Abstract
Objectives
Magnetic resonance imaging (MRI)-guided high intensity focused ultrasound surgery (MRgFUS) is a newly emerging non-invasive technique for the treatment of uterine fibroids. The purpose of this study is to review the clinical impact of MRgFUS.
Methods
This study examined 157 patients. The high intensity focused ultrasound (HIFU) utilized in this study was Philips Achieva 1.5 Tesla MR (Philips Healthcare, Best, the Netherlands) and Sonalleve HIFU system. The patients were followed in post-operative Month 1, Month 3, and Month 6 to investigate any change. Then, these were further classified according to the use of uterine stimulant (oxytocin) in parallel, Funaki Type of uterine fibroid, HIFU intensity, and non-perfused volume (NPV) ratio.
Results
When the uterine stimulant was utilized, the HIFU intensity was measured at significantly lower levels, compared with the group not using uterine stimulant, and treatment duration was significantly. The NPV ratio was found significantly higher in the group using uterine stimulant. Concerning the correlation between Funaki Type of uterine fibroid and average sonication power, it was found that the closer to Type I, the lower the sonication power, the shorter the treatment duration, and the higher the NPV ratio significantly.
Conclusions
In this study, it was found that the lower the Funaki Types of uterine fibroids, and the higher the NPV ratio immediately after the operation, the larger the uterine fibroid volume decrease and SSS change were. Also, if uterine stimulant was used in parallel in treatment, treatment duration and HIFU intensity could become shorter and lower.
Keywords: High-intensity focused ultrasound ablation, Leiomyoma, Myoma, Oxytocin
Introduction
Uterine fibroids are one of the most common tumor diseases in women, accounting for 20% to 25% of the female population. There are diverse ongoing procedures for uterine fibroids to treat profuse menstruation, menstrual pain increase, size increase, subfertility, postmenopausal size increase and others.1,2 Laparoscopic myomectomy, one of the treatment procedures of uterine fibroids, has been widely known for its effectiveness.3,4 In subfertility patients, significant differences have been reported in their post-myomectomy fertility.5,6 In menopausal women, however, it is reported that, under hormone treatment (estrogen-progestin therapy), their uterine fibroid sizes could increase temporarily and this is irrelevant to estrogen medication methods and progesterone addition.7,8 Recently, as a way of minimum invasion, single-incision laparoscope operation has been actively implemented, which utilizes only 1 of the existing 3 to 4 ports used in the previous laparoscope operation.9,10
However, more non-invasive techniques are sought after to avoid the risk of general anesthesia, longer hospitalization and recovery period. Recently, as a non-invasive technique, high intensity focused ultrasound (HIFU) has been introduced and diversely implemented. HIFU is a thermal therapy that increases the temperature of a focal point up to 57℃ to 80℃ to result in coagulative tissue necrosis while preserving uterus. HIFU does not require general anesthesia and hospitalization. Patients can leave the hospital on the very day of operation and return to their daily lives. HIFU also gets the limelight as a non-invasive treatment technique of adenomyosis in subfertility patients, which has had no effective therapy. HIFU technique is reported to promote the relief of symptoms due to adenomyosis.11,12 Also, studies have been going on about the impaired implantation and habitual miscarriage in subfertility cases due to adenomyosis.13,14
HIFU treatment is divided into ultrasound image-guided HIFU (USgHIFU) and magnetic resonance imaging (MRI)-guided HIFU (MRgHIFU) based on the operative uterine fibroid monitoring methods. The MRI-guided technique allows thermometry and easy monitoring of surrounding organs. But it is a quasi-real time technique. The ultrasound image-guided technique does not allow thermometry implementation and monitoring of surrounding organs and parts deeper inside. Still, the treatment is made within a relatively shorter period of time. HIFU cannot ultimately eradicate uterine fibroids and makes tissue inspection difficult.
This research by the Obstetrics and Gynecology (Ob/Gyn) of the Hospital introduces the clinical effectiveness of Sonalleve-MRgFUS by investigation the uterine myoma volume change, patients' clinical improvement status and re-intervention in uterine fibroid cases treated with Sonalleve-MRgFus.
Materials and Methods
1. Study subject and method
This study investigated 157 patients who had received MRgFUS at the Ob/Gyn of the Hospital from March 2015 to February 2016. This study was approved by the Institutional Review Board of the Hospital. All of the study treatment procedures were performed by the HIFU team consisting of 4 obstetricians, 2 medical imaging specialists, 2 radiotherapy specialists and 1 nurse. The MRI and HIFU utilized in this study were Philips Achieva 1.5 Tesla MR (Philips Healthcare, Best, The Netherlands) and Sonalleve HIFU system. Patients' information was collected via retrospective data analysis based on hospitalization record, outpatient record, and operational record. Patients' information includes age, past child delivery record, past medical operational history, HIFU indicant, adenomyosis existence, Funaki type of uterine fibroid, the number of treated uterine fibroids in multiple myoma cases, HIFU treatment hours, Modified blood-retinal barrier (BRB) use, average HIFU intensity, gaps between pre/post-operative changes in hemoglobin (Hb) and cancer antigen 125 (CA-125), post-operative pain and skin burn, complication existence such as intestinal or bladder damage, changes in uterine fibroid volumes in post-operative Month 1, Month 3, and Month 6, and post-operative changes in clinical symptoms. Concerning Funaki type of uterine fibroid, if a fibroid showed lower intensity image in the pre-operative T2-weighted image, compared with the skeletal muscle signal, such a case was defined as Type I,; higher than the skeletal muscle signal but lower than myometrium signal, Type II; equal to or higher than the myometrium signal, Type III To evaluate the seriousness of patients' symptoms.15 the Symptom severity score (SSS) was utilized in the study to measure their pre-operative status and post-operative Month 1, Month 3, and Month 6 status.16 SSS investigated whether the patients had profuse menstruation, blood clots during menstruation period, a long period, serious change in menstrual cycle, lower abdominal discomfort, dysuria, nocturia, and fatigue by scoring them from 1 to 5 points then sum the scores. Treatment duration was defined as the time of HIFU commencement to the time of final round of HIFU treatment. For pre/post-treatment blood tests, the measurements gained in the last pre-treatment blood test were utilized as well as the measurements in post-treatment Month 1. Month 3, and Month 6. Complication is defined as a physical status in need of additional medical treatment due to HIFU treatment. Complication cases include urogenital problems such as at least 5-day-long uterine hemorrhage, 3-day-long fever or longer, and hematuria; along with intestinal injury, nerve damage and skin burn. Temporary hematuria and skin burn not more serious than the first degree and not larger than 2 cm in its size were excluded.
2. Operation method
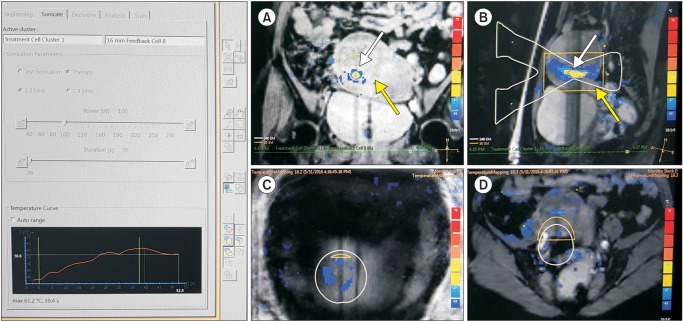
All of the patients fasted for 8 hours then visited the outpatient unit to do the general preparations for a surgical operation and moved to the MRI room. The patients took a prone position on the MRI bed. The HIFU coils were placed on the patients and MRI scan was implemented to adjust a precise focus. If the intestine was found in front of uterus in the first MRI test scan just before the treatment, modified BRP (bladder and rectum filling and bladder emptying) technique was conducted to avoid possible intestinal injury by high intensity ultrasound. Modified BRP technique is to change the position of uterus or intestine by filling in the bladder with normal saline or rectum with glycerin or through other diverse techniques in order not to expose intestine to HIFU for intestine damage prevention. To control patients' pain, Fentanyl (Fentanyl inj., 500 ug/10 mL) and Ketorolac (Keromin inj., 30 mg/1 mL) were injected through infusion pump. If the operation duration is longer than 60 minutes, epidural catheter was inserted then Fentanyl and Bupivacaine (Bupivacaine MYM inj., 50 mg/20 mL) were administered to control pain. The patients received uterine stimulant, Carbetocin (Duratocin inj., 100 ug/1 mL), together, if necessary. For the sake of safety, an emergency stop button was placed in the patients' hand. Then, HIFU treatment was conducted under real-time MRI scanning. The HIFU treatment was done under quasi real time MRI scanning. Based on thermometry, HIFU-caused temperature change in the focus area was observed along with the temperature change in surrounding organs (Fig. 1). The Hospital's Sonalleve HIFU system is linked to Philips Achieva 1.5 Tesla MR. Its treatable depth is up to 12 cm inside from patients' skin; frequency of high intensity ultrasound is 1.2 or 1.4 MHz; and output can reach up to 200 W. Sonalleve HIFU system employs the volumetric heating algorithm and uses 4 mm, 8 mm, 12 mm, 16 mm-diameter treatment cells.17 The Hospital set the goal of focal point temperature increase to at least 57℃ and 240 eqivalent minutes (EM) and has applied diverse cell sizes. By using the minimum output power reaching the targeted focal point temperature, skin burn was minimized. To measure the tissue temperature change, the MRI-thermometry was recorded based on proton resonance frequency shift.18,19 Every patient was scanned with pre-operative T2-, T1-weighted MRI and contrast enhanced T1-weighted MRI while receiving contrast enhanced T1-weighted MRI scanning immediately after the operation for non-perfused volume (NPV) check before completing the procedure (Fig. 2). Post-treatment NPV was calculated by measuring the circumference of sequential slice in the prolate ellipse (length × width × depth × 0.523) method.15
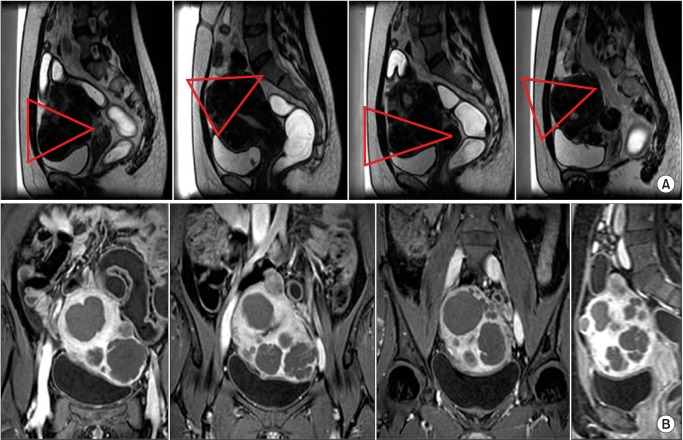
Fig. 1. Coronal (A) and sagittal (B) magnetic resonance (MR) thermometry images show temperature overlay and the magnetic resonance (MR); white lines, white arrow) during volumetric MR imaging (MRI)-guided high intensity focused ultrasound surgery (MRgFUS) ablation with a 16 mm treatment cell. X-shaped two white triangles are their presumed sonication pathway. Yellow box indicate the 30-240 EM area, the possible thermal damage (yellow arrow). MR thermometry show the temperature of abdominal skin (C) & tissues adjacent to sacrum (D).
Fig. 2. (A) T2-, (B) T1-weighted magnetic resonance (MR) image before high intensity focused ultrasound (HIFU), (C) Contrast enhanced T1-weighted MR image after HIFU show excellent non-perfused volume (NPV) and NPV ratio. Arow indicate the multiple fibroid of uterus.
3. Statistical analysis
For this study statistical analysis, SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was employed. Chi-square test or Fischer's exact test, independent sample t-test, and one-way analysis of variance (ANOVA) were utilized. If the significance probability (P value) was less than 0.05, such a case was deemed statistically significant in this study.
Results
The ages of 157 patients receiving MRI-guided HIFU procedure ranged widely from 25 to 66 but more of them were around the age of 42 with the mean age of 42.08 years old, standard deviation of 6.20 years, and median of 42.50 years. Only 3 of them were after menopause while most of them were before their menopause. In 35% of them, adenomyosis was also accompanied. Eight (5.1%) of them had experienced hysteroscopic myomectomy. The average number of uterine fibroids was 4.24 with the average size of 7.27 ± 2.63 cm and volume of 174.48 ± 206.97 cm3. Fifty-eight (36.9%) of them were Funaki type I; 43 (27.4%), Funaki type II; and 54 (34.4%), Funaki type III. Of them, 115 (73.2%) had intramural fibroids and their main symptom was profuse menstruation found in 31 (19.7%), the largest part of them. The average SSS was 27.24 ± 4.76 (Table 1).
Table 1. Clinical characteristics of 157 patients undergone magnetic resonance imaging-guided high intensity focused ultrasound (MRgFUS).
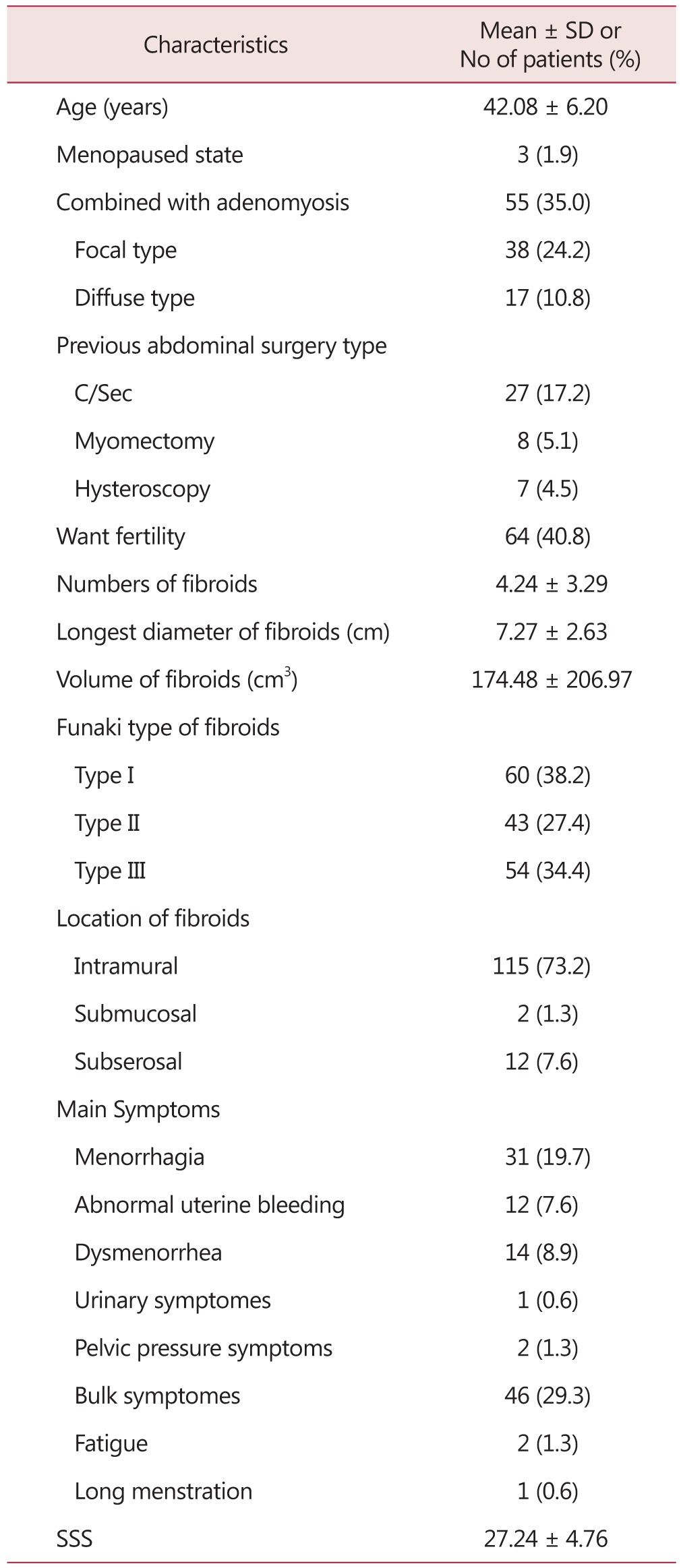
C/Sec: caesarian section, SSS: symptoms severity score, SD: standard deviation
The average treatment hours was 102.24 ± 56.24 minutes and average HIFU intensity was 115.22 ± 25.43 W. The average NPV was found 107.30 ± 132.52 and, average NPV ratio was 65.60 ± 22.73% (Table 2).
Table 2. Operative outcomes of 157 patients undergone magnetic resonance imaging-guided high intensity focused ultrasound (MRgFUS).
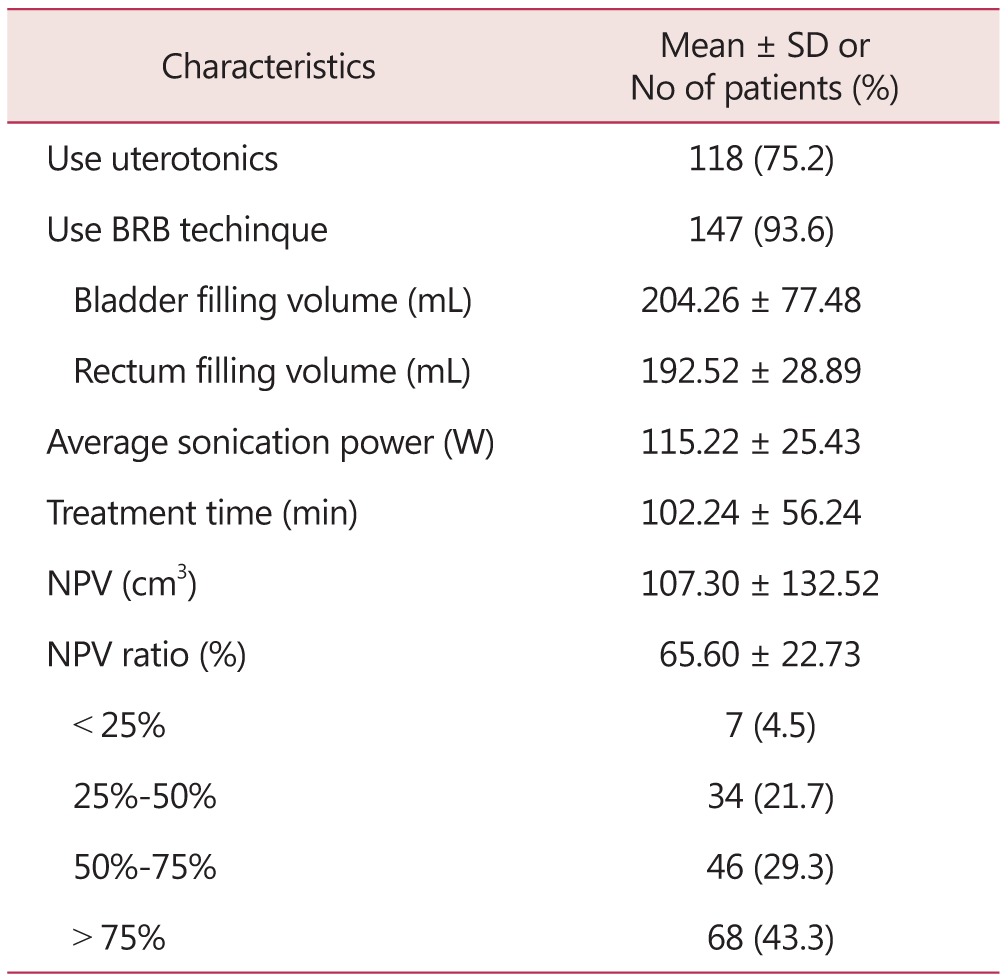
SD: standard deviation, BRB: blood–retinal barrier, min: minute, NPV: non-perfused volume
The post-operative SSS began to decrease fast to record, 72% in Month 1 and 45% in Month 3 (Table 3). In Month 6, however, the level was observed as high as 76%. This is deemed because many patients were not followed more than 3 months for their symptom relief while most of the patients followed for 6 months experienced insignificant symptom recovery due to the accompanied adenomyosis, etc. Uterine fibroid volume in Month 1 fell to 42%; Month 3, 35%; and Month 6, 25%. Post-MRgFUS Hb levels did not change much. It is deemed that this is because the levels were controlled or being controlled in most of the cases thanks to iron preparation taking or transfusion, etc. and most patients without a symptom refused blood collecting. CA-125 decreased from the average pre-operative level of 67.12 ± 120.90 to 39.08 ± 41.27 in post-operative Month 1 but rose to 75.29 ± 164.66 in Month 3; and 219.42 ± 265.56, in Month 6. It is also deemed because patients with recovery were not followed while only those with slow recovery due to accompanied adenomyosis, etc. were followed. Complications were found in 20 cases (12.7%). Minor burn was found in 19 cases and continued abnormal uterine hemorrhage, in 1 case. But no surgical operation, etc. were performed in any case. Severe burn, nerve damage or intestinal damage was not observed. In 13 cases, re-intervention was observed. In 6 cases, hysteroscopic myomectomy was performed; in 4 cases, mirena insertion; in 2 cases, laparoscopic myomectomy; and in 1 case, alparoscopic hysterectomy.
Table 3. Clinical outcomes of 157 patients undergone magnetic resonance imaging-guided high intensity focused ultrasound (MRgFUS).
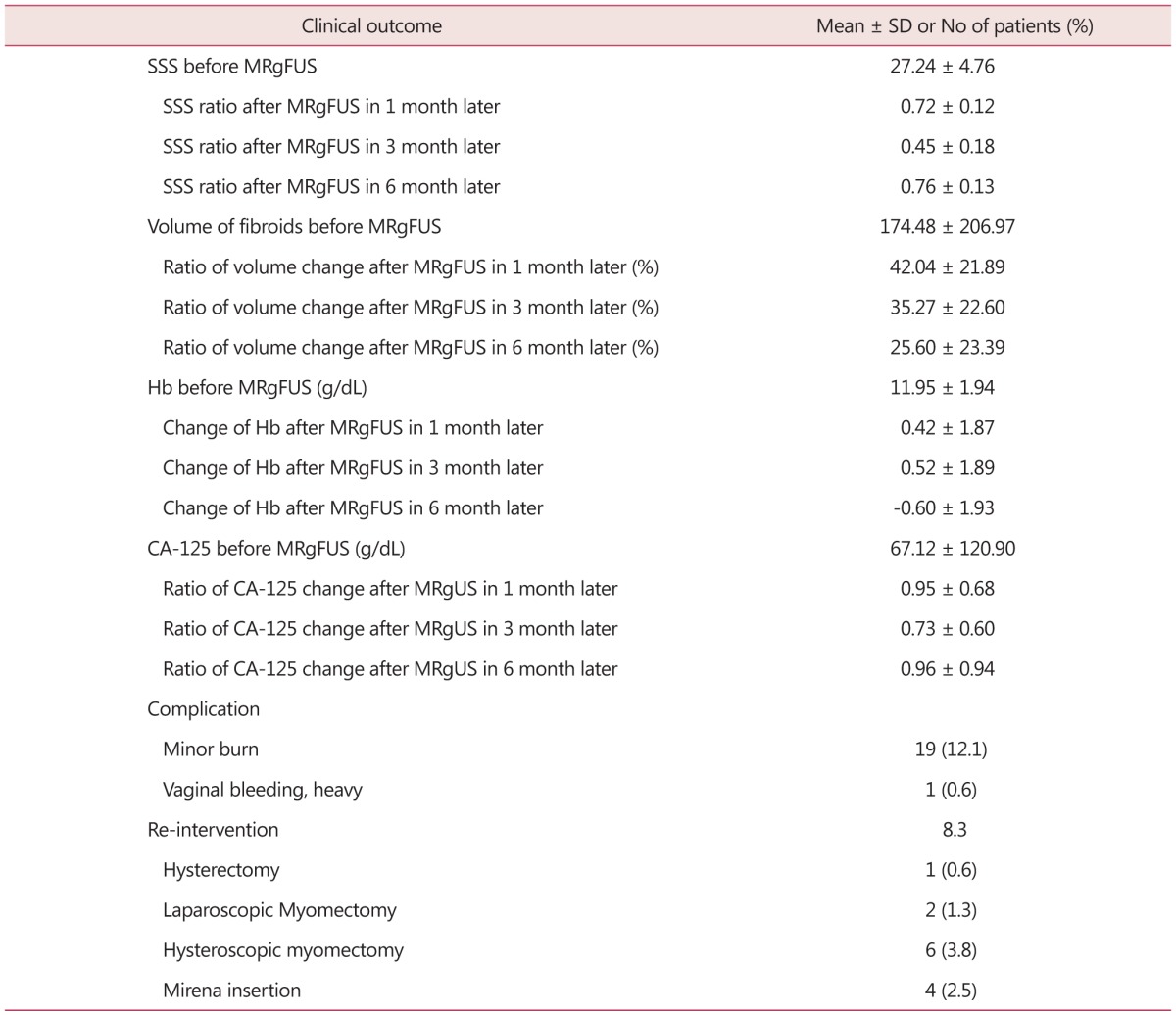
SD: standard deviation, SSS: symptom severity score, MRgFUS: magnetic resonance imaging-guided high intensity focused ultrasound, Hb: hemoglobin, CA-125: cancer antigen 125
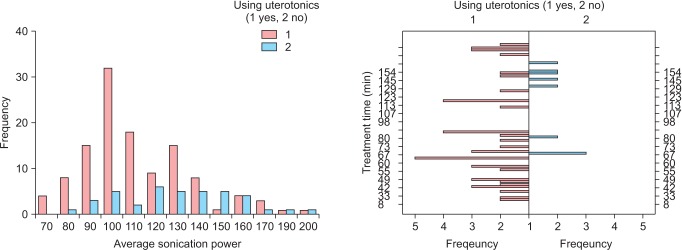
Among the patients receiving uterine fibroids ablation based on MRI-guided HIFU, uterine stimulant was used in parallel in 39 cases (Table 4). When the uterine stimulant was used, the group showed statistically significantly lower HIFU intensity (110.26 ± 22.60 vs. 130.51 ± 27.81 W) than the group not using uterine stimulant. And its treatment duration was found shorter as well (94.68 ± 54.53 vs. 127.76 ± 53.63 minutes) (Fig. 3). The NPV ratio was also found higher in the uterine stimulant-using group, indicating significantly higher therapeutic effectiveness (67.30 ± 21.59 vs. 59.87 ± 25.27). The SSS change ratio in post-operative Month 3 was also measured statistically significantly lower, indicating better performance of post-operative symptom ease in the uterine stimulant-using group (0.33 ± 0.10 vs. 0.43 ± 0.16). No other complication, uterine fibroid volume change, or re-intervention correlation was observed.
Table 4. Clinical outcomes of 157 patients undergone magnetic resonance imaging-guided high intensity focused ultrasound (MRgFUS) used with uterotonics.
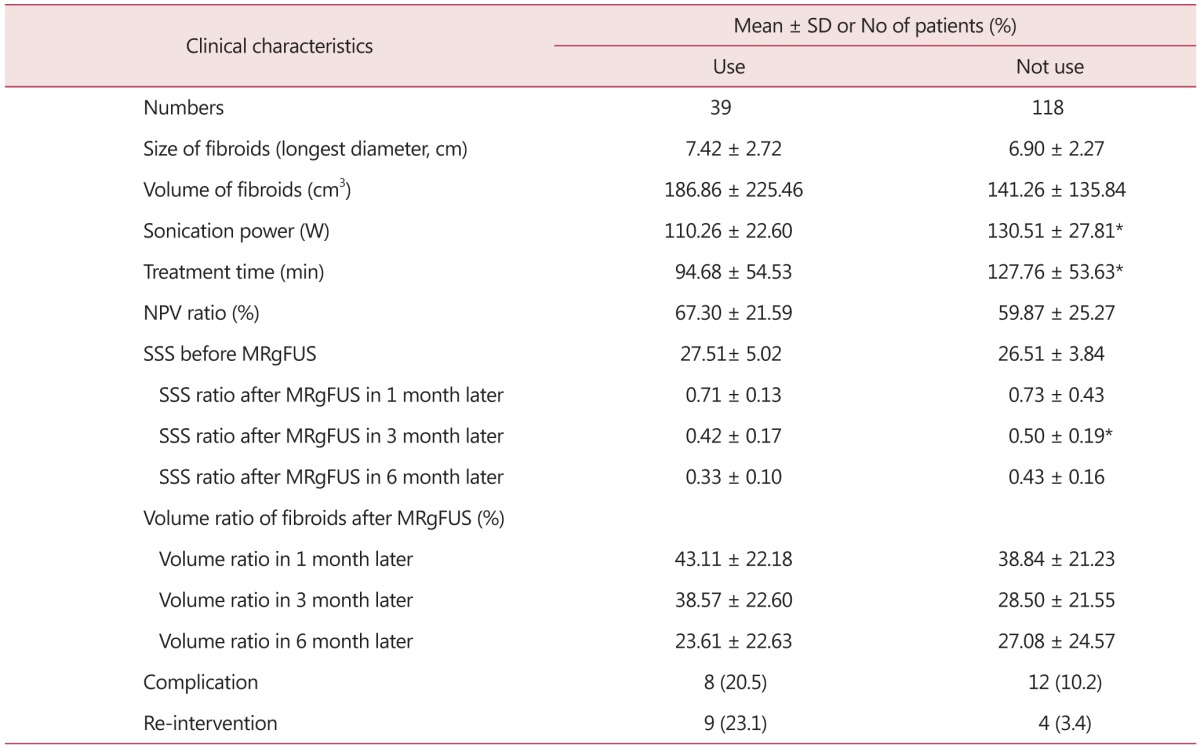
*P value < 0.05
SD: standard deviation, min: minute, NPV: non-perfused volume, SSS: symptom severity score, MRgFUS: magnetic resonance imaging-guided high intensity focused ultrasound
Fig. 3. Correlation between average sonication power, average treatment time, and using with uterotonics.* *Spearman correlation coefficient were 0.320 between the average sonication power and using or not using with uterotonics, -0.344 between treatment time and using or not using with uterotonics.
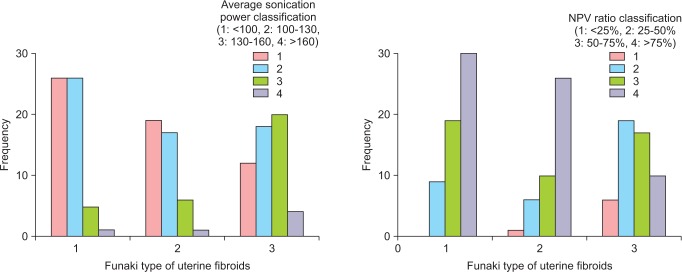
Concerning the correlation between Funaki type of uterine fibroid and average sonication power, it was found that the closer to Type I, the lower the sonication power was with statistical significance (spearman correlation coefficient 0.305). Regarding the correlation between treatment hours and NPV ratio, shorter treatment hours and higher NPV ratio were observed with statistical significance. The spearman correlation coefficient was 0.185, and correlation coefficient was -0.320, respectively (Table 5; Fig. 4). According to Funaki type of uterine fibroid, Type I and Type II showed significantly lower pre-operative SSS levels and the SSS change ratios were also significantly lower in all of the post-operative Month 1, Month 3, and Month 6. However, no correlation was found between Funaki type of uterine fibroid and uterine fibroid volume change, complication existence or re-intervention.
Table 5. Clinical outcomes of 157 patients undergone magnetic resonance imaging-guided high intensity focused ultrasound classified by Funaki type of fibroids.
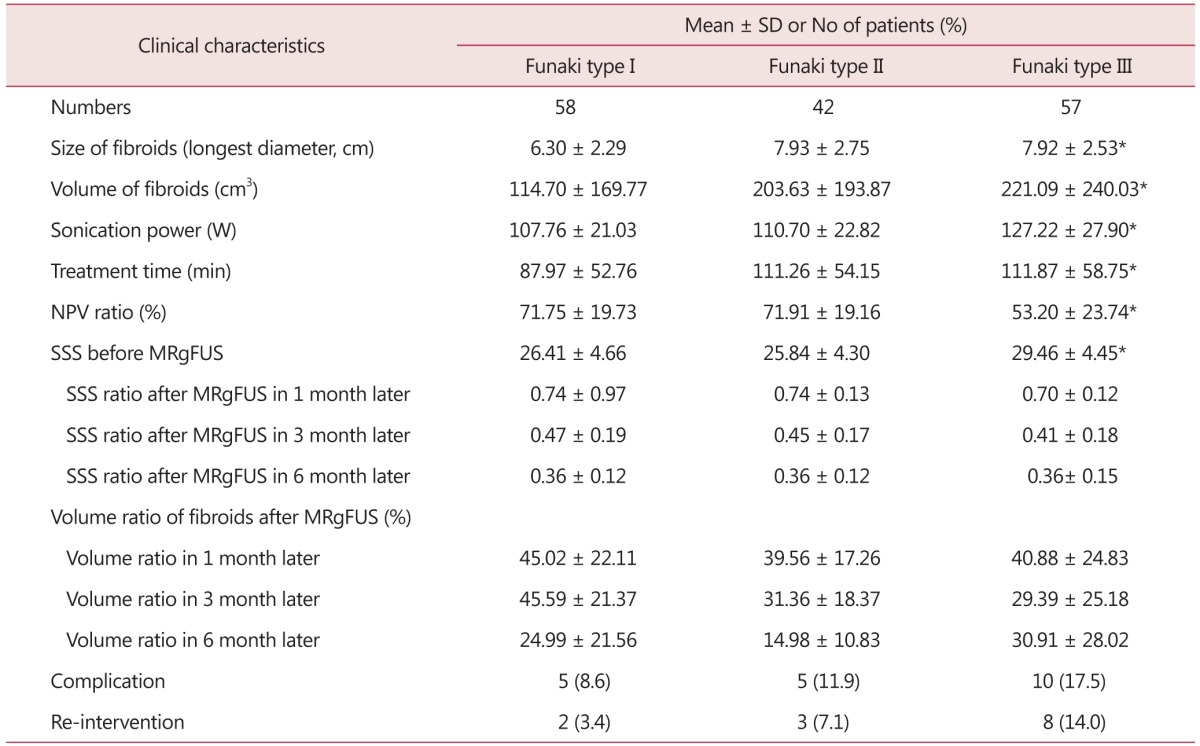
*P value < 0.05
SD: standard deviation, min: minute, NPV: non-perfused volume, SSS: symptom severity score, MRgFUS: magnetic resonance imaging-guided high intensity focused ultrasound
Fig. 4. Correlation between non-perfused volume (NPV) ratio, average sonication power and Funaki type of uterine fibroids after magnetic resonance imaging (MRI)-guided high intensity focused ultrasound surgery (MRgFUS). *The average sonication power was classified by 1: <100, 2 :100-130, 3 :130-160, 4: >160. Pearson correlation coefficient and Spearman correlation coefficient were 0.311 and 0.300 between the average sonication power and Funaki type of uterine fibroid, -0.344 and -0.320 between the NPV ratio and Funaki type of uterine fibroid in MRgFUS;both were statistically significant.
Depending upon HIFU intensity, the cases were classified into HIFU intensity not higher than 100 W, 100 to 130 W, 130 to 160 W, and not lower than 160 W. It was found that the lower the intensity, the shorter the average treatment hours. But no significant correlation was found with NPV ratio, uterine fibroid volume change, and SSS change (Table 6). Complication existence showed no statistically significant relation. But minor skin burn was found in 3/7 patients (17.6%) in less than 100 W output; 10/37 patients (27%) in 100 W to 130 W; 6/21 patients (28.6%) in 130 to 160 W; and 1/2 patients (50%) in 160 W or stronger, demonstrating the higher the HIFU intensity, the more the skin burn cases. Re-intervention showed no statistically significant result.
Table 6. Clinical outcomes of 157 patients undergone magnetic resonance imaging-guided high intensity focused ultrasound classified by sonication power level.
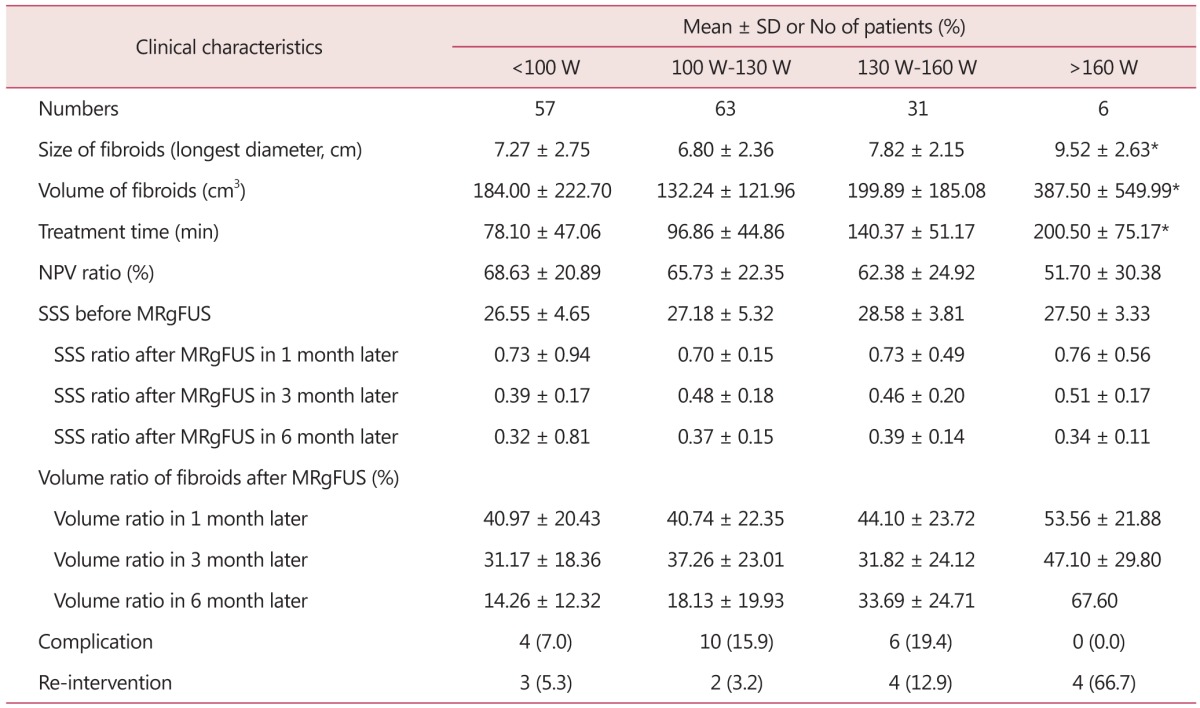
*P value < 0.05
SD: standard deviation, min: minute, NPV: non-perfused volume, SSS: symptom severity score, MRgFUS: magnetic resonance imaging-guided high intensity focused ultrasound
When the NPV ratio was classified into 25% or lower, 25% to 50%, 50% to 75%, and 75% or higher; it showed no correlation with average sonication power and treatment hours. But significant correlation was found with post-operative Month 1, Month 3 and Month 6 SSS change and Funaki type of uterine fibroid as well as post-MRgFUS Month 1 and Month 6 uterine fibroid volume change. No correlation was observed with complication existence and re-intervention (Table 7).
Table 7. Clinical outcomes of 157 patients undergone magnetic resonance imaging-guided high intensity focused ultrasound classified by non-perfused volume ratio.
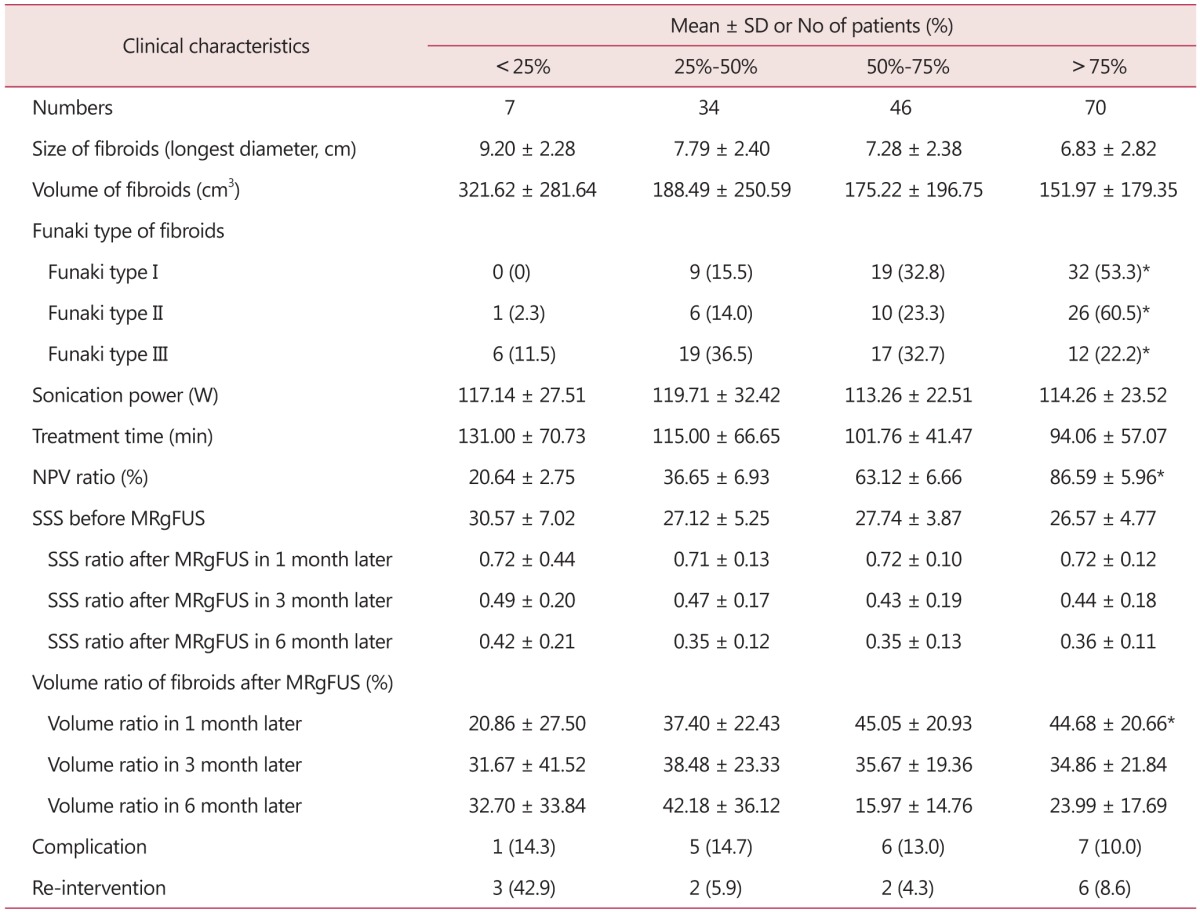
*P value < 0.05
SD: standard deviation, min: minute, NPV: non-perfused volume, SSS: symptom severity score, MRgFUS: magnetic resonance imaging-guided high intensity focused ultrasound
Discussion
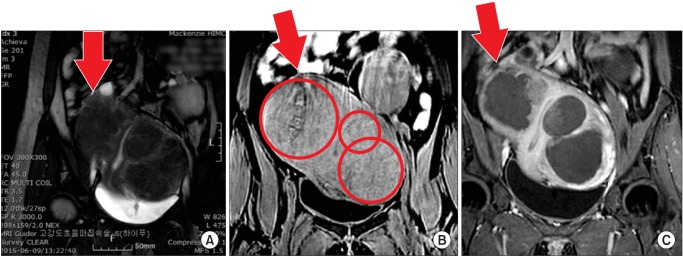
Uterine fibroid is one of the most common benign tumor disease in women. Its fundamental treatment involves hysterectomy, general anesthesia, long hospitalization and period, long recovery time. For uterine fibroid patients who want to keep their uterus, non-invasive preservation treatment methods have been continuously discussed. In this context, diverse techniques have been introduced such as medicine treatment, hysteroscopic myomectomy, laparoscopic myomectomy, single-incision laparoscopic myomectomy, uterine arterial embolization, radiofrequency myolysis, and HIFU.20,21 Of them, HIFU is advantageous as it can be applied to large-sized lesion or multiple lesions; repeated treatment application is possible; its treatment process can be observed during and after operation and its effectiveness can be assessed; and it does not require anesthesia and skin incision. In this sense, HIFU can be viewed as the most non-invasive technique while keeping the uterus unremoved (Fig. 5).
Fig. 5. (A) T2-weighted magnetic resonance (MR) image before high intensity focused ultrasound (HIFU), red triangle indicated the beam pathway, (B) Contrast enhanced T1-weighted MR image after HIFU show excellent non-perfused volume when using with multiple sonication beam pathway in the multiple fibroids, multipe fibroids on the same beam pathway was ablated simultaneously during one sonication.
HIFU treatment mechanism is divided into thermal effect and non-thermal effect such as cavitation. Thermal effect is generated by irreversible apoptosis through coagulative necrosis caused by raising temperature to at least 56℃ by exposing for 1 second.22 Tissue destruction by sound wave cavitation takes place as intracellular organelles and intra-organ fluid expand and contract due to sound wave pressure. It is known that as bubbles burst, sound wave pressure increases and pushes up the temperature to a high level instantly to destroy tissues. In other words, when human body is exposed to high intensity ultrasonic waves, the sound pressure effect of sound wave is known to make intracellular moisture into fine bubbles and if the bubbles grow larger enough to cause resonance, they suddenly burst and generate high pressure shock wave to destroy tissues.23
The Sonalleve HIFU system employs equivalent minutes (EM) unit as the standard for the biological effect of heat. Between 0 and 30 EM, it recognizes no thermal damage. Between 30 and 240 EM, thermal damages are recognized such as cellular wall destruction, edema, diverse blood vessel damages, etc. but most are reversible. At 240 EM or higher, it recognizes the possibility of irreversible coagulative necrosis. It is said that 240 EM is achieved for 15 minute exposure to 47℃; 1; 1 second to 57℃; 1/8 second to, 60℃; and 1/32 second to 62℃.
Kim et al.24 explained that the higher the uterine fibroids' T2-weighted signal, the higher the cellularity; and the better the perfusion, the higher the vascularity. Thus, they viewed it was difficult to perform HIFU treatment. In the case of hypercellularity and hypervascular fibroids, they said HIFU could be hardly implemented in over 2 cm-thick subcutaneous fat layer; and in the case of hypocellularity and hypovascular fibroids, it would be hard in over 2.5 to 3 cm subcutaneous fat layer.
In the Hospital, excluded cases are MRI contraindication, pregnancy, intestinal location suspicious of synechia of high intensity ultrasound beam pathway, fibroids with excessively bright T2-weighted signal, excessively heterogeneous myoma, calcificated myoma, myoma with serous internal necrosis, obesity with overly thick subcutaneous fat layers, 12 cm or longer distance between skin and myoma center, and uterus size of 24 weeks or over.
MRgFUS complications are skin burn, thermal damage of abdominal wall muscle and subcutaneous fat, sciatic nerve damage, hematuria, intestinal injury, abnormal uterine hemorrhage, etc. But many studies have not reported other than minor skin burns. The Hospital also observed complications in 20 cases (12.7%); minor burn in 19 cases; and continued abnormal uterine hemorrhage in only 1 case.
After MRgFUS, uterine fibroids showed volume reduction of 19% to 80% to record NPV ratio of 16.3% to 98%. Larger volume reduction is reported as NPV ratio increases. In the event of uterine fibroids ablation of 80% or over, volume decrease in Month 3 is reported to be 50% to 60%; Month 12, 30% to 40%.25
Gizzo et al.25 reported that every study utilized uterine fibroid symptom and quality of life (UFS-QOL) questionnaire to measure symptom relief and in overall data, symptom relief was shown in Month 6 from 56.3 to 31.0. They also reported that the higher the NPV ratio, the larger the symptom relief was. By comparing long term outcomes, Funaki et al.15 in 2007, reported that in the event of 40% uterine fibroid volume reduction, SSS score fell from 35 to 15, indicating improvement. They also found 14.0% of re-intervention rate within 2 years in Kunaki types I, and II; and 21.6% in Type III. In 2015, Mindjuk et al.26 studied 252 patients in a single institution and explained that at 80% NPV ratio or higher, uterine fibroid volume decrease was effective enough to produce excellent treatment performance.
The Hospital also found more significant correlation with post-operative Month 1 and Month 6 uterine fibroid volume decrease at higher NPV ratios. The NPV ratio showed significant correlation with Funaki Type. The NPV ratio rose higher as the type was closer to Type I. Post-MRgFUS SSS began to drop fast to record 72% in Month 1, and 45% in Month 3. In Month 6, however, the level went up back to 76%. This is deemed because the patients with symptom recovery were hardly followed and most of the patients followed for 6 months after the operation had accompanied adenomyosis, and others to experience only minor recovery. Uterine fibroid volume reduction in Month 1 was 42%; Month 3, 35%; and Month 6, 25%. The closer to Funaki Type I, the larger the reduction was.
Concerning the study on HIFU treatment using uterine stimulant in parallel, Zhang et al.27 reported that, in adenomyosis cases, observed significantly shorter high intensity ultrasound treatment duration along with high, NPV ratio, concluding that uterine stimulant use was effective in ablating adenomyosis. Regarding uterine fibroids, Huang et al.28 measured the sonication energy necessary to reach 60℃ in MRgFUS and reported its significant decrease when using uterine stimulant together. But no clinical study has conducted. In this present study, when uterine stimulant was utilized in parallel, HIFU intensity was measured significantly lower and the treatment duration was also found shorter (Fig. 4). The NPV ratio was found higher in group using uterine stimulant and SSS change ratio in post-operative Month 3 was also significantly lower to conclude high therapeutic effectiveness of using uterine stimulant together.
In this study, as the Funaki Type of uterine fibroid was closer to Type I, sonication power grew significantly lower; treatment hours grew shorter; and NPV ratio rose higher. (Fig. 5) From this finding, it can be inferred that when the type is Funaki Type III, vascularity would be higher in adenomyosis and response to the HIFU treatment would be inactive. In Funaki Type I and Type II, pre-operative SSS was found significantly lower. In post-operative Month 1, Month 3, and Month 6, SSS change ratio was significantly lower.
Dorenberg et al.29 performed MRgFUS by using the same equipment as that of the Hospital for the average duration of 156 minutes and average sonication frequency of 20 times. They reported to have found no side effect. Anne et al. also utilized the same HIFU equipment as that of the Hospital to follow up 36 patients for 21.4 months on average. As a result, they reported to have found clinical effectiveness in 61.1% of the patients and significant fall in SSS from 42.8 ± 16 to 25.4 ± 18 on average.30 Statistically significant recovery was found especially in symptoms such as pelvic heaviness in profuse menstruation and swelling. Uterine fibroid volume decreased by 27% and the researchers explained it was related with the NPV with statistical significance (r = 0.373, P = 0.028). Re-intervention in Month 12, Month 18, and Month 24 was 2.8%, 8.5%, and 21.6%, respectively.
In 2014, Quinn et al.31 reported overall re-intervention rate as high as 42.8% within 3 years and 59.3% within 5 years. They explained that, if NPV was lower than 50% or a uterine fibroid was Kunaki type III, the risk was higher. However, in 2014, Gorny et al.32 reported the re-intervention rate at 19% within 3 years and 23% within 4 years and explained the risk was higher if a patient was relatively younger, or showed heterogeneous or high signals in the T2-weighted images. Fukunishi, et al.33 stated that when using MRgFUS for uterine fibroid treatment, the marginal of uterine fibroids should be ablated sufficiently to reduce post-MRgFUS uterine fibroid regrowth. They also explained that recurrence would be higher if the uterine fibroid marginal with MRI non-perfusion looked comb-like structure immediately after the operation.
The Hospital observed post-MRgFUS re-intervention in 13 cases or 8.3% during the 12-month follow-up period; hysteroscopic myomectomy was implemented in 6 cases; mirena insertion in 4 cases; and intra-abdominal surgery in 3 cases.
The purpose of this study is to report the results of non-invasive uterine fibroid treatment based on Sonalleve-MRI-guided HIFU and compare them with multiple existing study indications with a view to inform a safer and more effective treatment technique.
This study, however, is limited by not controlling the skill of medical staff, age, uterine fibroid location, body mass index, and number of people in dependent variables such as menopause experience. Especially the number of participants in blood test of this study was less than many. Longer follow-up was not conducted to observe more about re-intervention.
It seems necessary for subsequent study to unify dependent variables, follow forward-looking strategy, conduct a long-term follow-up, and research the safety of MRgFUS for women with adenomyosis and who desire pregnancy.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Buttram VC, Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36:433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 2.Oh SR, Cho YJ, Han M, Bae JW, Park JW, Rha SH. Uterine lipoleiomyoma in peri or postmenopausal women. J Menopausal Med. 2015;21:165–170. doi: 10.6118/jmm.2015.21.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasson HM, Rotman C, Rana N, Sistos F, Dmowski WP. Laparoscopic myomectomy. Obstet Gynecol. 1992;80:884–888. [PubMed] [Google Scholar]
- 4.Dubuisson JB, Fauconnier A, Chapron C, Kreiker G, Nørgaard C. Reproductive outcome after laparoscopic myomectomy in infertile women. J Reprod Med. 2000;45:23–30. [PubMed] [Google Scholar]
- 5.Oliveira FG, Abdelmassih VG, Diamond MP, Dozortsev D, Melo NR, Abdelmassih R. Impact of subserosal and intramural uterine fibroids that do not distort the endometrial cavity on the outcome of in vitro fertilizationintracytoplasmic sperm injection. Fertil Steril. 2004;81:582–587. doi: 10.1016/j.fertnstert.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Bulletti C, D DEZ, Levi Setti P, Cicinelli E, Polli V, Stefanetti M. Myomas, pregnancy outcome, and in vitro fertilization. Ann N Y Acad Sci. 2004;1034:84–92. doi: 10.1196/annals.1335.010. [DOI] [PubMed] [Google Scholar]
- 7.Chang IJ, Hong GY, Oh YL, Kim BR, Park SN, Lee HH, et al. Effects of menopausal hormone therapy on uterine myoma in menopausal women. J Menopausal Med. 2013;19:123–129. doi: 10.6118/jmm.2013.19.3.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun S, Kim YM, Ji YI. Uterine adenomyosis which developed from hypoplastic uterus in postmenopausal woman with mayer-rokitansky-kuster-hauser syndrome: a case report. J Menopausal Med. 2013;19:135–138. doi: 10.6118/jmm.2013.19.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YW. Single port transumbilical total laparoscopic hysterectomy (TLH): initial experience in Korea. Korean J Obstet Gynecol. 2009;52:480–486. [Google Scholar]
- 10.Jeong JH, Kim YR, Kim EJ, Moon SH, Park MH, Kim JT, et al. Comparison of surgical outcomes according to suturing methods in single port access laparoscopic myomectomy. J Menopausal Med. 2015;21:47–55. doi: 10.6118/jmm.2015.21.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim KA, Yoon SW, Lee C, Seong SJ, Yoon BS, Park H. Short-term results of magnetic resonance imaging-guided focused ultrasound surgery for patients with adenomyosis: symptomatic relief and pain reduction. Fertil Steril. 2011;95:1152–1155. doi: 10.1016/j.fertnstert.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Fukunishi H, Funaki K, Sawada K, Yamaguchi K, Maeda T, Kaji Y. Early results of magnetic resonance-guided focused ultrasound surgery of adenomyosis: analysis of 20 cases. J Minim Invasive Gynecol. 2008;15:571–579. doi: 10.1016/j.jmig.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Hanstede MM, Tempany CM, Stewart EA. Focused ultrasound surgery of intramural leiomyomas may facilitate fertility: a case report. Fertil Steril. 2007;88:497.e5–497.e7. doi: 10.1016/j.fertnstert.2006.11.103. [DOI] [PubMed] [Google Scholar]
- 14.Zaher S, Lyons D, Regan L. Successful in vitro fertilization pregnancy following magnetic resonance-guided focused ultrasound surgery for uterine fibroids. J Obstet Gynaecol Res. 2011;37:370–373. doi: 10.1111/j.1447-0756.2010.01344.x. [DOI] [PubMed] [Google Scholar]
- 15.Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196:184.e1–184.e6. doi: 10.1016/j.ajog.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new diseasespecific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99:290–300. doi: 10.1016/s0029-7844(01)01702-1. [DOI] [PubMed] [Google Scholar]
- 17.Köhler MO, Mougenot C, Quesson B, Enholm J, Le Bail B, Laurent C, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys. 2009;36:3521–3535. doi: 10.1118/1.3152112. [DOI] [PubMed] [Google Scholar]
- 18.Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34:814–823. doi: 10.1002/mrm.1910340606. [DOI] [PubMed] [Google Scholar]
- 19.Enholm JK, Köhler MO, Quesson B, Mougenot C, Moonen CT, Sokka SD. Improved volumetric MR-HIFU ablation by robust binary feedback control. IEEE Trans Biomed Eng. 2010;57:103–113. doi: 10.1109/TBME.2009.2034636. [DOI] [PubMed] [Google Scholar]
- 20.Somigliana E, Vercellini P, Daguati R, Pasin R, De Giorgi O, Crosignani PG. Fibroids and female reproduction: a critical analysis of the evidence. Hum Reprod Update. 2007;13:465–476. doi: 10.1093/humupd/dmm013. [DOI] [PubMed] [Google Scholar]
- 21.Cho EA, Um MJ, Kim SA, Kim SJ, Jung H. Comparison of laparoscopic radiofrequency myolysis (LRFM) and ultrasonographic radiofrequency myolysis (URFM) in treatment of midline dysmenorrhea. J Menopausal Med. 2014;20:75–79. doi: 10.6118/jmm.2014.20.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill CR, Rivens I, Vaughan MG, ter Haar GR. Lesion development in focused ultrasound surgery: a general model. Ultrasound Med Biol. 1994;20:259–269. doi: 10.1016/0301-5629(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan MG, ter Haar GR, Hill CR, Clarke RL, Hopewell JW. Minimally invasive cancer surgery using focused ultrasound: a pre-clinical, normal tissue study. Br J Radiol. 1994;67:267–274. doi: 10.1259/0007-1285-67-795-267. [DOI] [PubMed] [Google Scholar]
- 24.Kim YS, Park MJ, Keserci B, Nurmilaukas K, Kohler MO, Rhim H, et al. Uterine fibroids: postsonication temperature decay rate enables prediction of therapeutic responses to MR imaging-guided high-intensity focused ultrasound ablation. Radiology. 2014;270:589–600. doi: 10.1148/radiol.13130380. [DOI] [PubMed] [Google Scholar]
- 25.Gizzo S, Saccardi C, Patrelli TS, Ancona E, Noventa M, Fagherazzi S, et al. Magnetic resonance-guided focused ultrasound myomectomy: safety, efficacy, subsequent fertility and quality-of-life improvements, a systematic review. Reprod Sci. 2014;21:465–476. doi: 10.1177/1933719113497289. [DOI] [PubMed] [Google Scholar]
- 26.Mindjuk I, Trumm CG, Herzog P, Stahl R, Matzko M. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317–1328. doi: 10.1007/s00330-014-3538-6. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Zou M, Zhang C, He J, Mao S, Wu Q, et al. Effects of oxytocin on high intensity focused ultrasound (HIFU) ablation of adenomysis: a prospective study. Eur J Radiol. 2014;83:1607–1611. doi: 10.1016/j.ejrad.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, He M, Liu YJ, Zhang L, Wang ZB. Effect of oxytocin on uterine fibroids treated by ultrasound ablation. Zhonghua Fu Chan Ke Za Zhi. 2011;46:412–415. [PubMed] [Google Scholar]
- 29.Dorenberg EJ, Courivaud F, Ring E, Hald K, Jakobsen JÅ, Fosse E, et al. Volumetric ablation of uterine fibroids using Sonalleve high-intensity focused ultrasound in a 3 Tesla scanner--first clinical assessment. Minim Invasive Ther Allied Technol. 2013;22:73–79. doi: 10.3109/13645706.2012.702672. [DOI] [PubMed] [Google Scholar]
- 30.Thiburce AC, Frulio N, Hocquelet A, Maire F, Salut C, Balageas P, et al. Magnetic resonance-guided highintensity focused ultrasound for uterine fibroids: Mid-term outcomes of 36 patients treated with the Sonalleve system. Int J Hyperthermia. 2015;31:764–770. doi: 10.3109/02656736.2015.1063169. [DOI] [PubMed] [Google Scholar]
- 31.Quinn SD, Vedelago J, Gedroyc W, Regan L. Safety and five-year re-intervention following magnetic resonanceguided focused ultrasound (MRgFUS) for uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2014;182:247–251. doi: 10.1016/j.ejogrb.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 32.Gorny KR, Borah BJ, Brown DL, Woodrum DA, Stewart EA, Hesley GK. Incidence of additional treatments in women treated with MR-guided focused US for symptomatic uterine fibroids: review of 138 patients with an average follow-up of 2.8 years. J Vasc Interv Radiol. 2014;25:1506–1512. doi: 10.1016/j.jvir.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Fukunishi H, Funaki K, Sawada K, Kato E, Maruo T. Therapy of uterine myomas using MR-guided focused ultrasound surgery (MRgFUS) Frauenheilkunde Geburtshilfe. 2007;67:336–340. [Google Scholar]