Abstract
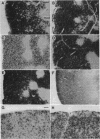
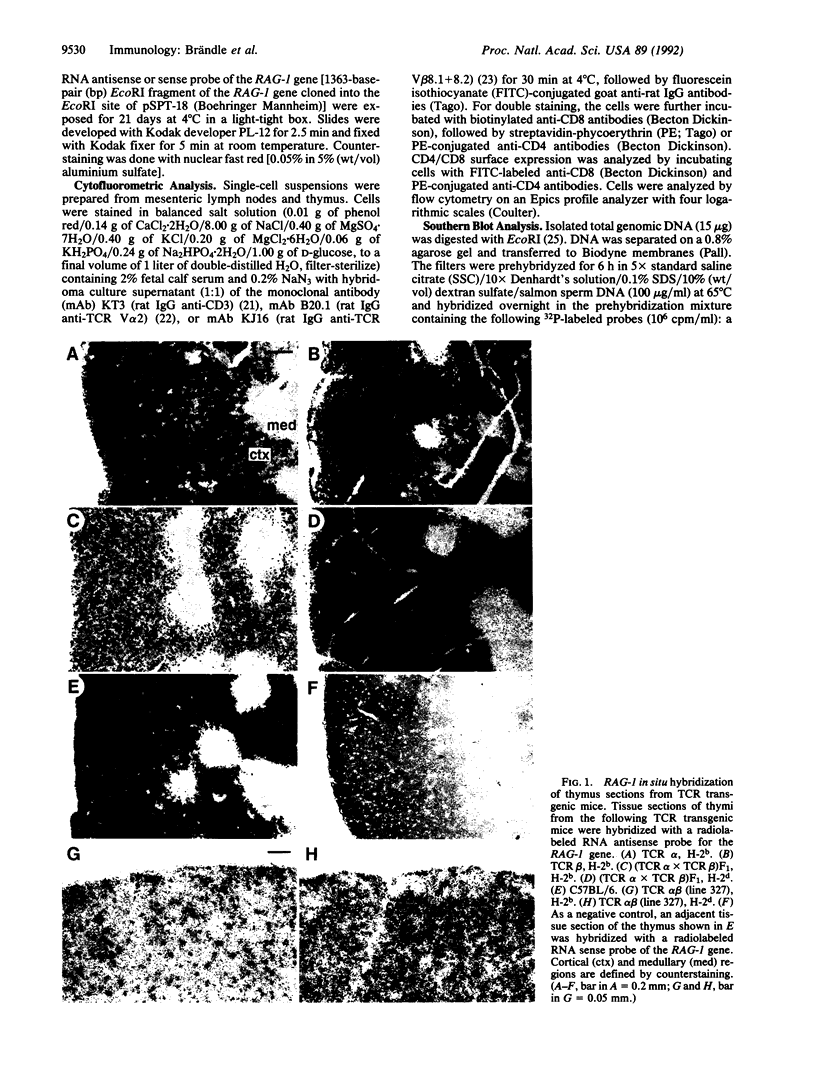
We have examined the expression of the recombination activating gene RAG-1 by in situ hybridization to thymi from mice bearing transgenes for the T-cell receptor (TCR) alpha chain, TCR beta chain, or both TCR alpha and beta chains. RAG-1 transcription was found in the thymic cortex of transgenic mice carrying a single TCR alpha- or TCR beta-chain transgene, comparable to normal mice. However, RAG-1 transcription was strikingly reduced in the thymic cortex from transgenic mice carrying both TCR alpha- and beta-chain genes and expressing major histocompatibility complex (MHC) class I (H-2b) molecules necessary for positive selection of the transgenic TCR. In contrast, thymi of transgenic mice also carrying both TCR alpha- and beta-chain genes but expressing MHC molecules (H-2d) that did not positively select the transgenic TCR displayed high levels of RAG-1 transcription. The low thymic RAG-1 expression coincided with high transgenic TCR alpha-chain surface expression and with inhibition of endogenous TCR alpha-chain rearrangement. Our findings suggest that binding of the TCR to self MHC molecules during positive selection down-regulates RAG-1 transcription in cortical thymocytes and thereby prevents further TCR alpha-chain rearrangements.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebischer T., Oehen S., Hengartner H. Preferential usage of V alpha 4 and V beta 10 T cell receptor genes by lymphocytic choriomeningitis virus glycoprotein-specific H-2Db-restricted cytotoxic T cells. Eur J Immunol. 1990 Mar;20(3):523–531. doi: 10.1002/eji.1830200310. [DOI] [PubMed] [Google Scholar]
- Blüthmann H., Kisielow P., Uematsu Y., Malissen M., Krimpenfort P., Berns A., von Boehmer H., Steinmetz M. T-cell-specific deletion of T-cell receptor transgenes allows functional rearrangement of endogenous alpha- and beta-genes. Nature. 1988 Jul 14;334(6178):156–159. doi: 10.1038/334156a0. [DOI] [PubMed] [Google Scholar]
- Borgulya P., Kishi H., Uematsu Y., von Boehmer H. Exclusion and inclusion of alpha and beta T cell receptor alleles. Cell. 1992 May 1;69(3):529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- Brändle D., Bürki K., Wallace V. A., Rohrer U. H., Mak T. W., Malissen B., Hengartner H., Pircher H. Involvement of both T cell receptor V alpha and V beta variable region domains and alpha chain junctional region in viral antigen recognition. Eur J Immunol. 1991 Sep;21(9):2195–2202. doi: 10.1002/eji.1830210930. [DOI] [PubMed] [Google Scholar]
- Casanova J. L., Romero P., Widmann C., Kourilsky P., Maryanski J. L. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J Exp Med. 1991 Dec 1;174(6):1371–1383. doi: 10.1084/jem.174.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Couez D., Malissen M., Buferne M., Schmitt-Verhulst A. M., Malissen B. Each of the two productive T cell receptor alpha-gene rearrangements found in both the A10 and BM 3.3 T cell clones give rise to an alpha chain which can contribute to the constitution of a surface-expressed alpha beta dimer. Int Immunol. 1991 Jul;3(7):719–729. doi: 10.1093/intimm/3.7.719. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Fenton R. G., Marrack P., Kappler J. W., Kanagawa O., Seidman J. G. Isotypic exclusion of gamma delta T cell receptors in transgenic mice bearing a rearranged beta-chain gene. Science. 1988 Aug 26;241(4869):1089–1092. doi: 10.1126/science.2970670. [DOI] [PubMed] [Google Scholar]
- Finkel T. H., Cambier J. C., Kubo R. T., Born W. K., Marrack P., Kappler J. W. The thymus has two functionally distinct populations of immature alpha beta + T cells: one population is deleted by ligation of alpha beta TCR. Cell. 1989 Sep 22;58(6):1047–1054. doi: 10.1016/0092-8674(89)90503-5. [DOI] [PubMed] [Google Scholar]
- Furutani M., Yanagi Y., Fujisawa I., Nakayama T., Kishimoto H., Kuida K., Asano Y., Tada T. Post-transcriptional allelic exclusion of two functionally rearranged T cell receptor alpha genes. Int Immunol. 1989;1(3):281–288. doi: 10.1093/intimm/1.3.281. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Haskins K., Hannum C., White J., Roehm N., Kubo R., Kappler J., Marrack P. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. VI. An antibody to a receptor allotype. J Exp Med. 1984 Aug 1;160(2):452–471. doi: 10.1084/jem.160.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue I., Trucy J., McCoy C., Couez D., Malissen B., Malissen M. A novel type of aberrant T cell receptor alpha-chain gene rearrangement. Implications for allelic exclusion and the V-J recombination process. J Immunol. 1990 Jun 1;144(11):4410–4419. [PubMed] [Google Scholar]
- Huesmann M., Scott B., Kisielow P., von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991 Aug 9;66(3):533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Efstratiadis A., O'Connell C., Maniatis T. The nucleotide sequence of the human beta-globin gene. Cell. 1980 Oct;21(3):647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- Malissen M., Trucy J., Letourneur F., Rebaï N., Dunn D. E., Fitch F. W., Hood L., Malissen B. A T cell clone expresses two T cell receptor alpha genes but uses one alpha beta heterodimer for allorecognition and self MHC-restricted antigen recognition. Cell. 1988 Oct 7;55(1):49–59. doi: 10.1016/0092-8674(88)90008-6. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 Mar 6;68(5):869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Müller C., Kägi D., Aebischer T., Odermatt B., Held W., Podack E. R., Zinkernagel R. M., Hengartner H. Detection of perforin and granzyme A mRNA in infiltrating cells during infection of mice with lymphocytic choriomeningitis virus. Eur J Immunol. 1989 Jul;19(7):1253–1259. doi: 10.1002/eji.1830190716. [DOI] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Pircher H., Bürki K., Zinkernagel R. M., Hengartner H. Distinct sequence of negative or positive selection implied by thymocyte T-cell receptor densities. Nature. 1990 Aug 30;346(6287):861–863. doi: 10.1038/346861a0. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Wallace V. A., Broughton H., Ohashi C. T., Ferrick D. A., Jost V., Mak T. W., Hengartner H., Pircher H. Specific deletion of the J-C delta locus in murine alpha/beta T cell clones and studies using transgenic mice. Eur J Immunol. 1990 Mar;20(3):517–522. doi: 10.1002/eji.1830200309. [DOI] [PubMed] [Google Scholar]
- Pircher H., Bürki K., Lang R., Hengartner H., Zinkernagel R. M. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989 Nov 30;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Pircher H., Mak T. W., Lang R., Ballhausen W., Rüedi E., Hengartner H., Zinkernagel R. M., Bürki K. T cell tolerance to Mlsa encoded antigens in T cell receptor V beta 8.1 chain transgenic mice. EMBO J. 1989 Mar;8(3):719–727. doi: 10.1002/j.1460-2075.1989.tb03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H., Moskophidis D., Rohrer U., Bürki K., Hengartner H., Zinkernagel R. M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990 Aug 16;346(6285):629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- Pircher H., Ohashi P., Miescher G., Lang R., Zikopoulos A., Bürki K., Mak T. W., MacDonald H. R., Hengartner H. T cell receptor (TcR) beta chain transgenic mice: studies on allelic exclusion and on the TcR+ gamma/delta population. Eur J Immunol. 1990 Feb;20(2):417–424. doi: 10.1002/eji.1830200227. [DOI] [PubMed] [Google Scholar]
- Pircher H., Rebaï N., Groettrup M., Grégoire C., Speiser D. E., Happ M. P., Palmer E., Zinkernagel R. M., Hengartner H., Malissen B. Preferential positive selection of V alpha 2+ CD8+ T cells in mouse strains expressing both H-2k and T cell receptor V alpha a haplotypes: determination with a V alpha 2-specific monoclonal antibody. Eur J Immunol. 1992 Feb;22(2):399–404. doi: 10.1002/eji.1830220217. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Thompson S. D., Pelkonen J., Rytkönen M., Samaridis J., Hurwitz J. L. Nonrandom rearrangement of T cell receptor J alpha genes in bone marrow T cell differentiation cultures. J Immunol. 1990 Apr 1;144(7):2829–2834. [PubMed] [Google Scholar]
- Tomonari K. A rat antibody against a structure functionally related to the mouse T-cell receptor/T3 complex. Immunogenetics. 1988;28(6):455–458. doi: 10.1007/BF00355379. [DOI] [PubMed] [Google Scholar]
- Turka L. A., Schatz D. G., Oettinger M. A., Chun J. J., Gorka C., Lee K., McCormack W. T., Thompson C. B. Thymocyte expression of RAG-1 and RAG-2: termination by T cell receptor cross-linking. Science. 1991 Aug 16;253(5021):778–781. doi: 10.1126/science.1831564. [DOI] [PubMed] [Google Scholar]
- Uematsu Y., Ryser S., Dembić Z., Borgulya P., Krimpenfort P., Berns A., von Boehmer H., Steinmetz M. In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell. 1988 Mar 25;52(6):831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- Wilson A., Pircher H., Ohashi P., MacDonald H. R. Analysis of immature (CD4-CD8-) thymic subsets in T-cell receptor alpha beta transgenic mice. Dev Immunol. 1992;2(2):85–94. doi: 10.1155/1992/45150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]