Abstract
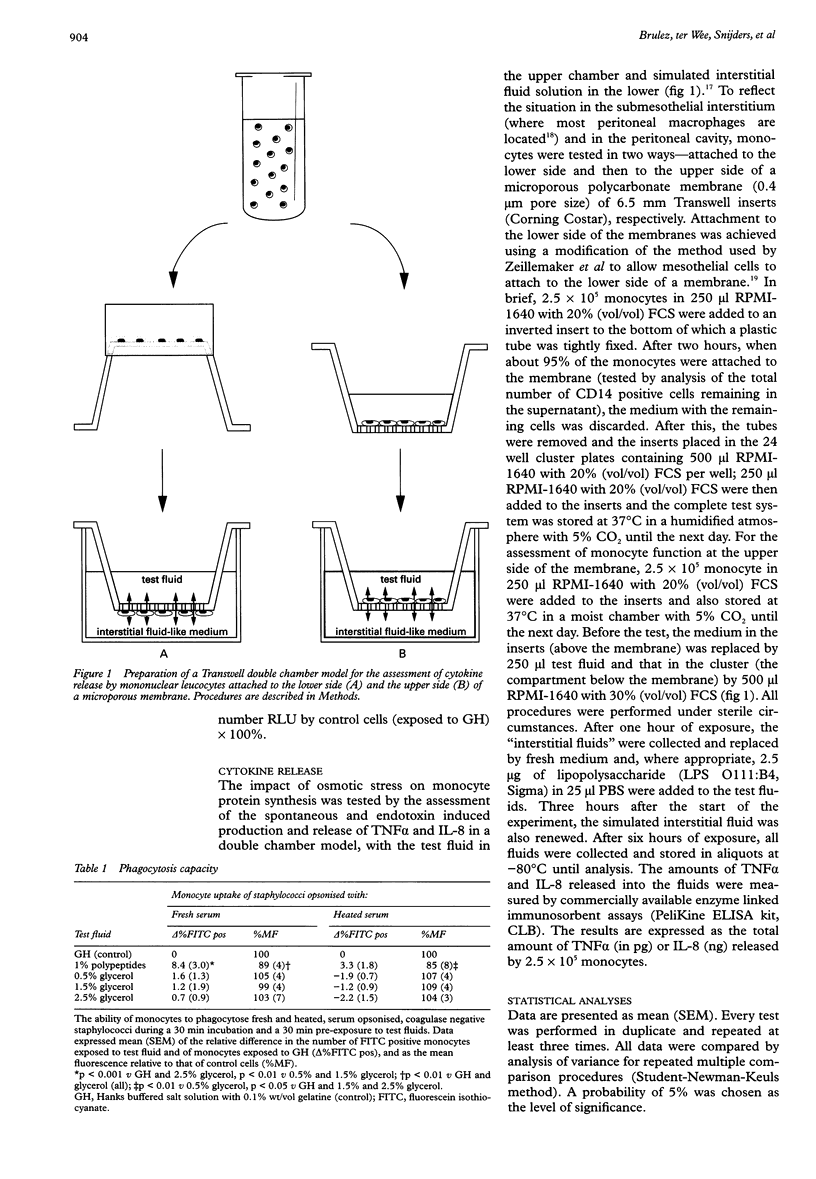
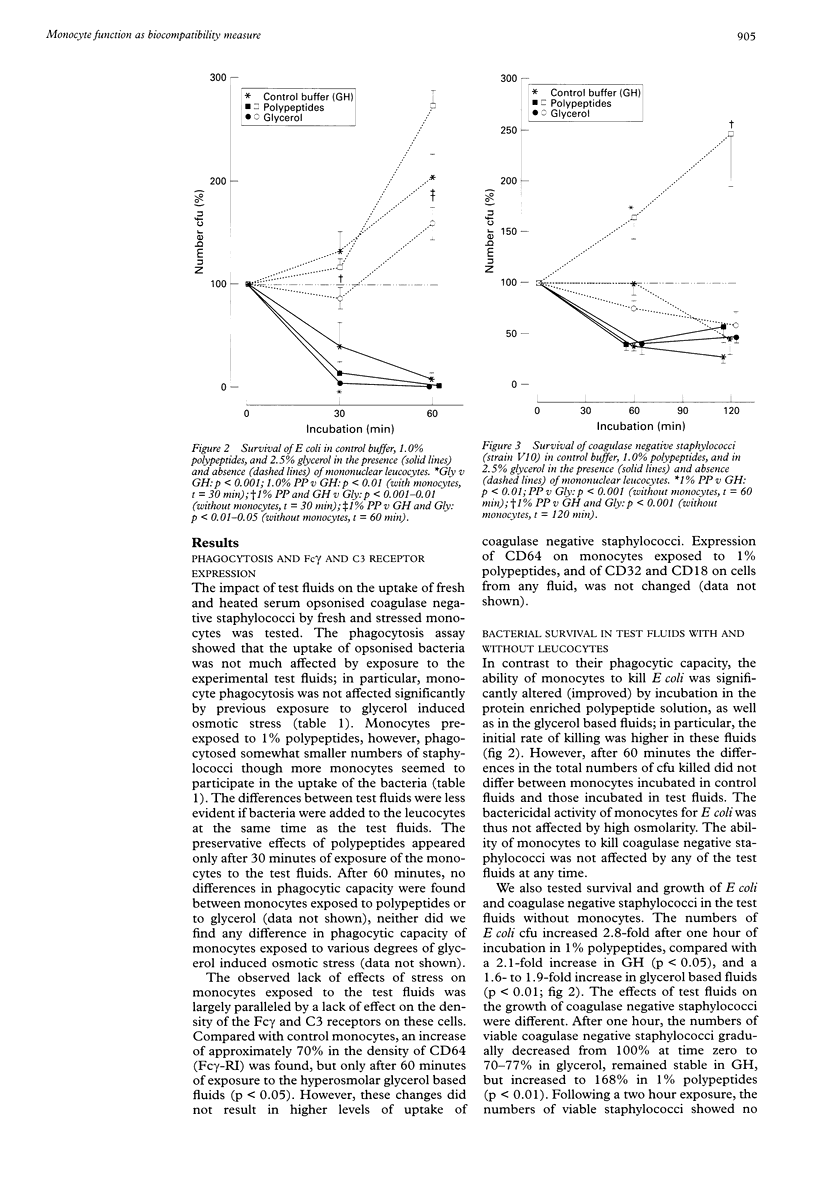
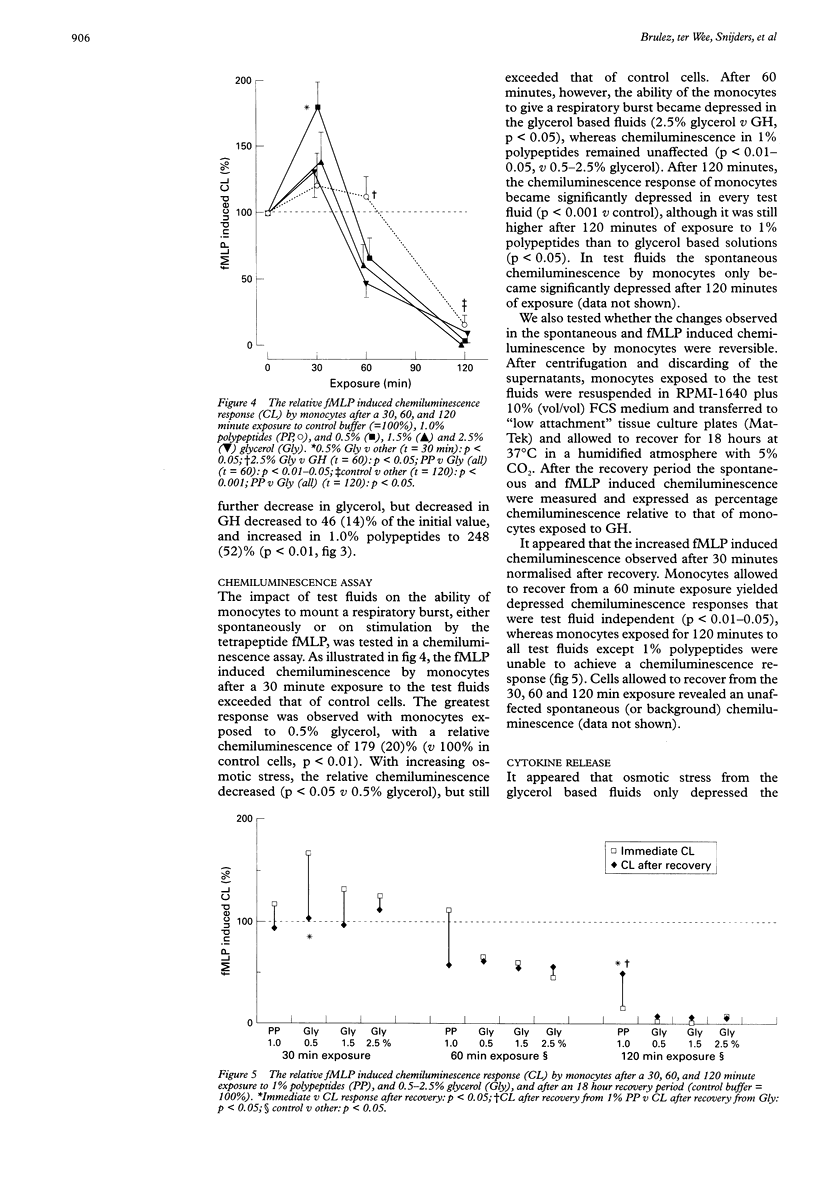
BACKGROUND: Previous studies showed that the currently used dextrose based peritoneal dialysis fluids impair several leucocyte functions. AIMS: To determine which in vitro mononuclear leucocyte (monocyte) function tests most clearly reflect the biocompatibility of peritoneal dialysis fluid. METHODS: Monocytes were tested for phagocytic capacity, bactericidal activity, Fc and C3 receptor expression, and chemiluminescence response, and by analysis of the release of interleukin 8 (IL-8) and tumour necrosis factor alpha (TNF alpha) in the presence of test fluids. Cytokine release was studied in an alternative dynamic in vitro peritoneal dialysis model in which monocytes were exposed to test fluid that was continuously equilibrated with an interstitial fluid-like medium through a microporous membrane. The chemiluminescence response by stressed monocytes was also tested after an 18 h recovery period. All tests were performed during or after exposure to different degrees of glycerol induced osmotic stress and after exposure to a 1% milk-whey derived, polypeptide enriched test fluid. Cells incubated in 0.1% gel Hanks buffer (GH) served as control. RESULTS: Osmotic stress induced impairment of leucocyte function was found by the chemiluminescence assay (mean (SEM): 179 (20)% v 138 (23)% after 30 minutes in 0.5% and 1.5% glycerol, respectively) and by the analysis of IL-8 released by monocytes (44 (9) ng in 0.7% glycerol v 40 (7) ng in 2.0% glycerol). Only the chemiluminescence assay showed a protective effect of polypeptides on leucocyte function (after > or = 60 minutes). If monocytes were allowed to recover in culture medium after exposure to test fluids, the changes in chemiluminescence response appeared to be reversible after a 30 minute exposure, but became more pronounced after 60 and 120 minutes. The phagocytosis and bacterial killing assays were less sensitive. The observations carried out with the phagocytosis assay did not correspond with the Fc or C3 receptor density data. CONCLUSIONS: The release of IL-8 by peripheral blood monocytes in a two compartment model and their chemiluminescence response are appropriate assays for the assessment of changes in leucocyte function in response to different peritoneal dialysis fluids.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alobaidi H. M., Coles G. A., Davies M., Lloyd D. Host defence in continuous ambulatory peritoneal dialysis: the effect of the dialysate on phagocyte function. Nephrol Dial Transplant. 1986;1(1):16–21. [PubMed] [Google Scholar]
- Brulez H. F., Heezius E. C., de Fijter C. W., Oe L. P., Verhoef J., Verbrugh H. A. In vitro compatibility of a 1.1% amino acid containing peritoneal dialysis fluid with phagocyte function. Adv Perit Dial. 1994;10:241–244. [PubMed] [Google Scholar]
- Brulez H. F., Verbrugh H. A. First-line defense mechanisms in the peritoneal cavity during peritoneal dialysis. Perit Dial Int. 1995;15(7 Suppl):S24–S34. [PubMed] [Google Scholar]
- Clerc C., Pibouin M., Ruelland A., Legras B., Chevrant-Breton J., Cloarec L. Cutaneous interstitial fluid protein concentrations in the inflammatory syndrome: pharmacological consequences. Clin Chim Acta. 1990 Aug 15;189(2):181–189. doi: 10.1016/0009-8981(90)90090-f. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Barke R. A., Ahrenholz D. H., Humphrey E. W., Simmons R. L. The adjuvant effect of peritoneal fluid in experimental peritonitis. Mechanism and clinical implications. Ann Surg. 1984 Jan;199(1):37–43. doi: 10.1097/00000658-198401000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duwe A. K., Vas S. I., Weatherhead J. W. Effects of the composition of peritoneal dialysis fluid on chemiluminescence, phagocytosis, and bactericidal activity in vitro. Infect Immun. 1981 Jul;33(1):130–135. doi: 10.1128/iai.33.1.130-135.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupke C. J., Zhang J., Rajpoot D., Wang J., Zhou X. J., Vaziri N. D. Effects of conventional peritoneal dialysates on leukocyte adhesion and CD11b, CD18 and CD14 expression. Kidney Int. 1996 Nov;50(5):1676–1683. doi: 10.1038/ki.1996.485. [DOI] [PubMed] [Google Scholar]
- Klein E., Ward R. A., Williams T. E., Feldhoff P. W. Peptides as substitute osmotic agents for glucose in peritoneal dialysate. ASAIO Trans. 1986 Jul-Sep;32(1):550–553. doi: 10.1097/00002480-198609000-00035. [DOI] [PubMed] [Google Scholar]
- Liberek T., Topley N., Jörres A., Coles G. A., Gahl G. M., Williams J. D. Peritoneal dialysis fluid inhibition of phagocyte function: effects of osmolality and glucose concentration. J Am Soc Nephrol. 1993 Feb;3(8):1508–1515. doi: 10.1681/ASN.V381508. [DOI] [PubMed] [Google Scholar]
- Lindholm B., Norbeck H. E. Serum lipids and lipoproteins during continuous ambulatory peritoneal dialysis. Acta Med Scand. 1986;220(2):143–151. doi: 10.1111/j.0954-6820.1986.tb02742.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., van der Auwera P., Watanabe Y., Tanaka M., Ogata N., Naito S., Kumazawa J. Neutrophil function in hyperosmotic NaCl is preserved by phosphoenol pyruvate. Urol Res. 1991;19(4):223–227. doi: 10.1007/BF00305299. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Schmeling D., Quie P. G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977 Oct;136(4):502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- Suassuna J. H., Das Neves F. C., Hartley R. B., Ogg C. S., Cameron J. S. Immunohistochemical studies of the peritoneal membrane and infiltrating cells in normal subjects and in patients on CAPD. Kidney Int. 1994 Aug;46(2):443–454. doi: 10.1038/ki.1994.292. [DOI] [PubMed] [Google Scholar]
- Topley N., Mackenzie R., Petersen M. M., Beavis M. J., Williams D., Thomas N., Faict D., Peluso F., Coles G. A., Davies M. In vitro testing of a potentially biocompatible continuous ambulatory peritoneal dialysis fluid. Nephrol Dial Transplant. 1991;6(8):574–581. doi: 10.1093/ndt/6.8.574. [DOI] [PubMed] [Google Scholar]
- Topley N. What is the ideal technique for testing the biocompatibility of peritoneal dialysis solutions? Perit Dial Int. 1995 Jul-Sep;15(6):205–209. [PubMed] [Google Scholar]
- Verbrugh H. A., Keane W. F., Hoidal J. R., Freiberg M. R., Elliott G. R., Peterson P. K. Peritoneal macrophages and opsonins: antibacterial defense in patients undergoing chronic peritoneal dialysis. J Infect Dis. 1983 Jun;147(6):1018–1029. doi: 10.1093/infdis/147.6.1018. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A. Organisms causing peritonitis. Contrib Nephrol. 1990;85:39–48. doi: 10.1159/000419061. [DOI] [PubMed] [Google Scholar]
- Weber G. F. The measurement of oxygen-derived free radicals and related substances in medicine. J Clin Chem Clin Biochem. 1990 Sep;28(9):569–603. [PubMed] [Google Scholar]
- XVIth Annual Conference on Peritoneal Dialysis. Seattle, Washington, February 21-23, 1996. Abstracts. Perit Dial Int. 1996;16 (Suppl 2):S1–106. [PubMed] [Google Scholar]
- Yang A. H., Chen J. Y., Lin Y. P., Huang T. P., Wu C. W. Peritoneal dialysis solution induces apoptosis of mesothelial cells. Kidney Int. 1997 Apr;51(4):1280–1288. doi: 10.1038/ki.1997.175. [DOI] [PubMed] [Google Scholar]
- Zeillemaker A. M., Mul F. P., Hoynck van Papendrecht A. A., Kuijpers T. W., Roos D., Leguit P., Verbrugh H. A. Polarized secretion of interleukin-8 by human mesothelial cells: a role in neutrophil migration. Immunology. 1995 Feb;84(2):227–232. [PMC free article] [PubMed] [Google Scholar]
- de Fijter C. W., Verbrugh H. A., Peters E. D., Oe P. L., van der Meulen J., Verhoef J., Donker A. J. In vivo exposure to the currently available peritoneal dialysis fluids decreases the function of peritoneal macrophages in CAPD. Clin Nephrol. 1993 Feb;39(2):75–80. [PubMed] [Google Scholar]
- van Bronswijk H., Verbrugh H. A., Heezius H. C., van der Meulen J., Oe P. L., Verhoef J. Dialysis fluids and local host resistance in patients on continuous ambulatory peritoneal dialysis. Eur J Clin Microbiol Infect Dis. 1988 Jun;7(3):368–373. doi: 10.1007/BF01962339. [DOI] [PubMed] [Google Scholar]


