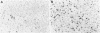Abstract
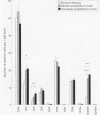
AIM: To quantify and compare decidual leucocyte subpopulations in complete and partial hydatidiform molar pregnancy with those in normal early pregnancy. METHODS: Decidual leucocytes were characterised using an avidin-biotin technique and a panel of monoclonal antibodies in formalin fixed, paraffin embedded decidual tissues from 10 normal first trimester pregnancy terminations and from 13 partial and 13 complete hydatidiform moles. Immunostained cells were fully quantitated and differences between subject groups were analysed using the Mann-Whitney test. T lymphocyte populations were further characterised using double immunohistochemical labelling. RESULTS: The numbers and percentages of CD3+ and CD4+ T cells were significantly increased in complete hydatidiform moles compared with partial moles and normal early pregnancy decidua. No differences were found in the number or percentage of CD8+ T cells. The CD8+ to CD4+ T cell ratio decreased significantly in complete mole compared with partial mole and normal decidua. The numbers and percentages of CD45RO+ cells increased significantly in both partial and complete hydatidiform mole compared with normal first trimester decidua. Double labelling confirmed that 50% of CD3+ T cells in complete and partial molar pregnancy coexpressed CD45RO, compared with 30% in normal pregnancy. Other leucocyte populations (eGLs, macrophages, B cells, and classical natural killer cells) did not differ between complete and partial mole and normal pregnancy decidua. CONCLUSIONS: The presence of an increased population of activated decidual CD45RO+ T cells in complete and partial molar pregnancy suggests altered maternal immune responses against molar trophoblast.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizadeh M., Legras C., Semana G., Le Bouteiller P., Genetet B., Fauchet R. Evidence for a polymorphism of HLA-G gene. Hum Immunol. 1993 Nov;38(3):206–212. doi: 10.1016/0198-8859(93)90542-9. [DOI] [PubMed] [Google Scholar]
- Berkowitz R. S., Mostoufizadeh GhM, Kabawat S. E., Goldstein D. P., Driscoll S. G. Immunopathologic study of the implantation site in molar pregnancy. Am J Obstet Gynecol. 1982 Dec 15;144(8):925–930. doi: 10.1016/0002-9378(82)90186-7. [DOI] [PubMed] [Google Scholar]
- Buckley J. D. The epidemiology of molar pregnancy and choriocarcinoma. Clin Obstet Gynecol. 1984 Mar;27(1):153–159. doi: 10.1097/00003081-198403000-00022. [DOI] [PubMed] [Google Scholar]
- Bulmer J. N., Johnson P. M., Sasagawa M., Takeuchi S. Immunohistochemical studies of fetal trophoblast and maternal decidua in hydatidiform mole and choriocarcinoma. Placenta. 1988 Mar-Apr;9(2):183–200. doi: 10.1016/0143-4004(88)90016-1. [DOI] [PubMed] [Google Scholar]
- Bulmer J. N., Johnson P. M. The T-lymphocyte population in first-trimester human decidua does not express the interleukin-2 receptor. Immunology. 1986 Aug;58(4):685–687. [PMC free article] [PubMed] [Google Scholar]
- Bulmer J. N., Morrison L., Longfellow M., Ritson A., Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991 Jul;6(6):791–798. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- Chen C. K., Huang S. C., Chen C. L., Yen M. R., Hsu H. C., Ho H. N. Increased expressions of CD69 and HLA-DR but not of CD25 or CD71 on endometrial T lymphocytes of nonpregnant women. Hum Immunol. 1995 Mar;42(3):227–232. doi: 10.1016/0198-8859(94)00105-y. [DOI] [PubMed] [Google Scholar]
- Clark D. A., Vince G., Flanders K. C., Hirte H., Starkey P. CD56+ lymphoid cells in human first trimester pregnancy decidua as a source of novel transforming growth factor-beta 2-related immunosuppressive factors. Hum Reprod. 1994 Dec;9(12):2270–2277. doi: 10.1093/oxfordjournals.humrep.a138436. [DOI] [PubMed] [Google Scholar]
- Ferry B. L., Starkey P. M., Sargent I. L., Watt G. M., Jackson M., Redman C. W. Cell populations in the human early pregnancy decidua: natural killer activity and response to interleukin-2 of CD56-positive large granular lymphocytes. Immunology. 1990 Aug;70(4):446–452. [PMC free article] [PubMed] [Google Scholar]
- Jokhi P. P., King A., Boocock C., Loke Y. W. Secretion of colony stimulating factor-1 by human first trimester placental and decidual cell populations and the effect of this cytokine on trophoblast thymidine uptake in vitro. Hum Reprod. 1995 Oct;10(10):2800–2807. doi: 10.1093/oxfordjournals.humrep.a135794. [DOI] [PubMed] [Google Scholar]
- Jokhi P. P., King A., Loke Y. W. Production of granulocyte-macrophage colony-stimulating factor by human trophoblast cells and by decidual large granular lymphocytes. Hum Reprod. 1994 Sep;9(9):1660–1669. doi: 10.1093/oxfordjournals.humrep.a138769. [DOI] [PubMed] [Google Scholar]
- Jokhi P. P., King A., Sharkey A. M., Smith S. K., Loke Y. W. Screening for cytokine messenger ribonucleic acids in purified human decidual lymphocyte populations by the reverse-transcriptase polymerase chain reaction. J Immunol. 1994 Nov 15;153(10):4427–4435. [PubMed] [Google Scholar]
- Kabawat S. E., Mostoufi-Zadeh M., Berkowitz R. S., Driscoll S. G., Goldstein D. P., Bhan A. K. Implantation site in complete molar pregnancy: a study of immunologically competent cells with monoclonal antibodies. Am J Obstet Gynecol. 1985 May 1;152(1):97–99. doi: 10.1016/s0002-9378(85)80188-5. [DOI] [PubMed] [Google Scholar]
- Kajii T., Ohama K. Androgenetic origin of hydatidiform mole. Nature. 1977 Aug 18;268(5621):633–634. doi: 10.1038/268633a0. [DOI] [PubMed] [Google Scholar]
- King A., Birkby C., Loke Y. W. Early human decidual cells exhibit NK activity against the K562 cell line but not against first trimester trophoblast. Cell Immunol. 1989 Feb;118(2):337–344. doi: 10.1016/0008-8749(89)90382-1. [DOI] [PubMed] [Google Scholar]
- King A., Jokhi P. P., Smith S. K., Sharkey A. M., Loke Y. W. Screening for cytokine mRNA in human villous and extravillous trophoblasts using the reverse-transcriptase polymerase chain reaction (RT-PCR). Cytokine. 1995 May;7(4):364–371. doi: 10.1006/cyto.1995.0046. [DOI] [PubMed] [Google Scholar]
- Mor G., Gutierrez L. S., Eliza M., Kahyaoglu F., Arici A. Fas-fas ligand system-induced apoptosis in human placenta and gestational trophoblastic disease. Am J Reprod Immunol. 1998 Aug;40(2):89–94. doi: 10.1111/j.1600-0897.1998.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Palmer J. R. Advances in the epidemiology of gestational trophoblastic disease. J Reprod Med. 1994 Mar;39(3):155–162. [PubMed] [Google Scholar]
- Parhar R. S., Yagel S., Lala P. K. PGE2-mediated immunosuppression by first trimester human decidual cells blocks activation of maternal leukocytes in the decidua with potential anti-trophoblast activity. Cell Immunol. 1989 Apr 15;120(1):61–74. doi: 10.1016/0008-8749(89)90174-3. [DOI] [PubMed] [Google Scholar]
- Piccinni M. P., Beloni L., Livi C., Maggi E., Scarselli G., Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998 Sep;4(9):1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- Risk J. M., Johnson P. M. Northern blot analysis of HLA-G expression by BeWo human choriocarcinoma cells. J Reprod Immunol. 1990 Sep;18(2):199–203. doi: 10.1016/0165-0378(90)90017-z. [DOI] [PubMed] [Google Scholar]
- Ritson A., Bulmer J. N. Isolation and functional studies of granulated lymphocytes in first trimester human decidua. Clin Exp Immunol. 1989 Aug;77(2):263–268. [PMC free article] [PubMed] [Google Scholar]
- Saito S., Nishikawa K., Morii T., Narita N., Enomoto M., Ichijo M. Expression of activation antigens CD69, HLA-DR, interleukin-2 receptor-alpha (IL-2R alpha) and IL-2R beta on T cells of human decidua at an early stage of pregnancy. Immunology. 1992 Apr;75(4):710–712. [PMC free article] [PubMed] [Google Scholar]
- Saito S., Nishikawa K., Morii T., Narita N., Enomoto M., Ito A., Ichijo M. A study of CD45RO, CD45RA and CD29 antigen expression on human decidual T cells in an early stage of pregnancy. Immunol Lett. 1994 Jun;40(3):193–197. doi: 10.1016/0165-2478(93)00019-a. [DOI] [PubMed] [Google Scholar]
- Shorter S. C., Vince G. S., Starkey P. M. Production of granulocyte colony-stimulating factor at the materno-foetal interface in human pregnancy. Immunology. 1992 Mar;75(3):468–474. [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M. Expression on cells of early human pregnancy decidua, of the p75, IL-2 and p145, IL-4 receptor proteins. Immunology. 1991 May;73(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- Sunderland C. A., Redman C. W., Stirrat G. M. Characterization and localization of HLA antigens on hydatidiform mole. Am J Obstet Gynecol. 1985 Jan 1;151(1):130–135. doi: 10.1016/0002-9378(85)90439-9. [DOI] [PubMed] [Google Scholar]
- Szulman A. E., Surti U. The syndromes of hydatidiform mole. I. Cytogenetic and morphologic correlations. Am J Obstet Gynecol. 1978 Jul 15;131(6):665–671. doi: 10.1016/0002-9378(78)90829-3. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. S., Satyaswaroop P. G. Sex steroid receptors in lymphoid cells of human endometrium. Am J Clin Pathol. 1989 Jun;91(6):656–663. doi: 10.1093/ajcp/91.6.656. [DOI] [PubMed] [Google Scholar]
- Todt J. C., Yang Y., Lei J., Lauria M. R., Sorokin Y., Cotton D. B., Yelian F. D. Effects of tumor necrosis factor-alpha on human trophoblast cell adhesion and motility. Am J Reprod Immunol. 1996 Aug;36(2):65–71. doi: 10.1111/j.1600-0897.1996.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Tranchot-Diallo J., Gras G., Parnet-Mathieu F., Benveniste O., Marcé D., Roques P., Milliez J., Chaouat G., Dormont D. Modulations of cytokine expression in pregnant women. Am J Reprod Immunol. 1997 Mar;37(3):215–226. doi: 10.1111/j.1600-0897.1997.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Vince G., Shorter S., Starkey P., Humphreys J., Clover L., Wilkins T., Sargent I., Redman C. Localization of tumour necrosis factor production in cells at the materno/fetal interface in human pregnancy. Clin Exp Immunol. 1992 Apr;88(1):174–180. doi: 10.1111/j.1365-2249.1992.tb03059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann T. G., Lin H., Guilbert L., Mosmann T. R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993 Jul;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Wake N., Araki T., Ichinoe K., Makoto K. Human lymphocyte antigen expression in hydatidiform mole: androgenesis following fertilization by a haploid sperm. Am J Obstet Gynecol. 1979 Nov 1;135(5):597–600. doi: 10.1016/s0002-9378(16)32983-0. [DOI] [PubMed] [Google Scholar]
- Yui J., Garcia-Lloret M., Wegmann T. G., Guilbert L. J. Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta. 1994 Dec;15(8):819–835. doi: 10.1016/s0143-4004(05)80184-5. [DOI] [PubMed] [Google Scholar]
- de Moraes-Pinto M. I., Vince G. S., Flanagan B. F., Hart C. A., Johnson P. M. Localization of IL-4 and IL-4 receptors in the human term placenta, decidua and amniochorionic membranes. Immunology. 1997 Jan;90(1):87–94. doi: 10.1046/j.1365-2567.1997.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]