Abstract
Background
In India, smokeless tobacco (SLT) use among pregnant women is high and its adverse effects on pregnancy outcomes have not been properly documented in.
Objectives
To collate available evidence on the association between SLT use and three adverse pregnancy outcomes, i.e. low birth weight, preterm birth and stillbirth among women in India.
Search Strategy
A systematic search was conducted in MEDLINE, IndMed, Web of Science, Google Scholar and major journals. Two authors independently reviewed the studies and extracted data.
Selection Criteria
Inclusion criteria were English articles published till December 2014, case control, case cohort or cohort, and exposure and outcome variables meeting predefined criteria. Exclusion criteria were case series, case reports, cross-sectional designs, risk estimate not restricted/adjusted for smoking with or without adjustment for other factors and duplicate data. Qualitative synthesis was followed by meta-analysis. Attributable burden was estimated using the population attributable fraction method.
Main Results
Pooled odds ratio was significant for all three outcomes: low birth weight (1.88, 95 % CI 1.38, 2.54), preterm birth (1.39: 1.01, 1.91) and stillbirth (2.85: 1.62, 5.01). We found that 0.87 million low birth weight babies, 0.19 million preterm births and 0.12 million stillbirths occurring annually in India could be attributed to maternal SLT use.
Conclusion
There was a suggestive evidence of SLT use associated with adverse pregnancy outcomes among women in India. Further studies in this field are required to generate more conclusive evidence.
Electronic supplementary material
The online version of this article (doi:10.1007/s13224-015-0821-7) contains supplementary material, which is available to authorized users.
Keywords: Adverse pregnancy outcomes, Smokeless tobacco, Systematic review, India
Introduction
The carcinogenic effects of smokeless tobacco (SLT) are relatively well established in India as well as globally [1–3]. India is home to a large number of SLT users and consequently bears a major burnt of its disease burden [4]. But research on non-carcinogenic effects of SLT has been almost non-existent. Among the non-carcinogenic ill effects adverse pregnancy outcomes are very important in India because of the high fertility rates and the millions of births occurring every year. Adverse pregnancy outcomes contribute to a huge disease burden, part of which can be potentially overcome by reducing SLT use among prospective mothers.
The effect of tobacco smoking on pregnancy outcomes has been well established over the last two decades [5]. Similar studies for smokeless tobacco are relatively rare, even at the global level. Research from Western countries has pointed out that preterm birth, low birth weight and stillbirth can be linked to SLT use [6]. Available evidence from Indian studies has not been systematically examined so far. Therefore, we conducted this systematic review to collate all available evidence in India on adverse effects of SLT use on pregnancy outcomes.
Materials and Methods
Literature Search
The eligibility criteria for inclusion of a study were designed in such a manner so as to include only high-quality articles which provide estimates that have been adjusted at least for tobacco smoking (Box S1). Two authors (DNS and RSA) independently carried out the literature search. Disagreements on study inclusion, quality assessment and data extraction were resolved by deliberation. We searched databases like PubMed, IndMED, Google Scholar, WHO SEARO reports, CDC tobacco reports, MOHFW India reports, Web of science, Science Citation Index, WHO Index Medicus of the South-East Asian Region and Open Grey. Details of the keywords used for PubMed are given in Box S2. Various combinations of the keywords were used for each condition to search Google Scholar, and its first 50 pages were screened for relevant and non-duplicate articles. Similarly, various combinations of the keywords were used in each of the databases and the same process repeated. Special efforts were taken to retrieve articles where smokeless tobacco use was also one of the factors, but not the main factor for which association was examined. Cross-references of all selected articles were scanned for additional studies. Attempts were made to retrieve grey literature like unpublished data, dissertations, and conference proceedings. To obtain publicly inaccessible data, a minimum of two email requests was sent to the corresponding author. If more than one article was published from a study, the article that provided the most updated data were selected. The last date of literature search was 31 July 2015. The review was carried out in accordance with the PRISMA guidelines.
Data Extraction
Using appropriate critical appraisal checklists, each article was assessed for quality by two authors. Study characteristics, such as first author, year of publication, date of data collection, place of study, study design, sample size, characteristics of cases and controls or cohort, methods of assessment of outcome and exposure, definitions of exposure, comparisons groups and risk estimates with 95 % CI were extracted onto pre-coded spreadsheets independently by the two authors (DNS and RSA). A risk estimate for SLT use was considered only if it was adjusted for at least tobacco smoking (optionally alcohol and other variables) or if the analysis was restricted to at least non-tobacco smokers (optionally non-drinkers).
Statistical Analysis
We performed a qualitative synthesis of the studies identified from the systematic search. Studies were appraised in terms of the quality of reporting, confounder adjustment, subgroup analysis and clinical and methodological characteristics. Based on the decision to combine the included studies, meta-analysis was conducted using invariance variance fixed effects model. Between studies heterogeneity was assessed by I square statistics. Forest plots were drawn to depict the individual and pooled effect sizes.
Results
Qualitative Synthesis
We found two studies that satisfied the selection criteria for adverse pregnancy outcomes. Pratinidhi et al. [7] had examined the relationship of SLT use with all three outcomes, low birth weight, preterm birth and still, whereas Gupta et al. [8] studied only low birth weight and preterm birth but a separate article [9] from the same population also discussed the association with stillbirth (Table S1, Figure S1).
The study by Pratinidhi et al. [7] was a cohort study involving about 700 pregnant women recruited from primary health centres in Pune. Tobacco use was self-reported, and outcomes were assessed at the time of delivery. There was no information on confounder adjustment, but the majority of the participants were probably non-smokers and therefore confounding due to tobacco smoking could be ruled out. They reported a significantly elevated risk of low birth weight and stillbirth but no significant association with preterm birth among tobacco chewers as compared to non-chewers.
The cohort study by Gupta et al. [8, 9] was carried out involving about 1100 pregnant women who were followed up till the termination of their pregnancy in Mumbai. Smokeless tobacco use by the participants was self-reported, and outcomes such as low birth weight, preterm birth and stillbirth were confirmed from medical records. Confounders adjusted were age, education, socio-economic status and antenatal care. Most of the women were non-smokers and so confounding due to smoking was also taken care of. They found a significantly increased risk of low birth weight (<2500 g), early preterm birth (<32 weeks), very early preterm birth (<28 weeks) and stillbirth among tobacco chewers as compared to non-chewers, but they reported a non-significant association with overall preterm birth.
Both these studies were adequately sized cohort studies carried out among pregnant women followed up till delivery. Confounding due to smoking was adjusted in both the studies by including non-smoking women. Both the studies reported similar results with regard to all three outcomes, i.e. significant positive association with low birth weight and stillbirth but no association with preterm birth. But Gupta PC et al. reported a significant association with early and very early preterm births that was not examined by Pratinidhi A et al.
Quantitative Synthesis
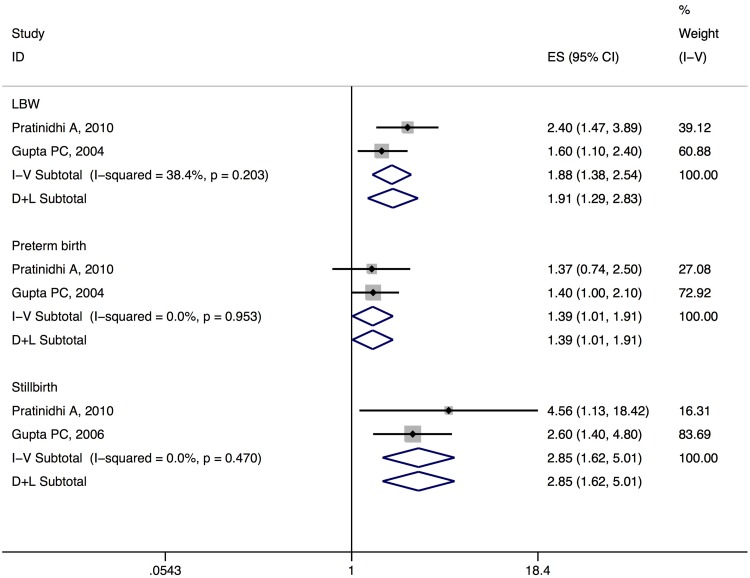
A fixed effects meta-analysis was conducted for the three conditions for which a minimum of two combinable studies was available. Inverse variance method was used to weight the studies. A significant pooled odds ratio was obtained for all three conditions. The results were as follows: for LBW summary OR 1.88 (95 % CI 1.29–2.83), I2 = 38 %, for preterm birth it was 1.39 (1.01–1.91), I2 = 0 % and for stillbirth it was 2.85 (1.62–5.01), I2 = 0 %. It can be seen that the relationship of SLT use with LBW and stillbirth was modest and significant, whereas for preterm birth it was only marginally significant (Table 1, Fig. 1).
Table 1.
Pooled odds ratio and attributable burden of adverse pregnancy outcomes due to smokeless tobacco use in India, 2010
| Pooled odds ratio (fixed effects) | I 2 (%) | Prevalence of SLT use in women aged 15–49 years (%) | Population attributable fraction (PAF) (95 % CI) | Total no. annual cases | SLT attributable annual cases (95 % CI) | |
|---|---|---|---|---|---|---|
| Low birth weight | 1.88 (1.38, 2.54) | 38.4 | 14.9 | 0.12 (0.05–0.19) | 75,00,000 | 8,70,951 (4,02,660–14,02,052) |
| Preterm birth | 1.39 (1.01, 1.91) | 0 | 14.9 | 0.06 (0.001–0.12) | 35,19,118 | 1,93,633 (5246–4,20,929) |
| Stillbirth | 2.85 (1.62, 5.01) | 0 | 14.9 | 0.22 (0.08–0.37) | 5,70,860 | 1,23,549 (48,365–2,13,781) |
Fig. 1.
Forest plots for the meta-analysis of adverse pregnancy outcomes (fixed and random effects model)
Attributable Burden
Now that a robust estimate of the role of SLT use, adjusted for confounders, in the causation of these adverse pregnancy outcomes was available; it was possible to estimate the population attributable burden. The total annual cases of low birth weight babies, preterm births and stillbirths were obtained from published sources [10–12]. National level prevalence of SLT use among pregnant women was not available; therefore, we calculated the prevalence of SLT use among women aged 15–49 years (reproductive years) from GATS India 2010 survey data, which was 14.9 % [13]. All estimates were as close to the year 2010 as possible. We found that 0.87 million low birth weight babies (12 % of LBW babies), 0.19 million preterm births (6 % of all preterm births) and 0.12 million stillbirths (22 % of all stillbirths) can be attributed to SLT use. They may be some overlap between attributable LBW and preterm births because 40 % of LBW babies are actually also born preterm (Table 1).
Discussion
It is fairly clear from the foregoing that research on the adverse pregnancy effects of SLT use has been minimal. Although only two studies were included for low birth weight (LBW), a number of Indian studies that have investigated the relation between tobacco chewing and LBW could not be included because of small size or cross-sectional nature of the study. For example, Mehta et al. [14] conducted a cross-sectional survey and found that prevalence of LBW was much higher in tobacco chewing mothers as compared to non-tobacco using mothers (65 vs. 36 %, p ≤ 0.001). Similar findings were also reported by other authors who conducted small size or cross-sectional studies [15–17]. Another Indian study by Deshmukh et al. [18] did not adjust the estimate for tobacco smoking. In other countries, a record based study conducted on Alaskan native women showed that mothers who used SLT had babies with a lower birth weight (birth weight reduced by 78 g) as compared to non-users [19].
Studies on preterm births were relatively few. A cross-sectional study in India by Kewal [15] showed that the odds of preterm birth was seven times more in SLT using mothers as compared to non-tobacco users (OR 7.08: 4.14–12.14). A cohort study from South Africa showed that snuff using mothers did not have increased risk of LBW as compared to non-users, but they did have increased risk of preterm births [20].
An Indian study by Rajaram et al. [21] showed that the risk of stillbirth was twice among those who used tobacco or alcohol as compared to non-users but since it did not provide separate estimates for tobacco chewers and it could not be included in this review. A study among women in Cambodia reported that tobacco chewing was associated with increased odds (OR 1.5: 1.1–2.1) of infant mortality as compared to non-users [22].
A couple of review articles on this topic have also concluded that tobacco chewing does lead to adverse pregnancy outcome like LBW, preterm birth and stillbirth [23, 24]. A comprehensive review by Ratsch and Bogossian [25] was conducted to identify the association between SLT use and adverse pregnancy outcomes. The review looked at outcomes like placental changes, stillbirth, birth weight, gestational age and after birth outcomes. It included studies mostly from Western and Asian countries. The authors pointed out several areas of knowledge gap and acknowledged the equivocal nature of evidence for each outcome. Another recently published systematic review examined the association of SLT use with LBW, preterm birth, stillbirth and small for gestational age [26]. They included studies without geographical and language restrictions, but they did not conduct a meta-analysis due to high heterogeneity between studies but concluded that there is evidence for harmful effects of SLT use on perinatal morbidity and mortality. Our findings are similar to their results as far as India is concerned, but in our review we deemed a meta-analysis to be appropriate for LBW, preterm birth and stillbirth.
Biological Plausibility
Mechanisms of action of the various components of SLT products, mainly nicotine have been studied in both animals and humans. A number of studies have demonstrated the harmful effects of nicotine and foetal development [27–29]. The effect is mainly mediated through nicotine, which enters the foetal circulation via the placenta and affects neuronal development and decreases foetal cell oxygenation [30, 31].
Implication of This Study
When compared to the several alternative interventions (such as regular antenatal visits, iron folate supplementation, improvement of maternal nutrition, high-risk screening for preterm birth and stillbirths and secondary curative care) available for improving newborn health [32], prevention of SLT use by pregnant women seems to be a simple and relatively cheap intervention tactic where all other interventions require some acts of commission, quitting tobacco use during pregnancy requires an act of omission and could be much easier to implement. The considerable gain obtained by this intervention should make it an absolutely essential part of antenatal care packages in India.
Strengths and Limitations
A number of adverse pregnancy outcomes have been explored in this review with special emphasis to evaluate the independent effect of SLT use. This review provides a baseline benchmark of what evidence already exists for each outcome and charts out the direction in which further research is needed from an Indian perspective.
This review bears a number of limitations. Firstly, for many studies that were excluded obtaining individual patient data might have made the estimate more robust. Secondly, the studies included were not representative of the entire country; studies were mostly carried out in the northern and western parts of the country. Therefore, any generalisation has to be made with caution. Finally, SLT comprises a range of diverse products the contents of which differ widely and consequently their health effects too. Here, it was not possible to study the effect of such diverse products due to lack of studies.
Conclusion and Recommendations
There is preliminary evidence to suspect the role of SLT use in the causation of low birth weight and stillbirth. However, the body of evidence is neither voluminous nor conclusive. A stark gap in the knowledge base in this area has been highlighted by this review. The cancer-producing effects of SLT have been widely researched and a conclusive body of evidence exists at the regional as well as the global level, but other health effects have gathered little to no attention from researchers. A new impetus is required to study in great depth the ‘other’ health effects of SLT in order to produce a comprehensive picture of the problem. Future studies should try to estimate the independent effect of SLT on various health outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author Contribution
RSA and DNS planned the study and performed the data retrieval. RSA performed the analysis and wrote the first draft of the manuscript. All authors read the final manuscript and approved it.
Dr. Rizwan A. Suliankatchi
is a medical doctor who graduated from the Madurai Medical College, Madurai. He also holds an Master degree in Community Medicine (Public Health) awarded by the prestigious All India Institute of Medical Sciences, New Delhi. His interest areas include epidemiology, reproductive and child health, non-communicable diseases, tobacco control and burden of disease estimation. He has more than 30 publications in the field of public health in international and national peer-reviewed journals and has been a contributing author to several books.
Compliance with Ethical Standards
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. Since this was a systematic review article no ethical approval was required.
Footnotes
Rizwan A. Suliankatchi is Assistant Professor of Community Medicine at Velammal Medical College Hospital and Research Institute; Dhirendra N. Sinha is Regional Adviser, Surveillance, (Tobacco Control) of Tobacco Free Initiative Unit at World Health Organization, Regional Office for South-East Asia.
Contributor Information
Rizwan A. Suliankatchi, Email: sarizwan1986@gmail.com
Dhirendra N. Sinha, Email: sinhad@who.int
References
- 1.Boffetta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol. 2008;9:667–675. doi: 10.1016/S1470-2045(08)70173-6. [DOI] [PubMed] [Google Scholar]
- 2.Lee PN, Hamling J. Systematic review of the relation between smokeless tobacco and cancer in Europe and North America. BMC Med. 2009;7:36. doi: 10.1186/1741-7015-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha DN, Rizwan SA, Gupta PC. Smokeless tobacco-associated cancers: a systematic review and meta-analysis of Indian studies. Int J Cancer. 2015 doi: 10.1002/ijc.29884. [DOI] [PubMed] [Google Scholar]
- 4.Sinha DN, Agarwal N, Gupta PC. Prevalence of smokeless tobacco use and number of users in 121 countries. Br J Med Med Res. 2015;9:1–20. doi: 10.9734/BJMMR/2015/16285. [DOI] [Google Scholar]
- 5.Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine Tob Res. 2008;10:267–278. doi: 10.1080/14622200701825908. [DOI] [PubMed] [Google Scholar]
- 6.England LJ, Kim SY, Tomar SL, et al. Non-cigarette tobacco use among women and adverse pregnancy outcomes. Acta Obstet Gynecol Scand. 2010;89:454–464. doi: 10.3109/00016341003605719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratinidhi A, Gandham S, Shrotri A, et al. Use of “Mishri” a smokeless form of tobacco during pregnancy and its perinatal outcome. Indian J Commun Med. 2010;35:14–18. doi: 10.4103/0970-0218.62547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta PC, Subramoney S, Sreevidya S. Smokeless tobacco use, birth weight, and gestational age: population based, prospective cohort study of 1217 women in Mumbai, India. BMJ. 2004;328:1538. doi: 10.1136/bmj.38113.687882.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta PC, Subramoney S. Smokeless tobacco use and risk of stillbirth: a cohort study in Mumbai, India. Epidemiology. 2006;17:47–51. doi: 10.1097/01.ede.0000190545.19168.c4. [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Health and Family Welfare, Government of India. India newborn action plan. New Delhi; 2014. p. 29. http://nrhm.gov.in/india-newborn-action-plan.html. Accessed 15 Aug 2015.
- 11.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 12.Cousens S, Blencowe H, Stanton C, et al. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet. 2011;377:1319–1330. doi: 10.1016/S0140-6736(10)62310-0. [DOI] [PubMed] [Google Scholar]
- 13.Ministry of Health and Family Welfare, Government of India. Global Adult Tobacco Survey: India Report 2009-10. New Delhi, India; 2010. http://whoindia.org/EN/Section20/Section25_1861.html. Accessed 13 Aug 2015.
- 14.Mehta A, Shukla S. Tobacco and pregnancy. J Obstet Gynaecol India. 1990;40:156–160. [Google Scholar]
- 15.Krishna K. Tobacco chewing in pregnancy. Br J Obstet Gynaecol. 1978;85:726–728. doi: 10.1111/j.1471-0528.1978.tb15591.x. [DOI] [PubMed] [Google Scholar]
- 16.Krishnamurthy S, Joshi S. Gender differences and low birth weight with maternal smokeless tobacco use in pregnancy. J Trop Pediatr. 1993;39:253–254. doi: 10.1093/tropej/39.4.253. [DOI] [PubMed] [Google Scholar]
- 17.Verma RC, Chansoriya M, Kaul KK. Effect of tobacco chewing by mothers on fetal outcome. Indian Pediatr. 1983;20:105–111. [PubMed] [Google Scholar]
- 18.Deshmukh JS, Motghare DD, Zodpey SP, et al. Low birth weight and associated maternal factors in an urban area. Indian Pediatr 1998;35:33–6. http://www.indianpediatrics.net/jan1998/33.pdf. Accessed 15 Aug 2015. [PubMed]
- 19.England LJ, Kim SY, Shapiro-Mendoza CK, et al. Maternal smokeless tobacco use in Alaska Native women and singleton infant birth size. Acta Obstet Gynecol Scand. 2012;91:93–103. doi: 10.1111/j.1600-0412.2011.01273.x. [DOI] [PubMed] [Google Scholar]
- 20.Steyn K, de Wet T, Saloojee Y, et al. The influence of maternal cigarette smoking, snuff use and passive smoking on pregnancy outcomes: the Birth To Ten Study. Paediatr Perinat Epidemiol. 2006;20:90–99. doi: 10.1111/j.1365-3016.2006.00707.x. [DOI] [PubMed] [Google Scholar]
- 21.Rajaram S, Zottarelli LK, Sunil TS. An assessment of fetal loss among currently married women in India. J Biosoc Sci. 2009;41:309–327. doi: 10.1017/S0021932008003222. [DOI] [PubMed] [Google Scholar]
- 22.Singh PN, Eng C, Yel D, et al. Maternal use of cigarettes, pipes, and smokeless tobacco associated with higher infant mortality rates in Cambodia. Asia Pac J Public Health. 2013;25:64S–74S. doi: 10.1177/1010539513493458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnamurthy S. Maternal tobacco use and adverse reproductive outcome. Natl Med J India 1997;10:2–4. http://nmji.in/approval/archive/Volume-10/issue-1/editorials-2.pdf. Accessed 15 Aug 2015. [PubMed]
- 24.Kumar S. Tobacco and areca nut chewing–reproductive impairments: an overview. Reprod Toxicol. 2013;36:12–17. doi: 10.1016/j.reprotox.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Ratsch A, Bogossian F. Smokeless tobacco use in pregnancy: an integrative review of the literature. Int J Public Health. 2014;59:599–608. doi: 10.1007/s00038-014-0558-6. [DOI] [PubMed] [Google Scholar]
- 26.Inamdar AS, Croucher RE, Chokhandre MK, et al. Maternal smokeless tobacco use in pregnancy and adverse health outcomes in newborns: a systematic review. Nicotine Tob Res. 2015;17:1058–1066. doi: 10.1093/ntr/ntu255. [DOI] [PubMed] [Google Scholar]
- 27.Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum Dev. 2007;83:699–706. doi: 10.1016/j.earlhumdev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Reeves S, Bernstein I. Effects of maternal tobacco-smoke exposure on fetal growth and neonatal size. Expert Rev Obstet Gynecol. 2008;3:719–730. doi: 10.1586/17474108.3.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers JM. Tobacco and pregnancy. Reprod Toxicol. 2009;28:152–160. doi: 10.1016/j.reprotox.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Yildiz D, Liu YS, Ercal N, et al. Comparison of pure nicotine- and smokeless tobacco extract-induced toxicities and oxidative stress. Arch Environ Contam Toxicol. 1999;37:434–439. doi: 10.1007/s002449900537. [DOI] [PubMed] [Google Scholar]
- 31.Stegmayr B, Johansson I, Huhtasaari F, et al. Use of smokeless tobacco and cigarettes—effects on plasma levels of antioxidant vitamins. Int J Vitam Nutr Res. 1993;63:195–200. [PubMed] [Google Scholar]
- 32.Bhutta ZA, Das JK, Bahl R, et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet. 2014;384:347–370. doi: 10.1016/S0140-6736(14)60792-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.