Abstract
Background
Fibroid or myoma is the commonest reported tumor of uterus, and is one of the important reasons for hysterectomy in our setting. Different drugs are available for medical management of fibroid uterus including mifepristone, a progesterone antagonist. Varying dosage regimen for mifepristone was studied as medical management of fibroid uterus. The aim of the project was to study the effect of mifepristone on the symptoms and size of fibroids, especially using a low-dose regimen for 6 months. In addition, any symptomatic improvement of menorrhagia and dysmenorrhea was also studied.
Methods
The study was designed as an observational prospective “before–after” study. Women diagnosed with uterine fibroids attending OPD of a tertiary care hospital were selected according to the inclusion criteria. A total of 36 patients were enrolled in the study. Sample size was calculated to study changes in various parameters after 6 months treatment with mifepristone 50 mg once a week. Baseline investigations were performed and menstrual blood loss was assessed using pictorial blood assessment charts. Fifty milligrams of mifepristone weekly was used, and patient assessed at 1 and 6 month. They were also further followed up till 3 months after stopping the drug to observe the changes in menstrual pattern, fibroid volume, hemoglobin and liver function tests. Baseline endometrial biopsy and another at 6 month on cessation of drug therapy were done for all patients.
Results
Majority of the study population comprised of perimenopausal women, i.e., 41–45 years (44 %). Fifty percent of the patients were Para 2 and belonged to the perimenopausal age-group (18 out of 36). The dominant presenting symptom was menorrhagia associated with dysmenorrhea and pelvic pain. After 6 months of treatment with mifepristone, the mean fibroid volume reduced from 204.33 to 113.16 cm3 (n = 33); p ≤ 0.001, and the percentage mean volume reduction of the fibroid in the study population was 44.57 % (range 1.10–100 %). Immediate reduction in bleeding PV was observed in 100 %, and 88.89 % (32/36) patients attained amenorrhea. The mean hemoglobin increased from 9.18 to 10.82 g/dl (p = 0.001). There was a transient rise in mean transaminases (AST/ALT) levels at 6 months which reverted to normal at 9 months follow-up.
Conclusion
To conclude, 6 months therapy with 50 mg of mifepristone given weekly is efficacious and acceptable for the treatment of symptomatic leiomyoma, especially in a select group of patients. Although its use as a primary medical therapy is limited due to recurrence of fibroid after stopping treatment, it is useful for perimenopausal women whose myoma would regress after menopause, and younger infertile patients with small-size deep intramural myomas not easily accessible to either hysteroscopic or laparoscopic surgery. It is also beneficial as a preoperative adjunct, in patients with preoperative severe anemia and large fibroids where surgery is technically difficult. Mode of surgery can be changed to a less-invasive vaginal hysterectomy rather than an abdominal procedure.
Keywords: Fibroid uterus, Mifepristone, Medical treatment, Hemoglobin, Endometrial hyperplasia
Introduction
Uterine fibroids, also called fibroid tumors, fibromyomas, myofibroma, leiomyofibroma, fibroleiomyoma, myoma, fibroma and leiomyoma, are benign tumors that develop in uterus. This is the most common tumor of the uterus and female pelvis [1]. The incidence is generally cited as 20–25 percent, but has been shown to be as high as 70–80 percent in studies using histologic and sonographic examinations. Majority of fibroids are asymptomatic; however, when symptomatic, patients present with menstrual disturbances, infertility, lump abdomen or pressure effects [2]. Management of fibroids can be surgical or medical. Among the surgical options, hysterectomy is viewed as the definitive management of symptomatic uterine fibroids. Myomectomy is an alternative for patients who desire childbearing, young patients, and for those who prefer to retain the uterus. Medical management may be given to minimize symptoms and signs, and for patients where surgery is contra-indicated. Drugs available are antifibrinolytic-tranexamic acid, danazol, GnRH analogs, progestogens and the antiprogesterone mifepristone. Mifepristone, an antiprogesterone was approved by FDA on September 28, 2000, with the trade name ‘Mifegyne’ as an abortifacient. The immune reactivity of progesterone receptors in the myoma and myometrial tissue was decreased significantly by this drug, suggesting the regression of these tumors may be obtained through a direct antiprogesterone effect. Varying dosage regimens have been suggested for treatment of fibroids though consensus is for a dose between 10 and 20 mg daily. Higher doses have not shown any increase in the beneficial effect while the side effects have been found to increase [3]. We studied the effect of mifepristone given once a week on uterine fibroids for 6 months. The available literature predominantly has studied effects of 3 months exposure with this drug, but in this study, we decided to study the effects of this drug when used for 6 months.
Materials and Methods
The study was designed as an observational prospective “before–after” study. A total of 36 patients were enrolled. Sample size was calculated to study changes in various parameters after 6 months treatment with mifepristone 50 mg once a week with the following specifications:
H0:µd = 0, H1:µd ≠ 0, IµI = 63.1
Level of significance = α = 5 %
Power of study = 1 − β = 80 %
Mean and range as per the available literature are tabulated in Table 1.
Table 1.
Mean and range as per the available literature
| Mifepristone treatment | Mean fibroid vol. | Range | Mean change |
|---|---|---|---|
| Before | 176.8 | 5.4-923 | 63.1 |
| After | 113.7 | 0.52-689 |
The expected mean reduction in fibroid size was taken to be 40 % and SD 24 %. Thus, the calculated sample size was 36. A total of 40 patients were recruited, of which 36 satisfied the inclusion/exclusion criteria. Inclusion criteria were fibroid size of 2.5 cm and above, those giving consent to be part of the study, reproductive age-group infertile patients or premenopausal patients, those accepting the use of barrier contraceptive during the period of the study, agreeing to have ultrasound examination at every follow-up or evaluation visit, agreeing to two endometrial biopsies—one before starting treatment and another within 10 days following treatment termination. Patients who were keen to become pregnant, breastfeeding patients, those on hormonal contraception or who had received any hormonal therapy in the last 3 months, any contraindications to receiving antiprogestins, adrenal insufficiency by history, sickle-cell disease, active liver disease (liver function tests greater than 1.5 times upper range of normal), severe respiratory disease (SpO2 < 92 %), renal disease (serum creatinine >1.5 mg/dl), coagulation defect (abnormal PT and PTT), thromboembolic disease (history of deep vein thrombosis or pulmonary embolus in the past) and those who refused to give consent were excluded from the study.
Permissions from institutional ethical committee were obtained. An informed consent was obtained from all the participants enrolled in the study. Baseline investigations were performed on all patients, which included CBC, LFT, RFT and thyroid function tests. Endometrial biopsy was done premenstrually to rule out endometrial hyperplasia, and transvaginal sonography was done to accurately document myoma volume. Menstrual blood loss was assessed using pictorial blood assessment chart (PBAC) scoring system.
The commercially available tablet of 200 mg was carefully broken into four equal parts (each part was 50 mg), and the monthly dose pack given to the patient with instruction to take 1/4th tablet on a fixed day each week. The patients were asked to take the drug for 6 months totally and called for review in the OPD at 1, 6 and 9 months. In the event of any adverse effects, they were told to stop the drug immediately and report to emergency unit/OPD. The patients were briefed about the possible side effects. They were also further followed up till 3 months after stopping the drug to observe the changes in menstrual pattern, fibroid volume, Hb and LFT. All patients were advised to use barrier contraception to avoid an inadvertent pregnancy. Patients who turned amenorrhoeic, were asked to report to the OPD where pregnancy was ruled out using urine βHCG. An endometrial biopsy was repeated at 6 month of therapy to rule out drug-induced hyperplasia.
Results
Demographically, majority of patients fell in 41–45 years age-group. Fifty percent were Para 2 (18 out of 36). The dominant symptom of our study group was menorrhagia associated with dysmenorrhea and pelvic pain. Seven out of 36 (19.4 %) of the patients initially presented with infertility as their main complaints and on further asking reported associated menorrhagia and dysmenorrhea (Table 2).
Table 2.
Characteristics of symptoms of patients
| Symptoms | Before treatment (%) |
|---|---|
| Menorrhagia | 32/36 (88.89 %) |
| Dysmenorrhea | 32/36 (88.89 %) |
| Pelvic pain | 34/36 (94.4 %) |
| Backache | 10/36 (27.8 %) |
| Urinary complaints | 3/36 (8.3 %) |
| Infertility | 7/36 (19.4 %) |
It was observed that after 6 months of treatment with mifepristone, the mean fibroid volume reduced from 204.33 to 113.16 cm3 (n = 33); p ≤ 0.001, and the percentage mean volume reduction of the fibroid in the study population was 44.57 % (range 1.10–100 %). However, at 9 months, the mean fibroid volume increased from 108.02 to 114.72 cm3 (n = 31); p value = 0.129, which was not statistically significant (Table 3).
Table 3.
Mean fibroid volume changes
| Parameter studied | N | Mean | SD | p value |
|---|---|---|---|---|
| Fibroid vol. | ||||
| Baseline | 33 | 204.08 | 135.63 | <0.001 |
| 6 month | 33 | 113.16 | 84.10 | |
| Fibroid vol. | ||||
| 6 month | 31 | 108.02 | 82.98 | 0.129 |
| 9 month | 31 | 114.72 | 98.39 | |
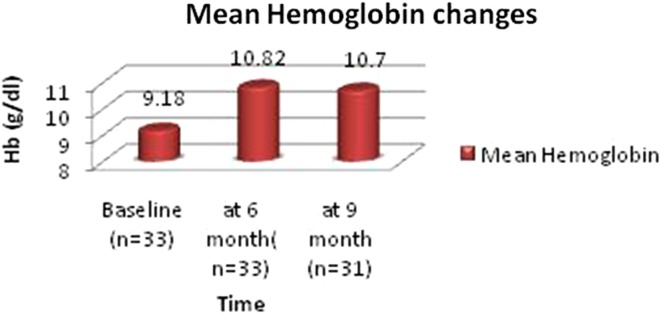
In the study population, immediate reduction in bleeding was observed in 100 %, and 88.89 % (32/36) patients attained amenorrhea. There was an improvement in general health and a feeling of well being primarily related to decreased menstrual blood loss and improvement in Haemoglobin. The mean Haemoglobin improved from 9.18 to 10.82 g/dl; p = 0.001 (n = 33), which was statistically significant. Subsequent follow-up at 9 months showed mean Hb increased from 10.70 g/dl at 6 month to 10.83 g/dl; p ≤ 0.001 (n = 31). This change was also statistically significant (Fig. 1).
Fig. 1.
Mean hemoglobin changes
Due to the antiprogesterone effect, an estrogen-dominant hormonal milieu is created at the endometrium, thereby causing endometrial changes following treatment with mifepristone (Table 4).
Table 4.
Effect on endometrium after treatment
| Endometrium | Before treatment (n = 36) | After treatment (n = 33) at 6 month |
|---|---|---|
| Normal | 35 (97 %) | 3 (9 %) |
| Atrophic | – | 2 (6 %) |
| Disordered proliferative | 1 (3 %) | 26 (79 %) |
| Proliferative | – | 1 (3 %) |
| Hyperplasia | – | – |
| Simple hyperplasia | – | – |
| Complex hyperplasia without atypia | – | 2 (6 %)a |
aOne patient opted for surgical therapy and underwent a total abdominal hysterectomy at 9½ month after initiation of the drug. Second patient was put on tab. MPA 10 mg TDS at 6 month after initiation of Mifepristone and was kept on follow-up
Assessment of mean blood loss by PBAC scoring system showed reduction from 111.70 (range 70–150) to 7.12 (range 0–70). Thirty-two out of 36 (88.89 %) of the patients at the end of 6 months attained amenorrhea, which is significant (p ≤ 0.001). As a consequence, the dysmenorrhea and pelvic pain were relieved, and subsequently, the hemoglobin rose, thereby reducing the requirement of blood transfusion.
Within the study period of 9 month, it was observed that there were two treatment failures where we had to resort to surgical treatment. One of the patients desired to conceive, and also the volume reduction of the fibroid was minimal, thus she underwent abdominal myomectomy during the study. The size of the fibroid was large (451.09 cm3 volume) and thus was not amenable to medical therapy. The second had to undergo total abdominal hysterectomy as she no longer desired fertility and symptoms showed no improvement. Histopathology of the specimen showed adenomyosis. The literature review also showed that adenomyosis is poorly responsive to mifepristone. One patient showed significant reduction in fibroid volume, but was found to have complex endometrial hyperplasia without atypia on EB. She was given high-dose progesterone for 3 months and was followed up with TVS monthly. She showed a thinned-out endometrium 3 months after stopping mifepristone.
Discussion
Mifepristone as a treatment option for myoma, was first reported by Murphy et al. [3]. Current studies support that growth of myoma is dependent on progesterone and therefore antiprogestins (mifepristone) and selective progesterone receptor modulators (SPRMs-Asoprisnil/Ulipristal) can be effective in treatment. Several clinical trials have been done since then with doses varying from 2.5 to 100 mg for 3–12 months [4]. Studies have found that a dose of 2.5 mg is not effective in reducing the myoma volume, but a dose as low as 5 mg per day and maximum as high as 50 mg per day was found effective [4, 5]. In the present observational prospective “before–after” study, 50 mg mifepristone given once a week was used for 6 months. The rationale of this dose was for ease of administration considering the tablet size commercially available, as well as to improve patient compliance with the once a week, Sunday-to-Sunday schedule. No other study has used this dose of 50 mg weekly. All studies reviewed suggest a daily dose regimen. The first dose was started within first 7 days of menstrual cycle. The drug was started once the endometrial biopsy report and pre-treatment investigations were available. It is known that if the drug is given in the early follicular phase, it starts acting before the development of the dominant follicle, and if given in the late follicular phase, it leads to collapse of the dominant follicle and withdrawal bleeding. In this study, the drug was started in early follicular phase for all patients. The mean myoma volume decreased by 44.57 % which is similar with 25 mg dose of Mukherjee et al. [6] and 5 and 10 mg for 6 months by Fiscella et al. [7]. Fibroid size of 2.5 cm was taken in our study as most fibroids below this cut-off are asymptomatic.
Mifepristone also caused significant improvement in leiomyoma-related symptoms. Mean blood loss (MBL) declined in 100 % of our patients. Of these, 88.89 % became amenorrhoeic and the rest showed a decreased MBL. However, MBL started increasing when observed at 9 months. Mean PBAC score reduced from 111.70 to 7.12 in the present study at 6 month. Kulshrestha et al. [8] found mean PBAC score reduced from 253 to 19.8 in 25 mg daily group and 289.2–10.4 in 10 mg group. In the present study, the change in fibroid volume showed a significant reduction of 44.57 % in fibroid volume at the end of therapy. The further follow-up at 3 months after cessation of therapy showed no further reduction. This suggests no residual effect of the drug. However, the lack of immediate increase in size of fibroids after stopping the drug also suggests that the progesterone receptors take some time to recover from the blockage by mifepristone before regrowth occurs. A total of seven patients had infertility as a presenting complaint in the study. Of these, one had primary infertility, and six had secondary infertility. All these patients, barring one, had small-sized symptomatic deep intramural fibroids not easily amenable to either hysteroscopic or laparoscopic removal. The rationale for use of this drug in these patients was to try and see if the size of the fibroid would reduce to such a point where it would not be a significant factor causing her symptoms and eventually her infertility. The one patient who had a large fibroid and infertility (volume 451 cm3) eventually did require a myomectomy.
Endometrial hyperplasia without atypia was found as one of the possible side effect of mifepristone therapy. Eisinger et al. [9] concluded that 28 % of subjects develop simple endometrial hyperplasia without atypia. However, these drug-induced endometrial changes are reversible as menstruation resumes on cessation of the drug. In the study, two of the patients developed complex hyperplasia of the endometrium without atypia (6 % of the study group).
Conclusion
Six months therapy with 50 mg of mifepristone given weekly is efficacious and acceptable for the treatment of symptomatic leiomyoma. Although its use as a primary medical therapy is limited due to recurrence after stopping treatment, it can be used especially for perimenopausal women whose myoma would regress after menopause and younger infertile patients with small-size deep intramural myomas not easily accessible to either hysteroscopic or laparoscopic surgery. It can also be beneficial as a preoperative adjunct, in patients with preoperative severe anemia and large fibroids where surgery is technically difficult. Mode of surgery can be changed to a less-invasive vaginal hysterectomy rather than an abdominal procedure.
Dr. Anupam Kapur
is Professor and HOD in Obstetrics and Gynecology at INHS Asvini, Mumbai. Previously, he was Professor in the Department of Obstetrics and Gynaecology, Armed Forces Medical College, Pune. He is a trained endoscopic surgeon with keen interest in hysteroscopic surgeries. He has taught undergraduate and postgraduate students for the last 20 years in armed forces. He has completed 12 research projects and published research papers in various journals. He regularly delivers lectures in various conferences, and was the resource person for various workshops and symposia. He is presently working on various risk factors associated with late-preterm birth and also pregnancy outcomes after hysteroscopic surgeries.
Compliance with Ethical Standards
Conflict of interest
Anupam Kapur, Ranjeeta Angomchanu and Madhusudan Dey declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standard of the responsible committee on human experimentation (national or institutional) and with the Helsinki declaration of 1975, as revised in 2008(5).
Informed Consent
Additional informed consent was obtained from all patients for whom identifying information is included in this article.
Footnotes
Anupam Kapur is a Prof and HOD at the Department of Obstetrics and Gynecology, INHS Asvini; Ranjeeta Angomchanu is a Graded Specialist at the Military Hospital; Madhusudan Dey is a Reader at the Department of Obstetrics and Gynecology, Armed Forces Medical College.
References
- 1.Breech LL, Rock JA. Leiomyomata uteri and myomectomy. In: Rock JA, Jones HW III, editors. Te Linde’s operative gynecology. 10. New Delhi: Wolters Kluwer Pvt Ltd; 2010. pp. 687–726. [Google Scholar]
- 2.Buttram VC, Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36:433–445. doi: 10.1016/S0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 3.Murphy AA, Kettel LM, Morales AJ, et al. Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J Clin Endocrinol Metab. 1993;76(2):513–517. doi: 10.1210/jcem.76.2.8432797. [DOI] [PubMed] [Google Scholar]
- 4.Carbonell JL, Acosta R, Perez Y, et al. Safety of 10 mg versus 5 mg of Mifepristone during 9 months for the treatment of uterine fibroids. Double-blind randomized clinical trial. Int J Women’s Health. 2013;5:115–124. doi: 10.2147/IJWH.S33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagaria M, Suneja A, Vaid NB, et al. Low-dose Mifepristone in treatment of uterine leiomyoma: a randomized double-blind placebo-controlled clinical trial. Aust N Z J Obstet Gynaecol. 2009;49:77–83. doi: 10.1111/j.1479-828X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee S, Chakraborty S. A study evaluating the effect of Mifepristone (RU 486) for the treatment of leiomyomata uteri. Niger Med J. 2011;52(3):150–152. doi: 10.4103/0300-1652.86123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiscella K, Eisinger SH, Meldrum S, et al. Effect of Mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol. 2006;108(6):1381–1387. doi: 10.1097/01.AOG.0000243776.23391.7b. [DOI] [PubMed] [Google Scholar]
- 8.Kulshrestha V, Kriplani L, Agarwal N, et al. Low dose Mifepristone in medical management of uterine leiomyoma—an experience from a tertiary care hospital from north India. Indian J Med Res. 2013;137:1154–1162. [PMC free article] [PubMed] [Google Scholar]
- 9.Eisinger SH, Bonfiglio T, Fiscella K, et al. Twelve month safety and efficacy of low-dose Mifepristone for uterine myomas. J Minim Invasive Gynecol. 2005;12(3):227–233. doi: 10.1016/j.jmig.2005.01.022. [DOI] [PubMed] [Google Scholar]