, ACG clinical guidelines for the diagnosis and management of CD published in 2013[11] recommend routine SBBs during EGD in populations where the probability for CD is 5% or more. As the prevalence of CD among patients with IDA is about 5%, duodenal biopsies and serology for tTG antibodies are therefore recommended[11]. In addition, the recently published guidelines regarding diagnosis and management of CD recommend that individuals who undergo EGD due to anemia, weight loss or diarrhea should have SBBs, irrespective of their serology status[2].
In the present study we compared two strategies in patients with IDA; routine SBBs during EGD in all patients regardless their celiac serology status (strategy A) vs SBBs only in patients with positive serology (strategy B). The main outcomes were quality adjusted life years (QALY), average cost and the incremental cost effectiveness ratio (ICER). In addition, we also looked at life expectancy (LE) and ran an acceptability model for our results.
MATERIALS AND METHODS
Model
We constructed a state transition Markov model[12,13] to evaluate whether there is an added benefit in terms of QALYs and ICER, as well as whether it is more cost-effective to perform routinely SBBs in patients with IDA. QALY is the most common outcome unit used in cost-effectiveness analysis and it incorporates both the quality of life and life expectancy, while ICER is a statistic used in cost-effectiveness analysis to summarize the cost-effectiveness of a health care intervention. We compared two strategies: routine SBBs in all patients, referred to EGD due to IDA (strategy A); or screening all patients with IDA for CD serology and SBBs only in those patients with positive serology (strategy B). Three possibilities were analyzed: IDA patients without CD; IDA patients who have CD but were misdiagnosed due to negative serological test, and IDA patients correctly diagnosed with CD. Patients were placed into one of the following health states in each cycle of the model; (1) No CD; (2) CD but undiagnosed (i.e., considered healthy); (3) potential CD, defined as positive serology for CD but without villous atrophy (normal mucosa, Marsh 1 or 2)[14]; (4) CD under normal diet; (5) CD under strict gluten free diet (GFD); and (6) Death.
We assumed that the gold standard for the diagnosis of CD is SBBs, therefore in patients who have normal SBBs, CD can be ruled out.
Prevalence of CD in patients with IDA
IDA presents in 80%-90% of patients with CD and has been described as the sole presentation of the disease even in the absence of gastrointestinal symptoms[15,16]. The prevalence of CD among IDA patients varies between 1.3%-14.6% in different countries[17-19]. We estimated a prevalence of 5% of CD in IDA with a range of 5%-10%.
Assumptions regarding diagnostic tests
In a systematic review of 42 studies published by Lewis et al[20] the pooled sensitivity and specificity of IgA tTG antibody test was 92.8% (95%CI: 91.9-93.6), and 98.1% (95%CI: 97.8-98.4) respectively. According to the National Institute of Clinical Excellence (NICE), using biopsy results as a reference (Marsh 3 only as positive diagnosis of CD), tTG antibody test had a sensitivity of 87.5% (95%CI: 66.5-96.7), and specificity of 89.5% (95%CI: 87.0-91.6)[21]. tTG antibody test has been recommended to be the first step in screening for CD, as it is less costly than IgA anti endomysial antibodies, and has similar diagnostic value for the diagnosis and/or exclusion of CD[22,23]. In patients with IgA deficiency, serology based on IgG (IgG deamidated gliadin peptide) should be taken.
For the sensitivity analysis, we selected these base-case values according to those reported by Lewis et al[22] (sensitivity value of 93% with a range of 89%-96%, and specificity of 99% with a range of 89%-99%).
Assumption regarding the hazard ratio for mortality
During 45 years of follow-up, all-cause mortality was greater in persons with undiagnosed CD compared to those who were sero-negative for CD, with a HR of 3.9 (95%CI: 2.0-7.5), P < 0.01[24]. Cottone et al[25] reported a 3.8 standardized mortality ratio (SMR) rate in patients with CD compared to the general population and this increased risk seemed to be due to non-Hodgkin's lymphoma. Corrao et al[26] documented increased SMRs of 2.5 (1.3-4.6) among patients with a diagnostic delay of 120 mo or more; of 2.9 (1.8-4.3) among patients with severe CD; and of 5.2 (3.4-7.8) among patients with poor adherence to a GFD[26]. West and others found that the overall HR for mortality in CD was 2.09 in the first year after diagnosis and declined to 1.1 after the first year of diagnosis[27]. Meta-analysis done by Tio et al[28] showed an increase risk for all-cause mortality in CD patients with an OR of 1.24 (95%CI: 1.19-1.3). On the contrary, other studies reported no excess in overall mortality in patients with undetected CD compared to the general population[29,30]. We used an HR of 1.39 in the base case with a range of 1.33-1.45.
Adherence rate to a gluten free diet
There are different definitions regarding adherence to gluten free diet (GFD), all intrinsically linked to the manner in which adherence was assessed and measured. The rate of a GFD in CD patients for over a period of 10 years was reported to be between 50%-80% while the rate of strict adherence to GFD ranges between 42%-91%[31,32]. We assumed that the annual transition probability from adherence to GFD to a normal diet is estimated to be 0.9 (range of 0.6-0.9), and that the transition probability from strict diet to normal diet is estimated to be 0.2 (range 0.2-0.6).
Transition probability from potential CD to mucosal flattening
Biagi et al[33] published the cumulative probability of mucosal flattening in patients with potential CD over a period of 24 mo. In order to extrapolate the transition probability for a longer time we assumed that cumulative incidence of mucosal flattening follows Weibull distribution. The parameters of the Weibull distribution were estimated using Nelder-Mead Algorithm to optimize the model parameters. For the Weibull distribution, the transition probability is given by: 1-StSt-u = 1 - exp[λt - uγexp(λtγ)]. Where u is the cycle time, λ is the scale parameter and γ is the shape parameter of the Weibull distribution.
Assumptions regarding utilities
Many CD patients suffer from persistent clinical symptoms and reduced health-related quality of life despite a strict GFD[34-36]. A Swedish study documented that patients with CD who had been under a GFD for 10 years, with variable adherence rate, had a calculated utility of 0.717 compared with an average of 0.726 for the general population[37]. We used in our sensitivity analysis, a utility value of 0.92 (range of 0.90-1) for newly diagnosed CD patients, and value of 0.99 (range 0.95-1) for CD patients after GFD. Since the aim of identifying CD in patients with IDA is to improve quality of life, we projected gains in QALYs and costs for the remainder of patients’ lives. This was accomplished by calculating remaining life expectancy as a function of age from the United States Life Tables[37].
Costs
We calculated costs of serology (tTG Antibody test only), EGD, SBBs and the cost of evaluating symptoms. The average payments for each coded procedure were based on the 2013 Medicare Fee Schedule (http://gi.org/wp-content/uploads/2013/02/2013ACGPaymentGrid.pdf) (Table 1). According to expert’s opinion we assumed that about 50% (range of 30%-80%) of the patients with a missed diagnosis of CD, will need a repeat investigation. We considered the cost of such an investigation to be $150. The calculations neglected the cost of EGD complications, as the risk of perforation, duodenal hematoma or death is very rare.
Table 1.
Variables costs
| Variable | Base | Low | High |
| Time horizon | Life-time | 70 | 100 |
| Age of patient, yr | 45 | 40 | 60 |
| Cost of Biopsy (including complication) | 60 | 60 | 80 |
| Cost of tTG Ab's test | 70 | 60 | 80 |
| Cost of evaluating symptoms | 150 | 100 | 300 |
| Cost of upper endoscopy | 350 | 300 | 400 |
| Increasing rate of cost of patient with undiagnosed CD | 1.25 | 1 | 1.4 |
All costs are in 2013 (USD).
Assumption regarding the percentage of undiagnosed CD who will undergo further investigation after one year
According to expert’s opinion, we assume that 50% of all patients with a missed diagnosis of CD (biopsies were not performed during the first EGD) will need further investigation within the next coming year. In the sensitivity analysis we used a value of 50%, ranging from 30% to 80%.
Sensitivity analyses
We performed one-way sensitivity analyses on all variables (transition probabilities, costs, and utilities) in the model, and two-way sensitivity analysis on selected variables. One-way sensitivity analyses were performed in order to identify variables that had an impact on the decision of which strategy is the dominant one. In addition, we conducted a Monte Carlo simulation of 100 sampling trials with 10000 patients. Each variable was tested separately to determine whether varying the particular variable over a broad range alters the ICER. In the base line analysis, we assumed a willingness-to-pay (WTP) threshold of $50000 per QALY as being cost-effective[38]. Table 2 summarizes the base-case values and ranges used in sensitivity analysis in our model.
Table 2.
Base-case values and ranges used in sensitivity analysis in our model
| Variable | Base | Low | High | Ref. |
| Transition probability continuing strict diet given the patient was on strict diet1 | 0.90 | 0.60 | 0.90 | [37,38] |
| Transition probability to normal diet from strict diet | 0.20 | 0.20 | 0.60 | Assumption |
| Transition probability continuing normal diet given the patient was on normal diet | 0.30 | 0.20 | 0.60 | Assumption |
| Probability finding celiac due to symptoms | 0.20 | 0.10 | 0.40 | Assumption |
| Prevalence of celiac in IDA patient | 0.05 | 0.05 | 0.10 | [18,20-23] |
| Discount rate of costs | 0.03 | 0.00 | 0.05 | Assumption |
| Specificity of serologic tests | 0.99 | 0.89 | 0.99 | [24-26] |
| Sensitivity of serologic tests | 0.93 | 0.89 | 0.96 | [24-26] |
| Utility of CD | 0.92 | 0.90 | 1.00 | [40-43] |
| Utility of GFD | 0.99 | 0.95 | 1.00 | Assumption |
| Rate of Overt CD | 0.80 | 0.75 | 0.90 | Assumption |
| of Weibull distribution | 0.2686 | 0.26 | 0.29 | Assumption |
| of Weibull distribution | 1.1668 | 1.15 | 1.18 | Assumption |
| HR mortality of CD patients vs general population | 1.39 | 1.33 | 1.45 | [29-34] |
Annual transitions probabilities. IDA: Iron deficiency anemia; CD: Celiac disease; GFD: Gluten free diet; HR: Hazard ratio.
RESULTS
QALYs outcome
The strategy to obtain SBBs regardless the serological results (strategy A) yielded 19.888 QALY, and dominated the strategy of conducting SBBs only in patients with a positive serological test (strategy B), which had 19.887 QALY. Figure 1A is a Tornado plot showing the different parameters that had an impact on the incremental QALY. The most influential parameters on the QALY outcome were the prevalence of CD in IDA patients, the utility of CD and the probability of identifying CD due to symptoms. As demonstrated in the one-way sensitivity analysis figures of these parameters (Figure 1B-D, respectively), the dominance of the strategy of performing SBBs in all IDA patients was robust.
Figure 1.
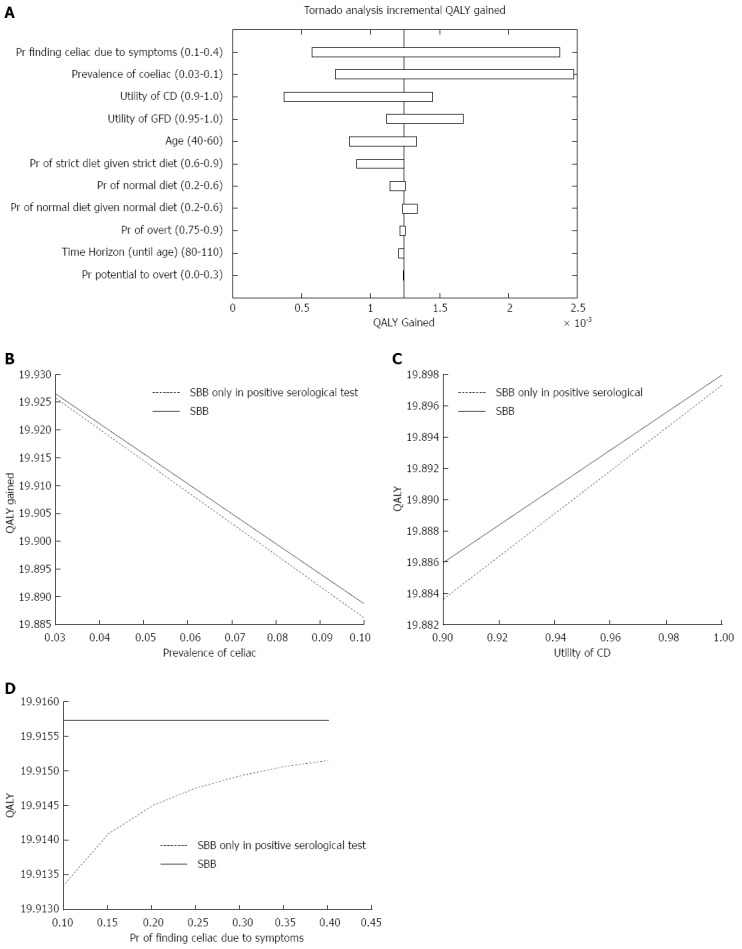
Quality adjusted life-years outcome. A: Influential parameters on the incremental quality adjusted life-years (QALY); B: One-way sensitivity analysis figure of the prevalence of celiac disease; C: One-way sensitivity analysis figure of the utility of celiac disease; D: One-way sensitivity analysis figure of the probability of diagnosing celiac disease due to symptoms. CD: Celiac disease; GFD: Gluten free diet; SBB: Small bowel biopsy.
Cost outcome
The average cost of strategy A was $218.10 vs $234.17 for strategy B. These results are explained by the lower cost of performing serological tests only in patients with positive CD biopsies compared to performing them in the whole study population. As shown in Figure 2A, the costs of serological tests, biopsies and patients’ symptoms evaluation had the greatest impact on the incremental average cost.
Figure 2.
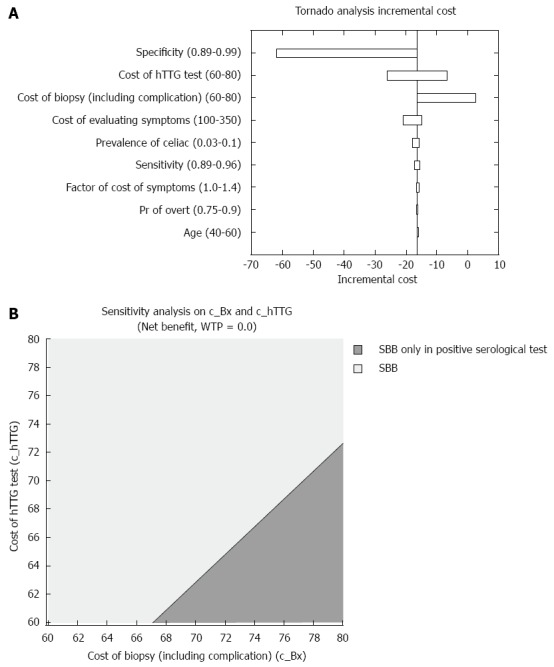
Cost outcome. A: Influential parameters on the incremental cost; B: Two-way sensitivity analysis depicting the less costly strategy, in regards to serological tests and small bowel biopsies prices.
We next tested the two-way sensitivity analysis with the costs of serological tests and SBBs. As shown in Figure 2B, as long as the cost of SBBs is less than $67, strategy A dominates strategy B. These results are independent of the costs of serological test within the range of $60-$80. However, when the cost of SBBs is greater than $67, the dominant strategy depends on both the cost of SBBs and the cost of the serological tests.
ICER
Analyzing the ICER for both strategies, we found that strategy A was more cost effective than strategy B. Figure 3A demonstrates the parameters that affected the ICER analysis. As shown in Figure 3B, for a cost of biopsy less than $77, the universal SBBs strategy dominated (i.e., costs less with higher QALY). However, when the cost of biopsy was greater than $77, the ICER increased up to almost $3000 per QALY and the strategy of conducting SBBs only in patients with positive serology dominated.
Figure 3.
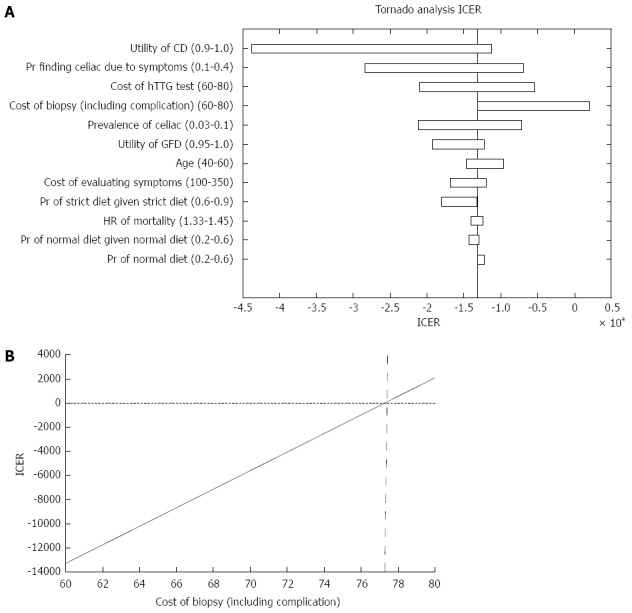
Incremental cost-effectiveness ratio outcome. A: Parameters that affected the incremental cost-effectiveness ratio (ICER) analysis; B: ICER of both strategies in regard to the cost of small bowel biopsies. CD: Celiac disease; GFD: Gluten free diet.
The results of the Monte Carlo simulation (Figure 4A) of 100 sampling trials with 10000 patients in each trial, demonstrate that strategy A yielded the same QALY with lower costs than strategy B. Finally, in order to verify our results, we performed an acceptability curve (Figure 4B). This figure demonstrates that as the willingness to pay for each QALY increases, the validity of our cost effectiveness study increases as well. For example, if the willingness to pay for 1 QALY is $10000 the probability of the validity of our results would be approximately 98%.
Figure 4.
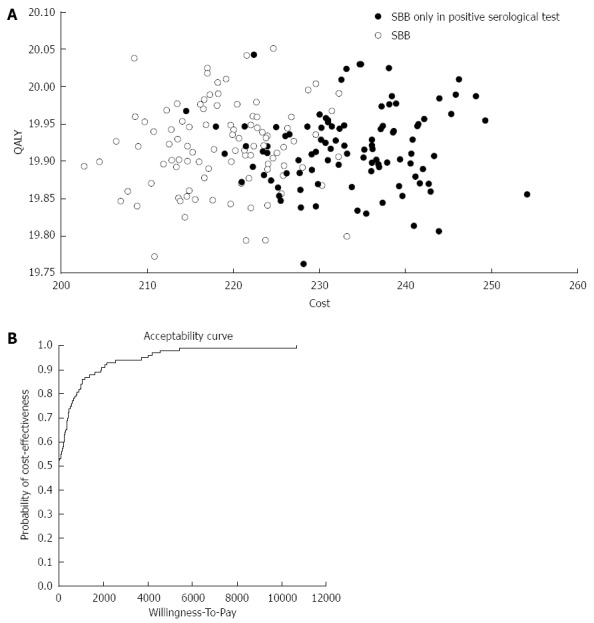
Validation. A: Monte Carlo simulation of 100 sample trials with 10000 patients in each trial; B: Acceptability curve that validate the cost effectiveness results of the study in relation to the willingness to pay for each quality adjusted life year (QALY). SBB: Small bowel biopsie.
DISCUSSION
The results of our model provide additional data that performing duodenal biopsies in patients with IDA, regardless of their celiac serology status or even in patients with IDA and negative serology, is a cost effective strategy with improved QALY. This strategy substantially dominated the strategy of performing SBBs only in patients with positive celiac serology.
We applied our model to patients aged ≥ 45 years, but routine duodenal biopsy for a diagnosis of CD was found to increase diagnostic yield even in patients over 65 years of age. (1) The role of performing routine endoscopic duodenal biopsies during the evaluation of IDA is increasingly emphasized, as the prevalence of CD in this population was reported to increase the diagnostic yield by 5%-14%[3,15]. There are several known macroscopic endoscopic markers for CD including: scalloping, mosaicism, fissuring and others[39]. As normal endoscopic looking mucosa does not exclude the diagnosis of CD, there are several new technology development of endoscopic tools and procedures proved to increase the yield of the diagnosis. These tools include: narrow band imaging, optical coherence tomography, water immersion technique, i-scan technology and confocal laser endomicroscopy[39-43]. However according to the published clinical guidelines of CD[11] SBB is still required for the diagnosis. Moreover, endoscopic lesions that may explain IDA should not preclude obtaining SBBs[2].
Recent studies have indicated that patients with untreated CD have significant morbidity and even severe complications[44]. These patients have a decreased quality of life, as the stigma of chronic disorder and the need for major dietary restrictions, increase the self-perceived burden of their illness[45]. As a result, in some studies, even well treated CD patients have failed to attain well-being compare to that of the general population[46,47]. The mean pre-treatment QALY score for the CD population in the literature was 0.66 and increased to 0.86 after initiating a GFD. A lower QALY score was associated with a delay over 2 years since the appearance of symptoms to diagnosis[48]. In our study QALY had a major impact on the final conclusion to perform SBBs in all IDA patients.
We also demonstrated that strategy A had a better ICER (less total costs with more QALY) suggesting that conducting SBBs as a first step in all IDA patients followed by tTG serology in patients with ≥Marsh 3a[11], would be a more cost effective strategy. However, not all CD patients have positive serology and lesser degree of villous atrophy is more frequently seen in sero-negative patients[49]. As such, there is a difficulty to identify this group of patients unless the physician maintains a high degree of clinical suspicion. Therefore, strategy B, of performing SBBs only in patients with positive serology may lead to under-diagnosis of CD[50,51].
Finally, we also measured life expectancy (LE) without a discount rate, for the two strategies (data not shown). We found that for a 45 old patient with IDA, strategy A had a longer LE compared to strategy B, 78.04843 years vs 78.04770 years respectively. The prevalence of CD in IDA patients was found to be the parameter with the greatest impact on LE.
Our study has several limitations: we compared conducting SBBs unrelated to serological results assuming that the patients did not have a serological test, which reduced the total cost. However, in real practice, most patients would have had a serologic test done before facing the question whether or not to conduct an EGD with SBBs. Consequently testing the same outcomes, we applied our model, to a strategy of conducting SBBs in patients with IDA and negative serology. As Goddard et al[10] estimated, if the pretest probability of CD in patients presenting only with IDA is 5%, the post-test probability of CD is 0.3%. This means there is a need of duodenal biopsies from about 330 tTG antibody-negative patients to detect one extra patient with CD at an estimated additional cost of £35000 (about $50000). If the cost of SBBs is $60, the additional cost would be $19800. Under the assumptions of our model, the average cost of "No SBBs" strategy was $83.1 compared to $141.1 in the "SBBs" strategy (additional of $57.98). The average QALY in the "No SBBs" strategy was 19.4399 vs 19.4412 QALY in the "SBBs" strategy. The ICER was $45393 per QALY, which is still considered cost-effective (< $50000)[38]. However, when the cost of SBBs is higher than $60 the ICER increases and yield a non-cost-effective ICER. Since the cost of SBBs in our model was lower compared to the analysis performed by Goddard et al[10] we challenged our model by calculating SBBs with a higher cost, ranging between $50 to $120 (Figure 5A). Under these costs the ICER ranges between $37000 to $95000 per QALY. Our results revealed that as long as the utility of CD is less than 0.92, the ICER would still be under $50000 for 1 QALY (Figure 5B).
Figure 5.
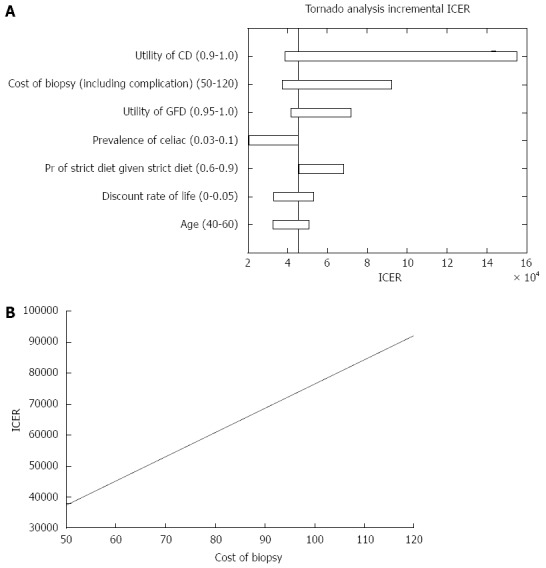
Incremental cost-effectiveness ratio outcome in patients with negative celiac serology. A: Parameters that affected the incremental cost-effectiveness ratio (ICER) analysis; B: A one way sensitivity analysis of ICER in regards to the cost of small bowel biopsies. CD: Celiac disease; GFD: Gluten free diet.
Our model was mainly prevalence dependent, and was not dependent solely on the sensitivity and specificity values of tTG antibodies. Therefore, we ran the model twice: first we used the values of sensitivity and specificity reported by the NICE 2012: 87% (95%CI: 65.3-96.6), and 96.9% (95%CI: 95.3-98.0) respectively. In the second run, we used the values used by the British Society of Gastroenterology guidelines for the management of IDA[10]. Analyzing both the Tornado plot and the sensitivity analysis in both cases showed stable results and proved that performing SBBs in IDA patients, regardless the tTG serology results, was still the dominant strategy. This model may miss potential CD patients, who will not be further evaluated for CD serology, due to normal histological findings. In these cases we presume that if IDA will be refractory and persistent serology for CD will ultimately be checked.
In conclusion our model suggests that, EGD with SBBs appears to be a cost-effective approach with improved QALYs in patients with IDA when the prevalence of CD is 5% or greater. SBBs should be a routine screening tool for CD among patients with IDA, regardless of their celiac antibody status.
COMMENTS
Background
Iron deficiency anemia (IDA) is a common presentation of celiac disease (CD) found in as many as 50% of the patients at the time of diagnosis. However, the need for routine duodenal biopsies in IDA patients, independent of their celiac serology results, is still debated. The latest clinical guidelines for the diagnosis and management of CD published in 2013 recommend routine SBBs during upper endoscopy in when the probability for CD is 5% or more. As the prevalence of CD among patients with IDA is about 5%, duodenal biopsies and serology for tTG antibodies are therefore recommended.
Research frontiers
According to recent studies, quality of life of celiac patients is inferior compared to healthy population. About 5% of IDA patients are diagnosed with CD. No studies were done to estimate the cost effectiveness of routine SBBs, regardless celiac serology status, in IDA patients in order to diagnose CD patients earlier and by that reduce morbidity and mortality. This is the first study which explore the cost effectiveness of performing routine SBBs to diagnose celiac in IDA patients. The authors measured, using a Markov model, quality adjusted life years (QALY), average cost and the incremental cost effectiveness ratio (ICER).
Innovations and breakthroughs
This model shows that routine SBBs, regardless of serology status, yielded higher QALY, lower cost and higher ICER than performing SBBs only in patients with positive serology. These results were valid as long as cost of SBBs stayed less than $67. In addition, the ICER of strategy A was preferable, providing the cost of biopsy stays under $77.
Applications
Upper endoscopy with routine SBBs is a cost-effective approach with improved QALYs in patients with IDA when the prevalence of CD is 5% or greater. SBBs should be a routine screening tool for CD among patients with IDA, regardless of their celiac antibody status.
Terminology
QALY - The most common outcome unit used in cost-effectiveness analysis is QALY, which incorporates both the quality of life and life expectancy. The quality of each health state is measured on a scale of 0 to 1 and is based on patient’s preferences over the health states. ICER - The incremental cost-effectiveness ratio is a statistic used in cost-effectiveness analysis to summarise the cost-effectiveness of a health care intervention, defined by the difference in cost between two possible interventions, divided by the difference in their effect.
Peer-review
The manuscript is well written, and the idea is interesting, as it shows a novel methodological approach to the management of celiac disease, with relevant saving of money.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Data sharing statement: No additional data are available.
Peer-review started: May 6, 2016
First decision: June 20, 2016
Article in press: August 1, 2016
P- Reviewer: Chen CY, Ianiro G S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Umbreit J. Iron deficiency: a concise review. Am J Hematol. 2005;78:225–231. doi: 10.1002/ajh.20249. [DOI] [PubMed] [Google Scholar]
- 2.Ludvigsson JF, Bai JC, Biagi F, Card TR, Ciacci C, Ciclitira PJ, Green PH, Hadjivassiliou M, Holdoway A, van Heel DA, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210–1228. doi: 10.1136/gutjnl-2013-306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catassi C, Gatti S, Fasano A. The new epidemiology of celiac disease. J Pediatr Gastroenterol Nutr. 2014;59 Suppl 1:S7–S9. doi: 10.1097/01.mpg.0000450393.23156.59. [DOI] [PubMed] [Google Scholar]
- 4.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–188. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 5.Mandal AK, Mehdi I, Munshi SK, Lo TC. Value of routine duodenal biopsy in diagnosing coeliac disease in patients with iron deficiency anaemia. Postgrad Med J. 2004;80:475–477. doi: 10.1136/pgmj.2003.014670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugai E, Moreno ML, Hwang HJ, Cabanne A, Crivelli A, Nachman F, Vázquez H, Niveloni S, Argonz J, Mazure R, et al. Celiac disease serology in patients with different pretest probabilities: is biopsy avoidable? World J Gastroenterol. 2010;16:3144–3152. doi: 10.3748/wjg.v16.i25.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmack SW, Genta RM. The diagnostic value of the duodenal biopsy: a clinico-pathologic analysis of 28,000 patients. Dig Liver Dis. 2010;42:485–489. doi: 10.1016/j.dld.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Riestra S, Domínguez F, Fernández-Ruiz E, García-Riesco E, Nieto R, Fernández E, Rodrigo L. Usefulness of duodenal biopsy during routine upper gastrointestinal endoscopy for diagnosis of celiac disease. World J Gastroenterol. 2006;12:5028–5032. doi: 10.3748/wjg.v12.i31.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goddard AF, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. British Society of Gastroenterology. Gut. 2000;46 Suppl 3-4:IV1–IV5. doi: 10.1136/gut.46.suppl_4.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309–1316. doi: 10.1136/gut.2010.228874. [DOI] [PubMed] [Google Scholar]
- 11.Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–676; quiz 677. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 13.Pauker SG, Kassirer JP. Decision analysis. N Engl J Med. 1987;316:250–258. doi: 10.1056/NEJM198701293160505. [DOI] [PubMed] [Google Scholar]
- 14.Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, Hadjivassiliou M, Kaukinen K, Kelly CP, Leonard JN, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavimandan A, Sharma M, Verma AK, Das P, Mishra P, Sinha S, Mohan A, Sreenivas V, Datta Gupta S, Makharia GK. Prevalence of celiac disease in nutritional anemia at a tertiary care center. Indian J Gastroenterol. 2014;33:114–118. doi: 10.1007/s12664-013-0366-6. [DOI] [PubMed] [Google Scholar]
- 16.Ehsani-Ardakani MJ, Rostami Nejad M, Villanacci V, Volta U, Manenti S, Caio G, Giovenali P, Becheanu G, Diculescu M, Pellegrino S, et al. Gastrointestinal and non-gastrointestinal presentation in patients with celiac disease. Arch Iran Med. 2013;16:78–82. [PubMed] [Google Scholar]
- 17.Gonen C, Yilmaz N, Yalcin M, Simsek I, Gonen O. Diagnostic yield of routine duodenal biopsies in iron deficiency anaemia: a study from Western Anatolia. Eur J Gastroenterol Hepatol. 2007;19:37–41. doi: 10.1097/01.meg.0000250583.07867.b7. [DOI] [PubMed] [Google Scholar]
- 18.Grisolano SW, Oxentenko AS, Murray JA, Burgart LJ, Dierkhising RA, Alexander JA. The usefulness of routine small bowel biopsies in evaluation of iron deficiency anemia. J Clin Gastroenterol. 2004;38:756–760. doi: 10.1097/01.mcg.0000139034.38568.51. [DOI] [PubMed] [Google Scholar]
- 19.Zamani F, Mohamadnejad M, Shakeri R, Amiri A, Najafi S, Alimohamadi SM, Tavangar SM, Ghavamzadeh A, Malekzadeh R. Gluten sensitive enteropathy in patients with iron deficiency anemia of unknown origin. World J Gastroenterol. 2008;14:7381–7385. doi: 10.3748/wjg.14.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis NR, Scott BB. Systematic review: the use of serology to exclude or diagnose coeliac disease (a comparison of the endomysial and tissue transglutaminase antibody tests) Aliment Pharmacol Ther. 2006;24:47–54. doi: 10.1111/j.1365-2036.2006.02967.x. [DOI] [PubMed] [Google Scholar]
- 21.Swallow K, Wild G, Sargur R, Sanders DS, Aziz I, Hopper AD, Egner W. Quality not quantity for transglutaminase antibody 2: the performance of an endomysial and tissue transglutaminase test in screening coeliac disease remains stable over time. Clin Exp Immunol. 2013;171:100–106. doi: 10.1111/cei.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis NR, Scott BB. Meta-analysis: deamidated gliadin peptide antibody and tissue transglutaminase antibody compared as screening tests for coeliac disease. Aliment Pharmacol Ther. 2010;31:73–81. doi: 10.1111/j.1365-2036.2009.04110.x. [DOI] [PubMed] [Google Scholar]
- 23.Collin P, Kaukinen K, Vogelsang H, Korponay-Szabó I, Sommer R, Schreier E, Volta U, Granito A, Veronesi L, Mascart F, et al. Antiendomysial and antihuman recombinant tissue transglutaminase antibodies in the diagnosis of coeliac disease: a biopsy-proven European multicentre study. Eur J Gastroenterol Hepatol. 2005;17:85–91. doi: 10.1097/00042737-200501000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, Brantner TL, Kim WR, Phelps TK, Lahr BD, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cottone M, Termini A, Oliva L, Magliocco A, Marrone C, Orlando A, Pinzone F, Di Mitri R, Rosselli M, Rizzo A, et al. Mortality and causes of death in celiac disease in a Mediterranean area. Dig Dis Sci. 1999;44:2538–2541. doi: 10.1023/a:1026655609906. [DOI] [PubMed] [Google Scholar]
- 26.Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C, Cottone M, Sategna Guidetti C, Usai P, Cesari P, Pelli MA, et al. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001;358:356–361. doi: 10.1016/s0140-6736(01)05554-4. [DOI] [PubMed] [Google Scholar]
- 27.West J, Logan RF, Smith CJ, Hubbard RB, Card TR. Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ. 2004;329:716–719. doi: 10.1136/bmj.38169.486701.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tio M, Cox MR, Eslick GD. Meta-analysis: coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment Pharmacol Ther. 2012;35:540–551. doi: 10.1111/j.1365-2036.2011.04972.x. [DOI] [PubMed] [Google Scholar]
- 29.Godfrey JD, Brantner TL, Brinjikji W, Christensen KN, Brogan DL, Van Dyke CT, Lahr BD, Larson JJ, Rubio-Tapia A, Melton LJ, et al. Morbidity and mortality among older individuals with undiagnosed celiac disease. Gastroenterology. 2010;139:763–769. doi: 10.1053/j.gastro.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canavan C, Logan RF, Khaw KT, West J. No difference in mortality in undetected coeliac disease compared with the general population: a UK cohort study. Aliment Pharmacol Ther. 2011;34:1012–1019. doi: 10.1111/j.1365-2036.2011.04811.x. [DOI] [PubMed] [Google Scholar]
- 31.Barratt SM, Leeds JS, Sanders DS. Factors influencing the type, timing and severity of symptomatic responses to dietary gluten in patients with biopsy-proven coeliac disease. J Gastrointestin Liver Dis. 2013;22:391–396. [PubMed] [Google Scholar]
- 32.Hall NJ, Rubin G, Charnock A. Systematic review: adherence to a gluten-free diet in adult patients with coeliac disease. Aliment Pharmacol Ther. 2009;30:315–330. doi: 10.1111/j.1365-2036.2009.04053.x. [DOI] [PubMed] [Google Scholar]
- 33.Biagi F, Trotta L, Alfano C, Balduzzi D, Staffieri V, Bianchi PI, Marchese A, Vattiato C, Zilli A, Luinetti O, et al. Prevalence and natural history of potential celiac disease in adult patients. Scand J Gastroenterol. 2013;48:537–542. doi: 10.3109/00365521.2013.777470. [DOI] [PubMed] [Google Scholar]
- 34.de Rosa A, Troncone A, Vacca M, Ciacci C. Characteristics and quality of illness behavior in celiac disease. Psychosomatics. 2004;45:336–342. doi: 10.1176/appi.psy.45.4.336. [DOI] [PubMed] [Google Scholar]
- 35.Häuser W, Gold J, Stein J, Caspary WF, Stallmach A. Health-related quality of life in adult coeliac disease in Germany: results of a national survey. Eur J Gastroenterol Hepatol. 2006;18:747–754. doi: 10.1097/01.meg.0000221855.19201.e8. [DOI] [PubMed] [Google Scholar]
- 36.Kurppa K, Collin P, Mäki M, Kaukinen K. Celiac disease and health-related quality of life. Expert Rev Gastroenterol Hepatol. 2011;5:83–90. doi: 10.1586/egh.10.81. [DOI] [PubMed] [Google Scholar]
- 37.Hallert C, Grännö C, Grant C, Hultén S, Midhagen G, Ström M, Svensson H, Valdimarsson T, Wickström T. Quality of life of adult coeliac patients treated for 10 years. Scand J Gastroenterol. 1998;33:933–938. doi: 10.1080/003655298750026949. [DOI] [PubMed] [Google Scholar]
- 38.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 39.Ianiro G, Gasbarrini A, Cammarota G. Endoscopic tools for the diagnosis and evaluation of celiac disease. World J Gastroenterol. 2013;19:8562–8570. doi: 10.3748/wjg.v19.i46.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh R, Nind G, Tucker G, Nguyen N, Holloway R, Bate J, Shetti M, George B, Tam W. Narrow-band imaging in the evaluation of villous morphology: a feasibility study assessing a simplified classification and observer agreement. Endoscopy. 2010;42:889–894. doi: 10.1055/s-0030-1255708. [DOI] [PubMed] [Google Scholar]
- 41.Gasbarrini A, Ojetti V, Cuoco L, Cammarota G, Migneco A, Armuzzi A, Pola P, Gasbarrini G. Lack of endoscopic visualization of intestinal villi with the “immersion technique” in overt atrophic celiac disease. Gastrointest Endosc. 2003;57:348–351. doi: 10.1067/mge.2003.116. [DOI] [PubMed] [Google Scholar]
- 42.Cammarota G, Cazzato A, Genovese O, Pantanella A, Ianiro G, Giorgio V, Montalto M, Vecchio FM, Larocca LM, Gasbarrini G, et al. Water-immersion technique during standard upper endoscopy may be useful to drive the biopsy sampling of duodenal mucosa in children with celiac disease. J Pediatr Gastroenterol Nutr. 2009;49:411–416. doi: 10.1097/MPG.0b013e318198ca88. [DOI] [PubMed] [Google Scholar]
- 43.Cammarota G, Ianiro G, Sparano L, La Mura R, Ricci R, Larocca LM, Landolfi R, Gasbarrini A. Image-enhanced endoscopy with I-scan technology for the evaluation of duodenal villous patterns. Dig Dis Sci. 2013;58:1287–1292. doi: 10.1007/s10620-012-2467-y. [DOI] [PubMed] [Google Scholar]
- 44.Fasano A. European and North American populations should be screened for coeliac disease. Gut. 2003;52:168–169. doi: 10.1136/gut.52.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paarlahti P, Kurppa K, Ukkola A, Collin P, Huhtala H, Mäki M, Kaukinen K. Predictors of persistent symptoms and reduced quality of life in treated coeliac disease patients: a large cross-sectional study. BMC Gastroenterol. 2013;13:75. doi: 10.1186/1471-230X-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636–651. doi: 10.1053/gast.2001.22123. [DOI] [PubMed] [Google Scholar]
- 47.Ivarsson A, Persson LA, Juto P, Peltonen M, Suhr O, Hernell O. High prevalence of undiagnosed coeliac disease in adults: a Swedish population-based study. J Intern Med. 1999;245:63–68. doi: 10.1046/j.1365-2796.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- 48.Norström F, Lindholm L, Sandström O, Nordyke K, Ivarsson A. Delay to celiac disease diagnosis and its implications for health-related quality of life. BMC Gastroenterol. 2011;11:118. doi: 10.1186/1471-230X-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abrams JA, Diamond B, Rotterdam H, Green PH. Seronegative celiac disease: increased prevalence with lesser degrees of villous atrophy. Dig Dis Sci. 2004;49:546–550. doi: 10.1023/b:ddas.0000026296.02308.00. [DOI] [PubMed] [Google Scholar]
- 50.Dahele AV, Aldhous MC, Humphreys K, Ghosh S. Serum IgA tissue transglutaminase antibodies in coeliac disease and other gastrointestinal diseases. QJM. 2001;94:195–205. doi: 10.1093/qjmed/94.4.195. [DOI] [PubMed] [Google Scholar]
- 51.Dickey W, Hughes DF, McMillan SA. Reliance on serum endomysial antibody testing underestimates the true prevalence of coeliac disease by one fifth. Scand J Gastroenterol. 2000;35:181–183. doi: 10.1080/003655200750024362. [DOI] [PubMed] [Google Scholar]


