Abstract
Introduction
The aim of this systematic review (SR) and meta-analysis was to assess the efficacy and safety of vedolizumab in the treatment of Crohn's disease (CD).
Material and methods
A systematic literature search was conducted in Medline/PubMed, Embase and Cochrane Library until 25 January, 2015. Included studies were critically appraised according to the PRISMA protocol. Assessment in specified subgroups of CD patients and meta-analysis with Revman software were performed.
Results
Two randomized controlled trial (RCTs) were included in a meta-analysis for the induction phase of therapy: GEMINI II and GEMINI III. The clinical response was significantly higher for patients who received vedolizumab compared to placebo in the general population (risk benefit (RB) = 1.48; p = 0.0006) and in both analyzed subgroups: patients with previous failure of anti-TNFs treatment (RB = 1.51; p = 0.006) and patients naive to earlier anti-TNFs (RB = 1.41; p = 0.001). The clinical remission in the general population and subpopulation of TNF-antagonist naive patients was significantly higher for patients who received vedolizumab compared to placebo (RB = 1.77; p = 0.003; RB = 2.29; p = 0.0004; respectively). Meta-analysis for adverse events, serious adverse events (SAEs) and serious infections, revealed that vedolizumab was as safe as placebo in the induction phase of therapy.
Conclusions
The clinical response was significantly higher for patients who received vedolizumab in the general population and in both analyzed subgroups of patients. The clinical remission in the general population and subpopulation of TNF-antagonist naive patients was significantly higher for vedolizumab, but no significant differences were revealed in the subgroup of patients with previous TNF antagonist failure.
Keywords: Crohn's disease, vedolizumab, MLN-002, meta-analysis, systematic review
Introduction
Crohn's disease (CD) is one of two main forms of inflammatory bowel disease (IBD) (the second one is ulcerative colitis) [1, 2]. The primary difference between the two forms of IBD is the location and nature of the inflammatory changes. Crohn's disease is characterized by the fact that it may affect any part of the gastrointestinal tract from mouth to anus, but most frequently involves the distal small intestine and proximal colon [1]. Crohn's disease is a relapsing systemic inflammatory disease with complications such as strictures, abscesses, sinus tracts and fistulae. Crohn's disease can result in a wide variety of symptoms including abdominal pain, diarrhea and weight loss; there also occur pain and fever, which can signify development of an abscess. Choice of the initial drug for CD depends on phenotype, comorbidities, disease activity, and other individual characteristics of a patient. In most cases, fast-acting, short-term use agents (such as steroids or anti-tumor necrosis factor (TNF)) are combined with thiopurines or methotrexate. The choice of treatment should consider efficacy, side-effects and long-term complications [2].
Vedolizumab is a humanized monoclonal IgG1 antibody which inhibits migration and adhesion of leukocytes (by deactivation of α4β7 integrin) into the gastrointestinal tract. Its activity is focused on α4
7 MAdCAM-1 binding, which is expressed mostly in the gastrointestinal tract, so use of vedolizumab could provide advantages compared with use of anti-α4 antibodies (e.g. natalizumab) due to lower risk of drug-related side effects [3–6].
The aim of the systematic review and meta-analysis was to examine the efficacy and safety of vedolizumab for the treatment of CD compared to placebo; additional analyses were performed for use of the drug in specified subgroups of CD patients.
Material and methods
Literature search strategy
The search strategy was based on the medical subject heading MeSH terms and EMTREE combined with Boole's logical operators (Table I). The systematic literature review was conducted using the following main electronic databases: Medline via PubMed, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL), until 25.01.2015. Additional relevant studies were identified by searching the Cochrane IBD/FBD Review Group Specialized Trials Register, as well as websites of the British Society of Gastroenterology (BSG) and the European Crohn's and Colitis Organization (ECCO). The Cochrane Database of Systematic Reviews (CDSR), and Medline via PubMed and Embase databases were also searched for review articles. The reference lists of included studies and review articles were screened to identify additional eligible studies. Randomized controlled trials (RCTs) comparing vedolizumab with placebo in patients with Crohn's disease were included.
Table I.
MeSH subject headings and EMTREE keywords used in constructed search strategy for the primary studies
| Key words (combined with Boole's Logical Operator, AND/OR) | |
|---|---|
| Medical condition | (Crohn disease OR Crohn's Disease OR Crohns Disease OR Crohn's Enteritis OR Regional Enteritis OR Enteritis Regionalis OR Regional Enterocolitis OR Intestinal tract, Regional enteritis OR Inflammatory Bowel Disease 1 OR Granulomatous Enteritis OR Ileocolitis OR Granulomatous Colitis OR Terminal Ileitis OR Regional Ileitides OR Regional Ileitis OR Cleron disease OR Morbus Crohn) |
| Intervention | (Vedolizumab OR mln0002 OR mln 0002 OR mln 02 OR mln02 OR monoclonal antibody mln 02 OR monoclonal antibody ldp 02 OR ldp 02, ldp02 OR entyvio) |
| Methodological limits | PubMed: humans; EMBASE: humans, Embase only; Cochrane: Cochrane Central Register of Controlled Trials: No limits applied, word variations have been searched |
| Language limits | Only English publications |
Selection criteria
Studies were identified using the search strategy by two independent reviewers (P.M. and B.SL.). When there was uncertainty regarding eligibility, a third reviewer was consulted (P.K.). Study selection was based on the title and abstract and, if necessary, full-text articles. References of identified studies were hand searched for other relevant studies. All eligible RCTs were critically appraised and analyzed according to the PRISMA Statement protocol [7]. Studies were selected for inclusion in this analysis based on the following criteria: 1) randomized control trial (RTC); 2) patients treated for CD; 3) the intervention assessed was vedolizumab. Results from non-randomized or uncontrolled or open-label studies were not incorporated into the dataset. Full-text articles were included if they contained required information about study population, treatment regimen and necessary data to extract. However, non-published studies were also taken into consideration. Abstracts or posters after screening were excluded. Non-English publications were excluded.
Data extraction
Data was also extracted independently by two reviewers (P.M. and P.K.) using pre-defined data extraction forms. The following data were extracted from studies that met the inclusion criteria: characteristics of participants, intervention and regimen details, clinical endpoints, follow-up period, and study design. For the efficacy analysis, data on clinical response and clinical remission were extracted. The safety analysis included the following data: any adverse events (AEs), any serious adverse events (SAEs) and serious infections. The methodological quality of eligible RCTs was assessed using the Collaboration's recommended tool for assessing risk of bias – domain-based evaluation. In assessing the risk of bias, “ + ” is granted in the case of low risk of bias, “–” indicates a high risk of bias, while “?” indicates unclear risk of bias [8].
Statistical analysis
Data were analyzed using intention-to-treat (ITT) results from the included studies. The influence of intervention was expressed as a relative benefit (RB; described as risk ratio in the graphs presented due to specific labeling of effect measurement in the statistical analysis software) to the positive outcomes (clinical response, clinical remission) or a relative risk (RR) to the negative outcomes (the other analyzed endpoints), respectively. Meta-analysis was performed only for homogeneous data determined by degree of clinical and statistical heterogeneity. Clinical heterogeneity was assessed on the basis of characteristics of the included studies, whereas the statistical heterogeneity of the trial results was evaluated using the χ2 test and the I2 test. Heterogeneity of study results was considered statistically significant at p < 0.1, and then the random-effects model was used, while at p ≥ 0.1 the fixed-effects model was applied. The analysis used a conservative approach, in which the value of the χ2 test (p-value) below the threshold of 0.1 (the value of the low sensitivity of the test) indicates that differences are not accidental. Additionally the rated parameter I2 evaluates heterogeneity of results. A value in the range 0–40% indicates likely insignificant heterogeneity, 30–60% moderate heterogeneity, 50–90% is high inhomogeneity, and heterogeneity is 75–100%, which allows the pooled analysis of the data only if being particularly critical in the interpretation of results [9]. For other calculations statistical significance was defined at p < 0.05. We performed a meta-analysis and all statistical tests and created forest plots using Review Manager 5.3 software. The graphic presentation of meta-analyses applied a forest plot in which the squares marked results of individual studies with the horizontal lines representing confidence intervals and a rhombus indicating the results of all studies pooled (meta-analysis).
Results
Literature search – description and quality of included studies
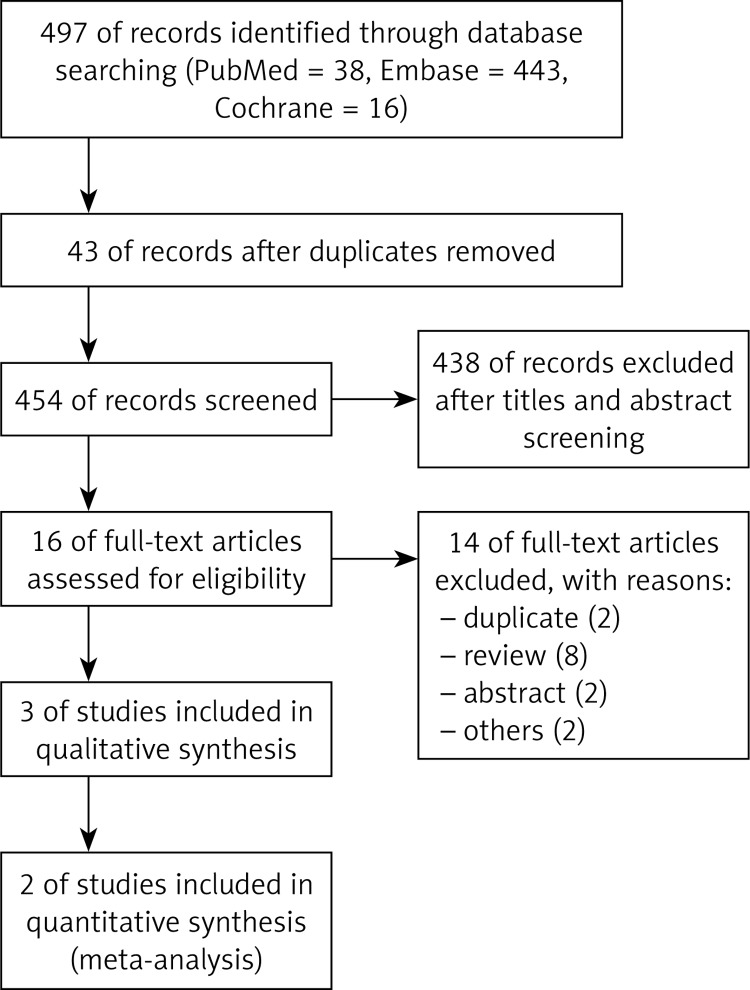
The search results of RCTs are summarized in Figure 1. A total of 497 potentially relevant studies were identified in the literature search; 43 of them were duplicates, and the other 438 studies were excluded after the screening of titles and abstracts.
Figure 1.
Search flow diagram
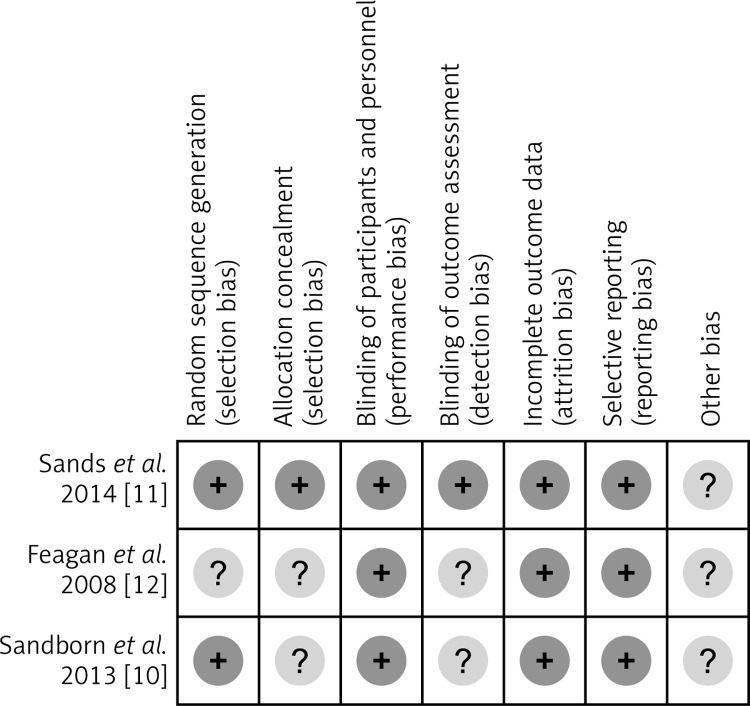
Thus, 16 full-text articles were reviewed, of which 13 were excluded and a total of 3 RCTs [10–12] were considered in the meta-analysis. Two of these three studies [10, 11] did not differ significantly in terms of population characteristics; the definitions of chosen endpoints – clinical response and clinical remission – were exactly the same and other methods were similar – these two studies were homogeneous enough to be meta-analyzed. Table II summarizes characteristic of trials included in the qualitative synthesis in the present study. The methodological quality of the meta-analyzed RCTs was evaluated as high. The probability of occurrence of bias in most studies and domains was considered low (Figure 2). Both trials were randomized and double blinded. They also provided data relating to the number of patient withdrawals.
Table II.
Characteristics of RCTs included in the systematic review (qualitative synthesis)
| Study | Participants | Intervention | Clinical endpoints | Follow-up period | Study design | Jadad quality score |
|---|---|---|---|---|---|---|
| Sandborn et al. 2013 [10] | Patients (age 18 to 80 years) with Crohn's disease for at least 3 months (score of 220 to 450 on the Crohn's Disease Activity Index (CDAI)) and with no response to or unacceptable side effects from one or more of the following: glucocorticoids, immunosuppressive agents or TNF antagonists | Vedolizumab 300 mg intravenously, n = 220 (in this group there were also patients with TNF antagonist failure, n = 105) vs. placebo intravenously, n = 148 (patients with TNF antagonist failure, n = 70); treatment was given at weeks 0 and 2 for induction therapy | Clinical remission (CDAI score of ≤ 150 points) Clinical response (CDAI-100 response: ≥ 100-point decrease in the CDAI score) |
6 weeks – induction phase (+ maintenance phase – 52 weeks) |
RCT, double-blind, placebo-controlled, phase III | 4 |
| Sands et al. 2014 [11] | Patients (age 19 to 77 years) with Crohn's disease for at least 3 months (score of 220 to 400 on the Crohn's Disease Activity Index (CDAI)) and with no response to or unacceptable side effects from one or more of the following: glucocorticoids, immunosuppressive agents or TNF antagonists | Vedolizumab 300 mg intravenously, n = 209 (in this group there were also patients with TNF antagonist failure, n = 158) vs. placebo intravenously, n = 207 (patients with TNF antagonist failure, n = 157); treatment was given at weeks 0, 2 and 6 for induction therapy | Clinical remission (CDAI score of ≤ 150 points) Clinical response (CDAI-100 response: ≥ 100-point decrease in the CDAI score) |
6 weeks – induction phase | RCT, double-blind, placebo-controlled, phase III | 4 |
| Feagan et al. 2008 [12] | Adult patients (age 23 to 52 years) with endoscopic, histopathologic, or radiologic documentation of Crohn's disease of the ileum and/or colon and a Crohn's Disease Activity Index (CDAI) score of 220 to 400 at screening | Vedolizumab 2.0 mg/kg (n = 65) or 0.5 mg/kg (n = 62) intravenously vs. an identical-appearing placebo (n = 58); treatment was given on days 1 and 29 | Primary end point: the rate of clinical response at day 57, defined as a 70-point or higher decrement in the CDAI score from baseline | 57 days | RCT, double-blind, placebo-controlled, phase II | 4 |
Patients with TNF antagonist failure – patients who reported no response and/or poor tolerance of prior treatment with TNF antagonists.
Figure 2.
Risk of bias summary
Clinical remission
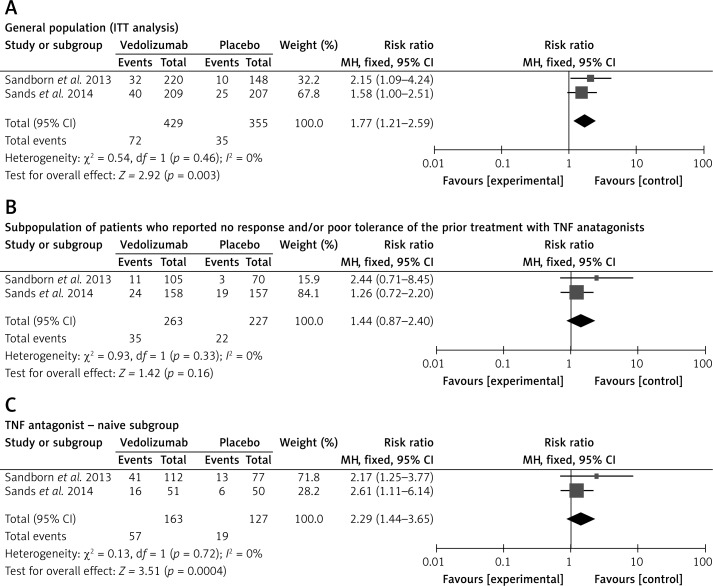
Clinical remission was defined in both reference studies as a Crohn's Disease Activity Index (CDAI) score of ≤ 150 points. There was no evidence of significant heterogeneity when data from the studies were pooled; therefore, a fixed-effect model was used for the analysis of clinical remission. The meta-analysis revealed that clinical remission was significantly higher for patients who received vedolizumab as compared to control patients (Figure 3 A). Subgroup analysis for the pooled clinical remission rates was performed according to the earlier response of patients to TNF antagonist treatment. The subpopulation of patients who previously reported no response and/or poor tolerance of the treatment with TNF antagonists was also analyzed; the results of the meta-analysis showed no statistically significant differences in clinical remission between groups treated with vedolizumab or placebo (Figure 3 B). The meta-analysis in the subpopulation of patients naive to earlier TNF antagonist treatment revealed that clinical remission was significantly higher for patients who received vedolizumab as compared to control patients (Figure 3 C).
Figure 3.
Forest plot of meta-analysis for vedolizumab vs. placebo of clinical remission: A – in the general population (ITT analysis) at week 6, B – in the subpopulation of patients who reported no response and/or poor tolerance of the prior treatment with TNF antagonists at week 6, and C – in the TNF antagonist-naive subgroup at week 6
Clinical response rate
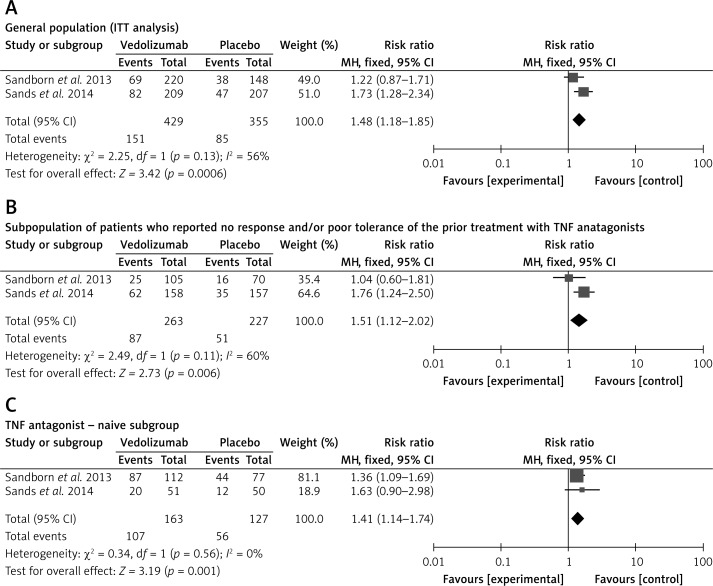
Clinical response was defined as a ≥ 100-point decrease in the CDAI score from output values in both analyzed studies. In the general population the clinical response to vedolizumab was higher than in the placebo groups (Figure 4). The fixed-effect model was used for analysis of the clinical response because there was no evidence of significant heterogeneity when data from the studies were pooled. Results of the meta-analysis showed that clinical remission was significantly higher for patients who received vedolizumab as compared to control patients (Figure 4 A). The meta-analysis in subpopulations of patients revealed that clinical response was significantly higher for patients who received vedolizumab as compared to control patients in both subgroups: patients with previous failure of TNF antagonist treatment (Figure 4 B) and patients naive to earlier TNF antagonist treatment (Figure 4 C).
Figure 4.
Forest plot of meta-analysis for vedolizumab vs. placebo of response to treatment A) in the general population (ITT analysis) at week 6, B) in the subpopulation of patients who reported no response and/or poor tolerance of the prior treatment with TNF antagonists at week 6, and C) in the TNF antagonist-naive subgroup at week 6
Adverse events
A summary of general adverse events (AEs) and serious adverse events (SAEs) is shown in Table III. Fewer patients treated with vedolizumab discontinued therapy due to AEs than those receiving placebo in the induction phase. The risk of discontinuation of the intervention due to SAEs was comparable between vedolizumab and control groups. Moreover, there were no differences between vedolizumab and placebo related to the risk of drug-related AEs and drug-related SAEs (Table III).
Table III.
Summary of safety profile
| End point | Reference | Vedolizumab n/N (%), N | Placebo n (%), N |
|---|---|---|---|
| Any adverse event | Sandborn et al. 2013 | 124/220 (56.0) | 88/148 (59.0) |
| Sands et al. 2014 | 117/209 (56.0) | 124/207 (60.0) | |
| Drug-related adverse event | Sandborn et al. 2013 | ND | ND |
| Sands et al. 2014 | 34/209 (16.0) | 34/207 (16.0) | |
| Discontinued because of adverse events | Sandborn et al. 2013 | ND | ND |
| Sands et al. 2014 | 4/209 (2.0) | 8/207 (4.0) | |
| Serious adverse events: | Sandborn et al. 2013 | 20/220 (9.0) | 9/148 (6.0) |
| Sands et al. 2014 | 13/209 (6.0) | 16/207 (8.0) | |
| Drug-related serious adverse event | Sandborn et al. 2013 | ND | ND |
| Sands et al. 2014 | 1/209 (< 1.0) | 1/207 (< 1.0) | |
| Discontinued because of serious adverse events | Sandborn et al. 2013 | ND | ND |
| Sands et al. 2014 | 4/209 (2.0) | 5/207 (2.0) | |
| Serious infection | Sandborn et al. 2013 | 1/220 (< 1.0) | 2/148 (1.0) |
| Sands et al. 2014 | 2/209 (< 1.0) | 0/207 (0.0) |
ND – no data.
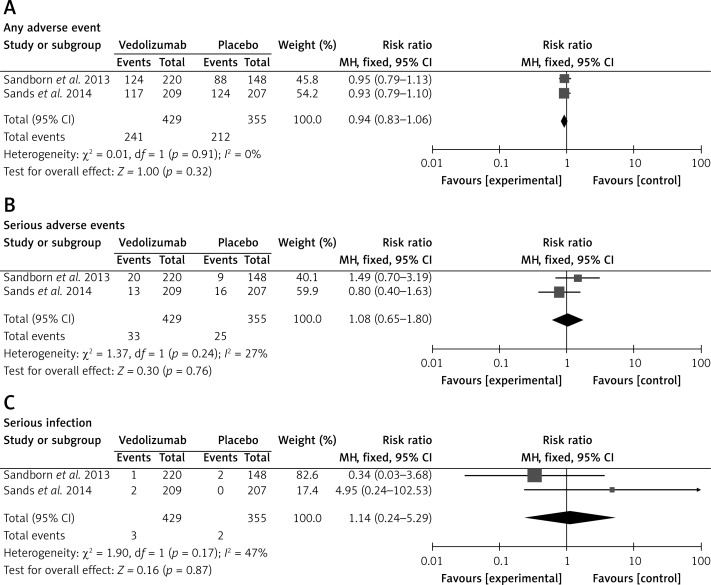
A statistical analysis was performed for AEs, SAEs and serious infections occurring during the induction phase of treatment of CD with vedolizumab compared with placebo. In the intervention groups, the frequency of any AEs was similar to the control groups (Figure 5). There was no evidence of significant heterogeneity when data from the studies were pooled; therefore a fixed-effect model was used for analysis of the AEs. The meta-analysis revealed that risk of AEs was not significantly different for patients who received vedolizumab as compared with control patients (Figure 5 A). In the case of SAEs and serious infections, the results of the meta-analysis showed no significant differences between the groups (Figures 5 B and C).
Figure 5.
Forest plot of meta-analysis of adverse events for vedolizumab vs. placebo: A – any adverse event, B – serious adverse events, C – serious infection (ITT analysis)
Discussion
Crohn's disease is difficult to treat, and a large percentage of patients with active Crohn's disease do not have a response to available treatments at all or have an initial response that is not sustained [13–16].
Although natalizumab, a humanized monoclonal antibody that inhibits α4 integrin, is effective in inducing remission in patients with CD [17, 18], it is used rarely [17–19] because of the risk of progressive multifocal leukoencephalopathy (PML) [20]. Vedolizumab is gut-specific [21, 22], and no cases of PML have been reported with its use [23], so it is one of the potential benefits in the safety profile over natalizumab in CD treatment.
The results of this systematic review and meta-analysis including two RCTs [10, 11] showed that the clinical response and remission were significantly higher for patients with CD treated with vedolizumab as compared to control patients, except for the subgroup of patients with previous TNF antagonist failure, for which no significant differences in clinical remission were revealed. Moreover, AEs, SAEs or serious infections were not more common in vedolizumab-treated patients than control patients.
Importantly, our study is the first to focus not only on use of vedolizumab in the general CD patient population but also in suitable subgroups of patients. The results of our study suggested that although vedolizumab can be an accurate therapeutic approach for CD patients, the best clinical effect will be observed for patients naive to previous TNF antagonist treatment, and unfortunately for patients with anti-TNF failure the results will not be so unequivocal. That fact may be a key in the future therapeutic approach of CD treatment.
Generally, the results of this study demonstrated the superiority of vedolizumab over placebo in patients with CD. Our analysis supports the use of vedolizumab for the treatment of CD.
It should be mentioned that the study by Feagan et al. [12] – which we excluded from the meta-analysis – was included in the meta-analysis by Wang et al. with the same two RCTs that were included by us [24]. The main reasons why we excluded the Feagan et al. study were the small number of study participants, the narrow age range of patients (23–52 years) and, what is the most important, the follow-up period and drug dosing regimen being completely inconsistent with the other two RCTs (administration of vedolizumab due to summary of product characteristics was applied only in studies 10-11; Table II); our meta-analysis revealed results of the therapy according to the authorized dosage and therapy regimen, unlike that of Wang et al. Heterogeneity in dosage and regimen between trials makes the results of the meta-analysis more biased and less relevant to therapy used in real everyday clinical practice.
Moreover, as has been mentioned before, the difference between the Wang et al. study of 2014 [24] and our study is that the former does not contain separate meta-analyses for efficacy of vedolizumab in subgroups of patients previously treated or untreated with TNF antagonists. This issue appears to be very important despite the fact that there is a significant difference between vedolizumab and placebo groups in clinical remission level for the general population of patients (RB = 1.77; 95% CI: 1.21–2.59; p = 0.003); there is no statistically significant difference in clinical remission for patients who previously reported no response and/or poor tolerance of the treatment with TNF antagonists (Figure 2). Aggregated results for clinical remission for the overall population were similar when comparing Wang et al. and our results (RR = 1.71 vs. 1.77, respectively), and we confirmed the conclusion of a significant difference between vedolizumab and placebo for the endpoint.
This systematic review and meta-analysis includes both limitations and strengths. The primary limitation is the fact that only two RCTs were available for inclusion. The calculations in the meta-analysis were based on published study results instead of individual patient data, which may generate bias. The included studies differed from each other in terms of the follow-up periods – but only results for the induction phase (6 weeks of treatment in both clinical trials) were aggregated and analyzed in our study. Another important limitation of this review is the length of the follow-up period in the case of safety profile (6 weeks), which was not long enough to evaluate all possible adverse events of therapy; further studies on this issue should be performed for a longer follow-up.
The strengths of this systematic review and meta-analysis include strict methodology based on the methods and recommendations from the PRISMA Statement [7] concerning a clear search strategy and predefined inclusion criteria for studies for the systematic review and meta-analysis. Data extraction and calculations were conducted independently by two authors. Furthermore, only RCTs were taken into consideration in this paper. The analyses were performed on the basis of ITT data, and depending on the heterogeneity of data, an appropriate statistical model was applied (fixed or random). Clinical outcomes were assessed using exactly the same definitions in both studies. Other important strengths of this meta-analysis concern the large number of patients included in the trials, and also the high quality of the eligible studies.
Despite the above-mentioned limitations, this systematic review and meta-analysis made it possible to compare the effectiveness of vedolizumab with placebo in patients with CD.
In conclusion, the results of this systematic review and meta-analysis demonstrated that vedolizumab therapy has a beneficial effect in the treatment of CD, when compared with placebo. Based on the safety analysis, there was no evidence for an increase in the incidence of any adverse events. This analysis supports the use of vedolizumab for the treatment of CD.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Assadsangabi A, Lobo AJ. Diagnosing and managing inflammatory bowel disease. Practitioner. 2013;257:13–8. [PubMed] [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 3.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 4.Yonekawa K, Harlan JM. Promises and limitations of targeting adhesion molecules for therapy. In: Ley K, editor. Adhesion molecules: function and inhibition. Basel (Switzerland): Birkh user Verlag AG; 2007. pp. 289–330. [Google Scholar]
- 5.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the madcam-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153:517–28. [PubMed] [Google Scholar]
- 6.Tilg H, Kaser A. Vedolizumab, a humanized mab against the alpha4beta7 integrin for the potential treatment of ulcerative colitis and crohn's disease. Curr Opin Investig Drugs. 2010;11:1295–304. [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Cochrane Collaboration and John Wiley; 2008. [Google Scholar]
- 9.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711–21. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 11.Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–27. doi: 10.1053/j.gastro.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Feagan BG, Greenberg GR, Wild G, et al. Treatment of active Crohn's disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 2008;6:1370–7. doi: 10.1016/j.cgh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med. 2007;357:239–50. doi: 10.1056/NEJMoa062897. [Erratum, N Engl J Med 2007; 357: 1357]. [DOI] [PubMed] [Google Scholar]
- 15.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomized trial. Lancet. 2002;359:1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 16.Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease: a meta-analysis. Ann Intern Med. 1995;123:132–42. doi: 10.7326/0003-4819-123-2-199507150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med. 2005;353:1912–25. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 18.Targan SR, Feagan BG, Fedorak RN, et al. Natalizumab for the treatment of active Crohn's disease: results of the encore trial. Gastroenterology. 2007;132:1672–83. doi: 10.1053/j.gastro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh S, Goldin E, Gordon FH, et al. Natalizumab for active Crohn's disease. N Engl J Med. 2003;348:24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- 20.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–80. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 21.Fedyk ER, Wyant T, Yang LL, et al. Exclusive antagonism of the alpha4 beta7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm Bowel Dis. 2012;18:2107–19. doi: 10.1002/ibd.22940. [DOI] [PubMed] [Google Scholar]
- 22.Haanstra KG, Hofman SO, Lopes Estêvão DM, et al. Antagonizing the alpha4beta1 integrin, but not alpha4beta7, inhibits leukocytic infiltration of the central nervous system in rhesus monkey experimental autoimmune encephalomyelitis. J Immunol. 2013;190:1961–73. doi: 10.4049/jimmunol.1202490. [DOI] [PubMed] [Google Scholar]
- 23.Gledhill T, Bodger K. New and emerging treatments for ulcerative colitis: a focus on vedolizumab. Biologics. 2013;7:123–30. doi: 10.2147/BTT.S30416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang MC, Zhang LY, Han W, et al. PRISMA – efficacy and safety of vedolizumab for inflammatory bowel diseases. A systematic review and meta-analysis of randomized controlled trials. Medicine. 2014;93:e326. doi: 10.1097/MD.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]