Abstract
Introduction
Local application of bisphosphonates has been proven to be safer than systemic administration to promote implant fixation. The objective of this study was to introduce such a simple, convenient and efficient method to enhance titanium (Ti) implant osseointegration in ovariectomized (OVX) rats.
Material and methods
Twenty female Sprague-Dawley rats sequentially underwent bilateral ovariectomy and tibia implantation, and injection of 30 µg/implant zoledronic acid (ZOL) at the site of implantation was performed. At the end of the study, the tibiae, mandibles, femurs and vertebrae were harvested for dual energy X-ray absorptiometry, histology and micro-computed tomography examination.
Results
Ovariectomized rats showed poor bone density, bone mass and trabecular microstructure. OVX + ZOL rats were characterized by significantly improved peri-implant bone area (1.72-fold), bone contact (2.30-fold), bone mineral density (1.57-fold) and bone mineral content (1.67-fold), as well as moderately increased bone volume to total volume ratio (1.34-fold), percentage osteointegration (1.54-fold), connectivity density (1.45-fold), and trabecular number (1.43-fold), but decreased trabecular separation (57.69%) when compared with the control levels (p < 0.05). No histological signs of jaw osteonecrosis were observed in the rats treated with ZOL, and there was no significant difference between the OVX group and OVX + ZOL group in the bone mass of the mandible, femur and 5th lumbar vertebra (p > 0.05). In addition, the overproduction of osteoporosis-induced advanced glycation end-products (AGEs) was completely prevented by local treatment with 30 µg/implant ZOL.
Conclusions
A local, one time, low-dose injection of ZOL at the site of implantation is able to promote the osseointegration of Ti implants following postmenopausal osteoporosis, and this action may be partly mediated by inhibition of the osteoporosis-induced AGE overproduction in the bone marrow.
Keywords: bisphosphonate, osteoporosis, implant, advanced glycation end products
Introduction
Titanium (Ti) is a widely used material in dental and orthopedic surgery, and can be used as plates, screws, rods, as well as joint replacements [1]. With favorable biocompatibility, bioactivity and osteoconductivity, as well as excellent resilience and mechanical properties, Ti implants have been shown to induce osteogenesis-related signaling pathway, stimulate osteoblast proliferation and differentiation, and generate an osteogenic microenvironment both in vitro and in vivo [2]. Success of implantation is related to the patient's overall general health, the bone quantity and density available at the site, and whether the patients are heavy smokers, or drug or alcohol abusers [3]. As one of the main risk factors for Ti implant failure, osteoporosis is increasingly becoming a major and escalating health care problem due to the ageing of the world's population [4, 5]. In the condition of osteoporosis, wear particles shed from Ti implants may induce more pro-inflammatory cytokine release by macrophages and intensify the peri-implant osteolysis, which accumulates into accelerated bone loss and compromises new bone formation [6, 7]. Thus, it is desirable to develop useful and simple methods to promote the osseointegration and mechanical stability of Ti implants, not only in normal people but also in the patients who are suffering from osteoporosis.
Bisphosphonates (BPs) are stable analogs of endogenous pyrophosphates that can inhibit bone loss, increase bone mass and diminish fracture risk associated with osteoporosis [8]. Since three decades ago, these drugs have been widely prescribed for clinical treatment of osteoporosis, Paget's disease, multiple myeloma, hypercalcemia of malignancy, and a variety of other skeletal abnormalities [9, 10]. Numerous studies have confirmed the clinical efficacy of systemic BP treatment in the success of implantation [11]. As first-line drugs for bone diseases associated with excessive bone resorption, BPs can reduce osteoclast-mediated bone resorption by affecting osteoclast recruitment, differentiation and resorption activity, improve osteoblast-mediated bone formation by inducing osteoblast proliferation, differentiation and osteogenesis activity, and promote peri-implant new bone formation by inhibiting the action of macrophages on bone and their products-related osteolysis both in vitro and in vivo [12]. However, despite their extraordinary potency, osteonecrosis of the jaw (ONJ), atrial fibrillation, bone and joint pain, and other undesirable effects associated with long-term high-dose exposure to BPs have brought increased controversy over the broad clinical practice of these drugs [10]. Recently, local application, directly acting on peri-implant osteoclasts and osteoblasts, has been proven to be safer and more useful [13–15].
The main objective of this study was to introduce a simple, safe and efficient local BP treatment method in osteoporotic bone. Using an ovariectomized (OVX) animal model, we investigated the potential effect of a local, one time, low-dose treatment of zoledronic acid (ZOL) on the osseointegration of Ti implants inserted in the tibiae, and assessed whether this local treatment is able to affect other bones such as the mandible, femur and vertebra. This method is simple, convenient, easy to implement compared with other coating methods, and does not require special equipment.
Material and methods
Animals
The animal experiment was approved by the Animal Care Committee of Shanxi Medical University. Twenty female Sprague-Dawley rats weighing 190–210 g were bred individually in cages, staying in identical environments and receiving standard rodent diets. After a 10-day acclimatization period, all rats underwent bilateral ovariectomy, as reported previously [16]. Three months later, they were randomly assigned to two groups of ten rats each for bilateral tibia implantation. Briefly, an incision was made to expose the proximal tibia metaphysis, and a pilot hole was drilled through the intercondylar eminence into the medullary canal. Then, a volume of 30 µl of either ZOL (1 mg/ml) (Novartis Pharma AG, Switzerland) or saline was slowly injected into the channel. Thirty seconds later, a custom-made Ti implant, measuring 1 mm in diameter and 10 mm in length, was inserted into the channel (Figure 1). Finally, the incision was closed. After the implantation, we further removed the right mandibular first molar and resected some gingivae at the extraction site to increase the likelihood of developing ONJ (Figure 1) [17, 18]. Another 3 months later, the rats were sacrificed and their tibiae along with the mandibles, femurs and vertebrae were harvested for dual energy X-ray absorptiometry (DXA), histology and micro-computed tomography (micro-CT) examination, as well as measurement of advanced glycation end-products (AGEs).
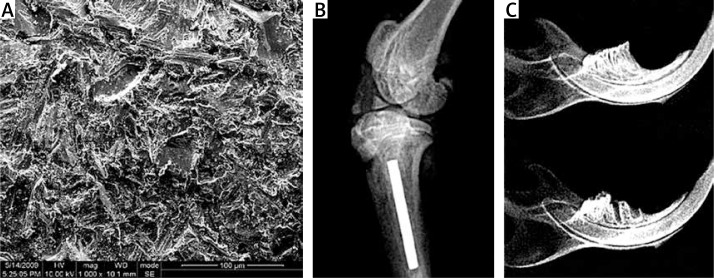
Figure 1.
A – Scanning electron micrograph of titanium (Ti) implant. B – Radiograph of Ti implant in tibia. C – Radiographs of mandibles with and without the right mandibular first molar
Dual energy X-ray absorptiometry
Samples were uniformly immersed in water, placed in identical positions, and scanned by DXA (Lunar PIXImus, GE Medical Systems, WI, USA) with small animal computer software. We defined the peri-implant zone of the tibia [14], as well as the mandible, femur and 5th lumbar vertebra (L5), as the regions of interest (ROI). Following scanning, the values of bone mineral content (BMC), bone mineral density (BMD) and bone area were calculated.
Histology
Tibiae were fixed in 10% neutral buffered formalin for 3 days, and demineralized in 10% ethylene-diamine-tetraacetic acid (EDTA) for 4 weeks. Then, the samples were trimmed, and Ti implants were pulled out. Tibiae without implants were embedded in paraffin. Finally, sections of 4 µm thickness were cut using a LEICA 2500E microtome (LEICA, Germany). The section at approximately 3.0 mm below the growth plate was selected as the one of interest, and stained with hematoxylin-eosin. Histomorphometry was performed to quantify the percentages of bone area (BA) and bone contact (BC) for each sample using a semi-automated digitizing image analyzer system, consisting of a Nikon ECLIPSE E600 stereomicroscope, a computer-coupled Nikon DXM1200 digital camera and NIS-Elements F 2.20 image software. BA was defined as the area percentage of the newly formed bone within a circle of 0.1 mm around the Ti implant to the whole area as described earlier [13], while BC was set as the length percentage of the direct bone-implant interface to total implant surface. Similarly, the mandibles, femurs and vertebrae were fixed, demineralized, embedded and stained with hematoxylin-eosin, and microscopic examinations were performed.
Micro-computed tomography
Proximal tibiae were positioned in a custom jig with water, and monitored craniocaudally by a micro-CT 80 scanner (Scanco Medical, Bassersdorf, Switzerland), at 70 kV, 114 mA and 700 ms integration time [19]. 18 mm of tibia was scanned from the proximal epiphysis to 1 mm below the Ti implant, including approximately 1200 images with a resolution of 2048 × 2048 pixels and an isotropic voxel size of 10 µm. Then, the binary images were reconstructed to 3D images for further qualitative and quantitative evaluations (σ = 1.2, support = 1, threshold for bone = 205, and threshold for Ti implants = 700). The volume of interest (VOI) was set as the 100 slices from 3.0 mm below the growth plate and limited in a ring from implant axis with a radius of 1.0 mm [19]. The following parameters were assessed: bone volume to total volume ratio (BV/TV), percentage osteointegration (%OI), connectivity density (Conn.D), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp) and trabecular number (Tb.N).
Measurement of advanced glycation end-products
Immunohistochemical staining (SABC detection system) was performed to evaluate the accumulation of AGEs in the bone marrow of the tibiae. In brief, bone sections of 4 µm thickness were deparaffinized with xylene and rehydrated with ethanol. Then, sodium citrate buffer was used for heat-mediated antigen retrieval, and 5% bovine serum albumin (BSA) was added for blocking at 37°C for 20 min. Sections were incubated with primary antibody at 4°C overnight. In this study, polyclonal rabbit anti-carboxymethyllysine (CML) antibody (1 : 150, Abcam) was used as the primary antibody, which showed stronger immunoreactivity in the bone marrow [20]. Staining was performed using DAB kits, and the sections were counterstained with hematoxylin.
Statistical analysis
Data are expressed as mean ± standard error (SE), and SPSS 12.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Comparison between the OVX group and OVX + ZOL group was performed using the t test, and p < 0.05 was considered as indicating statistical significance.
Results
DXA analysis
As expected, OVX induced poor peri-implant BMD (0.266 ±0.031 g/cm2) and BMC (0.102 ±0.023 g) at the end of the study, while both parameters were significantly increased in the rats locally treated with 30 µg/implant ZOL (0.417 ±0.026 g/cm2 and 0.170 ±0.015 g, respectively, p < 0.05; Table I). To assess the possible risks of local injection of ZOL, we further measured the BMD and BMC values of the mandible, femur and L5. However, there was no significant difference between the OVX group (mandible, 0.210 ±0.016 g/cm2 and 0.575 ±0.027 g; femur, 0.201 ±0.013 g/cm2 and 0.388 ±0.019 g; L5, 0.226 ±0.025 g/cm2 and 0.109 ±0.020 g) and OVX + ZOL group (mandible, 0.225 ±0.019 g/cm2 and 0.602 ±0.033 g; femur, 0.204 ±0.010 g/cm2 and 0.394 ±0.017 g; L5, 0.231 ±0.016 g/cm2 and 0.115 ±0.014 g; p > 0.05); 30 µg/implant ZOL did not change the skeletal mineralization of these bones.
Table I.
The BMD, BMC and area measured by DXA
| Parameter | OVX group | OVX + ZOL group | ||||||
|---|---|---|---|---|---|---|---|---|
| Peri-implant | Mandible | Femur | L5 | Peri-implant | Mandible | Femur | L5 | |
| BMD [g/cm2] | 0.266 ±0.031 | 0.210 ±0.016 | 0.201 ±0.013 | 0.226 ±0.025 | 0.417 ±0.026* | 0.225 ±0.019 | 0.204 ±0.010 | 0.231 ±0.016 |
| BMC [g] | 0.102 ±0.023 | 0.575 ±0.027 | 0.388 ±0.019 | 0.109 ±0.020 | 0.170 ±0.015* | 0.602 ±0.033 | 0.394 ±0.017 | 0.115 ±0.014 |
| Area [cm2] | 0.379 ±0.041 | 2.770 ±0.024 | 1.885 ±0.014 | 0.475 ±0.028 | 0.413 ±0.029 | 2.741 ±0.032 | 1.907 ±0.018 | 0.488 ±0.035 |
Bone mineral density (BMD), bone mineral content (BMC), and area are shown. Data are presented as mean ± SE, and comparison between OVX group and OVX + ZOL group was performed using t test.
p < 0.05 when compared to OVX group.
Histology evaluation
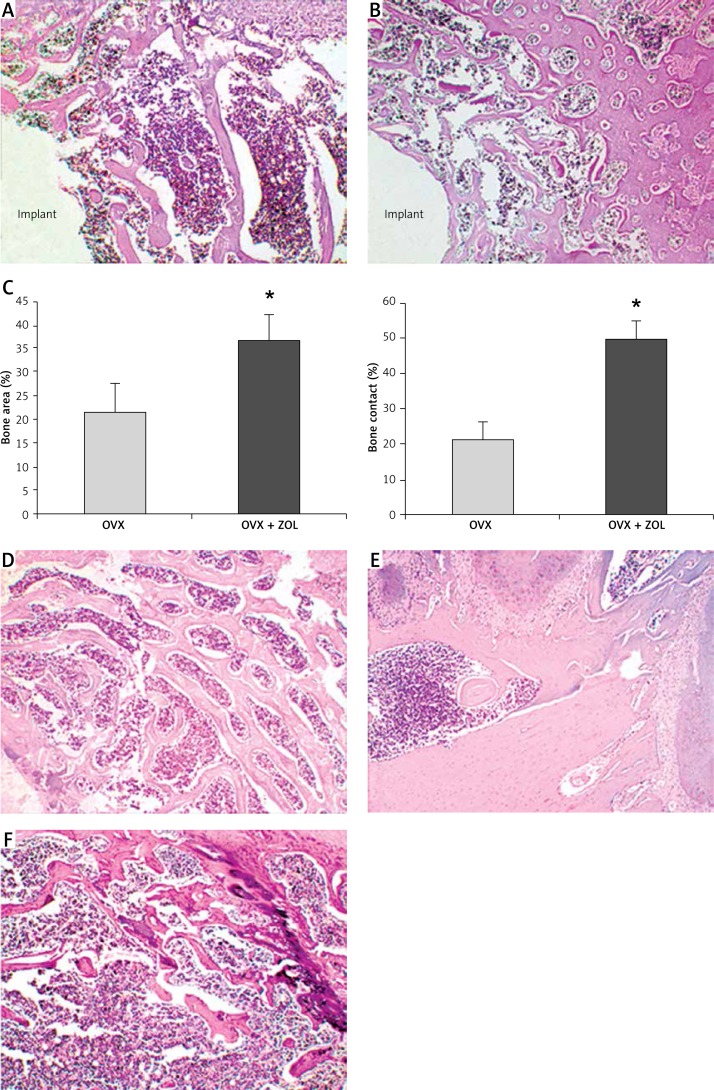
Histomorphometric analysis revealed that the normal skeletal features were destroyed in OVX rats, with lower BA (21.34 ±6.19%) and BC (21.56 ±4.32%). The route of local injection of 30 µg/implant ZOL effectively promoted the osseointegration and peri-implant new bone formation of Ti implants inserted in the tibiae of OVX rats. In the OVX + ZOL group, the values of BC were increased up to 2.30 times the control levels (49.52 ±5.11%), and the values of BA were up to 1.72 times the control levels (36.60 ±5.72%, p < 0.05; Figure 2). Three months after implantation, no clinical or histological signs of ONJ were observed in the rats locally treated with ZOL. All rats showed intact overlying mucosa, and no exposed bone appeared in the posterior mandibular region. Moreover, there was no significant difference between the OVX group and OVX + ZOL group in the bone mass of the mandible, femur and L5 (Figure 2).
Figure 2.
A – Representative light micrograph of the implant-bone interface from ovariectomized (OVX) rats (original magnification 10×). B – Representative light micrograph of the implant-bone interface from OVX rats treated with zoledronic acid (ZOL) (original magnification 10×). C – Effects of local ZOL treatment on the bone area (BA) and bone contact (BC) values. Data are expressed as mean ± SE, and comparison was determined with t test. *p < 0.05 comparison with OVX group. D – Representative light micrograph of the 5th lumbar vertebra (original magnification 10×). E – Representative light micrograph of the mandible (original magnification 10×). F – Representative light micrograph of the femur (original magnification 10×)
Micro-computed tomography evaluation
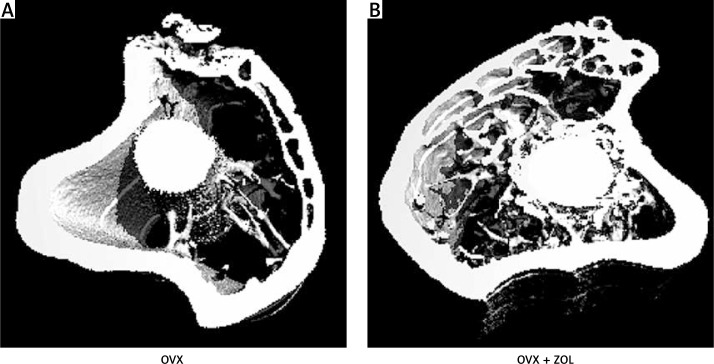
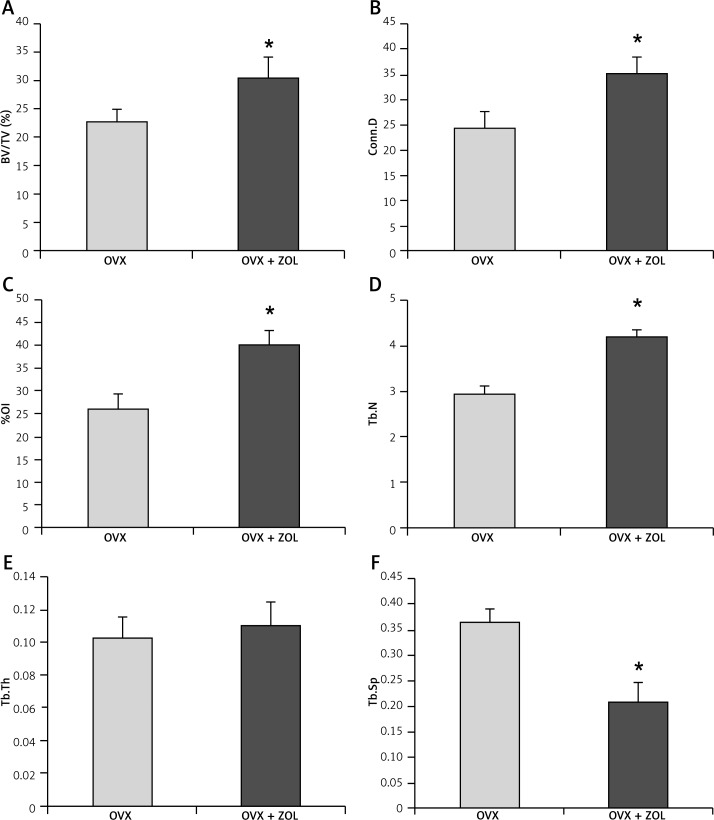
The high-resolution 3D images obtained from micro-CT clearly confirmed the results of histological analysis. Three months after implantation, local injection of 30 µg/implant ZOL exhibited a significant effect in promoting the peri-implant 3D bone volume (Figure 3). The OVX rats had lower values of BV/TV (22.61 ±2.46%), %OI (26.01 ±3.23%), Conn.D (24.34 ±3.42 mm–3), and Tb.N (2.93 ±0.20 mm–1), with little bone apposition around the Ti implants. OVX + ZOL rats were characterized by moderately increased BV/TV (1.34-fold),%OI (1.54-fold), Conn.D (1.45-fold), and Tb.N (1.43-fold), but decreased Tb.Sp (57.69%) when compared with the control levels. However, by contrast, no significant difference was found in the trabecular thickness between the two groups (p > 0.05; Figure 4).
Figure 3.
Representative 3D micro-CT images of the proximal tibia with titanium implants 3 months after implantation
Figure 4.
Effects of local zoledronic acid (ZOL) treatment on the microstructure parameters around titanium implants inserted in the tibiae of ovariectomized (OVX) rats. Data are expressed as mean ± SE, and comparison was determined with the t test. *p < 0.05 comparison with OVX group
BV/TV – bone volume/tissue volume, Conn.D – connectivity density, %OI – osseointegration, Tb.N – trabecular number, Tb.Th – trabecular thickness, Tb.Sp – trabecular separation.
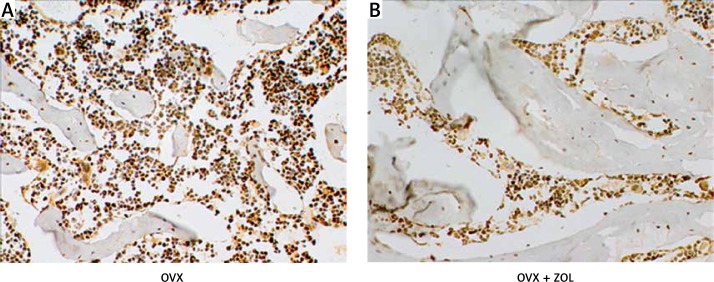
Immunohistochemical detection of advanced glycation end-products
Figure 5 shows the immunohistological staining of the proximal tibia from different groups. The AGE CML was seen brown in the bone marrow. In contrast, the accumulation of AGEs was significantly inversely correlated with the new bone formation around the Ti implants. OVX induced not only bone loss but also more AGE expression in vivo, while local treatment of 30 µg/implant ZOL evidently promoted peri-implant new bone formation and resulted in the downregulation of AGEs in the bone marrow. Our results indicated that the beneficial effects of ZOL local treatment on Ti implant osseointegration in OVX rats may be partly mediated by inhibition of the osteoporosis-induced AGE overproduction in the bone marrow.
Figure 5.
Effects of local zoledronic acid (ZOL) treatment on the advanced glycation end products (AGEs) expressed in the bone marrow of the proximal tibia (original magnification 40×)
Discussion
One of the mainstays of prevention and treatment of bone diseases associated with excessive bone resorption such as osteoporosis has been systemic use of BPs. However, oral or intravenous administration of high doses of BPs over long periods may impair the normal skeletal mineralization and induce osteomalacia, osteonecrosis of the jaw (ONJ) and femoral stress fractures [10], although these risks seem to be very low. In our previous studies, the method of immobilization of BPs onto hydroxyapatite (HA)-coated implants has been proven to be efficient to improve implant fixation in OVX rats, but the HA coating procedure by plasma spraying was complicated and time-consuming [13, 14]. Thus, the present study was designed to evaluate the efficiency of another local BP treatment method, which appears to be simple, convenient, and easy to implement. A local, one time, low-dose injection of BPs at the site of implantation was performed, which allows for sustained release of BPs from natural HA into the bone marrow and has been proven to be a useful modality to reduce the risk of implant loosening in skeletally mature animals [21–23]. The novelty of our study lies in introducing such a simple approach in OVX rats, an extensively used animal model for postmenopausal osteoporosis that is the most common indication for BP administration and a more challenging pathological condition for implantation success when compared with normal bone.
At 3 months after implantation, injection of 30 µg/implant ZOL at the site of implantation significantly normalized the high rate of bone turnover around the Ti implants and enhanced the bone-to-implant contact and peri-implant new bone formation. Specifically, no clear undesirable effects have yet emerged. There are three possible explanations for the efficiency and safety associated with local BP treatment in this study. First, the poor bioavailability of BPs was significantly improved. The BPs have a high affinity for natural HA, which is the major component of normal bone and therefore represents an ideal carrier for drugs [24]. BPs could be cleared very rapidly within 24 h from the circulation [25]; however, their skeletal half-life is extremely long, up to several years [26]. Second, the dosage of ZOL was 30 µg/implant, which was effective in this study but may be variable in other animal models, depending on the study design, the implant size, the channel size, the drug concentration, the surgical technique, and so on. Such a dosage was much lower when compared to those used for the treatment of osteoporosis. Our route resulted in more BPs retained by the skeleton and a lower proportion entering the circulation. A third possibility is that, when we injected ZOL into the channel drilled in the medullary canal, this drug selectively concentrated on the peri-implant sites, and directly targeted osteoclasts and osteoblasts in the bone marrow. Distribution of BPs depends on the rate of bone turnover; these drugs always bind selectively to resorption surfaces and have targeting effects [26]. In our animal model, OVX induced a larger contact region exposed to ZOL, and the activity of osteoclasts was much stronger than in normal bone [16]. Collectively, our local BP treatment method by virtue of natural HA as the carrier appears to be effective, safe, and easy to perform.
The BP used in this study was ZOL, which has been evaluated most extensively in vivo and in vitro. As was presented in these studies, ZOL has the greatest antiresorptive activity and is the most potent inhibitor of farnesyl pyrophosphate synthase activity [27]; ZOL is the only BP approved for the treatment of skeletal-related events in bone-metastatic prostate cancer across the USA and Europe [28]. Intravenous ZOL-related ONJ has been reported more commonly than other popular BPs [29, 30]. Thus, in this study we chose ZOL, which may represent a “best case scenario” for its efficiency and a “worst case scenario” for its risk. In our animal model, ZOL was slowly released from natural HA into the bone marrow, which in turn induced sustained and powerful suppression of bone destruction, even though given at a very low dose. Bilateral ovariectomy (surgically induced menopause) was believed to account for the bolstered action of intra-osseous ZOL [25], and could unmask more bone marrow cells responsive to ZOL, as described earlier [16]. On one hand, ZOL reduced bone marrow calcium, downregulated adhesion molecules on stromal cells, limited migration of inflammatory cells and secretion of pro-inflammatory cytokines, and impeded osteoclast-mediated bone loss [23, 31]. On the other hand, this drug also directly promoted bone marrow stromal cell proliferation and osteogenic differentiation, upregulated osteoblast maturation and osteoprotegerin production in the vicinity of Ti implants, and inhibited osteoblast and osteocyte apoptosis in vivo [24, 32, 33].
In our animal model, the osteoporosis-induced AGE overproduction in vivo was completely prevented by local treatment of 30 µg/implant ZOL. Gangoiti et al. investigated the action of BPs and AGEs on two osteoblast-like cell lines and found that BPs could reverse the deleterious actions of AGEs on osteoblastic cells in culture [34]. Yamagishi et al. further demonstrated that BPs not only inhibited AGE-induced oxidative stress generation but also prevented AGE-induced nuclear factor-κB activation [35]. In the present study, ZOL suppressed the accumulation of AGEs, affected bone collagen maturity and cross-linking, and in turn ameliorated bone remodeling around Ti implants in OVX rats. The possibility that blockage of the AGE signaling pathway through inhibition of protein farnesylation is involved in the action of ZOL has been suggested [36]. Interestingly, Tang et al. recently found that high doses of BPs increased the accumulation of AGEs in the cortical shaft, but at doses equivalent to those used for osteoporosis treatment there was no such effect [37]. Caution should be exercised when explaining these results. They involved oral application of BPs for one year to normal dogs, but not local, one time treatment of a very low dose of ZOL to osteoporotic animals; they focused on the AGE accumulation in the cortical bone, not in the bone marrow.
Our study had several limitations. The data were obtained from OVX rats, not from humans; the intramedullary Ti implants were unloaded; the BP investigated here was only ZOL. Additional studies are needed to obtain further insight into the benefits and the risks of BP therapy, which is important for the extensive clinical use of these drugs.
In conclusion, a local, one time, low-dose injection of ZOL at the site of implantation successfully promoted the osseointegration of Ti implants following postmenopausal osteoporosis, and this action may be partly mediated by inhibition of the osteoporosis-induced AGE overproduction in the bone marrow. These findings hold great promise for the in vivo local use of BPs, especially at the site of post menopausal osteoporosis, and suggest that AGEs may be novel therapeutic targets for postmenopausal osteoporotic patients scheduled for implantation.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Park JH, Olivares-Navarrete R, Baier RE, et al. Effect of cleaning and sterilization on titanium implant surface properties and cellular response. Acta Biomater. 2012;8:1966–75. doi: 10.1016/j.actbio.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munhoz EA, Bodanezi A, Cestari TM, et al. Long-term rabbits bone response to titanium implants in the presence of inorganic bovine-derived graft. J Biomater Appl. 2012;27:91–8. doi: 10.1177/0885328210396946. [DOI] [PubMed] [Google Scholar]
- 3.Jacobi-Gresser E, Huesker K, Schütt S. Genetic and immunological markers predict titanium implant failure: a retrospective study. Int J Oral Maxillofac Surg. 2013;42:537–43. doi: 10.1016/j.ijom.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Yazici S, Korkmaz U, Erkan M, et al. The effect of breastfeeding duration on bone mineral density in postmenopausal Turkish women: a population-based study. Arch Med Sci. 2011;7:486–92. doi: 10.5114/aoms.2011.23416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosoi T. Genetic aspects of osteoporosis. J Bone Miner Metab. 2010;28:601–7. doi: 10.1007/s00774-010-0217-9. [DOI] [PubMed] [Google Scholar]
- 6.Gabet Y, Müller R, Levy J, et al. Parathyroid hormone 1-34 enhances titanium implant anchorage in low-density trabecular bone: a correlative micro-computed tomographic and biomechanical analysis. Bone. 2006;39:276–82. doi: 10.1016/j.bone.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Jakob F, Ebert R, Ignatius A, et al. Bone tissue engineering in osteoporosis. Maturitas. 2013;75:118–24. doi: 10.1016/j.maturitas.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson JC. Prevention of osteoporosis: one step forward, two steps back. Menopause Int. 2011;17:137–41. doi: 10.1258/mi.2011.011112. [DOI] [PubMed] [Google Scholar]
- 9.Avramidis A, Polyzos SA, Moralidis E, et al. Scintigraphic, biochemical, and clinical response to zoledronic acid treatment in patients with Paget's disease of bone. J Bone Miner Metab. 2008;26:635–41. doi: 10.1007/s00774-008-0852-6. [DOI] [PubMed] [Google Scholar]
- 10.Kos M. Association of dental and periodontal status with bisphosphonate-related osteonecrosis of the jaws. A retrospective case controlled study. Arch Med Sci. 2014;10:117–23. doi: 10.5114/aoms.2014.40738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvas JSB, Pereira RMR, Caparbo VF, et al. A single dose of zoledronic acid reverses the deleterious effects of glucocorticoids on titanium implant osseointegration. Osteoporos Int. 2010;21:1723–9. doi: 10.1007/s00198-009-1125-5. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Hu J, Li Y, et al. Effects of ibandronate-hydroxyapatite on resorptive activity of osteoclasts. Arch Med Sci. 2011;7:53–60. doi: 10.5114/aoms.2011.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y, Luo E, Hu J, et al. Effect of combined local treatment with zoledronic acid and basic fibroblast growth factor on implant fixation in ovariectomized rats. Bone. 2009;44:225–32. doi: 10.1016/j.bone.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 14.Gao Y, Zou SJ, Liu XG, et al. The effect of surface immobilized bisphosphonates on the fixation of hydroxyapatite-coated titanium implants in ovariectomized rats. Biomaterials. 2009;30:1790–6. doi: 10.1016/j.biomaterials.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Lu L, Zhang W, Wu X, et al. A novel ropivacaine-loaded in situ forming implant prolongs the effect of local analgesia in rats. Arch Med Sci. 2013;9:614–21. doi: 10.5114/aoms.2012.30829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varkey M, Kucharski C, Doschak MR, et al. Osteogenic response of bone marrow stromal cells from normal and ovariectomized rats treated with a low dose of basic fibroblast growth factor. Tissue Eng. 2007;13:809–17. doi: 10.1089/ten.2006.0348. [DOI] [PubMed] [Google Scholar]
- 17.Kyrgidis A, Tzellos TG, Toulis K, et al. An evidence-based review of risk-reductive strategies for osteonecrosis of the jaws among cancer patients. Curr Clin Pharmacol. 2013;8:124–34. doi: 10.2174/1574884711308020005. [DOI] [PubMed] [Google Scholar]
- 18.Sharma D, Hamlet S, Petcu E, Ivanovski S. Animal models for bisphosphonate-related osteonecrosis of the jaws – an appraisal. Oral Dis. 2013;19:747–54. doi: 10.1111/odi.12067. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Zhu SS, Luo E, et al. Basic fibroblast growth factor suspended in Matrigel improves titanium implant fixation in ovariectomized rats. J Control Release. 2009;139:15–21. doi: 10.1016/j.jconrel.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Hein GE. Glycation endproducts in osteoporosis – is there a pathophysiologic importance? Clin Chim Acta. 2006;371:32–6. doi: 10.1016/j.cca.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Astrand J, Aspenberg P. Topical, single dose bisphosphonate treatment reduced bone resorption in a rat model for prosthetic loosening. J Orthop Res. 2004;22:244–9. doi: 10.1016/j.orthres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Yaffe A, Binderman I, Breuer E, et al. Disposition of alendronate following local delivery in a rat jaw. J Periodontol. 1999;70:893–5. doi: 10.1902/jop.1999.70.8.893. [DOI] [PubMed] [Google Scholar]
- 23.Skoglund B, Holmertz J, Aspenberg P. Systemic and local ibandronate enhance screw fixation. J Orthop Res. 2004;22:1108–13. doi: 10.1016/j.orthres.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Giger EV, Castagner B, Leroux JC. Biomedical applications of bisphosphonates. J Control Release. 2013;167:175–88. doi: 10.1016/j.jconrel.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Steinman RA, Brufsky AM, Oesterreich S. Zoledronic acid effectiveness against breast cancer metastases – a role for estrogen in the microenvironment? Breast Cancer Res. 2012;14:213–21. doi: 10.1186/bcr3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adami S, Zamberlan N. Adverse effects of bisphosphonates. Durg Safety. 1996;14:158–70. doi: 10.2165/00002018-199614030-00003. [DOI] [PubMed] [Google Scholar]
- 27.Sörensen TC, Arnoldi J, Procter P, et al. Bone substitute materials delivering zoledronic acid: physicochemical characterization, drug load, and release properties. J Biomater Appl. 2013;27:727–38. doi: 10.1177/0885328211424623. [DOI] [PubMed] [Google Scholar]
- 28.Carter JA, Botteman MF. Health-economic review of zoledronic acid for the management of skeletal related events in bone metastatic prostate cancer. Expert Rev Pharmacoecon Outcomes Res. 2012;12:425–37. doi: 10.1586/erp.12.31. [DOI] [PubMed] [Google Scholar]
- 29.Abrahamsen B. Bisphosphonate adverse effects, lessons from large databases. Curr Opin Rheumatol. 2010;22:404–9. doi: 10.1097/BOR.0b013e32833ad677. [DOI] [PubMed] [Google Scholar]
- 30.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–65. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 31.Viereck V, Emons G, Lauck V, et al. Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. Biochem Biophys Res Commun. 2002;291:680–6. doi: 10.1006/bbrc.2002.6510. [DOI] [PubMed] [Google Scholar]
- 32.Von Knoch F, Jaquiery C, Kowalsky M, et al. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials. 2005;26:6941–9. doi: 10.1016/j.biomaterials.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 33.Bellido T, Plotkin LI. Novel actions of bisphosphonates in bone: preservation of osteoblast and osteocyte viability. Bone. 2011;49:50–5. doi: 10.1016/j.bone.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gangoiti MV, Cortizo AM, Arnol V, et al. Opposing effects of bisphosphonates and advanced glycation end-products on osteoblastic cells. Eur J Pharmacol. 2008;600:140–7. doi: 10.1016/j.ejphar.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 35.Yamagishi S, Matsui T, Nakamura K, et al. Minodronate, a nitrogen-containing bisphosphonate, inhibits advanced glycation end product-induced vascular cell adhesion molecule-1 expression in endothelial cells by suppressing reactive oxygen species generation. Int J Tissue React. 2005;27:189–95. [PubMed] [Google Scholar]
- 36.Okamoto T, Yamagishi S, Inagaki Y, et al. Incadronate disodium inhibits advanced glycation end products-induced angiogenesis in vitro. Biochem Biophys Res Commun. 2002;297:419–24. doi: 10.1016/s0006-291x(02)02218-0. [DOI] [PubMed] [Google Scholar]
- 37.Tang S, Allen M, Phipps R, et al. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with resedronate or alendronate. Osteoporos Int. 2009;20:887–94. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]