Abstract
Introduction
Cardiovascular diseases are positively correlated with periodontal disease. However, the molecular mechanisms linking atherosclerosis and periodontal infection are not clear. This study aimed to determine whether Porphyromonas gingivalis lipopolysaccharide (Pg-LPS) altered the expression of genes regulating cholesterol metabolism in macrophages in the presence of low-density lipoprotein (LDL).
Material and methods
THP-1-derived macrophages were exposed to different concentrations (0.1, 1, 10 µg/ml) of LPS in the presence of 50 µg/ml native LDL. Macrophages were also incubated with 1 µg/ml LPS for varying times (0, 24, 48, or 72 h) in the presence of native LDL. Foam cell formation was determined by oil red O staining and cholesterol content quantification. CD36, lectin-like oxidized LDL receptor-1 (LOX-1), ATP-binding cassette G1 (ABCG1), and acetyl CoA acyltransferase 1 (ACAT1) expression levels were measured by western blot and qRT-PCR.
Results
Foam cell formation was induced in a time- and concentration-dependent manner as assessed by both morphological and biochemical criteria. Pg-LPS caused downregulation of CD36 and ABCG1 but upregulation of ACAT1, while LOX-1 expression was not affected (p = 0.137).
Conclusions
Pg-LPS appears to be an important link in the development of atherosclerosis by mechanisms targeting cholesterol homeostasis, namely, excess cholesterol ester formation via ACAT1 and reduced cellular cholesterol efflux via ABCG1.
Keywords: periodontitis, macrophages, CD36, acetyl CoA acyltransferase 1, ATP-binding cassette G1
Introduction
In recent decades, infection and inflammation have been shown to be involved in atherosclerosis initiation and progression [1]. Periodontal disease is one of the most prevalent infections found in humans [2]. Data from a meta-analysis [3] suggested that periodontal disease is an independent risk factor for cardiovascular diseases. Porphyromonas gingivalis (Pg), a major pathogen in periodontitis, is found in atherosclerotic plaques [4]. Moreover, Pg lipopolysaccharide (Pg-LPS) infection may exacerbate atherosclerosis pathogenesis [5]. These data suggest that Pg and Pg-LPS may invade the systemic bloodstream through the disrupted basement membrane, which is easily seen in severe periodontitis, thereby contributing to atherosclerosis progression.
The process of atherosclerosis begins with the recruitment of monocyte-derived macrophages into the arterial intima and their transformation into lipid-loaded foam cells through the uptake of modified low-density lipoprotein (LDL) [6]. An in vitro study [7] demonstrated that Pg and Pg-LPS can induce foam cell formation in murine macrophages, partly by promoting the association of LDL with macrophages and the modification of LDL, indicating a causal relationship between Pg-LPS and atherosclerosis.
Macrophage cholesterol homeostasis, which is regulated by cholesterol synthesis, influx, and efflux, plays a vital role in foam cell formation [8]. The influx pathway occurs by uptake of modified LDL mainly through scavenger receptors (SRs), among which SR-A and CD36 have been identified as being responsible for 75–90% of the uptake [9]. Additionally, lectin-like oxidized LDL receptor-1 (LOX-1), a new member of the scavenger receptor family, may also be involved in ox-LDL uptake and foam cell formation [10]. Upon excess free cholesterol (FC) delivery to the endoplasmic reticulum, FC is rapidly re-esterified by acetyl-CoA cholesterol acyltransferase 1 (ACAT1) and then stored in cytoplasmic lipid droplets, creating a characteristic “foamy” appearance [11]. Given that ACAT1-regulated re-esterification is initially beneficial for cells for preventing cell toxicity induced by FC, increased ACAT1 expression may lead to the transformation of macrophages into foam cells, accelerating atherosclerosis development in humans [12]. Apart from re-esterification, the FC efflux pathway is another major mechanism for removing cellular cholesterol. Cholesterol efflux to lipid-poor ApoA occurs specifically via ATP-binding cassette (ABC) transporter G1 (ABCG1) [13]. Pg-LPS alone has been demonstrated to induce foam cell formation by modifying cells at the level of gene expression [14, 15]. However, Pg-LPS-induced foam cell formation results from increased LDL uptake [7]. Despite the extensive studies on these topics, the underlying mechanisms responsible for foam cell formation in cells infected with LPS in the presence of LDL are not yet clear.
Here, we investigated the effects of Pg-LPS infection on the expression levels of key proteins supporting foam cell formation in the presence of native LDL in LPS-treated macrophages.
Material and methods
Cell culture
Human THP-1 monocytes obtained from the cell bank of Shanghai Institutes for Biological Sciences were seeded in 6-well plates at a density of 1 × 105 cells per well and cultured in RPMI1640 medium (Hyclone, USA) supplemented with 2 mM l-glutamine, 10% fetal bovine serum (FBS), and antibiotics at 37°C and 5% CO2. After the addition of 160 nmol/l phorbol 12-myristate 13-acetate (PMA) for 48 h, THP-1 cells were differentiated into macrophages for further studies.
Pg-LPS infection of THP-1-derived macrophages
Ultrapure LPS from Porphyromonas gingivalis (catalog #tlrl-pglps, version #10G20 MT) was purchased from InvivoGen (USA), and human LDL was purchased from Beijing Xiesheng Bio Company (China). To test the effects of Pg-LPS on foam cell formation, THP-1-derived macrophages were incubated with or without increasing concentrations of Pg-LPS (0.1, 1, 10 µg/ml) in the presence of LDL (50 µg/ml) for 48 h. In our preliminary experiments, macrophages were cultured in the presence of 50 µg/ml LDL alone, and no significant changes were found in the amount of Oil Red O-stained cells or in the content of cellular cholesterol after culture for as long as 72 h (data not shown). These data showed that LDL alone (without stimulation by LPS or PMA) did not induce foam cell formation. Therefore, cells were further stimulated with LDL (50 µg/ml) and Pg-LPS (1 µg/ml) at different time intervals (0, 24, 48, or 72 h).
Oil Red O staining
THP-1-derived macrophages were washed twice with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde for 15 min. Cells were then stained with Oil Red O (Sigma, USA) for 10 min and counterstained with hematoxylin for 1 min. The intracellular lipid droplets were stained red, while cell nuclei were stained blue under light microscopy. Macrophages with 10 or more Oil Red O-positive lipid droplets were defined as foam cells according to morphological criteria [16]. The number of foam cells was scored in three fields per well, and the percentage of foam cells was calculated according to the total number of cells counted.
Quantification of cellular cholesterol content
To further measure the formation of foam cells according to biochemical criteria, the amounts of total cholesterol (TC) and FC were quantified using the zymochemistry method as previously described [17]. Briefly, cells from each sample were disrupted by ultrasonication. Then the TC and FC contents were determined with a standard curve and expressed as µg cholesterol/mg protein. The cholesterol ester (CE) content was calculated by subtracting FC from TC. Foam cells were defined when the ratio of CE to TC was over 50% [18].
MTT assay
MTT assays were performed to determine the cytotoxicity of LPS in cells. Briefly, after 4 h of incubation with 20 µl MTT (5 mg/ml; Sigma, USA), culture medium was removed from each sample and dissolved in 150 µl of DMSO (Sigma). The absorbance was measured at 490 nm, and the viability of cells was calculated as the relative ratio of absorbance to that of control.
RNA isolation and real-time RT-PCR
Total RNA from each sample was isolated with TRIzol reagent (Invitrogen, USA) according to the manufacturer's instructions. cDNA was synthesized with M-MuLV transcriptase (Fermentas, Canada) in a 20-µl mixture containing 2 µg of RNA. The sequences of forward and reverse primers are given in Table I. Real-time PCR was then performed using SYBR premix EX Taq II buffer (Takara, Japan) on a LightCycler 480 system (Roche, USA). The amplification was carried out as follows: one cycle of 95°C for 30 s, followed by 40 cycles of 5 s at 95°C for denaturing, 20 s at 60°C for annealing, and 20 s at 75°C for extension. The relative levels of CD36, LOX-1, ACAT1, and ABCG1 were calculated according to the formula 2–ΔΔCt. The data are presented as fold changes in target gene expression normalized to β-actin and relative to the untreated control.
Table I.
Primer sequences of target genes and β-actin for real-time PCR
| Target gene | Sequences |
|---|---|
| β-actin | F 5′-CATTGCCGACAGGATGCAG-3′ |
| R 5′-CTCGTCATACTCCTGCTTGCTG-3′ | |
| CD36 | F 5′-GGTGATGATGGAGAATAAGCC-3′ |
| R 5′-AAGAGCCCAGAGTCGGAGTTG-3′ | |
| LOX-1 | F 5′-CTTGCTCGGAAGCTGAATG-3′ |
| R 5′-CCGTCCTCCCAGAGCCAT-3′ | |
| ACAT1 | F 5′-TTCTCATCCGCTGATCCGTT-3′ |
| R 5′-TAGCGACATAACCCCATCTTACAG-3′ | |
| ABCG1 | F 5′-CATTGCCGACAGGATGCAG-3′ |
| R 5′-CTCGTCATACTCCTGCTTGCTG-3′ |
Western blot analysis
After the cells were washed twice with PBS, they were lysed in lysis buffer (Beyotime, China) and incubated on ice for 30 min. The lysates were centrifuged at 12,000 rpm for 10 min at 4°C. The protein concentration was determined with the BCA assay (Beyotime). Twenty micrograms of protein from each sample was separated by electrophoresis on 10% sodium dodecyl sulfate (SDS) polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). The membranes were then probed with rabbit polyclonal anti-CD36 (1: 2000), anti-LOX-1 (1: 2000), anti-ACAT1 (1: 1500), anti-ABCG1 (1: 2000), or anti-β-actin (1: 2000) antibodies (Proteintech, China) overnight at 4°C. Subsequently, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1: 2000; Proteintech Inc) for 60 min at room temperature. Immunoreactivity was detected by enhanced chemiluminescence (ECL). The relative expression levels of target genes were normalized to the expression of β-actin.
Statistical analysis
All experiments were repeated at least three times, and data were presented as means ± standard deviations (SDs). Student's t tests were used for direct comparisons between two groups, while one-way analysis of variance (ANOVA) was used for comparisons of three or more groups, followed by Tukey's post hoc test. Differences with p-values of less than 0.05 (two-tailed) were considered statistically significant.
Results
Pg-LPS induced foam cell formation in a concentration- and time-dependent manner, according to morphological criteria
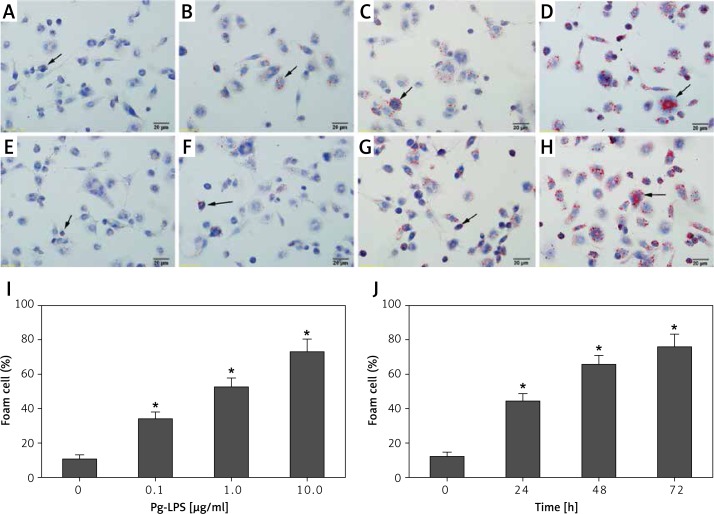
After treatment with 0.1 µg/ml Pg-LPS and LDL (Figure 1 B), foam cell formation was minimal, but was obviously higher than that obtained for the control (LDL alone), as demonstrated by Oil Red O staining of lipid droplets (Figure 1 A). However, in cells treated with higher Pg-LPS and LDL concentrations, large lipid droplet masses were observed (Figure 1 C and D). The number of foam cells significantly increased in all the Pg-LPS treated groups (Figure 1 I). These results indicated that LPS exposure caused foam cell formation in a concentration-dependent manner.
Figure 1.
Pg-LPS induced foam cell formation, according to morphological criteria. THP-1-derived macrophages were cultured with or without increasing concentrations of Pg-LPS in the presence of 50 µg/ml LDL for 48 h. A – Untreated macrophages as a control; B – 0.1 µg/ml; C – 1.0 µg/ml; D – 10.0 µg/ml. I – The number of foam cells induced by different concentrations of LPS. Moreover, incubation of macrophages with LDL (50 µg/ml) and Pg-LPS (1.0 µg/ml) was performed for different durations. E – 0 h as a control; F – 24 h; G – 48 h; H – 72 h. J – The number of foam cells induced by LPS for different durations. Black arrows indicate representative Oil Red O stained lipid droplets. Original magnification of microphotographs: 400×; scale bar = 20 µm
Next, macrophages were stimulated with 1 µg/ml Pg-LPS in the presence of LDL for increasing times. Compared to the results for uninfected macrophages (Figure 1 E), increasing numbers of Oil Red O-stained cells were observed with longer durations of treatment (Figures 1 F–H), suggesting that Pg-LPS induced foam cell formation in a time-dependent manner. Furthermore, the number of foam cells increased significantly with increase in infection times (Figure 1 J). MTT assays showed that the cell viability was greater than 95% at the highest dose and longest treatment time (data not shown), demonstrating that the treatment did not alter macrophage viability.
Pg-LPS induced foam cell formation in a concentration- and time-dependent manner, according to biochemical criteria
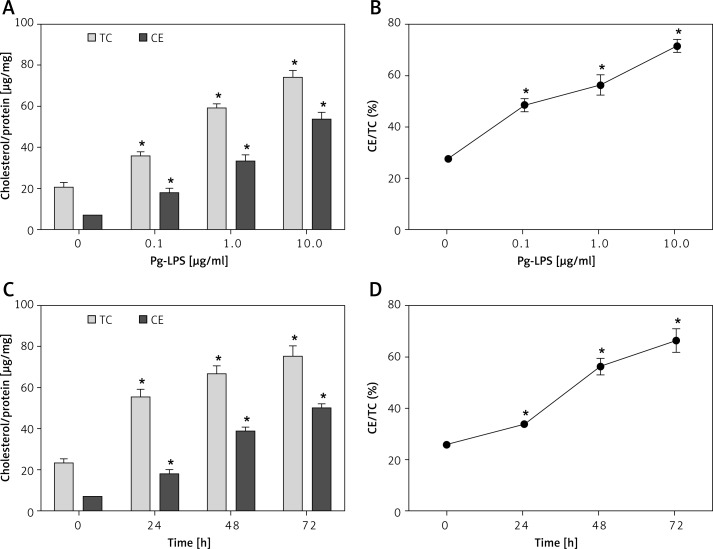
Given that excessive CE accounted for the elevated amount of lipid droplets, the cellular CE content was measured to investigate foam cell formation according to biochemical criteria. Incubation of LDL-treated macrophages with Pg-LPS caused a concentration-dependent increase in CE and TC accumulation (Figure 2 A). Moreover, treatment with 1 or 10 µg/ml LPS significantly induced foam cell formation (Figure 2 B), as assessed when using CE/TC > 50% for identification of foam cells [18]. As shown in Figure 2 C, Pg-LPS exposure induced a time-dependent increase in CE and TC content, and the CE/TC ratio was greater than 50% after 24 h of LPS exposure (Figure 2 D), which indicated foam cell formation according to biochemical criteria.
Figure 2.
Pg-LPS induced foam cell formation, according to biochemical criteria. Macrophages were exposed to increasing concentrations (0, 0.1, 1, 10 µg/ml) of Pg-LPS for 48 h in the presence of 50 µg/ml LDL (A and B). To measure time-dependent effects, cells were incubated with Pg-LPS (1.0 µg/ml) in the presence of LDL (50 µg/ml) for 0, 24, 48, or 72 h (C and D). A and C – The content of intracellular total cholesterol (TC) and cholesterol ester (CE) was normalized to protein levels and expressed as µg/mg. B and D – The ratios of CE to TC. Data are presented as means ± SDs; *P < 0.05
CD36 was downregulated by Pg-LPS treatment
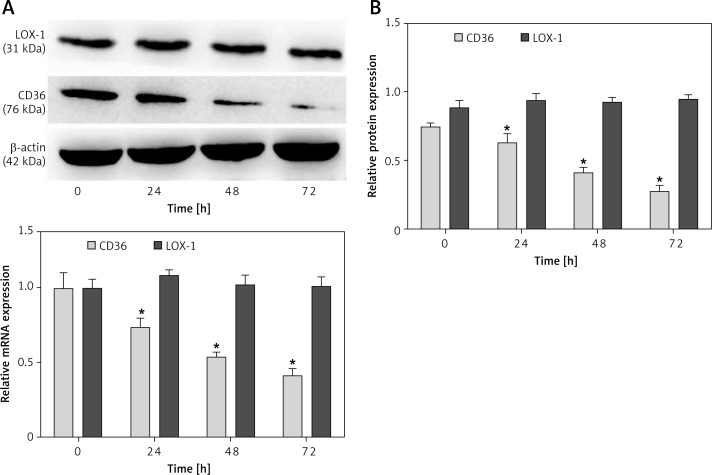
Since CD36 and LOX-1 regulate lipoprotein up-take, CD36 and LOX-1 mRNA and protein expression levels were measured to investigate the cholesterol influx pathway during Pg-LPS exposure in the presence of LDL. Pg-LPS treatment downregulated CD36 mRNA expression in a concentration-dependent manner, nearly reaching less than 50% of that of the control (10 µg/ml; Figure 3 A). Western blot analysis (Figure 3 B) revealed similar downregulation of CD36, with a dramatic 90% drop in comparison with that of the control at the highest concentration (10 µg/ml). No significant changes were found in the mRNA or protein expression of LOX-1, another SR family member, even when cells were exposed to the highest concentration of Pg-LPS applied in our study (p > 0.05).
Figure 3.
Measurement of concentration-dependent CD36 and LOX-1 expression in Pg-LPS-treated macrophages. Macrophages were exposed to increasing concentrations (0, 0.1, 1, 10 µg/ml) of Pg-LPS for 48 h in the presence of 50 µg/ml LDL. A – qRT-PCR data for CD36 and LOX-1 mRNA expression are presented as fold changes relative to the untreated control (0 µg/ml). B – Western blot analysis of CD36 and LOX-1 protein expression. The protein levels were normalized to those of β-actin. *P < 0.05
Because of treatment with Pg-LPS (1 µg/ml) in the presence of LDL, we observed a significant time-dependent decrease in CD36 expression. CD36 mRNA expression dropped to 40% of that of the control (Figure 4 A), while the protein expression fell to less than 40% of that of the control (Figure 4 B). However, there was no significant time-dependent difference in LOX-1 expression, which was consistent with the results in cells treated with different concentrations of Pg-LPS.
Figure 4.
Measurement of time-dependent CD36 and LOX-1 expression in Pg-LPS-treated macrophages. Cells were treated with Pg-LPS (1.0 µg/ ml) and LDL (50 µg/ml) for increasing durations (0, 24, 48, or 72 h). A – qRT-PCR data of CD36 and LOX-1 mRNA expression are presented as fold changes relative to the untreated control (0 h). B – Western blot analysis of CD36 and LOX-1 protein expression. The protein levels were normalized to β-actin. *P < 0.05
ACAT1 expression was upregulated, whereas ABCG1 expression was downregulated in response to Pg-LPS treatment
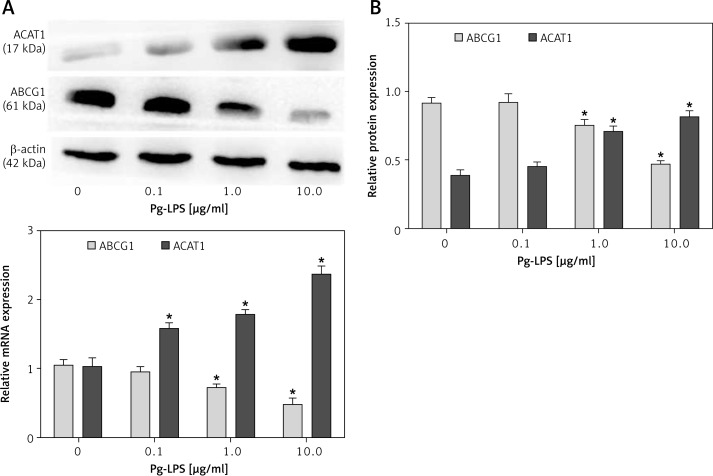
In order to determine the effects of Pg-LPS on the esterification of FC into CE and the efflux of FC [7] from macrophages co-cultured with LDL, the expression levels of ACAT1 and ABCG1 were analyzed. As shown in Figure 5 A, 10 µg/ml Pg-LPS upregulated ACAT1 mRNA expression to 2.3-fold that of the control, and this effect was concentration dependent. However, ABCG1 expression decreased to only 43% of that of the control when used at the highest concentration (10 µg/ml). Similar patterns of protein expression were observed by western blotting. At 0.1 µg/ml Pg-LPS, no changes in either ABCG1 or ACAT1 levels were observed (p > 0.05). However, the highest concentration of Pg-LPS resulted in approximately 2-fold upregulation in ACAT1 expression and 50% downregulation of ABCG1 expression (Figure 5 B).
Figure 5.
Measurement of concentration-dependent ACAT1 and ABCG1 expression in Pg-LPS-treated macrophages. Macrophages were exposed to increasing concentrations (0, 0.1, 1, 10 µg/ml) of Pg-LPS for 48 h in the presence of 50 µg/ml LDL. A – ACAT1 and ABCG1 mRNA levels are presented as fold changes relative to the untreated control (0 µg/ml). B – Western blot analysis of ACAT1 and ABCG1 protein expression. The protein levels were normalized against b-actin as an internal control. Data are presented as means ± SDs. *P < 0.05
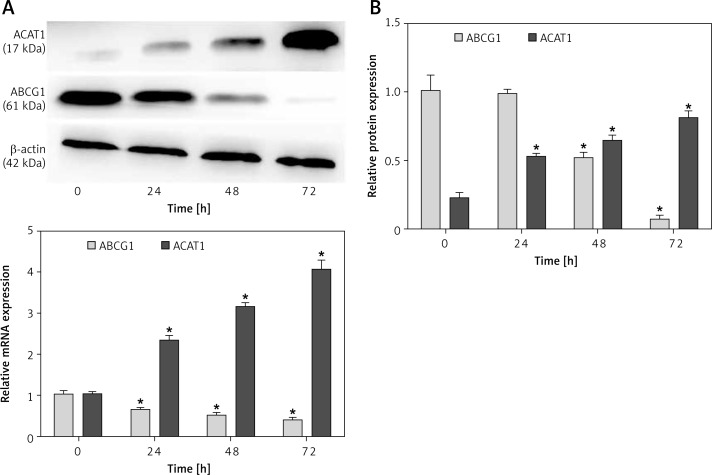
The incubation of THP-1-derived macrophages with 1 µg/ml Pg-LPS and LDL resulted in a maximal 4-fold change in ACAT1 mRNA expression and a 3-fold change in ACAT1 protein expression compared with the control level (Figure 6 A and B). In contrast, ABCG1 expression markedly decreased in a time-dependent manner. Specifically, ABCG1 protein expression was almost completely inhibited, with 93% reduction at 72 h.
Figure 6.
Measurement of time-dependent ACAT1 and ABCG1 expression in Pg-LPS-treated macrophages. Cells were treated with Pg-LPS (1.0 µg/ml) and LDL (50 µg/ml) for increasing durations (0, 24, 48, or 72 h). A – ACAT1 and ABCG1 mRNA levels are presented as fold changes relative to the untreated control (0 h). B – Western blot analysis of ACAT1 and ABCG1 protein expression. The protein levels were normalized to β-actin as an internal control. *P < 0.05
Discussion
Cumulative evidence published over the last two decades suggests a causal relationship between periodontal infection and cardiovascular diseases (CVDs). Chronic periodontitis is characterized by multiple but transient occurrences of bacteremia, which may result in the systemic spread of LPS from the oral cavity to atherosclerotic plaques, leading to the formation of foam cells. Because of the similarities between THP-1-derived macrophages and primary peripheral blood mononuclear cell-derived macrophages, THP-1-derived macrophages were exposed to LPS stimuli in the presence of low concentrations of LDL in our study. Our results indicated that Pg-LPS induced macrophage foam cell formation in a concentration- and time-dependent manner, according to both morphological and biochemical criteria, suggesting that Pg-LPS treatment increased the risk of cardiovascular damage. This was consistent with a previous finding that LPS purified from another Gram-negative bacterium, Chlamydia pneumoniae, could induce cholesterol ester accumulation and foam cell formation [19]. The LPS has been shown to increase LDL-R expression, which leads to excessive cholesterol ester accumulation [20]. Additionally, Pg-LPS can enhance the binding of LDL with macrophages and modification of LDL [7]. Collectively, these data may provide insights into the mechanisms of Pg-LPS-induced foam cell formation in the presence of LDL. Interestingly, in our study, LDL alone did not induce foam cell formation, which was inconsistent with a recent report. The different cell source may cause this inconsistency [21]. Our observation was consistent with another finding that PMA stimulated human monocyte derived-macrophage cholesterol accumulation, while there was no significant change in the total cholesterol content without PMA stimulation [22].
Thus, we proceeded to investigate the molecular mechanisms responsible for Pg-LPS-induced foam cell formation. Our study demonstrated that Pg-LPS induced a linear decrease in both CD36 gene and protein expression in a time- and concentration-dependent manner, while no significant changes were observed in LOX-1 expression. To our knowledge, this is the first study investigating the effects of Pg-LPS on LOX-1. On the other hand, some inconsistent reports describing the effects of LPS on CD36 expression in monocytes and macrophages have been published [14, 23]. The discordance in CD36 expression found between the previous study [14] and our study could be explained by the presence of LDL in our assays. First, LPS-mediated oxidative stress is known to cause LDL oxidation [24], and ox-LDL reduces LPS-mediated inflammatory effects [25], including CD36 upregulation [26]. Second, LPS has been shown to stimulate LDL-R expression and macropinocytosis in RAW264.7 cells to enhance foam cell formation in the presence of LDL [21]. CD36 downregulation may work as a compensatory mechanism for the excessive uptake of cholesterol-rich LDL via LPS-mediated LDL-R upregulation.
Upon uptake of LDL, excess FC in foam cells can either be re-esterified into CE by ACAT1 or removed from the cells through a receptor-mediated FC efflux pathway regulated by ABCA1 and ABCG1 [8]. Therefore, we further investigated the roles of ACAT1 and ABCG1 in Pg-LPS-induced foam cell formation. To the best of our knowledge, our study is the first to show that in vitro Pg-LPS treatment significantly increased ACAT1 mRNA and protein expression, but inhibited ABCG1 expression in a time- and concentration-dependent manner in THP-1-derived macrophages cocultured with LDL. More notably, LPS-induced foam cell formation and CE accumulation appeared to be associated with the upregulation of ACAT1 and downregulation of ABCG1, implying that LPS-induced foam cell formation may be influenced by enhanced synthesis of CE via ACAT1 and reduced efflux of cholesterol via ABCG1. No effects of LPS were observed on ABCG1 expression in a previous report [14]. However, our result was consistent with another previous study demonstrating that LPS infection significantly repressed ABCG1 expression [15].
Our previous study demonstrated that Pg-LPS can induce various pro-inflammatory cytokines in macrophages, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1 [27]. Many inflammatory components participate in atherosclerotic lesion development [28]. However, TNF-α has been demonstrated to specifically enhance ACAT1 expression to promote atherosclerosis [29]. Therefore, we can speculate that LPS-induced upregulation of ACAT1 may contribute to the inflammatory conditions produced by LPS.
Additionally, it is possible that ACAT1 upregulation and CD36 downregulation may be a defense mechanism against excess LDL in the culture medium; CD36 downregulation would reduce LDL uptake, and ACAT1 upregulation would help eliminate excess FC generated by LDL.
In conclusion, our study showed that a periodontal infectious agent altered the expression of key molecules regulating lipid uptake and cholesterol esterification and efflux, all of which facilitate the perturbation of cholesterol homeostasis and lead to CE accumulation and foam cell formation. This in vitro evidence further supported the positive correlation between periodontal disease and atherosclerosis via molecular mimicry. Therefore, the prevention and treatment of periodontal infection are of great importance for patients with CVDs.
Acknowledgments
Fen Liu and Yi Wang contributed equally to this paper.
This work was supported by grants from the National Natural Science Foundation of China (grant no. 81200795) and Zhejiang Provincial Natural Science Foundation of China (grant no. LY12H14003 and no. LQ12H09003).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ertek S, Cicero A. Impact of physical activity on inflammation: effects on cardiovascular disease risk and other inflammatory conditions. Arch Med Sci. 2012;8:794–804. doi: 10.5114/aoms.2012.31614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 2001;14:727–52. doi: 10.1128/CMR.14.4.727-752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphrey LL, Fu RW, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. 2008;23:2079–86. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford PJ, Gemmell E, Hamlet SM, et al. Cross-reactivity of GroEL antibodies with human heat shock protein 60 and quantification of pathogens in atherosclerosis. Oral Microbiol Immunol. 2005;20:296–302. doi: 10.1111/j.1399-302X.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu B, Cheng L, Liu D, et al. Role of p38 mitogen-activated protein kinase pathway in Porphyromonas gingivalis lipopolysaccharide-induced VCAM-1 expression in human aortic endothelial cells. J Periodontol. 2012;83:955–62. doi: 10.1902/jop.2011.110406. [DOI] [PubMed] [Google Scholar]
- 6.Yuan Y, Li P, Ye J. Lipid homeostasis and the formation of macrophage-derived foam cells in atherosclerosis. Protein Cell. 2012;3:173–81. doi: 10.1007/s13238-012-2025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi M, Miyakawa H, Kuramitsu HK. Porphyromonas gingivalis induces murine macrophage foam cell formation. Microb Pathog. 2003;35:259–67. doi: 10.1016/j.micpath.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Sekiya M, Osuga J, Igarashi M, Okazaki H, Ishibashi S. The role of neutral cholesterol ester hydrolysis in macrophage foam cells. J Atheroscler Thromb. 2011;18:359–64. doi: 10.5551/jat.7013. [DOI] [PubMed] [Google Scholar]
- 9.Kunjathoor VV, Febbraio M, Podrez EA, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–8. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka H, Kume N, Miyamoto S, et al. Expression of lectin like oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–7. doi: 10.1161/01.cir.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 11.Chang TY, Chang CC, Cheng D. Acyl-coenzyme A:cholesterol acyltransferase. Annu Rev Biochem. 1997;66:613–38. doi: 10.1146/annurev.biochem.66.1.613. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Germain SJ, Benfield PP, Gillies PJ. Gene expression of acyl-coenzyme-A:cholesterol-acyltransferase is upregulated in human monocytes during differentiation and foam cell formation. Arterioscler Thromb Vascular Biol. 1996;16:809–14. doi: 10.1161/01.atv.16.6.809. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy MA, Barrera GC, Nakamura K, et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–31. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Li XY, Wang C, Xiang XR, Chen FC, Yang CM, Wu J. Porphyromonas gingivalis lipopolysaccharide increases lipid accumulation by affecting CD36 and ATP-binding cassette transporter A1 in macrophages. Oncol Rep. 2013;30:1329–36. doi: 10.3892/or.2013.2600. [DOI] [PubMed] [Google Scholar]
- 15.Park Y, Pham TX, Lee J. Lipopolysaccharide represses the expression of ATP-binding cassette transporter G1 and scavenger receptor class B, type I in murine macrophages. Inflamm Res. 2012;61:465–72. doi: 10.1007/s00011-011-0433-3. [DOI] [PubMed] [Google Scholar]
- 16.Schaffner T, Taylor K, Bartucci EJ, et al. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980;100:57–80. [PMC free article] [PubMed] [Google Scholar]
- 17.Gamble W, Vaughan M, Kruth HS, Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978;19:1068–70. [PubMed] [Google Scholar]
- 18.Small DM. Cellular mechanisms for lipid deposition in atherosclerosis (first of two parts) New Engl J Med. 1977;297:873–7. doi: 10.1056/NEJM197710202971608. [DOI] [PubMed] [Google Scholar]
- 19.Kalayoglu MV, Byrne GI. A Chlamydia pneumoniae component that induces macrophage foam cell formation is chlamydial lipopolysaccharide. Infect Immun. 1998;66:5067–72. doi: 10.1128/iai.66.11.5067-5072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye Q, Chen Y, Lei H, et al. Inflammatory stress increases unmodified LDL uptake via LDL receptor: an alternative pathway for macrophage foam-cell formation. Inflamm Res. 2009;58:809–18. doi: 10.1007/s00011-009-0052-4. [DOI] [PubMed] [Google Scholar]
- 21.Morishita M, Ariyoshi W, Okinaga T, Usui M, Nakashima K, Nishihara T. A. actinomycetemcomitans LPS enhances foam cell formation induced by LDL. J Dent Res. 2013;92:241–6. doi: 10.1177/0022034512473309. [DOI] [PubMed] [Google Scholar]
- 22.Kruth HS, Huang W, Ishii I, Zhang WY. Macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2002;277:34573–80. doi: 10.1074/jbc.M205059200. [DOI] [PubMed] [Google Scholar]
- 23.Zamora C, Canto E, Nieto JC, Angels Ortiz M, Juarez C, Vidal S. Functional consequences of CD36 downregulation by TLR signals. Cytokine. 2012;60:257–65. doi: 10.1016/j.cyto.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Lopes-Virella MF. Interactions between bacterial lipopolysaccharides and serum lipoproteins and their possible role in coronary heart disease. Eur Heart J. 1993;14:118–24. [PubMed] [Google Scholar]
- 25.Kannan Y, Sundaram K, Aluganti Narasimhulu C, Parthasarathy S, Wewers MD. Oxidatively modified low density lipoprotein (LDL) inhibits TLR2 and TLR4 cytokine responses in human monocytes but not in macrophages. J Biol Chem. 2012;287:23479–88. doi: 10.1074/jbc.M111.320960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz-Masia D, Diez I, Calatayud S, et al. Induction of CD36 and thrombospondin-1 in macrophages by hypoxia-inducible factor 1 and its relevance in the inflammatory process. PloS One. 2012;7:e48535. doi: 10.1371/journal.pone.0048535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Wang H, Ye Q, et al. Co-regulation of LPS and tensile strain downregulating osteogenicity via c-fos expression. Life Sci. 2013;93:38–43. doi: 10.1016/j.lfs.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–76. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei L, Xiong Y, Chen J, et al. TNF-alpha stimulates the ACAT1 expression in differentiating monocytes to promote the CE-laden cell formation. J Lipid Res. 2009;50:1057–67. doi: 10.1194/jlr.M800484-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]