Abstract
Introduction
Right ventricular dysfunction (RVD) is an indicator of poor prognosis in normotensive patients with acute pulmonary embolism (APE). The aim of this study was to compare right ventricular (RV)/left ventricular (LV) ratio measured by echocardiography and multidetector computed tomography (MDCT) with tricuspid annulus plane systolic excursion (TAPSE) as a prognostic factor of APE-related 30-day mortality.
Material and methods
We examined 76 patients with confirmed APE, hemodynamically stable at admission. We evaluated the prognostic value of RV/LV ratio in the apical 4-chamber view and TAPSE measured at echocardiography and the MDCT RV/LV ratio.
Results
Thirty-day APE-related mortality was 10.5% (8 patients). The area under the curve (AUC) for TAPSE in the prediction of APE-related mortality was higher (p < 0.00001) (0.905, 95% CI: 0.828–0.983) than the AUC of the echo RV/LV ratio (0.427, 95% CI: 0.183–0.672) and MDCT RV/LV ratio (0.371, 95% CI: 0.145–0.598). In univariable Cox analysis, TAPSE was the only significant mortality predictor, with hazard ratio (HR) 0.73 (95% CI: 0.62–0.87, p = 0.0004). In multivariable Cox analysis TAPSE was the only significant mortality predictor, with HR 0.62 (95% CI: 0.46–0.85; p = 0.003), while age, heart rate, and RV/LV ratio in echo or MDCT were non-significant. TAPSE ≤ 15 mm was a significant predictor of APE-related mortality, with HR 26.2 (95% CI: 3.2–214.1; p = 0.002), PPV 44% and NPV 98%.
Conclusions
The TAPSE is preferable to echo and MDCT RV/LV ratio for risk stratification in initially normotensive patients with APE. The TAPSE ≤ 15 mm identifies patients with an increased risk of 30-day APE-related mortality.
Keywords: tricuspid annulus plane systolic excursion, acute pulmonary embolism, echocardiography, computed tomography, mortality risk
Introduction
The short-term prognosis of acute pulmonary embolism (APE) depends on hemodynamic status and underlying diseases [1, 2]. According to current guidelines, risk stratification in patients with APE is mainly based on the clinical presentation and the assessment of right ventricular dysfunction (RVD) and injury [3–5]. Right ventricular dysfunction is commonly assessed by echocardiography and is associated with an increased risk of in-hospital adverse outcome [6, 7]. However, various RVD criteria have been proposed. Recently, apart from right ventricular to left ventricular ratio (RV/LV) in echocardiography, decreased tricuspid annulus plane systolic excursion (TAPSE) was reported to be of prognostic value and is a preferable echocardiographic parameter for short-term prognosis of hemodynamically stable PE patients [8]. Currently multidetector contrast-enhanced computed tomography (MDCT) is widely used for the diagnosis of APE and allows the visualization and measurement of heart chambers. Thus MDCT can be an alternative to echocardiography for assessing right ventricular dysfunction [9–13]. However, so far there has been no direct comparison of the prognostic value of TAPSE and RV/LV measured by MDCT.
Therefore, we compared RV/LV measured by echocardiography and MDCT with TAPSE for 30-day pulmonary embolism-related mortality in initially normotensive APE patients.
Material and methods
In this prospective study, we enrolled a group of 76 consecutive patients with diagnosed APE. Acute pulmonary embolism was confirmed by MDCT when thromboemboli were visualized in at least a segmental pulmonary artery. On admission all patients were in a stable hemodynamic condition, had a systemic systolic blood pressure ≥ 90 mm Hg and showed no signs of peripheral hypoperfusion. According to ESC guidelines, intermediate risk APE was diagnosed when on admission patients were normotensive but with echocardiographic or MDCT signs of RV dysfunction or laboratory markers of RV dysfunction/injury were positive. Low risk APE was diagnosed when all checked RV dysfunction and myocardial injury markers were found negative [2]. Acute pulmonary embolism was diagnosed when symptoms of PE had been present for no longer than 14 days before the diagnosis. Patients with diagnosed chronic thromboembolic hypertension and participants in therapeutic clinical trials were not included in this study.
Echocardiography
Echocardiographic examination was performed with a Philips iE 33 machine (Andover, Md., USA) with 2.5–3.5 MHz transducers, as soon as possible after admission, and was digitally recorded. Patients were examined in the left lateral position. The dimensions of the right and left ventricles were measured in the parasternal long-axis view and apical four-chamber view at the level of the mitral and tricuspid valve tips in late diastole defined by the R wave of continuous ECG tracing. After recording of the tricuspid valve peak systolic velocity with continuous-wave Doppler echocardiography, the tricuspid regurgitation peak gradient (TRPG) was calculated using the simplified Bernoulli equation. Pulmonary ejection acceleration time (AcT) was measured using pulsed wave Doppler with the sample volume placed in the RV outflow tract just below the pulmonary valve. Measurements were averaged over 5 consecutive heart cycles. In M-mode presentation, right ventricular function was assessed by TAPSE measurement. We measured the distance (mm) of systolic excursion of the RV annular segment along its longitudinal plane, from a standard apical 4-chamber view.
Multidetector contrast-enhanced computed tomography (MDCT)
Standard contrast-enhanced protocols for the diagnosis of PE were used (image acquisition beginning 15–20 s after the start of contrast medium injection). Multidetector computed tomographies were digitally recorded and were evaluated by a panel (P.S., Ł.B., and M.W.) which included a radiologist expert in lung CT reading. Disagreement was resolved by consensus. The panel was unaware of the echocardiography results. Ventricular diameters were measured by identifying the maximal distance between the ventricular endocardium and the interventricular septum, perpendicular to the long axis of the heart, as previously described [14]. Measurements were performed at the valvular plane in two-dimensional axial transverse images, taking into account that the maximum dimension of the RV and LV may be found at slightly different levels. Intermediate-risk APE was diagnosed when RVD was revealed at echocardiography, whereas the remaining subjects formed the low-risk group. Pre-defined RVD was diagnosed when echocardiography showed: 1) RV free wall hypokinesis and RV/LV > 0.9 in the 4-chamber apical view; and/or 2) an elevated tricuspid valve pressure gradient exceeding 30 mm Hg with a shortened acceleration time of pulmonary ejection below 80 ms. The clinical end point of this study was defined as 30-day APE-related mortality. This observational study received Ethics Committee approval.
Statistical analysis
This is a prospective observational cohort study. Data characterized by a normal distribution are expressed as means followed by SD. Parameters without such a distribution are expressed as medians with maximum-minimum ranges. Student t test or Mann-Whitney U test was used for comparisons between 2 groups. The χ2 test was used to compare discrete variables (with Yates’ correction when needed). Receiver-operating characteristic (ROC) curves were analyzed to assess the optimal cut-off values of echocardiographic and MDCT parameters for 30-day APE-related mortality.
Two different cut-off values of TAPSE were defined: one that identifies patients with a good prognosis (i.e., with a high negative predictive value (NPV) for 30-day APE mortality), and another that identifies subjects at risk (i.e., with a high positive predictive value (PPV) for 30-day APE mortality).
Sensitivity, specificity, PPV, and NPV were calculated for the chosen cut-off value. Kaplan-Meier analysis was used to investigate 30-day survival. The impact of echocardiographic and MDCT parameters on APE-related mortality was evaluated using univariable Cox proportional-hazards regression. Forward stepwise selection with a 0.1 level for staying in the model was used to identify significant predictors in multivariable analysis. Areas under the ROC curves were compared pairwise according to DeLong et al. [15]. All tests were 2-sided. Data were considered significant at p < 0.05. Statistical calculations were performed using the Statistica data analysis software system (2011, version 10, StatSoft, Tulsa, Oklahoma) and MedCalc software (version 11.0.0.0, Ostend, Belgium).
Results
Patient characteristics and clinical course
The study group consisted of 76 consecutive patients with APE (35 men, 41 women, mean age: 64.6 ±18, median: 68 (19–94); years). Intermediate risk APE was diagnosed in 54 patients, low risk APE in 22 patients. Initially, all patients received body mass-adjusted low molecular weight heparin or activated partial thromboplastin time-adjusted unfractionated heparin intravenous infusion. Urgent thrombolysis for deteriorating hemodynamic status was performed in 3 (4%) intermediate risk APE patients, and all of them survived. The 30-day APE-related mortality was 10.5% (8 patients), and all-cause mortality was 13% (10 patients). The 2 non-APE-related deaths were due to neoplastic disease and gastrointestinal hemorrhage. Clinical characteristics of APE subjects are provided in Table I.
Table I.
Clinical characteristics of studied patients with APE
| Parameter | All patients (N = 76) | APE-related mortality (N = 8) | P-value | Remaining patients (N = 68) |
|---|---|---|---|---|
| Female/male | 41/35 | 6/2 | 0.44 | 35/33 |
| Age [years] | 68 (19–94) | 82 (57–94) | 0.002 | 66 (19–90) |
| HR [1/s] | 88 (54–180) | 110 (94–160) | 0.001 | 87 (54–180) |
| Systemic blood pressure [mm Hg] | 125 (95–220) | 110 (90–155) | 0.2 | 130 (95–220) |
| Comorbidities (COPD, CHF, neoplasm), n (%) | 23 (30) | 4 (50) | 0.38 | 19 (28) |
| Thrombolysis, n (%) | 3 (4) | 0 (0) | 0.78 | 3 (4) |
| Diuretics, n (%) | 32 (42) | 8 (100) | 0.0018 | 24 (35) |
| Ca-blockers, n (%) | 13 (17) | 0 (0) | 0.38 | 13 (19) |
| Troponin [ng/ml] | 0.03 (0–5.58) | 0.085 (0–0.93) | 0.356 | 0.03 (0–5.58) |
| Troponin positive, n (%) | 46 (61) | 5 (63) | 0.79 | 41 (60) |
| NT-proBNP [pg/ml] | 1957 (25–35000) | 9511 (4872–35000) | 0.005 | 1708 (25–25000) |
APE – acute pulmonary embolism, HR – heart rate, COPD – chronic obstructive pulmonary disease, CHF – congestive heart failure, NT-proBNP – N-terminal pro-B-type natriuretic peptide.
Patients who died were older, presented with increased heart rate and elevated plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP).
Echocardiographic and MDCT parameters of the study population are summarized in Table II.
Table II.
Echocardiographic and MDCT parameters of the study population
| Parameter | All patients (N = 76) | APE-related mortality (N = 8) | P-value | Remaining patients (N = 68) |
|---|---|---|---|---|
| RV4C [mm] | 41.0 (27–60) | 41.6 ±8.9 | 0.19 | 37.5 (20–48) |
| LV4C [mm] | 41.1 ±8.0 | 40.6 ±11.0 | 0.84 | 41.2 ±7.6 |
| RV/LV 4C | 0.93 (0.48–2.07) | 0.95 (0.48–1.68) | 0.51 | 0.93 (0.63–2.07) |
| RV CT [mm] | 44.2 (26.1–71.6) | 41.9 ±10.3 | 0.44 | 44.8 ±10.1 |
| LV CT [mm] | 36.2 (21.3–74) | 38.7 (24–74) | 0.31 | 36 (21.3–67.5) |
| RV/LV CT | 1.1 (0.53–2.77) | 1.06 (0.55–1.55) | 0.24 | 1.1 (0.53–2.77) |
| AcT [ms] | 79.5 (37–130) | 75 (50–111) | 0.72 | 79.5 (37–130) |
| TRPG [mm Hg] | 35.5 (10–100) | 45.5 (34–100) | 0.11 | 34 (10–94) |
| TAPSE [mm] | 19.4 ±5.6 | 12.3 ±3.6 | 0.0002 | 20.3 ±5.2 |
RV4C – right ventricle four-chamber diameter, LV4C – left ventricle four-chamber diameter, CT – computed tomography, AcT – acceleration time, TRPG – tricuspid regurgitant peak gradient, TAPSE – tricuspid annulus plane systolic excursion.
Dimensions of RV, LV and RV/LV ratio at echocardiography and MDCT did not differ between survivors and non-survivors. Interestingly, the mean value of TAPSE was significantly lower in non-survivors.
Hazard risk of predictors of APE mortality in univariable analysis
Univariable Cox proportional hazards regression analysis showed that only TAPSE significantly predicted clinical outcome (Table III).
Table III.
Univariable predictors of APE-related mortality in 76 initially normotensive patients
| Parameter | HR | 95% CI | P-value |
|---|---|---|---|
| RV/LV 4C | 0.44 | 0.03–6.48 | 0.547 |
| RV/LV CT | 0.26 | 0.03–2.0 | 0.197 |
| TAPSE | 0.73 | 0.62–0.87 | 0.0004 |
RV – right ventricle, LV – left ventricle, 4C – four-chamber view, CT – computed tomography, TAPSE – tricuspid annulus plane systolic excursion.
In multivariable Cox analysis TAPSE was the only significant mortality predictor with HR = 0.62 (95% CI: 0.46–0.85; p = 0.003), while age, heart rate, and RV/LV ratio in echo or MDCT were non-significant.
ROC curve analysis
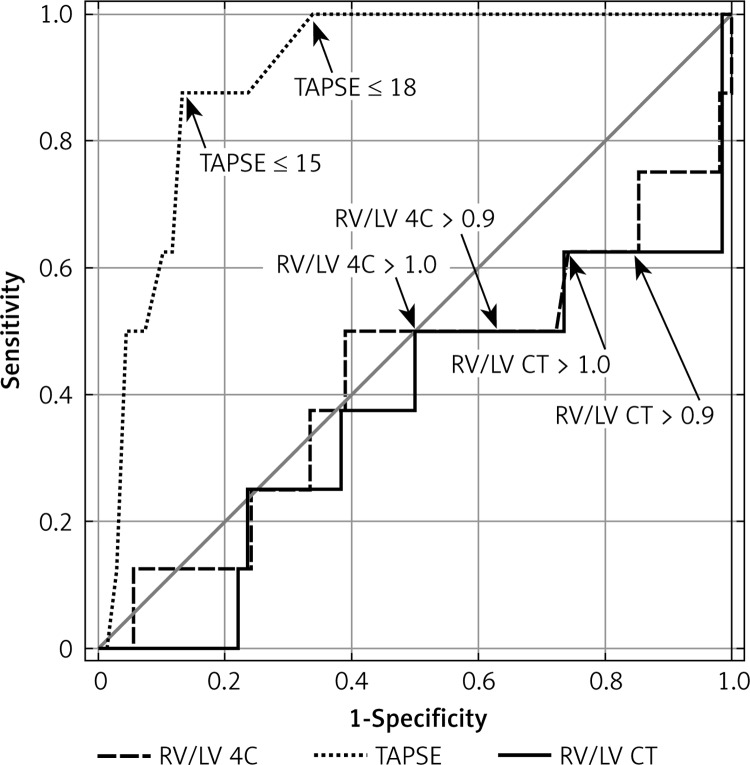
ROC analysis showed that the area under the curve (AUC) for TAPSE in the prediction of APE-related mortality was the highest (AUC = 0.905, 95% CI: 0.828–0.983, p < 0.0001) (Table IV, Figure 1).
Table IV.
ROC analysis of parameters in the prediction of APE-related mortality in study population
| Parameter | AUC | 95% CI | P-value | Cut-off point | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| RV/LV 4C | 0.427 | 0.183–0.672 | 0.5812 | 0.9 1.0 |
10.8 13.3 |
84 87.5 |
| RV/LV CT | 0.371 | 0.145–0.598 | 0.2937 | 0.9 1.0 |
8.1 8.9 |
78.6 85 |
| TAPSE | 0.905 | 0.828–0.983 | < 0.0001 | ≤ 15 ≤ 18 |
43.75 25.8 |
98.3 100 |
RV – right ventricle, LV – left ventricle, 4C – four-chamber view, CT – computed tomography, TAPSE – tricuspid annulus plane systolic excursion.
Figure 1.
ROC of TAPSE, RV/LV at echocardiography and MDCT for APE-related mortality in studied patients
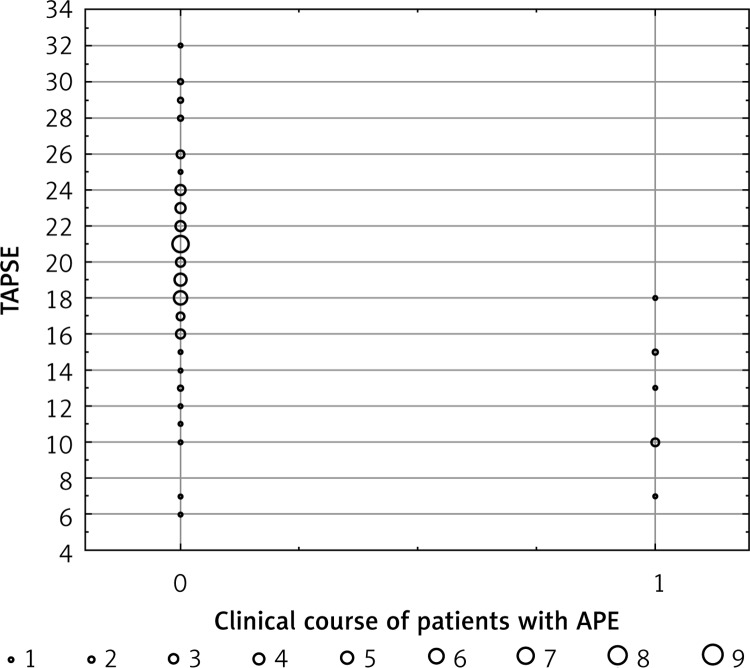
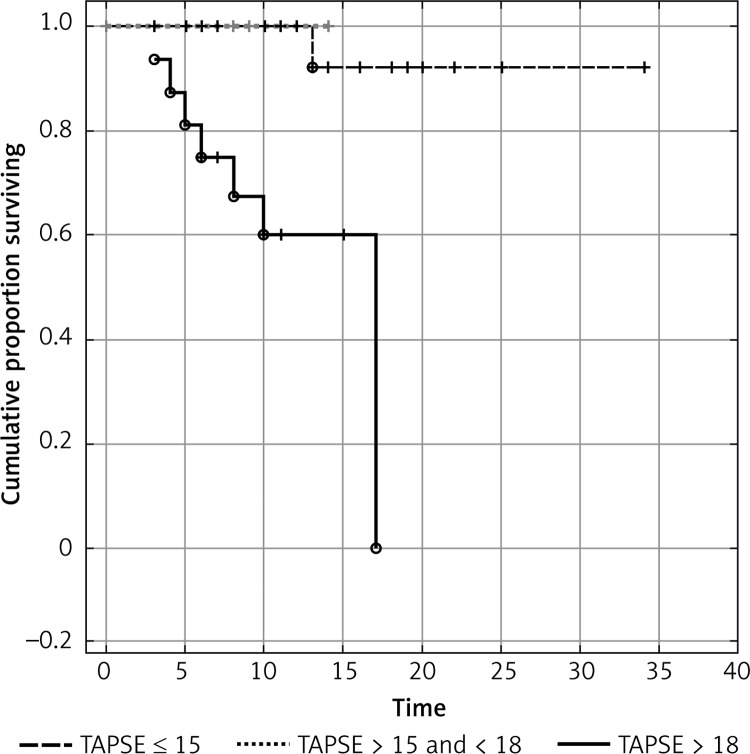
In order to determine prognostic value, we defined two cut-off values of TAPSE. The TAPSE ≤ 15 mm (21% of subjects) showed a PPV of 43.75% for APE-related mortality with NPV 98.3%. The TAPSE ≥ 18 mm had a PPV of 25.8% with a 100% NPV. All patients with TAPSE ≥ 18 were in the low-risk group with good prognosis. In initially normotensive patients, TAPSE ≤ 15 mm was associated with a 43.75% risk of APE-related death. Figure 2 shows individual TAPSE values in APE patients according to clinical course, whereas Figure 3 presents the survival analysis according to TAPSE.
Figure 2.
Distribution of individual TAPSE values in initially normotensive APE patients according to clinical course. 0 – Uncomplicated clinical course (n = 68), 1 – patients with APE-related mortality (n = 8)
Figure 3.
Kaplan-Meier survival analysis according to TAPSE in 76 initially normotensive patients with APE
Discussion
Acute pulmonary embolism is a relatively common life-threatening disorder. Pulmonary arterial bed obstruction can cause potentially reversible RV dysfunction. Immediate diagnosis is very important because it allows treatment to be started and significantly reduces mortality. Currently, MDCT is the method of choice for imaging the pulmonary arterial bed for suspected APE in routine practice. Moreover, MDCT allows assessment of the right-to-left ventricular dimension ratio. Many studies have shown prognostic significance of this parameter. Schoepf et al. published a study of 431 patients with APE. The RV/LV > 0.9 was present in 64% of them, and its NPV and PPV for 30-day mortality were 92.3% and 15.6%, respectively. The hazard ratio of RV/LV > 0.9 for predicting 30-day mortality was 5.17 (95% CI: 1.63–16.35; p = 0.005) after adjusting for other risk factors such as pneumonia, cancer, chronic obstructive pulmonary disease and age [9]. Becattini et al. analyzed the prognostic value of RV/LV ≥ 0.9 in MDCT in 457 APE patients. Right ventricular dysfunction in MDCT was an independent predictor for in-hospital death or clinical deterioration in the overall population (HR = 3.5, 95% CI: 1.6–7.7; p = 0.002) and in hemodynamically stable patients (HR = 3.8, 95% CI: 1.3–10.9; p = 0.007) [14]. In a recently published paper, Choi et al. retrospectively reviewed 657 consecutive patients with APE. Multivariable analysis showed that RV/LV ≥ 1 in MDCT is significantly predictive of an adverse outcome (OR = 3.00, 95% CI: 1.27–7.07, p = 0.012) [16]. Transthoracic echocardiography (TTE) is of little value in the diagnosis of normotensive APE patients because reported sensitivity is around 60–70% [17, 18]. On the other hand, TTE is very valuable in risk stratification. A meta-analysis published by ten Wolde et al. revealed a two-fold increase of risk of PE-related mortality in patients with echocardiographic signs of right ventricular dysfunction [19]. The unadjusted risk ratio of RV dysfunction, as assessed by echocardiography (five studies), for predicting death was 2.4 (95% CI: 1.3–4.4) in a meta-analysis published by Sanchez et al. [20]. Fremont at al. analyzed 1416 consecutive patients hospitalized for APE. Multivariate analysis showed RV/LV ratio > 0.9 to be an independent predictive factor for hospital mortality (OR = 2.66; p < 0.01) [6].
Unfortunately, echocardiographic criteria of RV dysfunction differ among published studies and include RV dilatation, hypokinesis, increased RV/LV diameter ratio, shortening of pulmonary artery acceleration time (AcT) and increased TRPG [7, 21, 22]. In a paper published by Kjaergard et al. the authors analyzed echocardiographic data of 283 non-massive APE patients. Among these patients shortened AcT was associated with increased mortality (HR = 0.84 per 10 ms increase, p = 0.0001) [23]. The TAPSE is a simple, reproducible echocardiographic parameter for estimating RV function. Recent guidelines for right heart assessment in adults recommended routine use of TAPSE as a simple method of estimating RV function with a lower reference value of 16 mm [24].
Normotensive patients with APE form a very heterogeneous group. Some of them are candidates for a short hospital stay or even outpatient treatment, but some require close monitoring and, if necessary, more aggressive therapy.
A simple prognostic parameter, easily obtained during routine echo or MDCT, is important for management of APE patients. However, there are no studies directly comparing the prognostic significance of TAPSE and RV/LV measured by MDCT. Therefore, we compared RV/LV measured by echocardiography and MDCT with TAPSE in relation to 30-day APE-related mortality in initially normotensive APE patients.
We analyzed the data of 76 normotensive APE patients. Diagnosis was confirmed by MDCT when thromboemboli were visualized in at least a segmental pulmonary artery. We assessed RV/LV in MDCT. All patients underwent echocardiography as soon as possible after hospital admission with TAPSE and RV/LV measurements. The 30-day APE-related mortality was 10.5% (8 patients) and all-cause mortality was 13% (10 patients). Univariable Cox proportional hazards regression analysis showed that only TAPSE significantly predicted clinical outcome (HR = 0.73, 95% CI: 0.62–0.87, p = 0.0004), while RV/LV in MDCT and echo was non-significant. Additionally, multivariable analysis showed that TAPSE (HR = 0.62, 95% CI: 0.46–0.85; p = 0.003) was the only independent predictor of 30-day APE-related mortality. We found that TAPSE ≤ 15 mm (21% of subjects) showed a PPV of 43.75% for APE-related mortality with NPV 98.3% while TAPSE ≥ 18 mm had a PPV 25.8% with a 100% NPV. All patients with TAPSE ≥ 18 formed a low-risk group with good prognosis. In initially normotensive patients TAPSE ≤ 15 mm was associated with a 43.75% risk of APE-related death.
The TAPSE, a simple echocardiographic parameter, may help stratify risk in normotensive APE patients and is superior to RV/LV in MDCT and echocardiography. Patients with TAPSE ≤ 15 mm should be admitted to an intensive care unit and closely monitored. In these patients, APE may lead to clinical deterioration with indications for fibrinolysis. Subjects with TAPSE ≥ 18 mm form a low-risk group with good prognosis and are candidates for a short hospital stay.
One of the major limitations of the present study is its small sample size, which might reduce the statistical significance of the results. Moreover, this is a single-centre observational study, and causes of death were not adjudicated. Therefore, our results should be validated in an external APE population.
In conclusion, TAPSE, an easily measurable echocardiographic parameter, is superior to echo and MDCT RV/LV ratio for risk stratification in initially normotensive patients with APE. The TAPSE ≤ 15 mm identifies patients with an increased risk of 30-day APE-related mortality.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 2.Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29:2276–315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 3.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007;116:427–33. doi: 10.1161/CIRCULATIONAHA.106.680421. [DOI] [PubMed] [Google Scholar]
- 4.Vedovati MC, Becattini C, Agnelli G, et al. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest. 2012;142:1417–24. doi: 10.1378/chest.11-2739. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinides S. Clinical practice. Acute pulmonary embolism. N Engl J Med. 2008;359:2804–13. doi: 10.1056/NEJMcp0804570. [DOI] [PubMed] [Google Scholar]
- 6.Fremont B, Pacouret G, Jacobi D, Puglisi R, Charbonnier B, de Labriolle A. Prognostic value of echocardiographic right/left ventricular end-diastolic diameter ratio in patients with acute pulmonary embolism: results from a monocenter registry of 1,416 patients. Chest. 2008;133:358–62. doi: 10.1378/chest.07-1231. [DOI] [PubMed] [Google Scholar]
- 7.Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med. 2005;165:1777–81. doi: 10.1001/archinte.165.15.1777. [DOI] [PubMed] [Google Scholar]
- 8.Pruszczyk P, Goliszek S, Lichodziejewska B, et al. Prognostic value of echocardiography in normotensive patients with acute pulmonary embolism. JACC Cardiovasc Imaging. 2014;7:553–60. doi: 10.1016/j.jcmg.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110:3276–80. doi: 10.1161/01.CIR.0000147612.59751.4C. [DOI] [PubMed] [Google Scholar]
- 10.Araoz PA, Gotway MB, Harrington JR, Harmsen WS, Mandrekar JN. Pulmonary embolism: prognostic CT findings. Radiology. 2007;242:889–97. doi: 10.1148/radiol.2423051441. [DOI] [PubMed] [Google Scholar]
- 11.van der Meer RW, Pattynama PM, van Strijen MJ, et al. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology. 2005;235:798–803. doi: 10.1148/radiol.2353040593. [DOI] [PubMed] [Google Scholar]
- 12.Quiroz R, Kucher N, Schoepf UJ, et al. Right ventricular enlargement on chest computed tomography: prognostic role in acute pulmonary embolism. Circulation. 2004;109:2401–4. doi: 10.1161/01.CIR.0000129302.90476.BC. [DOI] [PubMed] [Google Scholar]
- 13.Becattini C, Agnelli G, Germini F, Vedovati MC. Computed tomography to assess risk of death in acute pulmonary embolism: a meta-analysis. Eur Respir J. 2014;43:1678–90. doi: 10.1183/09031936.00147813. [DOI] [PubMed] [Google Scholar]
- 14.Becattini C, Agnelli G, Vedovati MC, et al. Multidetector computed tomography for acute pulmonary embolism: diagnosis and risk stratification in a single test. Eur Heart J. 2011;32:1657–63. doi: 10.1093/eurheartj/ehr108. [DOI] [PubMed] [Google Scholar]
- 15.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 16.Choi KJ, Cha SI, Shin KM, et al. Prognostic implications of computed tomographic right ventricular dilation in patients with acute pulmonary embolism. Thrombosis Res. 2014;133:182–6. doi: 10.1016/j.thromres.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Roy PM, Colombet I, Durieux P, Chatellier G, Sors H, Meyer G. Systematic review and meta-analysis of strategies for the diagnosis of suspected pulmonary embolism. BMJ. 2005;331:259. doi: 10.1136/bmj.331.7511.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grifoni S, Olivotto I, Cecchini P, et al. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000;101:2817–22. doi: 10.1161/01.cir.101.24.2817. [DOI] [PubMed] [Google Scholar]
- 19.ten Wolde M, Sohne M, Quak E, Mac Gillavry MR, Buller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med. 2004;164:1685–9. doi: 10.1001/archinte.164.15.1685. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29:1569–77. doi: 10.1093/eurheartj/ehn208. [DOI] [PubMed] [Google Scholar]
- 21.Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest. 2004;125:1539–45. doi: 10.1378/chest.125.4.1539. [DOI] [PubMed] [Google Scholar]
- 22.Sekhri V, Mehta N, Rawat N, Lehramn SG, Aronow WS. Management of massive and nonmassive pulmonary embolism. Arch Med Sci. 2012;8:957–69. doi: 10.5114/aoms.2012.32402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjaergard J, Sagaard P, Hassager C. Right ventricular strain in pulmonary embolism by Doppler tissue echocardiography. J Am Soc Echocardiogr. 2004;17:1210–2. doi: 10.1016/j.echo.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]